Abstract
Most drug labels do not contain dosing recommendations for a significant portion of real‐world patients for whom the drug is prescribed. Current label recommendations predominately reflect the population studied in pivotal trials that typically exclude patients who are very young or old, emaciated or morbidly obese, pregnant, or have multiple characteristics likely to influence dosing. As a result, physicians may need to guess the correct dose and regimen for these patients. It is now feasible to provide dose and regimen recommendations for these patients by integrating available scientific knowledge and by utilizing or modifying current regulatory agency‐industry practices. The purpose of this commentary is to explore several factors that should be considered in creating a process that will provide more effective, safe, and timely drug dosing recommendations for most, if not all, patients. These factors include the availability of real‐world data, development of predictive models, experience with the US Food and Drug Administration (FDA)’s pediatric exclusivity program, development of clinical decision software, funding mechanisms like the Prescription Drug Users Fee Act (PDUFA), and harmonization of global regulatory policies. From an examination of these factors, we recommend a relatively simple, efficient expansion of current practices designed to predict, confirm, and continuously improve drug dosing for more patients. We believe implementing these recommendations will benefit patients, payers, industry, and regulatory agencies.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ We know there are patient characteristic gaps in drug dosing (e.g., pregnancy, pediatrics, and obesity) and we also know how to create effective drug dosing recommendations for populations and individual patients.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Recommendations are provided to integrate prior knowledge (prediction), clinical trial experience, and real‐world data into a systematic way to create timely drug dosing for patient characteristics likely to influence outcome.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The inclusion of real‐world patient information provides an enhanced opportunity with the clinical trial patient experience to continuously improve drug efficacy and safety.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The patient rather than the clinical trial is placed at the center of the effort to deliver more effective and safe dosing. Clinical pharmacologists’ research will be more directly impactful on patient wellness.
Today clinicians may have to guess the drug dosing regimen if the patient is a neonate, very old, morbidly obese, emaciated, or has any number of other factors (e.g., unusual genotype, renal failure, and/or taking an interacting drug). It has been estimated that clinicians treating neonate patients may face this dilemma five times daily. 1 Relatively recent regulatory changes have provided a scientific and clinical basis for dosing pediatric patients for many new drugs. 2 Yet, drug dosing is still guesswork for clinicians treating the many patients who are not well‐represented in clinical trials upon which the regulatory approved label is primarily based. Current labels typically fall between one of two patient drug dosing extremes where either each patient’s dose is titrated to a biomarker (e.g., plasma glucose, cholesterol, or drug concentration), or one dosing regimen is approved for all adult patients without adequately understanding the limits based on age, size, organ function, genetics, or drug interactions.
At market approval, the new drug labelled dosing regimen usually reflects the dosing scheme used in the phase III pivotal studies, upon which the regulatory approval decision was based with additional dosing adjustments for pharmacokinetic (PK) and/or pharmacodynamic (PD) reasons. Dosing adjustment characteristics are either quantitative or directional (e.g., increase/decrease dose) and univariate (one factor, not combinations of factors). Although more recent legislation has provided incentives for pharmaceutical companies to provide dosing recommendations for children, much of this work is incomplete at initial approval with a multiple year gap until pediatric labeling is approved and incorporated. Further, adult dose selection may be guesswork for a patient who has characteristics at the extremes of size or age, is pregnant, or who is medically complex (polypharmacy or specific genotypes), as these types of patients typically are excluded from clinical trials. 3 , 4 , 5 However, it does not have to be this way. It is now feasible to decrease the uncertainty of dosing such patients by integrating available scientific knowledge, utilizing newly available real‐world patient (RWP) data‐analytics, and modifying current US Food and Drug Administration (FDA)‐industry practices.
The August 12, 2019, FDA‐University of North Carolina at Chapel Hill (UNC) public meeting on precision dosing highlighted the potential importance of improved dosing estimations for patients with drug‐disease targets in which the potential outcome from underdosing or overdosing could result in serious morbidity or even death. 6 One senior FDA physician indicated this (i.e., the age of dosing individualization) could be the third major milestone in drug development and regulation after the ages of safety in 1938 and efficacy in 1962. 7 The purpose of this commentary is to explore several factors that should be considered when creating a process for improving dosing recommendations that will provide more effective, safe, and timely drug dosing recommendations for most, if not all patients.
SIX CHANGES IMPACTING PRECISION DOSING
-
1.
Real‐world data (RWD) and real‐world evidence (RWE) from real‐world patients (RWP). The availability of patient RWD and RWE from RWPs is derived mainly from patient insurance claims and electronic health data sources over the past 10–15 years in the United States. RWD can come from a variety of sources but usually refers to either insurance claims data (e.g., Medicare) or electronic health record (EHR) data (e.g., Optum, Sentinel), as described in the FDA Framework for Real World Evidence Program. 8 This availability has created new opportunities to understand drug efficacy and safety and these insights are particularly useful when suboptimal dosing can result in death or severe morbidity (e.g., stroke). 9 Given that clinical trials may represent < 50% of the patient diversity present in the real‐world market population, availability of RWD provides an opportunity to understand drug benefit and risk beyond what is possible using data from registrational trials and to create drug dosing strategies, which improve patient outcomes when warranted. 10 Using atrial fibrillation as an example, the dabigatran phase III clinical trial had 18,113 patients on treatment (dabigatran 150 mg and 110 mg, warfarin) whereas a similar comparison or RWPs included 134,414 patients (dabigatran 150 mg and 75 mg, warfarin) with more diverse characteristics. 11 , 12 The overall drug outcome effects were quite similar. When subgroups were examined in the RWP investigation, results suggested that the benefit of dabigatran over warfarin may be diminished in very elderly (≥ 85) women and raised concerns regarding dosing in patients identified as having severe renal impairment. 12 As more comparisons between completed clinical efficacy‐safety trials and RWE become available, drug outcome effects will likely enable a richer understanding reflecting the true impact of dosing on all patients. 13 To assess the regulatory utility of RWE, the FDA has commissioned research to determine how well RWE can replicate the results of completed randomized clinical trials (e.g., phase III studies) focusing on published positive and negative studies in four disease areas (i.e., cardiovascular, endocrine, musculoskeletal, and pulmonary). 14 The FDA is also sponsoring research to determine the value of RWE in predicting results for randomized clinical trials that have not been completed. 15 Although there is growing evidence RWE will have value for some regulatory decisions, there are many bias‐related issues that need to be both defined and addressed. 16 The growth in RWD in data diversity, patient numbers, and data quality will have a major impact on understanding who is responding and how to use drugs more precisely.
Recommendation. The FDA and insurance payers should assess and share known phase III trial‐RWD drug efficacy‐safety gaps for major diseases and treatments
-
2.
Predictive model development. In developing drugs, far more progress has been made in predicting drug PKs (i.e., absorption, distribution, elimination, and exposure) than PDs (i.e., efficacy and safety). Because drug exposure often correlates with efficacy and safety, there is value in using these predictions to estimate dosing in patients not represented in phase III trials. For example, progress is being made in predicting relatively rare, serious drug safety issues (e.g., drug‐induced liver injury, 17 , 18 and Torsades de Pointes 19 ) and PKs (e.g., drug interactions, 20 pregnancy, 21 , 22 obesity, 23 children, 24 , 25 , 26 and the elderly 27 ) over the past 20 years. Although these predictions do not yet replace confirmation for certainty, they can be clinically useful to minimize risk and optimize benefit. A comparison of predicted to actual measured mean drug clearance across many different drugs in these special populations shows that clearance is within one‐ to two‐fold predictive in some instances. 28 , 29 Because drugs are primarily cleared or eliminated from the body by renal excretion and hepatic metabolism, the FDA has expected companies to perform PK studies in patients with either renal and/or hepatic impairment to determine if dosing adjustment is needed. Drug dosing regimens are included in drug labels designed to match the approximate drug concentration exposure from the clinical trials in patients with normal kidney and liver function. This practice assumes that kidney or liver impairment does not change drug PDs and is consistent with regulatory guidances on organ impairment, which focus on PK studies with only a brief mention to possible alterations in PDs.
With regard to predicting drug dosing for patients beyond the phase III trial experience, it is important to understand the following:
Disease. Various disease variables (e.g., symptoms, biomarkers, patient‐reported outcomes, and death) are used to measure efficacy in clinical trials and regulatory submissions. This information is useful in constructing disease models linking patient characteristics, biomarker measurement, outcomes, and interventions. Today, many drug‐disease models have been published or may be available in the FDA Clinical Pharmacology new drug application (NDA) review documents, available at Drugs@FDA.gov. Sponsor companies and FDA reviewers separately use disease models to help answer regulatory questions in the NDA or during the regulatory review. In addition to publications, this information can be found in the FDA Clinical Pharmacometrics review at Drugs@FDA.com. It is possible the drug exposure response (efficacy‐safety) relationship developed in the phase III trial will be different for special patient populations not studied (e.g., pediatrics and renal failure).
Drug. Clearance prediction is based on determining a correlation between the drug fractional clearance mechanisms and clinical measures (e.g., glomerular filtration rate, liver function, size, genotype, and sex). Even when drug efficacy has not been demonstrated in these “special” populations, using these predictions should have clinical utility. In fact, labels often reflect dosing adjustments for some cases of organ impairment based on observed PK alterations without having demonstrated the efficacy of these doses. Prediction accuracy and patient outcomes should be expected to improve with experience and continued research. Genomic data are being included in product labeling more regularly with a recent review noting that drug‐gene correlations accompanied by pharmacogenomic information were in 208 FDA product‐approved labeling. Additionally, there is an opportunity to have more consistent and optimal implementation across worldwide regulatory agencies. 30
Recommendation: Clinical specialist (e.g., cardiology, neurology, and clinical pharmacology‐pharmacy) societies should create a high priority drug‐disease target list where more precise dosing is likely to result in improved patient outcome. High priority criteria should be created by physicians and pharmacists who use the drugs to treat patients. General criteria to consider are drug‐disease targets dosing risks include death and/or significant morbidity from underdosing or overdosing and also where patient characteristics are known to significantly influence drug PKs and/or PDs. It is important to create/maintain a drug‐disease model public library, including computer code and prospective qualification testing. 31
-
3.
Pediatric efficacy‐safety drug dosing experience. The pediatric special population drug development experience over the past 20 years is also informative for other patient special groups (e.g., very old and pregnancy) and for prediction purposes. US legislation empowers the FDA to both require drug companies to perform pediatric studies leading to pediatric labeling and providesa company 6‐month additional patent exclusivity for completing specifically requested work. The clinical and scientific benefits from this program include providing a systemic mechanism for conducting pediatric research on new drugs, establishing an efficacy, safety, and PK basis for use and dosing for children (from premature infants through adolescents), as well as stimulating research on how best to conduct such studies. A major success was establishing a disease similarity link (i.e., extrapolation) between children and adults with partial onset seizures. This enables a less expensive and more successful PK bridging study to be used for approval. Other successes include the development of a regulatory approval pathway to extrapolate data from animal studies to children when clinical studies are not feasible, and providing useful pediatric labeling information for many drugs useful in seizures, diabetes, and HIV. 1 , 2 , 31 There have also been significant limitations to this approach, including the fact that it takes 7 or more years after a drug was initially approved for the label to reflect pediatric dosing and use, and the rate of requested study completion has been low (28.8% for efficacy studies and 55.9% for PK studies). Therefore, pediatric labeling changes for some priority indications may not occur. Based on data from completed studies, there is now a reasonably good ability to predict pediatric PKs for major clearance pathways. 26 Pharmaceutical companies have an estimated 680% median return on investment for doing requested pediatric studies as a result of gaining 6 months of additional patent exclusivity. 32 Based on this pediatric experience, some have suggested that we should replicate this regulatory model for the patient groups, such as those who are very old, pregnant, or patients at the extremes of body size. 33 , 34 If PK predictions can be applied assuming the exposure target is similar, dosing recommendations could be provided in the initial label when the drug is first approved. However, for these “special” patient populations, as well as in pediatrics, instead of requiring discrete prospective studies to detect differences in drug efficacy and safety, perhaps it would be feasible (or preferable) to focus on RWD from EHR and claims data. Funding clinical sites to collect enriched clinical RWD for regulatory efficacy, safety, and dosing questions might generate data from larger, more diverse patient groups than prospective clinical studies (e.g., Sentinel Network).
Recommendation: Because the pediatric efficacy, safety, and dosing project is a useful template for other special population groups, it is worthwhile to have the FDA decide what makes this program successful for children and to decide what learning can be transferred to other patient groups
-
4.
Clinical decision support (CDS) software. EHRs use a common library of clinical variable terms (e.g., demographics, disease, laboratory values, and outcomes) supporting patient care. CDS software should be seamlessly integrated with the drug‐disease models discussed above to facilitate dose individualization. The inputs to the models include patient attributes likely to influence drug exposure and response to treatment. These inputs can be seamlessly provided to the model from the patient’s EHR via the CDS software. This electronic health care environment enables CDS software to help clinicians better diagnose and treat disease, support financial health aspects (e.g., billing and cost efficiency), and enable researchers to better understand variables influencing disease outcome and optimal treatment. 35 Currently, about 90% of US hospitals and clinics have implemented EHRs, whereas this was only about 10% in 2008 after Congress’ approval of the 2009 Hitech Act along with the Affordable Care Act designed to increase access to health care. 36 , 37 Instead of paper prescriptions, for example, computerized physician order entry software enters the new drug and dosing regimen into the EHR and sends the prescription to the pharmacy. CDS software can be available at the point of prescribing to instantaneously assist with drug selection, dose selection, and identifying potential drug interactions. CDS drug dosing software has the ability to make the prescriber more aware of the patient’s characteristics, which could influence individual dosing requirements, along with existing knowledge about selecting the optimal drug and dosing regimen in the drug’s label, curated dosing programs, and the literature. For example, instead of the busy clinician needing to check the label online or perform a literature search on how to dose the drug in the eighth month of pregnancy, both the recommended dose and background information would be instantaneously available. The FDA has announced it intends to play a key role in certifying software quality as it relates to drug indications and dosing. 38
Recommendation: Software developers should design and implement drug‐dosing CDS software supporting efficacy‐safety and convenience needs of patients, practice, and education needs of prescribers. The FDA should develop a pathway that both facilitates implementation and that assures that the software will perform adequately
-
5.
Prescription Drug Users Fee Act (PDUFA). Since 1992 when the PDUFA was first approved, the FDA has charged the industry user fees for NDA reviews and various FDA‐sponsor drug regulatory meetings. 39 User fees allow the FDA to hire staff and perform research to better serve the public and meet pharmaceutical industry needs. The PDUFA must be reauthorized every 5 years based on a negotiated agreement with the pharmaceutical industry concerning new fee structure along with mutually agreed upon objectives to be completed over the next 5 years. Currently, for example, the PDUFA VI (2018‐22) includes deliverables, such as RWE regulatory utility, model informed drug development, and safety risk detection. 40 The PDUFA provides a framework and funding source to continuously improve how the FDA and the pharmaceutical industry develop drugs.
Recommendation: The PDUFA negotiations by the FDA and the pharmaceutical industry should support the process and research recommendations to develop better dosing for relevant old and new drugs and to translate this knowledge to prescribers and patients.
-
6.
International Drug Approval and Labeling. Although the primary basis for new drug approval in each country or region is usually the same pivotal clinical trial efficacy/safety experience, local drug regulatory agencies look through the lens of their local population’s disease prevalence, medical practice, genotypes, body size, and clinical trial experience characteristics in making approval and labeling decisions, including dose selection. In Japan for instance, of the 190 new drugs approved over an 8‐year period, the dose was higher in the United States for 60 drugs and higher in Japan for 13 drugs. 41 The most common reasons for these differences were more often due to issues like whether dose finding studies were conducted in both the United States and Japan and whether the US highest dose was selected for study in Japan, rather than genetic population differences. The International Council on Harmonization (ICH) publishes agreed drug development standard and practices enabling greater development efficiency for sponsors to meet local country needs with a common development plan. 42
Recommendation: Country differences in drug dose selection seem reasonable to expect when the decision is made based on the likelihood of providing the best opportunity for local patients to achieve efficacy without toxicity based on evidence. Several ways to achieve this are to include more local patients in global clinical pivotal studies, include adequate measures to characterize the patient, disease, and drug so that responders can be differentiated from nonresponders, and to use modeling to bridge the clinical trial results to local patient population characteristics. This may require revising ICH guidances on clinical trials, dose‐response, and ethnicity.
ADJUSTING DRUG DEVELOPMENT AND REGULATION TO ENABLE EFFECTIVE DOSING FOR ALL
Consideration should be given to making a few relatively minor changes to the current drug development‐regulatory paradigm based on three principles:
Commitment to provide drug dosing information supporting efficacy and safety for all patients likely to be prescribed the drug as close to market approval as possible and then continuously improve this information by incorporation of RWE.
Continuously invest in developing better prediction tools supporting efficacy, safety, and dosing across projects.
Specific company and the FDA actions taken to develop and implement precision dosing during the investigational new drug process are described in Figure 1 below. These eight steps are designed to determine the need for precision dosing for a new drug, develop the information and analysis needed to create dosing recommendations, and to systematically revise dosing recommendations over the product life cycle.
The eight steps are:
Sponsor shares with the FDA the potential RWP gap estimates between the anticipated pivotal efficacy clinical evidence (e.g., phase III trials) and the likely market population (see Table 1 ). A new FDA Guidance is needed to describe how to estimate the RWP gap and prevalence.
Declare risk of inadequate dosing precision and factors likely to alter PK or drug response in order to determine patient types in most need of precision dosing recommendations.
The FDA comments on and agrees with the Sponsor with final gap analysis and plans. The sponsor’s gap plan components should be included in the Guidance. This should include pivotal clinical trial considerations, such as how to recruit a more diverse phase III patient sample, dosing strategy, and population PKs plan.
Clinical trials will include collection and sampling time plans supporting analysis of efficacy, safety, and population PK/PD. The FDA should both agree with the plan and be authorized to require implementation.
The NDA submission will include the statistical and PK/PD analyses along with a modeling prediction report for dosing in patient groups not studied (or not well represented) in the pivotal study(s) (e.g., pregnancy, obesity, and complexity). (FDA Guidance needed.)
The FDA approved label will include dosing for all relevant patient characteristics based on either evidence or prediction where appropriate. CDS software consistent with the label will also be approved. The sponsor and the FDA should conduct a drug population model drug exposure prediction range for the RWP population characteristic ranges likely produce drug exposure extremes. These results could serve as an alert for dosing and labeling consideration. Sponsor and FDA pivotal trial population PK/PD analyses will comply with standards and will be available to the public in sufficient detail that the models can be replicated by future investigators (e.g., model code and assumptions underlying the model). The sponsor’s postmarket clinical predictions confirmation plan (e.g., pediatrics, obesity, and complexity) will be approved. Dosing recommendations for specific characteristics (e.g., weight and glomerular filtration rate) will indicate the limits based on label dosing recommendations. (FDA Guidance needed.)
-
RWP postmarket studies.
RWP drug efficacy‐safety studies will likely be conducted at various times postmarket to assess for the population in general along with patient subgroups variables, such as age, disease severity, comorbidities, socio‐economic socio‐geographic status, body size, organ function, genotype, and new drug formulations (e.g., line extensions and generics). It will be worthwhile to determine the value of applying a population PK model to predict the individual RWP trough steady‐state drug concentration (or other relevant PK metric) to be used as another analysis variable that can be related to efficacy, safety, and dosing. It will be useful for the FDA to include this option in their current research comparing new drug efficacy and safety from the phase III trial to RWP data evaluations. The first RWP efficacy‐safety assessment will be conducted by about 2 years postapproval to adjust dosing estimates if needed. (FDA Guidance needed.)
PK dosing predictions for RWP subgroups not represented in the pivotal trials will be prospectively assessed for prediction accuracy. This will need to be a relatively large study providing balance across the various groups enabling drug dosing recommendations to be adjusted (e.g., label and software) in conjunction with the above efficacy‐safety study results. The results will also be useful to revise the drug‐disease population PK or PK/PD model made available to the public. (FDA Guidance needed.)
Because new drugs are approved by international regulatory agencies (e.g., the European Medicines Agency (EMA), the Pharmaceuticals and Medical Devices Agency (PMDA), and the China Food and Drug Administration (CFDA)) consideration will need to be given to establishing a precision dosing guideline for the International Council for Technical Requirements for Pharmaceuticals for Human Use (ICH) covering the above technical requirements and standards. This should be the basis for standards for sharing RWP data, models, and tools as appropriate because we can learn from broader patient experience. In addition, these standards will make the work easier for companies to implement.
Figure 1.
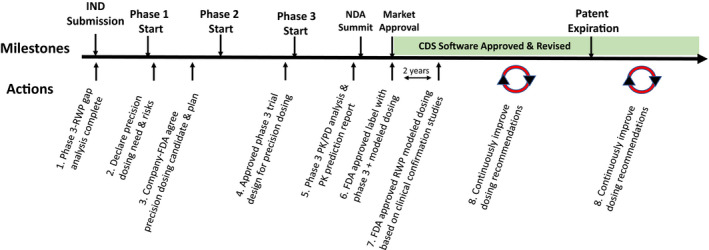
Precision drug dosing recommended actions over the investigational new drug (IND) and new drug application (NDA) Life Cycle. There are eight proposed actions over the research and development drug cycle designed to produce more precise dosing recommendations at market approval or soon thereafter and continuously improve dosing based on real‐world data (RWD). CDS, clinical decision support; FDA, US Food and Drug Administration; PD, pharmacodynamic; PK, pharmacokinetic; RWP, real‐world patient.
Table 1.
Drug dosing checklist for drug and patient’s characteristics likely to use this drug and affect drug exposure
|
RESEARCH PROJECTS AND INFRASTRUCTURE DEVELOPMENT
These changes provide a number of new research opportunities to provide more optimal dosing recommendations for all patients. It will be useful to establish working groups with diverse perspectives to create prioritized investment topics including the following:
Develop precision dosing recommendations for high priority off‐patent drugs. Lists need to be prepared with input from clinicians (e.g., physicians and pharmacists) and possibly patient advocacy groups. This could be coordinated by American Society for Clinical Pharmacology and Therapeutics (ASCPT) by therapeutic area possibly in conjunction with a disease centric society (e.g., American Cancer Society). These groups can participate in raising funding, approving a research plan and investigators, reviewing plans with the FDA Office of New Drugs therapeutic division for comment before starting, and, once the research is complete, filing a Citizen Petition to add more precise dosing to the label.
Create a regulatory path to revise drug labeling for generic drugs by including more precise dosing based on new research. This will likely require a Citizen Petition application to the FDA Office of New Drugs.
Better quantify the impact of individual patient characteristics on efficacy, safety, and dosing.
Better predict drug absorption and clearance for different mechanisms across patient‐disease diversity leading to better prediction tools.
Develop better disease models from clinical end points to biomarkers, including wearable devices and patient‐reported outcomes.
Develop CDS software that recommends optimal treatment choices in real time (e.g., drug dose and regimen) based on patient data in their chart (disease, phenotype, genotype, and laboratory data) and current drug optimal drug dosing information from broader sources.
Develop better patient drug exposure level sampling techniques/devices and assay technology offering greater analytical accuracy, speed, convenience, and cost‐effective data.
Develop and maintain clinical data and model warehouses/libraries facilitating both research and dosing tool development/improvement.
Development of new formulations that better support optimization of individual dosing.
Develop regulatory standards for what constitutes adequate predictive ability to be used for dosing recommendations.
FUNDING
Several funding sources to consider are the PDUFA, Generic Drug User Fee Amendment (GDUFA) user fees, and creating the FDA budget lines through the Center for Drug Evaluation and Research (CDER), and the Center for Devices and Radiological Health (CDRH) because they may each own a portion of this work. The PDUFA mechanism is particularly interesting in that there is an established mechanism for funding tied to goals and deliverables that is renewed every 5 years. Because CDS tools will most likely be developed and maintained by for‐profit companies, user fees can be established both for using the models curated by the FDA or academic sites along with regulatory tool approval.
CONCLUSION
Changes to current drug development processes are proposed that would constitute a relatively simple expansion of current practices extended to take advantage of the expanding promise of RWPs. The basic idea is to learn, predict, and confirm drug efficacy, safety, and dosing over the product life cycle in a manner that more patients subgroups are represented in an effective and timely manner. These changes will require new standards and regulatory guidances. They will create opportunities for more accurate and useful RWD and perhaps also create enriched clinical study designs. Because there will be multiple opportunities to quantitatively assess efficacy and safety, there will be more opportunities to create and evaluate predictive models. An assessment will be needed to determine the cost, benefit, and incentives required to execute these changes. Compared with the current pediatric experience, which is based on prospective clinical studies, the success rate should be greater and per patient costs much less. There will be opportunities to determine when prior experience warrants using prediction as the primary basis for dosing schemes. Likewise, there will be opportunities to determine when RWP type studies will be sufficient vs. when prospective trials are needed to answer specific questions. Proposed topics for the FDA, industry, and academic action are listed in Table 2 .
Table 2.
Topics requiring FDA, industry, and academic action
|
There should be ample benefits for patients, payers, companies, and the FDA. Patients will benefit from having more reliable information that the drug is efficacious and safe in more patients. Assuming bespoke drug treatment results in greater efficacy and safety for populations, payers (and patients) should have a lower cost burden. Assuming drugs will become more effective and safe for more patients, then the product should have greater value for the sponsor company. The FDA would now have a more wholistic drug development process from beginning to end that may be more effectively and reliably regulated. Early information would lead to decisions that would need to be revisited in a more consistent and quantitative manner over a product’s life cycle. These changes should lend greater value to developing high‐quality data that can be shared so that more useful analyses, predictions, and standards can be developed over time.
Upcoming PDUFA VII negotiations are a good vehicle to begin creating these changes. This is the age of individualization for disease mechanisms directing drug molecule design and dosing. The tools are currently available to build this new drug development future now enabling all patients to be served with better drugs and dosing.
Funding
No funding was received for this work.
Conflict of Interest
J.C. and R.P. are paid employees of Pfizer Inc. and F. Hoffmann la Roche, respectively, both pharmaceutical companies that may be affected by the views expressed in this article. All other authors declared no competing interests for this work.
Disclaimer
As an Associate Editor of Clinical Pharmacology and Therapeutics, Richard W. Peck was not involved in the review or decision process for this paper.
Author Contributions
J.R.P., J.C., Y.W., R.P., and D.W. wrote the manuscript. J.R.P. managed revisions.
Acknowledgment
The authors thank Philip D. Walson, MD, Department of Clinical Pharmacology, University Medical Center Göttingen, Göttingen, Germany, for his manuscript review and insightful comments.
References
- 1. Cohen‐Wolkowiez, M. & Benjamin, D.K. Development of therapeutics for children – a tricky balancing act. JAMA Pediatr. 173, 18–19 (2019). [DOI] [PubMed] [Google Scholar]
- 2. Hwang, T.J. , Orenstein, L. , Kesselheim, A.S. & Bourgeois, F.T. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatr. 173, 68–74 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emanuela Voinescu, P. et al Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy. Neurology 91, E1228–E1236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drenth‐van Maanen, A.C. , Wilting, I. & Jansen, P.A.F. Prescribing medicines to older people—How to consider the impact of ageing on human organ and body functions. Br. J. Clin. Pharmacol. 10.1111/bcp.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smit, C. et al Population pharmacokinetics of vancomycin in obesity: Finding the optimal dose for (morbidly) obese individuals. Br. J. Clin. Pharmacol. 86, 303–317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Office of Translational Sciences Precision Dosing: Defining the need and approaches to deliver individualized drug dosing in the real‐world setting ‐ 08/12/2019| FDA <https://www.fda.gov/drugs/precision‐dosing‐defining‐need‐and‐approaches‐deliver‐individualized‐drug‐dosing‐real‐world‐setting>. [DOI] [PMC free article] [PubMed]
- 7. McCaughan, M. New age thinking: US FDA’s temple sees third era of drug development <https://pink.pharmaintelligence.informa.com/PS140700/New‐Age‐Thinking‐US‐FDAs‐Temple‐Sees‐Third‐Era‐Of‐Drug‐Development> (2019).
- 8. US Food and Drug Administration (FDA) . Framework for FDA’s Real‐World Evidence Program. FDA; 1–49 <https://www.fda.gov/media/120060/download> (2019). [Google Scholar]
- 9. Gonzalez, D. et al Precision dosing: public health need, proposed framework, and anticipated impact. Clin. Transl. Sci. 10, 443–454 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spong, C.Y. & Bianchi, D.W. Improving public health requires inclusion of underrepresented populations in research. JAMA 319, 337–338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham, D.J. et al Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 131, 157–164 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Connolly, S.J. et al Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151 (2009). [DOI] [PubMed] [Google Scholar]
- 13. Sherman, R.E. et al Real‐world evidence – What is it and what can it tell us? N. Engl. J. Med. 375, 2293–2297 (2016). [DOI] [PubMed] [Google Scholar]
- 14. Gingery, D. Real‐World Data Could Get Boost From Trial Replication Project: Pink Sheet <https://pink.pharmaintelligence.informa.com/PS122984/RealWorld‐Data‐Could‐Get‐Boost‐From‐Trial‐Replication‐Project> (2018).
- 15. Hale, C. Using real‐world data, the FDA aims to predict randomized trial results before they’re done. FierceBiotech <https://www.fiercebiotech.com/medtech/using‐real‐world‐data‐fda‐aims‐to‐predict‐randomized‐trial‐results‐before‐they‐re‐done> (2019).
- 16. Franklin, J.M. , Glynn, R.J. , Suissa, S. & Schneeweiss, S. Emulation differences vs. biases when calibrating real‐world evidence findings against randomized controlled trials. Clin. Pharmacol. Ther. 107, 735–737 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woodhead, J.L. , Watkins, P.B. , Howell, B.A. , Siler, S.Q. & Shoda, L.K.M. The role of quantitative systems pharmacology modeling in the prediction and explanation of idiosyncratic drug‐induced liver injury. Drug Metab. Pharmacokinet. 32, 40–45 (2017). [DOI] [PubMed] [Google Scholar]
- 18. Yang, K. , Woodhead, J.L. , Watkins, P.B. , Howell, B.A. & Brouwer, K.L.R. Systems pharmacology modeling predicts delayed presentation and species differences in bile acid‐mediated troglitazone hepatotoxicity. Clin. Pharmacol. Ther. 96, 589–598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz, P.J. , Woosley, R.L. & Woosley, R.L. Predicting the Unpredictable: drug‐induced QT prolongation and Torsades de pointes. J. Am. Coll. Cardiol. 67, 1639–1650 (2016). [DOI] [PubMed] [Google Scholar]
- 20. Hsueh, C.H. , Hsu, V. , Pan, Y. & Zhao, P. Predictive performance of physiologically‐based pharmacokinetic models in predicting drug‐drug interactions involving enzyme modulation. Clin. Pharmacokinet. 57, 1337–1346 (2018). [DOI] [PubMed] [Google Scholar]
- 21. Dallmann, A. , Ince, I. , Coboeken, K. , Eissing, T. & Hempel, G. A physiologically based pharmacokinetic model for pregnant women to predict the pharmacokinetics of drugs metabolized via several enzymatic pathways. Clin. Pharmacokinet. 57, 749–768 (2018). [DOI] [PubMed] [Google Scholar]
- 22. Dallmann, A. et al Physiologically based pharmacokinetic modeling of renally cleared drugs in pregnant women. Clin. Pharmacokinet. 56, 1525–1541 (2017). [DOI] [PubMed] [Google Scholar]
- 23. Ghobadi, C. et al Application of a systems approach to the bottom‐up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin. Pharmacokinet. 50, 809–822 (2011). [DOI] [PubMed] [Google Scholar]
- 24. Zhou, W. et al Predictive performance of physiologically based pharmacokinetic and population pharmacokinetic modeling of renally cleared drugs in children. CPT Pharmacometrics Syst. Pharmacol. 5, 475–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cerruti, L. , Bleyzac, N. & Tod, M. Semi‐mechanistic model for predicting the dosing rate in children and neonates for drugs mainly eliminated by cytochrome metabolism. Clin. Pharmacokinet. 57, 831–841 (2018). [DOI] [PubMed] [Google Scholar]
- 26. Bi, Y. et al Role of model‐informed drug development in pediatric drug development, regulatory evaluation, and labeling. J. Clin. Pharmacol. 59, S104–S111 (2019). [DOI] [PubMed] [Google Scholar]
- 27. Chetty, M. et al Physiologically based pharmacokinetic modelling to guide drug delivery in older people. Adv. Drug Deliv. Rev. 135, 85–96 (2018). [DOI] [PubMed] [Google Scholar]
- 28. Yee, K.L. et al Evaluation of model‐based prediction of pharmacokinetics in the renal impairment population. J. Clin. Pharmacol. 58, 364–376 (2018). [DOI] [PubMed] [Google Scholar]
- 29. Edginton, A.N. , Schmitt, W. , Voith, B. & Willmann, S. A mechanistic approach for the scaling of clearance in children. Clin. Pharmacokinet. 45, 683–704 (2006). [DOI] [PubMed] [Google Scholar]
- 30. Koutsilieri, S. , Tzioufa, F. , Sismanoglou, D.C. & Patrinos, G.P. Unveiling the guidance heterogeneity for genome‐informed drug treatment interventions among regulatory bodies and research consortia. Pharmacol. Res. 153, 104590 (2020). [DOI] [PubMed] [Google Scholar]
- 31. Harnisch, L. , Matthews, I. , Chard, J. & Karlsson, M.O. Drug and disease model resources: a consortium to create standards and tools to enhance model‐based drug development. CPT Pharmacometrics Syst. Pharmacol. 2, e34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sinha, M.S. , Najafzadeh, M. , Rajasingh, E.K. , Love, J. & Kesselheim, A.S. Labeling changes and costs for clinical trials performed under the US Food and Drug Administration Pediatric Exclusivity Extension, 2007 to 2012. JAMA Intern. Med. 178, 1458–1466 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jadhav, P.R. et al A proposal for scientific framework enabling specific population drug dosing recommendations. J. Clin. Pharmacol. 55, 1073–1078 (2015). [DOI] [PubMed] [Google Scholar]
- 34. Liu, T. , Ghafoori, P. & Gobburu, J.V.S. Allometry is a reasonable choice in pediatric drug development. J. Clin. Pharmacol. 57, 469–475 (2017). [DOI] [PubMed] [Google Scholar]
- 35. Bright, T.J. et al Effect of clinical decision‐support systems: a systematic review. Ann. Intern. Med. 157, 29–43 (2012). [DOI] [PubMed] [Google Scholar]
- 36. Pawloski, P.A. , Brooks, G.A. , Nielsen, M.E. & Olson‐Bullis, B.A. A systematic review of clinical decision support systems for clinical oncology practice. J. Natl. Compr. Cancer Netw. 17, 331–338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hersh, W.R. et al Outcomes from health information exchange: systematic review and future research needs. JMIR Med. Informatics 3, e39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. US Food and Drug Administration (FDA) . Clinical Decision Support Software | FDA <https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/clinical‐decision‐support‐software> (2019).
- 39. Prescription Drug User Fee Act <https://en.wikipedia.org/wiki/Prescription_Drug_User_Fee_Act>.
- 40. Prescription Drug Users Fee Act . PDUFA reauthorization performance goals and procedures fiscal years 2018 through 2022 <https://www.fda.gov/media/99140/download>
- 41. Arnold, F.L. , Fukunaga, S. , Kusama, M. , Matsuki, N. & Ono, S. Assessment of factors associated with dose differences between Japan and the United States. Clin. Pharmacol. Ther. 95, 542–549 (2014). [DOI] [PubMed] [Google Scholar]
- 42. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use <https://www.ich.org/page/ich‐guidelines>.


