Abstract
Background
Effective pain control balanced with maintaining physical function and minimizing medication side effects is essential to accelerated recovery after total knee arthroplasty (TKA). Multimodal pain management regimens combining oral medications as well as local analgesia have shown promise in facilitating these goals. Some regimens use anesthetics delivered via a local infiltration catheter while others use periarticular injections (PAIs). However, it is uncertain if an infiltration catheter provides additional pain relief or decreases opioid consumption when compared with conventional PAI alone.
Methods
Fifty patients undergoing TKA at one institution were randomized equally into 2 groups for a prospective trial. Group I received an intraarticular catheter (On-Q∗) in combination with injection of 30 ml of 0.5% bupivacaine the day after surgery before removal. Group II received no pain catheter. Both groups received a conventional intraoperative PAI and postoperative oral pain medication. Pain scores were measured with visual analog scale and opioid medication consumption in morphine milligram equivalents (mgs).
Results
There were no differences in pain scores or opioid consumption in the first 48-hours postoperatively (P = .05). Reported maximum pain scores were low in both groups; 3.33 in group I and 2.97 in group II. Although not statistically significant in this cohort, there was increased opioid consumption in the catheter group: 14.78 mg vs 12.76 mg.
Conclusion
An intraarticular pain catheter in conjunction with a multimodal approach with intraoperative PAI after TKA does not improve 48-hour pain scores or opioid consumption compared with PAI alone in this randomized controlled trial. Overall pain scores were very low.
Keywords: Pain catheter, Opioid consumption, Total knee, Pain management
Introduction
Total knee arthroplasty (TKA) continues to be the gold standard surgical procedure for treating severe, symptomatic arthritis in the knee joint [1,2]. As such, demand for primary total knee replacement is projected to increase to almost 3.5 million procedures per year by 2030 [1]. With increasing numbers of patients undergoing this procedure, a shift toward an accelerated postoperative recovery has evolved in the care of the patient undergoing TKA. Notably, postoperative pain control has become increasingly important [[2], [3], [4]]. Several studies have highlighted the importance of pain control in regard to patient satisfaction and improved rehabilitation [2,5,6]. Of particular interest is how to balance effectively controlling pain while also maintaining function for physical activity and minimizing the side effects related to pain medications. Opioids not only increase the risk of constipation, nausea, vomiting, and poor participation in therapy but also carry significant potential for abuse and have been shown to decrease bacterial phagocytosis by host immune cells, increasing the risk of serious infections in certain patient populations [[7], [8], [9], [10]]. Recent research has supported the use of multimodal pain management that includes a combination of oral medications as well as local analgesia [[11], [12], [13]]. A variety of local anesthetic formulations have been used for periarticular injections (PAI) including liposomal bupivacaine (Exparel; Pacira Pharmaceuticals, San Diego, CA) and standard bupivacaine alone or in combination with varying admixtures of nonsteroidal anti-inflammatory drugs, corticosteroids, vasoconstrictors, and alpha-2 receptor agonists. These have been shown to be effective in decreasing pain, decreasing opioid consumption, and advancing the initiation of active motion in the joint [4,14]. Although large-scale randomized investigations such as the local infiltration analgesia with and without EXPAREL following total knee arthroplasty (PILLAR) study have revealed superiority of liposomal bupivacaine to traditional PAI in pain control metrics [15], a recent meta-analysis of randomized controlled trials found liposomal bupivacaine compared with traditional PAI yielded no difference in visual analog scale (VAS) after TKA. However, there was lower morphine equivalent consumption and lower incidence of nausea and vomiting in patients who received liposomal bupivacaine [16].
The use of anesthetic via local infiltration catheter connected to an infusion pump is a component of many pain management protocols after total joint arthroplasty [17]. Despite reports of potential drawbacks of intraarticular catheters (infection, additional nursing care, and the need to safely and completely remove the catheter) [[18], [19], [20]], this method of analgesia has the benefit of preserving neuromuscular function and allowing for early initiation of physical therapy. Although there are conflicting results in the literature regarding the use of local infiltrative analgesia and significant reduction in the requirement for opioid pain medications, some studies indicate a significant decrease in recovery time [21]. However, it is uncertain whether the current practice of using the infiltration catheter provides additional pain relief or enhances satisfaction compared with a conventional multimodal pericapsular analgesia injection alone. It is also unclear whether this practice offers any differences in postoperative outcomes. We sought to determine the benefits of an infiltration catheter used in conjunction with a conventional multimodal pericapsular injection. We hypothesized that the use of an infiltration catheter in addition to a pericapsular injection will provide pain reduction, decreased opioid use, and outcome measures equal or superior to pericapsular injection alone.
Material and methods
After obtaining approval from our institutional review board, all patients indicated for total knee replacement at our institution were screened for study eligibility. Inclusion criteria for this study included all patients aged 18 years or older who were indicated for and elected to proceed with unilateral total knee replacement for osteoarthritis. Patients indicated for bilateral total knee replacement were excluded. Additional exclusion criteria included those patients using opioid medications within 2 months of surgery, those with diagnosed chronic pain syndromes, those who had any other surgery in the 3 months preceding total knee replacement, and patients with inflammatory arthritis or those with significant osteoarthritis of the nonoperative knee or either hip.
Patients meeting eligibility requirements were recruited during the preoperative visit, and informed consent was obtained. Demographic data consisting of gender, age, smoking history, and body mass index were recorded. Included patients were then assigned to a group using computer-generated randomization.
A standardized unilateral TKA surgery was performed by a single experienced fellowship-trained arthroplasty surgeon using hypotensive spinal anesthesia without tourniquet. Patients were administered Tylenol (Johnson and Johnson, New Brusnswick, NJ) 925 mg, gabapentin (600 mg), and Celebrex (Pfizer, New York, NY) 400 mg in the preoperative holding area, but no preoperative opioids were provided. Intraoperatively, both groups received a multimodal pericapsular injection consisting of 49.25 mL ropivacaine (5 mg/mL) with 0.8 ml clonidine (0.1 mg/mL), 0.5 ml epinephrine (1 mg/mL), and 30 mg of ketorolac. Ketorolac was omitted in patients with contraindications to non-steroidal anti-inflammatory drugs (NSAIDs). For the treatment group, an intraarticular indwelling multiorifice infiltration catheter (On-Q∗ pain catheter; Halyard Health, Alpharetta, GA) was inserted into the knee during closure, instilling 2 ml of 0.5% bupivacaine solution per hour. The infusion rate is constant for the pain catheter and established by the manufacturer. The catheter was placed within the joint exiting the knee through the anterolateral suprapatellar pouch. It is our protocol to remove the catheter on the first postoperative day. Immediately before removal, a 30-mL dose of 0.5% bupivacaine solution was injected through the catheter. Patients in the control group received no indwelling infiltration catheter. Postoperative medicinal pain control regimens were standardized to include a scheduled opioid receptor agonist (tramadol 50 mg 1-2 tabs TID) if no history of seizure, celecoxib 200 mg BID, acetaminophen 925 mg TID, gabapentin 600 mg QD, and 1-2 mg hydromorphone or 5 mg of oxycodone oral formulation for breakthrough severe pain pending patient preference (nausea, itching).
During the hospital stay, members of each group were instructed to complete an initial VAS satisfaction survey after initial postoperative recovery and then every 4 hours while awake or when receiving pain medication per standard nursing protocol. Daily opioid pain medication consumption was tracked and recorded during the first postoperative week. Mean opioid use was converted to morphine milligram equivalents (mgs).
Descriptive statistics for both outcomes, pain level and drug dose, over time by treatment group were performed. To investigate the association between outcome and treatment group over time, linear mixed modeling was used. Linear mixed effects models take into account all available data and thus allow for missing data. Pain levels and drug dose were modeled separately. Owing to the nonlinear nature of both pain and drug dose over time, time was considered as a categorical variable. The effect of the treatment group was tested by including a time by treatment group interaction effect in the linear model. Both parametric and nonparametric statistical tests were used (2-sample T-test and Wilcoxon rank-sum tests, respectively), and P values are reported for each, with P < .05 set as the value for statistical significance. We performed a post hoc power analysis with power set as 80%, which allowed us to detect a difference of 1.1 units on the VAS scale or a 9.1-mg difference with regard to morphine milligram equivalents at the P < .05 level of significance.
Results
A total of 50 patients were randomized into either the catheter group or control group. All 50 patients were included in the analysis. Demographics were well-matched between the treatment group and the control group with no significant difference between metrics of age, gender distribution, BMI, and former smoking status (P > .05). Metrics are listed in Table 1. Patients in the control group had an average age of 66.4 years, and 56% were female. Treatment group average age was 68.9 years, and 60% were female. The mean BMI of the pain catheter group was 30.2, while the control group mean BMI was 30. Former smoking status was noted in 44% in the control group and 52% of patients in the treatment group.
Table 1.
Demographic data.
| Time | Control (no infusion catheter) |
|---|---|
| Average age | 66.4 |
| % Female | 56% |
| % Former smoker | 44% |
| Average BMI | 31 |
| Treatment (infusion catheter) | |
|---|---|
| Average age | 68.9 |
| % Female | 60% |
| % Former smoker | 52% |
| Average BMI | 30.2 |
Demographic data for group 1 (pain catheter) and group 2 (no pain catheter).
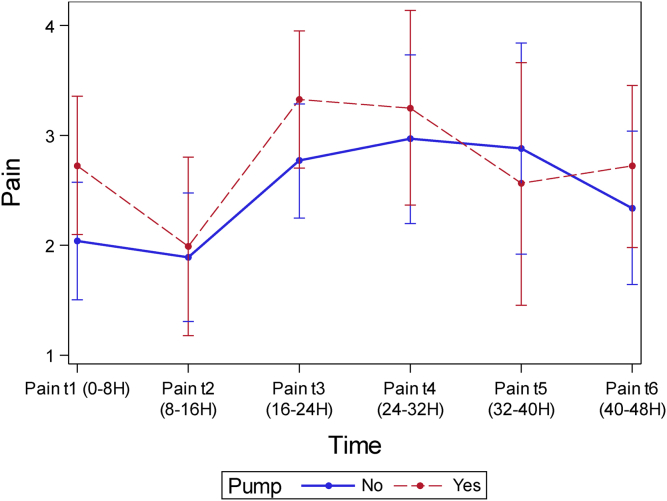
Mean VAS pain scores were comparable at all time-points to 48 hours (Table 2). Overall pain scores were low in both groups, with maximum VAS pain scores of 2.97 in the control group and 3.33 in the treatment group. Although not statistically significant, VAS scores were lower in the control group at all time-points except at 40 hours postoperatively (catheter group, 2.56; control group, 2.89; P = .64) (Fig. 1).
Table 2.
VAS scores.
| Time | Infusion catheter |
|||
|---|---|---|---|---|
| No | Yes | P value Wilcoxon | P value T-test | |
| t1 (0-8 h) | 2.04 | 2.73 | .1031 | 0.0911 |
| t2 (8-16 h) | 1.89 | 1.99 | .6918 | 0.8390 |
| t3 (16-24 h) | 2.77 | 3.33 | .1338 | 0.1608 |
| t4 (24-32 h) | 2.97 | 3.25 | .9627 | 0.6204 |
| t5 (32-40 h) | 2.89 | 2.56 | .3776 | 0.6419 |
| t6 (40-48 h) | 2.34 | 2.72 | .6227 | 0.4319 |
Postoperative visual analog scale (VAS) pain scores for group 1 (pain catheter) and group 2 (no pain catheter).
Figure 1.
Visual analog pain (VAS) scores for patients receiving an OnQ∗ pump and those not receiving an OnQ∗ pump plotted vs postoperative time interval.
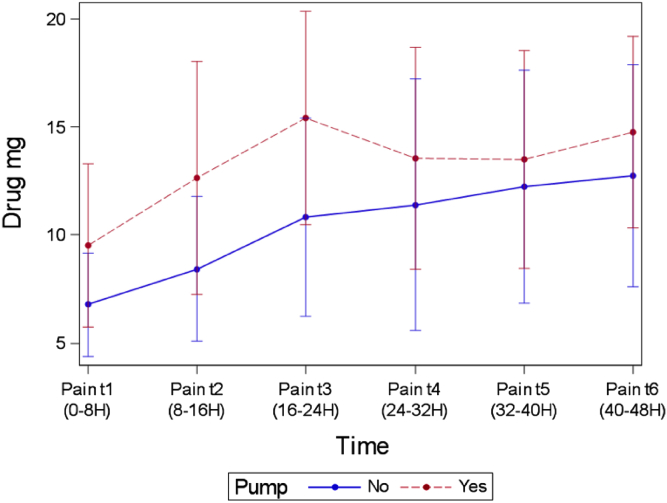
Mean opioid use as converted to morphine milligram equivalents (mg) was not statistically different between groups at any time-point (Table 3). The time-point that most closely approached significance was at 24 hours, with 10.8 mg in the control group and 15.4 mg in the treatment group (P = .16) (Fig. 2).
Table 3.
Opioid consumption.
| Time | Infusion catheter |
|||
|---|---|---|---|---|
| No | Yes | P value Wilcoxon | P value T-test | |
| t1 (0-8 h) | 6.77 | 9.54 | .3998 | 0.2105 |
| t2 (8-16 h) | 8.42 | 12.66 | .2556 | 0.1761 |
| t3 (16-24 h) | 10.81 | 15.42 | .1306 | 0.1652 |
| t4 (24-32 h) | 11.39 | 13.56 | .4567 | 0.5664 |
| t5 (32-40 h) | 12.24 | 13.50 | .7602 | 0.7182 |
| t6 (40-48 h) | 12.76 | 14.78 | .6287 | 0.5306 |
Postoperative oral opioid consumption (morphine equivalents) for group 1 (infusion catheter) and group 2 (no infusion catheter).
Figure 2.
Opioid consumption (mg morphine equivalents) for patients receiving an OnQ∗ pump and those not receiving an OnQ∗ pump plotted vs postoperative time interval.
Discussion
The findings in this study reveal no significant difference in pain scores or opioid consumption at any time-point in the first 48 hours postoperatively between the 2 groups. Overall, pain scores were low in both groups. This highlights the overall efficacy of a multimodal approach to perioperative pain management after TKA and that there is likely no need to supplement a conventional periarticular injection with an intraarticular pain catheter. Although a statistically significant difference was not observed, there was greater opioid consumption in the treatment group.
Prior studies have evaluated bupivacaine infusion for postoperative pain control after TKA and total hip arthroplasty without intraoperative pericapsular infiltration of analgesia [17,22]. No significant improvements in pain scores were noted with bupivacaine infusion alone. Our results reflect the findings seen in a previous study by Specht et al. that evaluated local infusion analgesia administered after total hip arthroplasty [23]. In this randomized double-blind study, 60 consecutive patients undergoing total hip arthroplasty received a standardized surgery and local infiltrative analgesia. Patients were randomized into a postoperative infusion group and a placebo group, and opioid consumption, pain, tiredness, and postoperative nausea and vomiting were evaluated. No difference was found in pain scores, tiredness, and opioid consumption at any time-point. However, postoperative nausea and vomiting was improved in their treatment group in the short term.
To our knowledge, this is the first prospective randomized controlled trial to compare pain control and opioid consumption between an intraarticular catheter combined with multimodal approach (including nonliposomal local anesthetic pericapsular injection) vs a multimodal protocol and nonliposomal anesthetic pericapsular injection alone after TKA. Williams et al. evaluated infusion with local bupivacaine and epinephrine injection (rather than a multimodal pericapsular injection) and found no significant improvement in pain scores from placebo in 48 hours [17]. However, the authors noted a trend toward decreased opioid consumption. More recently, an investigation by Smith and Kazarian compared periarticular liposomal bupivacaine injection (Exparel; Pacira Pharmaceuticals) to intraarticular catheter infusion of bupivacaine HCl with an On-Q∗ Pain Relief System pump [24]. In this randomized, controlled, double-blinded superiority trial of 200 patients, those receiving liposomal bupivacaine consumed a larger, but not statistically significant, amount of opioid than those treated with the On-Q∗. However, patients in the On-Q∗ cohort experienced less discomfort with activity.
An initially unexpected result of our study was increased opioid use in the treatment group. This finding stands in contrast to some prior investigations [17,21,22,25] but in agreement with the more recent comparative trial [24]. Although our results did not approach statistical significance, the patients with the infiltration catheter used more morphine milligram equivalents than did those without an infiltration catheter. This difference may be explained by the inability to blind the patient or ward staff to the treatment group in this study. The etiology of pain after surgery is multifaceted, and there may be an expectation of pain due to the visual reminder that a pain catheter provides. In addition, nursing staff may be more apt to provide more attention to patients with visible devices.
Strengths of this study include the prospective nature of the investigation, computerized randomization, and the demographic similarity of the control and treatment groups. A limitation of this study is the small sample size, which impacted our ability to detect statistically significant differences of less than 1.1 units on the VAS scale. Reassuringly, this almost fully mirrors the inherent limits of resolution of the VAS scale which are measured with unit differences of 1. The small sample size also impacted the ability to detect statistically significant differences in morphine consumption. However, although our study was powered to detect statistical significance at 9.1 mg, this is mitigated by the actual absolute difference in opioid consumption (maximum 4.6 mg), with the majority differing by approximately 1-2 mg. Future studies with pre hoc power analyses and larger sample sizes would help with establishment of statistical significance for smaller amounts, although this would be tempered by the natural limits of resolution with regard to the VAS scale which is graded by units of 1, and also by the fact that at smaller doses of opioids, these become increasingly clinically insignificant differences, even if they are statistically significant. Indeed, despite the relatively small sample size in this study, similar studies reassuringly contain similarly sized patient groups [17,[21], [22], [23]]. Another limitation, as mentioned previously, was the lack of a sham/placebo catheter. This may have altered the behavior of those involved in the care of the patient and in the mindset of the patient themselves. Thus, a treatment-by-indication bias might have inadvertently been introduced in the outcomes, especially the outcome trend toward increased opioid use in the infusion catheter group [26].
Conclusions
In our prospective, randomized-controlled trial, we found postoperative On-Q∗ use in addition to a multimodal approach with intraoperative pericapsular injection after TKA does not improve 48-hour pain scores or opioid consumption compared with a conventional pericapsular injection alone. Therefore, we believe the use of such a modality is not necessary after TKA when a treatment protocol consists of an intraoperative multidrug pericapsular injection and standardized medicinal approach.
Conflict of interests
P.M. Lichstein is a board member for AAHKS Advocacy Committee. W. Fitz received royalties from Conformis Inc., is a paid consultant for Conformis Inc., and has stock or stock options in Conformis Inc..
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2020.11.021.
Supplementary data
References
- 1.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Ranawat A.S., Ranawat C.S. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22:12. doi: 10.1016/j.arth.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Ranawat C.S., Ranawat A.S., Mehta A. Total knee arthroplasty rehabilitation protocol: what makes the difference? J Arthroplasty. 2003;18:27. doi: 10.1054/arth.2003.50080. [DOI] [PubMed] [Google Scholar]
- 4.Lamplot J.D., Wagner E.R., Manning D.W. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29:329. doi: 10.1016/j.arth.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Parvizi J., Miller A.G., Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93:1075. doi: 10.2106/JBJS.J.01095. [DOI] [PubMed] [Google Scholar]
- 6.Brokelman R.B.G., van Loon C.J.M., Rijnberg W.J. Patient versus surgeon satisfaction after total hip arthroplasty. J Bone Joint Surg Br. 2003;85:495. [PubMed] [Google Scholar]
- 7.Wiese A.D., Griffin M.R., Stein C.M., Mitchel E.F., Grijalva C.G. Opioid analgesics and the risk of serious infections among patients with rheumatoid arthritis: a self-controlled case series study. Arthritis Rheumatol. 2016;68:323. doi: 10.1002/art.39462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ninković J., Roy S. Morphine decreases bacterial phagocytosis by inhibiting actin polymerization through cAMP-, rac-1-, and p38 MAPK-dependent mechanisms. Am J Pathol. 2012;180:1068. doi: 10.1016/j.ajpath.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert T.J., Cohn J.C., Rothman J.S., Springstead J., Rothman R.H., Booth R.E. Patient-controlled analgesia in a postoperative total joint arthroplasty population. J Arthroplasty. 1991;6(Suppl):S23. doi: 10.1016/s0883-5403(08)80052-6. [DOI] [PubMed] [Google Scholar]
- 10.Vendittoli P.-A., Makinen P., Drolet P. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006;88:282. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]
- 11.Joo J.-H., Park J.-W., Kim J.-S., Kim Y.-H. Is intra-articular multimodal drug injection effective in pain management after total knee arthroplasty? A randomized, double-blinded, prospective study. J Arthroplasty. 2011;26:1095. doi: 10.1016/j.arth.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 12.Peters C.L., Shirley B., Erickson J. The effect of a new multimodal perioperative anesthetic regimen on postoperative pain, side effects, rehabilitation, and length of hospital stay after total joint arthroplasty. J Arthroplasty. 2006;21:132. doi: 10.1016/j.arth.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Parvataneni H.K., Shah V.P., Howard H., Cole N., Ranawat A.S., Ranawat C.S. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22:33. doi: 10.1016/j.arth.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J., Teng Y., Fan Z., Khan M.S., Cui Z., Xia Y. The efficacy of periarticular multimodal drug injection for postoperative pain management in total knee or hip arthroplasty. J Arthroplasty. 2013;28:1882. doi: 10.1016/j.arth.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Mont M.A., Beaver W.B., Dysart S.H., Barrington J.W., Del Gaizo D.J. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33:90. doi: 10.1016/j.arth.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Zeng Y., Zeng J., Li M., Wei W., Shen B. The efficacy of liposomal bupivacaine compared with traditional peri-articular injection for pain control following total knee arthroplasty: an updated meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2019;20(1):306. doi: 10.1186/s12891-019-2660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams D., Petruccelli D., Paul J., Piccirillo L., Winemaker M., de Beer J. Continuous infusion of bupivacaine following total knee arthroplasty: a randomized control trial pilot study. J Arthroplasty. 2013;28:479. doi: 10.1016/j.arth.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 18.DeWeese F.T., Akbari Z., Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001:226. doi: 10.1097/00003086-200111000-00028. [DOI] [PubMed] [Google Scholar]
- 19.Badner N.H., Bourne R.B., Rorabeck C.H., MacDonald S.J., Doyle J.A. Intra-articular injection of bupivacaine in knee-replacement operations. Results of use for analgesia and for preemptive blockade. J Bone Joint Surg Am. 1996;78:734. doi: 10.2106/00004623-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 20.O’Neil S., Danielson K., Johnson K., Matelic T. ON-Q infusion pump linked to increased hospital stay after total knee arthroplasty. J Orthop. 2018;15:666. doi: 10.1016/j.jor.2018.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne C., Copp S., Reden L., Pulido P., Colwell C. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty. 2004;19:377. doi: 10.1016/j.arth.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Nechleba J., Rogers V., Cortina G., Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg. 2005;18:197. doi: 10.1055/s-0030-1248181. [DOI] [PubMed] [Google Scholar]
- 23.Specht K., Leonhardt J.S., Revald P. No evidence of a clinically important effect of adding local infusion analgesia administrated through a catheter in pain treatment after total hip arthroplasty. Acta Orthop. 2011;82:315. doi: 10.3109/17453674.2011.570671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith E.B., Kazarian G.S., Maltenfort M.G., Lonner J.H., Sharkey P.F., Good R.P. Periarticular liposomal bupivacaine injection versus intra-articular bupivacaine infusion catheter for analgesia after total knee arthroplasty: a double-blinded, randomized controlled trial. J Bone Joint Surg Am. 2017;99:1337. doi: 10.2106/JBJS.16.00571. [DOI] [PubMed] [Google Scholar]
- 25.Bianconi M., Ferraro L., Traina G.C. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth. 2003;91:830. doi: 10.1093/bja/aeg277. [DOI] [PubMed] [Google Scholar]
- 26.Slutsky D.J. Statistical errors in clinical studies. J Wrist Surg. 2013;2:285. doi: 10.1055/s-0033-1359421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.