Abstract
Background
Treatment response for psoriasis is typically evaluated using clinical scores. However, patients can relapse after clinical clearance, suggesting persistent inflammation. Dermoscopy, reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can non‐invasively improve treatment response assessment.
Objectives
To compare the clinical and non‐invasive microscopic features in a psoriatic target lesion treated with clobetasol cream or calcipotriol/betamethasone dipropionate foam (Cal/BD foam).
Methods
Prospective, unicentric, open, randomized clinical trial comparing clinical data [total clinical score (TCS)] and microscopic data (dermoscopy, RCM and OCT) in psoriasis patients treated with clobetasol or Cal/BD foam.
Results
We included 36 adult patients (22 men). At week 4, more patients treated with Cal/BD foam achieved TCS ≤1 than with clobetasol (63.2% vs. 18.8%, P = 0.016). Treatment satisfaction was higher with Cal/BD foam (P < 0.03). Microscopically, Cal/BD foam induced more reduction in epidermal thickness at week 4 (P < 0.049). Dilated horizontal blood vessels were more common with clobetasol than with Cal/BD foam at week 8 (69.2% vs. 31.2%, P = 0.159). If epidermal hyperplasia was noted at baseline, the response was poorer with clobetasol (P = 0.029).
Limitations
Small sample size, open study, imaging sampling bias.
Conclusion
Cal/BD foam is more effective than clobetasol, has better patient satisfaction and induces greater reduction in the hyperkeratosis/acanthosis, regardless of baseline epidermal hyperplasia.
Introduction
The effectiveness of topical treatments for plaque psoriasis is typically evaluated using clinical scores such as psoriasis area severity index (PASI) or physician global assessment (PGA). However, sometimes patients relapse short after withdrawing their treatments besides showing clinical clearance using these scores, thus suggesting persistence of underlying inflammation not visible to the naked eye. In addition, classical topical treatments such as potent corticosteroids can lead to side‐effects if used for long periods of time, which are difficult to anticipate when using conventional clinical examination. In this sense, ancillary tests, ideally non‐invasive ones, may help evaluate the treatment response and assess the side‐effects.
Non‐invasive imaging technologies such as dermoscopy, reflectance confocal microscopy (RCM) or optical coherence tomography (OCT) have been extensively used to diagnose skin cancers 1 , 2 , 3 , 4 , 5 , 6 and to assess skin cancer treatment responses. 7 , 8 , 9 Since these technologies allow the visualization of structures not visible with the naked eye and have direct histopathological correlates, 10 , 11 , 12 they seem ideal to better define the effective clearance and assess the side‐effects in psoriatic patients undergoing topical treatments.
The current study aims to describe and compare the clinical and non‐invasive microscopic features in a psoriatic target lesion in a prospective cohort of psoriatic patients before, during and after the treatment with two topical treatments for mild to moderate psoriasis: clobetasol cream vs. a fixed combination of calcipotriol/betamethasone dipropionate foam (Cal/BD foam).
Materials and methods
After approval of the Ethics Committee of Hospital Clínic de Barcelona, from March 2018 to March 2019, we prospectively included consecutive patients with mild to moderate plaque psoriasis who were amenable to be treated with topical treatments [total body surface area (BSA) <10%]. Inclusion and exclusion criteria are found in Tables S1 and S2.
After written informed consent, patients were randomized 1:1 using a predefined randomization table to apply clobetasol cream 0.5 mg/g (Clovate®; Industrial Farmacéutica Cantabria, Santander, Spain) once daily 2–4 weeks according to clinical response or Cal/BD foam 50 µg/g + 0.5 mg/g (Enstilar®; LEO Pharma, Copenhagen, Denmark) once daily for 4 weeks. The treatment arm was not blind since the researchers randomizing and following the patients (OY, NM), and the patients knew the treatment. These same researchers decided whether it was necessary to continue or stop the treatment based on clinical response and/or the presence of adverse events. Patients were evaluated at baseline, at week 2, at week 4 and at week 8 (1 month without treatment) (Fig. S1).
Clinical evaluation
We obtained high‐resolution clinical pictures (Canon Powershot G15, Canon Inc, Tokyo, Japan) of the patient’s most severe plaque at baseline. Later, pictures were obtained at the same location using the same camera settings and illumination parameters. Furthermore, based on these images, we determined the Total Clinical Score (TCS), which grades erythema (0–3), induration (0–3) and scaling (0–3). The image evaluation was performed at the end of the study by three investigators (SE, LC and SZ) who were blinded to the treatment arm. The study primary end point was to achieve a TCS ≤1 at week 4. Adverse events were also noted, and if they posed a risk to the patient, treatment was discontinued. At the last visit, patients were also asked to rate their treatment satisfaction using a Visual Analogic Scale from 0 to 10, being 0 very dissatisfied and 10 extremely satisfied.
Microscopic evaluation
We obtained dermoscopic pictures using DermLite Foto (3Gen, San Juan Capistrano, CA, USA), handheld RCM images (Vivascope 3000; MAVIG/Caliber ID, Rochester, NY, USA) and dynamic OCT image rasters (Vivosight, Michelson Diagnostics, Maidstone, UK) at the centre of the target lesion. The images were later evaluated by three investigators (SE, LC and SZ), who did not collect the images, and were blinded to the treatment arm. The imaging features evaluated are summarized in Table S3 and included quantitative variables and semiquantitative variables (0–3). The definition of the features evaluated can be found elsewhere. 13
Sample size calculation and statistical analysis
A sample size of 16 patients in each group was determined in order to detect differences of two units (SD = 2.25) in the reduction of plaque psoriasis descriptors (TCS) between both groups. We considered a power of 80% and a significance level of 0.05. Considering a loss of evaluable individuals (loss of follow‐up, missing data, etc.) of 10%, a total of 18 patients in each group (36 patients in total) were deemed necessary to achieve statistically significant results.
Descriptive statistics were used to describe the distribution of clinical and microscopic data. Fisher’s exact test was used to compare qualitative variables. Mann–Whitney test was used to compare quantitative and qualitative variables that were not normally distributed. Two‐sided P values <0.05% were considered statistically significant. Analyses were performed using SPSS Statistics v. 22 (IBM Corporation, Armonk, NY, USA).
Results
Cohort characteristics
We included 36 patients (22 men, 14 women) with a median age of 47.5 years (range 28–80 years) (Table 1). The median weight, height, abdominal perimeter and body mass index were 75.5 kg (range 49–103 kg), 170 cm (range 152–185 cm), 96 cm (range 65–117 cm) and 24.94 kg/m2 (range 18.81–38.1 kg/m2), respectively. Regarding treatments in the previous 4 months, three patients had only used emollients, two had received phototherapy, and 30 patients used one or more topical treatments.
Table 1.
Baseline demographics and clinical characteristics of each group
| Median | Clobetasol | Cal/BD foam |
|---|---|---|
| Age | 49.5 years | 43 years |
| Weight | 75.7 kg | 73 kg |
| Height | 170 cm | 171 cm |
| Abdominal perimeter | 96 cm | 93 cm |
| BMI | 24.94 kg/cm2 | 24.91 kg/cm2 |
| TCS at baseline | 4.5 | 5 |
The majority of the target lesions were located on the elbows (n = 15) followed by the buttocks/lumbosacral area (n = 8), the abdomen (n = 5), the knees (n = 4), other parts of the legs (n = 3) and the back (n = 1). One patient was withdrawn from the study due to pregnancy, although the patient later delivered a healthy baby besides incidental application of topical Cal/BD foam for less than a week. In eight occasions, patients missed a follow‐up appointment, and among them, three did not come to the last follow‐up appointment and were considered lost to follow‐up. One patient in the clobetasol arm applied the treatment for 2 weeks due to complete response (TCS = 0) whereas the remaining patients applied the treatment for 4 weeks.
Clinical results
When assessing the lesions at baseline, there were no statistical differences in the median TCS before treatment between the clobetasol group (median 4.5) and the Cal/BD foam group (median 5) (P = 0.235), making the two groups comparable. Compared to the baseline TCS evaluation, at 4 weeks, there was a greater TCS reduction using Cal/BD foam compared to clobetasol (80% vs. 43.4% reduction; median TCS 1 vs. 3, P < 0.022) (Fig. 1). At week 4, there was a significant difference in the number of patients who achieved the study primary end point (TCS ≤ 1): 63.2% in the Cal/BD foam group vs. 18.8% in the clobetasol group (P = 0.016) (Fig. 2). At week 8, the TCS increased in both groups but remained lower with Cal/BD foam: median TCS with Cal/BD 1.5 vs. median TCS with clobetasol 3 (P = 0.568) (Fig. 1). No severe adverse events were noted during the study. Regarding the adverse events related to the treatment, only itch at the application site was reported in two patients applying Cal/BD foam and in one applying clobetasol. Four patients in the Cal/BD foam group reported that the treatment was messy to apply. However, patient satisfaction with the treatment was significantly higher with Cal/BD foam compared to clobetasol [median 9, mean 9.06 (range 7–10) vs. median 7, mean 6.5 (range 1–10), P < 0.03; Fig. S2].
Figure 1.
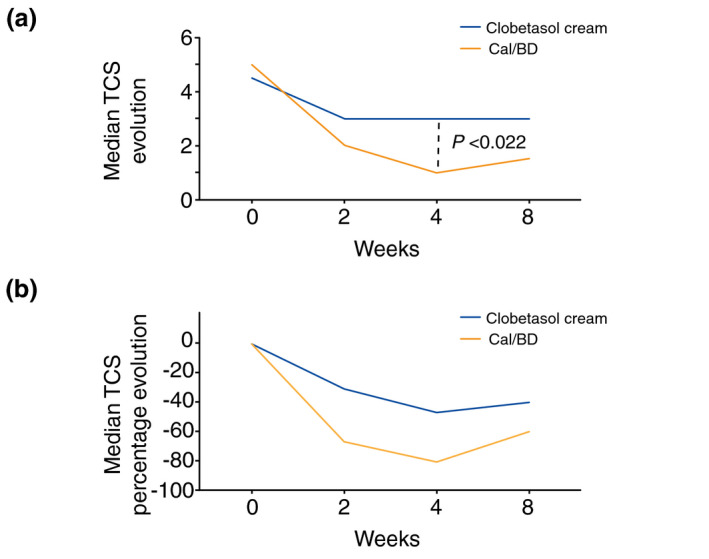
Line graphs showing the evolution of total clinical score (TCS) in both treatment groups. Panel (a) shows the median TCS evolution. Panel (b) shows the percentage median TCS evolution.
Figure 2.
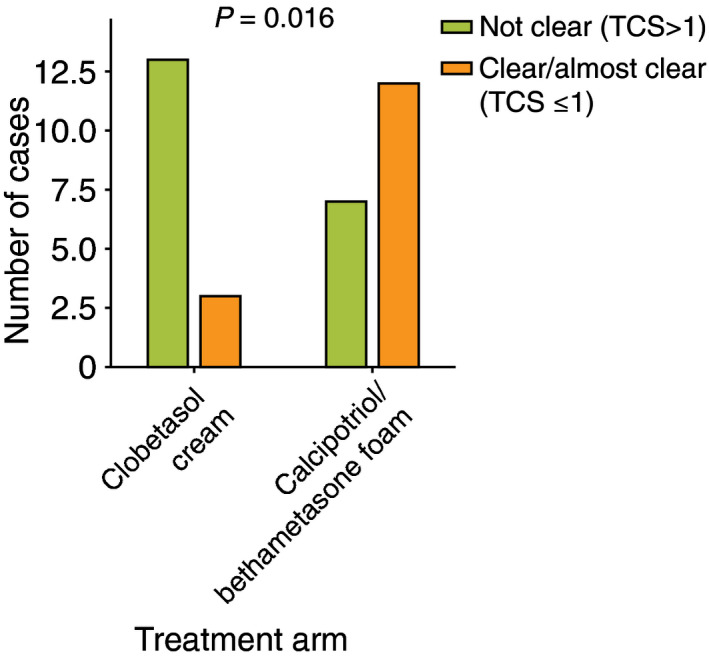
Graph bar showing cases achieving the study primary’s end point (TCS ≤ 1) at W4 (orange bars) in both treatment groups.
Microscopic results
Cal/BD foam induced a faster and greater reduction in epidermal thickness at weeks 2, and four identified with OCT (P < 0.049, Fig. 3a). Inflammatory cells identified with RCM increased with Cal/BD foam at week 2 and later decreased, being lower than clobetasol after week 4 (P < 0.05, Fig. 3b).
Figure 3.
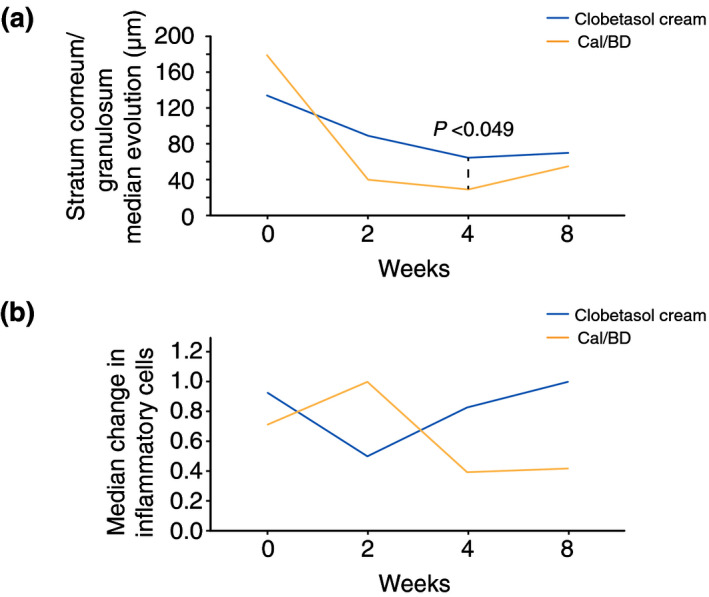
Line graphs showing the evolution of relevant microscopic findings. Panel (a) shows the evolution of the epidermal thickness measured with OCT. Panel (b) shows the evolution of inflammatory cells identified using RCM.
Linear blood vessels were identified using dermoscopy in 28.6% of clobetasol cases vs. 37.5% of Cal/BD foam cases at week 8 (P = 0.709). When assessing these vessels with RCM and OCT, we identified dilated horizontal vessels in the upper dermis (vessels >70 µm) in 69.2% of clobetasol cases vs. 31.2% of Cal/BD foam cases at week 8 (P = 0.159, Fig. 4).
Figure 4.
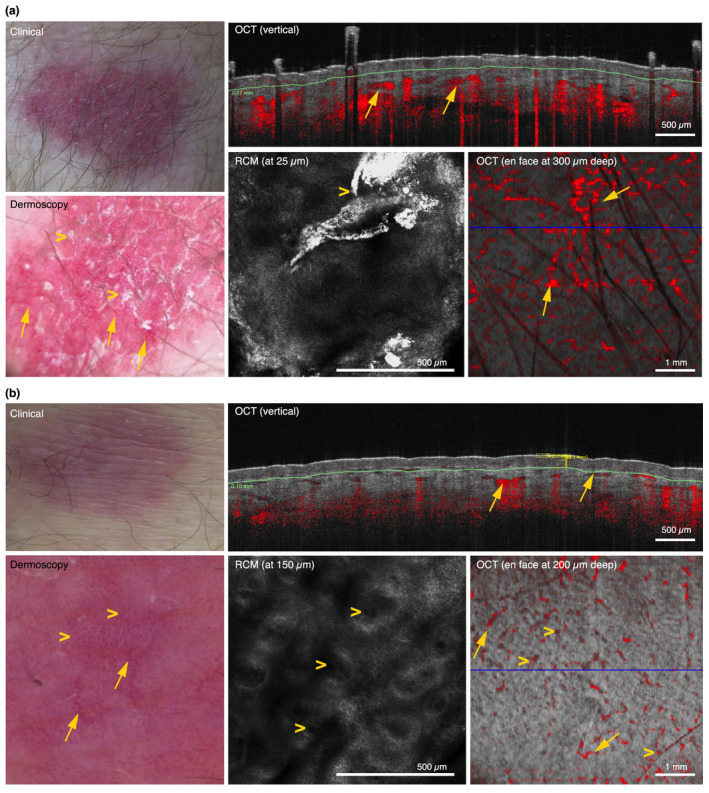
Clinical, dermoscopic, RCM and OCT images at week 8 in a case treated with clobetasol (panel a) and in a case treated with Cal/BD foam (panel b). (a) Clobetasol case at week 8 showing TCS 2, linear vessels (arrows) and scale (arrowheads) on dermoscopy, and parakeratosis (arrowheads) and dilated horizontal blood vessels (arrows) on RCM and OCT. (b) Cal/BD foam case at week 8 showing TCS 1, linear (arrows) and dotted vessels (arrowheads) on dermoscopy, increased numbers of vertical dilated blood vessels (arrowheads) and slightly dilated horizontal blood vessels (arrows) on RCM and OCT.
Regarding the microscopic features that were associated with sustained response (week 8), we saw that if epidermal hyperplasia was noted at baseline, the response was poorer with clobetasol (P = 0.029), whereas the presence or absence of epidermal hyperplasia did not affect the treatment response at week 8 with Cal/BD foam (P = 0.542; Fig. 5). Although it did not achieve statistical significance, we observed similar results regarding the presence of scale on dermoscopy; when important scaling was observed at baseline, the lesions would be less likely to continue clear at week 8 with clobetasol (11 not clear vs. 2 clear, P = 0.489), whereas with Cal/BD foam, more cases with intense scaling on dermoscopy maintained a TCS ≤1 at week 8 (10 not clear vs. 7 clear, P = 0.211).
Figure 5.
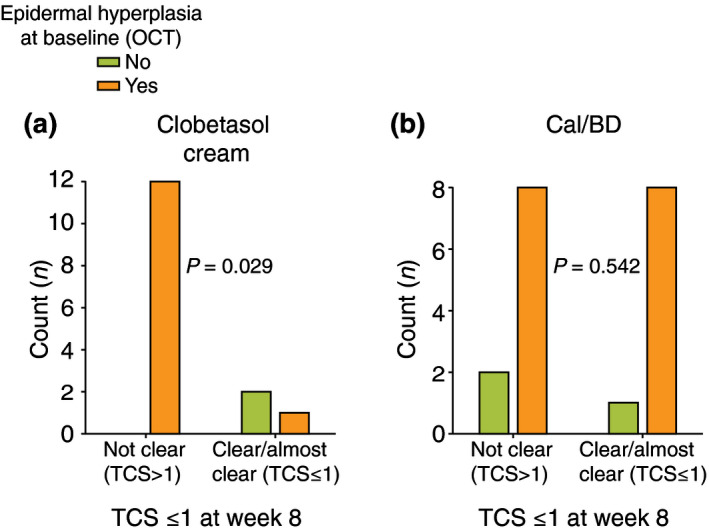
Graph bars comparing TCS clearance status at the end of the study (week 8) based on the presence or absence of epidermal hyperplasia at baseline. Panel (a) shows the results for the patients treated with clobetasol cream, and panel (b) shows the results for the patients treated with Cal/BD foam.
We did not see significant differences between both treatments regarding the changes in parakeratosis, spongiosis, irregular epidermis or dilated vertical blood vessels.
Discussion
Our results suggest that Cal/BD foam is more effective than clobetasol after 4 weeks of treatment, shown both clinically and microscopically, is potentially safer than clobetasol by inducing less iatrogenic vessels and has higher patient satisfaction. Clinically, we have shown that Cal/BD foam induces a faster response compared to clobetasol cream with a mean reduction of 80% vs. 43.4% (P < 0.022) of the TCS at 4 weeks. Additionally, more patients achieved total or almost total lesion clearance (TCS ≤ 1) at week 4 with Cal/BD foam compared to clobetasol (63.2% vs. 18.8%, P = 0.016). Furthermore, more patients maintained a TCS ≤1 at week 8 (1 month without treatment) in the Cal/BD foam arm, although it did not reach statistical significance (P = 0.568).
Regarding the microscopic findings, we identified a faster reduction in the scaling observed with dermoscopy when using Cal/BD foam, as well as a faster reduction in the hyperkeratosis/acanthosis in OCT with Cal/BD foam. This correlates well with another of our findings which shows that if more epidermal hyperplasia was noted with OCT at baseline, the lesions would be less likely to respond with clobetasol. This suggests that clobetasol cream may not penetrate deep enough in the psoriatic plaque if this is thick, whereas Cal/BD foam can be still effective regardless of the plaque thickness, even though the vehicle is a foam. The reason for this increased effectivity may be that the current formulation of Cal/BD foam is a novel aerosol foam which when sprayed does not crystalize and allows the rapid evaporation of propellants thus leaving a supersaturated layer of Cal/BD on the skin. 14 In fact, previous studies have shown that Cal/BD penetrates deeper and exerts a more intense action when formulated in this novel foam compared to the same concentrations of Cal/BD but formulated as an ointments. 15 , 16 , 17
Another interesting microscopic finding we identified was related to the presence of dilated horizontal vessels at the end of the study. Although our sample size is small and the results did not reach statistical significance, our results suggest that clobetasol induces more dilated linear vessels compared to Cal/BD foam at week 8 (69.2% of clobetasol cases vs. 31.2% of Cal/BD foam cases, P = 0.159). Hence, OCT and RCM allow the visualization of these dilated vessels better than the naked eye or dermoscopy. These vessels indicate the potential iatrogenic effect of corticosteroids, 18 , 19 thus suggesting non‐invasive microscopic methods may be necessary to assess the early onset of adverse events related to treatment.
Interestingly, we have also seen a temporary increase in the numbers of inflammatory cells with RCM when using Cal/BD foam after 2 weeks of treatment (Fig. 3b). An explanation for this finding could be that Cal/BD induces an increase in CD8+ lymphocytes, which restores the CD4+ and CD8+ lymphocyte epidermal counts thus normalizing the skin microenvironment. 20 However, to confirm this hypothesis, biopsies may have been needed to perform immunohistochemical studies. Another possible explanation for this increase may be due to a certain degree of irritation related to the vehicle (a foam) or to calcipotriol itself. 14 In fact, two patients reported itch at the application site of Cal/BD foam. Nevertheless, this did not seem relevant and did not reduce treatment adherence since patients continued to apply the treatment and actually reported higher patient satisfaction with Cal/BD foam compared to clobetasol (Fig. S2). This increase in inflammatory cells was transient, and later, the inflammatory cell counts decreased to levels lower than with clobetasol. Hence, probably this early increase in inflammatory may correlate with a better treatment response, suggesting that microscopic data may not only improve the detection of early adverse events, but can better grade the treatment response.
Non‐invasive microscopic assessment using tools such as dermoscopy, RCM or OCT has been used extensively in the fields of dermato‐oncology and more recently to diagnose inflammatory dermatoses 21 , 22 , 23 , 24 and to grade treatment response. 13 , 25 The advantage of these technologies is that they allow a fast assessment without the need of being invasive. Dermoscopy uses a magnifying lens (commonly 10×) which allows the visualization of structures not visible to the naked eye but not reaching the cellular level. 12 RCM uses a near‐infrared light laser which allows the visualization of en face images with cellular resolution; 26 , 27 however, because of this high resolution, RCM can only image up to ~250 µm (superficial dermis). OCT uses also a laser light source which allows a deeper penetration, up to 1 cm, but at the cost of a worse resolution. OCT can assess the architecture and in some devices the visualization of blood vessels (dynamic OCT). 6 , 28 , 29
As stated in the previous paragraph, the use of these imaging technologies has some limitations; although we used different imaging technologies to maximize the information obtained, some degree of sampling error may have occurred, since psoriatic plaques are typically in the range of centimetres, much larger than the field of view of the technologies used. Nevertheless, we tried to minimize this issue by imaging the centre of the target lesion, although sometimes identifying the same exact area was hard since the morphology of psoriatic plaques may change throughout the treatment process.
Another challenge we encountered was the assessment of vessels using dynamic OCT. Vessel quantification in OCT needs to be done in the different planes and can vary depending on anatomical factors, vasodilation due to exogenous factors (temperature, elevation of the area imaged, irritation), among others. In addition, the software does not provide an objective measurement of the quantity of vessels, which makes its assessment difficult. Because of this, we started the development of an informatics algorithm to automatically quantify the vessel density. However, this is not an easy endeavour since vessels in dynamic OCT need to be adjusted by the blood flow in healthy skin, which can also change depending on the anatomic area. Additionally, dynamic OCT induces a ‘shadow’ artefact in which under a given vessel the device cannot assess correctly the blood flow. Another aspect to take into consideration is that vessels need to be measured at the same vertical plane to be compared, but since psoriatic plaques changed their thickness, comparison over time was challenging. This may explain why we have identified a tendency in increased dilated blood vessels with clobetasol, but the difference did not reach statistical significance. Another limitation which can also explain the latter includes a small sample size, since it was calculated to evaluate differences in the TCS. Another limitation of our study is that the patients and investigators acquiring the images were not blind to the treatment and that although the analysis of the images was performed by three evaluators who were blind to the treatment arm, subjective interpretation of the images may have impacted the results.
Regarding the last point, efforts have been made to provide objective, automated or semi‐automated image assessment using artificial intelligence (AI). Currently, different AI algorithms have shown to have high diagnostic accuracy to diagnose multiple dermatoses, at the same level or superior to dermatologists. 30 , 31 Thus, it seems intuitive that AI algorithms may allow an objective accurate objective evaluation in psoriasis. Several studies have attempted to do so by using colour calibration, 32 and in fact semi‐automated and automated PASI calculation algorithms have been developed using calibrated images acquired in order to provide more objective disease quantification than manual PASI or PGA calculations performed by physicians. 33 , 34 , 35 Hence, such methods could also be applied in other fields of dermatological imaging in order to improve disease quantification and to better determine response and management of side‐effects. 36 , 37
Conclusion
To sum up, we have shown that Cal/BD foam is more effective than clobetasol cream after 4 weeks of treatment, has better patient satisfaction than clobetasol and induces a faster and greater reduction in the hyperkeratosis/acanthosis, regardless of the initial epidermal hyperplasia status. Our results also suggest that clobetasol may induce more iatrogenic telangiectasias compared to Cal/BD foam. However, our sample was small and lacks automated measurements. Hence, future studies using AI and multimodal imaging methods are necessary to obtain objective data regarding treatment response and prediction of early adverse events in psoriatic patients treated with topical treatments.
Supporting information
Table S1 . Study’s inclusion criteria.
Table S2 . Study’s exclusion criteria.
Table S3 . Description of the microscopic variables analyzed in this study.
Figure S1 . Scheme of the treatment visits.
Figure S2 . Box plot comparing patient satisfaction with clobetasol vs. calcipotriol/betamethasone dipropionate foam, showing an overall higher satisfaction with the latter.
Acknowledgements
We would like to thank all the physicians at the dermatology department at Hospital Clínic, as well as family physicians at CAP Casanova for contributing patients to the study.
Conflicts of interest
OY has served as a consultant to Almirall and has received honoraria for this. JM has served as a consultant to Almirall, Amgen, Canfield and Pierre Fabre and has received honoraria for this. PI has served as a consultant to Almirall and has received honoraria for this. SP has served as a consultant to Almirall, Avene, ISDIN, La Roche Posay and Leo Pharma, and has received honoraria for this. The other authors do not have conflicts of interest relevant to the current manuscript.
Funding source
This research was funded by Leo Pharma. The sponsor did not influence on the study design or planning.
IRB: This study was performed under the Hospital Clínic de Barcelona’s Ethics Committee‐approved protocol number HC‐ENS‐2017‐01.
References
- 1. Alarcon I, Carrera C, Palou J et al Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol 2014; 170: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borsari S, Pampena R, Lallas A et al Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol 2016; 152: 1093. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez S, Sanchez V, Gonzalez‐Rodriguez A, Parrado C, Ullrich M. Confocal microscopy patterns in nonmelanoma skin cancer and clinical applications. Actas Dermosifiliogr 2014; 105: 446–458. [DOI] [PubMed] [Google Scholar]
- 4. Pellacani G, Cesinaro AM, Seidenari S. Reflectance‐mode confocal microscopy of pigmented skin lesions–improvement in melanoma diagnostic specificity. J Am Acad Dermatol 2005; 53: 979–985. [DOI] [PubMed] [Google Scholar]
- 5. Markowitz O, Schwartz M, Feldman E et al Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol 2015; 8: 14–20. [PMC free article] [PubMed] [Google Scholar]
- 6. Ulrich M, Themstrup L, de Carvalho N et al Dynamic optical coherence tomography in dermatology. Dermatology 2016; 232: 298–311. [DOI] [PubMed] [Google Scholar]
- 7. Alarcon I, Carrera C, Alos L et al In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol 2014; 71: 49–55. [DOI] [PubMed] [Google Scholar]
- 8. Hibler B, Yélamos O, Cordova M et al Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis 2017; 99: 346–352. [PMC free article] [PubMed] [Google Scholar]
- 9. Manubens E, Barreiro A, Bennassar A et al Fast evaluation and monitoring of ingenol mebutate treatment of multiple basal cell carcinomas by in vivo hand‐held reflectance confocal microscopy. J Eur Acad Dermatol Venereol 2017; 31: e284–e286. [DOI] [PubMed] [Google Scholar]
- 10. Ardigo M, Longo C, Gonzalez S. Multicenter study on inflammatory skin diseases from The International Confocal Working Group (ICWG): specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol 2016; 175: 364–374. [DOI] [PubMed] [Google Scholar]
- 11. Agozzino M, Gonzalez S, Ardigo M. Reflectance confocal microscopy for inflammatory skin diseases. Actas Dermosifiliogr 2016; 107: 631–639. [DOI] [PubMed] [Google Scholar]
- 12. Yélamos O, Braun R, Liopyris K et al Dermoscopy/dermatoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol 2019; 80: 341–363. [DOI] [PubMed] [Google Scholar]
- 13. Agozzino M, Noal C, Lacarrubba F, Ardigo M. Monitoring treatment response in psoriasis: current perspectives on the clinical utility of reflectance confocal microscopy. Psoriasis (Auckl) 2017; 7: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puig L, Carretero G. Update on topical treatments for psoriasis: the role of calcipotriol plus betamethasone dipropionate aerosol foam. Actas Dermosifiliogr 2019; 110: 115–123. [DOI] [PubMed] [Google Scholar]
- 15. Queille‐Roussel C, Bang B, Clonier F, Lacour JP. Enhanced vasoconstrictor potency of the fixed combination calcipotriol plus betamethasone dipropionate in an innovative aerosol foam formulation vs. other corticosteroid psoriasis treatments. J Eur Acad Dermatol Venereol 2016; 30: 1951–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollesen Basse L, Olesen M, Lacour J, Queille‐Roussel C. Enhanced in vitro skin penetration and antipsoriatic effect of fixed combination calcipotriol plus betamethasone dipropionate in an innovative foam vehicle. J Invest Dermatol 2014; 134.S33:abst:192. [Google Scholar]
- 17. Koo J, Tyring S, Werschler WP et al Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris–a randomized phase II study. J Dermatolog Treat 2016; 27: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vazquez‐Lopez F, Marghoob AA. Dermoscopic assessment of long‐term topical therapies with potent steroids in chronic psoriasis. J Am Acad Dermatol 2004; 51: 811–813. [DOI] [PubMed] [Google Scholar]
- 19. Grajdeanu IA, Statescu L, Vata D et al Imaging techniques in the diagnosis and monitoring of psoriasis. Exp Ther Med 2019; 18: 4974–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satake K, Amano T, Okamoto T. Calcipotriol and betamethasone dipropionate synergistically enhances the balance between regulatory and proinflammatory T cells in a murine psoriasis model. Sci Rep 2019; 9: 16322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moscarella E, Longo C, Zalaudek I et al Dermoscopy and confocal microscopy clues in the diagnosis of psoriasis and porokeratosis. J Am Acad Dermatol 2013; 69: e231–e233. [DOI] [PubMed] [Google Scholar]
- 22. Agozzino M, Berardesca E, Donadio C et al Reflectance confocal microscopy features of seborrheic dermatitis for plaque psoriasis differentiation. Dermatology 2014; 229: 215–221. [DOI] [PubMed] [Google Scholar]
- 23. Ardigo M, Maliszewski I, Cota C et al Preliminary evaluation of in vivo reflectance confocal microscopy features of Discoid lupus erythematosus. Br J Dermatol 2007; 156: 1196–1203. [DOI] [PubMed] [Google Scholar]
- 24. Astner S, Gonzalez E, Cheung A, Rius‐Diaz F, Gonzalez S. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol 2005; 53: 986–992. [DOI] [PubMed] [Google Scholar]
- 25. Paganelli A, Ciardo S, Odorici G, Pellacani G, Conti A. Efficacy of ustekinumab after failure of infliximab CT‐P13 in a HLA‐Cw6‐positive patient affected by pityriasis rubra pilaris: monitoring with reflectance confocal microscopy (RCM) and optical coherence tomography (OCT). J Eur Acad Dermatol Venereol 2017; 31: e249–e251. [DOI] [PubMed] [Google Scholar]
- 26. Rajadhyaksha M, Gonzalez S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol 1999; 113: 293–303. [DOI] [PubMed] [Google Scholar]
- 27. Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH,Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol 1995; 104: 946–952. [DOI] [PubMed] [Google Scholar]
- 28. Schuh S, Holmes J, Ulrich M et al Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther (Heidelb) 2017; 7: 187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ulrich M, Themstrup L, de Carvalho N et al Dynamic optical coherence tomography of skin blood vessels – proposed terminology and practical guidelines. J Eur Acad Dermatol Venereol 2018; 32: 152–155. [DOI] [PubMed] [Google Scholar]
- 30. Esteva A, Kuprel B, Novoa RA et al Dermatologist‐level classification of skin cancer with deep neural networks. Nature 2017; 542: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marchetti MA, Codella NCF, Dusza SW et al Results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol 2018; 78: 270–277.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raina A, Hennessy R, Rains M et al Objective measurement of erythema in psoriasis using digital color photography with color calibration. Skin Res Technol 2016; 22: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fink C, Alt C, Uhlmann L et al Precision and reproducibility of automated computer‐guided Psoriasis Area and Severity Index measurements in comparison with trained physicians. Br J Dermatol 2019; 180: 390–396. [DOI] [PubMed] [Google Scholar]
- 34. Fink C, Fuchs T, Enk A, Haenssle HA. Design of an algorithm for automated, computer‐guided PASI measurements by digital image analysis. J Med Syst 2018; 42: 248. [DOI] [PubMed] [Google Scholar]
- 35. Fink C, Uhlmann L, Klose C, Haenssle HA. Automated, computer‐guided PASI measurements by digital image analysis versus conventional physicians’ PASI calculations: study protocol for a comparative, single‐centre, observational study. BMJ Open 2018; 8: e018461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. George Y, Aldeen M, Garnavi R. Psoriasis image representation using patch‐based dictionary learning for erythema severity scoring. Comput Med Imaging Graph 2018; 66: 44–55. [DOI] [PubMed] [Google Scholar]
- 37. Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. A novel and robust Bayesian approach for segmentation of psoriasis lesions and its risk stratification. Comput Methods Programs Biomed 2017; 150: 9–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 . Study’s inclusion criteria.
Table S2 . Study’s exclusion criteria.
Table S3 . Description of the microscopic variables analyzed in this study.
Figure S1 . Scheme of the treatment visits.
Figure S2 . Box plot comparing patient satisfaction with clobetasol vs. calcipotriol/betamethasone dipropionate foam, showing an overall higher satisfaction with the latter.


