Abstract
The aim of the present study was to investigate the effects of 10 Hz repetitive transcranial magnetic stimulation (rTMS) of the right dorsolateral prefrontal cortex (DLPFC) during vegetative state (VS). Between May 2017 and November 2018, 95 patients were treated in the Coma Recovery Department of the Central Hospital of Jinzhou. According to the inclusion and exclusion criteria, a total of 32 patients in VS caused by brain injury were enrolled. The patients were assigned into rTMS and control groups in a non-randomized manner. All patients received JFK Coma Recovery Scale-Revised (CRS-R) scores and underwent motor evoked potential (MEP) latency and central motor conduction time (CMCT) measurement before the first treatment and after 20 days of treatment, which was the end of the study. Following 20 days of treatment, a significant increase was observed in the CRS-R scores of patients in the rTMS group compared with those obtained at pretreatment (P<0.001). An increase in the CRS-R scores of the control group was also observed compared with the pretreatment scores (P=0.035). The change in CRS-R scores (P<0.001) and improved conscious state rate (P=0.0016) were significantly different between the two groups. A significant decrease in MEP (P<0.001) and CMCT (P<0.001) was observed in the rTMS group compared with measurements obtained at pretreatment, whereas no significant decrease was observed in the control group (P=0.693; P=0.070). The changes in MEP (P<0.001) and CMCT (P<0.001) between the two groups were statistically significant. In conclusion, 10 Hz rTMS of the right DLPFC in early disorders of consciousness is feasible and efficient. rTMS treatment could improve patient state of awareness and accelerate patient recovery in VS.
Keywords: repetitive transcranial magnetic stimulation, disorder of consciousness, vegetative state recovery, non-invasive brain stimulation, right dorsolateral prefrontal cortex
Introduction
Coma induced by severe brain injury is a self-limiting state that typically resolves within 2 weeks (1). Severe and diffuse lesions of the cortex or underlying white matter, bilateral thalamic damage and brainstem injury account for the primary reasons of comas (1). After two weeks, comas are classified into disorders of consciousness (DOC), which can be divided into 2 subgroups: Vegetative state (VS) and minimally conscious state (MCS) (1). VS is a condition of a wakeful unconscious state. In VS, patients can spontaneously open their eyes, but without repetitive behavioral responses to verbal comprehension, verbal or gestural communication, and purposeful situations of visual, auditory, tactile and harmful stimuli (1,2). MCS is a condition of severely altered consciousness characterized by functional communication or functional use of objects (1,2). Patients with MCS showed a range of behavioral signs of inconsistent awareness and reliable communication (1,2). The use of multiple treatments for DOC recovery in different stages is necessary, including invasive and non-invasive treatments. Invasive methods, including deep brain and spinal cord stimulation, have ethical and procedural limitations (3,4) and are not suitable for early DOC. A report of non-invasive measures using electrical and magnetic stimulation of the brain, spinal cord and roots was endorsed by the first International Federation of Clinical Neuroelectrophysiology (IFCN) 30 years ago. Among developed neurostimulation techniques, non-invasive measures, including transcranial magnetic stimulation and transcranial direct current stimulation, may be promising for early DOC therapeutic intervention (5). However, there are still no sufficient evidence-based methods to treat DOC to date. During the past decade, several studies and clinical trials have demonstrated potential therapeutic applications of non-invasive brain stimulation, particularly for transcranial magnetic stimulation (TMS), which is non-invasive, safe, painless and effective in treating DOC (6-8).
A magnetic stimulating coil placed on the human scalp can generate a strong magnetic field by a rapid pulse current, which can penetrate the skull, causing secondary induction current at adjacent nerve tissues (9). The current acts on the cell membrane of cerebral cortical neurons, generating excitatory or inhibitory postsynaptic potentials to stimulate neurons that lead to altered cortical function (10,11). TMS can be applied one stimulus at a time (single-pulse TMS), in pairs of stimuli separated by a variable interval (paired-pulse TMS) or in trains repetitive TMS (rTMS) (3,11). rTMS focuses on a particular cortical site to improve neurophysiological functions and has been proven to have a neuromodulatory effect (3,7,11). Furthermore, its repetitive effect has been proven to be more effective than single-pulse and paired-pulse TMS in the treatment of DOC (12-16). rTMS is currently used for the treatment of several diseases such as depression, psychiatric illness, epilepsy, Parkinson's disease and cognitive dysfunction, and achieves satisfactory curative effects (16-20).
However, studies using rTMS to treat DOC have shown conflicting results. Louise-Bender Pape et al (6) reported a trend toward significant neurobehavioral gains by rTMS to stimulate the right dorsolateral prefrontal cortex (DLPFC) in a traumatic patient with VS/unresponsive wakefulness syndrome. Xie and Zhang (8) reported that rTMS treatment could improve consciousness disturbance in 10 patients with stroke as detected by quantitative electroencephalography spectral power analysis. Naro et al (21) found that a single session of 10 Hz rTMS over the right DLPFC may improve consciousness and partially restore the connectivity within several cortical areas, but no clinical effects were observed between the test and control groups. Liu et al (22) reported no behavioral improvement following one session of primary motor cortex (M1) 20 Hz rTMS for 10 min in 10 patients with DOC. However, hemodynamic functions were improved in the MCS group but not in the unresponsive wakefulness syndrome group. Bai et al (23) reported that an increased Coma Recovery Scale-Revised (CRS-R) score was observed in a female with intracranial hemorrhage for 9 months prior to receiving rTMS 30 times. No effect of rTMS on the DOC was reported in another study. Cincotta et al (24) reported a double-blinded randomized controlled trial comprising 74 patients with DOC. No behavioral improvements were found following five repeated sessions at 20 Hz applied over the M1 for 10 min in 11 patients with unresponsive wakefulness syndrome. For the diagnosis of a consciousness disorder, the target of rTMS stimulation may impact the efficacy of rTMS on DOC. The present study hypothesized that high-frequency rTMS stimulation on patients in VS could improve the consciousness state (CS) and CRS-R scores. The aim of the present study was to investigate the efficacy of rTMS on VS patients who received 10 Hz rTMS on the right DLPFC.
Materials and methods
Patients
In the present retrospective cohort study, all patients were recruited from the Departments of Neurosurgery and Coma Recovery (Central Hospital of Jinzhou, Jinzhou, China) and between May 2017 to November 2018. The Coma Recovery Department of Jinzhou Central Hospital is the first standard coma recovery and rehabilitation ward in northeast China. The inclusion criteria were as follows: i) Accepted coma treatments 2 weeks after admission; ii) aged 18-75 years; iii) vital signs were stable; iv) their CRS-R score was assessed by international criteria and they fulfilled the diagnostic criteria of VS (1); v) had no seizures or brain edema; vi) received no sedatives and anti-epilepsy drugs within the last 7 days; vii) informed consent was signed by patients of their legally authorized representative to participate in the present study. The exclusion criteria were as follows: i) No motor evoked potentials (MEP) were elicited; ii) severe dysfunction of heart, liver or kidney; iii) extreme complications, such as pneumonia and deep venous thrombosis; iv) seizures or skull scalp injury during treatment; v) craniotomy or metallic implantation on the right side of the head; vi) previous neurological or psychiatric disorders; vii) no 20 consecutive rTMS treatments in the rTMS group. There were two groups in the present study. The patients whose legally authorized representatives accepted rTMS treatment were allocated into the rTMS group and those who refused rTMS treatment were allocated into the control group. Finally, patient data was collected from 15 patients from the rTMS group and 17 patients from the control group. All patients received similar routine medication and rehabilitation course in our coma recovery ward.
Study design and stimulation protocol
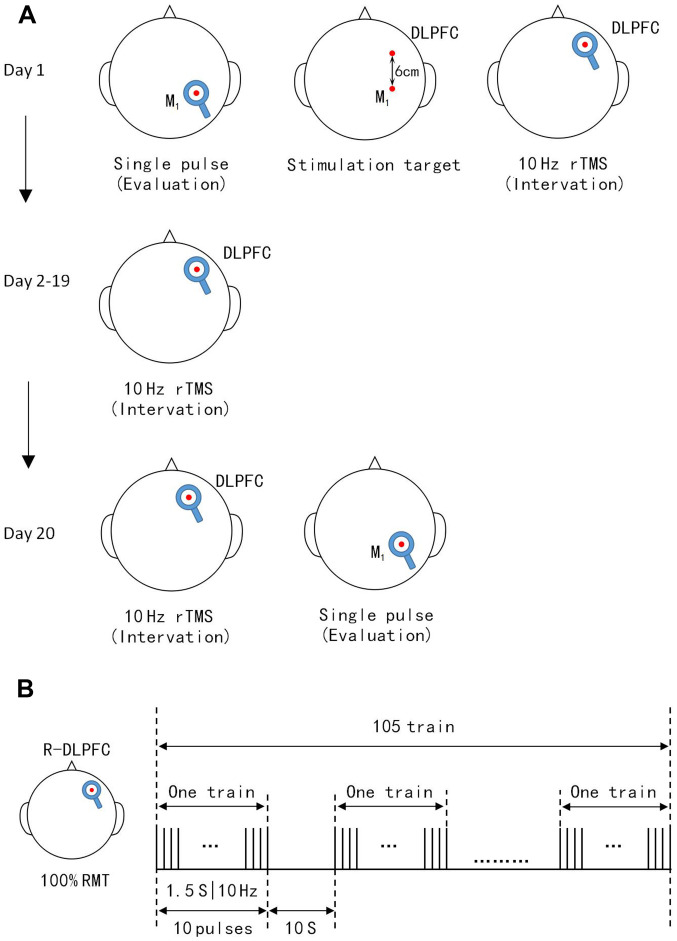
The effect of rTMS is dependent on its frequency. Low-frequency rTMS (<1 Hz) can reduce neuronal excitability, local metabolism levels and cerebral blood flow, and inhibit cortical activity (13,25), whereas high-frequency rTMS (5-20 Hz) exerts the opposite effects. In addition, rTMS can affect the transmission of monoamine neurotransmitters and genetic expression of neuronal excitability in the brain (11,26). The patients in the rTMS group received active 10 Hz rTMS on the right DLPFC once per day over 20 consecutive days. TMS pulses were delivered using a Yiruide CCY-IA stimulator and a circular coil (Wuhan Yiruide Medical Equipment New Technology Co., Ltd.), which can produce a pulse stimulation frequency of 0.01-100 Hz and a 2.0-T maximum magnetic field strength. The circular coil had a diameter of 10 cm, where stimulation is most effective under the coil. The minimal stimulation is in the center of the ring (15). The consciousness is dependent on the function of the cerebral cortex, which affects high-level central neural system function and bilateral hemisphere functional cortices that compete and suppress together, and the ascending reticular activating system that affects regular awakening (27,28). The key point for rTMS is the site of stimulation, which has been discussed in several studies. The DLPFC (6-8) and M1 (24,29,30) are the most common stimulation sites of choice. Several studies have chosen the left (7) or right (8) DLPFC as the site of stimulation, with positive outcomes. The right DLPFC is closely linked to the brain network structure, with the ascending reticular activating system connecting to the DLPFC and even the entire cortex, via the thalamic relay and raphe nuclei, which are the most important for DOC recovery (6). The maintenance of sustained arousal and attention are also cardinal functions of the right DLPFC (6). Based on these reasons, the right DLPFC was selected as the stimulation site in the present study. The stimulation target is the most important parameter for rTMS treatment (27-30). The right hemisphere motor cortex was determined by MEP and the 5+1 cm prefrontal cortex in front of the right M1 was chosen as the right DLPFC (Fig. 1A) (31). The coil was placed tangentially toward the scalp over the right DLPFC for stimulation. Stimulation intensity is the second most important parameter for each patient and was determined based on the resting motor threshold (RMT). The stimulation intensity of RMT is also undefined. Xia et al (7) administered 90% RMT to patients with chronic DOC. Xie and Zhang (8) used 100% RMT to treat patients with DOC with stroke. However, high levels of intensity and frequency could trigger seizures. Cavinato et al (32) reported that 20 Hz rTMS treatment could trigger frequent seizures. Most rTMS studies used 10 Hz and 100% RMT and had a lower risk of epilepsy (5,6,8). Based on the above consideration, 10 Hz and 100% RMT were chosen as the treatment parameters in the present study. According to the IFCN Committee recommendations (15), RMT was defined as the lowest TMS intensity to evoke at least 5/10 electromyogram with an amplitude of >50 µV peak-to-peak in the relaxed first dorsal interosseous muscle of the right hand (7,15). During RMT measurement, earplugs were inserted into the ears of patients, which continuously played a masking noise to prevent the interference of auditory potentials with TMS discharge. When the total number of pulses per treatment was >1,000 and the stimulation intensity was ≥100% RMT, the cure rate of patients was significantly increased. A single treatment of 1,000-2,000 pulses is safe and effective according to TMS guidelines (15). A single daily session of stimulation consisted of 1,575 pulses (10 Hz trains for 1.5 sec; repeated 105 times with an inter-train interval of 10 sec; total session, 20 min and 8 sec) at an intensity of 100% RMT (Fig. 1B) was applied in the present study. Meanwhile, patient routine medication and rehabilitation courses continued as usual during rTMS treatment.
Figure 1.
TMS protocol for patients. (A) Single pulse TMS-MEP recordings before the protocol were used to collect data and locate the M1. DLPFC was 6 cm anterior to the M1 paralleled to the sagittal line. TMS-MEP evaluations were not conducted on days 2-19. Single pulse TMS-MEP recorded immediately after the 20 sessions were used to assess protocols. (B) Illustration of a single daily session of stimulation consisted of 10 Hz trains for 1.5 sec. These were repeated 105 times with an inter-train interval of 10 sec. Total sessions were 20 min and 8 sec. TMS-MEP, transcranial magnetic stimulation-motor evoked potentials; DLPFC, dorsolateral prefrontal cortex.
Outcome evaluation
All patients received CRS-R scores and MEP latency and central motor conduction time (CMCT) measurements prior to the first treatment and after 20 days of treatment, which was the end of the study.
Statistical analysis
All statistical analysis was performed using GraphPad Prism 7.0 (GraphPad Software, Inc.). The Mann-Whitney U test, Wilcoxon matched pairs signed rank test, paired t-test, unpaired t-test and Fisher's exact probability tests were used for comparison between groups, as appropriate. Normally distributed data are expressed as the mean ± SD. Skewed distributed data are expressed as the median with interquartile range. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient data in rTMS and control groups
All patients remained in a stable clinical state during routine medication and rehabilitation course. There were no focal lesions in the right DLPFC in all patients, as evidenced by their brain scans. Detailed clinical characteristics of participants are shown in Tables I and II. The mean age of the patients was 60.5±1.8 (range, 49-70) years in the rTMS group and 59.7±2.1 (range, 39-72) years in the control group (P>0.05). There were eight males and seven females in the rTMS group, including seven with ICH and eight with TBI and 10 males and seven females in the control group, including nine with ICH and eight with TBI. The admission time was 4.6±0.8 h in the rTMS group and 4.4±0.7 h in the control group (P>0.05). The mean CRS-R score was 3.7±0.7 (3-5) in the rTMS group and 3.8±0.8 (3-5) in the control group (P>0.05). The mean MEP prior to treatment was 29.87±0.96 msec in the rTMS group and 30.02±0.98 msec in the control group (P>0.05). The mean CMCT before treatment was 11.04±0.24 msec in the rTMS group and 11.14±0.23 msec in the control group (P>0.05). Baseline data, including age, sex, etiology, admission time, admission Glasgow Coma Scale score, CRS-R score, MEP and CMCT prior to treatment were not significantly different between the rTMS and control groups (Fig. 2).
Table I.
Clinical data of the repetitive transcranial magnetic stimulation group.
| CRS-R | MEP latency, msec | CMCT, msec | CS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age years | Sex | Etiology | Admission time, h | Admission GCS | Day 1 | Day 20 | Day 1 | Day 20 | Day 1 | Day 20 | Day 20 | |
| 1 | 65 | M | ICH | 4 | 4 | 3 | 5 | 31.04 | 27.96 | 12.45 | 12.03 | VS | |
| 2 | 55 | M | TBI | 3 | 5 | 4 | 8 | 29.81 | 29.44 | 11.09 | 10.21 | MCS- | |
| 3 | 58 | F | ICH | 4 | 4 | 3 | 6 | 30.83 | 27.15 | 11.97 | 11.12 | MCS- | |
| 4 | 62 | F | TBI | 2 | 5 | 4 | 7 | 29.13 | 28.13 | 10.54 | 9.86 | MCS- | |
| 5 | 49 | M | TBI | 3 | 4 | 3 | 7 | 30.56 | 29.84 | 11.23 | 10.14 | MCS- | |
| 6 | 52 | M | TBI | 2 | 4 | 3 | 6 | 31.27 | 27.45 | 12.37 | 11.84 | MCS- | |
| 7 | 68 | F | TBI | 4 | 4 | 4 | 6 | 28.97 | 29.21 | 10.21 | 9.75 | MCS- | |
| 8 | 50 | F | ICH | 4 | 4 | 3 | 7 | 30.07 | 27.46 | 11.08 | 10.26 | MCS- | |
| 9 | 70 | M | ICH | 5 | 6 | 5 | 8 | 28.75 | 30.01 | 10.11 | 9.84 | MCS- | |
| 10 | 65 | F | TBI | 3 | 4 | 3 | 5 | 31.66 | 27.48 | 12.58 | 11.46 | VS | |
| 11 | 57 | M | ICH | 6 | 6 | 5 | 9 | 28.97 | 28.04 | 9.75 | 9.43 | MCS- | |
| 12 | 60 | F | TBI | 3 | 4 | 4 | 8 | 29.11 | 28.96 | 10.87 | 10.22 | MCS- | |
| 13 | 63 | M | ICH | 5 | 5 | 4 | 7 | 29.35 | 27.26 | 10.34 | 9.94 | MCS- | |
| 14 | 65 | F | ICH | 4 | 5 | 4 | 9 | 29.45 | 27.88 | 10.89 | 9.75 | MCS- | |
| 15 | 69 | M | TBI | 3 | 5 | 4 | 8 | 29.14 | 27.88 | 10.11 | 10.03 | MCS- | |
ICH, intracerebral hemorrhage; TBI, traumatic brain injury; GCS, Glasgow Coma Scale; CRS-R, JFK Coma Recovery Scale-Revised; MEP, motor evoked potentials; CMCT, central motor conduction time; CS, consciousness state; M, male; F, female; VS, vegetative state; MCS, minimally conscious state; -, negative.
Table II.
Clinical data of the control group.
| CRS-R | MEP latency, msec | CMCT, msec | CS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age years | Sex | Etiology | Admission time, h | Admission GCS | Day 1 | Day 20 | Day 1 | Day 20 | Day 1 | Day 20 | Day 20 | |
| 1 | 68 | F | ICH | 4 | 4 | 3 | 3 | 31.84 | 31.55 | 12.67 | 12.58 | VS | |
| 2 | 65 | M | ICH | 5 | 4 | 3 | 4 | 30.95 | 31.01 | 12.14 | 11.99 | VS | |
| 3 | 52 | M | TBI | 3 | 5 | 4 | 5 | 30.13 | 30.05 | 11.75 | 11.37 | VS | |
| 4 | 63 | F | ICH | 5 | 4 | 4 | 5 | 29.45 | 29.34 | 10.98 | 10.56 | VS | |
| 5 | 59 | F | TBI | 3 | 5 | 4 | 6 | 29.63 | 29.55 | 11.04 | 10.88 | MCS- | |
| 6 | 39 | M | ICH | 3 | 6 | 4 | 7 | 29.97 | 28.88 | 11.34 | 10.01 | MCS- | |
| 7 | 56 | F | TBI | 2 | 5 | 5 | 7 | 28.74 | 28.05 | 10.13 | 9.59 | MCS- | |
| 8 | 57 | M | TBI | 3 | 4 | 4 | 6 | 29.03 | 28.93 | 10.21 | 10.09 | MCS- | |
| 9 | 69 | M | ICH | 5 | 4 | 3 | 4 | 31.37 | 32.01 | 12.45 | 12.56 | VS | |
| 10 | 71 | F | ICH | 4 | 3 | 3 | 3 | 30.87 | 32.54 | 11.97 | 12.01 | VS | |
| 11 | 62 | M | TBI | 4 | 4 | 3 | 4 | 31.41 | 30.61 | 12.47 | 12.06 | VS | |
| 12 | 72 | M | ICH | 6 | 5 | 5 | 5 | 28.87 | 28.64 | 10.11 | 10.13 | VS | |
| 13 | 49 | F | TBI | 4 | 4 | 3 | 6 | 30.21 | 29.15 | 10.87 | 10.22 | MCS- | |
| 14 | 60 | M | TBI | 4 | 4 | 3 | 4 | 29.95 | 29.74 | 10.54 | 10.45 | VS | |
| 15 | 54 | M | ICH | 4 | 5 | 4 | 5 | 29.87 | 28.93 | 10.89 | 10.57 | VS | |
| 16 | 56 | F | ICH | 5 | 4 | 5 | 3 | 28.98 | 29.88 | 9.89 | 10.51 | VS | |
| 17 | 62 | M | TBI | 3 | 4 | 5 | 3 | 29.01 | 30.13 | 9.97 | 11.06 | VS | |
ICH, intracerebral hemorrhage; TBI, traumatic brain injury; GCS, Glasgow Coma Scale; CRS-R, JFK Coma Recovery Scale-Revised; MEP, motor evoked potentials; CMCT, central motor conduction time; CS, consciousness state; M, male; F, female; VS, vegetative state; MCS, minimally conscious state; -, negative.
Figure 2.
Baseline data for the rTMS and control groups. (A) Etiology, (B) sex, (C) sex distribution in the rTMS group, (D) sex distribution in the control group, (E) number of males in the rTMS and control groups, (F) number of females in the rTMS and control groups, (G) age, (H) admission time, (I) admission GCS score, (J) CRS-R score, (K) MEP and (L) CMCT prior to treatment were not significantly different between rTMS and control groups. CRS-R, JFK Coma Recovery Scale-Revised; MEP, motor evoked potentials; CMCT, central motor conduction time; rTMS, repetitive transcranial magnetic stimulation; pre, prior to treatment; ICH, intracerebral hemorrhage; TBI, traumatic brain injury; GCS, Glasgow Coma Scale.
Effects of the 10 Hz rTMS treatment protocol as determined by CRS-R scores on day 20
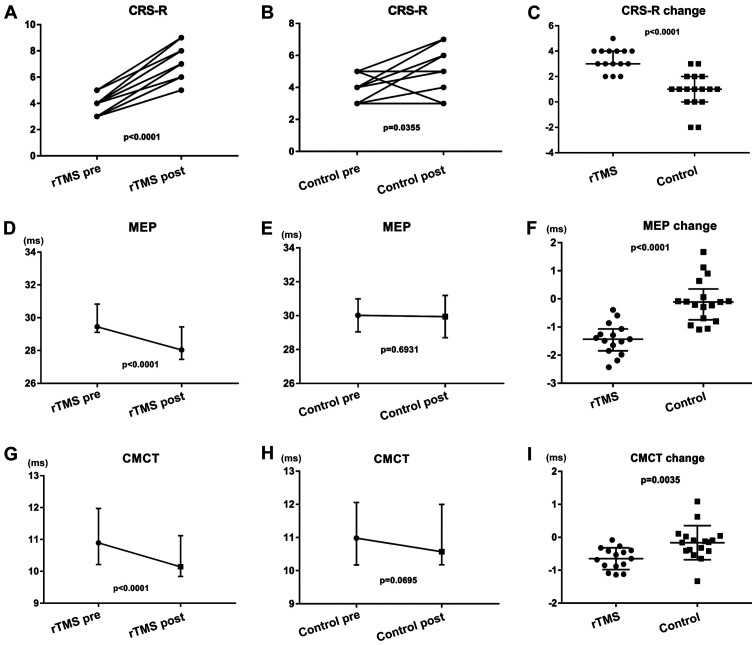
A total of 15 patients in the rTMS group and 17 patients in the control group completed the treatment (Tables I and II), with no specific side effects recorded. There were no statistical differences between the two groups prior to treatment (Fig. 2). A significant increase in the CRS-R scores (Fig. 3A; P<0.001) in the rTMS group was observed 20 days later compared with those at pretreatment. An increase in the CRS-R scores (Fig. 3B; P=0.035) in the control group was also observed compared with those at pretreatment. The CRS-R scores were increased in all patients in the rTMS group. A total of seven patients showed improved CRS-R scores by >4 points in the rTMS group. Only two patients in the control group improved their CRS-R scores by 3 points; however, no significant increase was observed in most patients. The median CRS-R score change in the rTMS group improved by 3 points, whereas the control group improved by 1 point (Fig. 3C; P<0.001). Significant differences were observed in the improvement of the CS rate. In the rTMS group, 13 VS patients (86.7%) turned MCS-, with only five patients (29.4%) in the control group (P=0.0016). In the rTMS group, the changes in CRS-R scores of seven patients were ≥4 points at day 20 (Table III).
Figure 3.
Data following 20 days of treatment for the rTMS and control groups. (A) A significant increase in the CRS-R scores compared with pretreatment in the (A) rTMS and (B) control groups. (C) The changes in CRS-R scores between the two groups were statistically significant. (D) A significant decrease in the MEP of the rTMS group compared with pretreatment. (E) No significant decrease in the MEP of the control group compared with pretreatment. (F) The changes in MEP between the two groups were statistically significant. (G) A significant decrease in the CMCT of the rTMS group compared with pretreatment. (H) No significant decrease in the CMCT of the control group compared with pretreatment. (I) The changes in CMCT between the two groups were statistically significant. CRS-R, JFK Coma Recovery Scale-Revised; MEP, motor evoked potentials; CMCT, central motor conduction time; rTMS, repetitive transcranial magnetic stimulation; pre, prior to treatment; post, after treatment; ms, milliseconds.
Table III.
Changes of ≥4 points in CRS-R scores at day 20 in the rTMS group.
| CRS-R | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Day | Auditory | Visual | Motor | Oro-motor | Comm | Arousal | Total | CS |
| 2 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 4 | VS |
| 20 | 1 | 1 | 3 | 1 | 0 | 2 | 8 | MCS- | |
| 5 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 3 | VS |
| 20 | 1 | 1 | 3 | 0 | 0 | 2 | 7 | MCS- | |
| 8 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 3 | VS |
| 20 | 1 | 1 | 3 | 0 | 0 | 2 | 7 | MCS- | |
| 11 | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 5 | VS |
| 20 | 2 | 1 | 3 | 1 | 0 | 2 | 9 | MCS- | |
| 12 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 4 | VS |
| 20 | 1 | 1 | 3 | 1 | 0 | 2 | 8 | MCS- | |
| 14 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 4 | VS |
| 20 | 2 | 1 | 3 | 1 | 0 | 2 | 9 | MCS- | |
| 15 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 4 | VS |
| 20 | 1 | 1 | 3 | 1 | 0 | 2 | 8 | MCS- | |
Comm, communication; CS, consciousness state; CRS-R, JFK Coma Recovery Scale-Revised; VS, vegetative state; MCS, minimally conscious state; -, negative.
Effects of the 10 Hz rTMS treatment protocol as determined by MEP latency and CMCT measurements
The two groups of patients were examined using magnetic stimulation evoked potentials, the main indicators of which were MEP and CMCT. There were no statistical differences between the two groups prior to treatment (Fig. 2K; P=0.662; Fig. 2L; P=0.758). A significant decrease in the MEP and CMCT in the rTMS group were observed 20 days later compared with those at pretreatment (Fig. 3D; P<0.001; Fig. 3G; P<0.001), with no significant decrease in the control group (Fig. 3E; P=0.693; Fig. 3H; P=0.070). The changes in MEP and CMCT between the two groups were statistically significant (Fig. 3F; P<0.00; Fig. 3I; P<0.01).
Discussion
The aim of the present study was to investigate the effects of 10 Hz rTMS at the right DLPFC in patients with VS. The present results demonstrated that 10 Hz rTMS at the right DLPFC could improve CS from VS to MCS-, increase the CRS-R score and decrease MEP and CMCT.
CS was the main factor that could affect the outcome evaluation, since VS and MCS were fundamentally different in DOC (1,22,33). Functional neuroimaging has previously verified that MCS+ patients preserve greater metabolic activity and resting state functional connectivity in the language network (33,34). It is important to distinguish VS form MCS, as misdiagnosis can affect the outcome of a study (35). It was confirmed that functional outcome was significantly more favorable for patients in MCS relative to those in VS, especially for traumatic brain injury (36,37). All participants were in VS, as determined by their CRS-R (measured three times), in our departments. The CRS-R score was the highest score assessed by three trained physicians. Patients who had recently used antiepileptics and sedatives were excluded from the current study, as these drugs can have a significant impact on the accurate scoring of patients. After 20 days of treatment, an increase in the CRS-R scores in the control group was also observed compared with the pretreatments cores that may be caused by an insufficient number of cases and insufficient self-recovery following brain injury in certain patients. However, the change in CRS-R scores was significantly different between the two groups. Patient no. 14 from the rTMS group was used as a sample. Prior to treatment, she had an occasional response to sound and a startle response when the physician's finger approached her eyeballs. Flexing was observed when her upper limbs were stimulated; however, she could not open her eyes. Her CRS-R scores for auditory, motor and oro-motor function had increased by 1 point 20 days after treatment. Her arousal score increased by 2 points. She could open her eyes spontaneously, locate sound and respond to pain in her upper limbs. She was also occasionally able to put the surface of her tongue between her lips. Her behavior had improved and the increased CRS-R score was 5. Behavioral improvements were also observed in 13 VS patients from in the rTMS group. Auditory and motor functions and arousal are major aspects of behavioral improvement (8). The findings of the present study were similar to those in the reports of Louise-Bender Pape et al (6) and Xie and Zhang (8). The choice of DLPFC as the stimulating target might be the reason for the significant increase in arousal. It was shown that 10 Hz rTMS of the right DLPFC in early VS is feasible and efficient.
MEP latency and amplitude, as well as CMCT, were used as evaluation indicators by Barker et al (9) in 1985. Cakar et al (38) reported that MEP latency and amplitude, as well as CMCT, exhibited a correlation with clinical parameters and daily life functionality in 22 chronic post-stroke patients. Shorter MEP latency and faster CMCT were positively correlated with improved clinical measurements. The data of the present study, which demonstrated that rTMS treatment could increase the conduction velocity of the central nervous system. Additionally, treatment enhanced the excitability of central neurons and the function of the cerebral cortex to activate the ascending reticular activating system, which affects regular awakening, were similar to the results obtained by Cakar et al (38). The present study focused on recovery outcomes of early VS patients, which is rarely reported in current studies. Early functional recovery could enhance the confidence of doctors and family members to continue treatment, avoiding abandonment of treatment for some VS patients who might recover. The present study was not, however, without its limitations. The present study had a relatively small sample size and was a monocentric retrospective cohort study, lacking randomization and a sham group. It lacked measures of the patient long term outcome as many factors affected long term prognosis after treatment including family, economy and follow-up treatment levels. The data for 3- and 6-month follow-ups were incomplete for various reasons. A multicenter, randomized and sham treatment study should be conducted in the future.
In conclusion, 10 Hz rTMS at the right DLPFC in early DOC is feasible and efficient. rTMS treatment could significantly improve patient state of awareness and accelerate their recovery in early VS.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Scientific Technology Research Projects of Jinzhou (grant no. 16B1G37).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XG designed the study. XG, YZ and XL drafted the manuscript. YZ, TX and XL collected and statistically analyzed data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Central Hospital of Jinzhou (Jinzhou, China). Written informed consent was obtained from each patient's legally authorized representative.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: The state of the science. Nat Rev Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- 2.Wu DY, Cai G, Yuan Y, Liu L, Li GQ, Song WQ, Wang MB. Application of nonlinear dynamics analysis in assessing unconsciousness: A preliminary study. Clin Neurophysiol. 2011;122:490–498. doi: 10.1016/j.clinph.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Guerra A, Costantini EM, Maatta S, Ponzo D, Ferreri F. Disorders of consciousness and electrophysiological treatment strategies: A review of the literature and new perspectives. Curr Pharm Des. 2014;20:4248–4267. [PubMed] [Google Scholar]
- 4.Shin SS, Dixon CE, Okonkwo DO, Richardson RM. Neurostimulation for traumatic brain injury. J Neurosurg. 2014;121:1219–1231. doi: 10.3171/2014.7.JNS131826. [DOI] [PubMed] [Google Scholar]
- 5.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, et al. Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 6.Louise-Bender Pape T, Rosenow J, Lewis G, Ahmed G, Walker M, Guernon A, Roth H, Patil V. Repetitive transcranial magnetic stimulation-associated neurobehavioral gains during coma recovery. Brain Stimul. 2009;2:22–35. doi: 10.1016/j.brs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Xia X, Bai Y, Zhou Y, Yang Y, Xu R, Gao X, Li X, He J. Effects of 10 Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in disorders of consciousness. Front Neurol. 2017;8(182) doi: 10.3389/fneur.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Y, Zhang T. Repetitive transcranial Magnetic Stimulation improves Consciousness disturbance in stroke patients: A quantitative electroencephalography spectral power analysis. Neural Regen Res. 2012;7:2465–2472. doi: 10.3969/j.issn.1673-5374.2012.31.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possoble mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front Hum Neurosci. 2015;9(303) doi: 10.3389/fnhum.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- 13.Klomjai W, Lackmy-Vallée A, Roche N, Pradat-Diehl P, Marchand-Pauvert V, Katz R. Repetitive transcranial magnetic stimulation and transcranial direct current stimulation in motor rehabilitation after stroke: An update. Ann Phys Rehabil Med. 2015;58:220–224. doi: 10.1016/j.rehab.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125:2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips AL, Burr RL, Dunner DL. rTMS effects in patients with co-morbid somatic pain and depressive mood disorders. J Affect Disord. 2018;241:411–416. doi: 10.1016/j.jad.2018.08.065. [DOI] [PubMed] [Google Scholar]
- 17.Kozel FA. Clinical repetitive transcranial magnetic stimulation for posttraumatic stress disorder, generalized anxiety disorder, and bipolar disorder. Psychiatr Clin North Am. 2018;41:433–446. doi: 10.1016/j.psc.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Kimiskidis VK, Valentin A, Kälviäinen R. Transcranial magnetic stimulation for thediagnosis and treatment of epilepsy. Curr Opin Neurol. 2014;27:236–241. doi: 10.1097/WCO.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 19.Randver R. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex to alleviate depression and cognitive impairment associated with Parkinson's disease: A review and clinical implications. J Neurol Sci. 2018;393:88–99. doi: 10.1016/j.jns.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Nardone R, Tezzon F, Höller Y, Golaszewski S, Trinka E, Brigo F. Transcranial magnetic stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer's disease. Acta Neurol Scand. 2014;129:351–366. doi: 10.1111/ane.12223. [DOI] [PubMed] [Google Scholar]
- 21.Naro A, Russo M, Leo A, Bramanti P, Quartarone A, Calabrò RS. A single session of repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in patients with unresponsive wakefulness syndrome: Preliminary results. Neurorehabil Neural Repair. 2015;29:603–613. doi: 10.1177/1545968314562114. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Gao J, Pan S, Meng F, Pan G, Li J, Luo B. Effects of High-frequency repetitive transcranial magnetic stimulation on cerebral hemodynamics inpatients with disorders of consciousness: A Sham-controlled study. Eur Neurol. 2016;76:1–7. doi: 10.1159/000447325. [DOI] [PubMed] [Google Scholar]
- 23.Bai Y, Xia X, Kang J, Yin X, Yang Y, He J, Li X. Evaluating the effect of repetitive transcranial magnetic stimulation on disorders of consciousness by using TMS-EEG. Front Neurosci. 2016;10(473) doi: 10.3389/fnins.2016.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cincotta M, Giovannelli F, Chiaramonti R, Bianco G, Godone M, Battista D, Cardinali C, Borgheresi A, Sighinolfi A, D'Avanzo AM, et al. No effects of 20 Hz-rTMS of the primary motor cortex in vegetative state: A randomised, sham-controlled study. Cortex. 2015;71:368–3676. doi: 10.1016/j.cortex.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Hosono Y, Urushihara R, Harada M, Morita N, Murase N, Kunikane Y, Shimazu H, Asanuma K, Uguisu H, Kaji R. Comparison of monophasic versus biphasic stimulation in rTMS over premotor cortex: SEP and SPECT studies. Clin Neurophysiol. 2008;119:2538–2545. doi: 10.1016/j.clinph.2008.07.279. [DOI] [PubMed] [Google Scholar]
- 26.Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, McDonald WM, Li X, Bernhardt ER, Haynor DR, Holtzheimer PE III, et al. Prefrontal rTMS for treating depression: Location and intensity results from the OPT-TMS Multi-site clinical trial. Brain Stimul. 2013;6:108–117. doi: 10.1016/j.brs.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 28.Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19:322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Piccione F, Cavinato M, Manganotti P, Formaggio E, Storti SF, Battistin L, Cagnin A, Tonin P, Dam M. Behavioral and neurophysiological effects of repetitive transcranial magnetic stimulation on the minimally conscious state: A case study. Neurorehabil Neural Repair. 2011;25:98–102. doi: 10.1177/1545968310369802. [DOI] [PubMed] [Google Scholar]
- 30.Manganotti P, Formaggio E, Storti SF, Fiaschi A, Battistin L, Tonin P, Piccione F, Cavinato M. Effect of high-frequency repetitive transcranial magnetic stimulation on brain excitability in severely Brain-injured patients in minimally conscious or vegetative state. Brain Stimul. 2013;6:913–921. doi: 10.1016/j.brs.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 32.Cavinato M, Iaia V, Piccione F. Repeated sessions of Sub-threshold 20-Hz rTMS. Potential cumulative effects in a Brain-injured patient. Clin Neurophysiol. 2012;123:1893–1895. doi: 10.1016/j.clinph.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 33.Thibaut A, Bodien YG, Laureys S, Giacino JT. Minimally conscious state ‘plus’: Diagnostic criteria and relation to functional recovery. J Neurol. 2020;267:1245–1254. doi: 10.1007/s00415-019-09628-y. [DOI] [PubMed] [Google Scholar]
- 34.Bruno MA, Majerus S, Boly M, Vanhaudenhuyse A, Schnakers C, Gosseries O, Boveroux P, Kirsch M, Demertzi A, Bernard C, et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol. 2012;259:1087–1098. doi: 10.1007/s00415-011-6303-7. [DOI] [PubMed] [Google Scholar]
- 35.Annen J, Filippini MM, Bonin E, Cassol H, Aubinet C, Carrière M, Gosseries O, Thibaut A, Barra A, Wolff A, et al. Diagnostic accuracy of the CRS-R index in patients with disorders of consciousness. Brain Inj. 2019;33:1409–1412. doi: 10.1080/02699052.2019.1644376. [DOI] [PubMed] [Google Scholar]
- 36.Lammi MH, Smith VH, Tate RL, Taylor CM. The minimally conscious state and recovery potential: A follow-up study 2 to 5 years after traumatic brain injury. Arch Phys Med Rehabil. 2005;86:746–754. doi: 10.1016/j.apmr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Bodien YG, Carlowicz CA, Chatelle C, Giacino JT. Sensitivity and specificity of the coma recovery Scale-revised total score in detection of conscious awareness. Arch Phys Med Rehabil. 2016;97:490–492.e1. doi: 10.1016/j.apmr.2015.08.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cakar E, Akyuz G, Durmus O, Bayman L, Yagci I, Karadag-Saygi E, Gunduz OH. The relationships of Motor-evoked potentials to hand dexterity, motor function, and spasticity in chronic stroke patients: A transcranial magnetic stimulation study. Acta Neurol Belg. 2016;116:481–487. doi: 10.1007/s13760-016-0633-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.