Abstract
This phase 1, open‐label, single‐center study evaluated the pharmacokinetics (PK), pharmacodynamics, safety, and tolerability of single‐dose emicizumab in healthy Chinese males. Overall, 16 subjects received a single subcutaneous dose of 1‐mg/kg emicizumab. Blood samples were obtained before dosing on day 1 and at regular intervals over 16 weeks after dosing for PK evaluation. A single 1‐mg/kg subcutaneous dose of emicizumab was safe and well tolerated in healthy Chinese male subjects in the study. Mean (± standard deviation) area under the concentration‐time curve from time 0 to infinity and maximum concentration were 287 ± 74.2 μg⋅d/mL and 7.11 ± 1.77 μg/mL, respectively, with a terminal half‐life of 26.7 (±4.3) days. Emicizumab administration did not show significant impact on pharmacodynamic markers tested, which mostly remained stable throughout the study. One subject tested positive for antidrug antibody, with no impact on his PK or safety profile. Compared with results from healthy Japanese and Caucasian subjects receiving the same dose in previous clinical trials, the current results further indicated the absence of difference of emicizumab PK profile across Chinese, Japanese, and Caucasian subjects, validating the use of similar therapeutic doses in Asian and non‐Asian populations.
Keywords: Chinese, emicizumab, healthy subjects, pharmacodynamics, pharmacokinetics
Hemophilia A is an X‐linked recessive bleeding disorder that occurs in approximately 1 in 5000 live male births. Patients with hemophilia A have a deficiency or absence of blood coagulation factor VIII (FVIII), an essential component of the coagulation cascade. 1 , 2 The absence or functional deficiency of FVIII leads to a lifelong bleeding tendency. Regular prophylaxis treatment using FVIII products with either plasma‐derived or recombinant FVIII molecules has been the standard of care for decades. However, frequent intravenous infusions of FVIII products often pose a mental and physical burden. Moreover, with the development of anti‐FVIII neutralizing alloantibodies (inhibitors), patients face an increased risk of bleeding and complicated clinical management procedures.
Emicizumab (also known as ACE910, RO5534262, and HEMLIBRA) is a recombinant, humanized, bispecific, immunoglobulin G4 antibody that binds with activated factor IX (FIXa) and factor X (FX), mimicking the cofactor function of activated FVIII (FVIIIa). In so doing, it restores the function of missing FVIIIa, which is needed for effective hemostasis. Owing to its unique structure, emicizumab is not expected to be affected by existing FVIII inhibitors (as it shares no sequence homology with FVIII) or to induce new development of such inhibitors. 3 , 4 , 5
Emicizumab is the first commercially available non–factor‐replacement drug. 6 The drug was initially developed by Chugai Pharmaceutical and demonstrated hemostatic activity in a nonhuman primate model of hemophilia A. 4 , 7 In a small phase I/II study, once‐weekly subcutaneous (SC) administration of emicizumab markedly reduced the rate of bleeding episodes among patients with hemophilia A irrespective of the presence of FVIII inhibitors. 8
The possibility of SC administration removes the need for venous access. Of note, the absolute bioavailability following SC administration is high, between 80.4% and 93.1% depending on the site of absorption. 9
Because of the pharmacokinetic (PK) and pharmacodynamic (PD) properties of emicizumab, 10 use of emicizumab enables a dosing interval of once every week, every 2 weeks, or every 4 weeks. Using these dosing regimens, efficacy and safety were demonstrated in phase III clinical trials. Emicizumab prophylaxis significantly reduced annualized bleeding rates compared with no prophylaxis in adolescents and adults with hemophilia A with or without inhibitors, 11 , 12 , 13 and prevented or substantially reduced bleeding in children with hemophilia A with or without inhibitors. 14 , 15
Emicizumab is approved in many countries, including the United States and Japan, for the routine prophylaxis of bleeding episodes in hemophilia A patients with or without inhibitors against FVIII. In the European Union, it is approved for the routine prophylaxis of bleeding episodes in patients with hemophilia A with inhibitors or in severe hemophilia A without inhibitors. 6
As part of the efforts to bring emicizumab to Chinese patients with hemophilia A, this study was conducted to assess the PK of emicizumab in healthy Chinese subjects following a single SC administration of emicizumab. In addition, the PD, safety, and tolerability of emicizumab in healthy Chinese subjects were also assessed.
Methods
The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization guidelines for Good Clinical Practice. The protocol was approved by the ethics committee of the participating institution (Ethics Committee of Peking University Third Hospital, Beijing, China). All subjects provided written informed consent before participation in any study procedures. The study is registered with ClinicalTrials.gov (NCT03380780).
Subjects
Eligible subjects were healthy Chinese men, aged 20 to 45 years inclusive at the time of screening, who must have had Chinese parents and grandparents, all of whom were born in China. The eligible subjects must have had a body mass index between 19 and 24 kg/m2, inclusive. Healthy status was defined by absence of evidence of any active or chronic disease following a detailed medical and surgical history, a complete physical examination including vital signs, 12‐lead electrocardiogram (ECG), hematology, blood chemistry, coagulation, viral serology, urinalysis, and immunology.
Use of any prescribed or over‐the‐counter medication or herbal medicine taken within 14 days prior to dosing or within 5 times the elimination half‐life of the medication prior to dosing (whichever is longer) were prohibited. During the study, no concomitant medications (including herbal products and vitamins) were permitted, with the exception of necessary medications to treat adverse events. The rationales for exceptions were to be discussed and clearly documented between the investigator and sponsor. Subjects were excluded if they had history of drug abuse or alcohol dependence within the past 2 years or confirmed positive results for drugs abuse or alcohol breath test at screening or day –1; if they smoked more than 10 cigarettes per day or the equivalent amount of tobacco; or if they had a condition or disease that, in the judgment of the investigator, would place them at undue risk; interfere with the absorption, distribution, metabolism, and excretion of emicizumab; or interfere with the ability of the subject to complete the study. Subjects with previous or concomitant thromboembolic disease such as deep vein thrombosis or signs of thromboembolic disease, or family history of thromboembolic disorder such as serious deep vein thrombosis at high risk for thrombotic microangiopathy (TMA) (eg, had a previous medical or family history of TMA) were also excluded. Subjects were excluded if protein C activity, protein S activity, antithrombin III activity, FIX activity, FX activity, lupus anticoagulant (T1/T2 ratio), or anticardiolipin‐β2 glycoprotein I complex antibody levels outside the reference range at screening, or FVIII activity ≥120 U/dL at screening. Subjects with previous or concomitant autoimmune or connective tissue disease, or history of tuberculosis or active tuberculosis with a positive T‐SPOT®. TB test result at screening were also excluded.
Study Design and Emicizumab Administration
This was a single‐center and open‐label study to evaluate the PK, PD, safety, and tolerability of emicizumab following a single SC administration in healthy Chinese subjects. Following a 28‐day screening period, subjects were admitted to the clinical research unit on day –1. On the morning of day 1, subjects received a single 1‐mg/kg SC dose of emicizumab in the abdomen. The study medication was administered after fasting from midnight until at least 8 am. A standard lunch was provided 4 hours after dosing. Subjects were allowed to leave the unit 72 hours after dosing and were asked to return to the unit for the collection of blood samples and for remaining assessments. Subjects were scheduled to have regular ambulatory visits for 16 weeks.
Assessments
Blood samples for the determination of plasma concentrations of emicizumab were collected before dosing on day 1 and at time matching predose on days 2, 4, 6, 8, 11, 15, 22, 29, 36, 43, 50, 57, 71, 85, and 113. Blood samples for the PD biomarker of coagulation (ie, activated partial thromboplastin time [aPTT], prothrombin time/international normalized ratio [PT/INR], fibrinogen, d‐dimer, prothrombin fragments 1 and 2 [PF1+2] and FVIII activity), were collected at baseline, before dosing and corresponding to PK sampling times with the exception of 2 time points (days 6 and 36). Blood samples for measurement of FVIII inhibitors were collected on day 1 before dosing and on day 113. Plasma samples for testing of anti‐emicizumab antibodies (ADA) were obtained before dosing on day 1 and on days 57 and 113.
Subjects were monitored throughout the study for safety signals. Safety assessments consisted of monitoring and recording adverse events (AEs), including serious AEs and nonserious AEs of special interest (ie, thromboembolic events, TMA, or systemic hypersensitivity reactions), standard laboratory assessments, coagulation tests, vital signs, ECG, and physical examinations. AEs were coded using the Medical Dictionary for Regulatory Activities version 21.1 terminology for AEs and diseases.
Bioanalytical Assays
Emicizumab plasma concentrations were measured with a specific and validated enzyme‐linked immunosorbent assay (ELISA) at QPS Netherlands B.V. (Groningen, the Netherlands). The ELISA detects binding‐competent drug by the use of anti‐idiotypic antibodies directed against both target binding sites of emicizumab. A high‐binding plate was coated overnight with rAJ540‐rbtIgG (capture antibody). Samples containing emicizumab react with the coated capture antibody. After a wash, rAQ8‐mIgG2b (detector antibody) was added to the plate and incubated. The bound detector antibody can be detected by using a peroxidase‐labeled goat anti‐mouse immunoglobulin G. After a wash to remove unbound reagent, ABTS ((2,2'‐Azino‐bis(3‐ethylbenzthiazoline‐6‐sulfonic acid)) was added and incubated. The absorbance is proportional with the emicizumab concentration in the sample (wavelength at 405 nm and 490 nm as reference). The method has a quantification range between 100 ng/mL (lower limit of quantification) and 6400 ng/mL. The precision and accuracy of the assay, as determined from the analysis of quality control samples were satisfactory throughout this portion of the study and ranged from 6.7% to 8.2% (precision) and from 99.6% to 112.0% (accuracy).
All biomarker analyses were performed by Medpace Reference Laboratories (Cincinnati, Ohio). Both aPTT 16 and the exploratory safety biomarkers (d‐dimer, 17 PF1+2, 18 fibrinogen, 19 PT 20 ) were analyzed using commercial test kits approved for in vitro diagnostic use (STA‐PTT A [aPTT], STA‐LIATEST D‐DI [d‐dimer], STA‐Fibrinogen [fibrinogen], STA‐Neoplastine CI PLUS [PT]; all from Diagnostica Stago and analyzed on automated coagulation analyzer; Enzygnost F1+2 ELISA [Siemens]) according to the manufacturers’ kit inserts. The PT was reported as the INR (reference range, 0.9‐1.1).
FVIII activity was measured using 2 different chromogenic assays. 21 , 22 A validated CE‐marked chromogenic assay containing human FIXa and FX (Hyphen Biomed, Neuville‐sur‐Oise, France) and an assay approved for in vitro diagnostic use containing bovine FIXa and FX (Siemens, Erlangen, Germany) were performed on automated coagulation analyzer (Diagnostica Stago).
Anti‐FVIII antibodies (inhibitors) were analyzed using a validated Chromogenic Bethesda Assay (CBA) 23 at Medpace Reference Laboratories (Cincinnati, Ohio). Titer values ≥0.6 chromogenic Bethesda units/mL were defined as positive. During validation, assay precision (coefficient of variation [%CV]) was 10.3% to 18.8%, while accuracy (comparison to manufacturer's labeled titer) was ‐37.4% to –1.8%.
A validated bridging ELISA method was used to analyze ADA in plasma. The analysis was performed at QPS Netherlands B.V. at QPS Netherlands B.V. (Groningen, Netherlands). The sensitivity of the method was 6.04 ng/mL. Assay precision (screening assay) ranged between 2.4% and 3.8%.
Statistical Analysis
Predose samples below the limit of quantification were set to 0. Postdose samples below the limit of quantification were set to 0 for calculation of means. PK parameters (eg, area under the concentration‐time curve [AUC], maximal plasma concentration [Cmax], time to maximum observed plasma concentration [tmax], apparent clearance, apparent volume of distribution, and terminal half‐life [t½]) were obtained by noncompartmental analysis methods using WinNonLin 6.4 (Pharsight Corporation, Certara USA, Princeton, New Jersey). The Cmax and tmax were read directly from the time‐concentration data. The t½ was estimated by ln(2)/λz, where λz was the terminal elimination rate constant. AUC from time 0 to the last measurable plasma concentration time point (AUClast) was estimated by the linear trapezoidal rule. AUC extrapolated to infinity (AUC0–∞) was determined as follows: AUClast + Clast/λz, where Clast was the last measurable plasma concentration. The apparent oral clearance was estimated by the ratio Dose/AUC0–∞. Data are presented as median (range) for tmax and mean (standard deviation [SD]) for all other parameters. The primary emicizumab PK parameters were Cmax and AUC0‐∞. All other PK parameters were regarded as secondary.
Results
A total of 16 healthy Chinese male subjects were enrolled at Peking University Third Hospital, Beijing, China. All 16 subjects received a single abdominal SC injection of 1 mg/kg emicizumab under fasting conditions. All 16 subjects completed the study and were included in the analysis of PK, PD, immunogenicity, and safety. Baseline characteristics of the 16 subjects enrolled are reported in Table 1.
Table 1.
Baseline Demographics of Study Subjects
| N = 16 | |
|---|---|
| Age, y, median (range) | 29.5 (21‐44) |
| Male, n (%) | 16 (100.0) |
| Race, n (%) | |
| Asian (Chinese) | 16 (100.0) |
| Weight, kg, median (range) | 60.1 (51.8‐75.1) |
| Height, cm, median (range) | 168 (159‐181) |
| Body mass index, kg/m2, median (range) | 21.8 (19.3‐24.0) |
Pharmacokinetics
Following a single SC dose of emicizumab 1 mg/kg in healthy Chinese subjects, mean (± SD) emicizumab plasma concentration increased with mean Cmax (7.11 ± 1.77 μg/mL) reached around 7 days (median tmax; range, 3‐15 days) after dosing. The emicizumab concentration then declined in a monophasic manner, with a mean t1/2 of 26.7 (±4.25) days (range, 20.2‐35.3 days) (Figure 1, Table 2). The mean AUC0‐∞ was 287 ± 74.2 μg⋅day/mL. The %CVs associated with Cmax and AUCs were 24% to 26%. Emicizumab concentrations were above the lower limit of quantification in all postdose samples.
Figure 1.
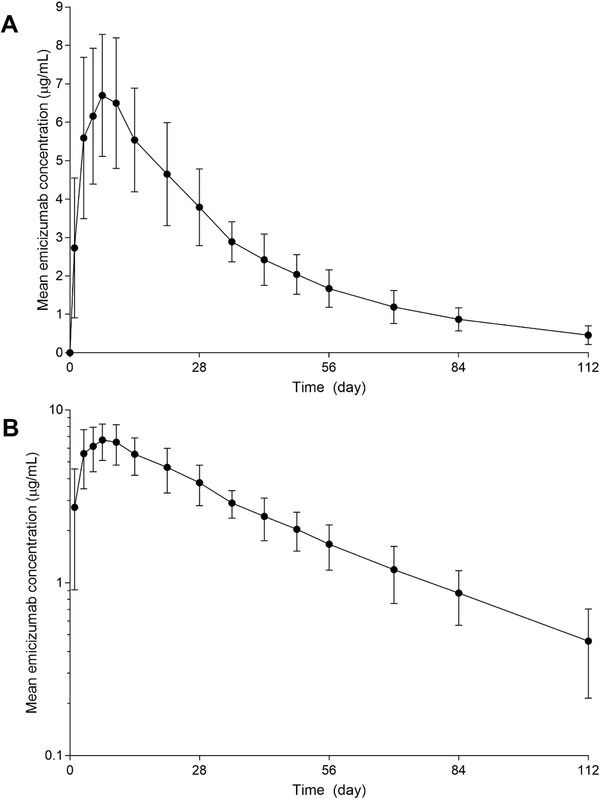
Mean ± standard deviation emicizumab plasma concentration versus time profile in healthy Chinese male subjects. (A) Linear scale; (B) log‐linear scale.
Table 2.
Emicizumab Pharmacokinetic Parameters Following a Single 1‐mg/kg Abdominal Subcutaneous Administration in Healthy Chinese Male Subjects (n = 16)
| Parameters | tmax (day) | Cmax (μg/mL) | AUC0‐∞ (μg⋅d/mL) | AUClast (μg⋅d/mL) | t½ (d) | CL/F (mL/d)a | V/F (mL)a |
|---|---|---|---|---|---|---|---|
| N = 16 | 7.01 (3.00‐15.0) | 7.11 (1.77) | 287 (74.2) | 268 (63.7) | 26.7 (4.25) | 235 (88.0) | 8870 (2950) |
AUC0‐∞, area under the concentration‐time curve from time 0 to infinity; AUClast, area under the concentration‐time curve from time 0 to last measurable concentration time point; Cmax, maximum plasma concentration; SD, standard deviation; t½, terminal half‐life; CL/F, apparent clearance; tmax, time to maximum plasma concentration; V/F, apparent volume of distribution.
Data are presented as median (range) for tmax and mean (SD) for all other parameters.
aAbsolute bioavailability (F) of SC administration is between 0.804‐0.9319
Pharmacodynamics
Individual and mean values over time for aPTT following a single 1‐mg/kg emicizumab SC administration in healthy Chinese subjects are displayed in Figure 2. A slight decrease in aPTT (16% decrease from baseline to day 10) was observed in all participants after dosing. aPTT values returned to baseline over time and, despite the slight initial decrease, remained within the normal range (23.9‐40.0 seconds) for all subjects throughout the study.
Figure 2.
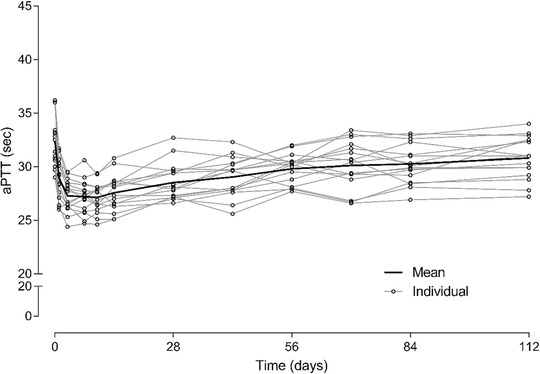
Time course of aPTT following a single 1 mg/kg emicizumab subcutaneous administration in healthy Chinese subjects. aPTT, activated partial thromboplastin time.
Individual and mean values over time for FVIII activity following a single 1‐mg/kg emicizumab SC administration in healthy Chinese subjects are displayed in Figure 3A using a chromogenic assay with human FIXa and FX (Hypen Biophen). FVIII activity levels remained within the normal range (40‐150 U/dL) with individual fluctuations with levels between 44 and 135 U/dL, in healthy subjects. Similar results were observed for FVIII activity using a chromogenic assay with bovine FIXa and FX (Siemens) (Figure 3B).
Figure 3.
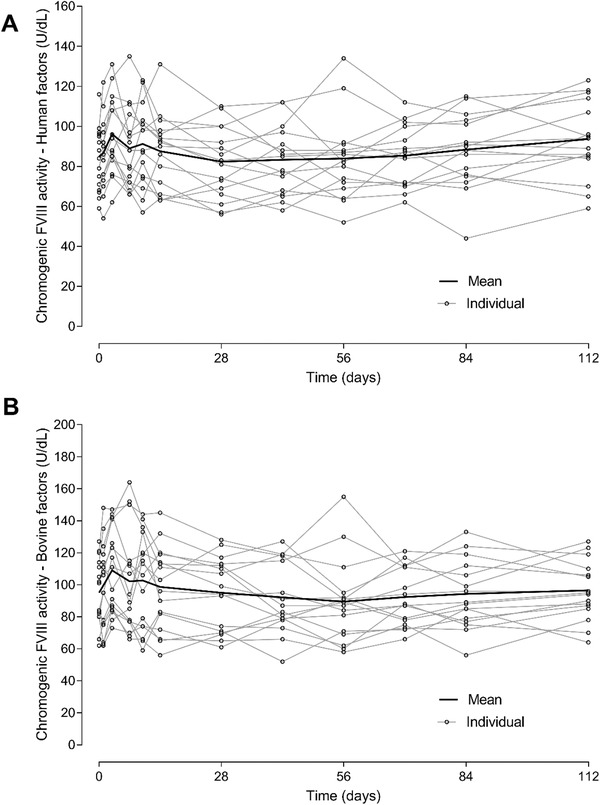
Time course of FVIII activity following a single subcutaneous dose of 1‐mg/kg emicizumab in healthy Chinese subjects. (A) Hyphen Biophen assay (human factors); (B) Siemens assay (bovine factors). FVIII, factor VIII.
The safety biomarkers, d‐dimer, PF1+2, fibrinogen, and PT (INR) remained stable during the study.
Immunogenicity
FVIII Inhibitor
Following a single 1‐mg/kg SC administration of emicizumab in healthy Chinese subjects, FVIII inhibitor titers remained negative for all but 1 subject who had a borderline positive value of 0.6 chromogenic Bethesda units/mL at the follow‐up visit (day 113). The subject's FVIII activity remained stable and in the normal range (114 U/dL and 119 U/dL on day 113 measured with the Hyphen and Siemens assays, respectively).
Anti‐emicizumab Antibody
All subjects tested negative at all visits for ADA with 1 exception. One subject (different from subject with positive FVIII inhibitor) tested positive for ADA with a low titer of 10 on a single occasion at the follow‐up visit on day 113. His PK profile did not differ from other subjects. His safety profile was also consistent with other subjects with no serious AEs reported. All the subject's AEs (nasal obstruction, decreased caucasian blood cell count, and oropharyngeal pain) were recovered before study day 57, when the ADA testing was still negative. No AEs were reported between study day 57 and day 113, when the ADA became positive. No further testing for ADAs was carried out after the follow‐up visit on day 113.
Safety
The safety analyses included all subjects (16 subjects) who received emicizumab. A total of 47 AEs were reported by 14 of 16 subjects (87.5%) in the study. All AEs were of low (grade 1 or 2) intensity. Of the 47 AEs, 9 AEs in 4 of 16 subjects (25%) were considered to be related to the study drug by the investigator. The events were decreased white blood cell count (N = 3), flatulence, upper respiratory tract infection, increased blood bilirubin, dry throat, nasal obstruction, and oropharyngeal pain (N = 1 each). None of these related AEs occurred in >1 subject. The most commonly reported AEs (≥2 subjects) were increased blood creatine phosphokinase, upper respiratory tract infection, oropharyngeal pain, and increased C‐reactive protein. No AEs of grade ≥3 intensity were reported. There were no serious AEs or deaths. No subjects experienced AEs of special interest as specified in the protocol. No subjects were withdrawn from study because of AEs. No clinically significant changes in laboratory test values, vital signs, or ECG findings were observed with emicizumab administration.
Discussion
This study evaluated the PK of emicizumab in healthy Chinese subjects following a single SC administration of emicizumab with assessment of PD, safety, and tolerability. The PK profile of a single dose of emicizumab in healthy Chinese subjects was characterized by a slow absorption following SC injection (median tmax of 7 days), and a slow monophasic elimination (mean t½ of 26.7 days) with moderate variability (%CV 24%‐26% for both Cmax and AUC). The PK characteristics of emicizumab have been previously evaluated in multiple clinical trials, in both healthy subjects and patients with hemophilia A. The first‐in‐human study 24 (ACE001JP) examined the safety, tolerability, PK, and PD of emicizumab in healthy male Japanese and Caucasian subjects receiving a single SC injection of emicizumab (Japanese: 0.001, 0.01, 0.1, 0.3, or 1 mg/kg; Caucasian: 0.1, 0.3, or 1 mg/kg; n = 6 per dose group). In this study, emicizumab exhibited a linear PK and had an elimination half‐life of ∼4 to 5 weeks. Emicizumab exposure in healthy Japanese subjects was comparable to that in Caucasians, suggesting no ethnicity differences in emicizumab PK. This study also indicated that single SC doses of up to 1 mg/kg (maximal tested dose) emicizumab were well tolerated in healthy male Chinese subjects with profiles consistent with Japanese and caucasian subjects. Results from a first‐in‐human study, together with emicizumab PK properties, did not reveal or support the likelihood of ethnicity differences in emicizumab PK. Thus, the maximal tested dose in healthy subjects (1 mg/kg) had been selected for the current study to assess the emicizumab PK profile in a healthy Chinese population and to compare the results with those from Caucasian and Japanese populations. Results from current study with a single SC dose of emicizumab 1 mg/kg in healthy male Chinese subjects are consistent with what have been observed in healthy male Japanese and Caucasian subjects. 9 , 24 The emicizumab PK profile and overall exposure (1 mg/kg single dose) in healthy Chinese subjects are generally similar to those in Japanese and Caucasian subjects with the same dose, indicating similar PK properties of emicizumab in Asian and non‐Asian populations (Figure 4, Table 3).
Figure 4.
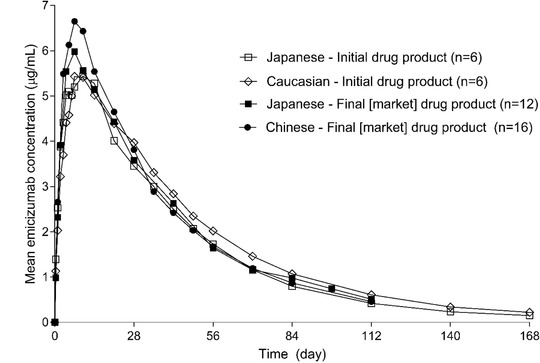
Emicizumab pharmacokinetic profiles following a single subcutaneous dose of 1‐mg/kg emicizumab in different ethnic healthy subjects.
Table 3.
Comparison of Emicizumab Pharmacokinetic Parameters Following a Single 1‐mg/kg Emicizumab Abdominal Subcutaneous Administration in Healthy Male Subjects
| Chinese | Japanese 18 | Japanese 17 | Caucasian 17 | |
|---|---|---|---|---|
| N | 16 | 12 | 6 | 6 |
| Formulation | Final (market) | Final (market) | Initial | Initial |
| tmax, d | 7.01 (3.00‐15.0) | 6.97 (3.98‐14.0) | 10.1 (4.00‐14.2) | 8.53 (7.00‐15.2) |
| Cmax, μg/mL | 7.11 ± 1.77 | 6.26 ± 1.26 | 5.92 ± 1.24 | 5.56 ± 0.812 |
| AUC0‐∞, μg⋅d/mL) | 287 ± 74.2 | 274 ± 53.3 | 266 ± 50.0 | 304 ± 79.3 |
| t½, d | 26.7 ± 4.25 | 28.0 ± 5.53 | 29.0 ± 3.26 | 32.2 ± 6.68 |
| Body weight (kg) | 62.6 (6.7) | 66.2 (9.8) | 61.2 (5.0) | 74.8 (9.2) |
AUC0‐∞, area under the concentration‐time curve from time 0 to infinity; Cmax, maximum plasma concentration; SD, standard deviation; t½, terminal half‐life; tmax, time to maximum plasma concentration.
Data are presented as mean ± SD for Cmax, AUC0‐∞, t1/2, or median (range) for tmax.
Furthermore, the administration of 1 mg/kg emicizumab in healthy Chinese subjects had no relevant impact on the safety biomarkers, such as d‐dimer levels, PF1+2, fibrinogen, or PT/INR. In line with emicizumab mechanism of action, a minor decrease within the normal range (23.9‐40.0 seconds) of aPTT was noticeable at time around tmax. FVIII activity was measured by chromogenic assays using bovine (Siemens) or human coagulation factors (Hyphen Biomed). The assay containing bovine coagulation factors (Siemens) is insensitive to emicizumab and can be used to monitor endogenous or infused FVIII activity. In contrast, the chromogenic assay with human factors (Hyphen Biomed) is sensitive to both emicizumab and FVIII; hence, it could potentially overestimate the true FVIII activity in the test plasma. However, the current study results did not show elevated FVIII activity using chromogenic assay with human factors in healthy subjects who received emicizumab. This most likely reflects the high endogenous FVIII activity in healthy subjects, the relatively low emicizumab concentration, and the competition between FVIII and emicizumab for their targets, FIXa and FX (in vitro, emicizumab did not increase thrombin generation at physiological concentrations of FVIII [data on file]). The 2 chromogenic assays using bovine or human coagulation factors are different and results cannot be quantitatively compared. Nevertheless, FVIII activity measured with both chromogenic assays remained within normal ranges throughout the study duration.
All subjects tested negative for ADA, except 1 subject who tested positive at follow‐up visit on day 113. This result is consistent with previous studies in healthy volunteers. 9 , 24 This result is also consistent with the knowledge of low prevalence of ADA potential for emicizumab. 25 In addition, this subject's PK profile did not differ from other subjects, and the safety profile was consistent with other subjects with no serious events reported. One subject tested borderline positive for FVIII inhibitors at follow‐up visit. This is likely to be a false positive as the subject's FVIII activity was stable and within the normal range. Overall, the safety profile was consistent with the safety profiles of the single‐dose study of emicizumab in healthy Japanese and Caucasian subjects.
In conclusion, the current study results further indicated the absence of ethnicity difference of emicizumab PK profile across Chinese, Japanese, and Caucasian subjects. Altogether, this further validates the use of similar therapeutic dose of emicizumab in Asian and non‐Asian populations.
Conflicts of Interest
W.Z., C.P., L.L., E.F., A.K., S.F., W.H., L.L., and C.S. were employees of F. Hoffmann–La Roche at the time of study conduct. H.L. and Y.W. declare no conflicts of interest.
Funding
This study was sponsored by F. Hoffmann‐La Roche Ltd.
References
- 1. Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773‐1779. [DOI] [PubMed] [Google Scholar]
- 2. Franchini M, Mannucci PM. Hemophilia A in the third millennium. Blood Rev. 2013;27(4):179‐184. [DOI] [PubMed] [Google Scholar]
- 3. Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18(10):1570‐1574. [DOI] [PubMed] [Google Scholar]
- 4. Muto A, Yoshihashi K, Takeda M, et al. Anti‐factor IXa/X bispecific antibody (ACE910): hemostatic potency against ongoing bleeds in a hemophilia A model and the possibility of routine supplementation. J Thromb Haemost. 2014;12(2):206‐213. [PubMed] [Google Scholar]
- 5. Sampei Z, Igawa T, Soeda T, et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS One. 2013;8(2):e57479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blair HA. Emicizumab: a review in haemophilia A. Drugs. 2019;79(15):1697‐1707. [DOI] [PubMed] [Google Scholar]
- 7. Young G, Callaghan M, Dunn A, et al. Emicizumab for hemophilia A with factor VIII inhibitors. Expert Rev Hematol. 2018;11(11):835‐846. [DOI] [PubMed] [Google Scholar]
- 8. Shima M, Hanabusa H, Taki M, et al. Factor VIII‐mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374(21):2044‐2053. [DOI] [PubMed] [Google Scholar]
- 9. Kotani N, Yoneyama K, Kawakami N, et al. Relative and absolute bioavailability study of emicizumab to bridge drug products and subcutaneous injection sites in healthy volunteers. Clin Pharmacol Drug Dev. 2019;8(6):702‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoneyama K, Schmitt C, Kotani N, et al. A pharmacometric approach to substitute for a conventional dose‐finding study in rare diseases: example of phase III dose selection for emicizumab in hemophilia A. Clin Pharmacokinet. 2018;57(9):1123‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809‐818. [DOI] [PubMed] [Google Scholar]
- 12. Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811‐822. [DOI] [PubMed] [Google Scholar]
- 13. Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open‐label, non‐randomised phase 3 study. Lancet Haematol. 2019;6(6):e295‐e305. [DOI] [PubMed] [Google Scholar]
- 14. Young G, Liesner R, Sidonio RF, et al. A multicenter, open‐label, phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood 2019;134 (24):2127‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shima M, Nogami K, Nagami S, et al. A multicentre, open‐label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia. 2019;25(6):979‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Margolis J. The kaolin clotting time: a rapid one‐stage method for diagnosis of coagulation defects. J Clin Pathol. 1958;11(5):406‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oger E, Leroyer C, Bressollette L, et al. Evaluation of a new, rapid, and quantitative d‐dimer test in patients with suspected pulmonary embolism. Am J Respir Crit Care Med. 1998;158(1):65‐70. [DOI] [PubMed] [Google Scholar]
- 18. Pelzer H, Schwarz A, Stüber W. Determination of human prothrombin activation fragment 1+2 in plasma with an antibody against a synthetic peptide. Thromb Haemost. 1991;65:153‐159. [PubMed] [Google Scholar]
- 19. Clauss A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens [Coalic physiological quick method for the determination of fibrinogen]. Acta Haematol. 1957;17:237‐246. [DOI] [PubMed] [Google Scholar]
- 20. Quick AJ. On the quantitative estimation of prothrombin. Am J Clin Pathol. 1945;15:560‐566. [DOI] [PubMed] [Google Scholar]
- 21. Peyvandi F, Oldenburg J, Friedman KD. A critical appraisal of one‐stage and chromogenic assays of factor VIII activity. J Thromb Haemost. 2016;14(2):248‐261. [DOI] [PubMed] [Google Scholar]
- 22. Wagenvoord RJ, Hendrix HH, Hemker HC. Development of a simple chromogenic factor VIII assay for clinical use. Haemostasis. 1989;19(4):196‐204. [DOI] [PubMed] [Google Scholar]
- 23. Miller CH, Rice AS, Boylan B, et al. Comparison of clot‐based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study. J Thromb Haemost. 2013;11(7):1300‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uchida N, Sambe T, Yoneyama K, et al. A first‐in‐human phase 1 study of ACE910, a novel factor VIII‐mimetic bispecific antibody, in healthy subjects. Blood. 2016;127(13):1633‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paz‐priel I, Chang T, Asikanius E, et al. Immunogenicity of emicizumab in people with hemophilia A: results from the HAVEN 1–4 studies. Blood. 2018;132:633. [Google Scholar]


