Abstract
Objective
We aim to compare the cost‐effectiveness of the old cytology programme with the new high‐risk human papillomavirus (hrHPV) screening programme, using performance indicators from the new Dutch hrHPV screening programme.
Design
Model‐based cost‐effectiveness analysis.
Setting
The Netherlands.
Population
Dutch 30‐year‐old unvaccinated females followed up lifelong.
Methods
We updated the microsimulation screening analysis (MISCAN) model using the most recent epidemiological and screening data from the Netherlands. We simulated both screening programmes, using the screening behaviour and costs observed in each programme. Sensitivity analyses were performed on screening behaviour, utility losses and discount rates.
Main outcome measures
Cervical cancer incidence and mortality rates, number of screening tests and repeat tests, colposcopy referrals by lesion grade, costs from a societal perspective, quality‐adjusted life years (QALYs) gained and cost‐effectiveness.
Results
The new Dutch cervical cancer screening programme decreased the cervical cancer mortality by 4% and the incidence by 1% compared with the old programme. Colposcopy referrals of women without cervical intra‐epithelial neoplasia grade 2 or worse, increased by 172%, but 13% more QALYs were still achieved. Total costs were reduced by 21%, mainly due to fewer screening tests. Per QALY gained, the hrHPV programme cost 46% less (€12,225) than the cytology programme (€22,678), and hrHPV‐based screening remained more cost‐effective in all sensitivity analyses.
Conclusions
The hrHPV‐based screening programme was found to be more effective and cost‐effective than the cytology programme. Alternatives for the current triage strategy should be considered to lower the number of unnecessary referrals.
Tweetable abstract
First results after implementation confirm that HPV screening is more cost‐effective than cytology screening.
Keywords: Cost‐benefit analysis, early detection of cancer, human papillomavirus DNA tests, Netherlands, uterine cervical neoplasms
Tweetable abstract
First results after implementation confirm that HPV screening is more cost‐effective than cytology screening.
Introduction
In January 2017, the Dutch population‐based cervical cancer screening programme switched the primary screening test from cytology to the high‐risk human papillomavirus (hrHPV) test. Women can now choose either to have a cervical smear taken by their general practitioner (GP) or to use a self‐sampling kit. The latter option was added as an alternative screening method to increase attendance rates in women who feel uncomfortable with taking a test at their GP. The implementation of this new programme was based on, among other considerations, cost‐effectiveness analyses showing that primary hrHPV screening is more cost‐effective than primary cytology screening. 1 , 2 However, as no other country had implemented primary hrHPV screening up to that time, many model inputs had to be based on assumptions, potentially biasing the results. 1 , 2
The Dutch cervical cancer screening programme has been monitored for decades, using high‐quality data. 3 However, information on important performance indicators (such as the participation rate, use of self‐sampling, positivity rates, referral rates, precancerous cervical intra‐epithelial neoplasia [CIN] detection rates and costs) of the primary hrHPV‐based screening programme has only recently been published. 4 Some key indicators were found to be unfavourable for the effectiveness of the new programme, such as a drop of three percentage points in screening participation as well as a lower adherence to triage testing. 4 , 5 This unique information from the implementation of hrHPV‐based screening can now be used as reliable model input for a cost‐effectiveness analysis to compare the new programme with the old cytology‐based screening programme.
Using this newly available monitoring data, we aimed to answer the following research question: What are the costs, effects and cost‐effectiveness of the newly implemented cervical cancer screening programme using primary hrHPV testing compared with the old cytology‐based screening programme? We will simulate scenarios where a 30‐year‐old cohort of unvaccinated women are offered either the full cytology‐based programme or the full hrHPV‐based programme and follow these women up until death. For these women, we will present costs per life years gained and costs per quality‐adjusted life years (QALYs) gained as the main outcome. The number of referrals to a gynaecologist and detected CIN, most of which will not progress to cancer, will be presented, as these are considered to be important harmful effects of screening. 6 , 7 These results are useful for policymakers of similar countries to decide whether a switch to primary hrHPV screening is beneficial for their country.
Methods
To estimate the effects of both the cytology screening programme and the hrHPV‐based screening programme in The Netherlands, the MISCAN‐Cervix (Microsimulation Screening Analyses‐Cervix) model was used. 1 , 8 , 9 , 10 An extensive model description can be found in Appendix S1. In short, MISCAN‐Cervix is a microsimulation model, coded in Borland Delphi 7, that simulates the natural history of cervical cancer in a hypothetical population. Women have an age‐specific risk to acquire one or multiple hrHPV infections which may or may not progress sequentially to CIN1, CIN2 and CIN3 or regress at any time. A CIN3 may progress to a micro‐invasive cancer and later to more invasive cancer stages before it is clinically detected. Different screening strategies can be simulated in this population to quantify and compare the harms and benefits of each strategy (Appendix S1: Figures S1–S4, Table S1). As described in Appendix S1, many model assumptions are based on high‐quality data from the Netherlands Cancer Registry (NCR) and the nationwide network and registry of histo‐ and cytopathology in the Netherlands (PALGA), both having a national coverage. 11 , 12 To reduce the impact of random variability on the predicted outcomes, the model simulates a large population of 10 million women and applies the same sequence of random numbers in each simulation.
Model updates
For this analysis, we extended and recalibrated (Appendix S1: Figures S6–S9) the existing model using the most recent cancer and screening data from the NCR and PALGA 11 , 12 in order to incorporate three new features compared with the previously published model. First, hrHPV infections in the model are now type‐specific, allowing for different progression probabilities per hrHPV type. Four groups of hrHPV types were defined based on their oncogenicity and their presence in different HPV vaccines: 13 , 14 HPV16, HPV18, other hrHPV types covered by the nonavalent vaccine (HPV‐31/33/45/52/58) and the remaining seven hrHPV types (HPV‐35/39/51/56/59/66/68). Second, FIGO2+ cancers were split up into FIGO2, FIGO3 and FIGO4, as survival probabilities differ between those stages. Third, the test characteristics of both cytology and the hrHPV test were updated based on evidence from published literature and to be able to fit well to observed data on interval cancers and false‐positive rates by hrHPV status. 15 , 16 , 17 , 18 In this updated version, 12% of existing precancerous lesions are consistently missed by cytology and the probability of an abnormal cytological result is higher in hrHPV‐positive women (calibrated parameters, see Table S3 in Appendix S1). Multiple studies found the concordance between hrHPV tests from different manufacturers to be lower in lower grade lesions (≤CIN1), suggesting that more hrHPV infections are missed. 15 , 19 Therefore, we now assume that for hrHPV‐positive women, the sensitivity of the hrHPV test increases with the severity of their lesion.
Screening programmes
In the Dutch cytology programme, women aged 30–60 years were invited for screening every 5 years. Women with a high‐grade squamous intra‐epithelial lesion (HSIL) or worse were directly referred to colposcopy, while women with a low‐grade cytological abnormality (i.e. atypical squamous cells of undetermined significance or low‐grade squamous intra‐epithelial lesion) were invited for a repeat test after 6 months. The vast majority of the executive laboratories analysed those cervical smears using both cytology and hrHPV testing, although some still used cytology only. 20 When an HSIL or worse was found at this co‐test, the woman was referred to colposcopy. Women testing hrHPV‐positive were also referred to colposcopy if they had a low‐grade cytological abnormality result. Women testing negative on both tests were discharged from follow up. The remaining women were invited for a repeat cytology test after 12 months (Appendix S1: Figure S5).
In the hrHPV‐based screening programme, women are still invited every 5 years at the ages 30–60; however, the screening interval has been extended to 10 years for women testing hrHPV‐negative at age 40 or 50 and there is an extra invitation at age 65 for women testing hrHPV‐positive at age 60. After a positive hrHPV test, the sample is analysed with cytology, after which women with abnormal cytology results are referred to a gynaecologist, whereas women with normal cytology are invited for a repeat cytology test after 6 months (Appendix S1: Figure S5). Women who are uncomfortable with taking a test at their GP, can request a self‐sampling kit, although if their test result is hrHPV‐positive, they still need to have a smear taken by their GP to test for cytological abnormalities.
In the Netherlands, primary screening and follow‐up tests are fully paid for by the government. If a woman is referred for colposcopy, health insurance covers the diagnosis and treatment costs. Health insurance is obligatory in the Netherlands and each insured person is also liable for an excess.
Model assumptions – demographic characteristics, epidemiology and natural history
A cohort of 10 million women was simulated and followed until death. This cohort represents 30‐year‐old Dutch women in 2019 with regard to their remaining life expectancy (54.3 years), hysterectomy probabilities, hrHPV epidemiology and progression probabilities to CIN and cancer as described in Appendix S1.
Model assumptions – screening behaviour
The screening behaviour of all women in The Netherlands is registered on an individual level in PALGA. Based on these observations, we were able accurately to model the screening behaviour in both programmes. As described in more detail in Appendix S1, the screening behaviour during the cytology programme was based on all women invited in 2015 and the screening behaviour during the hrHPV programme was based on all women invited in 2017.
Participation by age
The age‐specific attendance at the primary test differs between the programmes. Table 1 shows the percentage of the female population without a hysterectomy who participates in screening; in the hrHPV‐based programme this can either be the regular GP test or a self‐sampling kit.
Table 1.
Modelled screening behaviour by type of screening programme: base‐case assumptions
| Screening behaviour | Cytology‐based screening programme | hrHPV‐based screening programme |
|---|---|---|
| GP test participation by age in all women of the population * | ||
| 30 years | 52.3% | 43.4% |
| 35 years | 57.9% | 49.3% |
| 40 years | 64.3% | 56.4% |
| 45 years | 67.6% | 15.6%** |
| 50 years | 70.4% | 61.5% |
| 55 years | 69.6% | 12.7%** |
| 60 years | 66.8% | 60.3% |
| 65 years | NA | 3.1%*** |
| Self‐sampling participation by age * | ||
| 30 years | NA | 5.5% |
| 35 years | NA | 4.8% |
| 40 years | NA | 4.5% |
| 45 years | NA | 0.9%** |
| 50 years | NA | 4.6% |
| 55 years | NA | 1.0%** |
| 60 years | NA | 5.7% |
| 65 years | NA | 0.2%*** |
| Adherence to cytology after a positive self‐sample | NA | 90.1% |
| Adherence to triage testing | ||
| 6 months after primary test | 92.2% | 77.1% |
| 6 months after primary self‐sample | NA | 41.6% |
| 18 months after primary test | 67.3% | NA |
| Adherence to a referral for colposcopy after a | ||
| Direct referral (ASC‐US/LSIL) | NA | 88.4% |
| Direct referral (HSIL) | 97.0% | 96.9% |
| Referral at 6 months after primary test (ASCUS/LSIL) | 97.5% | 88.4% |
| Referral at 6 months after primary test (HSIL) | 97.5% | 96.9% |
| Referral at 18 months after primary test | 52.4% | NA |
ASC‐US, atypical squamous cells of undetermined significance; hrHPV, high‐risk human papillomavirus; HSIL, high‐grade squamous intra‐epithelial lesion; LSIL, low‐grade squamous intra‐epithelial lesion; NA, not applicable.
Simulated participation rate in all women excluding those who have had a hysterectomy and those with a prevalent diagnosed cancer.
Participation in the general population is much lower at ages 45 and 55 because significantly fewer women are invited for screening at these ages (i.e. only those who do not participate or test hrHPV‐positive in the preceding screening round).
Participation in the general population is much lower at age 65 because significantly fewer women are invited for screening at this age (i.e. only those who test hrHPV‐positive at age 65).
For most ages, the attendance rates could be directly observed in the first screening round after implementation of hrHPV screening. However, in this first screening round, all women aged 45 and 55 were invited for screening, whereas in future rounds, only those who tested hrHPV‐positive in the preceding round or did not participate the preceding round will be invited. Therefore, fewer women will participate at those ages than currently observed in the first screening round (calculations presented above Appendix S1: Table S2). Also, in this first screening round, no women aged 65 were invited as yet as they first had to test hrHPV‐positive at age 60. Therefore, we assumed the participation rate for women at age 65, who tested hrHPV‐positive at age 60, to be the same as at age 60.
Distribution of screenings across the population
The chance that an individual woman participates in a screening round is not entirely random; the total attendance is assumed to be distributed among 90% of the female population who potentially participate in screening, whereas the remaining 10% never attend a GP test (‘never attenders’) and have a 2.6 times higher background risk for acquiring an hrHPV infection (calibrated parameter, see Appendix S1). Also, if a woman attends one screening round, she is more likely to attend the next round and vice versa.
Of all self‐sampling users in the new screening programme, 10.6% were assumed to have been never attenders in the old screening programme, based on screening histories in the previous two screening rounds (calculations described in Appendix S1). As women aged 30 or 35 were not invited at least twice before, the proportion of young women taking a self‐sample that would otherwise be never attenders was assumed to be equal to the weighted average proportion of 40‐ to 60‐year‐old women. For all ages, the total screening attendance was higher in the cytology‐based programme than in the hrHPV‐based programme (Table 1).
Participation in triage testing and colposcopy
Adherence to triage testing and colposcopy was monitored in both programmes. In the hrHPV‐based programme, the adherence to triage testing was lower in self‐sampling users (41.6%) than in women who attended the primary test at their GP (77.1%). Also, adherence to colposcopy was higher in women with HSIL (96.1%) than in women with lower grade cytology results (88.4%). In the cytology‐based programme, the adherence to triage testing at 18 months after the primary test (67.3%) was lower than at 6 months after the primary test (92.2%). The adherence to colposcopy at 18 months (52.4%) was considerably lower than after a direct referral or a referral 6 months after the primary test (97 and 97.5%, respectively).
Model assumptions – test characteristics, costs and utilities
The sensitivity and specificity of both cytology and the hrHPV test are presented in Table S3 of Appendix S1. The test characteristics of cytology were calibrated to observed data, whereas the test characteristics of the hrHPV test were derived from the literature 15 , 21 as described in Appendix S1.
All costs and utilities applied are presented in Table 2. The cost‐effectiveness analysis was performed using a societal perspective. All costs presented are in euros (€) and are indexed to the year 2019. 22 , 23 The utilities for screening and disease are obtained from an empirical Dutch study by de Kok et al. 24 using the SF‐6D questionnaire. Costs and effects were discounted annually by 3% as suggested by Sanders et al. 25 in their recommendations for cost‐effectiveness analyses.
Table 2.
Base‐case assumptions on costs and disutilities applied for screening, diagnosis and treatment
| Disutility (%) 24 | Duration of disutilty (months) 24 | Costs | ||
|---|---|---|---|---|
| € (2019) | Source | |||
| Screening | ||||
| Primary cytology programme | ||||
| Primary cytology test | 0 | 0 | 70 | Dutch public health subsidy scheme 22 |
| Repeat cytology test | 0.03 | 15 | 51 | |
| Reflex hrHPV test after cytology repeat test | 0 | 0 | 139 | |
| Primary hrHPV test programme | ||||
| Primary hrHPV test | 0 | 0 | 58 | Dutch public health subsidy scheme 22 |
| Primary hrHPV self‐sampling kit | 0 | 0 | 43 | |
| Reflex cytology after hrHPV test | 0 | 0 | 26 | |
| Repeat cytology after hrHPV self‐sampling | 0.03 | 1 | 52 | |
| Repeat cytology after 6 months | 0.03 | 6 | 53 | |
| Diagnosis and treatment | ||||
| No CIN detected | 0.03 | 1 | 316 | Report on the effects and costs of cervical cancer screening in the Netherlands in 2006 23 |
| CIN1 | 0.03 | 1 | 986 | |
| CIN2 | 0.03 | 1 | 1461 | |
| CIN3 | 0.03 | 1 | 1710 | |
| FIGO1A | 0.08 | 12 | 5601 | |
| FIGO1B | 0.08 | 12 | 13,283 | |
| FIGO2+ clinically detected | 0.14 | 12 | 12,226 | |
| FIGO2+ screen detected | 0.14 | 12 | 13,092 | |
| Cancer survivor | 0.03 | 120 | 0* | |
| Palliative care | 0.5 | 12 | 29,745 | |
CIN, cervical intra‐epithelial neoplasia; FIGO, International Federation of Gynaecology and Obstetrics; HPV, human papillomavirus.
Costs included in treatment costs.
Sensitivity analyses
Multiple univariate sensitivity analyses have been performed. First, we assumed the screening attendance and/or triage adherence as observed in the cytology‐based programme (Table 1) to hold also for the hrHPV‐based programme. Second, we used an alternative published disutility set. 1 Last, we performed the analyses using discount rates of 4% for costs and 1.5% for effects, as is the guideline of the National Health Care Institute in The Netherlands. 26
Core outcomes
Outcomes of interest were total number of screening tests, referrals to colposcopy, cancer incidence, cancer mortality, costs, life years gained and QALYs gained compared with a situation without screening. All outcomes will be presented per 100 000 30‐year‐old women followed for their remaining life.
Ethics approval and patient involvement
Ethical approval by a medical ethical committee was not required under Dutch law as no patients were involved in the development of the research, and only non‐identifiable data were used for this study.
Funding
This study was funded by the EU‐Framework Programme (Horizon 2020; project reference 634753; PI: prof HJ de Koning, MD PhD, Erasmus MC) of the European Commission and by the Dutch National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu).
Results
Model calibration
After calibration, the model outcomes fitted the observed age‐specific cervical cancer incidence rates, cervical cancer stage distribution, detection rates of CIN and cervical cancer, hrHPV positivity rates and the hrHPV‐type distribution by age and by lesion grade (Appendix S1: Figures S10–S15). The model also validated well with age‐specific cervical cancer mortality rates observed in the Netherlands in 2004–2013 (Appendix S1: Figure S16).
Effects, costs and cost‐effectiveness
Table 3 presents the base‐case results per 100 000 30‐year‐old women followed for their remaining life. The table includes the predicted effects, costs and cost‐effectiveness of offering either no cervical cancer screening at all, the cytology screening programme or the hrHPV‐based screening programme. Compared to the cytology programme, the hrHPV‐based programme used fewer screening tests (−18%), referred more women to colposcopy (+66%), decreased the cancer incidence (−1%) and mortality (−4%) and reduced the total costs (−21%). The extra referrals to colposcopy were predominantly among women with ≤CIN1 and the decrease in total costs was mainly due to the lower number of screening tests.
Table 3.
Base‐case results per 100 000 women simulated lifelong
| Screen strategy | Difference between hrHPV and cytology (%) | |||
|---|---|---|---|---|
| No screening | Cytology | hrHPV | ||
| Effects (numbers, undiscounted) | ||||
| Total screening tests | – | 444 356 | 364 306 | −18 |
| Primary screening tests (GP) | – | 422 959 | 281 710 | −33 |
| Primary self‐samples | – | – | 25 797 | NA |
| Reflex cytology after positive GP test | – | – | 33 906 | NA |
| Cytology smear after positive self‐sample | – | – | 3384 | NA |
| Tests 6 or 18 months after primary test | – | 21 397 | 19 509 | −9 |
| Referrals to colposcopy | – | 7746 | 12 841 | +66 |
| No lesion present | – | 1458 | 5242 | +260 |
| CIN 1 | – | 1514 | 2851 | +88 |
| CIN 2 | – | 1523 | 2039 | +34 |
| CIN 3/AIS | – | 3070 | 2509 | −18 |
| Screen detected cervical cancer | – | 181 | 200 | +10 |
| Clinically detected cervical cancers | 1157 | 522 | 496 | −5 |
| Total cervical cancers | 1157 | 704 | 697 | −1 |
| Cervical cancer mortality | 440 | 215 | 206 | −4 |
| Life years gained compared to no screening | – | 5163 | 5250 | +2 |
| QALY's gained compared to no screening | – | 4580 | 5161 | +13 |
| Costs (€ millions, undiscounted) | ||||
| Screening tests | – | 33 | 19 | −41 |
| Diagnosis and treatment of precancerous lesions and false‐positive referrals | – | 9 | 12 | +24 |
| Diagnosis and treatment of cervical cancer | 14.7 | 8 | 8 | −2 |
| Palliative care | 13.1 | 6 | 6 | −4 |
| Total costs | 27.7 | 57 | 45 | −21 |
| Cost‐effectiveness (in €, discounted yearly by 3% for both costs and effects) | ||||
| Costs per life year gained compared to no screening | – | 15,247 | 10,890 | −29 |
| Costs per QALY gained compared to no screening | – | 22,678 | 12,225 | −46 |
AIS, adenocarcinoma in situ; CIN, cervical intra‐epithelial neoplasia; GP, general practitioner; hrHPV, high‐risk human papillomavirus; NA, not available because this was not contained in the cytology programme; QALY, quality‐adjusted life‐year.
The cost‐effectiveness of the hrHPV‐based programme was more favourable than that of the cytology‐based programme. When compared to no screening, the cytology programme cost €22,678 per QALY gained, while this was €12,225 (−46%) for the hrHPV‐based programme. Per life year gained, the cytology programme cost €15,247, while this was €10,890 (−29%) for the hrHPV‐based programme.
Sensitivity analyses
Figure 1 shows that when the attendance rates at primary screening in the hrHPV programme were assumed to be equal to those of the cytology programme, the cost‐effectiveness of the hrHPV‐based programme slightly deteriorated from €12,225 to €12,951 per QALY gained. Assuming the same adherence in the triage across both programmes also slightly deteriorated the cost‐effectiveness of the hrHPV‐based programme (€13,108 per QALY gained). When both equal screening attendance and equal triage adherence were assumed, the cost‐effectiveness would deteriorate to €13,757. Nevertheless, under these assumptions, the hrHPV‐based programme would still remain more cost‐effective than the cytology‐based programme (€22,678 per QALY gained).
Figure 1.
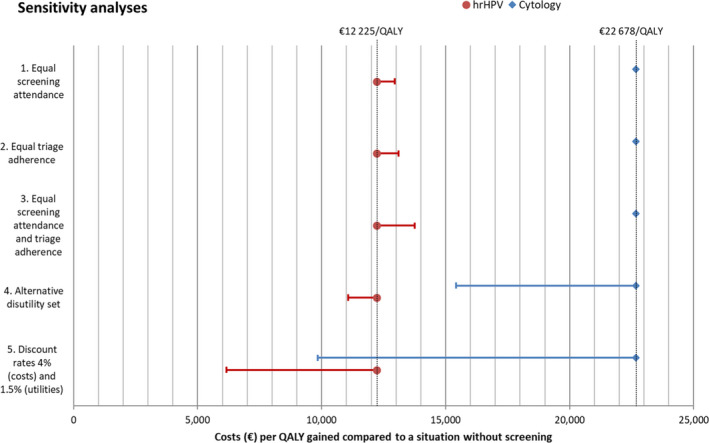
Results of the sensitivity analyses. The red dots and blue diamonds indicate the base‐case cost‐effectiveness of the hrHPV‐based programme and the cytology‐based programme, respectively. The horizontal lines indicate how the cost‐effectiveness of each programme would change if: (1) the attendance to primary screening in the hrHPV‐based programme is equal to that of the cytology programme; (2) the adherence to triage testing is equal to that of the cytology programme; (3) both the attendance to primary screening and the adherence to triage testing are equal to that of the cytology programme; (4) an alternative published utility set is applied to the results of both programmes 1 ; (5) costs are discounted with 4% annually and utilities with 1.5% annually, as is recommended in The Netherlands. 26 hrHPV, high‐risk human papillomavirus; QALY, quality‐adjusted life year.
Using the alternative set of disutilities or discount rates improved the cost‐effectiveness of both programmes substantially; however, the hrHPV‐based programme remained the most cost‐effective option of the two in both cases.
Discussion
Main findings
According to our modelling analyses, the recent switch from cytology to hrHPV testing in the Dutch cervical cancer screening programme will improve its cost‐effectiveness. Compared to the lifetime cytology‐based screening programme, the lifetime hrHPV‐based programme is expected to incur considerably fewer costs (−21%) for a modestly higher number of life years (+2%) gained and 13% more QALYs gained.
The reduction in total costs by switching to the hrHPV‐based screening programme is almost completely due to the reduction in screening costs. The predicted increase in life years gained is explained by the lower cancer incidence and cancer mortality in the hrHPV‐based programme, in which more precancerous lesions are detected and treated despite lower attendance rates. We found that the increase in detection of low‐grade precancerous lesions is substantial. As most low‐grade lesions will not progress to cancer, the number of women who are referred to a gynaecologist unnecessarily increases as well, causing anxiety for these women and potentially leading to overtreatment. However, the reduction in QALYs resulting from unnecessary referrals does not outweigh the QALYs gained because of the lower cancer incidence and cancer mortality.
The number of detected CIN3 lesions does not increase with the switch to hrHPV screening. Although the HPV test is more sensitive for CIN3 lesions than cytology, fewer CIN3 lesions are prevalent at screening because more low‐grade lesions are picked up before progression towards CIN3. The number of cancers detected by screening does increase, which is caused by the introduction of the extended screening intervals, allowing more lesions to progress to cancer before the next screening round.
Sensitivity analyses on screening behaviour and utilities consistently showed a more favourable cost‐effectiveness of the hrHPV‐based programme.
Limitations and strengths
In the Netherlands, the first cohort of women vaccinated against HPV‐16 and HPV‐18 will enter the screening programme in 2023. We compared the effects of hrHPV‐based screening with those of cytology‐based screening for unvaccinated women only. The results of both programmes are likely to be different for vaccinated and unvaccinated women in vaccinated cohorts. 10 , 27 Therefore, the cost‐effectiveness of screening in vaccinated populations needs further investigation.
Also, the attendance for primary screening for women aged 45, 55 or 65 could not yet be observed in the first round of the hrHPV‐based programme, as the eligibility for screening at those ages normally depends on the results of the preceding round.
Furthermore, we compared the cost‐effectiveness between both programmes by dividing the total costs by the total QALYs gained. Although this method does capture the overall cost‐effectiveness of each programme, different cost types might be allocated to different parties depending on how the programme is funded. Because of that, costs may rise for some parties, especially those paying for diagnosis and treatment of low‐grade lesions. If more costs would be allocated to participating women, this may lead to different screening behaviour.
To the authors' knowledge, this is the first modelling study to use observed data from an implemented hrHPV‐based organised screening programme as model inputs. The national pathology database, PALGA, which was the main source for calibrating the model and obtaining model inputs for this analysis, contains high‐quality data on an individual level about results of both hrHPV testing and cytology. Because this detailed, robust data could be used, the screening behaviour in both programmes could be modelled very accurately, thereby reducing uncertainty of the outcomes.
Furthermore, univariate sensitivity analyses were performed varying several important assumptions. The hrHPV‐based screening programme remained more cost‐effective in all sensitivity analyses.
Interpretation
The main reason the hrHPV‐based screening programme was found to be more cost‐effective than the cytology‐based programme is because the hrHPV‐based screening programme has lower screening costs while retaining the protection for cervical cancer. These screening costs are lower due to the reduced number of screening rounds combined with lower unit costs for primary hrHPV testing versus cytology. The retained protection at longer intervals has also been demonstrated by follow‐up studies of the POBASCAM trial and the ARTISTIC trial. 28 , 29 Therefore, reducing the number of screening rounds can be concluded to be a safe way to improve the cost‐effectiveness of hrHPV‐based screening programmes.
The finding that the hrHPV‐based screening programme is more cost‐effective than the cytology‐based screening programme is in line with previous modelling studies assessing the cost‐effectiveness of comparable cytology‐based and hrHPV‐based screening programmes. 1 , 2 , 27 , 30 Although the methods and assumptions used in previous studies vary widely, none of them used inputs that were observed after implementation of an hrHPV‐based programme. Because of that, the same screening attendance was assumed for both programmes. When comparing the difference in effects between both programmes in this study with that of previous studies, one should be aware of this difference in assumptions on screening behaviour.
The lower observed attendance rate in the hrHPV‐based screening programme might be directly related to specific organisational changes that were implemented in conjunction with the switch in screening protocol. 4 For example, GPs are no longer able personally to invite women for screening. Therefore, the lower attendance rates in hrHPV‐based screening might not be applicable to other countries implementing hrHPV‐based screening.
Previous studies showed that offering hrHPV self‐sampling could increase the participation in women who would otherwise not attend screening. 31 Now that the hrHPV self‐sampling kit has been found to be non‐inferior to a GP test, 19 offering hrHPV self‐sampling could improve the effectiveness of screening programmes. 19 , 32 This is dependent on the proportion of regular attendees that would switch to self‐sampling and the proportion of never attenders, with a higher background risk, that will now participate in self‐sampling. 32
We showed that the switch to the hrHPV‐based screening programme leads to an increase in the detection rates of low‐grade CIN lesions. Most of the detected low‐grade lesions will not progress. 7 Previous studies on triage strategies have shown that the number of unnecessary referrals to colposcopy could be reduced by the use of genotyping. 33 Genotyping is not used in the current Dutch hrHPV‐based screening programme but should be considered to reduce the number of colposcopies.
Conclusion
Even though lower participation in primary screening and lower adherence to triage testing were observed after the introduction of the hrHPV‐based screening programme in the Netherlands, the cost‐effectiveness is still estimated to be more favourable in the hrHPV‐based programme than in the old cytology‐based programme. However, there is a substantial increase in the number of women who are unnecessarily referred to a gynaecologist, so alternatives to the currently used triage strategy should be investigated.
Disclosure of interests
All authors report grants from the National Institute for Public Health and the Environment. EELJ, HJK, MB and IMCMK report grants from the European Commission during the conduct of the study. HJK reports speaker’s fees from University of Zürich/MSD outside the submitted work. Completed disclosure of interest forms are available to view online as supporting information.
Contribution to authorship
EELJ, HJK, IMCMK, MB and SKN contributed to the study concept and design. Model design was by EELJ, IMCMK, MB and SKN. CAA and EELJ interpreted the observational data used for model inputs. Model analyses were by EELJ, IMCMK and SKN. The manuscript was drafted by EELJ and IMCMK and all authors contributed to revising the manuscript and approved the final version of the manuscript.
Details of ethics approval
Ethical approval by a medical ethical committee is not required under Dutch law as no patients were involved in the development of the research and only non‐identifiable data were used for this study.
Funding
This study was funded by the EU‐Framework Programme (Horizon 2020; project reference 634753; PI: prof HJ de Koning, MD PhD, Erasmus MC) of the European Commission and by the Dutch National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu). The grants did not include external peer review or priority assessment. The funders were not involved in the study design, performance of the analyses or writing of the paper.
Supporting information
Appendix S1. MISCAN‐Cervix model description.
Figure S1. Basic structure of MISCAN‐Cervix.
Figure S2. The natural history structure of MISCAN‐Cervix.
Figure S3. Life histories with and without disease.
Figure S4. Life history with screening.
Figure S5. Triage in the cytology (left) and the hrHPV (right) screening programmes.
Figure S6. Calibration cycle.
Figure S7. Calibrated durations and probabilities during step 1 of the calibration process.
Figure S8. Calibrated durations and probabilities during step 2 of the calibration process.
Figure S9. Calibrated probabilities during step 3 of the calibration process.
Figure S10. Model fit on age‐specific cervical cancer incidence rates.
Figure S11. Model fit cervical cancer stage distribution by age.
Figure S12. Model fit on age‐specific detection rates.
Figure S13. Model fit on age‐specific hrHPV test‐positivity rates.
Figure S14. Model fit on age‐specific hrHPV‐type distribution among hrHPV‐positive women.
Figure S15. Model fit on lesion‐specific hrHPV‐type distribution among hrHPV‐positive women.
Figure S16. Model fit on age‐specific mortality rates.
Table S1. Durations of health states.
Table S2. Modelled screening behaviour by type of screening programme: base‐case assumptions.
Table S3. Test characteristics of the cytology test and the hrHPV test by disease status.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgement
The authors wish to thank Erhan Demirel for his role as a programmer with the development of scripts used for analysing model outcomes.
Jansen EEL, Naber SK, Aitken CA, de Koning HJ, van Ballegooijen M, de Kok IMCM. Cost-effectiveness of HPV-based cervical screening based on first year results in the Netherlands: a modelling study. BJOG 2021; 128:573–582.
Linked article: This article is commented on by TJ Palmer, p. 583 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.16449.
References
- 1. van Rosmalen J, de Kok IM, van Ballegooijen M. Cost‐effectiveness of cervical cancer screening: cytology versus human papillomavirus DNA testing. BJOG 2012;119:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Kok IM, van Rosmalen J, Dillner J, Arbyn M, Sasieni P, Iftner T, et al. Primary screening for human papillomavirus compared with cytology screening for cervical cancer in European settings: cost effectiveness analysis based on a Dutch microsimulation model. BMJ 2012;344:e670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rebolj M, van Ballegooijen M, Berkers LM, Habbema D. Monitoring a national cancer prevention program: successful changes in cervical cancer screening in the Netherlands. Int J Cancer 2007;120:806–12. [DOI] [PubMed] [Google Scholar]
- 4. Aitken CA, van Agt H, Siebers AG, van Kemenade F, Niesters HGM, Melchers WJG, et al. Introduction of primary screening using high‐risk HPV DNA detection in the Dutch cervical cancer screening programme: a population‐based cohort study. BMC Med 2019;17:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huijsmans CJ, Geurts‐Giele WR, Leeijen C, Hazenberg HL, van Beek J, de Wild C, et al. HPV prevalence in the Dutch cervical cancer screening population (DuSC study): HPV testing using automated HC2, cobas and Aptima workflows. BMC Cancer 2016;16:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Velentzis LS, Caruana M, Simms KT, Lew JB, Shi JF, Saville M, et al. How will transitioning from cytology to HPV testing change the balance between the benefits and harms of cervical cancer screening? Estimates of the impact on cervical cancer, treatment rates and adverse obstetric outcomes in Australia, a high vaccination coverage country. Int J Cancer 2017;141:2410–22. [DOI] [PubMed] [Google Scholar]
- 7. Ciavattini A, Serri M, Di Giuseppe J, Liverani CA, Gardella B, Papiccio M, et al. Long‐term observational approach in women with histological diagnosis of cervical low‐grade squamous intraepithelial lesion: an Italian multicentric retrospective cohort study. BMJ Open 2019;9:e024920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Habbema JD, van Oortmarssen GJ, Lubbe JT, van der Maas PJ. The MISCAN simulation program for the evaluation of screening for disease. Comput Methods Programs Biomed 1985;20:79–93. [DOI] [PubMed] [Google Scholar]
- 9. van den Akker‐van Marle ME, van Ballegooijen M, van Oortmarssen GJ, Boer R, Habbema JD. Cost‐effectiveness of cervical cancer screening: comparison of screening policies. J Natl Cancer Inst 2002;94:193–204. [DOI] [PubMed] [Google Scholar]
- 10. Naber SK, Matthijsse SM, Rozemeijer K, Penning C, de Kok IM, van Ballegooijen M. Cervical cancer screening in partly HPV vaccinated cohorts – a cost‐effectiveness analysis. PLoS One 2016;11:e0145548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007;29:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Netherlands Cancer Registry (NCR) [www.iknl.nl/en/ncr]. Accessed 26 November 2019.
- 13. Vesikari T, Brodszki N, van Damme P, Diez‐Domingo J, Icardi G, Petersen LK, et al. A randomized, double‐blind, phase III study of the immunogenicity and safety of a 9‐valent human papillomavirus L1 virus‐like particle vaccine (V503) versus Gardasil(R) in 9–15‐year‐old girls. Pediatr Infect Dis J 2015;34:992–8. [DOI] [PubMed] [Google Scholar]
- 14. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus‐like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004;364:1757–65. [DOI] [PubMed] [Google Scholar]
- 15. Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Differential detection of human papillomavirus genotypes and cervical intraepithelial neoplasia by four commercial assays. J Clin Microbiol 2016;54:2669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population‐based study in routine clinical practice. Lancet Oncol 2011;12:663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seoud M, Tjalma WA, Ronsse V. Cervical adenocarcinoma: moving towards better prevention. Vaccine 2011;29:9148–58. [DOI] [PubMed] [Google Scholar]
- 18. Jansen E, Matthijsse S, Naber S, Aitken C, Agt HV, Ballegooijen MV, et al. New evidence with regard to test characteristics from a modelling study [Abstract]. Amsterdam: Eurogin; 2017. [Google Scholar]
- 19. Polman NJ, Ebisch RMF, Heideman DAM, Melchers WJG, Bekkers RLM, Molijn AC, et al. Performance of human papillomavirus testing on self‐collected versus clinician‐collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen‐positive, non‐inferiority trial. Lancet Oncol 2019;20:229–38. [DOI] [PubMed] [Google Scholar]
- 20. Erasmus MC and PALGA . Bevolkingsonderzoek Baarmoederhalskanker Monitor 2016 [www.rivm.nl/sites/default/files/2018‐11/LEBAmonitor2016.pdf]. Accessed 14 November 2019.
- 21. Bulk S, Bulkmans NW, Berkhof J, Rozendaal L, Boeke AJ, Verheijen RH, et al. Risk of high‐grade cervical intra‐epithelial neoplasia based on cytology and high‐risk HPV testing at baseline and at 6‐months. Int J Cancer 2007;121:361–7. [DOI] [PubMed] [Google Scholar]
- 22. Overheid.nl . Subsidieregeling publieke gezondheid [https://wetten.overheid.nl/BWBR0018743/2019‐01‐01#HoofdstukII_Paragraaf2_Artikel42]. Accessed 17 May 2019.
- 23. Van Ballegooijen M, Rebolj M, Essink‐Bot ML, Meerding WJ, Berkers LM, Habbema JDF. De effecten en kosten van het bevolkingsonderzoek naar baarmoederhalskanker in Nederland na de herstructurering. Rotterdam: Erasmus MC; 2006. [Google Scholar]
- 24. de Kok I, Korfage IJ, van den Hout WB, Helmerhorst TJM, Habbema JDF, Essink‐Bot ML, et al. Quality of life assumptions determine which cervical cancer screening strategies are cost‐effective. Int J Cancer 2018;142:2383–93. [DOI] [PubMed] [Google Scholar]
- 25. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost‐effectiveness analyses: Second Panel on Cost‐Effectiveness in Health and Medicine. JAMA 2016;316:1093–103. [DOI] [PubMed] [Google Scholar]
- 26. Kompas F. Farmaco‐economie [www.farmacotherapeutischkompas.nl/algemeen/farmaco‐economie]. Accessed 24 September 2019.
- 27. Lew JB, Simms K, Smith M, Lewis H, Neal H, Canfell K. Effectiveness modelling and economic evaluation of primary HPV screening for cervical cancer prevention in New Zealand. PLoS One 2016;11:e0151619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dijkstra MG, van Zummeren M, Rozendaal L, van Kemenade FJ, Helmerhorst TJ, Snijders PJ, et al. Safety of extending screening intervals beyond five years in cervical screening programmes with testing for high risk human papillomavirus: 14 year follow‐up of population based randomised cohort in the Netherlands. BMJ 2016;355:i4924. [DOI] [PubMed] [Google Scholar]
- 29. Kitchener HC, Gilham C, Sargent A, Bailey A, Albrow R, Roberts C, et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur J Cancer 2011;47:864–71. [DOI] [PubMed] [Google Scholar]
- 30. Burger EA, Ortendahl JD, Sy S, Kristiansen IS, Kim JJ. Cost‐effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. Br J Cancer 2012;106:1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gok M, Heideman DA, van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JW, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ 2010;340:c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rozemeijer K, de Kok IM, Naber SK, van Kemenade FJ, Penning C, van Rosmalen J, et al. Offering self‐sampling to non‐attendees of organized primary HPV screening: when do harms outweigh the benefits? Cancer Epidemiol Biomarkers Prev 2015;24:773–82. [DOI] [PubMed] [Google Scholar]
- 33. Cook DA, Smith LW, Law J, Mei W, van Niekerk DJ, Ceballos K, et al. Aptima HPV assay versus hybrid capture((R)) 2 HPV test for primary cervical cancer screening in the HPV FOCAL trial. J Clin Virol 2017;87:23–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. MISCAN‐Cervix model description.
Figure S1. Basic structure of MISCAN‐Cervix.
Figure S2. The natural history structure of MISCAN‐Cervix.
Figure S3. Life histories with and without disease.
Figure S4. Life history with screening.
Figure S5. Triage in the cytology (left) and the hrHPV (right) screening programmes.
Figure S6. Calibration cycle.
Figure S7. Calibrated durations and probabilities during step 1 of the calibration process.
Figure S8. Calibrated durations and probabilities during step 2 of the calibration process.
Figure S9. Calibrated probabilities during step 3 of the calibration process.
Figure S10. Model fit on age‐specific cervical cancer incidence rates.
Figure S11. Model fit cervical cancer stage distribution by age.
Figure S12. Model fit on age‐specific detection rates.
Figure S13. Model fit on age‐specific hrHPV test‐positivity rates.
Figure S14. Model fit on age‐specific hrHPV‐type distribution among hrHPV‐positive women.
Figure S15. Model fit on lesion‐specific hrHPV‐type distribution among hrHPV‐positive women.
Figure S16. Model fit on age‐specific mortality rates.
Table S1. Durations of health states.
Table S2. Modelled screening behaviour by type of screening programme: base‐case assumptions.
Table S3. Test characteristics of the cytology test and the hrHPV test by disease status.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


