Abstract
Maternal obesity and/or high‐fat diet during pregnancy predispose the offspring to metabolic disease. It is however unclear how pre‐natal and post‐natal exposure respectively affect the risk of hepatic steatosis and the trajectory towards non‐alcoholic steatohepatitis in the offspring. We investigate hepatic lipid metabolism and how these factors are related to metabolic outcome in new born and young rats. Rat dams were exposed to a high‐fat/high sucrose (HFHS) diet for 17 weeks prior to mating and during pregnancy. After birth, female offspring were killed and male offspring were cross‐fostered, creating four groups; Control‐born pups lactated by control (CC) or HFHS dams (CH) and HFHS‐born pups lactated by control (HC) or HFHS dams (HH). At 4 weeks of age, pups were killed and metabolic markers in plasma were assayed, together with hepatic lipid composition and expression of relevant genes. Female HFHS neonates had smaller livers at birth (P < .05), a reduced hepatic lipid content (P < .05) and altered lipid composition. The post‐natal environment dominated the metabolic profile in the male offspring at 4 weeks of age. Offspring exposed to a HFHS environment post‐natally had increased adiposity (P < .0001), increased hepatic triacylglycrol accumulation (P < .0001), and an altered lipid profile with elevated n‐6 polyunsaturated fatty acid (PUFA) levels (P < .0001) and a reduction in ceramide (P < .001) and monounsaturated fatty acid (MUFA) (P < .0001). In summary, maternal HFHS diet during gestation affects the hepatic lipid profile in neonates. The pre‐natal exposure becomes less pronounced in young male offspring at 4 weeks of age, where the post‐natal diet has the largest impact.
Keywords: fetal programming, hepatic lipid profile, obese‐prone rats, steatosis
1. INTRODUCTION
Obesity and the associated metabolic disorders have become a worldwide epidemic. Consequently, an increasing number of children are born by obese mothers. 1 , 2 Human epidemiological studies reveal that an unhealthy diet during pregnancy increases the risk of obesity, type‐2 diabetes, hyperlipidaemia, hypertension and non‐alcoholic fatty liver disease (NAFLD) in the offspring. 1 , 3 , 4 Exposure to maternal obesity and/or high‐fat diet during fetal life causes very early aberrations in lipid metabolism, manifested as hepatic lipid accumulation, which has been observed in human neonates, 5 , 6 as well as in animal models. 7 , 8 Thus, from the time of birth, these offspring have an ectopic lipid accumulation, with a potential trajectory towards NAFLD and metabolic disease later in life.
Hepatic steatosis is per se considered a benign and reversible condition, but it can lead to accumulation of lipotoxic intermediates, such as ceramides and free fatty acids (FFA). 9 Ceramides attenuate insulin signalling, induce apoptosis, can cause formation of reactive oxygen species and are involved with inflammatory response in the liver. 10 Increased ceramide accumulation has therefore been suggested to be a major factor involved in the progress from NAFLD, to the pathological state non‐alcoholic steatohepatitis (NASH). 10 Elevated circulating palmitate (saturated fatty acids, FA) levels also attenuate hepatic insulin‐receptor signalling, 11 while palmitoleate (monounsaturated fatty acid, MUFA) improves insulin actions. 12 Oleate (MUFA) has both been shown to reduce insulin signalling when perfused into rat livers ex vivo, 11 but also to improve the detrimental effect of palmitate on hepatic insulin signalling in vitro. 13 This suggests that early impaired programming of hepatic lipid homeostasis may affect long‐term hepatic insulin sensitivity. Therefore, only considering effects on overall hepatic triacylglycrol (TAG) levels, and not the single bioactive lipids, may be misleading when evaluating metabolic disease risks.
In many animal studies of early‐life programming, the maternal diets during pregnancy are maintained throughout lactation, however exposure during various critical windows during fetal and post‐natal development result in different phenotypic outcomes. 14 , 15 Here, we used a cross‐fostering model to discriminate between pre‐natal and post‐natal effects from the maternal high‐fat/high sucrose (HFHS) diet on metabolic parameters in the offspring. We investigate accumulation and composition of hepatic lipids, the expression of genes involved in hepatic lipid and fatty acid metabolism and how these factors are related to the metabolic outcome at 4 weeks of age.
2. RESULTS
2.1. Maternal metabolic phenotype
Female rats were fed a control or a HFHS diet for 17 weeks prior to mating. During this period, the HFHS and control rats had a comparable weight gain, and no differences in blood glucose, plasma insulin, leptin or TAG were seen at week 17 (Table S1). Weight gain during pregnancy as well as body weight and adiposity at weaning, were alike in the two groups. Blood glucose and plasma insulin were also similar at weaning, although there was a tendency toward increased leptin (4.54 ± 1.43 ng/mL vs 7.07 ± 2.19 ng/mL, P = .06) and decreased plasma TAG (0.97 ± 0.23 mmol/l vs 0.66 ± 0.15 mmol/l, P = .07) in the HFHS group (Table S1).
2.2. Metabolic phenotype of female offspring at birth
All pups were weighed the morning after birth (female control n = 33; female HFHS n = 13; male control n = 35; male HFHS n = 20). Females were smaller than males (P < .01), but HFHS‐ and control‐born pups had similar weights (Figure 1A). The HFHS female pups had smaller livers than female controls (199 ± 35 mg vs 173 m ± 31 mg, P < .05), but weights of brown adipose tissue (BAT) and the heart were comparable to controls (Table S2). The maternal HFHS diet had a substantial impact on circulating metabolic markers in the female neonates. Female HFHS pups had a 25% increase in blood glucose (P < .05) without affecting the circulating insulin level, five‐fold increase in plasma leptin (P < .05) and finally plasma TAG concentration was 72% reduced (P < .05) compared to the control‐born offspring (Figure 1B).
Figure 1.
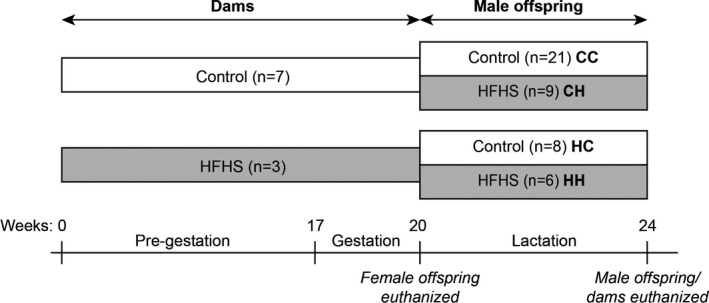
The metabolic profile of female offspring at birth. A, Body weight of offspring at birth (female control, n = 32; male control, n = 27; female HFHS, n = 12; male HFHS, n = 19). B, Blood glucose and plasma TAG, leptin and insulin levels in the female offspring the day after birth (for glucose: control, n = 30; HFHS, n = 12; for TAG, leptin and insulin: control, n = 7–9; HFHS, n = 2–4). C, Lipid profiles in livers from two female pups/dam (TAG, FFA and PL content and composition, and ceramide content, see Figure S1 and Table S2 for additional data) (control, n = 12; HFHS, n = 6). D,E, Comparison of the hepatic lipid composition of offspring and the lipid profile of their mother's milk in a PLS analysis. Gender and diet effects on body weight were estimated by 2‐way ANOVA, and groups compared by t‐test. * P < .05
2.3. Hepatic lipid metabolism in female offspring at birth
Due to the change in liver size between HFHS and control‐born pups, we investigated their hepatic lipid composition. Hepatic TAG and ceramide content were reduced by 69% (P < .05) and 20% (P < .05), respectively (Figure 1C), in the neonates from HSHF‐fed dams compared to control born, without changes in FFA or phospholipid (PL) content (Table S2). The hepatic lipid composition in TAG, PL and FFA differed between the HFHS and control neonates (a detailed hepatic lipid composition can be found in Figure S1), but it appeared to be closely related to the lipid composition of their mother's milk, which we sampled from the stomach content of the offspring. We therefore compared the hepatic and milk lipid composition in a PLS regression analysis (Figure 1D‐E, data included in the analysis can be found in Table S2). The PLS demonstrated that n‐6 polyunsaturated fatty acid (PUFA) content in the TAG, PL and FFA lipid fractions dominated the hepatic lipid profile of HFHS offspring and the HFHS milk composition. Additionally, higher levels of MUFA in TAG, PL and FFA, and saturated FFA in TAG appeared in offspring liver and mother's milk of controls. Generally, the milk and TAG lipid composition was very similar in all lipid classes (MUFA, n‐3 PUFA, n‐6 PUFA and saturated FA). Even though the lipid composition of the milk varied, the total fatty acid content (243 ± 34 mg/g in control and 242 ± 19 mg/g in HFHS) and leptin levels (2.92 ± 1.06 pg/mg in control and 3.52 ± 1.22 pg/mg in HFHS) were alike.
2.4. Metabolic phenotype of male offspring at 4 weeks of age
After birth, male offspring were cross‐fostered; control‐born pups were lactated by control‐ (CC) or HFHS‐fed (CH) dams and HFHS‐born pups were lactated by control‐ (HC) or HFHS‐fed dams (HH) (Figure 2). The litter size was normalised for each dam (4–6 pups/dam), since offspring growth is litter size dependent. 16 We also evaluated and confirmed that litter size was a non‐significant covariant on body weight in the offspring at weaning in this study (ANCOVA P = .30). At 4 weeks of age, all pups were killed, blood was collected for plasma analysis, and livers were obtained for lipid profiling and gene expression. All data were analysed in a principal component analysis (PCA). The PCA was setup to investigate: (i) if the four offspring groups differed from each other in metabolic profiles, and if so, (ii) which groups had the most similar metabolic profiles and (iii) which parameters characterise the metabolic profile in each of the groups. The following data were loaded into the analysis: body weight, organ weight (liver, epididymal adipose tissue), plasma parameters (glucose, insulin, leptin, TAG), hepatic lipid profile of FFA, TAG and PL (a detailed lipid profile for TAG, FFA and PL can be found in Figure S2), ceramide content, hepatic lipid metabolism gene expression (Cd36, Acaca, Acox, Scd1, Fasn, Fads1, Fads2, Elovl5, Cpt1a, Mlxipl(ChREBP), Srebf1) and hepatic inflammatory gene expression (Il1b, Tnf, Ccl2(MCP‐1), Cd68, Cd163) (Figure 3A, all data included in the PCA can be found in Table S3). The analysis showed that the post‐natal environment had the strongest impact on the metabolic profile at weaning, since CC/HC and CH/HH separated along PC1 on the x‐axis. However, the pre‐natal environment was also reflected along PC2, as shown by CC/CH separating from HC/HH on the y‐axis (Figure 3B).
Figure 2.
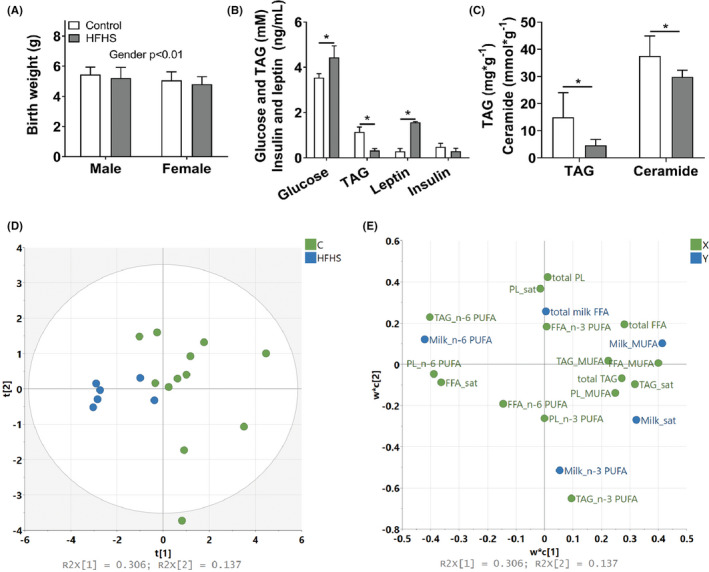
Study design. Female rats were fed a control or high‐fat/high sucrose diet for 17 wk prior to mating. Female offspring were killed the day after birth, and male offspring were cross‐fostered between chow and HFHS‐fed dams. Male offspring and dams were killed at weaning. Blood and tissues were collected from all for analysis
Figure 3.
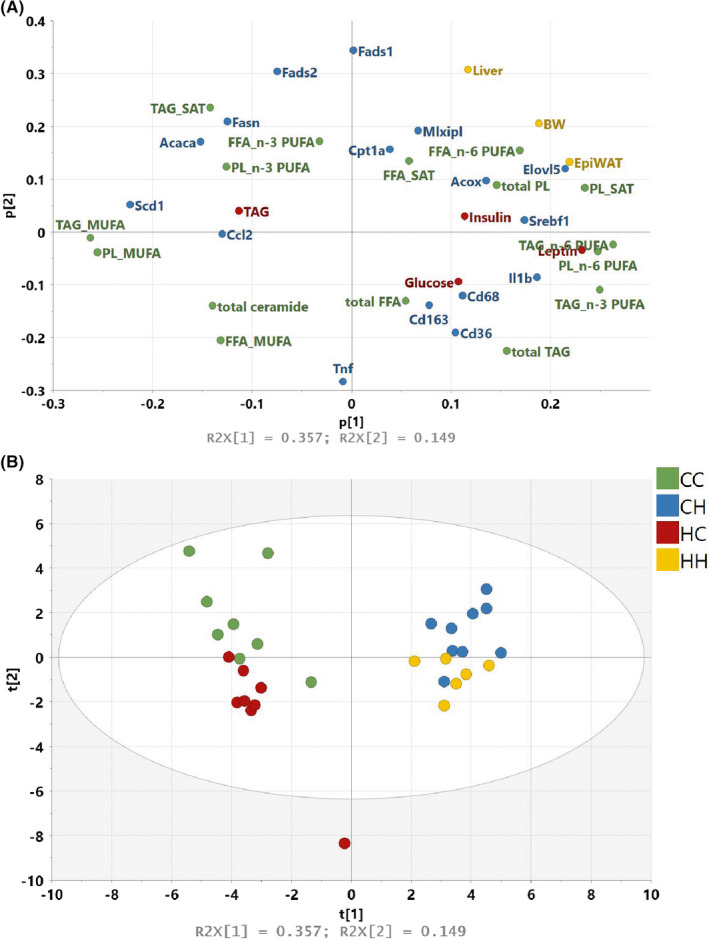
PCA analysis of all data from male offspring at 4 wk of age. A, The loading plot contains all data included in the analysis. Parameters are coloured according to data origin: yellow, body and organ weights; red, plasma markers; green, hepatic lipid profile; blue, hepatic gene expression. B, The score plot demonstrates how the male offspring separate according to their overall metabolic profile (CC, n = 8; CH, n = 9; HC, n = 8; HH, n = 6)
2.5. The influence of post‐natal environment on the metabolic profile
The post‐natal environment separated the data along PC1 in the score plot and is therefore the strongest driver of the metabolic profile in the male offspring at weaning. When examining the loading plot (Figure 3A), the HFHS post‐natal profile was characterised by increased adiposity (P < .0001) and body weight (P < .0001, Figure 4A,B), however, pre‐natal exposure to a HFHS diet resulted in a reduced adipogenic response post‐natally (CH vs HH, P < .05 and CC vs HC, P < .05, pre‐natal effect in 2‐way ANOVAP < .0001). Elevated adiposity was associated with increased circulating leptin levels in the post‐natal HFHS offspring (P < .0001, Figure 4C). Insulin levels were also elevated in HFHS post‐natal groups (P < .0001), and the CH offspring had furthermore increased blood glucose (Figure 4D,E). On the contrary, HC offspring also had elevated blood glucose, but this impairment did not result in raised insulin levels.
Figure 4.
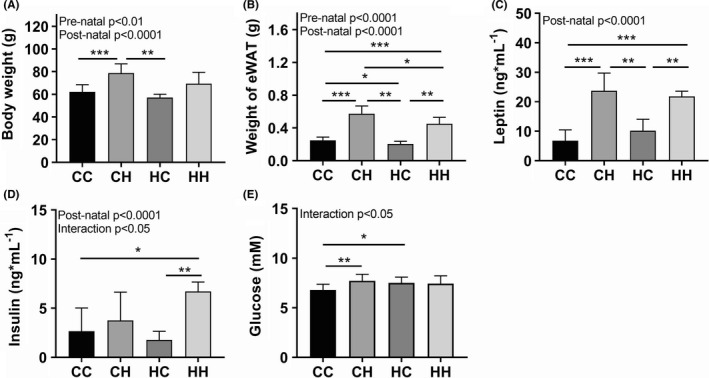
Metabolic phenotype of male offspring at 4 wk of age. Male offspring stayed with the indicated foster dams until 4 wk of age, where the study was terminated. A, Body weight and B, weight of epididymal adipose tissue. Circulating levels of (C) leptin, (D) insulin and (E) glucose (CC, n = 21; CH, n = 9; HC, n = 8; HH, n = 6). For additional metabolic data see Table S3. Pre‐natal and post‐natal effects were estimated by 2‐way ANOVA and group‐wise comparisons by t‐test. * P < .05, ** P < .01, *** P < .001
High fat/high sucrose lactated offspring had ectopic TAG accumulation in the liver (Figure 5A), which was associated with an elevation in expression of inflammatory genes according to the PCA results (Figure 3A). However, many specific lipid species were also affected by the post‐natal HFHS diet. The PCA analysis highlighted n‐6 PUFA in FFA, TAG and PL, n‐3 PUFA in TAG and saturated fatty acids in PL as being the most affected lipids. The n‐6 PUFA content was elevated in the post‐natal HFHS groups (Figure 5B and Table S3). Along with the changes in PUFAs, we also observed elevated Elovl5mRNA expression in post‐natal HFHS (Figure 5C), which is the enzyme responsible for elongating LC‐PUFAs. MUFA content in FFA, TAG and PL was decreased in the post‐natal HFHS offspring (Figure 5D and Table S3), and the reduction was associated with a complete absence of Scd1 mRNA level (Figure 5E), which encodes the enzyme that converts saturated fatty acids to MUFA. Finally, hepatic ceramide content appeared to be decreased in the post‐natal HFHS pups, without an apparent effect from the pre‐natal environment (Figure 5F).
Figure 5.
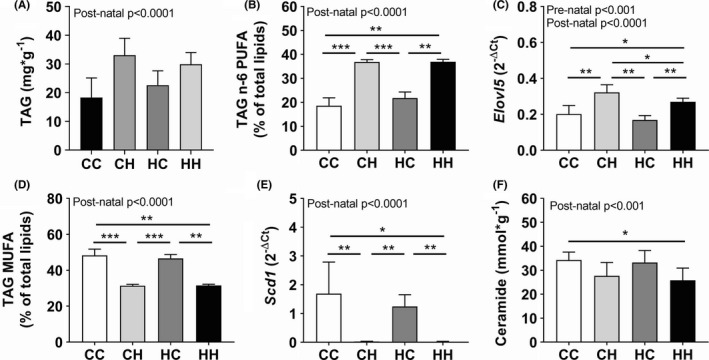
Hepatic lipid profile in male offspring at 4 wk of age. Livers were collected and a lipid profile was performed. A, TAG content, B, n‐6 PUFA content in TAG and C, and the expression of Elovl5, the gene responsible for elongating LC‐PUFA. D, MUFA content in TAG and E, and the expression of Scd1, that encodes the enzyme which convert saturated FA to MUFA. F, Ceramide content. (CC, n = 8; CH, n = 9; HC, n = 8; HH, n = 6). For additional lipid data see Table S3. Pre‐natal and post‐natal effects were estimated by 2‐way ANOVA and group‐wise comparisons by t‐test * P < .05, ** P < .01, *** P < .001
2.6. The influence of pre‐natal environment on the metabolic profile
While most parameters were influenced primarily by the post‐natal environment, the pre‐natal environment hid a subtle effect on the metabolic profile, which only became apparent when investigating the data in a multivariate fashion. The pre‐natal diet separated clearly the dietary groups along PC2, and mRNA expression levels of Fads1 and Fads2 were affected purely by pre‐natal exposure with lower levels of both genes in HFHS pre‐natal exposed offspring (Figure 6A,B).
Figure 6.
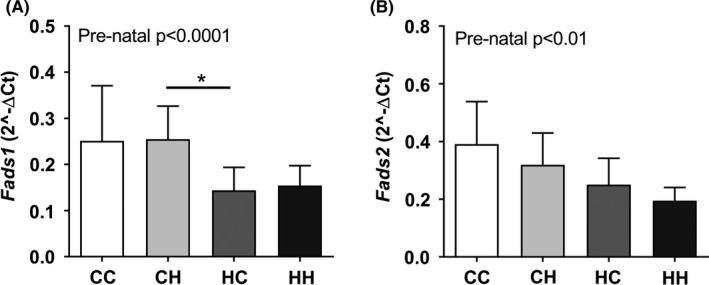
Parameters influenced only by the pre‐natal exposure in male offspring at 4 wk of age. Hepatic expression of (A) Fads1 and (B) Fads2 were the only parameters affected by the pre‐natal exposure alone
3. DISCUSSION
Maternal high‐energy diet during gestation and lactation increases the risk of metabolic dysfunction in the offspring later in life. The hepatic lipid profile is affected already at birth, 7 but it is unknown if the maternal diet during lactation can modulate and rescue these changes. In this study, we used a maternal HFHS rat model with cross‐fostering during lactation to investigate how the pre‐ vs post‐natal environment influences the hepatic lipid composition in offspring. Acute effects both at birth and weaning are reported.
To investigate a maternal high‐energy diet, we chose a hypercaloric diet high in fat supplemented with sucrose water, since it mimics the human caféteria diet. Additionally, we also used an obese‐prone rat strain. Despite 17 weeks on the HFHS diet, there was no apparent impact on the metabolic profile in the dams compared to the control. We suspect that the high sucrose content of the meal is causing the lack of changes between the groups, since a high sucrose diet is less obesogenic than one high in fat. 17 Furthermore, the dams were known breeders and had delivered a litter prior to this study. Pregnancy and lactation give rise to hyperphagia and a negative energy balance, 18 , 19 which could also influence the body weight and metabolic profile of the dams. Since the HFHS diet had no apparent effect on the dams, we assume that the offspring phenotype was primarily caused by differences in nutrient exposure between groups.
All offspring were weighed the day after birth, and despite a similar body weight between control and HFHS offspring, the HFHS females had smaller livers than female controls. The smaller livers in the HFHS offspring were associated with lowered TAG accumulation and reduced ceramide content, which contradicts previous findings in neonates born by obese rodents. 7 , 20 The hepatic lipid profile in HFHS also diverged from the control group, which contrasts with a study by Cerf et al that reports very few changes in hepatic lipid composition in rat fetuses exposed to a HF diet. 21 Our PLS analysis demonstrated that the hepatic lipid composition in our study was associated to the lipid profile of the mother's milk, which were sampled from the pups’ stomachs. We hypothesise that the fetal hepatic lipid profile is affected by the consumed milk or, that the lipids in both fetal liver and mother's milk reflects the maternal lipid profile.
The post‐natal HFHS offspring showed a greater weight gain at 4 weeks of age compared to control lactated offspring, but surprisingly the fatty acid content of the mother's milk was alike between groups. Other studies have found elevated lipid content in breast milk from HF fed dams, 21 , 22 but they investigated the milk in mid and late gestation, and we sampled colostrum at birth. The nutrient content in breast milk varies over the course of lactation in rats, 23 so the fatty acid content could potentially have differed between HFHS and control dams in the later lactational stages.
Increased adiposity in HFHS lactated offspring was associated with impaired insulin sensitivity and increased hepatic TAG accumulation according to the PCA. Hepatic TAG accumulation in HF exposed offspring has previously been reported, 20 , 24 however we also observed a concurrent decrease in overall hepatic ceramide content. Normally, elevated hepatic TAG leads to steatosis, increased ceramide accumulation and subsequent inflammation. 10 But we have previously observed elevated hepatic TAG alongside with decreased ceramide in pregnant HF fed mice on gestation day 18. 7 In the present data, hepatic TAG was associated with increased inflammatory markers in the liver (Cd68, Cd163 and Il1b) in the PCA, but total hepatic ceramide did not cluster with the inflammatory parameters. Hepatocytes can secrete excess ceramide as a protective mechanism against lipotoxicity, when ceramide synthesis is induced by fatty acid oversupply. 25 A study in LDLr knock‐out mice has previously demonstrated that hepatic ceramide levels in mice fed a Western diet can be similar to control fed mice, while a 6‐fold increase in ceramide is found in plasma. 26 Thus, an increased hepatic ceramide secretion, might explain the observed decrease in hepatic ceramide in the pups lactated by HFHS dams.
The lipid profiles in mother's milk and livers from neonates and young offspring showed a high degree of similarity by their elevated levels of n‐6 PUFA and decreased MUFA content. Synthesis of long‐chained PUFA takes place through desaturation and elongation of the dietary essential fatty acids, linoleic and α‐linolenic acid. The desaturation is catalysed by the Δ5‐ and Δ6‐desaturase (Fads1 and Fads2, respectively), and elongation by the elongase (Elovl5), 27 , 28 but we found here that only Elovl5 expression correlated with n‐6 PUFA levels. Part of the HFHS post‐natal n‐6 PUFA accumulation in CH and HH livers might therefore originate from the elevated n‐6 PUFA in the breast milk. PUFA, but also leptin, is known to down‐regulate Scd1 expression, 29 which in our study is completely inhibited in livers from HFHS lactated offspring. Scd1 encodes the enzyme that converts saturated FAs to MUFA. The decreased MUFA content in the HFHS lactated offspring is therefore very likely a result of a low endogenous MUFA production along with a reduction in dietary MUFA from the milk. MUFAs are important to maintain normal metabolic function. Palmitoleic acid, a MUFA, increases muscle insulin sensitivity and suppress hepatic steatosis in mice. 12 Furthermore, chronic administration of palmitoleate reduces insulin resistance and hepatic lipid accumulation, as well as increases satiety and the release of appetite‐reducing hormones in rats, when compared to other similar FAs. 30 , 31 Our results point in the same direction, since we observed an association between decrease in hepatic MUFA and increase in hepatic TAG, insulin and blood glucose levels in the PCA.
The pre‐natal exposure leaves an imprint in neonate rat, which become less pronounced at 4 weeks of age. However, the pre‐natal effect is evident at 4 weeks of age, when all data are considered with a multivariate data analysis approach. The latest nutritional exposure, which in this case is the post‐natal environment, dominates the general metabolic phenotype at 4 weeks of age. The reduced litter size in this study might, however, exacerbate the influence from the post‐natal environment. A combination of maternal HFD and small litter sizes amplifies the metabolic outcome in the offspring, 32 , 33 so we can speculate that our post‐natal profiles might have been more subtle, if pups had been weaned in a regular litter size. Thus, altogether we find that maternal HFHS diet during lactation leads to obesity, increased hepatic TAG levels and impaired glucose homeostasis even in young offspring, while maternal HFHS diet during pregnancy showed less impact on the metabolic phenotype in offspring at 4 weeks of age. Many early metabolic impairments are reported to be maintained into adulthood, 34 , 35 but whether they can be reversed needs further investigation.
4. METHODS
4.1. Animal experiment
The study was conducted according to Danish regulation and with protocols approved by the Danish Animal Ethics Committee. Female rats (OP‐CD, Charles River, Kingston, NY, USA) 8 weeks of age were fed a high‐fat/high sucrose (HFHS; 60E% fat, D12492, Research Diets, Brogaarden, Denmark, supplemented with 15% (w/vol) sucrose water) or control diet (10E% fat, D12450B, Research Diets, Brogaarden, Denmark) for 17 weeks (diet composition is found in Table S4). The HFHS diet mimicked a typical human “junk” food diet by combining high‐fat food with sucrose‐sweetened beverages. This combination was chosen, because sucrose as beverage is supposed to be more adipogenic than sucrose in solid food. 36 The female rats were assigned to dietary intervention, so that body weight was similar between the two groups at study initiation (n = 9–11 per group). Group sizes were chosen based on previous experience, 7 since this was a pilot study. During the 17 weeks of dietary exposure, rats were impregnated by strain and age matched males, which were fed a chow diet (Altromin, Brogaarden, Denmark) and gave birth to a first generation of offspring (manuscript in preparation). The female rats were mated again (17 weeks on experimental diets at this time point) and offspring from the second round of breeding was investigated in this study. Here, we only report metabolic status of the female rats that got pregnant during the second breeding (n = 7 in control groups, n = 3 in HFHS group). Two control dams littered during the same night in the same cage, so these litters were considered as one in the following analysis. Female pups were killed the day after birth. Blood and tissues were collected and treated as described below. The male pups were cross‐fostered (Figure 2), which resulted in four male offspring groups; born and lactated by control dams (CC); born by control dams and lactated by HFHS dams (CH); born by HFHS dams and lactated by control dams (HC); and born and lactated by HFHS dams (HH). The male offspring were assigned to groups randomly and blinded. Litters were standardized to 4–6 pups per dam, and dams were maintained on their respective diets throughout mating, gestation and lactation. After 4 weeks of lactation, the male offspring and dams were killed. Blood and tissues were collected and treated as described below.
Animals were group housed (3 female rats per cage before mating, 1 male and 1–2 females per cage during mating, 1–2 females per cage during gestation and 1 dam with 4–6 pups per cage post gestation) under 12:12 light‐dark cycle in humidity and temperature‐controlled rooms. All had ad libitum food and access to water. Rats receiving sucrose water were additionally supplemented with fresh water.
4.2. Weighing and blood sampling
Dams were weighed and blood was collected in EDTA tubes (BD Microtainer, BD Biosciences, Oxford, UK) from the tail vein before second mating and at weaning after an overnight fast. Offspring were weighed at birth and subsequently on a weekly basis throughout lactation. Blood was collected from the female neonates by decapitation. Glucose was measured immediately on whole blood and the remaining blood was pooled from 2–3 female littermates for plasma isolation. At 4 weeks of age, the male offspring were short fasted for 4 hours, due to their young age, for blood glucose measurement. The offspring were then anaesthetized with a ketamine/xylazine cocktail (200 mg/kg body weight ketamine and 10 mg/kg body weight xylazine) and blood was collected by cardiac puncture for plasma analyses.
4.3. Blood glucose and plasma parameters
Blood glucose was analysed by a glucometer (On Call Plus, ACON Laboratories, San Diego, CA, USA). Plasma triglycerides were analysed on a Cobas Mira Plus with a Horiba ABX Pentra kit (Montpellier, France). Plasma insulin and leptin were analysed on a Meso Scale Selector Imager 6000 with Meso Scale Discovery kits according to manufacturer's instructions (Meso Scale Discovery, Rockville, MD, USA).
4.4. Lipid composition and leptin content of milk bolus and experimental diets
Stomachs from the female offspring contained milk boluses and were collected the day after birth, snap‐frozen in liquid nitrogen and stored in −80°C until analysis.
The stomach content (n = 2 offspring/dam) were analysed to determine milk composition (total stomachs per group C = 12, HFHS = 6). Proteins were extracted from mother's milk by homogenizing half the milk‐bolus in 100 µL of water containing a protease inhibitor, followed by 2 minutes of centrifugation (20.000× g). The supernatants were analysed for leptin content on a Meso Scale Selector Imager 6000 with a Meso Scale Discovery kit according to manufacturer's instructions (Meso Scale Discovery).
Lipids were extracted from the remaining milk‐bolus and the experimental diets by the Folch method, 37 after addition of glyceryl trinonadecanoyl (Sigma, St. Louis, MO, USA) as internal standard and milliQ‐H2O to obtain the required ratio between CHCl3:MeOH:H2O (8:4:3) in the final extract. Fatty acid content and composition in the final lipid extract was analysed as previously described. 38
4.5. Lipid composition of livers from offspring
Livers were snap‐frozen in liquid N2 and stored in −80°C until analysed. Hepatic lipids were extracted using the Folch procedure in the presence of internal standards for FFA, TAG and phospholipids (PL) as previous described 38 (for female neonates; n = 2 offspring/dam, total livers per group C = 12, HFHS = 6). In addition, ceramide C17:1 long‐chained base (Avanti Polar Lipids, Alabaster, AL, USA) was added as internal standard for ceramide quantification prior to extraction. One aliquot of the lipid extract was used for PL, TAG and FFA analysis, 38 and one aliquot used for quantification of ceramides, as described in Drachmann et al, 39 but using the above mentioned C17:1 ceramide as internal standard.
4.6. RT‐qPCR
Liver samples were collected from female offspring the day after birth and male offspring at weaning, immersed in RNA later at 4°C for 24 hours and stored at −80°C until RNA extraction. RNA was extracted by a RNeasy kit (Qiagen, Hilden, Germany) according to manufacturer's instructions and converted to cDNA by a High Capacity cDNA Reverse Transcription kit (Invitrogen, Taastrup, Denmark). Quantitative real‐time PCR was run on a 7900HT Fast Real‐time PCR system (Applied Biosystems, Naerum, Denmark) with a Taqman Fast Universal PCR master mix (Applied Biosystems) and primers/probers from Integrated DNA Technologies (Coralville, IA, USA) (Table S5). Results from genes of interest were normalised to two housekeeping genes (Gapdh and B2m) and displayed as 2^‐delta(Ct).
4.7. Statistical analysis
Data are represented as mean ± SD in all graphs, and no data points were excluded from the statistical analysis. Pre‐natal effects the day after birth were evaluated by a two‐sided two sample permutation test in the “coin” package in R (http://www.r‐project.org/, R version 3.2.3). 17 Pre‐natal and post‐natal effects at weaning were analysed by a permutated 2‐way ANOVA in the “lmperm” package in R as described by Anderson (2001). 40 Post‐hoc tests were performed as a pair‐wise comparison between groups by a two‐sided two sample permutation test and the P‐values were adjusted for multiple testing by the Holm method. 41 The influence of lactating litter size on the offspring's bodyweight at weaning was analysed by a permutated ANCOVA in the “lmperm” package in R. Effects were considered significant when P < .05. Principal component analysis (PCA) and partial least square (PLS) regression were performed in SIMCA (SIMCA version 14, Umetrics, Sartorius Stedim Data Analytics AB, Umeå, Sweden).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/1440‐1681.13396.
Supporting information
Figure S1
Figure S2
Table S1
Table S2
Table S3
Table S4
Table S5
ACKNOWLEDGEMENTS
This work was supported by the Danish Council for Strategic Research (grant no. 09‐067124). The Danish Council of Strategic Research had no role in the design, analysis or writing of this article.
Ingvorsen C, Lelliott CJ, Brix S, Hellgren LI. Effects of maternal high‐fat/high sucrose diet on hepatic lipid metabolism in rat offspring. Clin Exp Pharmacol Physiol. 2021;48:86–95. 10.1111/1440-1681.13396
REFERENCES
- 1. Poston L. Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Best Pract Res Clin Endocrinol Metab. 2012;26(5):627‐639. [DOI] [PubMed] [Google Scholar]
- 2. Dodd JM, Grivell RM, Nguyen A‐M, Chan A, Robinson JS. Maternal and perinatal health outcomes by body mass index category. Aust N Zeal J Obstet Gynaecol. 2011;51(2):136‐140. [DOI] [PubMed] [Google Scholar]
- 3. Hochner H, Friedlander Y, Calderon‐Margalit R, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow‐up Study. Circulation. 2012;125(11):1381‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869‐873. [DOI] [PubMed] [Google Scholar]
- 5. Brumbaugh DE, Tearse P, Cree‐Green M, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatrics. 2013;162(5):930‐U77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modi N, Murgasova D, Ruager‐Martin R, et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res. 2011;70(3):287‐91. [DOI] [PubMed] [Google Scholar]
- 7. Ingvorsen C, Thysen AH, Fernandez‐Twinn D, et al. Effects of pregnancy on obesity‐induced inflammation in a mouse model of fetal programming. Int J Obes (Lond). 2014;38(10):1282‐9. [DOI] [PubMed] [Google Scholar]
- 8. McCurdy CE, Bishop JM, Williams SM, et al. Maternal high‐fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119(2):323‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farese RV, Zechner R, Newgard CB, Walther TC. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 2012;15(5):570‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab. 2012;23(8):365‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderwald C, Brunmair B, Stadlbauer K, Krebs M, Fuernsinn C, Roden M. Effects of free fatty acids on carbohydrate metabolism and insulin signalling in perfused rat liver. Eur J Clin Invest. 2007;37(10):774‐82. [DOI] [PubMed] [Google Scholar]
- 12. Cao HM, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ricchi M, Odoardi MR, Carulli L, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24(5):830‐40. [DOI] [PubMed] [Google Scholar]
- 14. Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod Toxicol. 2005;20(3):345‐52. [DOI] [PubMed] [Google Scholar]
- 15. Symonds ME, Sebert SP, Budge H. The impact of diet during early life and its contribution to later disease: critical checkpoints in development and their long‐term consequences for metabolic health. Proc Nutr Soc. 2009;68(4):416‐21. [DOI] [PubMed] [Google Scholar]
- 16. Fiorotto ML, Burrin DG, Perez M, Reeds PJ. Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am J Physiol. 1991;260(6 Pt 2):R1104‐13. [DOI] [PubMed] [Google Scholar]
- 17. Jensen VS, Hvid H, Damgaard J, et al. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague‐Dawley rats. Diabetol Metab Syndr. 2018;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol. 2002;23(3):225‐56. [DOI] [PubMed] [Google Scholar]
- 19. Rolls BJ, van Duijvenvoorde PM, Rowe EA. Effects of diet and obesity on body weight regulation during pregnancy and lactation in the rat. Physiol Behav. 1984;32(2):161‐8. [DOI] [PubMed] [Google Scholar]
- 20. Kjaergaard M, Nilsson C, Rosendal A, Nielsen MO, Raun K. Maternal chocolate and sucrose soft drink intake induces hepatic steatosis in rat offspring associated with altered lipid gene expression profile. Acta Physiol (Oxf). 2014;210(1):142‐53. [DOI] [PubMed] [Google Scholar]
- 21. Cerf ME, Louw J, Herrera E. High fat diet exposure during fetal life enhances plasma and hepatic Omega‐6 fatty acid profiles in fetal Wistar rats. Nutrients. 2015;7(9):7231‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franco JG, Fernandes TP, Rocha CPD, et al. Maternal high‐fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J Physiol. 2012;590(21):5503‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholas KR, Hartmann PE. Milk secretion in the rat: progressive changes in milk composition during lactation and weaning and the effect of diet. Comp Biochem Physiol A Comp Physiol. 1991;98(3–4):535‐42. [DOI] [PubMed] [Google Scholar]
- 24. Benatti RO, Melo AM, Borges FO, et al. Maternal high‐fat diet consumption modulates hepatic lipid metabolism and microRNA‐122 (miR‐122) and microRNA‐370 (miR‐370) expression in offspring. Br J Nutr. 2014;111(12):2112‐22. [DOI] [PubMed] [Google Scholar]
- 25. Watt MJ, Barnett AC, Bruce CR, Schenk S, Horowitz JF, Hoy AJ. Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia. 2012;55(10):2741‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasumov T, Li L, Li M, et al. Ceramide as a mediator of non‐alcoholic Fatty liver disease and associated atherosclerosis. PLoS One. 2015;10(5):e0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gregory MK, Gibson RA, Cook‐Johnson RJ, Cleland LG, James MJ. Elongase reactions as control points in long‐chain polyunsaturated fatty acid synthesis. PLoS One. 2011;6(12):e29662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heird WC, Lapillonne A. The role of essential fatty acids in development. Annu Rev Nutr. 2005;25(1):549‐571. Palo Alto: Annual Reviews. [DOI] [PubMed] [Google Scholar]
- 29. Mauvoisin D, Mounier C. Hormonal and nutritional regulation of SCD1 gene expression. Biochimie. 2011;93(1):78‐86. [DOI] [PubMed] [Google Scholar]
- 30. Yang ZH, Takeo J, Katayama M. Oral administration of omega‐7 palmitoleic acid induces satiety and the release of appetite‐related hormones in male rats. Appetite. 2013;65:1‐7. [DOI] [PubMed] [Google Scholar]
- 31. Yang Z‐H, Miyahara H, Hatanaka A. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK‐Ay Mice with genetic type 2 diabetes. Lipids Health Dis. 2011;10(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149(11):5348‐56. [DOI] [PubMed] [Google Scholar]
- 33. Chen H, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS One. 2009;4(7):e6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R528‐38. [DOI] [PubMed] [Google Scholar]
- 35. Buckley AJ, Keseru B, Briody J, Thompson M, Ozanne SE, Thompson CH. Altered body composition and metabolism in the male offspring of high fat‐fed rats. Metab Clin Exp. 2005;54(4):500‐7. [DOI] [PubMed] [Google Scholar]
- 36. DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obesity. 2000;24(6):794‐800. [DOI] [PubMed] [Google Scholar]
- 37. Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem. 1957;226:497‐509. [PubMed] [Google Scholar]
- 38. Pedersen MH, Lauritzen L, Hellgren LI. Fish oil combined with short chain fatty acids synergistically prevent tissue accumulation of free fatty acids during weight loss in obese mice. Br J Nutr. 2011;106(10):1449‐56. [DOI] [PubMed] [Google Scholar]
- 39. Drachmann T, Mathiassen JH, Pedersen MH, Hellgren LI. The source of dietary fatty acids alter the activity of secretory sphingomyelinase in the rat. Eur J Lipid Sci Technol. 2007;109(10):1003‐9. [Google Scholar]
- 40. Anderson MJ. Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquatic Sci. 2001;58(3):626‐39. [Google Scholar]
- 41. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Table S1
Table S2
Table S3
Table S4
Table S5


