Abstract
Background
Prosthetic joint infection (PJI) of total hip (THA) or total knee arthroplasty (TKA) after dental procedures is uncommon, and antibiotic prophylaxis remains controversial. For high-risk patients, the American Academy of Orthopedic Surgeons recommends amoxicillin prophylaxis. However, no systematic review of the literature of PJIs associated with dental procedures explores if amoxicillin is suitable for the reported organisms.
Methods
A librarian-assisted search of the major databases (PubMed, Medline, Embase, Scopus) identified 954 articles. Only case reports, case series, and reviews with patient level data were included. After exclusions, 79 articles were fully reviewed.
Results
Forty-four PJIs after dental procedures were identified, 22 in primary THA, 20 in primary TKA, one in revision THA, and one in a hip resurfacing procedure. Antibiotic prophylaxis was documented for 5 patients. The dental procedure was invasive in 35 (79.5%). Comorbidities were present in 17 patients (38.7%). The organisms reported were Streptococcus spp. in 44%, other aerobic gram-positives in 27%, anaerobic gram-positives in 18%, and gram-negative organisms in 11%. An estimated 46% of organisms may be resistant to amoxicillin. The outcomes of treatment were reported for 35 patients (79.5%). Twenty-seven patients (61.4%) had no clinical signs of PJI at the final follow-up visit.
Conclusions
Lower extremity PJI associated with dental procedures is often caused by organisms unlikely to be prevented with amoxicillin. Additional studies are warranted to determine the choice and efficacy of antibiotic prophylaxis to prevent dental-associated PJI in the highest risk patients. Insufficient data exist to recommend the optimal treatment for patients with PJI in THA and TKA associated with dental procedures.
Keywords: Hip arthroplasty, Knee arthroplasty, Prosthetic joint infection, Dental prophylaxis, Antibiotic prophylaxis
Prosthetic joint infection (PJI) of a total hip arthroplasty (THA) and total knee arthroplasty (TKA) is a devastating complication that occurs in less than 2% of patients, with a small subset attributed to hematogenous spread [1,2]. PJI after THA and TKA may be temporally related to dental work and associated with organisms usually found in the oral cavity. Controversy exists over the association between dental work and lower extremity PJI. Bacteremia is a common occurrence, after both noninvasive and invasive dental procedures, but is transient and likely not a greater risk than daily activities including chewing or brushing [2].
Antibiotic prophylaxis for patients with THA and TKA who undergo dental procedures is also controversial, in part due to the uncertain pathophysiology. Before 2015, both the American Academy of Orthopedic Surgeons (AAOS) and the American Dental Association were in agreement on antibiotic prophylaxis before dental work in all patients with THA or TKA within 2 years of implantation [3]. Thereafter, a schism occurred between these 2 organizations, with the American Dental Association advising against prophylaxis in general and the AAOS creating specific, narrowed guidelines [4,5]. Despite these recommendations, many health-care providers continue to routinely provide antibiotic prophylaxis before dental work for these patients [6,7].When antibiotic prophylaxis is indicated, the 2016 AAOS guideline recommends using amoxicillin; ampicillin, cephalosporins, and macrolides are listed as alternatives for patients allergic to penicillin or ampicillin or unable to tolerate oral medication [4,8].
Whether or not antibiotic prophylaxis is given before dental procedures, there are reports of PJI temporally associated with dental work [9,10]. To our knowledge, no systematic review of the literature exists of patients who developed a lower extremity PJI temporally associated with dental procedures. The aims of this study were to determine the frequency of this complication, the underlying comorbidities of the patients involved, the use of antibiotic prophylaxis, the type of organisms isolated and antibiotic sensitivities, and outcomes of treatment.
Material and methods
Search strategy
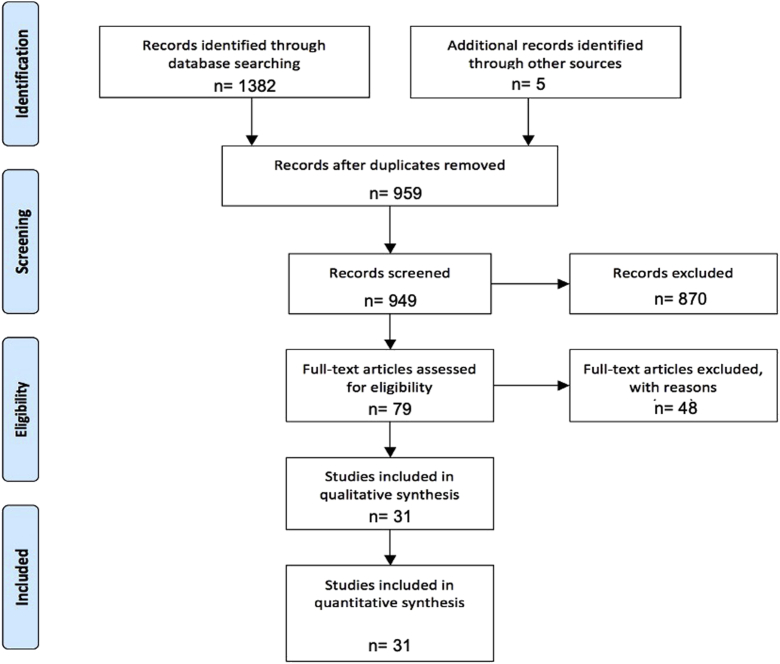
An electronic search of the literature was conducted in MEDLINE (PubMed), Embase (Elsevier), and Web of Science (Clarivate Analytics) from inception to February 7, 2020, according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines [11]. An updated search was conducted on February 12, 2020. The search was conducted by a professional medical librarian and included a mix of keywords and controlled vocabulary representing prosthetic joints, infection, and dental procedures. Editorials and comments were excluded. References were uploaded into Covidence, a systematic review screening tool, with which 2 independent reviewers screened titles and abstracts. Conflicts were resolved by a third independent reviewer. Raters were not blinded to citation identifiers (eg, author, institution, year of publication). PRISMA chart can be found in the Appendix section.
Appendix.
PRISMA Flowchart.
Inclusion and exclusion criteria
The studies included provided data regarding a PJI of a THA or TKA around the time of a dental procedure. These studies included contained patient-level information regarding type of arthroplasty, time from dental procedure, organism isolated, and the demographics of sex, age, and presence of comorbidities. Studies were excluded if they did not include patient-level information, the specific organism isolated, or timing from dental procedure or were written in languages other than English.
Data extraction and analysis
Two reviewers independently extracted data from the identified studies, and conflicts were identified and resolved by a third independent reviewer. Extracted data included author, date of publication, and patient-level data regarding infection episode. Dental procedures were categorized as invasive or noninvasive based on the AAOS Appropriate Use Criteria statement, with invasive procedures defined as those that involved manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa [4]. For the purposes of analysis, treatment was categorized as nonsurgical, debridement, antibiotics, and implant retention (DAIR), one-stage exchange arthroplasty, or two-stage exchange arthroplasty. Summary statistics were generated using Stata or MP 13.0 (StataCorp LP, College Station, TX).
Estimating amoxicillin susceptibility
The organisms reported were sorted by common clinical grouping, family, and genus. The susceptibilities of group or family or genus were then estimated by one author, a specialist of infectious diseases, using the John Hopkins Antibiotic Guide, the Sanford Guide to Antimicrobial Therapy, and a literature review for rare genera [[12], [13], [14], [15], [16], [17], [18], [19], [20]]. An overall estimated amoxicillin susceptibility rate was determined from a weighted mean calculated using the frequency of organisms and the estimated amoxicillin susceptibility by genus or species.
Results
The literature search identified 954 citations, of which 79 met the predefined screening criteria. Of the 79 studies, 31 had data that could be extracted (Fig. 1). These 31 studies had patient-level data for 44 patients, and the years of publication ranged from 1976 to 2019. The number of cases of PJI in THA or TKA associated with dental procedures analyzed was 44.
This cohort included 22 female patients (50.0%) with a mean age 64.1 years (SD, 9.7 years; range, 44 to 84 years) and 20 male patients (45.5%) with a mean age of 62.0 years (SD, 13.0 years; range, 39 to 92 years) (Table 1). The age and sex were not reported for 2 patients. The index procedure was a primary TKA in 20 patients (45.5%) and a primary THA in 22 patients (50.0%), a revision THA in one patient (2.3%), and a hip resurfacing in 1 patient (2.3%). Data on comorbidities were reported for 17 patients (38.6%), including 6 patients with rheumatoid arthritis, 5 with hypertension, one with chronic obstructive pulmonary disease, and one with chronic kidney disease. The dental procedures performed were described as invasive in 35 patients (79.5%), specifically listed as extractions in 18 patients, root canals in 7, gingival procedures in 5, irrigation and debridement of an abscess in 3, and unspecified invasive in 2. The dental procedure performed was described as noninvasive in 6 patients (13.6%), specifically listed as routine cleanings in 5 patients and dental manipulation in one. In 3 patients (6.8%), the data were insufficient to classify the dental procedures as invasive or noninvasive. The dental procedure associated with the PJI occurred at a mean of 4.8 years (range, 4 days to 20 years; SD, 4.6 years) after the index joint replacement. In 8 patients (18.2%), the dental procedure occurred within 1 year of index surgery. Twenty-two patients (50.0%) were specifically noted to have not received antibiotic prophylaxis, and 5 patients (11.4%) were noted to have received antibiotic prophylaxis (amoxicillin in 2, penicillin in one, erythromycin in one, and lincomycin in one). For 17 patients (38.6%), data regarding antibiotic prophylaxis were not provided or were insufficient for analysis. The first symptoms of PJI after the dental procedure were noted at a mean of 27.4 days (range, 1 day to 5 months; SD, 35.4 days).
Table 1.
Published reports of dental procedure–associated lower extremity PJI (N = 45).
| Author | Year | Sex | Age | Procedure | Time from surgery | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Rubin [21] Case #1 | 1976 | F | 68 | THA | 5.5 y | Three I + Ds | Died of pneumonia before 2 stage revision |
| Rubin Case #2 | 1976 | F | 58 | THA | 5 y | “Replacement” | NR |
| Rubin Case #3 | 1976 | M | 62 | THA | 30 m | 3 arthroscopic I + D s | No infection at 1 y f/u |
| Schurman [22] | 1976 | F | 61 | TKA | 19 m | I + D; then 2 stage exchange arthroplasty | No infection at 52 m f/u |
| Jacobsen [23] | 1980 | -- | -- | THA | 2 y | NR | NR |
| Lindqvist [24] Case #1 |
1985 | M | 67 | THA | 2 y | I + D | No infection at 37 m f/u |
| Lindqvist Case #2 | 1985 | F | 66 | THA | 4d | I + D and polyethylene exchange | No further significant events |
| Lindqvist Case #3 | 1985 | F | 84 | THA | 4 m | I + D and polyethylene exchange | No infection at 12 m f/u |
| Strazzeri [25] | 1986 | F | 61 | THA | 10 y | I + D (declined explant) | No infection at 12 m f/u |
| Grogan [26] | 1986 | NR | NR | TKA | 5 m | Exchange arthroplasty | No infection at 38 m f/u |
| Pravda [27] | 1989 | F | 78 | TKA | 14 m | Exchange arthroplasty | No infection at 27 m f/u |
| Sullivan [28] | 1990 | F | 44 | THA | 5 y | Exchange arthroplasty | No infection at 50 m f/u |
| Manian [29] | 1991 | M | 73 | TKA | 6 y | “Revision” | NR |
| Skiest [30] | 1995 | M | 39 | THA | 5 y | Resection arthroplasty | NR |
| Waldman [31] Case #1 | 1997 | F | 76 | TKA | NR | NR | NR |
| Waldman Case #2 | 1997 | F | 71 | TKA | NR | 2 stage exchange arthroplasty | No infection at 4 m f/u; died of C. difficile at 6 m |
| Waldman Case #3 | 1997 | M | 61 | TKA | NR | Declined surgery | Scant culture negative knee drainage at 2 y |
| Waldman Case #4 | 1997 | M | 62 | TKA | NR | 2 stage exchange arthroplasty | No infection at 24 m f/u |
| Waldman Case #5 | 1997 | F | 56 | TKA | NR | I + D without polyethylene exchange | 5 m after procedure, sustained fall, revision, still infected - lifelong antibiotics |
| Waldman Case #6 | 1997 | M | 57 | TKA | NR | Arthroscopic I + D | No infection at 2 m f/u |
| Waldman Case #7 | 1997 | F | 70 | TKA | NR | DAIR after antibiotics only failed | NR |
| Waldman Case #8 | 1997 | F | 67 | TKA | NR | 2 stage exchange arthroplasty | No infection at 4 m f/u |
| LaPorte [32] Case #1 | 1999 | F | 63 | THA | NR | Implant removal and antibiotics | Free of pain at 3 m |
| LaPorte Case #2 | 1999 | M | 72 | THA | NR | Implant removal and antibiotics | No infection at 3 m f/u |
| LaPorte Case #3 | 1999 | F | 78 | THA | NR | Surgery canceled for cardiac reasons | Continued hip pain |
| Kaar [33] | 2000 | M | 67 | Revision THA | NR | I + D | No infection at 12 w f/u |
| Nadlacan [34] | 2001 | M | 44 | TKA | 11 m | I + D; polyethylene exchange | NR |
| Jellicoe [35] | 2002 | F | 78 | THA | -- | 2 stage exchange arthroplasty | No infection at 6 m after reimplantation |
| Bartz [36] | 2005 | F | 63 | THA | 1 y | 2 stage exchange arthroplasty | No infection at 3 m f/u |
| Klingler [37] | 2005 | M | 44 | TKA | 9 y | 2 stage exchange arthroplasty | No infection at 4 m f/u |
| Trivedi [38] | 2005 | F | 53 | TKA | 8 y | Exchange arthroplasty | No infection at 2.1 y f/u |
| Michels [39] | 2007 | M | 59 | THA | -- | I + D then exchange arthroplasty | No infection at 2.8 y f/u |
| Waqar [40] | 2008 | M | 49 | TKA | 22 m | Exchange arthroplasty | No infection at 10.1 y f/u |
| Brown [41] | 2012 | M | 59 | THA | 5 y | Exchange arthroplasty | No infection at 2.4 y f/u |
| Mahobia [42] | 2013 | F | 75 | TKA | 8 m | Exchange arthroplasty | No infection at 3.5 y f/u |
| Mougari [43] | 2013 | M | 55 | TKA | 20 y | Arthroscopic I + D | No infection at 8.7 y f/u |
| Al-Himdani [44] | 2015 | F | 55 | Hip resurfacing | 10 y | Exchange arthroplasty | No infection at 7.8 y f/u |
| Klein [45] | 2015 | F | 65 | TKA | 11 y | Exchange arthroplasty | No infection at 4.8 y f/u |
| Aweid [46] | 2016 | M | 81 | THA | 5 y | Exchange arthroplasty | No infection at 2.4 y f/u |
| Quenard [47] | 2017 | M | 75 | THA | 7 y | I + D and antibiotic spacer | Later cultured Aspergillus |
| Bartash [48] | 2017 | M | 54 | THA | 3 y | I + D, then exchange | Died from “other issues” |
| Kansara [49] | 2019 | F | 64 | TKA | 295 d | Antibiotics and 2 stage exchange arthroplasty | NR |
| Olson [50] | 2019 | M | 92 | THA | 2 w | Sinus tract I + D; later revision | NR |
| Rieber [51] | 2019 | M | 68 | THA | NR | I + D; polyethylene exchange | Staged revision |
d, day(s); DAIR, debridement, antibiotics, and implant retention; F, female; M, male; m, month(s); f/u, follow up; I + D, irrigation and debridement; N, total number of cases; NR, not reported; PJI, prosthetic joint infection; THA, total hip arthroplasty; TKA, total knee arthroplasty; w, week(s); y, year(s).
Forty-four bacterial organisms were reported from 44 PJI. Two of the 44 PJI were polymicrobial. One PJI was caused by Staphylococcus aureus and Proteus mirabilis, and both organisms were included the analysis. The other polymicrobial PJI was reported as mixed gram-positive and gram-negative organisms and excluded from sensitivity analysis.
The causative organisms were diverse (Table 2). The most frequently isolated organisms were viridans group streptococcal bacteria in 17 (37.8%) cases. Overall, aerobic gram-positive bacteria were isolated in 31 patients (70.5%), anaerobic gram-positive organisms in 8 patients (18.2%), and gram-negative bacteria in 5 patients (11.4%). Among aerobic gram-positive organisms, 5 were Staphylococcus aureus, 2 were pyogenic streptococci, 17 were viridans group streptococci, 2 were Granulicatella adiacens (formally nutritionally variant streptococci), 4 were Rothia spp., and one was a Micrococcus species (sp.). The anaerobic gram-positive bacteria included 5 Peptostreptococcus spp., 3 Actinomyces spp., and one Slackia exigua. The gram-negative organisms included 3 Haemophilus parainfluenzae and 2 Enterobacteriaceae. Using the previously described methods, aggregate sensitivity to amoxicillin was estimated to be 54% for these organisms (Table 2).
Table 2.
Organisms reported in dental procedure–associated lower extremity PJI and estimated sensitivity to amoxicillin (N = 45a).
| Common clinical group | Genus or Species | No. | Estimated % amoxicillin susceptibility for group [[12], [13], [14], [15], [16], [17], [18], [19], [20]] | % Total for group |
|---|---|---|---|---|
| Aerobic gram-positive organisms | ||||
| Staphylococcus aureus | Staphylococcus aureus | 5 | 0 | 11.4 |
| Pyogenic streptococci | Group C Streptococcus | 1 | 100 | 4.5 |
| Group G Streptococcus | 1 | |||
| Viridans group streptococci | S. salivarius | 1 | 50 | 38.6 |
| S. anginosus group (S. intermedius) | 1 | |||
| S. mitis group | 2 | |||
| S. oralis | 1 | |||
| S. gordonii | 1 | |||
| S. mutans | 1 | |||
| viridans group Streptococcus, NOS | 10 | |||
| Formally nutritionally variant streptococci | Granulicatella adiacens | 2 | 50 | 4.5 |
| Micrococcaceae | Rothia dentocariosa | 1 | 60 | 11.4 |
| Rothia mucilaginosa | 1 | |||
| Rothia aeria | 1 | |||
| Rothia sp. | 1 | |||
| Micrococcus sp. | 1 | |||
| Anaerobic gram-positive organisms | ||||
| Peptostreptococcus spp. | Peptostreptococcus micros | 1 | 95 | 18.1 |
| Peptostreptococcus (including former Peptococcus) spp. | 4 | |||
| Actinomyces spp. | Actinomyces israelii | 1 | ||
| Actinomyces sp. | 1 | |||
| Coriobacteriaceae | Slackia exigua | 1 | ||
| Gram-negative organisms | ||||
| Fastidious, anaerobic gram-negative | Haemophilus parainfluenzae | 3 | 50 | 11.4 |
| Enterobacteriaceae | Serratia marcescens | 1 | 0 | |
| Proteus mirabilis | 1 | 0 | ||
N, total number of organisms; NOS, not otherwise specified; PJI, prosthetic joint infection.
Forty-four organisms were reported from 44 PJI. One PJI had both Staphylococcus aureus and Proteus mirabilis. One PJI was reported as mixed gram-positive and gram-negative organisms and is excluded from Table 2.
The PJI treatment for these patients varied greatly (Table 1). Thirteen patients (29.5%) were treated definitively with some form of irrigation and debridement, with or without polyethylene exchange. Eight patients (18.2%) had an exchange arthroplasty, and 7 patients (15.9%) had 2-stage exchange arthroplasty. Two patients had irrigation and debridement followed by exchange arthroplasty, with one patient having irrigation and debridement followed by two-stage exchange. Two patients had implant removal and antibiotics for definitive treatment.
The outcomes of treatment were reported for 35 patients (79.5%), with a mean follow-up of 29 months (range, 2 months to 10 years) (Table 1). Twenty-seven patients (61.4%) had no clinical signs of PJI at the final follow-up visit, and 3 patients had persistent PJI treated with lifelong antibiotic suppression. One patient had recurrent PJI at 1 year after treatment. Three deaths were reported; one because of community-acquired pneumonia before planned two-stage procedure, one from Clostridioides difficile infection, and one because of “unrelated issues” after exchange arthroplasty. One patient had no further intervention because of cardiac reasons. The clinical outcome of treatment was not reported for 9 patients (20.4%). Based on this systematic review alone, insufficient data exist to recommend the optimal treatment for patients with PJI in THA and TKA associated with dental procedures. In the 35 patients with treatment and outcome reported and success defined (by the authors) as patient alive and implant retained, the rate of success was 94.3% overall and 85.7% with 2-stage revisions.
Discussion
PJI associated with dental procedures in patients with THA and TKA is uncommon, and the use of antibiotic prophylaxis for some or all patients remains controversial. The 2016 AAOS multidisciplinary panel evaluated 64 scenarios to determine the “appropriate use” of antimicrobial prophylaxis for these patients having dental work. An appropriate use calculator considered 5 variables, including invasiveness of dental procedure, immunocompromised status of patient, glycemic control, history of infection requiring operation, and the time elapsed since arthroplasty. Of the 64 scenarios devised, prophylaxis was “rarely appropriate” in 61%, “may be appropriate” in 27%, and “appropriate” in only 12% [4]. Data on the organisms causing dental-associated PJI and the efficacy of antimicrobial prophylaxis to prevent PJI remain insufficient.
This systematic review of reported PJI in patients undergoing THA and TKA associated with dental procedures noted that an estimated 46% of the organisms isolated are likely to be resistant to amoxicillin, including many viridans group streptococci, some Granulicatella and Rothia spp., and all Staphylococcus aureus. Under 13% of the patients in this analysis had documented receipt of antibiotic prophylaxis. Thus, it seems unlikely that antibiotic given had a notable effect on the causative organisms.
The frequency of amoxicillin-resistant organisms questions the recommendation of amoxicillin as the optimal prophylaxis to prevent PJI in THA and TKA patients undergoing dental procedure. The recommendation for amoxicillin as the preferred prophylaxis is based on the low number needed to treat of 1.8 to prevent cases of dental-related bacteremia as well as a low rate of adverse effects with this antibiotic [[52], [53], [54]]. As many as 625 to 1250 courses of antibiotics may be needed to prevent one PJI [55]. Prophylaxis has been deemed cost-effective by some investigators only when the risk of PJI after dental treatment is at least 1.2% or if prophylaxis was 100% effective, both of which may not be realistic numbers [2,56]. In 1990, it was estimated that prevention of one PJI case with antibiotic prophylaxis would cost the health-care system nearly $480,000, and the cost to spare a year of life was closer to $500,000, both figures which are likely higher today [57]. The judicious use of antibiotic prophylaxis for endocarditis in high-risk patients undergoing high-risk procedures suggested a financial saving of between 5.5 and 8.2 million Euros, with a gain of 2687 quality-adjusted life years annually [58]. However, given differences in morbidity and mortality and the characteristics of patients who receive prophylaxis, it is unclear whether such estimated savings from endocarditis can be extrapolated to PJI. With the current available data, many countries advise against antibiotic prophylaxis for PJI prevention, while a few recommend use in only the highest risk patients [59,60].
The current AAOS Appropriate Use Criteria guidelines suggest that antibiotic prophylaxis be reserved for the highest risk patients undergoing invasive procedures because of the estimated low efficacy of antimicrobial prophylaxis to prevent PJI from dental procedure–related bacteremia [4,8]. In addition, since its inception in 1996, the idea of antibiotic stewardship and the need for judicious use of antibiotics to delay or limit resistance has been at the center of most decision trees regarding antibiotic prophylaxis [61]. No ideal oral antibiotic with a high rate of susceptibility against all potential oral pathogens is available either. The use of an oral first-generation cephalosporin may provide greater coverage for gram-positive aerobes but less efficacy for gram-positive anaerobes. This type of “trade-off” occurs with all oral antibiotics. For patients with very high-risk comorbidities who cannot tolerate an oral antibiotic, the use of intravenous or intramuscular prophylaxis with a broader spectrum antibiotic, such as with ceftriaxone, could be considered for future study. A systematic review of endocarditis prophylaxis reported that intravenous amoxicillin-clavulanic acid promoted a considerable reduction in bacteremia compared with other recommended prophylaxis [1]. However, this study recommended prophylaxis only in a very selected group of patients with high levels of dental infection undergoing invasive dental procedures under general anesthesia [62]. With ceftriaxone, concern exist for an increased risk of adverse reactions and a greater disruption of the microbiome [63,64]. Conversely, few serious adverse reactions and almost no deaths would be expected with single prophylactic dose of amoxicillin [52,65]. Thus, amoxicillin remains the recommended prophylaxis despite insufficient data to confirm the benefit of such prophylaxis before dental procedures in patients with THA and TKA. Other antibiotics that have efficacy against oral bacteria are also not without their side effect profiles. Other antibiotics with efficacy against oral bacteria are not without side effects: tetracyclines, macrolides, lincosamides (clindamycin), and carbapenems are frequently associated with gastrointestinal adverse effects, the most concerning of which is Clostridioides difficile colitis. Macrolides have the potential for acute hepatitis and allergic reactions, and clindamycin can potentially cause neutropenia and enterocolitis, among other concerns [66]. Therefore, the decision to use antibiotic prophylaxis is not simply a decision regarding efficacy but instead a tradeoff on the potential benefits versus risks for the patient.
This study is not without limitations. First, the number of reported cases of dental procedure–associated PJI is very low, likely underestimating the true frequency of this complication. All the included studies are case reports and case series, making the overall quality of the evidence low with significant risk of bias. Specifically, the reported cases are highly susceptible to publication bias, which likely predisposes to publication of the most complicated cases or perhaps the most unusual pathogens. Therefore, the reported cases and organisms may not be representative of PJI associated with dental procedures in patients undergoing THA and TKA in the general population. Other limitations include an inadequate level of reporting in aggregated case studies and the lack of antibiotic susceptibilities for individual pathogens. An infectious disease specialist estimated antibiotic susceptibility based on two widely accepted reference guides and a review of the literature, but this may not accurately reflect the case-by-case susceptibility. Unfortunately, accurate susceptibility ranges are not possible to estimate with any real degree of confidence because of the vast differences in the literature among studies for even a single species. Overall, while our estimate is imprecise, we feel that it does not detract from the overall message that amoxicillin is far from 100% active against the variety of organisms that colonize the oropharynx. Finally, the available literature will likely underestimate amoxicillin-sensitive organisms if some cases of PJI are prevented by amoxicillin or another antibiotic. In spite of these limitations, this study adds to the published literature as a comprehensive review of the published reports on dental procedure–associated PJI and provides an estimated amoxicillin susceptibility of the causative organisms.
Conclusions
This systematic review of the reported cases of PJI in patients undergoing THA and TKA suggests that dental procedure–associated PJI is an uncommon occurrence with or without prophylaxis. The organisms isolated from these cases were quite varied, with an overall estimated resistance to amoxicillin of 46%. These findings suggest that additional studies are needed to determine the optimal antibiotic and route of administration for the highest risk patients with THA and TKA undergoing one or multiple invasive dental procedures. Based on the individual risk profile of the patient and the provider’s level of concern for risk of infection, antibiotic prophylaxis may only be appropriate in the highest risk patients. The decision to use an antibiotic other than amoxicillin for prophylaxis must balance an increased adverse effect profile with the overall patient history of infection and comorbidities and the potential morbidity and mortality from a PJI associated with dental procedures. This study had insufficient cases to determine the optimal treatment and outcomes of PJI related to dental procedures, and obtaining such data would require a multicenter prospective study with PJI from known dental procedures that ideally includes antibiotic susceptibility information.
Declaration of interests
The authors declare there are no conflicts of interest.
Appendix
Supplementary data
References
- 1.Beam E., Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am. 2018;32(4):843. doi: 10.1016/j.idc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Rademacher W.M.H., Walenkamp G., Moojen D.J.F., Hendriks J.G.E., Goedendorp T.A., Rozema F.R. Antibiotic prophylaxis is not indicated prior to dental procedures for prevention of periprosthetic joint infections. Acta Orthop. 2017;88(5):568. doi: 10.1080/17453674.2017.1340041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Your joint replacement, dental procedures and antibiotics. J Am Dent Assoc. 2003;134(7):899. [Google Scholar]
- 4.Quinn R.H., Murray J.N., Pezold R., Sevarino K.S. The American Academy of orthopaedic surgeons appropriate use criteria for the management of patients with orthopaedic implants undergoing dental procedures. J Bone Joint Surg Am. 2017;99(2):161. doi: 10.2106/JBJS.16.01107. [DOI] [PubMed] [Google Scholar]
- 5.Sollecito T.P., Abt E., Lockhart P.B. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: evidence-based clinical practice guideline for dental practitioners--a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2015;146(1):11.e18. doi: 10.1016/j.adaj.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Colterjohn T., de Beer J., Petruccelli D., Zabtia N., Winemaker M. Antibiotic prophylaxis for dental procedures at risk of causing bacteremia among post-total joint arthroplasty patients: a survey of Canadian orthopaedic surgeons and dental surgeons. J Arthroplasty. 2014;29(6):1091. doi: 10.1016/j.arth.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 7.McNally C.M., Visvanathan R., Liberali S., Adams R.J. Antibiotic prophylaxis for dental treatment after prosthetic joint replacement: exploring the orthopaedic surgeon's opinion. Arthroplast Today. 2016;2(3):123. doi: 10.1016/j.artd.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Orthopaedic Surgeons Appropriate use criteria: management of patients with orthopaedic implants undergoing dental procedures. https://aaos.webauthor.com/go/auc/terms.cfm?actionxm=Terms&auc_id=224965
- 9.Friedlander A.H. Presence of staphylococci in mouth and presence of streptococci in late infections of knee and hip joint prostheses: antibiotic prophylaxis, a conundrum. Spec Care Dentist. 2009;29(6):226. doi: 10.1111/j.1754-4505.2009.00108.x. [DOI] [PubMed] [Google Scholar]
- 10.Dyck H., Huggett S., Hille E., Dierk O., Sardemann W. A case report: infection of a knee prosthesis after dental treatment - approaches to prevention. Hyg Medizin. 2006;31(12):569. [Google Scholar]
- 11.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert D.N., Eliopoulos G.M., Chambers H.F., Saag M.S., Pavia A.T. Antimicrobial Therapy Inc; Sperryville, VA: 2019. The Sanford guide to antimicrobial Therapy. [Google Scholar]
- 13.Alberti M.O., Hindler J.A., Humphries R.M. Antimicrobial susceptibilities of Abiotrophia defectiva, Granulicatella adiacens, and Granulicatella elegans. Antimicrobial Agents Chemother. 2016;60(3):1411. doi: 10.1128/AAC.02645-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao C.-H., Teng L.-J., Hsueh P.-R. Nutritionally variant streptococcal infections at a university hospital in Taiwan: disease emergence and high prevalence of β-lactam and macrolide resistance. Clin Infect Dis. 2004;38(3):452. doi: 10.1086/381098. [DOI] [PubMed] [Google Scholar]
- 15.Droz S., Zbinden R. Rothia dentocariosa. In: Yu V.L., Weber R., Raoult D., editors. 2nd ed. Vol. I. Apple Trees Productions; New York: 2003. http://www.antimicrobe.org/b230.asp (Antimicrobial therapy and vaccines). Microbes. [Google Scholar]
- 16.Magee J.T., Burnett I.A., Hindmarch J.M., Spencer R.C. Micrococcus and Stomatococcus spp. from human infections. J Hosp Infect. 1990;16(1):67. doi: 10.1016/0195-6701(90)90050-x. [DOI] [PubMed] [Google Scholar]
- 17.Abro S., Ali A. Antibiogram of the Micrococcus luteus, Pseudomonas aeruginosa, Staphylococcus epidermidis and Staphylococcus intermedius isolated from the bovine frozen semen. Pure Appl Biol. 2016;5:204. [Google Scholar]
- 18.von Eiff C., Herrmann M., Peters G. Antimicrobial susceptibilities of stomatococcus mucilaginosus and of Micrococcus. Antimicrobial Agents Chemother. 1995;39(1):268. doi: 10.1128/aac.39.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K.-S., Rowlinson M.-C., Bennion R. Characterization of Slackia exigua isolated from human wound infections, including abscesses of intestinal origin. J Clin Microbiol. 2010;48(4):1070. doi: 10.1128/JCM.01576-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auwaerter P.G., editor . The Johns Hopkins University; Baltimore, MD: 2020. The Johns Hopkins POC-IT ABX Guide 2020. [Google Scholar]
- 21.Rubin R., Salvati E.A., Lewis R. Infected total hip replacement after dental procedures. Oral Surg Oral Med Oral Pathol. 1976;41(1):18. doi: 10.1016/0030-4220(76)90247-4. [DOI] [PubMed] [Google Scholar]
- 22.Schurman D.J., Aptekar R.G., Burton D.S. Infection in total knee joint replacement, secondary to tooth abscess. West J Med. 1976;125(3):226. [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen P.L., Murray W. Prophylactic coverage of dental patients with artificial joints: a retrospective analysis of thirty-three infections in hip prostheses. Oral Surg Oral Med Oral Pathol. 1980;50(2):130. doi: 10.1016/0030-4220(80)90199-1. [DOI] [PubMed] [Google Scholar]
- 24.Lindqvist C., Slatis P. Dental bacteremia–a neglected cause of arthroplasty infections? Three hip cases. Acta Orthop Scand. 1985;56(6):506. doi: 10.3109/17453678508993046. [DOI] [PubMed] [Google Scholar]
- 25.Strazzeri J.C., Anzel S. Infected total hip arthroplasty due to Actinomyces israelii after dental extraction. A case report. Clin Orthop Relat Res. 1986;210:128. [PubMed] [Google Scholar]
- 26.Grogan T.J., Dorey F., Rollins J., Amstutz H.C. Deep sepsis following total knee arthroplasty. Ten-year experience at the University of California at Los Angeles Medical Center. J Bone Joint Surg Am. 1986;68(2):226. [PubMed] [Google Scholar]
- 27.Pravda J., Habermann E. Hemophilus parainfluenzae complicating total knee arthroplasty. A case report. Clin Orthop Relat Res. 1989;243:169. [PubMed] [Google Scholar]
- 28.Sullivan P.M., Johnston R.C., Kelley S.S. Late infection after total hip replacement, caused by an oral organism after dental manipulation. A case report. J Bone Joint Surg Am. 1990;72(1):121. [PubMed] [Google Scholar]
- 29.Manian F.A. Prosthetic joint infection due to haemophllus paralnfluenzae after dental surgery. South Med J. 1991;84(6):807. doi: 10.1097/00007611-199106000-00044. [DOI] [PubMed] [Google Scholar]
- 30.Skiest D.J., Coykendall A.L. Prosthetic hip infection related to a dental procedure despite antibiotic prophylaxis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(5):661. doi: 10.1016/s1079-2104(05)80112-x. [DOI] [PubMed] [Google Scholar]
- 31.Waldman B.J., Mont M.A., Hungerford D.S. Total knee arthroplasty infections associated with dental procedures. Clin Orthop Relat Res. 1997;(343):164. [PubMed] [Google Scholar]
- 32.LaPorte D.M., Waldman B.J., Mont M.A., Hungerford D.S. Infections associated with dental procedures in total hip arthroplasty. J Bone Joint Surg Br. 1999;81(1):56. doi: 10.1302/0301-620x.81b1.8608. [DOI] [PubMed] [Google Scholar]
- 33.Kaar T.K., Bogoch E.R., Devlin H.R. Acute metastatic infection of a revision total hip arthroplasty with oral bacteria after noninvasive dental treatment. J Arthroplasty. 2000;15(5):675. doi: 10.1054/arth.2000.4331. [DOI] [PubMed] [Google Scholar]
- 34.Nadlacan L.M., Hirst P. Infected total knee replacement following a dental procedure in a severe haemophiliac. Knee. 2001;8(2):159. doi: 10.1016/s0968-0160(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 35.Jellicoe P.A., Cohen A., Campbell P. Haemophilus parainfluenzae complicating total hip arthroplasty: a rapid failure. J Arthroplasty. 2002;17(1):114. doi: 10.1054/arth.2002.29314. [DOI] [PubMed] [Google Scholar]
- 36.Bartz H., Nonnenmacher C., Bollmann C. Micromonas (Peptostreptococcus) micros: unusual case of prosthetic joint infection associated with dental procedures. Int J Med Microbiol. 2005;294(7):465. doi: 10.1016/j.ijmm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Klingler E.T., Verma P., Harris A. Infection of a total knee arthroplasty with Rothia dentocariosa: brief report and review of the literature. Infect Dis Clin Pract. 2005;13(4):195. [Google Scholar]
- 38.Trivedi M.N., Malhotra P. Rothia prosthetic knee joint infection. J Microbiol Immunol Infect. 2015;48(4):453. doi: 10.1016/j.jmii.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Michels F., Colaert J., Gheysen F., Scheerlinck T. Late prosthetic joint infection due to Rothia mucilaginosa. Acta Orthop Belg. 2007;73(2):263. [PubMed] [Google Scholar]
- 40.Waqar S., Hussain T., Bawarish M.A. Knee sepsis caused by Streptococcus mitis after dental hygiene therapy in a patient with total knee replacement: a case report. Curr Orthop Pract. 2008;19(5):586. [Google Scholar]
- 41.Brown M.L., Drinkwater C.J. Hematogenous infection of total hip arthroplasty with Actinomyces following a noninvasive dental procedure. Orthopedics. 2012;35(7):e1086. doi: 10.3928/01477447-20120621-27. [DOI] [PubMed] [Google Scholar]
- 42.Mahobia N., Chaudhary P., Kamat Y. Rothia prosthetic knee joint infection: report and mini-review. New Microbes New Infect. 2013;1(1):2. doi: 10.1002/2052-2975.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mougari F., Jacquier H., Bercot B. Prosthetic knee arthritis due to Granulicatella adiacens after dental treatment. J Med Microbiol. 2013;62(Pt 10):1624. doi: 10.1099/jmm.0.058263-0. [DOI] [PubMed] [Google Scholar]
- 44.Al-Himdani S., Woodnutt D. Group C streptococcal septic arthritis of a prosthetic hip joint following dental treatment. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-211203. bcr2015211203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein R., Dababneh A.S., Palraj B.R. Streptococcus gordonii prosthetic joint infection in the setting of vigorous dental flossing. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-210695. bcr2015210695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aweid O., Sundararajan S., Teferi A. Granulicatella adiacens prosthetic hip joint infection after dental treatment. JMM Case Rep. 2016;3(3):e005044. doi: 10.1099/jmmcr.0.005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quénard F., Seng P., Lagier J.C., Fenollar F., Stein A. Prosthetic joint infection caused by Granulicatella adiacens: a case series and review of literature. BMC Musculoskelet Disord. 2017;18(1):276. doi: 10.1186/s12891-017-1630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartash R., Guo Y., Pope J.B. Periprosthetic hip joint infection with Aspergillus terreus: a clinical case and a review of the literature. Med Mycol Case Rep. 2017;18:24. doi: 10.1016/j.mmcr.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kansara T., Pernia M., Kim Y., Saeed M. Rare occurrence of prosthetic knee septic arthritis due to Streptococcus viridans in the background of a dental procedure. Cureus. 2019;11(10):e5980. doi: 10.7759/cureus.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olson L.B., Turner D.J., Cox G.M., Hostler C.J. Streptococcus salivarius prosthetic joint infection following dental cleaning despite antibiotic prophylaxis. Case Rep Infect Dis. 2019;2019:8109280. doi: 10.1155/2019/8109280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rieber H., Frontzek A., Schmitt H. Slackia exigua, an anaerobic Gram-positive rod and part of human oral microbiota associated with periprosthetic joint infection of the hip. First case and review of the literature. Anaerobe. 2019;56:130. doi: 10.1016/j.anaerobe.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Lee P., Shanson D. Results of a UK survey of fatal anaphylaxis after oral amoxicillin. J Antimicrob Chemother. 2007;60(5):1172. doi: 10.1093/jac/dkm315. [DOI] [PubMed] [Google Scholar]
- 53.Wilson W., Taubert K.A., Gewitz M. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation. 2007;116(15):1736. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 54.Watters W., 3rd, Rethman M.P., Hanson N.B. Prevention of orthopaedic implant infection in patients undergoing dental procedures. J Am Acad Orthop Surg. 2013;21(3):180. doi: 10.5435/JAAOS-21-03-180. [DOI] [PubMed] [Google Scholar]
- 55.Sendi P., Uckay I., Suva D., Vogt M., Borens O., Clauss M. Antibiotic prophylaxis during dental procedures in patients with prosthetic joints. J Bone Joint Infect. 2016;1:42. doi: 10.7150/jbji.16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slover J.D., Phillips M.S., Iorio R., Bosco J. Is routine antibiotic prophylaxis cost effective for total joint replacement patients? J Arthroplasty. 2015;30(4):543. doi: 10.1016/j.arth.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson J.J., Schweitzer S., DePorter D.J., Lee J.J. Antibiotic prophylaxis for dental patients with joint prostheses? A decision analysis. Int J Technol Assess Health Care. 1990;6(4):569. doi: 10.1017/s0266462300004220. [DOI] [PubMed] [Google Scholar]
- 58.Franklin M., Wailoo A., Dayer M.J. The cost-effectiveness of antibiotic prophylaxis for patients at risk of infective endocarditis. Circulation. 2016;134(20):1568. doi: 10.1161/CIRCULATIONAHA.116.022047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uckay I., Pittet D., Bernard L., Lew D., Perrier A., Peter R. Antibiotic prophylaxis before invasive dental procedures in patients with arthroplasties of the hip and knee. J Bone Joint Surg Br. 2008;90(7):833. doi: 10.1302/0301-620X.90B7.20359. [DOI] [PubMed] [Google Scholar]
- 60.Daly C.G. Antibiotic prophylaxis for dental procedures. Aust Prescr. 2017;40(5):184. doi: 10.18773/austprescr.2017.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charani E., Holmes A. Antibiotic stewardship-twenty years in the making. Antibiotics (Basel) 2019;8(1):7. doi: 10.3390/antibiotics8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lafaurie G.I., Noriega L.A., Torres C.C. Impact of antibiotic prophylaxis on the incidence, nature, magnitude, and duration of bacteremia associated with dental procedures: a systematic review. J Am Dent Assoc. 2019;150(11):948. doi: 10.1016/j.adaj.2019.06.017. e944. [DOI] [PubMed] [Google Scholar]
- 63.Shalviri G., Yousefian S., Gholami K. Adverse events induced by ceftriaxone: a 10-year review of reported cases to Iranian Pharmacovigilance Centre. J Clin Pharm Ther. 2012;37(4):448. doi: 10.1111/j.1365-2710.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- 64.de Vries-Hospers H.G., Tonk R.H., van der Waaij D. Effect of intramuscular ceftriaxone on aerobic oral and faecal flora of 11 healthy volunteers. Scand J Infect Dis. 1991;23(5):625. doi: 10.3109/00365549109105188. [DOI] [PubMed] [Google Scholar]
- 65.Salvo F., Polimeni G., Moretti U. Adverse drug reactions related to amoxicillin alone and in association with clavulanic acid: data from spontaneous reporting in Italy. J Antimicrob Chemother. 2007;60(1):121. doi: 10.1093/jac/dkm111. [DOI] [PubMed] [Google Scholar]
- 66.Heta S., Robo I. The side effects of the most commonly used group of antibiotics in periodontal treatments. Med Sci (Basel) 2018;6(1):6. doi: 10.3390/medsci6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.