Abstract
Background
Chronic rhinosinusitis with or without nasal polyps (CRSwNP/CRSsNP) seriously impairs health‐related quality of life (HRQoL). This analysis describes the impact of the exhalation delivery system with fluticasone (EDS‐FLU) on HRQoL, assessed by the 36‐item Short‐Form Health Survey version 2 (SF‐36v2), and on utilities, assessed via the Short‐Form 6‐Dimension (SF‐6D), in patients with CRSwNP.
Methods
Post hoc analysis of pooled randomized clinical trial data (NAVIGATE I and II; N = 643) to examine change from baseline in SF‐36v2 and SF‐6D at end‐of‐double‐blind (EODB: 16 weeks) and end‐of‐open‐label (EOOL: 24 weeks; following 8 weeks of open‐label treatment) for EDS‐FLU vs placebo (EDS‐PBO). Baseline characteristics predictive of change in SF‐36 and SF‐6D scores were assessed.
Results
Mean baseline SF‐36v2 scores were below population norms. At EODB, mean improvement was greater for all SF‐36v2 domain and component scores with EDS‐FLU (range: 2.9 [physical functioning] to 5.11 [bodily pain {BP}]) vs EDS‐PBO (range: 0.81 [mental health] to 2.87 [BP]) (each comparison p < 0.01); physical and mental component score improvements within the EDS‐FLU group exceeded the minimal clinically important difference (MCID). Clinically meaningful and statistically significant improvements in SF‐6D utility scores were seen in EDS‐FLU–treated patients compared to EDS‐PBO–treated patients (0.058 vs 0.023, respectively, p < 0.001). At EOOL, SF‐36v2 and SF‐6D mean scores were at or above population norms, with clinically meaningful and statistically significant improvements from baseline.
Conclusion
In this pooled analysis of 2 large pivotal EDS‐FLU trials, health domain and health utilities improvements were significantly greater with EDS‐FLU than EDS‐PBO and were comparable to population norms.
Keywords: nasal polyps, quality of life, intervention study, health status, population health, quality improvement
Chronic rhinosinusitis with or without nasal polyps (CRSwNP and CRSsNP, respectively) is a debilitating chronic inflammatory disease affecting approximately 12% of adults in the United States. 1 CRS is characterized by persistent congestion, often with rhinorrhea, facial pain, and dysosmia, lasting 12 weeks or longer, and is frequently associated with flares of acute sinusitis, increased healthcare resource utilization, 2 , 3 and increased antibiotic prescriptions. 4 A recent claims‐based study estimated that an antibiotic is prescribed in approximately 70% of CRS visits, and that CRS is responsible for 7.1% of all antibiotic prescriptions, more than any other primary diagnosis. 4 In addition, CRS has been shown to be associated with significant impairments in sleep, mood, and work productivity. 5 , 6 , 7 , 8
Not surprisingly, studies have found that patients with CRS report decreased health‐related quality‐of‐life (HRQoL) and health status. 9 , 10 , 11 , 12 QoL instruments are classified into 2 main types: general (or generic) and disease‐specific, and the measurement of both of these perspectives has proven instrumental in understanding the impact of diseases and conditions on patients’ QoL. The measurement of the impact on general QoL can help better understand the relative burden of diseases, and is useful in evaluating the impact of healthcare interventions relative to general population health levels. 13
For U.S. patients with CRS, general QoL domains and mean health utility levels (as measured by the Short Form‐36 Health Survey, version 2 (SF‐36v2, standard 4‐week recall) and the Short‐Form 6‐Dimension (SF‐6D), respectively, have been reported to be below U.S. norms, and its effect on health utilities has been shown to be comparable to other serious chronic conditions. 11 , 14 Significant improvements in general QoL and health utility levels have been observed following successful medical or surgical therapy. 15
CRS has been shown to be responsive to systemic steroid therapy, 16 and many patients respond to conventional nasal steroid sprays. 17 However, due to the limited deposition of conventional nasal steroid medication at the level of the ostiomeatal complex (OMC) and deeper portions of the nasal cavity Fig. (1A), 18 patients with more severe disease may not experience adequate symptom control. 17 Recent treatment strategies for these failures include surgery and alternative methods to deliver steroids to sinus tissue where polyps grow. 17 Furthermore, polyps typically originate in the upper regions of the nasal cavity, contributing to continued CRS symptoms, and exacerbations of CRS. Conventional medical treatments are frequently insufficient for patients with more severe disease due to this inability to deliver medication throughout the sinonasal cavity.
FIGURE 1.
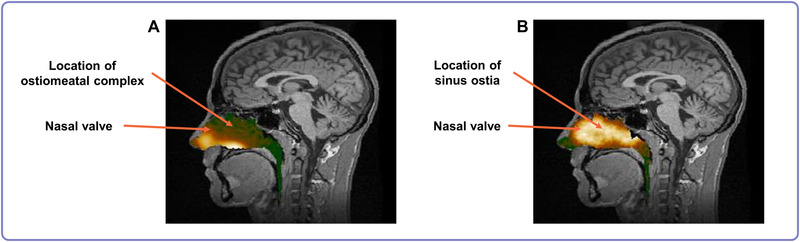
Gamma camera image deposition information (logarithmic hot‐iron intensity scale) from the nasal cavity that is superimposed on the corresponding sagittal MRI section. The image represents deposition 0 to 2 minutes after delivery using a conventional liquid spray (A) and an exhalation delivery system (B). In the first image (A), deposition of spray was greatest in the lower anterior regions of the nose, whereas in the second image (B), deposition of liquid was greatest in the upper posterior regions of the nose. The images were from the same healthy subject after each method of administration (Image used with permission from Djupesland 18 ). MRI = magnetic resonance imaging.
Although functional endoscopic sinus surgery (FESS) has been shown to improve general and disease‐specific QoL in patients with CRS, 19 , 20 it does not specifically treat the underlying inflammation of CRS. For many patients surgery is not curative, with approximately 50% reporting a return of CRS symptoms and polyps within 18 months 21 , 22 and approximately 20% requiring revision surgery within 5 years. 19
The Exhalation Delivery System with Fluticasone (EDS‐FLU, XHANCE®) uses a different approach to intranasal drug delivery shown to achieve high/deep deposition for treatment of nasal polyps (Fig. 1B). 18 , 23 EDS‐FLU has demonstrated a broad improvement in all 4 defining symptoms of CRS (congestion, rhinorrhea, facial pain/pressure, hyposmia) and total 22‐item Sino‐Nasal Outcome Test (SNOT‐22) score. 24 , 25 However, its impact upon general HRQoL and health utility has not been described. Therefore, the objective of this study was to describe the impact of EDS‐FLU on general HRQoL and health utility in patients with CRS with nasal polyps (CRSwNP). Secondary aims were to evaluate the impact of baseline characteristics on changes in HRQoL and health utility.
Patients and methods
Study population
In this post hoc analysis, data from patients participating in 2 identically‐designed, double‐blind, randomized, controlled trials were pooled and analyzed (NAVIGATE I and NAVIGATE II). The countries from which patients were recruited was the only difference between the NAVIGATE I and II studies. Pooling the unit data across studies provided wider geographic representation and greater statistical precision. Figure 2 provides a brief description of the study designs, which are described in detail elsewhere. 24 , 25
FIGURE 2.
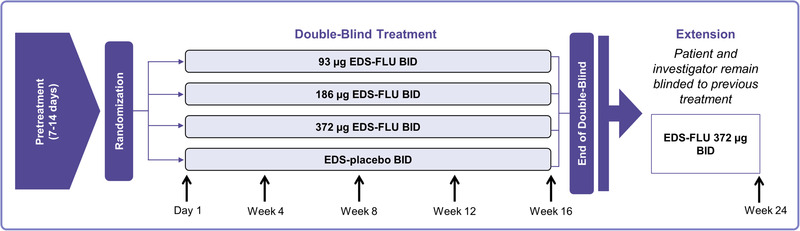
NAVIGATE I and II study design. BID = twice daily; EDS‐FLU = exhalation delivery system with fluticasone.
Briefly, patients were randomized to a double‐blind, 16‐week, EDS‐placebo controlled phase followed by an 8‐week open‐label extension without revealing prior treatment allocation. Eligible patients with CRSwNP were 18 years old and required to have moderate or severe nasal congestion/obstruction as reported by the patient (morning score ≥2 [0 = none, 1 = mild, 2 = moderate, 3 = severe] for at least 5 days during the 7‐day period leading up to screening) and a total nasal polyp grade of 2 or greater (minimum polyp grade of 1 in each nasal cavity). Exclusion criteria included complete nasal cavity obstruction or inability to achieve bilateral nasal airflow, perforated septum, >5 prior sinonasal surgeries, or sinonasal surgery within 6 months of screening.
Outcome measures
Change from baseline in HRQoL (measured by the SF‐36v2, standard 4‐week recall) and health utility (measured by the SF‐6D) was calculated at end of double‐blind (EODB: week 16) and end of open label (EOOL: week 24).
HRQoL
The SF‐36v2 is a validated, patient‐reported outcomes instrument widely used to measure HRQoL in patients with a wide variety of health conditions, and enables comparisons to established general population HRQoL norms. 26 The 36 items are presented as 8 individual domain scores (physical function [PF], role limitations due to physical health [RP], bodily pain [BP], general health [GH], vitality [VT], social function [SF], role limitations due to emotional problems [RE], and mental health [MH]) and summarized via 2 component scores: the Physical Component Summary (PCS) and the Mental Component Summary (MCS).
All SF‐36v2 scores used in the analysis are normed using the 2009 U.S. population, and were assessed using the QualityMetric Scoring Software v5.1 (QualityMetric, Lincoln, RI). Scores of 50 + minimal clinically important difference (MCID) indicate HRQoL similar to general population. Scores greater than 50 + MCID indicate better HRQoL than the general population. Scores less than 50 – MCID indicate worse HRQoL than the general population. The MCID for the MCS, PCS, and 8 domains is as follows: MCS 3; PCS 2; GH 2; PF 3; RP 3; BP 3; VT 2; SF 3; RE 4; and MH 3. 26
Health utility
The SF‐6D is a health status measure representing a preference‐based score, or “health utility,” with a value between 0 (worst health state) and 1 (best health state). It is derived from a subset of 11 SF‐36v2 questions and calculated from UK population nonparametric Bayesian preference weights. It is used to calculate quality‐adjusted life years (QALYs) in economic evaluations. 27 U.S. population norms range between 0.76 and 0.80. 28 The MCID for the SF‐6Dv2 is 0.03 points. 29
Analysis
All patients with CRSwNP receiving EDS‐FLU were pooled in 1 treatment arm (All EDS‐FLU) and compared to placebo (EDS‐PBO). SF‐36v2 (domain and component) and SF‐6D scores were classified relative to U.S. population norms (ie, at, below, or above population norms) at baseline, EODB, and EOOL. Change from baseline in SF‐36v2 and SF‐6D scores at EODB and EOOL was also classified according to their clinical meaningfulness using MCID levels. Statistical significance of changes from baseline between EDS‐FLU and EDS‐PBO was assessed using an analysis of covariance (ANCOVA) model that included the corresponding SF‐36v2 or SF‐6D baseline score, treatment group (EDS‐PBO, All EDS‐FLU), and country. To assess the baseline characteristics associated with change from baseline in SF‐36v2 and SF‐6D scores at EODB, the following baseline characteristics were added to the ANCOVA model: age, sex, race; history of FESS, asthma, or allergic rhinitis; baseline polyp grade and SNOT‐22 score; current FESS eligibility (using criteria established for the studies by a panel of rhinology experts 24 , 25 ), and study. No imputation of missing values was undertaken.
Results
Patient demographics
In the pooled population of 643 patients with CRSwNP, 161 were in the EDS‐PBO arm and 482 in the All EDS‐FLU arm (161 in 93 µg twice per day [BID], 160 in 186 µg BID, and 161 in 372 µg BID). Of these, 633 had data available at baseline and EODB, and 575 had data at baseline and EOOL. Table 1 shows the demographic profile of the study population.
TABLE 1.
Baseline demographic characteristics of the pooled study population
| Characteristic | EDS‐PBO (n = 161) | All EDS‐FLU (n = 482) | Total (N = 643) |
|---|---|---|---|
| Age (years), mean ± SD | 46.0 ± 12.47 | 45.2 ± 12.77 | 45.4 ± 12.69 |
| % Female | 51.6 | 44.2 | 46.0 |
| Race, n (%) | |||
| White | 143 (88.8) | 441 (91.5) | 584 (90.8) |
| Black/African American | 11 (6.8) | 28 (5.8) | 39 (6.1) |
| Asian | 5 (3.1) | 4 (0.8) | 9 (1.4) |
| Other | 2 (1.2) | 9 (1.9) | 11 (1.7) |
| Any surgical history, n (%) | 93 (57.8) | 264 (54.8) | 357 (55.5) |
| Polypectomy | 63 (39.1) | 172 (35.7) | 235 (36.5) |
| FESS | 57 (35.4) | 175 (36.3) | 232 (36.1) |
| SNOT‐22 total score, mean ± SD | 52.9 ± 18.90 | 49.0 ± 19.75 | 49.9 ± 19.60 |
EDS‐FLU = exhalation delivery system with fluticasone; EDS‐PBO = exhalation delivery system with placebo; FESS = functional endoscopic sinus surgery; SD = standard deviation; SNOT‐22 = Sino‐Nasal Outcome Test.
SF‐36v2 domain and component summary score changes
Baseline to EODB
At baseline, scores were comparable between all EDS‐FLU and EDS‐PBO, with mean scores within 1 MCID between groups (Table 2). The mean improvement from baseline to EODB for all SF‐36v2 domain and component scores was significantly higher in the All EDS‐FLU arm compared to EDS‐PBO (all p < 0.01), with the EODB means in the All EDS‐FLU arm at or above population norms. These improvements in the All EDS‐FLU arm exceeded the MCID in all domains and components except PF and RE. Although all domains and components showed some improvement in the EDS‐PBO arm, none exceeded the MCID. The magnitude of the change in each mean SF‐36v2 domain and component score was between 1.8 times (PF) and 4 times (SF) greater for All EDS‐FLU vs EDS‐PBO‐treated patients. The absolute increase in SF‐36v2 from baseline to week 16 relative to the MCID is shown in Figure 3.
TABLE 2.
Change in SF‐36v2 domain and component summary scores over time*
| SF‐36 domain (MCID) | Treatment arm | Baseline | EODB (week 16) | LS mean change (BL – EODB) | p (All EDS‐FLU vs EDS‐PBO) | EOOL (week 24) | LS mean change (BL – EOOL) |
|---|---|---|---|---|---|---|---|
| General health (2) | EDS‐PBO | 44.8 | 46.5 | 1.66 | 0.0003 | 50 | 5.23 |
| All EDS‐FLU | 45.1 | 48.9 | 3.98 | 50.1 | 5.20 | ||
| Physical functioning (3) | EDS‐PBO | 47.9 | 49.8 | 1.56 | 0.01 | 52.7 | 4.67 |
| All EDS‐FLU | 48.8 | 51.7 | 2.90 | 52.7 | 4.27 | ||
| Role physical (3) | EDS‐PBO | 45.1 | 47.1 | 1.53 | <0.0001 | 50.9 | 5.44 |
| All EDS‐FLU | 46.0 | 50.3 | 4.44 | 50.8 | 5.15 | ||
| Bodily pain (3) | EDS‐PBO | 46.6 | 50.2 | 2.87 | 0.0016 | 54.2 | 6.89 |
| All EDS‐FLU | 47.6 | 52.8 | 5.11 | 53.9 | 6.34 | ||
| Vitality (2) | EDS‐PBO | 47.6 | 49.5 | 1.71 | <0.0001 | 54.4 | 6.33 |
| All EDS‐FLU | 48.3 | 52.6 | 4.43 | 54.0 | 5.78 | ||
| Social functioning (3) | EDS‐PBO | 45.4 | 47.4 | 0.99 | <0.0001 | 51.2 | 4.90 |
| All EDS‐FLU | 47.0 | 51.0 | 3.95 | 51.6 | 4.93 | ||
| Role emotional (4) | EDS‐PBO | 45.0 | 47.5 | 1.57 | 0.0046 | 49.9 | 4.45 |
| All EDS‐FLU | 46.9 | 50.0 | 3.41 | 50.5 | 4.24 | ||
| Mental health (3) | EDS‐PBO | 46.6 | 47.8 | 0.81 | 0.0006 | 50.8 | 4.36 |
| All EDS‐FLU | 47.2 | 50.3 | 3.08 | 51.0 | 4.08 | ||
| MCS (3) | EDS‐PBO | 45.9 | 47.4 | 0.98 | 0.0003 | 50.6 | 4.42 |
| All EDS‐FLU | 47.2 | 50.4 | 3.28 | 50.9 | 4.17 | ||
| PCS (2) | EDS‐PBO | 46.7 | 49.1 | 2.09 | <0.0001 | 52.8 | 5.77 |
| All EDS‐FLU | 47.3 | 51.4 | 4.17 | 52.5 | 5.36 |
*Change in SF‐36v2 at EODB (week 16) and at EOOL (week 24). Improvements in all individual SF‐36v2 domains and components were significant at EODB (week 16) for EDS‐FLU vs EDS‐PBO patients. After week 16 all patients received open‐label treatment with EDS‐FLU 386 µg BID for an additional 8 weeks. Both groups (initial EDS‐PBO and initial EDS‐FLU) experienced clinically‐meaningful improvements from BL in all SF‐36v2 domains and components following open‐label treatment.
BID = twice per day; BL = baseline; EDS‐FLU = exhalation delivery system with fluticasone; EDS‐PBO = exhalation delivery system with placebo; EODB = end of double blind; EOOL = end of open label; LS = least squares; MCID = minimal clinically important difference; MCS = Mental Component Summary; PCS = Physical Component Summary; SF‐36v2 = 36‐item Short‐Form Health Survey version 2.
FIGURE 3.
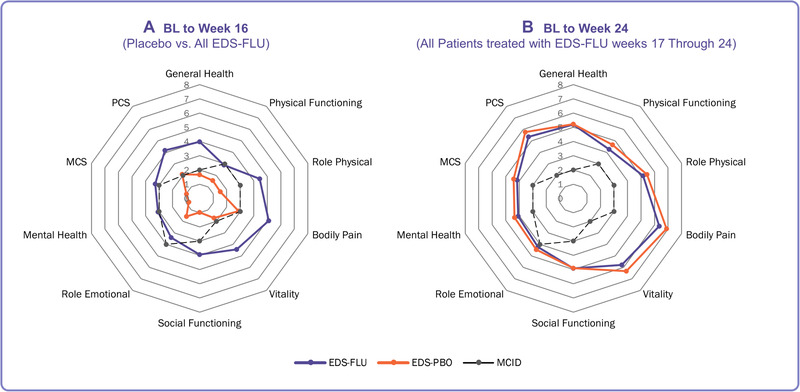
SF‐36v2 domain score change over time. Magnitude of the change in SF‐36 at week 16 and week 24, relative to the MCID. (A) Mean improvement in the EDS‐PBO arm did not exceed the MCID for any domain or component, in contrast to the EDS‐FLU arm, where the mean improvements in nearly every domain and component were greater than the MCID. after week 16 all patients received open‐label treatment with EDS‐FLU 386 µg BID for 8 weeks. (B) After open‐label treatment, patients initially treated with EDS‐PBO experienced achieved similar levels of improvement in SF‐36v2 domains and components as patients initially treated with EDS‐FLU. BID = twice daily; EDS‐FLU = exhalation delivery system with fluticasone; EDS‐PBO = exhalation delivery system with placebo; MCID = minimal clinically important difference; SF‐36 = 36‐item Short‐Form Health Survey; SF‐36v2 = 36‐item Short‐Form Health Survey version 2.
Baseline to EOOL
After all patients received 8 weeks of open‐label treatment following the end of the double‐blind phase, patients experienced an additional improvement in mean SF‐36v2 domain and component scores (Table 2). Patients previously treated with EDS‐PBO appeared to generally catch up to those who had been on drug for 24 weeks. All mean scores at EOOL were at or above U.S. population norms, and improvements from baseline were clinically meaningful, ranging from 1.1 times the MCID (RE) to 2.7 times the MCID (PCS).
SF‐6D utility changes
Baseline to EODB
At baseline, all treatment arms had SF‐6D utility scores well below population norms (EDS‐PBO, 0.680; EDS‐FLU, 0.686) (Table 3). The mean improvement from baseline to EODB for SF‐6D was significantly higher in the All EDS‐FLU arm compared to EDS‐PBO (0.058 vs 0.023, respectively, p = 0.0001). The mean SF‐6D utility at EODB in the EDS‐FLU arm did not reach a value within the population norms; however, the magnitude of the change from baseline for the All EDS‐FLU arm was clinically meaningful, 1.9 times the MCID, and was over 2.5 times greater than the change observed in the EDS‐PBO arm.
TABLE 3.
Change in utility (SF‐6D) scores over time
| Treatment arm | Baseline | EODB (week 16) | LS mean change (BL – EODB) | p (All EDS‐FLU vs EDS‐PBO) | EOOL (week 24) | LS mean change (BL – EOOL) |
|---|---|---|---|---|---|---|
| EDS‐PBO | 0.680 | 0.714 | 0.028 | 0.0001 | 0.761 | 0.080 |
| All EDS‐FLU | 0.686 | 0.747 | 0.060 | 0.765 | 0.080 |
Change in SF‐6D at EODB (week 16) and EOOL (week 24). Improvement in SF‐6D was significant at the EODB (week 16) for EDS‐FLU vs EDS‐PBO patients. After week 16 all patients received open‐label treatment with EDS‐FLU 386 µg BID for an additional 8 weeks. Both groups (initial EDS‐PBO and initial EDS‐FLU) experienced clinically‐meaningful improvements from BL in SF‐6D score following open‐label treatment.
BID = twice per day; BL = baseline; EDS‐FLU = exhalation delivery system with fluticasone; EDS‐PBO = exhalation delivery system with placebo; EODB = end of double blind; EOOL = end of open label; LS = least squares; SF‐6D = Short‐Form 6‐Dimension.
Baseline to EOOL
By the end of the open‐label treatment phase, both treatment groups experienced additional improvement in mean SF‐6D utility from the EODB, achieving a clinically meaningful improvement from baseline (Table 3) to levels comparable to population norms. The change from baseline to EOOL in both treatment arms was more than 2.6 times the MCID for the SF‐6D.
ANCOVA model of baseline characteristics associated with change in SF‐36v2 and SF‐6D
Results of the multivariate regression analysis of baseline characteristics on changes in QoL and health utilities at week 16 are shown in Table 4. In addition to treatment with EDS‐FLU, predictors of improvement in PCS were lower age, male gender, and white race. The additional predictor of improvement in MCS was no history of ESS. For health utilities at week 16 the additional predictor was male gender. All other baseline characteristics assessed were not associated with change in PCS, MCS, or SF‐6D.
TABLE 4.
Baseline characteristics associated with change in health‐related quality of life and health utilities, from baseline to EODB (week 16)*
| Baseline characteristics | PCS coefficient (95% CI); p | MCS coefficient (95% CI); p | SF‐6D coefficient (95% CI); p |
|---|---|---|---|
| Age | −0.066 (−0.106 to −0.027); 0.0010 | 0.015 (−0.033 to 0.063); 0.5452 | −0.001 (−0.001 to 0.000); 0.1575 |
| Sex (Reference: male) | 1.174 (0.178 to 2.169); 0.0209 | −0.517 (−1.748 to 0.714); 0.4097 | 0.017 (0.000 to 0.035); 0.0530 |
| Race (Reference: white) | |||
| Black/African American | −2.570 (−4.525 to −0.615); 0.0101 | −0.176 (−2.579 to 2.227); 0.8858 | −0.024 (−0.058 to 0.011); 0.1785 |
| Other | 1.511 (−1.155 to 4.176); 0.2660 | −0.579 (−3.873 to 2.714); 0.7298 | −0.024 (−0.071 to 0.024); 0.3292 |
| ESS history | −1.173 (−2.885 to 0.539); 0.1788 | −1.781 (−3.889 to 0.328); 0.0977 | −0.012 (−0.042 to 0.018); 0.4367 |
| ESS Eligibility | 0.100 (−0.927 to 1.126); 0.8484 | −0.690 (−1.949 to 0.569); 0.2822 | −0.006 (−0.025 to 0.012); 0.5127 |
| Polyp grade score | 0.051 (−0.429 to 0.530); 0.5006 | −0.003 (−0.593 to 0.587); 0.9915 | −0.002 (−0.010 to 0.007); 0.6846 |
| Asthma | −0.777 (−1.770 to 0.216); 0.1247 | 0.414 (−0.802 to 1.630); 0.5037 | −0.003 (−0.021 to 0.015); 0.7363 |
| Allergic rhinitis | −1.098 (−2.838 to 0.642); 0.2159 | 1.565 (−0.578 to 3.708); 0.1520 | 0.013 (−0.018 to 0.044); 0.3953 |
| SNOT‐22 score | −0.010 (−0.037 to 0.018); 0.5006 | −0.006 (−0.042 to 0.030); 0.9915 | 0.000 (−0.001 to 0.000); 0.7391 |
*Results presented are from an ANCOVA model for PCS, MCS, or SF‐D6 EODB change from baseline including covariates for: PCS, MCS, or SF‐6D baseline score; treatment group (EDS‐PBO, All EDS‐FLU); age; sex; race; history of ESS; asthma, or allergic rhinitis; baseline polyp grade; SNOT‐22 score; and current ESS eligibility. Only the covariates of interest (ie, excluding baseline score, treatment, and study) that were significant at the 10% level (ie, p < 0.1) are presented and are shown in bold.
ANCOVA = analysis of covariance; CI = confidence interval; EDS‐FLU = exhalation delivery system with fluticasone; EDS‐PBO = exhalation delivery system with placebo; EODB = end of double blind; ESS = endoscopic sinus surgery; MCS = mental component summary; PCS = physical component summary; SF‐6D = Short‐Form 6‐Dimension; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test.
Discussion
A large and growing body of evidence demonstrates that patients with CRSwNP have significantly decreased QoL. 6 , 8 , 10 , 11 , 14 This impairment leads patients to seek treatment and has dramatic economic impacts with impaired patient productivity. 7 , 8 Similar to prior reports, patients in this study reported baseline general HRQoL and health state utility scores significantly below population norms. The severity of this impairment has an impact comparable to other chronic illnesses such as asthma, coronary artery disease requiring percutaneous coronary intervention, and end‐stage renal disease with hemodyalisis. 10 In addition to objective improvements in endoscopic or computed tomography (CT) grading, it is also critical that any therapy for CRSwNP results in meaningful improvement in QoL. At the completion of the study, all patients receiving EDS‐FLU reported significant improvement in HRQoL and mean health utility scores, with both measures returning to or exceeding population norms.
In addition to being able to compare the health impact of various diseases, health state utility data also allows comparisons of treatment efficacy across these different disease states. These data can be important for policymakers and those who must make decisions on how to apportion healthcare resources. The change in health utility with EDS‐FLU is similar or greater than that reported for many commonly utilized treatments for chronic disease, including anti–tumor necrosis factor (TNF) drugs for psoriasis, joint replacement surgery, and coronary angioplasty (Fig. 4). 10 With regard to CRS, change in SF‐6D scores were similar to those reported after ESS for CRS, a treatment widely considered to have significant patient‐reported benefit. Although it may be tempting to suggest that EDS‐FLU is equivalent to surgery based on this data, it is not appropriate to make direct comparisons, as surgery is typically reserved for those who have failed a medical therapy such as EDS‐FLU. However, the similarity in absolute change does provide context for providers as they counsel patients who may be familiar with the extent of patient‐reported benefit typically seen after surgery.
FIGURE 4.
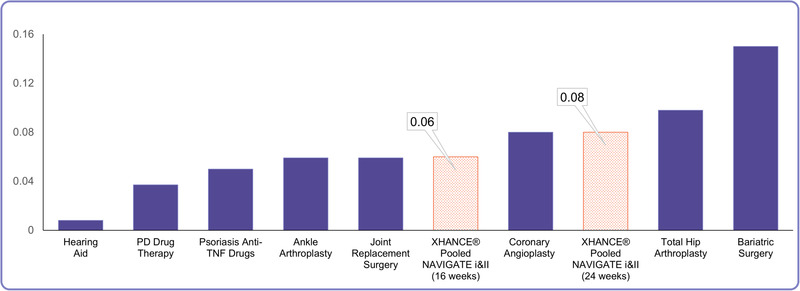
Comparison of change in SF‐6D with different medical and surgical interventions. Comparison of change in SF‐6D health status scores between EDS‐FLU (orange bars = after 16 and 24 weeks of therapy), and selected common medical and surgical procedures (adapted from Soler et al. 10 ). EDS‐FLU = exhalation delivery system with fluticasone; SF‐6D = Short‐Form 6‐Dimension.
Much of the data regarding patient‐reported treatment outcomes for CRS comes from uncontrolled observational cohorts. The weakness of these studies is that one cannot entirely discount the possibility of the placebo effect, wherein a patient's perception of their HRQoL is influenced by their belief in the treatment's efficacy. Furthermore, uncontrolled treatments can suffer from bias related to regression to the mean and/or fluctuations based on natural history of disease. The randomized, double‐blind placebo‐controlled design of this study reduces or eliminates these potential concerns. These improvements in patient‐reported outcome measures are further corroborated by objective reduction in polyp grade with EDS‐FLU. Thus, this data demonstrates both biologic responsiveness, as evidenced by improved polyp scores, as well as clinically relevant improvements in HRQoL.
A secondary aim of this study was to determine whether the impact of EDS‐FLU varied based on baseline characteristics including age, sex, race, polyp grade, SF‐36/6D scores, history of surgery/allergy/asthma, or study‐specific surgical eligibility at baseline. This question has direct clinical relevance as penetration of topical medication can be influenced by prior surgery, which typically improves access to sinus mucosa, as well as by other patient characteristics. Importantly, these baseline characteristics had minimal impact upon HRQoL or health utility improvements with EDS‐FLU.
Strengths of this study include the randomized, double‐blind design, and use of validated general HRQoL and health utility surveys. However, there are several caveats worth keeping in mind. Patients enrolled in these clinical trials had a relatively high burden of disease, as evidenced by high baseline SNOT‐22 scores. Therefore, the magnitude of response might not be uniformly seen across patients who report less baseline QoL impairment. The study was also carried out within the confines of a tightly controlled clinical trial, wherein compliance is expected to be high and treatment breaks are prohibited, in contrast to real‐world settings where patients can run into logistical hurdles acquiring and using their medications regularly. Last, the total study duration was just under 6 months. Although this was clearly adequate to assess the efficacy and safety of treating CRSwNP, it does not allow for conclusions of treatment efficacy beyond this time period. Considering CRSwNP is a chronic disease without a known cure, understanding long‐term treatment impacts on general HRQoL and health utility would be important, particularly regarding future comparative effectiveness research.
Safety findings
The most commonly reported adverse events (AEs) in the active treatment groups were associated with local effects at the site of administration in the nasal cavity (epistaxis, nasal congestion, erythema, and nasal septum ulceration) or associated with the underlying disease (acute sinusitis or nasopharyngitis). The majority of these AEs were mild and are known to have resolved with continued use of study drug. 24 , 25
Conclusion
In this post hoc analysis of patients with CRSwNP treated with EDS‐FLU, clinically meaningful and statistically significant improvements in HRQoL and health utility were observed after the 16‐week double‐blind period, and additional clinically meaningful and statistically significant improvements were observed during the open‐label extension phase. Age, sex, and race were predictive of change in some but not all SF‐36 domains/components; medical history and ESS eligibility/history were not predictive of change in SF‐36/6D scores. The magnitude of change in the SF‐6D with EDS‐FLU was comparable in magnitude to other medical and surgical interventions, such as the pharmacological treatment of Parkinson's disease, anti‐TNF psoriasis treatments, joint replacement therapy, and coronary angioplasty.
Acknowledgments
We thank the investigators and patients who participated in the clinical trials for EDS‐FLU.
How to Cite this Article:Soler ZM, Colman S, Velez FF, Schlosser RJ. Exhalation delivery system with fluticasone improves quality of life and health status: pooled analysis of phase 3 trials NAVIGATE I and II. Int Forum Allergy Rhinol. 2020;10:848–855.
Funding source for the study: Optinose US, Inc. The sponsor executed clinical trials for U.S. Food and Drug Administration (FDA)‐approval of EDS‐FLU, and provided financial support for analysis of clinical trial data.
Potential conflict of interest: F.F.V. is an employee and equity holder of OptiNose US, Inc. S.C. is an employee of Covance Market Access, a research firm that conducted the present post hoc data analysis for OptiNose US, Inc. Z.M.S. and R.J.S. have been paid as consultants for Optinose in the past but received no reimbursement for their work on this article.
Parts presented in part at the Academy of Allergy, Asthma, and Immunology/World Allergy Organization Joint Congress on March 2‐5, 2018, in Orlando, FL.
References
- 1. Palmer JN, Messina JC, Biletch R, Grosel K, Mahmoud RA. A cross‐sectional population‐based survey of US adults with symptoms of chronic rhinosinusitis. Allergy Asthma Proc. 2018;39:1‐9. [DOI] [PubMed] [Google Scholar]
- 2. Caulley L, Thavorn K, Rudmik L. Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: results of the US Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2015; 136:1517‐1522. [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharyya N, Villeneuve S, Joish VN, et al. Cost burden and resource utilization in patients with chronic rhinosinusitis and nasal polyps. Laryngoscope. 2019;129:1969‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith SS, Evans CT, Tan BK, Chandra RK, Smith SB, Kern RC. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132:1230‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope. 2013;123:2364‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schlosser RJ, Gage SE, Kohli P, Soler ZM. Burden of illness: a systematic review of depression in chronic rhinosinusitis. Am J Rhinol Allergy. 2016;30:250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125:1547‐1556. [DOI] [PubMed] [Google Scholar]
- 8. Rudmik L, Smith TL, Schlosser RJ, Hwang PH, Mace JC, Soler ZM. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope. 2014;124:2007‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang PC, Tai CJ, Lin MS, et al. Quality of life in Taiwanese adults with chronic rhino‐sinusitis. Qual Life Res. 2003;12:443‐448. [DOI] [PubMed] [Google Scholar]
- 10. Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121:2672‐2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alobid I, Benítez P, Bernal‐Sprekelsen M, et al. Nasal polyposis and its impact on quality of life: comparison between the effects of medical and surgical treatments. Allergy. 2005;60:452‐458. [DOI] [PubMed] [Google Scholar]
- 12. Ference EH, Stubbs V, Lidder AK, et al. Measurement and comparison of health utility assessments in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schlenk EA, Erlen JA, Dunbar‐Jacob J, et al. Health‐related quality of life in chronic disorders: a comparison across studies using the MOS SF‐36. Qual Life Res. 1997;7:57. [DOI] [PubMed] [Google Scholar]
- 14. Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104‐109. [DOI] [PubMed] [Google Scholar]
- 15. Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life; a prospective randomized controlled trial. Rhinology. 2010;48:305‐311. [DOI] [PubMed] [Google Scholar]
- 16. Lee SH. Mechanisms of glucocorticoid action in chronic rhinosinusitis. Asthma Immunol Res. 2015;7:534‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:S22‐S209. [DOI] [PubMed] [Google Scholar]
- 18. Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective—a review. Drug Deliv Transl Res. 2013;3:42‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hopkins C, Slack R, Lund V, et al. Long‐term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. 2009;119:2459‐2465. [DOI] [PubMed] [Google Scholar]
- 20. Rudmik L, Mace J, Soler ZM, et al. Long‐term utility outcomes in patients undergoing endoscopic sinus surgery. Laryngoscope. 2014;124:19‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127:550‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mascarenhas JG, da Fonseca VM, Chen VG, et al. Long‐term outcomes of endoscopic sinus surgery for chronic rhinosinusitis with and without nasal polyps. Braz J Otorhinolaryngol. 2013;79:306‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. OptiNose US, Inc . XHANCE™ [Prescribing Information]. Yardley, PA: OptiNose US, Inc.; 2017. https://www.xhance.com/files/XHANCE_Full_Prescribing_Information.pdf. Accessed May 10, 2020. [Google Scholar]
- 24. Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo‐controlled, double‐blind trial of the exhalation delivery system with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33:69‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leopold, DA , Elkayam D, Messina JC, et al. NAVIGATE II: Randomized, double‐blind trial of the exhalation delivery system with fluticasone for nasal polyposis. J Allergy Clin Immunol. 2019;143:126‐134. [DOI] [PubMed] [Google Scholar]
- 26. Maruish ME, ed. User's Manual for the SF‐36 v2 Health Survey. 3rd ed. Lincoln, RI: QualityMetric, Inc.; 2011. [Google Scholar]
- 27. Brazier J, Roberts J, Deverill M. The estimation of a preference‐based measure of health from the SF‐36. J Health Econ. 2002;21:271‐292. [DOI] [PubMed] [Google Scholar]
- 28. Fryback DG, Dunham NC, Palta M, et al. U.S. norms for six generic health‐related quality‐of‐life indexes from the national health measurement study. Med Care. 2007;45: 1162‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF‐6D. Health Qual Life Outcomes. 2003;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]


