Abstract
The European Commission requested the EFSA Panel on Plant Health to prepare and deliver risk assessments for commodities listed in Commission Implementing Regulation (EU) 2018/2019 as ‘High risk plants, plant products and other objects’. This Scientific Opinion covers the plant health risks posed by the following commodities: (i) dormant and free of leaves 1‐year‐old bare rooted plants and (ii) free of leaves 1‐year‐old liners of Ficus carica imported from Israel, taking into account the available scientific information, including the technical information provided by Israel. The relevance of any pest for this opinion was based on evidence following defined criteria. Four EU quarantine pests, Euwallacea fornicatus, Hypothenemus leprieuri, Scirtothrips dorsalis and Spodoptera frugiperda, and 11 EU non‐regulated pests fulfilled all relevant criteria and were selected for further evaluation. For these pests, the risk mitigation measures proposed in the technical dossier from Israel were evaluated separately for bare rooted plants and for liners, taking into account the possible limiting factors. For these pests, an expert judgement was given on the likelihood of pest freedom taking into consideration the risk mitigation measures acting on the pest, including uncertainties associated with the assessment. The estimated degree of pest freedom varied among the pests evaluated. Aonidiella orientalis and Russellaspis pustulans were the most frequently expected pests on the imported bare rooted plants, and Scirtothrips dorsalis on liners. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,585 and 10,000 bare rooted plants per 10,000 would be free of Aonidiella orientalis and Russellaspis pustulans and between 9,456 and 10,000 liners per 10,000 would be free of Scirtothrips dorsalis.
Keywords: common fig, plants for planting, bare rooted plants, liners, European Union
1. Introduction
1.1. Background and Terms of Reference as provided by European Commission
1.1.1. Background
The new Plant Health Regulation (EU) 2016/20311, on the protective measures against pests of plants, has been applied from December 2019. Provisions within the above Regulation are in place for the listing of ‘high risk plants, plant products and other objects’ (Article 42) on the basis of a preliminary assessment, and to be followed by a commodity risk assessment. A list of ‘high risk plants, plant products and other objects’ has been published in Regulation (EU) 2018/20192. Scientific opinions are therefore needed to support the European Commission and the Member States in the work connected to Article 42 of Regulation (EU) 2016/2031, as stipulated in the terms of reference.
1.1.2. Terms of Reference
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/20023, the Commission asks EFSA to provide scientific opinions in the field of plant health.
In particular, EFSA is expected to prepare and deliver risk assessments for commodities listed in the relevant Implementing Acts as ‘High risk plants, plant products and other objects’. Article 42, paragraphs 4 and 5, establishes that a risk assessment is needed as a follow‐up to evaluate whether the commodities will remain prohibited, removed from the list and additional measures will be applied or removed from the list without any additional measures. This task is expected to be on‐going, with a regular flow of dossiers being sent by the applicant required for the risk assessment.
Therefore, to facilitate the correct handling of the dossiers and the acquisition of the required data for the commodity risk assessment, a format for the submission of the required data for each dossier is needed.
Furthermore, a standard methodology for the performance of ‘commodity risk assessment’ based on the work already done by Member States and other international organisations needs to be set.
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002, the Commission asks EFSA to provide scientific opinion in the field of plant health for Ficus carica from Israel taking into account the available scientific information, including the technical dossier provided by Israel.
1.2. Interpretation of the Terms of Reference
The European Food Safety Authority (EFSA) Panel on Plant Health (hereafter referred to as ‘the Panel’) was requested to conduct a commodity risk assessment of Ficus carica from Israel following the Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019).
The European Union (EU) quarantine pests that are regulated as a group in the Commission Implementing Regulation (EU) 2019/2072 were considered and evaluated separately at species level. The references to ‘non‐European’ refer to all territories with exception of the EU territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
The criteria used in this opinion to determine if Scolytidae spp. (non‐European) is considered as potentially quarantine for the EU followed the proposal and criteria specified in EFSA PLH Panel (2020), i.e. a non‐EU Scolytinae is defined by its geographical distribution outside of the EU territory. As such, Scolytinae not reported from the EU and occurring only outside of the EU territory are considered as non‐EU Scolytinae. Furthermore, Scolytinae occurring outside the EU and having only a limited presence in the EU (reported from up to three EU Member States, with restricted distribution) are also considered as non‐EU.
Pests listed for F. carica as ‘Regulated Non‐Quarantine Pest’ (RNQP) in Annex IV of the Commission Implementing Regulation (EU) 2019/2072, and deregulated pests (i.e. pests which were listed as quarantine pests in the Council Directive 2000/29/EC and were deregulated by Commission Implementing Regulation (EU) 2019/2072) were not considered for further evaluation.
In its evaluation, the Panel:
Checked whether the provided information in the technical dossier (hereafter referred to as ‘Dossier’) provided by the applicant (Israel Ministry of Agriculture and Rural Development, Plant Protection and Inspection Services – PPIS) was sufficient to conduct a commodity risk assessment. When necessary, additional information was requested to the applicant.
Selected the relevant EU quarantine pests and protected zone quarantine pests (as specified in Commission Implementing Regulation (EU) 2019/20724, hereafter referred to as ‘EU quarantine pests’) and other relevant pests present in Israel and associated with the commodity.
For those Union quarantine pests for which specific measures are in place for the import of the commodity from Israel in Commission Implementing Regulation (EU) 2019/2072 and/or in the relevant legislative texts for emergency measures and provided that the specific country is in the scope of those emergency measures, the assessment was restricted to whether or not the applicant country implements those measures. The effectiveness of those measures was not assessed.
For those Union quarantine pests for which no specific measures are in place for the import of the commodity from Israel and other relevant pests present in Israel and associated with the commodity, the effectiveness of the measures described in the Dossier was assessed.
Risk management decisions are not within EFSA's remit. Therefore, the Panel provided a rating based on expert judgement regarding the likelihood of pest freedom for each relevant pest given the risk mitigation measures proposed by the PPIS.
2. Data and methodologies
2.1. Data provided by the PPIS
The Panel considered all the data and information (hereafter called ‘Dossier’) provided by PPIS of Israel in October 2019 including the additional information provided by PPIS of Israel on 14 June 2020, after EFSA's request. The Dossier is managed by EFSA.
The structure and overview of the Dossier are shown in Table 1. The number of the relevant section will be indicated in the opinion when referring to a specific part of the Dossier.
Table 1.
Structure and overview of the Dossier
| Dossier section | Overview of contents | Filename |
|---|---|---|
| 1.0 | Technical dossier | 1. Ficus information for EFSA 10 |
| 2.0 | Pest list on Ficus carica | 2. Pest list for Ficus carica |
| 3.0 | Reference for Batocera rufomaculata | 3. Batocera rufomaculata datasheet |
| 4.0 | Reference for Eutetranychus orientalis | 4. Eutetranychus orientalis datasheet |
| 5.0 | Reference for Pauropsylla buxtoni | 5. Pauropsylla buxtoni datasheet |
| 6.0 | Reference for Retithrips syriacus | 6. Retithrips syriacus datasheet |
| 7.0 | Reference for Scirtothrips dorsalis | 7. Scirtothrips dorsalis datasheet |
| 8.0 | Reference for Spodoptera littoralis | 8. Spodoptera littoralis datasheet |
| 9.0 | Additional information provided by PPIS on 14 June 2020 | Answers to EFSA questions Ficus April 2020 |
The data and supporting information provided by PPIS of Israel formed the basis of the commodity risk assessment.
Table 2 shows the databases used by PPIS to compile the Dossier. Additional information used by PPIS and details on literature searches along with full list of references can be found in Dossier Section 2.0.
Table 2.
Database sources used by PPIS when preparing the Dossier
| Acronym/short title | Database name and service provider | URL of database | Justification for choosing database |
|---|---|---|---|
| CABI | Name: CABI Crop Protection CompendiumProvider: CAB International | https://www.cabi.org/cpc/ | A database that draws together scientific information on all aspects of crop protection, including extensive global coverage of pests, diseases, weeds and their natural enemies, the crops that are their hosts, and the countries in which they occur. |
| Catalogue of Life | Name: Catalogue of LifeProvider: Species 2000 | https://www.catalogueoflife.org/ | This database provides information on world's known species of animals, plants, fungi and microorganisms. |
| EPPO | Name: EPPO Global DatabaseProvider: European and Mediterranean Plant Protection Organization | https://gd.eppo.int/ | This database provides all pest‐specific information that has been produced or collected by EPPO. |
| Fauna Europaea | Name: Fauna EuropaeaProvider: Museum für Naturkunde in Berlin | https://fauna-eu.org/ | A database which lists main zoological taxonomic index in Europe. |
| Forest Pests of North America | Forest Pests of North AmericaProvider: The University of Georgia ‐ Center for Invasive Species and Ecosystem Health | https://www.forestpests.org/insects_main.cfm | Native and non‐native insects, diseases and weeds of urban, managed and natural forests. |
| GBIF | Name: Global Biodiversity Information FacilityProvider: Secretariat in Copenhagen, established on the recommendation of OECD | https://www.gbif.org/ | This database provides information about biodiversity of the world. |
| PPME | Name: Plant Pests of the Middle EastProvider: The Robert H. Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem | http://www.agri.huji.ac.il/mepests/ | This database provides considerable information of the different pest species, their biology, host range and how to control them. |
| Scalenet | Name: ScalenetProvider: García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y, Hardy NB | http://scalenet.info/associates/ | This database provides information on scale insects, their taxonomic diversity, nomenclatural history, biogeography, ecological associations and economic importance. |
2.2. Literature searches performed by EFSA
Literature searches were undertaken by EFSA to prepare a list of pests potentially associated with F. carica. Following searches were combined: (i) a general search to identify pests of F. carica in different databases and (ii) a tailored search to identify whether these pests are present or not in Israel and the EU. The general search was run between 13 November and 2 December 2019. No language, date or document type restrictions were applied in the search strategy.
The Panel used the databases indicated in Table 3 to compile the list of pests associated with the F. carica. As for Web of Science, the literature search was performed using a specific, ad hoc established search string (see Appendix B). The string was run in ‘All Databases’ with no range limits for time or language filters. This is further explained in Section 2.3.2.
Table 3.
Databases used by EFSA for the compilation of the pest list associated with Ficus carica
Additional searches, limited to retrieve documents, were run when developing the opinion. The available scientific information including previous EFSA opinions on the relevant pests and diseases (see pest datasheets in Appendix A) and the relevant literature and legislation (e.g. Regulation (EU) 2016/2031; Commission Implementing Regulations (EU) 2018/2019; (EU) 2018/2018, (EU) 2019/2072, (EU) 2018/6385 and (EU) 2020/12016) were taken into account.
2.3. Methodology
When developing the opinion, the Panel followed the EFSA Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019).
In the first step, pests associated with the commodity in the country of origin (EU‐regulated pests and other pests) that may require risk mitigation measures are identified.
In this opinion, relevant EU non‐regulated pests were selected based on evidence for their potential impact for the EU. After the first step, all the relevant pests that may need risk mitigation measures are identified.
In the second step, the overall efficacy of the proposed risk mitigation measures for each pest is evaluated. A conclusion on the pest freedom status of the commodity for each of the relevant pests is achieved and uncertainties are identified. Pest freedom was assessed by estimating the number of infested/infected plants out of 10,000 exported plants. Further details on the methodology used to estimate the likelihood of pest freedom are provided in Section 2.3.3.
2.3.1. Commodity data
Based on the information provided by the PPIS of Israel, the characteristics of the commodity were summarised.
2.3.2. Identification of pests potentially associated with the commodity
To evaluate the pest risk associated with the importation of F. carica from Israel, a pest list was compiled. The pest list is a compilation of all identified plant pests associated with F. carica. The search strategy and search syntax were adapted to each of the databases listed in Table 3, according to the options and functionalities of the different databases and CABI keyword thesaurus.
The scientific name of the host plants (i.e. Ficus carica) was used when searching in the EPPO Global database and CABI Crop Protection Compendium. The same strategy was applied to the other databases excluding EUROPHYT and Web of Science.
EUROPHYT was investigated by searching for the interceptions associated with F. carica commodities imported from Israel and from other countries different from Israel from 1995 to November 2019. For the pests selected for further evaluation, a search in the EUROPHYT was performed for the interceptions from the whole world on any other host species, from 1995 to November 2019.
The search strategy used for Web of Science Databases was designed combining common names for pests and diseases, terms describing symptoms of plant diseases and the scientific and common names of the commodity. All of the pests already retrieved using the other databases were removed from the search terms in order to be able to reduce the number of records to be screened. Also, other Ficus species were excluded from the search.
The established search string is detailed in Appendix B, and was run on 15 November 2019.
The titles and abstracts of the scientific papers retrieved were screened and the pests associated with F. carica were included in the pest list.
All the pests retrieved using the different databases are included in a Microsoft Excel® file which was eventually further compiled with other relevant information (e.g. EPPO code per pest, taxonomic information, categorisation, distribution) useful for the selection of the pests relevant for the purposes of this opinion.
Finally, the list was complemented by those pests mentioned in the Dossier if they were not found using the source of information listed above.
The compiled pest list (see Microsoft Excel® file in Appendix E) includes all identified pests that use F. carica as host, potentially including predators and parasitoids of insects and saprophytic microbes, which can be associated with F. carica.
The evaluation of the compiled pest list was done in two steps: first, the relevance of the EU quarantine pests is evaluated (Section 4.1); second, the relevance of any other plant pests is evaluated (Section 4.2).
Pests for which limited information was available on one or more criteria used to identify them as relevant for this opinion are listed in Appendix C (List of potential pests not further assessed).
2.3.3. Listing and evaluation of risk mitigation measures
The proposed risk mitigation measures were listed and evaluated separately for the commodities considered in the opinion, which are bare rooted plants (BRP) and liners as specified in Section 3.1. When evaluating the potential pest freedom of the commodity, the following types of potential infection sources for F. carica plants in export nursery and relevant risk mitigation measures were considered (see also Figure 1):
pest entry from surrounding areas,
pest entry with new plants/seeds,
pest spread within the nursery.
Figure 1.
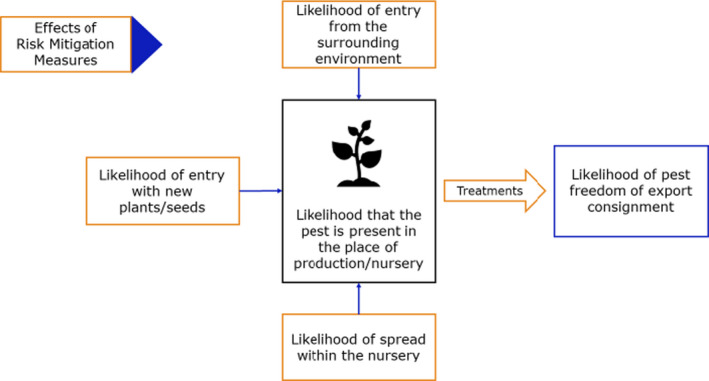
General factors considered for the estimation of pest freedom
The risk mitigation measures proposed by PPIS of Israel were evaluated.
Information on the biology, likelihood of entry of the pest to the export nursery, of its spread inside the nursery and the effect of the measures on the specific pest on the commodities (bare rooted plants and/or liners) were summarised in pest sheets for each pest selected for further evaluation (see Appendix A).
To estimate the level of pest freedom of the commodities, a semi‐formal expert knowledge elicitation (EKE) was performed following Annex B.8 on semi‐formal EKE of the EFSA opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment (EFSA Scientific Committee, 2018). The specific question for the semi‐formal EKE was defined as follows: ‘Taking into account i) the risk mitigation measures listed in the Dossier, and ii) other relevant information, how many of 10,000 F. carica plants (i.e. bare rooted plants or liners) will be infested with the relevant pest/pathogen when arriving in the EU?’. The EKE question was common for all the pests that were assessed.
When the biology of the pest, the production systems and the risk mitigation measures suggested similar likelihood of pest freedom for both commodities, the EKE was performed together for bare rooted plants and liners. The differences between the commodities were included in the uncertainty assessment. When these conditions were not met, the EKE was performed separately for the two commodities by a comparative assessment focusing on the differences between the commodities.
The uncertainties associated with the EKE (expert judgements) on the pest freedom of the commodity for each pest were taken into account and quantified in the probability distribution applying the semi‐formal method described in Section 3.5.2 of the EFSA PLH Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Finally, the results were reported in terms of the likelihood of pest freedom. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
The risk assessment uses individual plants as most suitable granularity. Following reasoning is given:
There is no quantitative information available regarding clustering plants during production.
For most pests under consideration a cross‐contamination during transport is not likely.
Individual plants will be finally sold via nurseries and retail to the consumer.
3. Commodity data
3.1. Description of the commodity
The commodity to be imported is both bare rooted plants and liners of F. carica (common name: common fig; family: Moraceae). According to Dossier Section 9.0, F. carica varieties that are exported from Israel to the EU are: ‘Brown Turkey’, ‘Ice Crystal’, ‘Jordan’, ‘Kadota’, ‘Nazareth’, ‘Penashe’, ‘Switzerland’.
Bare rooted plants: Dormant plants. Roots are rinsed, leaves are removed. The age of plants is 1 year. Plants are grown either in soil in open fields or in commercial growing medium (Klasmann‐Deilmann GmbH or Kekkila professional peat substrate) in net house. According to Dossier Section 9.0 in all fig varieties, bare rooted plants are 20–100 cm tall, with base diameter of up to 2 cm. The net of the net house is for shading and the net house is open on the sides. In addition, plants are washed, and soil is removed regardless if they are cultivated in open field or in the net house. Therefore, the Panel in its evaluation regarding the level of risk did not differentiate between the bare rooted plants grown in soil in open field from the bare rooted plants grown in the in commercial growing medium in net house.
Liners: One‐year‐old rooted cuttings in growing medium. Cultivated in commercial growing medium in pots (Klasmann‐Deilmann GmbH or Kekkila professional peat substrate) in a net house. According to Dossier Section 9.0 in all fig varieties, liners are about 10 cm high and with ~ 1 cm base diameter. Liners have leaves removed, and the plant and substrate are cleaned of plant debris. The growing medium is not changed when the liners are sent to the EU.
3.2. Description of the production areas
The crops designated for export, are grown in different fields from the crops designated for the local market. According to Dossier Section 9.0, the export nursery does not produce fig plants for the local market but sells export surplus locally.
Figure 2 presents the two current sites of F. carica cultivation in Israel: Bitzaron and Kfar Yehoshua (the southern and the northern spots on the map, respectively).
Figure 2.
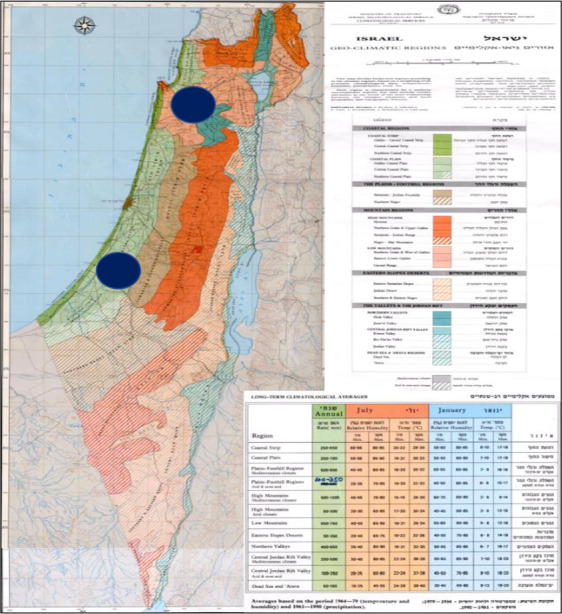
Current sites of Ficus carica cultivation in Israel
According to the additional information (Dossier Section 9.0), there are two F. carica cultivation nurseries, located in two sites. However, only one nursery is active in export at present and plans to export in the future (both bare rooted plants and liners), which is the site in Bitzaron, coordinates, 31.795942, 34.745201. The other cultivation site reported in the original Dossier to EFSA (in Kfar Yehoshua) has ceased to export and does not plan to export fig products at any time soon. No additional fig nursery cultivation sites for export to the EU are presently planned. Therefore, the Panel considered in its evaluation only the nursery in Bitzaron.
Based on the global Köppen–Geiger climate zone classification (Kottek et al., 2006), the climate of the production site of F. carica in Israel is similar to that found in some regions of southern EU (subgroup Csa, Mediterranean hot summer climates, see Figure 3).
Figure 3.
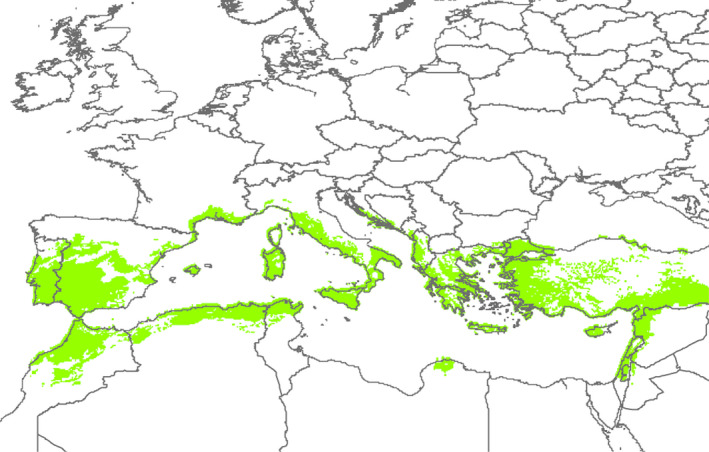
Distribution of Köppen–Geiger climate subgroup Csa (Mediterranean hot summer climates) areas in the Mediterranean basin (MacLeod and Korycinska, 2019)
According to Dossier Section 9.0, the minimum distance between fig trees cultivated for export and for the local market is over 1 km. Agricultural crops in a radius of 2 km from the fig cultivation includes cotton, tubers of various ornamental plants as well as persimmon, pomegranate, Brassica spp., watermelon. In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated at a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus and acacia. Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. However, no information was provided to the Panel on the occurrence of fig plants in private gardens or urban areas.
The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km far from the nursery (Dossier Section 9.0).
3.3. Production and handling processes
3.3.1. Growing conditions
Bare rooted plants are grown/rooted either in soil in open fields or in commercial growing medium (Klasmann‐Deilmann GmbH or Kekkila professional peat substrate) in sack containers in net house. In summer, before a new crop cycle, the open fields are treated with solarisation. According to Dossier Section 9.0, the fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants.
Liners as rooted cuttings are cultivated in the same commercial growing medium as above in pots in a net house. According to Dossier Section 9.0, the growing medium that is used for the exported fig products is always new at the beginning of each production cycle. According to Dossier Section 9.0, other plants are grown in the fig export nursery: Lagerstroemia indica and Morus alba, with a distance of a few dozens of metres between them and the fig plants.
The Dossier Section 9.0 states that the water that is used for irrigation is regular tap water, that goes through a 120‐mesh filter to remove rough dirt like sand and stones. Liners are irrigated by sprinklers, and bare rooted plants receive drip irrigation.
According to Dossier Section 9.0:
-
–
The coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
-
–
The nursery maintains appropriate sanitation measures to ensure there are no non‐cultivated herbaceous plants in the vicinity of the cultivated fig plants, including the access areas.
-
–
There are no shelter plants or hedges around the fig nursery.
-
–
The net is designed for shading – 40% shade and the net house is not entirely sealed.
3.3.2. Source of planting material
The mother plants are grown in a mother plant stock and treated in the same manner as the young plants.
According to Dossier Section 9.0, all propagation material come from a single mother orchard located within the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
3.3.3. Production cycle
The propagation protocol is described in the Dossier Section 1.0 as follows:
-
–
Summer – Open field soil preparation – Solarisation
-
–
March – Bare rooted plants: Rooting F. carica in soil, either in the open fields or in commercial growing medium in sack containers. Liners: Rooting the cuttings in commercial growing medium. According to Dossier Section 9.0, the plants are not removed from the growing medium in which they are initially planted, throughout the cultivation process.
-
–
The mother plants are grown in a mother plant stock and treated in the same manner as the young plants:
-
–
During the growing season, production fields (open fields and net house) are treated in a 3‐week cycle with preventative treatments, i.e. rotation of the following pesticide: Atlas (Bifenthrin), Ipon (Dinotefuran), Imidan (Phosmet) and EOS (Eco Oil Spray). Each pesticide is used every 9 weeks, and 2–3 times per season. These substances were selected for being effective in prevention against a range of insect pests, including borers, and are permitted for use in fig plants in Israel (Dossier Section 9.0). The Panel interprets that EOS is sprayed during winter and the remaining three pesticides are sprayed in alternation during the growing season.
-
–
The nursery treats the plants with appropriate fungicides in the case of any early signs of fungal infection (Dossier Section 9.0).
-
–
Against nematodes: treatment with Nemakor (Fenamiphos).
-
–
Weeds are treated with Faster (Glufosinate ammonium).
-
–
The nursery staff monitors all production fields on a weekly basis.
-
–
Soil and root samples are tested for nematodes.
-
–
-
–
December
-
–
lifting the bare rooted plants from the open field, washing the soil off the roots, selecting, grading and packing them in boxes. Storing them in cold storage at 2°C. The Panel assumes that the bare rooted plants grown in commercial growing medium are handled in the same way.
-
–
Packaging of liners (for details, see Section 3.3.5).
-
–
However, the production protocol was further clarified in Dossier Section 9.0. as follows.
Plants are topped before export to improve foliar growth and to obtain uniformity of growth.
Soil solarisation is performed by covering the soil with transparent polyethylene for two months – July and August (normally the time of highest radiation). The polyethylene sheet is spread after the soil has been cleaned from the previous crop and has been processed for the next cycle. The polyethylene in the sheets is supplemented with ‘antidrip’ or ‘antifog’ substances which prevent water condensation and accumulation on the sheet, thus improving treatment efficacy by raising the under‐sheet temperature by 4–5°C compared with regular polyethylene sheets. The maximum temperature in the top 20 cm of the soil is 44–48°C daily, for the duration of 2 months. The sheets are maintained clean and intact through the treatment duration, and the soil moisture is maintained to the field capacity level, by weekly irrigation with water.
3.3.4. Pest monitoring during production
According to Dossier Section 1.0, the nursery staff monitors all production fields on a weekly basis. All fields are under control and inspection of a PPIS inspector during the growing and delivery season. No particular pest or disease problem has occurred in the cultivation of F. carica propagation material in Israel, as seen in regular monitoring of the production fields. However, some pests are associated with this species in agriculture and in nature in Israel.
Growers wishing to export plants and propagation material (PPM) from Israel must be part of the PPM Export Programme. This programme consists of three different subprogrammes: export to the EU, export to the USA and export of plant PPIS‐certified tissue culture. PPIS has set a comprehensive overall system for the inspection of production fields. The system forms a part of a concept of inspection developed to ensure that the requirements of all importing countries are met. Specific official inspection and treatment can be carried out according to specific import requirements of the importing country. PPIS is responsible for the registration of producers of plants for planting, which is carried out via its website.
All plants for planting exported from Israel originate from nurseries that are approved by PPIS and are under PPIS inspection.
In the exporting nursery, PPIS inspection is carried out every 45 days.
Further to the PPIS inspection, the producers carry out regular comprehensive self‐inspections, once a week. This inspection is performed by the nursery agronomists and according to the PPIS inspector's instructions. The results are recorded in the nursery logbook and every adverse finding is reported immediately to the inspector. The logbook is regularly reviewed during the inspector visits to the site.
Whenever a harmful organism of interest is found at any production site, the grower is required to inform PPIS and to treat the site as appropriate. According to Dossier Section 9.0, diseases have not been detected and reported in the cultivation of fig plants for export; a pest that is found time to time in the fig cultivation is Aphis gossypii, and additional insecticide treatment is applied on top of the regular insecticide cycle. During consecutive inspections, if there is no further evidence to the presence of the pest, the PPIS considers the site of production to be free from this harmful organism (Dossier Section 1.0).
Furthermore, virus‐like symptoms are taken into account during the phytosanitary inspection throughout the cultivation process. No virus problems have been reported in the cultivation of figs for export. Importantly, preventative insecticide treatment is regularly applied, which prevents the establishment of potential virus vectors in the nursery, too. In the case that such symptoms do occur, the management must include this in its report to the PPIS, and must sample for pest identification and destroy the symptomatic plants (Dossier Section 9.0).
Further diagnostic procedures may be performed according to requirements of the importing country and following inspection findings that necessitate identification of a causative agent.
Root samples with attached soil are tested for nematodes once during autumn both for bare rooted plants and liners. Sampling includes 10 plants from each field, and 10 soil samples per field that represent the entire production field area.
3.3.5. Post‐harvest processes and export procedure
The following information on the post‐harvest and export procedure was provided by PPIS of Israel (Dossier Section 1.0).
The bare rooted plants are rinsed with regular tap water (not amended with chemicals) in a designated machine (Dossier Section 9.0). The bare rooted plants are then soaked in Captan 0.5% and stored in chilled storage rooms at a temperature of 2°C and 70% humidity. The plants are transferred from the storage rooms directly to a reefer container which maintains 2–4°C. The container is loaded onto the ship and unloaded when with the customers in the EU, so that the refrigerated conditions are maintained throughout the shipment. The bare rooted plants are packed, after Captan has evaporated to dryness, in 180 μm nylon bags, ~ 30 plants per bag.
Concerning liners, these are extracted from plastic trays. Then, the substrate and plants are cleaned of any scraps. Finally, liners are packed in 60 μm nylon bags and placed in cardboard boxes (120 × 50 × 25 cm), 200 plants per box (Dossier Section 9.0). The Panel assumes that the rinsing does not apply in the case of liners.
Both bare rooted plants and liners are topped, cleaned of any plant scraps and dried plant parts, checked individually for selecting and grading and scanned for pest damage. A plant with obvious pest symptoms is destroyed. Plants with suspected symptoms are gathered in a designated place within the packing house, for further inspection with magnifying glasses and sampled for diagnosis, if needed, then destroyed based on findings.
Bare rooted plants and liners are exported to the EU during the months of January and February. Plants are delivered to nurseries located in the EU (Dossier Sections 1.0 and 9.0). These nurseries transfer the plants into larger pots and grow them to their desired product size for sale within the EU (Dossier Section 9.0).
4. Identification of pests potentially associated with the commodity
The search for potential pests associated with F. carica rendered 782 pests (see Microsoft Excel® file in Appendix E).
4.1. Selection of relevant EU quarantine pests associated with the commodity
The EU listing of EU quarantine pests and protected zone quarantine pests (Commission Implementing Regulation (EU) 2019/2072) is based on assessments concluding that the pests can enter, establish, spread and have potential impact in the EU.
Twenty‐five EU quarantine pests that are reported to use F. carica as a host plant were evaluated (see Table 4) for their relevance of being included in this opinion.
Table 4.
Overview of the evaluation of the 25 EU quarantine pests known to target Ficus carica as a host plant for their relevance for this opinion
| Number | Pest name according to EU legislationa | EPPO code | Group | Pest present in Israel | Ficus carica confirmed as a host (reference) | Pest can be associated with Bare Rooted Plantsb | Pest can be associated with Linersb | Pest relevant for the opinion |
|---|---|---|---|---|---|---|---|---|
| 1 | Anastrepha fraterculus | ANSTFR | Insects | No | Yes (CABI, online) | Not evaluated | Not evaluated | No |
| 2 | Anastrepha ludens | ANSTLU | Insects | No | Yes (EPPO, online) | Not evaluated | Not evaluated | No |
| 3 | Anastrepha suspensa | ANSTSU | Insects | No | Yes (CABI, online) | Not evaluated | Not evaluated | No |
| 4 | Anoplophora chinensis | ANOLCN | Insects | No | Yes (CABI, online) | Not evaluated | Not evaluated | No |
| 5 | Bactrocera zonata | DACUZO | Insects | Yes | Yes (CABI, online; EPPO, online) | No | No | No |
| 6 | Bemisia tabaci (non‐European populations) | BEMITA | Insects | Yes | Yes (EUROPHYT, online) | Noc | Noc | No |
| 7 | Diaphorina citri | DIAACI | Insects | No | Yes (EPPO, online) | Not evaluated | Not evaluated | No |
| 8 | Eotetranychus lewisi | EOTELE | Mites | No | Yes (EPPO, online) | Not evaluated | Not evaluated | No |
| 9 | Euwallacea fornicatus as Scolytidae spp. (non‐European) | XYLBFO | Insects | Yes | Yes (Cooperband et al., 2016) | Yes | Yes | Yes |
| 10 | Euwallacea interjectus as Scolytidae spp. (non‐European) | XYLBIN | Insects | No | Yes (Kajii et al., 2013) | Not evaluated | Not evaluated | No |
| 11 | Hypocryphalus scabricollis as Scolytidae spp. (non‐European) | CRYHSC | Insects | No | Yes (Gaaliche et al., 2018) | Not evaluated | Not evaluated | No |
| 12 | Hypothenemus leprieuri as Scolytidae spp. (non‐European) | HYOTLE | Insects | Yes | Yes (Mifsud et al., 2012) | Yes | Yes | Yes |
| 13 | Lopholeucaspis japonica | LOPLJA | Insects | No | Yes (García Morales et al., online) | Not evaluated | Not evaluated | No |
| 14 | Oemona hirta | OEMOHI | Insects | No | Yes (EPPO, online) | Not evaluated | Not evaluated | No |
| 15 | Phymatotrichopsis omnivora Synonym: Phymatotrichum omnivorum | PHMPOM | Fungi | No | Yes (CABI, online) | Not evaluated | Not evaluated | No |
| 16 | Pterandrus rosa Synonym: Ceratitis rosa | CERTRO | Insects | No | Yes (CABI, online; EPPO, online) | Not evaluated | Not evaluated | No |
| 17 | Ripersiella hibisci as Premnotrypes spp. (non‐European) | RHIOHI | Insects | No | Yes (EPPO, online) | Not evaluated | Not evaluated | No |
| 18 | Scirtothrips dorsalis | SCITDO | Insects | Yes | Yes (Dossier) | Yes | Yes | Yes |
| 19 | Spodoptera frugiperda | LAPHFR | Insects | Yes | Yes (Schmidt‐Durán et al., 2015) | No | Yes | Yes |
| 20 | Spodoptera litura | PRODLI | Insects | No | Yes (Robinson et al., online) | Not evaluated | Not evaluated | No |
| 21 | Thrips palmi | THRIPL | Insects | No | Yes (EPPO, online) | Not evaluated | Not evaluated | No |
| 22 | Xiphinema americanum sensu stricto | XIPHAA | Nematode | No | Yes (Ferris, online) | Not evaluated | Not evaluated | No |
| 23 | Xyleborus bispinatus as Scolytidae spp. (non‐European) | XYLBBI | Insects | No | Yes (Faccoli et al., 2016) | Not evaluated | Not evaluated | No |
| 24 | Xylella fastidiosa | XYLEFA | Bacteria | Yes | Yes (EPPO, online) | Yes | Yes | Nod |
| 25 | Zeugodacus cucurbitae Synonym: Bactrocera cucurbitae | DACUCU | Insects | No | Yes (CABI, online) | Not evaluated | Not evaluated | No |
Commission Implementing Regulation (EU) 2019/2072.
The question if the pest can be associated with the commodity is evaluated only if the questions on the presence in Israel and the association with F. carica were answered with ‘yes’.
Bemisia tabaci is associated with leaves, therefore it was not considered as a relevant pest, because the plants are imported without leaves.
Although both commodities can act as a pathway for X. fastidiosa the rating for association of the commodities as pathway is set to ‘No’ because F. carica plants for export are produced in officially approved pest‐free areas (Confirmed by PPIS in Dossier Section 2.0 and the relevant valid document can be found at the official website of the European Union in the section ‘Declarations from non‐EU countries concerning the status of X. fastidiosa’ using the following link https://ec.europa.eu/food/sites/food/files/plant/docs/ph_biosec_decl_xylella_isr_20190703.pdf).
The relevance of an EU quarantine pest for this opinion was based on evidence that:
the pest is present in Israel;
Ficus carica is a host of the pest;
one or more life stages of the pest can be associated with the specified commodity.
Pests that fulfilled all three criteria were selected for further evaluation.
Of the 25 EU quarantine pests evaluated, four pests (Euwallacea fornicatus, Hypothenemus leprieuri, Scirtothrips dorsalis and Spodoptera frugiperda) present in Israel and known to be associated with the commodities were selected for further evaluation (see Table 4).
Considering that the production nursery is located in a Xylella fastidiosa pest‐free area and Commission Implementing Regulation (EU) 2020/1201 indicates specific measures for X. fastidiosa, this pest was not considered for further assessment by EKE.
4.2. Selection of other relevant pests (not‐regulated in the EU) associated with the commodity
The information provided by PPIS of Israel, integrated with the search EFSA performed, was evaluated to assess whether there are other potentially relevant pests of F. carica present in the country of export. For these potential pests not regulated in the EU, pest risk assessment information on the probability of introduction, establishment, spread and impact is usually lacking. Therefore, these pests that are potentially associated with F. carica were also evaluated to determine their relevance for this opinion based on evidence that:
the pest is present in Israel;
the pest (i) is absent or (ii) has a limited distribution in the EU (no more than three EU Member States);
Ficus carica is a host of the pest;
one or more life stages of the pest can be associated with the specified commodity;
the pest may have an impact in the EU.
Pests that fulfilled all five criteria were selected for further evaluation.
Based on the information collected, 757 potential pests not regulated in the EU, known to be associated with F. carica were evaluated for their relevance to this opinion. Pests were excluded from further evaluation when at least one of the conditions listed above (1–5) was not met. Details can be found in the Appendix E (Microsoft Excel® file). Of the evaluated EU non‐regulated pests, six insects (Aonidiella orientalis, Icerya aegyptiaca, Nipaecoccus viridis, Phenacoccus solenopsis, Retithrips syriacus, Russellaspis pustulans), one mite (Oligonychus mangiferus), three fungi (Colletotrichum siamense, Neocosmospora euwallaceae and Neoscytalidium dimidiatum) and one plant (Plicosepalus acaciae) were selected for further evaluation because they met all of the selection criteria. More information on these 11 pests can be found in the pest datasheets (Appendix A).
Considering that the production nursery is located in a Maconellicoccus hirsutus pest‐free area (Dossier Section 9.0), the pest was not considered relevant for further assessment.
4.3. Overview of interceptions
Data on the interception of harmful organisms on plants of F. carica can provide information on some of the organisms that can be present on F. carica despite the proposed measures taken.
According to EUROPHYT online (Accessed: 6 December 2019), there were four interceptions of plants for planting and other living plants of F. carica from Israel due to the presence of harmful organisms (see Table 5) between the years 1995 and November 2019. Other three interceptions were from Iran and Tunisia (see Table 6). Two of these intercepted harmful organisms, Bemisia tabaci and species from genus Xiphinema, are EU quarantine pests.
Table 5.
Overview of harmful organisms intercepted on Ficus carica plants (excluding fruits) from Israel (1995 to November 2019), based on notifications of interceptions by EU Member States [based on EUROPHYT (online), Accessed: 6 December 2019]
| Name of harmful organism | Group | Intercepted on plants of F. carica | Total |
|---|---|---|---|
| Bemisia tabaci | Insect | plants for planting, already planted; other living plants | 3 |
| Pratylenchus | Nematode | plants for planting, already planted | 1 |
Table 6.
Overview of harmful organisms intercepted on Ficus carica plants (excluding fruits) from other countries than Israel (1995 to November 2019), based on notifications of interceptions by EU Member States [based on EUROPHYT (online), Accessed: 6 December 2019]
| Name of harmful organism | Group | Intercepted on plants of F. carica | Total |
|---|---|---|---|
| Bemisia tabaci | Insect | Plants for planting, already planted | 1 |
| Xiphinema sp. | Nematode | Plants for planting, not yet planted | 1 |
| Diaspididae | Insect | Plants for planting, others | 1 |
According to Dossier Section 9.0, around 7,000 fig plants per year over the last 10 years were exported and this is the future expectation, too.
4.4. List of potential pests not further assessed
From the list of pests not selected for further evaluation, the Panel highlighted three pests (see Appendix C) for which the currently available evidence provides no reason to select these pests for further evaluation in this opinion. The detailed reason is provided for each pest in Appendix C.
4.5. Summary of pests selected for further evaluation
The 15 pests identified to be present in Israel and considered to be reasonably likely to be associated with F. carica are listed in Table 7. For these selected pests, the proposed risk mitigation measures for the two commodities under consideration (i.e. bare rooted plants and liners) were evaluated.
Table 7.
List of relevant pests selected for further evaluation
| Number | Current scientific name | EPPO code | Name used in the EU legislation | Taxonomic information | Group | Regulatory status |
|---|---|---|---|---|---|---|
| 1 | Aonidiella orientalis | AONDOR | – | Hemiptera, Diaspididae | Insects | Not quarantine in the EU |
| 2 | Colletotrichum siamense | COLLSM | – | Phyllachorales, Glomerellaceae | Fungi | Not quarantine in the EU |
| 3 | Euwallacea fornicatus | XYLBFO | Scolytidae spp. (non‐European) | Coleoptera, Curculionidae, Scolytinae | Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 4 | Hypothenemus leprieuri | HYOTLE | Scolytidae spp. (non‐European) | Coleoptera, Curculionidae, Scolytinae | Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 5 | Icerya aegyptiaca | ICERAE | – | Hemiptera, Monophlebidae | Insects | Not quarantine in the EU |
| 6 | Neocosmospora euwallaceae | FUSAEW | – | Hypocreales, Nectriaceae | Fungi | Not quarantine in the EU |
| 7 | Neoscytalidium dimidiatum | HENLTO | – | Botryosphaeriales | Fungi | Not quarantine in the EU |
| 8 | Nipaecoccus viridis | NIPAVI | – | Hemiptera, Pseudococcidae | Insects | Not quarantine in the EU |
| 9 | Oligonychus mangiferus | – | – | Acarida, Tetranychidae | Mites | Not quarantine in the EU |
| 10 | Phenacoccus solenopsis | PHENSO | – | Hemiptera, Pseudococcidae | Insects | Not quarantine in the EU |
| 11 | Plicosepalus acaciae | – | – | Santalales, Loranthaceae | Plants | Not quarantine in the EU |
| 12 | Retithrips syriacus | RETTSY | – | Thysanoptera, Thripidae | Insects | Not quarantine in the EU |
| 13 | Russellaspis pustulans | ASTLPU | – | Hemiptera, Asterolecaniidae | Insects | Not quarantine in the EU |
| 14 | Scirtothrips dorsalis | SCITDO | Scirtothrips dorsalis | Thysanoptera, Thripidae | Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 15 | Spodoptera frugiperda * | LAPHFR | Spodoptera frugiperda | Lepidoptera, Noctuidae | Insects | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
The Panel is aware that S. frugiperda could not be included in the Dossier as the pest was discovered to be present in Israel very recently, after the submission of the Dossier. Nevertheless, the Panel evaluated the pest based on the procedures described in the Dossier.
5. Risk mitigation measures
The information used for the evaluation of the effectiveness of the risk mitigation measures is summarised in pest datasheets (see Appendix A).
5.1. Possibility of pest presence in the export nursery
For each pest, the Panel evaluated the likelihood that the pest could be present in a F. carica nursery by evaluating the possibility that F. carica in the export nursery are infected either by:
introduction of the pest (e.g. insects, spores) from the environment surrounding the nursery,
introduction of the pest with new plants/seeds,
spread of the pest within the nursery.
5.2. Risk mitigation measures proposed
The Dossier Section 1.0 contains information on the regulations and inspection systems related to the plant of interest (F. carica) where it has been reported:
-
–
The Law of Supervision of Plant and Plant Product Export – 1954, https://fs.knesset.gov.il//2/law/2_lsr_208430.PDF (In Hebrew, no English version).
-
–
The Israeli Plant and Plant Products Exportation Supervision Regulations – 1979, https://www.moag.gov.il/ppis/Laws/Regulation/Pages/1979-%20pikuah%20al%20yatzu.aspx (in Hebrew, no English version).
-
–
ISPM standards (adopted) https://www.ippc.int/en/core-activities/standards-setting/ispms/
-
–
General requirements as required by the Supervision Law – 1954: regulations about export of propagation material.
-
–
Specific official inspection and treatment can be carried out according to specific import requirements of the importing country.
-
–
Instructions for sampling of nematodes (internal).
-
–
Process for inspection of the nursery that exports plants, propagation material and ornamentals:
-
o
Growers wishing to export plants and propagation material (PPM) from Israel must be part of the PPM Export Programme.
-
o
This programme consists of three different subprogrammes: export to the EU, export to the USA and export of plant PPIS‐certified tissue culture.
-
o
PPIS has established a comprehensive overall system for the inspection of places of production for plants for planting. The system forms a part of a concept of inspection developed to ensure that the requirements of all importing countries are met. PPIS is responsible for the registration of producers of plants for planting, which is carried out via PPIS website.
-
o
-
–
Procedure for checking and approval of shipments for export of propagation material, https://www.moag.gov.il/Procedures/Documents/ishur_mishkochim_ribui.pdf (in Hebrew, no English version).
-
–
Procedure for issuance and application of phytosanitary certificates for plants and plant products, https://www.moag.gov.il/Procedures/Documents/hanpaka_teudot_briut_zmachim.pdf (in Hebrew, no English version).
-
–
Instructions for sampling of nematodes (internal).
In Dossier Section 9.0, a clarification is provided stating that ‘destruction of plants is common practice in preventative sanitation in fig plants. Cuttings that do not root or wither for any reason, e.g. lack of irrigation, are removed and destroyed. In the fig cultivation, no infection or contamination of plants has occurred that required decontamination.’
With the information provided by PPIS (Dossier Sections 1.0 and 9.0), the Panel summarised the risk mitigation measures (Table 8) that are proposed in the production nursery.
Table 8.
Overview of proposed risk mitigation measures for Ficus carica plants designated for export to the EU from Israel
| Risk mitigation measure | Implementation in Israel | |
|---|---|---|
| 1 | Characteristics of the production field | The crops designated for export, are grown in different fields from the crops designated for the local market.Bare rooted plants. Plants are grown either in soil in open fields or in commercial growing medium in sack containers in net house.Liners: Rooted cuttings in growing medium. Cultivated in the same commercial growing medium as above in pots in a net house.According to Dossier Section 9.0, the growing medium that is used for the exported fig products is always new at the beginning of the production cycle.According to Dossier Section 9.0, the net is designed for shading – 40% shade and the net house is not entirely sealed.The Dossier Section 9.0 states ‘The water that is used for irrigation is regular tap water, that goes through a 120‐mesh filter to remove rough dirt like sand and stones. Liners are irrigated by sprinklers, and bare rooted plants receive drip irrigation’. |
| 2 | Soil treatment | Summer – open field soil preparation – before a new crop cycle, the field is treated with solarisation. Dossier Section 9.0 clarifies that solarisation is performed by covering the soil with transparent polyethylene for 2 months – July and August (normally the time of highest radiation). The polyethylene sheet is spread after the soil has been cleaned from the previous crop and has been processed for the next cycle. The polyethylene in the sheets is supplemented with ‘antidrip’ or ‘antifog’ substances which prevents water condensation and accumulation on the sheet, so improving treatment efficacy against pests by raising the under‐sheet temperature by 4–5°C compared with regular polyethylene sheets. The maximum temperature in the top 20 cm of the soil is 44–48°C daily, for the duration of 2 months. The sheets are maintained clean and intact through the treatment duration, and the soil moisture is maintained to the field capacity level, by weekly irrigation with water. |
| 3 | Rotation of the growing fields | Rotation of the growing fields between different locations in the manner of a ‘growing cycle’. |
| 4 | Insecticide treatment | During the growing season, production fields and mother plants are treated in a 3‐week cycle with preventative treatments, i.e. rotation of the following pesticides in alternation: Atlas (Bifenthrin), Ipon (Dinotefuran), Imidan (Phosmet) and EOS (Eco Oil Spray). Each pesticide is used every 9 weeks, and 2 or 3 times per season. These substances were selected for being effective in preventing a range of insect pests, including borers, and are permitted for use in fig plants (Dossier Section 9.0). The Panel interprets that EOS is sprayed during winter and the remaining three pesticides are sprayed in alternation during the growing season.The Dossier Section 1.0 provides a further list of pesticides (Deltamethrin, Lambda cyhalothrin, Spinetoram and Cyhexatin), which are sprayed periodically in a preventative manner. However, they are not included in the above cycle of preventative treatments.The routine, preventative insecticide treatment scheme is sufficient to maintain the cultivated figs free of mealybugs. In the unlikely case that mealybug reproduction is detected in the figs, additional treatment with one of the routine insecticides may be provided (Dossier Section 9.0). |
| 5 | Fungicide treatment | The nursery treats the plants with appropriate fungicides following any early signs of fungal infection (Dossier Section 9.0), which are very rarely encountered in the nursery fig cultivation (Dossier Section 1.0).The Dossier Section 9.0 states further that before rooting cuttings are immersed in Merpan (Captan).Post‐harvest treatment: The bare rooted plants are rinsed and soaked in Captan 0.5% and stored at 2°C. The plants are packed after Captan has evaporated to dryness. |
| 6 | Nematicide treatment | Against nematodes: treatment with Nemakor (Fenamiphos) and Bacillus firmus. |
| 7 | Treatment against weeds | Weeds are treated with Faster (Glufosinate ammonium).According to Dossier Section 9.0, the nursery maintains appropriate sanitation measures to ensure that there are no non‐cultivated herbaceous plants in the vicinity of the cultivated fig plants, including the access areas. |
| 8 | Plant treatment before export | Bare rooted plants: December – lifting the bare rooted plants from the open field, washing the soil off the roots, selecting, grading and packing them in boxes. Bare rooted plants are washed with regular tap water (not amended with chemicals) in a designated machine and leaves are removed (Dossier Section 9.0). The commodity is then stored at 2°C. The Panel assumes that the bare rooted plants grown in commercial growing medium are handled in the same way.Liners: December – Packaging of liners. Liners have leaves removed, and the plant and substrate are cleaned of plant debris (Dossier Section 9.0).Dossier Section 9.0 clarifies that the plants arrive at the packing house after rinsing. Each plant is topped, cleaned of any plant scraps and dried plant parts, and scanned for pest damages. A plant with obvious pest symptoms is destroyed. Plants with suspected symptoms are gathered in a designated place within the packing house, for further inspection with magnifying glasses and sampled for diagnosis, if needed, then destroyed based on findings.The Panel assumes that rinsing applies only to the bare rooted plants. |
| 9 | Sampling and testing | Soil and root samples are tested for nematodes as described in Dossier Section 9.0.Root samples with attached soil are tested for nematodes once during the active growth, during autumn. Sampling includes 10 plants from each field, and 10 soil samples per field that represent the entire field area.A soil sample is taken per 0.5–1 hectare, consists of 5–7 sampling points that are 5–30 cm deep and contains roots.Bare rooted plants and liners are collected with their substrate, wrapped in moist paper and placed in nylon bags.If any necrosis, galls or malformations are seen, they should be included in the sample. |
| 10 | Inspections during the production | All fields are under the control and inspection of a PPIS inspector every 45 days during the growing and delivery season which include a review of the nursery logbook for any pest and management reports, and searching the net houses and fields for any disease symptoms, pests and pest signs, weeds and anything that may carry risk to the plants for export. Nevertheless, species specific inspection schemes are not applied (Dossier Section 9.0).All plants for planting exported from Israel originate from nurseries that are approved by PPIS and are under PPIS inspection.Further to the PPIS inspection, the producers carry out regular comprehensive self‐inspections, once a week. This inspection is performed by the nursery agronomists and according to the PPIS inspector's instructions. According to Dossier Section 9.0 virus‐like symptoms are taken into account during the phytosanitary inspection throughout the cultivation process. Small pests such as thrips and mites produce obvious symptoms that indicate activity of these pests, and the regular inspection seeks any such symptoms. Further to this, the fields are scanned in an X route, by which 50 leaves are lifted for detection of small pests. The PPIS inspector has a magnifying glass with which any suspicious symptoms can be magnified. In addition, the root system of plants is checked after removing the plants from the pot to identify pests, including mealybugs.Whenever a harmful organism of interest is found at any production site, the grower is required to inform the PPIS and to treat the site as appropriate. During consecutive inspections, if there is no further evidence of the presence of the pest, the PPIS considers the site of production to be free from this harmful organism.According to Dossier Section 9.0, destruction of plants is common practice in preventative sanitation in fig plants. Cuttings that do not root or wither for any reason, e.g. lack of irrigation, are removed and destroyed. In the fig cultivation, no infection or contamination of plants has occurred that required decontamination.Further diagnostic procedures may be performed according to requirements of the importing country and following inspection findings that necessitate identification of a causative agent.Additional information on the applied phytosanitary procedures in plants destined for export in Israel, can be found in the European Commission report of an audit performed in Israel in March 2018, on the export controls of plants. Report No. 2018‐6493. |
| 11 | Inspections before export | Before export the plants, both bare rooted plants and liners are checked individually for pest damages (see risk mitigation measure no 8). |
| 12 | Surveillance and monitoring | No information available on specific surveys in the natural environment or the surrounding environment of the production areas (i.e. inspections outside production fields). |
5.3. Evaluation of the proposed measures for the selected relevant pests including uncertainties
For each pest, the relevant risk mitigation measures acting on the pest were identified. Any limiting factors on the effectiveness of the measures were documented. All the relevant information including the related uncertainties deriving from the limiting factors used in the evaluation are summarised in a pest datasheet provided in Appendix A.
Based on this information, for each relevant pest, an expert judgement has been given for the likelihood of pest freedom of commodities taking into consideration the risk mitigation measures acting on the pest and their combination.
For a given pest, whenever the measures were expected to affect the likelihood of pest freedom for bare rooted plants and liners similarly, a common EKE was performed for both commodities. This means the assessed distribution is valid for the likelihood of pest freedom for bare rooted plants as well as for liners. Remaining differences are covered by the uncertainty.
If measures were expected to affect the likelihood of pest freedom for bare rooted plants and liners differently, two separated EKE were performed for the two commodities. The result of the assessment of BRP is described by the likelihood of pest freedom for BRP and the result of the assessment of liners is described by the likelihood of pest freedom for liners. The reasons to differentiate the distributions are described in the justification of the distributions for each pest in the Appendix A. An overview of the evaluation of each relevant pest is given in the sections below (Sections 5.3.1, 5.3.2, 5.3.3, 5.3.4, 5.3.5, 5.3.6, 5.3.7, 5.3.8, 5.3.9, 5.3.10, 5.3.11, 5.3.12, 5.3.13, 5.3.14). The outcome of EKE on pest freedom after the evaluation of the proposed risk mitigation measures is summarised in Section 5.3.15.
The explanation of pest freedom categories used to rate the likelihood of pest freedom in the Sections 5.3.1–5.3.14 is shown in Table 9.
Table 9.
Explanation of pest freedom categories used to rate the likelihood of pest freedom
| Pest freedom category | Pest‐free plants out of 10,000 |
|---|---|
| Sometimes pest free | < 5,000 |
| More often than not pest free | 5,000 to – < 9,000 |
| Frequently pest free | 9,000 to – < 9,500 |
| Very frequently pest free | 9,500 to – < 9,900 |
| Extremely frequently pest free | 9,900 to – < 9,950 |
| Pest free with some exceptional cases | 9,950 to – < 9,990 |
| Pest free with few exceptional cases | 9,990 to – < 9,995 |
| Almost always pest free | 9,995 to – 10,000 |
5.3.1. Overview of the evaluation of Aonidiella orientalis
| Overview of the evaluation of Aonidiella orientalis for bare rooted plants and liners | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free plants | 9,585 out of 10,000 plants | 9,815 out of 10,000 plants | 9,910 out of 10,000 plants | 9,964 out of 10,000 plants | 9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 6 out of 10,000 plants | 36 out of 10,000 plants | 90 out of 10,000 plants | 185 out of 10,000 plants | 415 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of A. orientalis between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy on the bark, which can be covered by leaves, the detection of crawlers during inspection and the lack of information on the density of the pest in the surrounding areas. | ||||
5.3.2. Overview of the evaluation of Colletotrichum siamense
| Overview of the evaluation of Colletotrichum siamense for bare rooted plants | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,932 out of 10,000 plants | 9,956 out of 10,000 plants | 9,973 out of 10,000 plants | 9,986 out of 10,000 plants | 9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 6 out of 10,000 plants | 14 out of 10,000 plants | 27 out of 10,000 plants | 44 out of 10,000 plants | 68 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pathogen has been reported from Israel and can be present around the nursery because suitable host plants may be present. The pathogen may enter into the nursery and spread within the nursery by means of airborne and water splashed spores. Colletotrichum spp. are known to be associated with nursery plants.Measures taken against the pest and their efficacyThe measures taken against the pest (fungicide treatments and inspections) could be effective, however symptoms have never been described on F. carica and this may hamper a prompt detection and the application of fungicides. Moreover, fungicide treatments are only applied if symptoms are observed.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of C. siamense between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe application of fungicides is based on symptoms. However, symptoms are not expressed in case of latent infections as observed in other plants species. The symptoms on F. carica have not been described yet. All these aspects may lead to shortcomings in the control.Main uncertaintiesThe level of susceptibility of F. carica to the pathogen is the main uncertainty together with the lack of information on the density of the pathogen in the surrounding areas. | ||||
| Overview of the evaluation of Colletotrichum siamense for liners | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,834 out of 10,000 plants | 9,890 out of 10,000 plants | 9,930 out of 10,000 plants | 9,960 out of 10,000 plants | 9,984 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 16 out of 10,000 plants | 40 out of 10,000 plants | 70 out of 10,000 plants | 110 out of 10,000 plants | 166 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pathogen has been reported from Israel and can be present around the nursery because suitable host plants may be present. The pathogen may entry into the nursery and spread within the nursery by means of airborne and water splashed spores. Colletotrichum spp. are known to be associated with nursery plants. Sprinkling of liners could favour infection and spread of the pathogen and the soil could become contaminated by infected leaves.Measures taken against the pest and their efficacyThe measures taken against the pest (fungicide treatments and inspections) could be effective, however symptoms have never been described on F. carica and this may hamper a prompt detection and the application of fungicides. Moreover, fungicide treatments are only applied if symptoms are observed.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of C. siamense between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe application of fungicides is based on symptoms. However, symptoms are not expressed in case of latent infections as observed in other plants species. The symptoms on F. carica have not been described yet. All these aspects may lead to shortcomings in the control.Main uncertaintiesThe level of susceptibility of F. carica to the pathogen is the main uncertainty together with the lack of information on the density of the pathogen in the surrounding areas. | ||||
5.3.3. Overview of the evaluation of Euwallacea fornicatus and Neocosmospora euwallaceae
| Overview of the evaluation of Euwallacea fornicatus and Neocosmospora euwallaceae for bare rooted plants | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,961 out of 10,000 plants | 9,981 out of 10,000 plants | 9,989 out of 10,000 plants | 9,994 out of 10,000 plants | 9,997 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 3 out of 10,000 plants | 6 out of 10,000 plants | 11 out of 10,000 plants | 19 out of 10,000 plants | 39 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodity Euwallacea fornicatus is present in Israel on different host plants with a high biotic potential, so it can spread to and within the nursery. Ficus carica is host plant for E. fornicatus although unclear whether reproductive or non‐reproductive. It can be colonised in the nursery although the diameter of the plants is at the lower limit for colonisation. Neocosmospora euwallaceae is present in Israel and can be transmitted by the insect.Measures taken against the pests and their efficacyThe measures taken against E. fornicatus (inspections and pesticide applications) have limited efficacy because the insect is difficult to detect in the early phase of the colonisation and because it lives protected within the wood. The measures taken against N. euwallaceae are not expected to be fully effective.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of E. fornicatus and N. euwallaceae between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. Rinsing of the bare rooted plants before inspection before export may remove the frass and therefore make the detection very difficult.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy against insects and fungi in the wood. Other uncertainties concern the lack of information on the density of the pests in the surrounding areas, the rinsing effect on bare rooted plants before inspection before export, the suitability of plant size for beetle colonisation. | ||||
| Overview of the evaluation of Euwallacea fornicatus and Neocosmospora euwallaceae for liners | |||||
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,976 out of 10,000 plants | 9,991 out of 10,000 plants | 9,996 out of 10,000 plants | 9,999 out of 10,000 plants | 10,000 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 0 out of 10,000 plants | 1 out of 10,000 plants | 4 out of 10,000 plants | 9 out of 10,000 plants | 24 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodity Euwallacea fornicatus is present in Israel on different host plants with a high biotic potential, so it can spread to and within the nursery. Ficus carica is host plant for E. fornicatus although it is unclear whether reproductive or non‐reproductive. It can be colonised in the nursery although the diameter of the plants is at the very lower limit for colonisation. Neocosmospora euwallaceae is present in Israel and can be transmitted by the insect.Measures taken against the pest and their efficacyThe measures taken against E. fornicatus (inspections and pesticide applications) have limited efficacy because the insect is difficult to detect in the early phase of the colonisation and because it lives protected within the wood. The measures taken against N. euwallaceae are not expected to be fully effective.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of E. fornicatus and N. euwallaceae between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy against insects and fungi in the wood. Other uncertainties concern the lack of information on the density of the pests in the surrounding areas and the suitability of plant size for beetle colonisation. | ||||
5.3.4. Overview of the evaluation of Hypothenemus leprieuri
| Overview of the evaluation of Hypothenemus leprieuri for bare rooted plants and liners | |||||
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,995 out of 10,000 plants | 9,996 out of 10,000 plants | 9,998 out of 10,000 plants | 9,999 out of 10,000 plants | 10,000 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 0 out of 10,000 plants | 1 out of 10,000 plants | 2 out of 10,000 plants | 4 out of 10,000 plants | 5 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present in Israel on F. carica so it can spread to and within the nursery. Ficus carica can be colonised in the nursery.Measures taken against the pest and their efficacyThe measures taken against the pest (inspections and pesticide applications) have limited efficacy because the insect is difficult to detect in the early phase of the colonisation and because it lives protected under the bark.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of H. leprieuri between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. Rinsing of the bare rooted plants before export inspection may remove the frass and therefore make the detection very difficult.Main uncertaintiesThe main uncertainties are related to the absence of scientific information on life history and impacts and on pesticide applications that may have limited efficacy against insects under the bark. Other uncertainties concern the lack of information on the density of the pest in the surrounding areas, the rinsing effect on bare rooted plants before inspection before export, the suitability of plant size for beetle colonisation. | ||||
5.3.5. Overview of the evaluation of Icerya aegyptiaca
| Overview of the evaluation of Icerya aegyptiaca for bare rooted plants and liners | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,855 out of 10,000 plants | 9,934 out of 10,000 plants | 9,967 out of 10,000 plants | 9,986 out of 10,000 plants | 9,998 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 2 out of 10,000 plants | 14 out of 10,000 plants | 33 out of 10,000 plants | 66 out of 10,000 plants | 145 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a host plant for the pest at the bark level and it can be colonised in the nursery.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective, although there could be issues related to reaching the scales when hidden in crevices or wax covered.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of I. aegyptiaca between the years 1995 and November 2019(EUROPHYT, online). Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy on the bark, which can be covered by leaves, and in crevices. Other uncertainties concern the detection of crawlers during inspection and the lack of information on the density of the pest in the surrounding areas. |
||||
5.3.6. Overview of the evaluation of Neoscytalidium dimidiatum
| Overview of the evaluation of Neoscytalidium dimidiatum for bare rooted plants | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,871 out of 10,000 plants | 9,919 out of 10,000 plants | 9,954 out of 10,000 plants | 9,979 out of 10,000 plants | 9,993 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 7 out of 10,000 plants | 21 out of 10,000 plants | 46 out of 10,000 plants | 81 out of 10,000 plants | 129 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pathogen has been reported from Israel and can be present around the nursery because several suitable host plants are present. The pathogen may entry into the nursery and spread within the nursery by means of conidia disseminated by water and wind. Neoscytalidium dimidiatum is known to be associated with nursery plants.Measures taken against the pest and their efficacyThe measures taken against the pest (fungicide treatments and inspections) could have limited effects. The fungus may be present endophytically hampering its detection. If symptoms are not expressed because the fungus is endophytically associated with the host, fungicides are not applied. In any case the application of fungicides may not be completely effective.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of N. dimidiatum between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe application of fungicides is based on early symptoms. Therefore, if the fungus is present endophytically the treatments are not carried out.Main uncertaintiesThe level of susceptibility of F. carica to the pathogen is the main uncertainty together with the lack of information on the density of the pathogen in the surrounding areas. | ||||
| Overview of the evaluation of Neoscytalidium dimidiatum for liners | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,833 out of 10,000 plants | 9,884 out of 10,000 plants | 9,930 out of 10,000 plants | 9,968 out of 10,000 plants | 9,992 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 8 out of 10,000 plants | 32 out of 10,000 plants | 70 out of 10,000 plants | 116 out of 10,000 plants | 167 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pathogen has been reported from Israel and can be present around the nursery because several suitable host plants are present. The pathogen may enter into the nursery and spread within the nursery by means of conidia disseminated by water and wind. Neoscytalidium dimidiatum is known to be associated with nursery plants. Sprinkling of liners could favour the spread of the fungus. The soil could get contaminated with plants debris, mainly woody, carrying pathogen inoculum.Measures taken against the pest and their efficacyThe measures taken against the pest (fungicide treatments and inspections) could have limited effects. The fungus may be present endophytically hampering its detection. If symptoms are not expressed because the fungus is endophytically associated with the host, fungicides are not applied. In any case, the application of fungicides may not be completely effective. Symptoms of root rots on roots may not be detected before export.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of N. dimidiatum between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe application of fungicides is based on early symptoms. Therefore, if the fungus is present endophytically the treatments are not carried out.Main uncertaintiesThe level of susceptibility of F. carica to the pathogen is the main uncertainty together with the lack of information on the density of the pathogen in the surrounding areas. | ||||
5.3.7. Overview of the evaluation of Nipaecoccus viridis
| Overview of the evaluation of Nipaecoccus viridis for bare rooted plants | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,844 out of 10,000 plants | 9,923 out of 10,000 plants | 9,958 out of 10,000 plants | 9,981 out of 10,000 plants | 9,995 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 5 out of 10,000 plants | 19 out of 10,000 plants | 42 out of 10,000 plants | 77 out of 10,000 plants | 156 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment, weed control and inspections) are efficient and effective, although there could be issues related to reaching the mealybugs when hidden in crevices or in soil.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of N. viridis between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The soil inspection for mealybugs is difficult to perform.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy on the bark, which can be covered by leaves, and in crevices/soil. Other uncertainties concern the detection of crawlers during inspection and the lack of information on the density of the pest in the surrounding areas. | ||||
| Overview of the evaluation of Nipaecoccus viridis for liners | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,740 out of 10,000 plants | 9,849 out of 10,000 plants | 9,919 out of 10,000 plants | 9,965 out of 10,000 plants | 9,990 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 10 out of 10,000 plants | 35 out of 10,000 plants | 81 out of 10,000 plants | 151 out of 10,000 plants | 260 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective, although there could be issues related to reaching the mealybugs when hidden in crevices or in soil.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of N. viridis between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The soil inspection for mealybugs is not carried out.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy on the bark, which can be covered by leaves, and in crevices/soil. Other uncertainties concern the detection of crawlers during inspection and the lack of information on the density of the pest in the surrounding areas. | ||||
5.3.8. Overview of the evaluation of Oligonychus mangiferus
| Overview of the evaluation of Oligonychus mangiferus for bare rooted plants and liners | |||||
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,732 out of 10,000 plants | 9,818 out of 10,000 plants | 9,897 out of 10,000 plants | 9,957 out of 10,000 plants | 9,992 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 8 out of 10,000 plants | 43 out of 10,000 plants | 103 out of 10,000 plants | 182 out of 10,000 plants | 268 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present in Israel on different host plants, so it can spread to and within the nursery. Ficus carica is host plant for the pest. It can be colonised in the nursery although the overwintering stage is unclear.Measures taken against the pest and their efficacyThe measures taken against the pest (inspections and pesticide applications) have high efficacy because the mite is exposed on the upper side of the leaves.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of O. mangiferus between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings.Main uncertaintiesThe main uncertainties are the scarce information on the life history, especially overwintering stages. Other uncertainties concern the lack of information on the density of the pest in the surrounding areas. Mites are difficult to detect on bark at low density. | ||||
5.3.9. Overview of the evaluation of Phenacoccus solenopsis
| Overview of the evaluation of Phenacoccus solenopsis for bare rooted plants | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,788 out of 10,000 plants | 9,900 out of 10,000 plants | 9,941 out of 10,000 plants | 9,965 out of 10,000 plants | 9,983 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 17 out of 10,000 plants | 35 out of 10,000 plants | 59 out of 10,000 plants | 100 out of 10,000 plants | 212 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment, weed control and inspections) are efficient and effective, although there could be issues related to reaching the mealybugs when hidden in crevices or in soil.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of P. solenopsis between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The root inspection for mealybugs is difficult to perform.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy on the bark, which can be covered by leaves, and in crevices/soil. Other uncertainties concern the detection of crawlers during inspection and the lack of information on the density of the pest in the surrounding areas. | ||||
| Overview of the evaluation of Phenacoccus solenopsis for liners | |||||
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,586 out of 10,000 plants | 9,768 out of 10,000 plants | 9,880 out of 10,000 plants | 9,948 out of 10,000 plants | 9,979 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 21 out of 10,000 plants | 52 out of 10,000 plants | 120 out of 10,000 plants | 232 out of 10,000 plants | 414 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective, although there could be issues related to reaching the mealybugs when hidden in crevices or in soil.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of P. solenopsis between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The root inspection for mealybugs is not carried out.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy on the bark, which can be covered by leaves, and in crevices/soil. Other uncertainties concern the detection of crawlers during inspection and the lack of information on the density of the pest in the surrounding areas. | ||||
5.3.10. Overview of the evaluation of Plicosepalus acaciae
| Overview of the evaluation of Plicosepalus acaciae for bare rooted plants and liners | |||||
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,991 out of 10,000 plants | 9,994 out of 10,000 plants | 9,997 out of 10,000 plants | 9,999 out of 10,000 plants | 10,000 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 0 out of 10,000 plants | 1 out of 10,000 plants | 3 out of 10,000 plants | 6 out of 10,000 plants | 9 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe mistletoe has been reported from Israel and can be present around the nursery because suitable host plants (Acacia) are present. In addition, the main vector of the mistletoe spectacled bulbul (Pycnonotus xanthopygos) is also present in the surrounding of the nursery. The size of the commodities is not limiting for an infection to occur.Measures taken against the pest and their efficacyThere are no specific measures taken against the mistletoe except general phytosanitary inspections. Inspection may allow the detection of the mistletoe although early infestation may be overlooked.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of P. acaciae between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe production of the commodities take place in open fields or in net houses which are intended for shading rather than protecting the production from animals.Main uncertaintiesThe main uncertainty is the presence of the mistletoe in the area and its level of association with nursery plants of F. carica. | ||||
5.3.11. Overview of the evaluation of Retithrips syriacus
| Overview of the evaluation of Retithrips syriacus for bare rooted plants | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,820 out of 10,000 plants | 9,887 out of 10,000 plants | 9,940 out of 10,000 plants | 9,975 out of 10,000 plants | 9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 6 out of 10,000 plants | 25 out of 10,000 plants | 60 out of 10,000 plants | 113 out of 10,000 plants | 180 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective, although there could be issues related to finding the thrips in soil.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of R. syriacus between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The soil inspection for thrips is difficult to perform.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy in cases of resistance development. Other uncertainties concern the lack of information on the density of the pest in the surrounding areas. | ||||
| Overview of the evaluation of Retithrips syriacus for liners | |||||
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,634 out of 10,000 plants | 9,747 out of 10,000 plants | 9,850 out of 10,000 plants | 9,930 out of 10,000 plants | 9,975 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 25 out of 10,000 plants | 70 out of 10,000 plants | 150 out of 10,000 plants | 253 out of 10,000 plants | 366 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective, although there could be issues related to finding the thrips in soil.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of R. syriacus between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The soil inspection for thrips is difficult to perform or is not performed in liners.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy in cases of resistance development. Other uncertainties concern the lack of information on the density of the pest in the surrounding areas. | ||||
5.3.12. Overview of the evaluation of Russellaspis pustulans
| Overview of the evaluation of Russellaspis pustulans for bare rooted plants and liners | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,585 out of 10,000 plants | 9,815 out of 10,000 plants | 9,910 out of 10,000 plants | 9,964 out of 10,000 plants | 9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 6 out of 10,000 plants | 36 out of 10,000 plants | 90 out of 10,000 plants | 185 out of 10,000 plants | 415 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants and can spread to and within the nursery. Ficus carica is a secondary host plant for the pest and it can be colonised in the nursery.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of R. pustulans between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy on the bark, which can be covered by leaves, detection of crawlers during inspection and the lack of information on the density of the pest in the surrounding areas. | ||||
5.3.13. Overview of the evaluation of Scirtothrips dorsalis
| Overview of the evaluation of Scirtothrips dorsalis for bare rooted plants | |||||
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,635 out of 10,000 plants | 9,766 out of 10,000 plants | 9,878 out of 10,000 plants | 9,954 out of 10,000 plants | 9,989 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 11 out of 10,000 plants | 46 out of 10,000 plants | 122 out of 10,000 plants | 234 out of 10,000 plants | 365 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants with a high biotic potential, so it can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective, although there could be issues related to finding the thrips in soil, including the commercial growing medium, in litter and in plant parts not exposed to pesticides.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of S. dorsalis between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The soil inspection for thrips is difficult to perform.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy in cases of resistance development. Other uncertainties concern the lack of information on the density of the pest in the surrounding areas. | ||||
| Overview of the evaluation of Scirtothrips dorsalis for liners | |||||
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,456 out of 10,000 plants | 9,606 out of 10,000 plants | 9,741 out of 10,000 plants | 9,855 out of 10,000 plants | 9,939 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 61 out of 10,000 plants | 145 out of 10,000 plants | 259 out of 10,000 plants | 394 out of 10,000 plants | 544 out of 10,000 plants |
| Summary of the information used for the evaluation | Possibility that the pest could become associated with the commodityThe pest is present around the nursery on different host plants with a high biotic potential, so it can spread to and within the nursery. Ficus carica is a well‐known host plant for the pest and the pest can be associated with the bark.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections) are efficient and effective, although there could be issues related to finding the thrips in the commercial growing medium, in litter and in plant parts not exposed to pesticides.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of S. dorsalis between the years 1995 and November 2019 (EUROPHYT, online).Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The soil inspection for thrips is difficult to perform or is not performed in liners.Main uncertaintiesThe main uncertainties are the pesticide applications that may have limited efficacy in cases of resistance development. Other uncertainties concern the lack of information on the density of the pest in the surrounding areas. | ||||
5.3.14. Overview of the evaluation of Spodoptera frugiperda
| Overview of the evaluation of Spodoptera frugiperda for liners | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,922 out of 10,000 plants | 9,961 out of 10,000 plants | 9,979 out of 10,000 plants | 9,990 out of 10,000 plants | 9,998 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 2 out of 10,000 plants | 10 out of 10,000 plants | 21 out of 10,000 plants | 39 out of 10,000 plants | 78 out of 10,000 plants |
| Summary of the information used for the evaluation | The Panel is aware that S. frugiperda could not be included in the Dossier as the pest was discovered to be present in Israel very recently, after the submission of the Dossier. Nevertheless, the Panel evaluated the pest based on the procedures described in the Dossier.Possibility that the pest could become associated with the commodityMissing data on the distribution of the pest in the surrounding and on the suitability of nursery fig plants to the pest result in a high level of uncertainties for infestation. Detection of the pest before the export is unlikely, because soil is not checked.Measures taken against the pest and their efficacyThe measures taken against the pest (pesticide treatment and inspections during the production) are efficient. However, inspection before export will fail in detecting the pest in the soil.Interception recordsIn the EUROPHYT database, there are no records of notification of F. carica plants for planting from Israel due to the presence of S. frugiperda between the years 1995 and November 2019 (EUROPHYT, online). However, the pest has been intercepted several times on other hosts.Shortcomings of current measures/proceduresThe fields designated for export are not isolated from other fields in the nursery and from the surroundings. The soil inspection for the pest is not performed.Main uncertaintiesThe main uncertainties concern the lack of information on the density of the pest in the surrounding areas. | ||||
5.3.15. Outcome of Expert Knowledge Elicitation
Table 10 and Figure 4 show the outcome of the EKE on pest freedom of bare rooted plants after the evaluation of the proposed risk mitigation measures for the selected pests.
Table 10.
Assessment of the likelihood of pest freedom following evaluation of proposed risk mitigation measures against selected relevant pests on Ficus carica bare rooted plants designated for export to the EU. In panel A, the median value for the assessed level of pest freedom for each pest is indicated by ‘M’, the 5% percentile is indicated by ‘L’ and the 95% percentile is indicated by ‘U’. The percentiles together span the 90% uncertainty range on pest freedom. The pest freedom categories are defined in panel B of the table
| No. | Group | Pest name | Sometimes pest free | More often than not pest free | Frequently pest free | Very frequently pest free | Extremely frequently pest free | Pest free with some exceptional cases | Pest free with few exceptional cases | Almost always pest free |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Insects | Aonidiella orientalis | L | M | U | |||||
| 2 | Fungi | Colletotrichum siamense | L | M | U | |||||
| 3, 4 | Insects/Fungi | E. fornicatus and N. euwallaceae | LM | U | ||||||
| 5 | Insects | Hypothenemus leprieuri | LMU | |||||||
| 6 | Insects | Icerya aegyptiaca | L | M | U | |||||
| 7 | Fungi | Neoscytalidium dimidiatum | L | M | U | |||||
| 8 | Insects | Nipaecoccus viridis | L | M | U | |||||
| 9 | Mites | Oligonychus mangiferus | LM | U | ||||||
| 10 | Insects | Phenacoccus solenopsis | L | M | U | |||||
| 11 | Plants | Plicosepalus acaciae | L | MU | ||||||
| 12 | Insects | Retithrips syriacus | L | M | U | |||||
| 13 | Insects | Russellaspis pustulans | L | M | U | |||||
| 14 | Insects | Scirtothrips dorsalis | LM | U | ||||||
| PANEL A | ||||||||||
| Pest freedom category | Pest free plants out of 10,000 | Legend of marked pest freedom categories | ||
|---|---|---|---|---|
| Sometimes pest free | < 5,000 | L | Pest freedom category includes the elicited lower bound of the 90% uncertainty range | |
| More often than not pest free | 5,000 to – < 9,000 | |||
| Frequently pest free | 9,000 to – < 9,500 | M | Pest freedom category includes the elicited median | |
| Very frequently pest free | 9,500 to – < 9,900 | U | Pest freedom category includes the elicited upper bound of the 90% uncertainty range | |
| Extremely frequently pest free | 9,900 to – < 9,950 | |||
| Pest free with some exceptional cases | 9,950 to – < 9,990 | |||
| Pest free with few exceptional cases | 9,990 to – < 9,995 | |||
| Almost always pest free | 9,995 to – 10,000 | |||
| PANEL B | ||||
Figure 4.
Elicited certainty (y‐axis) of the number of pest free Ficus carica bare rooted plants (x‐axis; log‐scaled) out of 10,000 bare rooted plants designated for export to the EU introduced from Israel for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
Table 11 and Figure 5 show the outcome of the EKE on pest freedom of liners after the evaluation of the proposed risk mitigation measures for the selected pests.
Table 11.
Assessment of the likelihood of pest freedom following evaluation of proposed risk mitigation measures against selected relevant pests on Ficus carica liners designated for export to the EU. In panel A, the median value for the assessed level of pest freedom for each pest is indicated by ‘M’, the 5% percentile is indicated by ‘L’ and the 95% percentile is indicated by ‘U’. The percentiles together span the 90% uncertainty range on pest freedom. The pest freedom categories are defined in panel B of the table
| No. | Group | Pest name | Sometimes pest free | More often that not pest free | Frequently pest free | Very frequently pest free | Extremely frequently pest free | Pest free with some expectational cases | Pest free with few expectational cases | Almost always pest free |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Insects | Aonidiella orientalis | L | M | U | |||||
| 2 | Fungi | Colletotrichum siamense | L | M | U | |||||
| 3, 4 | Insects/Fungi | E. fornicatus and N. euwallaceae | L | MU | ||||||
| 5 | Insects | Hypothenemus leprieuri | LMU | |||||||
| 6 | Insects | Icerya aegyptiaca | L | M | U | |||||
| 7 | Fungi | Neoscytalidium dimidiatum | L | M | U | |||||
| 8 | Insects | Nipaecoccus viridis | L | M | U | |||||
| 9 | Mites | Oligonychus mangiferus | LM | U | ||||||
| 10 | Insects | Phenacoccus solenopsis | LM | U | ||||||
| 11 | Plants | Plicosepalus acaciae | L | MU | ||||||
| 12 | Insects | Retithrips syriacus | LM | U | ||||||
| 13 | Insects | Russellaspis pustulans | L | M | U | |||||
| 14 | Insects | Scirtothrips dorsalis | L | M | U | |||||
| 15 | Insects | Spodoptera frugiperda | L | M | U |
| PANEL A | ||||
|---|---|---|---|---|
| Pest freedom category | Pest free plants out of 10,000 | Legend of marked pest freedom categories | ||
| Sometimes pest free | < 5,000 | L | Pest freedom category includes the elicited lower bound of the 90% uncertainty range | |
| More often than not pest free | 5,000 to – < 9,000 | |||
| Frequently pest free | 9,000 to – < 9,500 | M | Pest freedom category includes the elicited median | |
| Very frequently pest free | 9,500 to – < 9,900 | U | Pest freedom category includes the elicited upper bound of the 90% uncertainty range | |
| Extremely frequently pest free | 9,900 to – < 9,950 | |||
| Pest free with some exceptional cases | 9,950 to – < 9,990 | |||
| Pest free with few exceptional cases | 9,990 to – < 9,995 | |||
| Almost always pest free | 9,995 to – 10,000 | |||
| PANEL B | ||||
Figure 5.
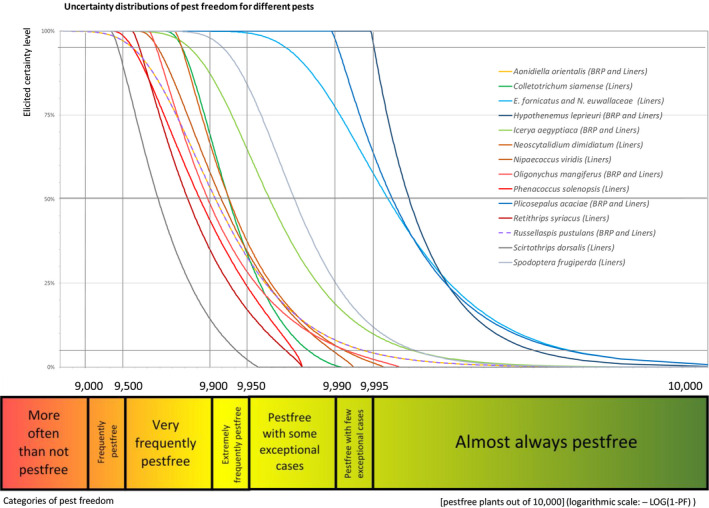
Elicited certainty (y‐axis) of the number of pest‐free Ficus carica liners (x‐axis; log‐scaled) out of 10,000 liners designated for export to the EU introduced from Israel for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
Figure 6 provides an explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the proposed risk mitigation measures for bare rooted plants and liners designated for export to the EU based on the example of Aonidiella orientalis.
Figure 6.
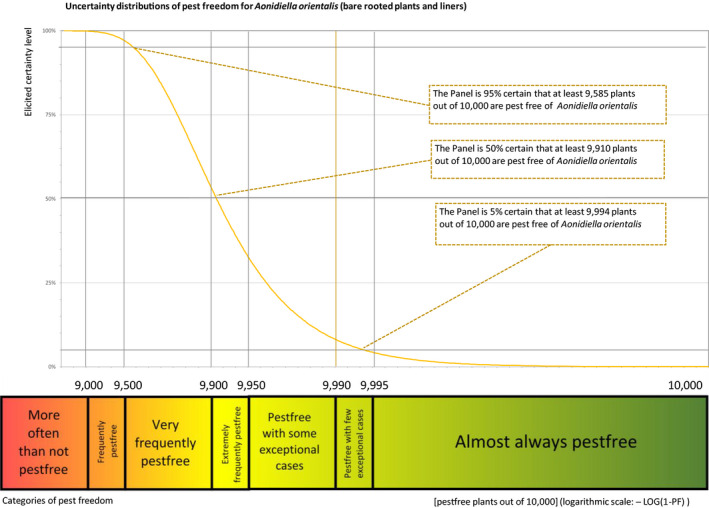
Explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the proposed risk mitigation measures for bare rooted plants and liners designated for export to the EU based on the example of Aonidiella orientalis
The Panel is 95% sure that:
-
–
9,585 or more bare rooted plants per 10,000 will be free from Aonidiella orientalis,
-
–
9,585 or more bare rooted plants per 10,000 will be free from Russellaspis pustulans,
-
–
9,635 or more bare rooted plants per 10,000 will be free from Scirtothrips dorsalis,
-
–
9,732 or more bare rooted plants per 10,000 will be free from Oligonychus mangiferus,
-
–
9,788 or more bare rooted plants per 10,000 will be free from Phenacoccus solenopsis,
-
–
9,820 or more bare rooted plants per 10,000 will be free from Retithrips syriacus,
-
–
9,844 or more bare rooted plants per 10,000 will be free from Nipaecoccus viridis,
-
–
9,855 or more bare rooted plants per 10,000 will be free from Icerya aegyptiaca,
-
–
9,871 or more bare rooted plants per 10,000 will be free from Neoscytalidium dimidiatum,
-
–
9,932 or more bare rooted plants per 10,000 will be free from Colletotrichum siamense,
-
–
9,961 or more bare rooted plants per 10,000 will be free from Euwallacea fornicatus and Neocosmospora euwallaceae,
-
–
9,991 or more bare rooted plants per 10,000 will be free from Plicosepalus acaciae,
-
–
9,995 or more bare rooted plants per 10,000 will be free from Hypothenemus leprieuri.
The Panel is 95% sure that:
-
–
9,456 or more liners per 10,000 will be free from Scirtothrips dorsalis,
-
–
9,585 or more liners per 10,000 will be free from Aonidiella orientalis,
-
–
9,585 or more liners per 10,000 will be free from Russellaspis pustulans,
-
–
9,586 or more liners per 10,000 will be free from Phenacoccus solenopsis,
-
–
9,634 or more liners per 10,000 will be free from Retithrips syriacus,
-
–
9,732 or more liners per 10,000 will be free from Oligonychus mangiferus,
-
–
9,740 or more liners per 10,000 will be free from Nipaecoccus viridis,
-
–
9,833 or more liners per 10,000 will be free from Neoscytalidium dimidiatum,
-
–
9,834 or more liners per 10,000 will be free from Colletotrichum siamense,
-
–
9,855 or more liners per 10,000 will be free from Icerya aegyptiaca,
-
–
9,922 or more liners per 10,000 will be free from Spodoptera frugiperda,
-
–
9,976 or more liners per 10,000 will be free from Euwallacea fornicatus and Neocosmospora euwallaceae,
-
–
9,991 or more liners per 10,000 will be free from Plicosepalus acaciae,
-
–
9,995 or more liners per 10,000 will be free from Hypothenemus leprieuri.
5.4. Evaluation of the application of specific measures
Commission Implementing Regulation 2019/2072, Annex VII, Point 1 specifies special requirements for import of growing medium, attached to or associated with plants, intended to sustain the vitality of the plants. Based on the information provided in the Dossier, the use of new growing medium (Klasmann‐Deilmann GmbH or Kekkila professional peat substrate) may fulfil the requirements specified in Annex VII, Item 1, (a) (ii). The requirements of Item 1 (b) (i) are partially fulfilled in that the water used for irrigation is regular tap water, that goes through a 120‐mesh filter (see Table 8 for description of the implemented mitigation measures). The Panel does not have sufficient information in the Dossier or additional information to make any statement about physical isolation and hygiene measures.
Commission Implementing Regulation (EU) 2020/1201 specifies measures to prevent the introduction into and the spread within the EU of Xylella fastidiosa (Wells et al.).
Specific measures regarding X. fastidiosa which are in place for the import of F. carica plants from Israel are specified in the Article 29 of the Commission Implementing Regulation (EU) 2020/1201, which allows introduction into the Union of host plants originating in a pest‐free area of an infected country.
Based on the information provided in the Dossier including the additional information provided by PPIS of Israel on 14 June 2020, after EFSA's request, the commodities under consideration (i.e. bare rooted plants and liners) are declared to be produced in a pest‐free area. This meets partly the requirements specified in point (a) and (b) of Commission Implementing Regulation (EU) 2020/1201. Details of the surveillance sampling scheme were not provided in the Dossier. The National Plant Protection Organisation of Israel has communicated in writing to the Commission the name of that area. The relevant document can be found at the official website of the European Union in the section ‘Declarations from non‐EU countries concerning the status of Xylella fastidiosa’ using the following link https://ec.europa.eu/food/sites/food/files/plant/docs/ph_biosec_decl_xylella_isr_20190703.pdf.
For other measures considered in point (a) and other points of the Implementing Regulation, the Panel does not have sufficient information in the Dossier or the additional information to make any statement. The Panel is aware that the current implemented regulation was adopted after the initial Dossier submission by Israel and after the reply to the request for additional information by EFSA.
6. Conclusions
There are 15 pests relevant for this opinion, of which 14 are associated with both bare rooted plants and liners of F. carica (Aonidiella orientalis, Colletotrichum siamense, Euwallacea fornicatus, Hypothenemus leprieuri, Icerya aegyptiaca, Neocosmospora euwallaceae, Neoscytalidium dimidiatum, Nipaecoccus viridis, Oligonychus mangiferus, Phenacoccus solenopsis, Plicosepalus acaciae, Retithrips syriacus, Russellaspis pustulans, Scirtothrips dorsalis) and one (Spodoptera frugiperda) only with liners. The Panel is aware that S. frugiperda could not be included in the Dossier as the pest was discovered to be present in Israel very recently, after the submission of the Dossier. Nevertheless, the Panel evaluated the pest based on the procedures described in the Dossier.
For these pests, the likelihood of the pest freedom after the evaluation of the proposed risk mitigation measures relevant for the specific commodity of F. carica designated for export to the EU was estimated.
For Aonidiella orientalis, the likelihood of pest freedom for bare rooted plants and liners following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,585 and 10,000 plants per 10,000 will be free from A. orientalis.
For Colletotrichum siamense, the likelihood of pest freedom for bare rooted plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,932 and 10,000 bare rooted plants per 10,000 will be free from C. siamense. The likelihood of pest freedom for liners was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,834 and 10,000 liners per 10,000 will be free from C. siamense.
For Euwallacea fornicatus and Neocosmospora euwallaceae, the likelihood of pest freedom for bare rooted plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,961 and 10,000 bare rooted plants per 10,000 will be free from E. fornicatus and N. euwallaceae. The likelihood of pest freedom for liners was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,976 and 10,000 liners per 10,000 will be free from E. fornicatus and N. euwallaceae.
For Hypothenemus leprieuri, the likelihood of pest freedom for bare rooted plants and liners following evaluation of proposed risk mitigation measures was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘almost always pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,995 and 10,000 plants per 10,000 will be free from H. leprieuri.
For Icerya aegyptiaca, the likelihood of pest freedom for bare rooted plants and liners following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,855 and 10,000 plants per 10,000 will be free from I. aegyptiaca.
For Neoscytalidium dimidiatum, the likelihood of pest freedom for bare rooted plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,871 and 10,000 bare rooted plants per 10,000 will be free from N. dimidiatum. The likelihood of pest freedom for liners was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,833 and 10,000 liners per 10,000 will be free from N. dimidiatum.
For Nipaecoccus viridis, the likelihood of pest freedom for bare rooted plants following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,844 and 10,000 bare rooted plants per 10,000 will be free from N. viridis. The likelihood of pest freedom for liners was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,749 and 10,000 liners per 10,000 will be free from N. viridis.
For Oligonychus mangiferus, the likelihood of pest freedom for bare rooted plants and liners following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,732 and 10,000 plants per 10,000 will be free from O. mangiferus.
For Phenacoccus solenopsis, the likelihood of pest freedom for bare rooted plants following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,788 and 10,000 bare rooted plants per 10,000 will be free from P. solenopsis. The likelihood of pest freedom for liners was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,586 and 10,000 liners per 10,000 will be free from P. solenopsis.
For Plicosepalus acaciae, the likelihood of pest freedom for bare rooted plants and liners following evaluation of proposed risk mitigation measures was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with few exceptional cases’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,991 and 10,000 plants per 10,000 will be free from P. acaciae.
For Retithrips syriacus, the likelihood of pest freedom for bare rooted plants following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,820 and 10,000 bare rooted plants per 10,000 will be free from R. syriacus. The likelihood of pest freedom for liners was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,634 and 10,000 liners per 10,000 will be free from R. syriacus.
For Russellaspis pustulans, the likelihood of pest freedom for bare rooted plants and liners following evaluation of proposed risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,585 and 10,000 plants per 10,000 will be free from R. pustulans.
For Scirtothrips dorsalis, the likelihood of pest freedom for bare rooted plants following evaluation of proposed risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,635 and 10,000 bare rooted plants per 10,000 will be free from S. dorsalis. The likelihood of pest freedom for liners was estimated as ‘very frequently pest free’ with the 90% uncertainty range spanning from ‘frequently pest free’ to ‘extremely frequently pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,456 and 10,000 liners per 10,000 will be free from S. dorsalis.
For Spodoptera frugiperda, the likelihood of pest freedom for liners following evaluation of proposed risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,922 and 10,000 liners per 10,000 will be free from S. frugiperda.
Glossary
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017).
- Measures
Control (of a pest) is defined in ISPM 5 (FAO 2017) as ‘Suppression, containment or eradication of a pest population’ (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate Risk Reduction Options that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017).
- Protected zones (PZ)
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017).
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017).
- Risk mitigation measure
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A risk mitigation measure may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017).
Abbreviations
- BRP
Bare rooted plants
- CABI
Centre for Agriculture and Bioscience International
- EKE
Expert Knowledge Elicitation
- EOS
Eco Oil Spray
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- ISPM
International Standards for Phytosanitary Measures
- NPPO
National Plant Protection Organisation
- PLH
Plant Health
- PPIS
Israel Ministry of Agriculture and Rural Development, Plant Protection and Inspection Services
- PPM
Plants and Propagation Material
- PRA
Pest Risk Assessment
- RNQPs
Regulated Non‐Quarantine Pests
Appendix A – Datasheets of pests selected for further evaluation via Expert Knowledge Elicitation
A.1. Aonidiella orientalis
A.1.1. Organism information
| Taxonomic information | Current valid scientific name: Aonidiella orientalisSynonyms: Aonidiella cocotiphagus, Aonidiella taprobana, Aspidiotus cocotiphagus, Aspidiotus orientalis, Aspidiotus osbeckiae, Aspidiotus pedronis, Aspidiotus taprobanus, Chrysomphalus orientalis, Chrysomphalus pedroniformis, Chrysomphalus pedronis, Evaspidiotus orientalis, Furcaspis orientalisName used in the EU legislation: –Order: HemipteraFamily: DiaspididaeCommon name: oriental yellow scale, oriental red scale, oriental scale, cochineal scaleName used in the Dossier: Aonidiella orientalis |
| Group | Insects |
| EPPO code | AONDOR |
| Regulated status | Aonidiella orientalis is not regulated in the EU neither is listed by EPPO.The pest is quarantine in Morocco (EPPO, online). |
| Pest status in Israel | Present in Israel (CABI, online; García Morales et al., online). It has been reported as a mango pest in Israel (Wysoki et al., 1993).The pest was first recorded at the Arava valley (from the Gulf of Elat to the Dead sea), in the south of Israel (Ben‐Dov, 1985). Over the years the pest spread to the north of the country where it was found around Lake Kinneret (Sea of Galilee) and, as reviewed by Wysoki et al. (1993) is now widely distributed in Israel. |
| Pest status in the EU | Absent in the EU (CABI, online; García Morales et al., online). In 2013, it was collected on leaves of Cocos nucifera in the Botanical Garden of Padova, in Italy (Pellizzari and Porcelli, 2014). |
| Host status on Ficus carica | Ficus carica is a host to A. orientalis (García Morales et al., online). |
| PRA information | Pest Risk Assessments available from EFSA:
|
| Other relevant information for the assessment | |
| Biology | Aonidiella orientalis is an armoured scale, which originates from Oriental region and it is now widely distributed in tropical countries (Waterhouse and Sands, 2001).Aonidiella orientalis reproduces sexually; adult females probably produce species‐specific sex pheromone to attract adult males (Naturalis Biodiversity Center, online). Parthenogenetic and viviparous forms of reproduction were also observed (Wagner et al., 2008). Aonidiella orientalis can have from three generations (in India) up to six generations (in Australia) each year (Naturalis Biodiversity Center, online; Waterhouse and Sands, 2001).Females and males develop through four life stages: an egg, two larval instars and an adult. The larval instars of males are called pre‐pupa and pupa. Adult males have wings and females are wingless (Waterhouse and Sands, 2001).As reviewed by Elder and Smith (1995), males need ~ 19.5 days to develop from the crawler stage to adult at 25°C, while females need on average 44 days from the crawler stage to production of the first crawler of the subsequent generation at the same temperatureFemales can lay about 200 eggs in a generation (Waterhouse and Sands, 2001). They are protected by waxy covering (Wagner et al., 2008). After hatching, the larvae (first‐instar crawlers) migrate to settle on the leaves, fruits and stems of the host plant where they remain until maturity. Crawlers may be carried to neighbouring plants by wind (Waterhouse and Sands, 2001) or by hitchhiking on clothing, equipment or animals (Leathers, 2016).According to Hennessey et al. (2013), the percentage of crawlers settling on a tree from an infested fruit is higher when the infested commodity (e.g. a fruit) is in contact with the tree than when it is placed 2 m away. Most of the stages of A. orientalis remain attached to a host during most of their lives. The only mobile stage is the first instar‐nymph (i.e. crawler stage), but it is not considered to be a good coloniser of new environments because it is small, fragile, not able to fly and slow in movements (Hennessey et al., 2013). Additionally, crawlers tend to remain and feed on plants close to the one they hatched on. |
| Symptoms | Main type of symptoms | Main symptoms are yellowing or death of the leaves and consequent defoliation, dieback of twigs, fruit discoloration and early drop (Rajagopal and Krishnamoorthy, 1996).Due to the pest feeding on leaves, characteristic chlorotic streaks, depressions, discoloration and distortion of leaves can be observed. Plant vigour is reduced (CABI, online).Heavy infestations cause drying of leaves and give the tree a burnt appearance. The seeds quantity and quality are also affected (Ensaf et al., 2016). |
| Presence of asymptomatic plants | Plant damage might not be obvious in early infestation, but the presence of scales on the plants could be observed. | |
| Confusion with other pathogens/pests | Aonidiella orientalis belongs to a group of many similar species not easy to be distinguished. These include A. aurantii, A. comperei, A. eremocitri, A. inornate, A. citrina and A. taxus (EPPO, 2005). A microscope observation is needed for identification. |
| Host plant range | Aonidiella orientalis is a polyphagous pest with a wide host range, including ~ 74 families and 163 genera (García Morales et al., online) except conifers. A. orientalis is reported as a host of Persian silk tree (Albizia julibrissin), Bottle brush (Callistemon lophanthus), apple of sodom (Calotropis procera), locust bean (Ceratonia siliqua), citrus (Citrus spp.), coconut (Cocos nucifera), sebesten (Cordia myxa), North Indian rosewood (Dalbergia sissoo), fig (Ficus spp.), crape myrtle (Lagerstroemia indica), mango (Mangifera indica), sapodilla (Manilkara zapota), white mulberry (Morus alba), banana (Musa sapientum), common myrtle (Myrtus communis), nerium (Nerium oleander), date palm (Phoenix dactylifera), Ghaf (Prosopis spicigera), common guava (Psidium guajava), pomegranate (Punica granatum), willow (Salix spp.), clove (Syzygium aromaticum), tamarind (Tamarindus indica), tamarisk (Tamarix indica), Christ's thorn jujube (Ziziphus spina‐christi) (García Morales et al., online; Moghaddam, 2013).It has been described as an economically important pest due to damage on citrus, fig, mango, papaya, bananas and palm trees. In Israel, it has been reported as a serious pest of mango (Wysoki et al., 1993). |
| Pathways | Possible pathways of entry for A. orientalis are plants for planting and fruits.The pest is mainly found on leaves, but in heavy infestations also on branches, trunks, shoots and fruits of the host plants (CABI, online) where all life stages can be found.The dispersal of the crawlers may also occur on cloths of orchard workers and tools (Hennessey et al., 2013) or associated with air currents or winds. |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. |
A.1.2. Possibility of pest presence in the nursery
A.1.2.1. Possibility of entry from the surrounding environment
Aonidiella orientalis is widely distributed in Israel, mainly in mango production areas (Wysoki et al., 1993). If mango or any other host plant is grown in the neighbourhood of the export nursery transfer of the insect may be possible.
The main dispersal stage is the first (crawling) instar, which can be dispersed naturally by wind (Waterhouse and Sands, 2001) or by hitchhiking on clothing, equipment or animals (Leathers, 2016). After selecting a feeding site, the scale becomes sessile and no further dispersal occurs. Crawlers tend to remain and feed on plants close to the one they hatched on. Human activities can facilitate a long‐distance dispersal of the crawlers (Hennessey et al., 2013).
In the Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Diospyros, Punica granatum, Populus alba, Populus euphratica, Acacia saligna, Eucalyptus and Ricinus communis are hosts of A. orientalis (García Morales et al., online).
Uncertainties:
-
–
There are uncertainties about the presence and population pressure of the pest in the areas surrounding the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by wind and human accidental transportation.
A.1.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located within the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media. Plants are produced inside the nursery and the scale insects are not associated with soil growing media.
A.1.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
According to the Dossier Section 9.0, following plants known to be hosts of the pest are grown in the fig liner export nursery: Lagerstroemia indica and Morus alba, with a distance of a few dozens of metres between them and the fig liners.
Therefore, it is possible for A. orientalis to reproduce within the nursery on F. carica and other host plants, which are present.
The dispersal within the nursery may be due to passive transportation by workers and tools and can be also caused by air currents.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible either by wind or accidental transfer within the nursery.
A.1.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of A. orientalis between the years 1995 and November 2019 (EUROPHYT, online).
Since 1996 A. orientalis has been intercepted several times in the Great Britain, mostly on imported mango and guava fruits and recorded also in a greenhouse on Dictyosperma and Cocos (Pellizzari and Porcelli, 2014).
A.1.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on A. orientalis is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:
|
The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:
|
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Pesticide sprays are generally effective against crawlers and less effective against the fixed stages of A. orientalis because of the wax covering of its body. Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty if the pesticide can effectively reach all the bark parts where the scales are located because of the barrier effect of the leaves. | Pesticide sprays are generally effective against crawlers and less effective against the fixed stages of A. orientalis because of the wax covering of its body. Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty if the pesticide can effectively reach all the bark parts where the scales are located because of the barrier effect of the leaves. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | Yes | Rinsing of the plants is not removing the pest.Scales can be easily found during inspection with magnifying glasses, which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. | Cleaning of plant debris is not removing the pest.Scales can be easily found during inspection with magnifying glasses, which is triggered by the observation of suspected symptoms.Uncertainties: – There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | A. orientalis are generally detectable except at the crawler stage.Uncertainties: – There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. | A. orientalis are generally detectable except at the crawler stage.Uncertainties: – There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. |
| 11 | Inspections before export | Yes | Scales can be easily found during inspection with magnifying glasses, which is triggered by the observation of suspected symptoms.Uncertainties: – There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. | Scales can be easily found during inspection with magnifying glasses, which is triggered by the observation of suspected symptoms.Uncertainties: – There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented, however A. orientalis is common in Israel.Uncertainties: – There is no information on the density of A. orientalis in the surrounding areas. | Surveillance in the surrounding area is not implemented, however A. orientalis is common in Israel.Uncertainties: – There is no information on the density of A. orientalis in the surrounding areas. |
A.1.5. Overall likelihood of pest freedom for bare rooted plants and liners
A.1.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants and liners
Although A. orientalis is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to wind and human activity. Inspections are expected to be effective because sessile stages of the insect are visible. Ficus carica is deemed as a minor host. Insecticide treatments are expected to be conducted at the right timing to target unprotected life stages of the insect. Mother plants are kept healthy as well by using treatments.
A.1.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants and liners
Aonidiella orientalis is widespread in Israel, the scenario assumes a high pest pressure from outside and strong transfer from the surrounding due to wind and intensive human activity. Inspections are expected to be ineffective because of the presence of hidden stages. Ficus carica is deemed as a major host. Insecticide treatments are expected to be conducted at timing when the insect is protected by wax. Mother plants are infested despite treatments and may contribute spreading the pest within the nursery.
A.1.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants and liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by wind and human activity, the weak information on the degree of susceptibility of F. carica, the internal spread and the absence of reported problems within the nursery and at EU borders, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.1.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, and unclear host suitability of F. carica, it results in high level of uncertainties for infestation rates below the median. Otherwise, detection of the pest especially before the export is likely, which gives less uncertainties for rates above the median.
A.1.5.5. Elicitation outcomes of the assessment of the pest freedom for Aonidiella orientalis on bare rooted plants and liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.1) and pest freedom (Table A.2).
Table A.1.
Elicited and fitted values of the uncertainty distribution of pest infestation by Aonidiella orientalis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2.00 | 40.0 | 80.0 | 200 | 500 | ||||||||||
| EKE | 1.07 | 2.81 | 5.88 | 12.5 | 22.2 | 35.7 | 51.1 | 89.7 | 145 | 185 | 242 | 315 | 415 | 516 | 651 |
The EKE results are the Weibull (0.9556, 131.58) distribution fitted with @Risk version 7.6.
Table A.2.
The uncertainty distribution of plants free of Aonidiella orientalis per 10,000 plants calculated by Table A.1
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,500 | 9,800 | 9,920 | 9,960 | 9,998 | ||||||||||
| EKE results | 9,349 | 9,484 | 9,585 | 9,685 | 9,758 | 9,815 | 9,855 | 9,910 | 9,949 | 9,964 | 9,978 | 9,988 | 9,994.1 | 9,997.2 | 9,998.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.2.
Figure A.1.
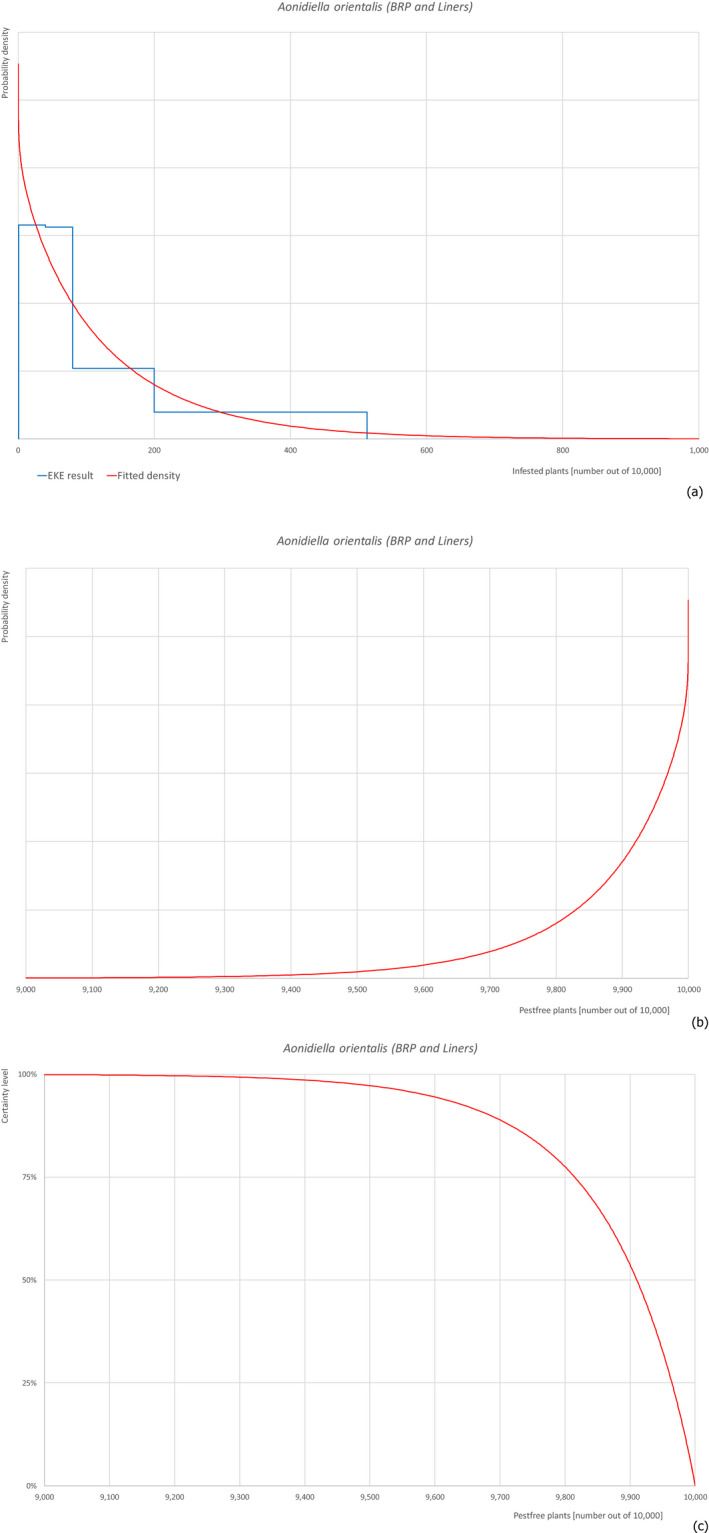
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.1.6. Reference list
Ben‐Dov Y, 1985. Further observations on scale insects (Homoptera: Coccoidea) of the Middle East. Phytoparasitica, 13, 185–192. https://doi.org/10.1007/bf02980667
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Aonidiella orientalis (oriental yellow scale). Available online: https://www.cabi.org/isc/datasheet/5852#todistributionDatabaseTable [Accessed: 27 February 2020].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques MA, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Chatzivassiliou E, Debode J, Manceau C, de la Peña E, Gardi C, Mosbach‐Schulz O, Preti S and Potting R, 2020a. Scientific Opinion on the commodity risk assessment of Albizia julibrissin plants from Israel. EFSA Journal 2020;18(1):5941, 49 pp. https://doi.org/10.2903/j.efsa.2020.5941
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques MA, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Chatzivassiliou E, Debode J, Manceau C, Gardi C, Mosbach‐Schulz O and Potting R, 2020b. Scientific Opinion on the commodity risk assessment of Jasminum polyanthum plants from Israel. EFSA Journal 2020;18(8):6225, 78 pp. https://doi.org/10.2903/j.efsa.2020.6225
Elder RJ and Smith D, 1995. Mass rearing of Aonidiella orientalis (Newstead) (Hemiptera: Diaspididae) on butternut gramma. Journal of the Australian Entomological Society, 34, 253–254. https://doi.org/10.1111/j.1440-6055.1995.tb01333.x
Ensaf SIM, Inaam AE and Manal HE, 2016. Outbreak of oriental yellow scale insect, Aonidiella orientalis (Newstead) (Homoptera: Diaspididae), on neem in Sudan. EPPO Bulletin, 46, 125–128. https://doi.org/10.1111/epp.12264
EPPO (European and Mediterranean Plant Protection Organization), 2005. PM 7/51. Aonidiella citrina. OEPP/EPPO Bulletin, 35, 327–330.
EPPO (European and Mediterranean Plant Protection Organization), online. Aonidiella orientalis (AONDOR), Categorization. Available online: https://gd.eppo.int/taxon/AONDOR/categorization [Accessed: 27 February 2020]
EUROPHYT, online. European Union Notification System for Plant Health Interceptions – EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 27 February 2020].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: A literature‐based model of scale insect biology and systematics, Aonidiella orientalis. Available online: http://scalenet.info/catalogue/Aonidiella%20orientalis/ [Accessed: 27 February 2020].
Hennessey MK, Peña JE, Zlotina M and Santos K, 2013. Likelihood of dispersal of the armored scale, Aonidiella orientalis (Hemiptera: Diaspididae) to avocado trees from infested fruit discarded on the ground, and observations on spread by handlers. In: Peña JE (ed.). Potential Invasive Pests of Agricultural Crops. CAB International, USA. pp. 401–411. https://doi.org/10.1079/9781845938291.0401
Leathers J, 2016. Aonidiella orientalis (newstead): oriental scale. Pest Rating Proposal and Final Ratings. Available online: https://blogs.cdfa.ca.gov/Section3162/?p=2035 [Accessed: 8 October 2019].
Moghaddam M, 2013. An annotated checklist of the scale insects of Iran (Hemiptera, Sternorrhyncha, Coccoidea) with new records and distribution data. Zookeys, 334, 1–92. https://doi.org/10.3897/zookeys.334.5818
Naturalis Biodiversity Center, online. Aonidiella orientalis. Diaspididae of the World 2.0. Available online: https://diaspididae.linnaeus.naturalis.nl/linnaeus_ng/app/views/species/taxon.php?id=113045&epi=155 [Accessed: 8 October 2019].
Pellizzari G and Porcelli F, 2014. Alien scale insects (Hemiptera Coccoidea) in European and Mediterranean countries: the fate of new and old introductions. Phytoparasitica, 42, 713–721. https://doi.org/10.1007/s12600-014-0414-5
Rajagopal D and Krishnamoorthy A, 1996. Bionomics and management of oriental yellow scale, Aonidiella orientalis (Newstead) (Homoptera: Diaspididae): an overview. Agricultural Reviews (Karnal), 17, 139–146.
Wagner MR, Cobbinah JR and Bosu PP, 2008. Forest entomology in West Tropical Africa: forest insects of Ghana. Springer Science & Business Media. 305 pp.
Waterhouse DF and Sands DPA, 2001. Classical Biological Control of Arthropods in Australia. CSIRo Entomology, Canberra, Australia. 560 pp.
Wysoki M, Ben‐Dov Y, Swirski E and Izhar Y, 1993. The arthropod pests of mango in Israel. Acta Horticulturae, 341, 452–466. https://doi.org/10.17660/actahortic.1993.341.50
A.2. Colletotrichum siamense
A.2.1. Organism information
| Taxonomic information | Current valid scientific name: Colletotrichum siamenseSynonyms: Colletotrichum communis, Colletotrichum dianesei, Colletotrichum endomangiferae, Colletotrichum hymenocallidis, Colletotrichum jasmini‐sambac, Colletotrichum melanocaulon (Farr and Rossman, online)Name used in the EU legislation: – Order: Phyllachorales Family: Glomerellaceae Common name: –Name used in the Dossier: – |
| Group | Fungi |
| EPPO code | COLLSM |
| Regulated status | Colletotrichum siamense is not regulated in the EU neither is listed by EPPO. |
| Pest status in Israel | Colletotrichum siamense has been reported on avocado in Israel (Sharma et al., 2017). |
| Pest status in the EU | According to CABI CPC (online) there are no records on the pathogen in the EU. However, C. siamense is reported to be present in Italy according to other sources (Farr and Rossman, online; Jayawardena et al., 2016). |
| Host status on Ficus carica | Colletotrichum siamense was isolated from F. carica in Australia (Farr and Rossman, online; James et al., 2014). |
| PRA information | Available Pest Risk Assessment from EFSA:– Commodity risk assessment of Jasminum polyanthum plants from Israel (EFSA PLH Panel, 2020). |
| Other relevant information for the assessment | |
| Biology | Colletotrichum siamense belongs to Colletotrichum gloeosporioides species complex and to Musae clade (Weir et al., 2012). This complex has hemibiotrophic lifestyle with intracellular hemibiotrophy. Penetration and colonisation of host tissues start with the germination of conidia and the formation of appressoria, which enter through the host cuticle and epidermal cell walls. Primary infection is biotrophic, the pathogen remains inside the living plant tissue and actively absorbs plant metabolites for its growth without killing the plant's cells. It is followed by a necrotrophic stage in which secondary infection hyphae invade and kills adjacent cells (de Silva et al., 2017).Colletotrichum species go through a quiescent stage. During this period, pathogen is dormant inside the plant tissues. The quiescence turns into the necrotrophic stage (de Silva et al., 2017).The life cycle of Colletotrichum species include both sexual/teleomorph and asexual/anamorph stages. The asexual stage is associated with disease symptoms. The sexual stage of the C. gloeosporioides species complex produces perithecia. Perithecia liberates ascospores, which germinate, infect plant tissues and develop into acervuli. Acervuli produces conidia. Conidia are dispersed by rain splash or wind onto healthy plant tissues. The pathogen continues to produce conidia throughout the season, resulting in polycyclic disease. Senescence of the host plant tissues can induce sexual stage of the pathogen and restart its life cycle (de Silva et al., 2017).Dispersal and disease development of Colletotrichum species are favoured by warm, humid and wet conditions (Coates et al., 2015). The pathogen can survive as saprophyte on dead branches, old injuries, fruits and remaining parts in the soil, and sporulates when there are conditions of high temperature and humidity. Pathogen spread resulting in secondary inoculum occurs through splash dispersal of conidia from sporulating lesions due to rain or overhead irrigation dependent on weather factors such as rain intensity, wind and raindrop size (Da Silva and Michereff, 2013).The pathogen can survive and overwinter on fresh leaves, fresh twigs and fallen leaves (Sharma et al., 2017). Colletotrichum species can overwinter as mycelium, sclerotia or even as perithecia (de Silva et al., 2017). |
| Symptoms | Main type of symptoms | Main symptoms of Colletotrichum anthracnose are:– Leaf blight– Lesions/spots on leaves, flowers, buds, stems and fruits– Canker of stems, shoots and twigs resulting in diebacks– Rot of cuttings– Blight of flowers– Fruit rot (Coates et al., 2015)Colletotrichum siamense causes fruit and foliar disease called anthracnose on many host plants (Meng et al., 2019). On leaves of Hymenocallis sp., the anthracnose appears as brown ellipsoid spots with orange conidial masses, without setae (Yang et al., 2009).The fungi can infect fruits, fresh leaves, fresh twigs, dry leaves and dry twigs (Sharma et al., 2017).Main symptom of C. gloeosporioides species complex on avocado is anthracnose, which causes leaf and fruit drop. On fruits large spreading lesion, dark brown to black, can occur (Marais, 2004).Information on symptoms on F. carica is not available. |
| Presence of asymptomatic plants | The plants are symptomless at early stages of primary (biotrophic) infection (de Silva et al., 2017).Quiescent infections can occur both in fruits and leaves. The pathogen infects young fruits but enters a dormant phase until the fruit maturity (Marais, 2004).Colletotrichum siamense can be present asymptomatically on leaves (James et al., 2014) and has been described as endophyte in different hosts including coffee berry tissues (Wikee et al., 2009), and on leaves of Piper nigrum leaves (Munasinghe et al., 2017), Centella asiatica (Radiastuti et al., 2019), Artocarpus sericicarpus, A. heterophyllus, Coffea canephora, Eriobotrya japonica, Ficus carica, Mentha sp., Rosmarinus officinalis, Theobroma cacao (James et al., 2014) or Cymbopogon citratus (Manangoda et al., 2013). | |
| Confusion with other pests | Colletotrichum siamense can be easily confused with other Colletotrichum species.Morphological features such as colony growth rate, colour of cultures, conidial size and shape and shape of appressoria can be used for identification of Colletotrichum species. However, many of the morphological features are not always available, and they can change with repeated sub‐culturing or vary under different growing conditions (Weir et al., 2012). Thus, molecular methods should be used for proper identification. However, the identification of Colletotrichum spp. is complicated by the occurrence of species complexes that are not easily resolved by morphological and single loci sequence approaches (James et al., 2014; Weir et al., 2012). Partial actin (ACT), b‐tubulin (TUB2), calmodulin (CAL), glutamine synthetase (GS), glyceraldehyde‐3‐phosphate dehydrogenase (GPDH) genes and the complete rDNAITS (ITS) region was used by Prihastuti et al. (2009) and Wikee et al. (2011) to identify Colletotrichum spp. from coffee berries and Jasminum sambac, respectively. |
| Host plant range | Colletotrichum siamense is a pathogen of apple (Malus domestica), Arabian jasmine (Jasminum sambac), arabica coffee (Coffea arabica), asoka‐tree (Saraca indica), avocado (Persea americana), blueberry (Vaccinium macrocarpon), chilli (Capsicum sp.) citrus (Citrus sp.) coconut (Cocos nucifera), dayflower (Commelina sp.), elephant grass (Pennisetum purpureum), fig (Ficus elastica), grapevine (Vitis vinifera), guava (Psidium guajava), jessamine (Murraya sp.), lemon grass (Cymbopogon citrates), mango (Mangifera indica), mountain ebony (Bauhinia variegata), olive (Olea europaea), pawpaw (Carica papaya), pistachio (Pistacia vera), spider lily (Hymenocallis sp.), strawberry (Fragaria × ananassa), tea (Camellia sinensis) and yam (Dioscorea rotundata) (Jayawardena et al., 2016).It has been isolated from asymptomatic leaves of F. carica in Australia (James et al., 2014). |
| Pathways | Possible pathways of entry for Colletotrichum gloeosporioides species complex are infected nursery stock, contaminated soil and fruits (Australian Government, 2020). The Panel considers that the same pathways could apply to C. siamense. |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. |
A.2.2. Possibility of pest presence in the nursery
A.2.2.1. Possibility of entry from the surrounding environment
Colletotrichum siamense has a wide host range, including fruits, vegetables and ornamentals (Weir, 2012; Meng et al., 2019). The major source of inoculum is from infected plant material, which can be leaves, twigs and fruits of the affected plant species. Splash dispersal from rain or sprinkler irrigation water is required to dislodge the conidia from the acervuli of the fungus, subsequent drying of the water droplets can lead to airborne inoculum, which can be further dispersed by wind. Therefore, the presence of host species or weeds in the environment can be a factor for the possible migration of inoculum.
In the Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
Of these plant species, Persea americana is a host of C. siamense (Jayawardena et al., 2016).
Uncertainties:
-
–
The presence and abundance of suitable host plants and C. siamense inoculum in the area surrounding of the nursery are unknown.
-
–
It is uncertain whether the fungus may be associated with tissues other than leaves on F. carica.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nursery. The airborne inoculum may enter the nursery from infected host plants present in the surrounding and cause bark infections. Bark infections have been reported as associated with Colletotrichum species.
A.2.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Although the inoculum of C. siamense can be present in the soil, according to Dossier Sections 1.0 and 9.0, the growing medium that is used for the exported fig products is a commercial growing medium and is always new.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers it is not possible that the pathogen could enter the nursery with new plants/seeds or soil growing media.
A.2.2.3. Possibility of spread within the nursery
According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants. Therefore, if the pathogen is present inside the nursery, rain splash may easily spread the disease to neighbouring plants. The pathogen may sporulate on fallen leaves and cause bark infection. The Dossier Section 9.0 states ‘The water that is used for irrigation is regular tap water, that goes through a 120‐mesh filter to remove rough dirt like sand and stones. Liners are irrigated by sprinklers, and bare rooted plants receive drip irrigation’. Irrigation by sprinklers may enhance splash dispersal of inoculum leading to new infections.
Lagerstroemia indica and Morus alba are grown in the nursery (Dossier Section 9.0) but are not hosts of the pest.
According to Dossier Section 9.0, the growing medium is peat substrate (EU‐made). Liners are rooted directly in pots, in the same growing medium as used for the bare rooted plants. Soil solarisation is performed by covering the soil with transparent polyethylene for 2 months – July and August (normally the time of highest radiation). The polyethylene sheet is spread after the soil has been cleaned from the previous crop and has been processed for the next cycle. The polyethylene in the sheets is supplemented with ‘antidrip’ or ‘antifog’ substances which prevents water condensation and accumulation on the sheet, so improving treatment efficacy by raising the under‐sheet temperature by 4–5°C compared with regular polyethylene sheets. The max temperature in the top 20 cm of the soil is 44–48°C daily, for the duration of 2 months. The sheets are maintained clean and intact through the treatment duration, and the soil moisture is maintained to the field capacity level, by weekly irrigation with a water volume that parallels 1 m3 water/dunam per day. Soil solarisation may reduce the inoculum of C. siamense present in soil.
Uncertainties:
-
–
There is uncertainty about the presence and population density of the pathogen in the nursery.
-
–
There is uncertainty regarding the suitability of F. carica to act as leave and bark host of C. siamense.
-
–
There is uncertainty on the level the fungus can fruit on nursery F. carica plants.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nursery is possible. The plantation density is high enough to ensure an easy dispersal of inoculum by means of water splash from one plant to the other.
A.2.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of C. siamense between the years 1995 and November 2019 (EUROPHYT, online).
A.2.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on C. siamense is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The characteristics of the production field should not affect significantly the pathogen in open field.The use of commercial growing medium always new in sack containers should prevent the entry of pathogen inoculum with the growing medium. However, the medium may become contaminated during production as a result of inoculum dispersal and incorporation of infected plant tissues.The net of the net house is designed for shading and it is not expected to prevent or reduce the entry of pathogen inoculum.Uncertainties:– There is uncertainty whether fallen leaves are periodically removed.– There is uncertainty on the level of viability of the inoculum in the soil (both the persistence in the soil and the ability to fruit). | The use of commercial growing medium always new should prevent the entry of pathogen inoculum with the growing medium. However, the medium may become contaminated during production as a result of inoculum dispersal and incorporation of infected plant tissues.Irrigation by sprinklers may enhance splash dispersal of inoculum leading to new infections.The net of the net house is designed for shading and it is not expected to prevent or reduce the entry of pathogen inoculum.Uncertainties:– There is uncertainty whether fallen leaves are periodically removed.– There is uncertainty on the level of viability of the inoculum in the soil (both the persistence in the soil and the ability to fruit). |
| 2 | Soil treatment | Yes, for bare rooted plants | Soil solarisation may reduce the inoculum of C. siamense present in soil.Uncertainties:– The level of reduction of inoculum in the soil as a result of solarisation is unknown. | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | No | Not applicable | Not applicable |
| 5 | Fungicide treatment | Yes | The Panel assumes that fungicide treatment with Myclobutanil or other appropriate fungicides occurs in the case of any early signs of infection. Therefore, if plants are symptomless fungicide treatment will not be carried out.Post‐harvest treatment is targeting infection from root pathogens and it is not expected to eradicate or to kill the pathogen if present in the plant.Uncertainties:– The level of effectiveness of the fungicides against the pathogen is unknown especially in association with bark infection. | The Panel assumes that fungicide treatment with Myclobutanil or other appropriate fungicides occurs in the case of any early signs of infection. Therefore, if plants are symptomless fungicide treatment will not be carried out.Uncertainties:– The level of effectiveness of the fungicides against the pathogen is unknown especially in association with bark infection. |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | Yes | Leaves removal will reduce the probability of carrying the pathogen. However, the pathogen can also be associated with other plant tissues.Uncertainties:– The level of the association of the pathogen with other plant tissues in F. carica. | Leaves removal and cleaning of the plant debris will reduce the probability of carrying the pathogen. However, the pathogen can also be associated with other plant tissues.Uncertainties: – The level of the association of the pathogen with other plant tissues in F. carica. |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | The inspections during the production should allow a prompt detection of any visible symptoms of any disease. However, low level of infections might be overlooked. Asymptomatic plants will go undetected.Uncertainties:– Symptoms on F. carica are currently unknown and might be different from those described on other hosts, thereby hampering a prompt detection.– Symptoms of the disease on plants tissues other than leaves may be more difficult to be detected. | The inspections during the production should allow a prompt detection of any visible symptoms of any disease. However, low level of infections might be overlooked. Asymptomatic plants will go undetected.Uncertainties:– Symptoms on F. carica are currently unknown and might be different from those described on other hosts, thereby hampering a prompt detection.– Symptoms of the disease on plants tissues other than leaves may be more difficult to be detected. |
| 11 | Inspections before export | Yes | The inspections before export should allow the observation of any visible symptoms of the disease. However, low level of infections might be overlooked. Asymptomatic plants will go undetected.Uncertainties:– Symptoms on F. carica are currently unknown and might be different from those described on other hosts, thereby hampering a prompt detection.– Symptoms of the disease on plants tissues other than leaves may be more difficult to be detected. | The inspections before export should allow the observation of any visible symptoms of the disease. However, low level of infections might be overlooked. Asymptomatic plants will go undetected.Uncertainties:– Symptoms on F. carica are currently unknown and might be different from those described on other hosts, thereby hampering a prompt detection.– Symptoms of the disease on plants tissues other than leaves may be more difficult to be detected. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, C. siamense is present in Israel.Uncertainties:– There is no information on the presence and density of C. siamense in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, C. siamense is present in Israel.Uncertainties:– There is no information on the presence and density of C. siamense in the surrounding areas. |
A.2.5. Overall likelihood of pest freedom for bare rooted plants
A.2.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare rooted plants
There is low pest pressure from the surroundings and F. carica is poorly susceptible. Infections occur only on leaves without an endophytic stage. Contaminations of the soil by infected fallen leaves rarely occur and are effectively controlled by soil solarisation. Inspections and control measures with fungicides are effective.
A.2.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare rooted plants
There is high pest pressure from the surroundings and F. carica is a susceptible host. The fungus mainly infects the leaves and other host tissues asymptomatically as an endophyte. Contaminations of the soil by fallen leaves frequently occur and soil solarisation is only partially effective. Because the pathogen rarely causes clear symptoms, it is difficult to detect during inspections. There are no fungicide treatments without observation of symptoms.
A.2.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare rooted plants (Median)
The median is closer to lower values because there is little evidence that F. carica is a main host of the pathogen and the pressure from the surroundings is most likely low.
A.2.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There are main uncertainties about the susceptibility of the F. carica and the endophytic (latent) stage of the pathogen.
A.2.5.5. Elicitation outcomes of the assessment of the pest freedom for Colletotrichum siamense on bare rooted plants
The following tables show the elicited and fitted values for pest infestation/infection (Table A.3) and pest freedom (Table A.4).
Table A.3.
Elicited and fitted values of the uncertainty distribution of pest infestation by Colletotrichum siamense per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5.00 | 15.0 | 25.0 | 45.0 | 80.0 | ||||||||||
| EKE | 4.80 | 5.29 | 6.15 | 7.99 | 10.6 | 14.1 | 17.9 | 26.6 | 37.2 | 43.7 | 51.5 | 59.6 | 67.9 | 73.9 | 79.5 |
The EKE results are the BetaGeneral (0.94316, 2.1577, 4.5, 90) distribution fitted with @Risk version 7.6.
Table A.4.
The uncertainty distribution of plants free of Colletotrichum siamense per 10,000 plants calculated by Table A.3
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,920 | 9,955 | 9,975 | 9,985 | 9,995 | ||||||||||
| EKE results | 9,921 | 9,926 | 9,932 | 9,940 | 9,949 | 9,956 | 9,963 | 9,973 | 9,982 | 9,986 | 9,989 | 9,992.0 | 9,993.8 | 9,994.7 | 9,995.2 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.4.
Figure A.2.
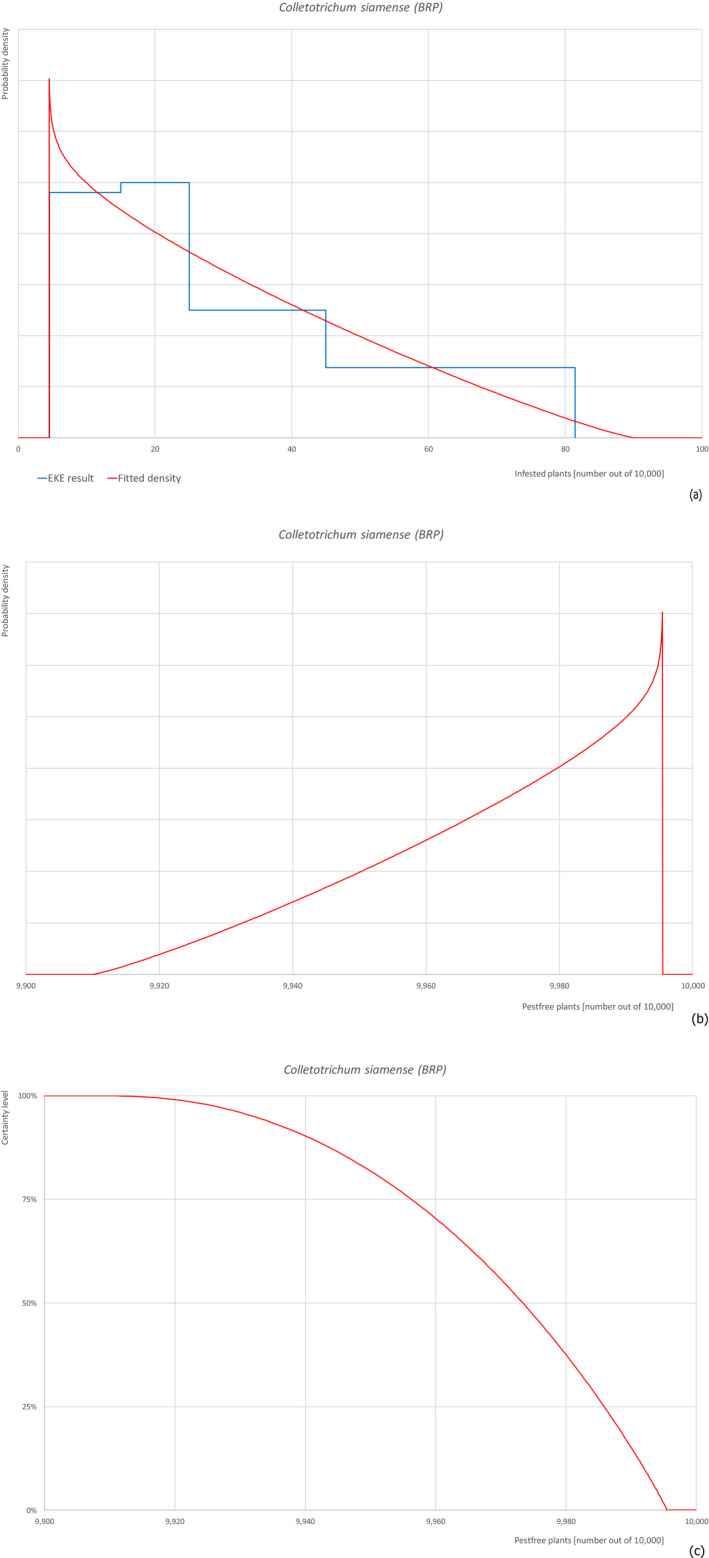
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.2.6. Overall likelihood of pest freedom for liners
A.2.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected liners
There is low pest pressure from the surroundings and F. carica is poorly susceptible. Infections only occur on leaves without an endophytic stage. Growth conditions are not very suitable for infections. Contaminations of the soil by infected fallen leaves rarely occur. Inspections and control measures with fungicides are effective.
A.2.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected liners
There is high pest pressure from the surroundings and F. carica is a susceptible host. The fungus mainly infects the leaves and other host tissues asymptomatically as an endophyte. Sprinkling of the liners favours infections. Contaminations of the soil by fallen leaves frequently occur. Because the pathogen rarely causes clear symptoms, it is difficult to detect during inspections. There are no fungicide treatments without observation of symptoms.
A.2.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected liners (Median)
The median is closer to the lower values because there is little evidence that F. carica is a main host of the pathogen and the pressure from the surroundings is most likely low. It is higher than for bare rooted plants because sprinkling is expected to favour leaf infections and fallen leaves could contaminate the substrate.
A.2.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There are main uncertainties about the susceptibility of the F. carica, the endophytic (latent) stage of the pathogen and the degree of suitable conditions for infections during production of the liners.
A.2.6.5. Elicitation outcomes of the assessment of the pest freedom for Colletotrichum siamense on liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.5) and pest freedom (Table A.6).
Table A.5.
Elicited and fitted values of the uncertainty distribution of pest infestation by Colletotrichum siamense per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 10.0 | 40.0 | 70.0 | 110 | 200 | ||||||||||
| EKE | 10.7 | 12.9 | 16.2 | 22.3 | 30.1 | 39.7 | 49.4 | 70.4 | 95.0 | 110 | 128 | 146 | 166 | 181 | 196 |
The EKE results is the BetaGeneral (1.2034, 2.6361, 8.75, 230) distribution fitted with @Risk version 7.6.
Table A.6.
The uncertainty distribution of plants free of Colletotrichum siamense per 10,000 plants calculated by Table A.5
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,800 | 9,890 | 9,930 | 9,960 | 9,990 | ||||||||||
| EKE results | 9,804 | 9,819 | 9,834 | 9,854 | 9,872 | 9,890 | 9,905 | 9,930 | 9,951 | 9,960 | 9,970 | 9,978 | 9,984 | 9,987 | 9,989 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.6.
Figure A.3.
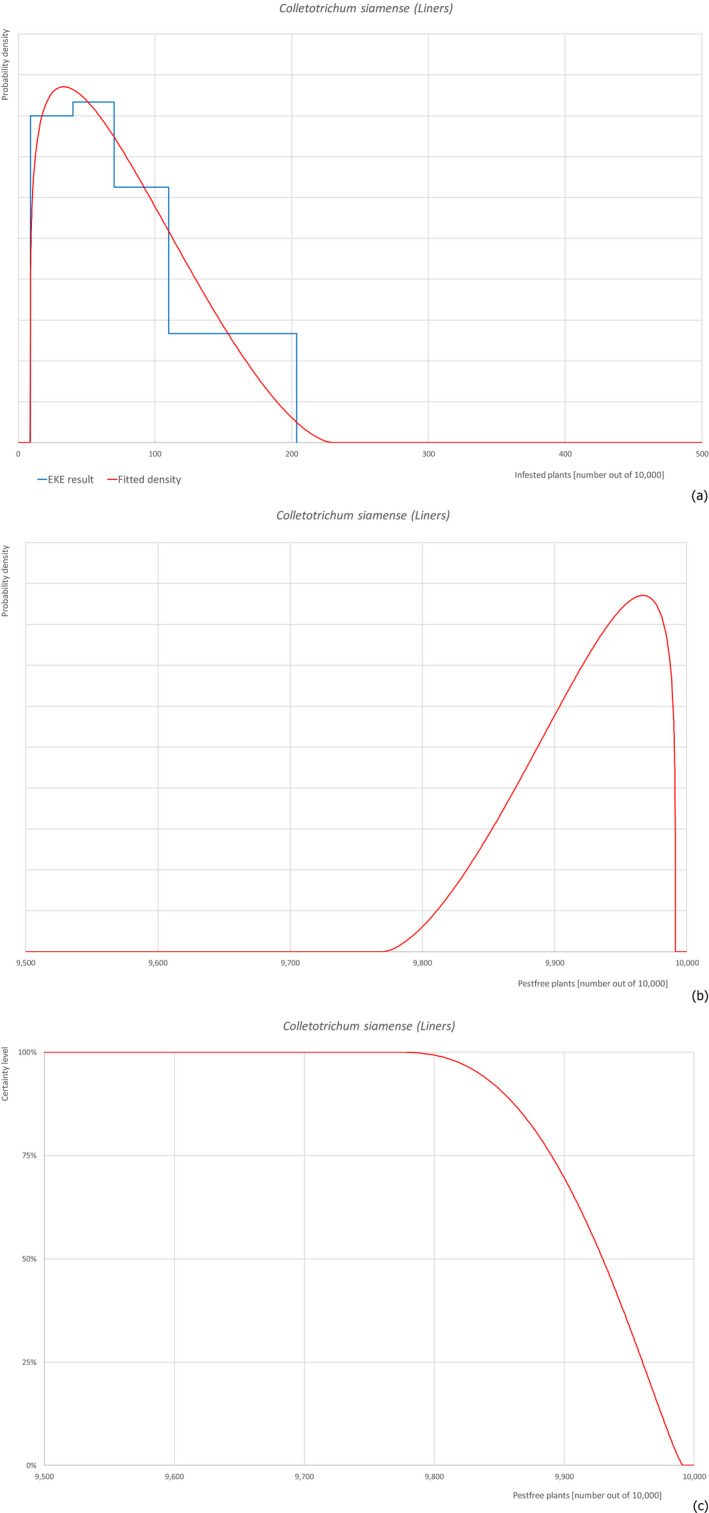
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
Figure A.4.
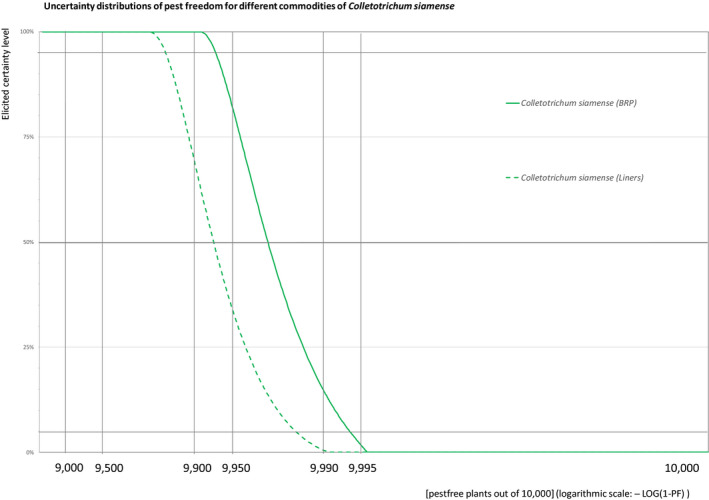
Elicited certainty (y‐axis) of the number of bare rooted plants (BRP) or liners of Ficus carica pest free from Colletotrichum siamense (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU introduced from Israel as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
A.2.7. Reference list
Australian Government, 2020. Final report for the review of biosecurity import requirements for fresh strawberry fruit from Japan. Department of Agriculture, Water and the Environment, Canberra, Commonwealth of Australia. 216 pp.
CABI CPC (Centre for Agriculture and Bioscience International), online. Colletotrichum siamense. Available online: https://www.cabi.org/cpc/ [Accessed: 8 June 2020].
Coates L, Cooke T and Forsberg L, 2015. The biology and management of Colletotrichum diseases in production nurseries. Agri‐science Queensland, Department of Agriculture, Fisheries and Forestry. Plant health, biosecurity, risk management and capacity building for the nursery industry (NY11001). Available online: https://www.greenlifeindustry.com.au/Attachment?Action=Download&Attachment_id=1829
Da Silva DCFB and Michereff SJ, 2013. Biology of Colletotrichum spp. and epidemiology of the anthracnose in tropical fruit trees. Rev. Caatinga, 26, 130–138.
de Silva DD, Crous PW, Ades PK, Hyde KD and Taylor PW, 2017. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biology Reviews, 31, 155–168. https://doi.org/10.1016/j.fbr.2017.05.001
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques MA, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Chatzivassiliou E, Debode J, Manceau C, Gardi C, Mosbach‐Schulz O and Potting R, 2020. Scientific Opinion on the commodity risk assessment of Jasminum polyanthum plants from Israel. EFSA Journal 2020;18(8):6225, 78 pp. https://doi.org/10.2903/j.efsa.2020.6225
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 5 May 2020].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA, Colletotrichum siamense. Available online: https://nt.ars-grin.gov/fungaldatabases/fungushost/new_frameFungusHostReport.cfm [Accessed: 23 April 2020].
James RS, Ray J, Tan YP and Shivas RG, 2014. Colletotrichum siamense, C. theobromicola and C. queenslandicum from several plant species and the identification of C. asianum in the Northern Territory, Australia. Australasian plant disease notes, 9, 138. https://doi.org/10.1007/s13314-014-0138-x
Jayawardena RS, Hyde KD, Damm U, Cai L, Liu M, Li XH, Zhang W, Zhao WS and Yan JY, 2016. Notes on currently accepted species of Colletotrichum. Mycosphere, 7, 1192–1260. https://doi.org/10.5943/mycosphere/si/2c/9
Marais L, 2004. Avocado diseases of major importance worldwide and their management. In: Naqvi SAMH (ed.). Diseases of Fruits and Vegetables, Vol. 2, Berlin, Springer. pp. 1–36.
Meng Y, Gleason ML, Zhang R and Sun G, 2019. Genome sequence resource of the wide‐host‐range anthracnose pathogen Colletotrichum siamense. Molecular Plant‐Microbe Interactions, 32, 931–934. https://doi.org/10.1094/mpmi-01-19-0010-a
Munasinghe MVK, Kumar NS, Jayasinghe L and Fujimoto Y, 2017. Indole‐3‐acetic acid production by Colletotrichum siamense, an endophytic fungus from Piper nigrum leaves. Journal of Biologically Active Products from Nature, 7, 475–479. https://doi.org/10.1080/22311866.2017.1408429
Prihastuti H, Cai L, Chen H, McKenzie E and Hyde KD, 2009. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity, 39, 89–109.
Radiastuti N, Bahalwan HA and Susilowati DN, 2019. Phylogenetic study of endophytic fungi associated with Centella asiatica from Bengkulu and Malaysian accessions based on the ITS rDNA sequence. Biodiversitas, 20, 1248–1258. https://doi.org/10.13057/biodiv/d200503
Sharma G, Maymon M and Freeman S, 2017. Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Scientific reports, 7, 1–16.
Yang YL, Liu ZY, Cai L, Hyde KD, Yu ZN and McKenzie EHC, 2009. Colletotrichum anthracnose of Amaryllidaceae. Fungal Diversity, 39, 123–146.
Weir BS, Johnston PR and Damm U, 2012. The Colletotrichum gloeosporioides species complex. Studies in Mycology, 73, 115–180. https://doi.org/10.3114/sim0011
Wikee S, Cai L, Pairin N, McKenzie EHC, Su YY, Chukeatirote E, Thi HN, Bahkali A, Moslem M, Abd‐Elsalam KA and David Hyde K, 2011. Colletotrichum species from Jasmine (Jasminum sambac). Fungal Diversity, 46, 171–182. https://doi.org/10.1007/s13225-010-0049-x
A.3. Euwallacea fornicatus and Neocosmospora euwallaceae
A.3.1. Organism information
| Taxonomic information | Insect Euwallacea fornicatus (Eichhoff, 1868)In the EPPO Global Database, Euwallacea fornicatus (polyphagus shot hole borer – PSHB) is considered as a species complex which includes: E. fornicatus sensu stricto, E. fornicatior, E. whitfordiodendrus and E. kuroshio. However, a recent taxonomic review of the species complex by Smith et al. (2019) proposed the following classification: Euwallacea fornicatus (= E. tapatapaoensis (Schedl, 1951); = E. whitfordiodendrus (Schedl, 1942)) syn. res.); E. fornicatior (Eggers, 1923) (= E. schultzei (Schedl, 1951) syn. nov.); E. kuroshio (Gomez and Hulcr, 2018) and E. perbrevis (Schedl, 1951) stat. res, see also discussion in EPPO, 2020.This pest sheet refers to Euwallacea fornicatus species complex, E. fornicatus sensu lato according to EPPO (2017).Name used in the EU legislation: Listed as EU‐quarantine pest as Scolytidae spp. (non‐European) [1SCOLF].EPPO code: XYLBFOOrder: ColeopteraFamily: CurculionidaeSubfamily: ScolytinaeCommon name: Polyphagous Shot Hole Borer (PSHB), Avocado ambrosia beetle, Tea Shot Hole Borer (TSHB), Kuroshio Shot Hole Borer (KSHB), Shot Hole Borers (SHB)Name used in the Dossier: Euwallacea fornicatus FungusCurrent valid name: Neocosmospora euwallaceae (S. Freeman, Z. Mendel, T. Aoki & O'Donnell) Sandoval‐Denis, L. Lombard & Crous in (Sandoval‐Denis et al., 2019)Synonyms: Fusarium euwallaceaeEPPO code: FUSAEWOrder: HypocrealesFamily: NectriaceaeName used in the Dossier: Fusarium euwallaceae | |
|---|---|---|
| Regulated status | The insect E. fornicatus is listed in Annex II/A of Regulation (EU) 2019/2072 as Scolytidae spp. (non‐European) [1SCOLF].The fungus N. euwallaceae is not currently regulated in the EU.Both, E. fornicatus and N. euwallaceae are listed in the EPPO A2 list (i.e. recommended for regulation). | |
| Pest status in Israel | Euwallacea fornicatus and N. euwallaceae are present in Israel (EPPO, online_a,b; Gomez et al., 2018). | |
| Pest status in the EU | Euwallacea fornicatus is reported as ‘Transient, under eradication’ in Italy (Europhyt Oubreaks database, online) and ‘Absent, pest eradicated’ in Poland (EPPO, online_a).Neocosmospora euwallaceae is not reported in the EU (EPPO, online_b). | |
| Host status on Ficus carica | Ficus carica is a host of E. fornicatus (Cooperband et al., 2016) and N. euwallaceae (Freeman et al., 2013; de Beer and Paap, 2019). The fungus has been reported on F. carica in Israel (Freeman et al., 2013).Some plant species are reported to be used only as feeding hosts by E. fornicatus, where reproductive life stages (e.g. tunnelling larvae, male beetles) are not reported (non‐reproductive host). In the USA, F. carica is categorised as a reproductive host for E. fornicatus (Cooperband et al., 2016; Greer et al., 2018). But according to Eskalen et al. (2013) and de Beer and Paap (2019), F. carica is a non‐reproductive host where beetles are not able to successfully breed.The fungus is transported in mycangia and could be vectored irrespective on host condition. | |
| Pest Risk Analysis information | Available Pest Risk Assessments:
|
|
| Other relevant information for the assessmentAccording to Dossier Section 9.0, bare rooted plants are 20–100 cm tall, with base diameter of up to 2 cm. Liners are about 10 cm high and with ~ 1 cm base diameter. | ||
| Biology | Euwallacea fornicatus is native to Asia, somewhere between northern Thailand and southern Japan (Coleman et al., 2013). Euwallacea fornicatus has a complex association with symbiotic fungi, particularly with N. euwallaceae (Paap et al., 2018) which is a plant pathogen. The beetle is also associated with Graphium euwallaceae and Paracremonium pembeum that are considered as nutritional fungi (Freeman et al., 2013). E. fornicatus sensu lato can infest healthy plants (EPPO, 2020). The beetle is a major pest having killed thousands of box elder trees (Acer negundo) in the Israel and California (Mendel et al., 2012).Euwallacea fornicatus has four life stages: egg, larvae (3 instars), pupa and adult. The total length of life cycle is about 42 days (including longevity) under optimal conditions, and there are several generations per year (multivoltinism), depending on temperature. Females live for ~ 7.9 days and male for 5.8 days (Kumar et al., 2011). Males are flightless, smaller than females and never leave the gallery (Browne, 1961). The adult female of E. fornicatus is 2.0–2.8 mm long and about twice as long as it is wide (CABI, online). Females have wings and remain in the galleries for several days. It is considered that the beetle (only females can fly) is able to fly up to about 457 m (EPPO, 2017). The mating takes place within the gallery between male and female offspring (Walgama, 2012). After mating, females emerge through the original entrance tunnel and fly to new hosts (CABI, online). They create galleries in the trees, where they introduce the symbiotic fungus (being transported through the mandibular mycangia), which colonises gallery walls, becoming a food source for developing larvae and adult beetles (Paap et al., 2018). After the attack of the beetle, the fungus invades the vascular tissue of the tree and contributes to cause symptoms. Eggs are laid in groups inside the galleries. Pupation takes place inside the galleries of twigs (Kumar et al., 2011). The ratio of male to female is ~ 1:3 (Judenko, 1956). Overwintering occurs in the woody parts of the trees in any developmental stage.Successful reproduction occurs in twigs, stems and branches (from 2 to > 30 cm in diameter) (Kirkendall and Ødegaard, 2007; Mendel et al., 2012). If larger branches are colonised, the beetle can survive for longer periods, and may produce more generations before moving to a new breeding site (branch, tree or plantation) (Ministerio de Agricultura, Alimentacion y Medio Ambiente, 2015).In Italy during the outbreak, the entry holes of the beetle were observed to be present also on branches with a diameter less than 2 cm (Europhyt Oubreaks database, online). | |
| Symptoms | Main type of symptoms | Main symptoms are brownish staining of the xylem, cambial necrosis, branch dieback and in the worst‐case scenario, the death of the tree (Ministerio de Agricultura, Alimentacion y Medio Ambiente, 2015). In general, there is a correlation between severity of the beetle attack (which therefore increases severity of infection by Fusarium sp.) and the observed dieback (Eskalen et al., 2013).N. euwallaceae infections can be associated with an abundant production of blue to brownish macroconidia (Freeman et al., 2013). The symptoms include also leaf yellowing and wilting of the branches, which, when there is heavy yield, break down at the section where the beetle galleries are located. Those symptoms, together with the ones caused by the fungus associated to the beetle, could lead to the death of young and mature trees (Ministerio de Agricultura, Alimentacion y Medio Ambiente, 2015; EPPO, 2016; EPPO, 2017).A good description of symptoms on several host plant species is given by the California Department of Fish and Wildlife (online).The symptoms caused by the beetle on a tree depend on the response of the plants to the fungus infection and vary among hosts species. |
| Presence of asymptomatic plants | Initial phases of infestation are associated with few external symptoms. While there is hardly visible injury in the bark at early stage of colonisation, later frass is produced and the attack becomes obvious. Examination of the wood under the infested spot bored by the beetle reveals the brownish staining of the xylem and necrosis caused by the fungus (Mendel et al., 2012). | |
| Confusion with other pathogens/pests | Euwallacea fornicatus is a species complex (see above) and it can be confounded with other ambrosia beetles and needs to be identified using morphological description and molecular methods. | |
| Host plant range | Eskalen et al. (2013) reported that, in the USA, more than 200 tree species were used as a host plant by E. fornicatus and of these species, 113 were reported as a host for the fungus, thereby supporting the breeding of the beetle (reproductive hosts). Other host plants may allow the beetle feeding, but not breeding, because the fungus does not establish (non‐reproductive hosts) (EPPO, 2020). Fungal infection is most likely due to susceptibility of the tree to the fungus, if the beetle is able to penetrate the cambium layer (Eskalen et al., 2013).Based on impact, major hosts classified by EPPO are Acacia melanoxylon, Camellia sinensis and Persea americana, while other hosts are classified as minor (EPPO, online_a). However, Acer has been reported as heavily damaged in Israel (EPPO, 2020). According to EPPO, a non‐complete list of E. fornicatus host plants include: Acer buergerianum, Acer macrophyllum, Acer negundo, Acer palmatum, Acer paxii, Albizia julibrissin, Alectryon excelsus, Ailanthus altissima, Alnus rhombifolia, Castanospermum australe, Cercidium floridum, Erythrina corallodendrum, Eucalyptus ficifolia, Ilex cornuta, Liquidambar styraciflua, Parkinsonia aculeata, Persea americana, Platanus racemosa, Platanus x acerifolia, Populus fremontii, Populus trichocarpa, Prosopis articulata, Quercus suber, Quercus agrifolia, Quercus engelmannii, Quercus lobata, Quercus robur, Ricinus communis, Salix babylonica, Salix gooddingii, Salix laevigata, Wisteria floribunda (EPPO, 2016; EPPO, 2017). Pinus massoniana is reported as an incidental host (EPPO, online_a). In Israel, avocado (Persea americana) is the host reporting the most significant economic damage, but several ornamental species are also affected, such as Ricinus communis, Acer negundo, Quercus pedunculiflora, Quercus robur, Platanus occidentalis, Platanus orientalis and Acer buergerianum (Mendel et al., 2017).Neocosmospora euwallaceae causes serious damage to more than 20 tree species, and, according to Eskalen et al. (2013) it was isolated from 113 different plant species. An attempted beetle attack may serve as an infection site for the fungus in both reproductive and non‐reproductive hosts of E. fornicatus, however in some cases the infection is not successful (Eskalen et al., 2013).The fungus has been reported on F. carica in Israel (Freeman et al., 2013). | |
| Pathways | According to EPPO (2020), the main pathways of entry are: plants for planting (except seeds), wood, wood packaging material, wood chips, hogwood, processing wood residues and possibly cut branches. | |
| Surveillance information | No surveillance information for these pests is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.3.2. Possibility of pest presence in the nursery
A.3.2.1. Possibility of entry from the surrounding environment
Neocosmospora euwallaceae can be introduced into the nursery only by the insect vector E. fornicatus. There are divergences in the literature about the flying capacity of Euwallacea sp. It is considered that the beetle (only females can fly) is able to fly up to about 457 m (EPPO, 2017). Calnaido (1965) reported an estimated flight distance of 864 m without external help (e.g. wind) while Owens et al. (2019) found a maximum dispersal distance of 400 m. In any case, only a few insects fly this distance. Wind speed and direction can have a great effect on the number of beetles that disperse as well as on the distance they can cover within a single flight (Owens et al., 2019).
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Punica granatum, Platanus acerifolia, Platanus mexicana, Platanus racemosa, Populus fremontii, Populus nigra, Populus trichocarpa, Quercus agrifolia, Quercus engelmanni, Quercus lobate, Quercus robur, Eucalyptus ficifolia, Acacia spp., Ricinus communis and Persea americana are reproductive hosts of E. fornicatus (EPPO, 2020).
Based on the presence of suitable hosts of the pests in the surrounding, the Panel assumes that both pests can be present in the production areas of F. carica destined for export to the EU.
According to Dossier Section 9.0, bare rooted plants are 20–100 cm tall, with base diameter of up to 2 cm. Liners are about 10 cm high and with ~ 1 cm base diameter. The diameter of 2 cm is the lower limit for the colonisation by E. fornicatus.
Uncertainties:
-
–
There is no surveillance information on the presence or population pressure of the pests in the area where the nursery is located.
-
–
No information available on the distance of the nursery to sources of pests in the surrounding environment.
-
–
There is an uncertainty on the level of susceptibility of plants to the beetle attack based on their diameter.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the insect and pathogen to enter the nursery from the surrounding area. It is possible because suitable hosts are present in the surrounding and F. carica plants may be attacked although their size is at the lower limit.
A.3.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the insect and the pathogen could enter the nursery with new plants/seeds or soil growing media.
A.3.2.3. Possibility of spread within the nursery
The crops designated for export are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
The possibility of spread of the pests within the nursery based on sources present in the nursery is dependent on whether the commodity and the mother plants may act as reproductive hosts of the beetle. In the USA, F. carica is categorised as a reproductive host for E. fornicatus (Cooperband et al., 2016; Greer et al., 2018). But according to Eskalen et al. (2013) and de Beer and Paap (2019) F. carica is a non‐reproductive host where beetles are not able to successfully breed. There is no information about the condition (reproductive or non‐reproductive host) of nursery F. carica trees. But for the sake of this analysis, there is no difference between them. The fungus is transported in mycangia and could be vectored irrespective on host condition.
Lagerstroemia indica and Morus alba are grown in the nursery (Dossier Section 9.0) but the host status is uncertain.
Spread within the nursery through the movement of soil, water, equipment, tools and humans is not relevant. Females of E. fornicatus can fly and hence spread together with the fungus.
Uncertainties
-
–
There is no information on the presence or population pressure of the pests in the nursery.
-
–
There is uncertainty on the suitability of F. carica (including nursery plants and mother plants inside the nursery) to act as reproductive host.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the insect and the pathogen within the nursery is possible.
A.3.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of E. fornicatus and N. euwallaceae between the years 1995 and November 2019 (EUROPHYT, online).
A.3.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on E. fornicatus and N. euwallaceae is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | Beetles may immigrate the production fields from the surrounding environment and attack plants grown in open fields. Plants grown in net house are not protected by the net.Uncertainties:– No uncertainties | Plants grown in net house are not protected by the net.Uncertainties:– No uncertainties |
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Residual efficacy of the applied insecticides may not protect the plants until the next application.Uncertainties:– It is not certain whether the beetle can be affected by the use of pesticides. | Residual efficacy of the applied insecticides may not protect the plants until the next application.Uncertainties:– It is not certain whether the beetle can be affected by the use of pesticides. |
| 5 | Fungicide treatment | Yes | Although the application of Myclobutanil is probably not targeting N. euwallaceae, the fungicide could have some effects in preventing pest establishment due to the fact that it is systemic. Based on the way this fungicide spread within the plant (it generally goes up), there is little chance that a spray application to the leaves could result in a significant effect down in the stem. The repeated use of myclobutanil may result in the development of resistance to the pesticide in the populations of the fungus.Captan is a preventative treatment. Therefore, it has no effects on plants that are already infected. Chilling storage is not expected to kill the fungus inside the plant.Uncertainties:– There is uncertainty on the level to which Myclobutanil sprayed on leaves may be translocated down into the plant thereby preventing or curing infections. | Although the application of Myclobutanil is probably not targeting N. euwallaceae, the fungicide could have some effects in preventing pest establishment due to the fact that it is systemic. Based on the way this fungicide spread with the plant (it generally goes up), there is little chance that a spray application to the leaves could result in a significant effect down in the stem. The repeated use of myclobutanil may result in the development of resistance to the pesticide in the populations of the fungus.Captan is a preventative treatment. Therefore, it has no effects on plants that are already infected. Chilling storage is not expected to kill the fungus inside the plant.Uncertainties:– There is uncertainty on the level to which Myclobutanil sprayed on leaves may be translocated down into the plant thereby preventing or curing infections. |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | Yes, for bare rooted plants | Rinsing the plants may remove the frass which is an evidence of beetle presence in the wood.Uncertainties:– There is no information whether the plants carrying frass are removed from the lot before rinsing. | Not applicable |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Given the inspection frequency, it is likely that the frass caused by beetle and wilting caused by the fungus is detected. However, newly infested trees may be difficult to detect.Uncertainties:– Considering the small size of the plants, it is difficult to find frass.– It is not known if the pesticide application is reliable for controlling the pest inside the wood. | Given the inspection frequency, it is likely that the frass caused by beetle and wilting caused by the fungus is detected. However, newly infested trees may be difficult to detect.Uncertainties:– Considering the even smaller size of the plants, it is unlikely to find frass.– It is not known if the pesticide application is reliable for controlling the pest inside the wood. |
| 11 | Inspections before export | Yes | It is likely that the beetle is detected based on frass. However, newly infested trees may be difficult to detect.Uncertainties:– There is no information whether the plants carrying frass are removed from the lot before rinsing. | It is likely that the beetle is detected based on frass. However, newly infested trees may be difficult to detect.Uncertainties:– Considering the even smaller size of the plants it is unlikely to find frass. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, E. fornicatus is common in Israel.Uncertainties:– There is no information on the density of E. fornicatus in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, E. fornicatus is common in Israel.Uncertainties:– There is no information on the density of E. fornicatus in the surrounding areas. |
A.3.5. Overall likelihood of pest freedom for bare rooted plants
A.3.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants
Although both E. fornicatus and N. euwallaceae are present in Israel, the scenario assumes a low pest pressure from outside, and a short distance dispersal of the vector. The Panel also considers that, due to the small size of the plants, they are too small to be attractive for the beetles. The diameter of the bare rooted plants in the nursery is mainly below the threshold of 2 cm of suitability for colonisation. Inspections are expected to be effective because frass originated by beetles is clearly visible.
A.3.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants
Euwallacea fornicatus and N. euwallaceae are present in Israel. E. fornicatus has a very high biotic potential, and N. euwallaceae has been reported on Ficus in Israel. The scenario assumes a high pest pressure from outside so that the beetle is pushed to colonise small trees. Diameter of bare rooted plants is big enough to be colonised, as colonisations in diameters of 1.5 cm have been reported from Bolzano outbreak. It is also taken into consideration the quick growth of F. carica. Pesticide treatments and inspections are expected not to be effective because of beetles are mainly inside the wood.
A.3.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants (Median)
Even when there is a high uncertainty regarding the pest pressure from outside, the Panel considers that the pest could be present in the surrounding and could also enter the nursery, although it is not likely that small trees are attractive for the beetle. In consequence, the Panel assumes a lower central scenario which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.3.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery results in high level of uncertainties for infestation rates below the median. Otherwise small trees are less attractive for the pest and the diameter is around the threshold of suitability for colonisations, which gives less uncertainties for rates above the median.
A.3.5.5. Elicitation outcomes of the assessment of the pest freedom for Euwallacea fornicatus and Neocosmospora euwallaceae on bare rooted plants
The following tables show the elicited and fitted values for pest infestation/infection (Table A.7) and pest freedom (Table A.8).
Table A.7.
Elicited and fitted values of the uncertainty distribution of pest infestation by Euwallacea fornicatus and Neocosmospora euwallaceae per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2.00 | 6.00 | 10.0 | 20.0 | 50.0 | ||||||||||
| EKE | 2.08 | 2.29 | 2.64 | 3.35 | 4.34 | 5.66 | 7.13 | 10.7 | 15.7 | 19.1 | 24.0 | 30.2 | 38.5 | 46.9 | 57.8 |
The EKE results is the Gamma (1.0454, 11.877, RiskShift (1.93)) distribution fitted with @Risk version 7.6.
Table A.8.
The uncertainty distribution of plants free of Euwallacea fornicatus and Neocosmospora euwallaceae per 10,000 plants calculated by Table A.7
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,950 | 9,980 | 9,990 | 9,994 | 9,998 | ||||||||||
| EKE results | 9,942 | 9,953 | 9,961 | 9,970 | 9,976 | 9,981 | 9,984 | 9,989 | 9,992.9 | 9,994.3 | 9,995.7 | 9,996.7 | 9,997.4 | 9,997.7 | 9,997.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.8.
Figure A.5.
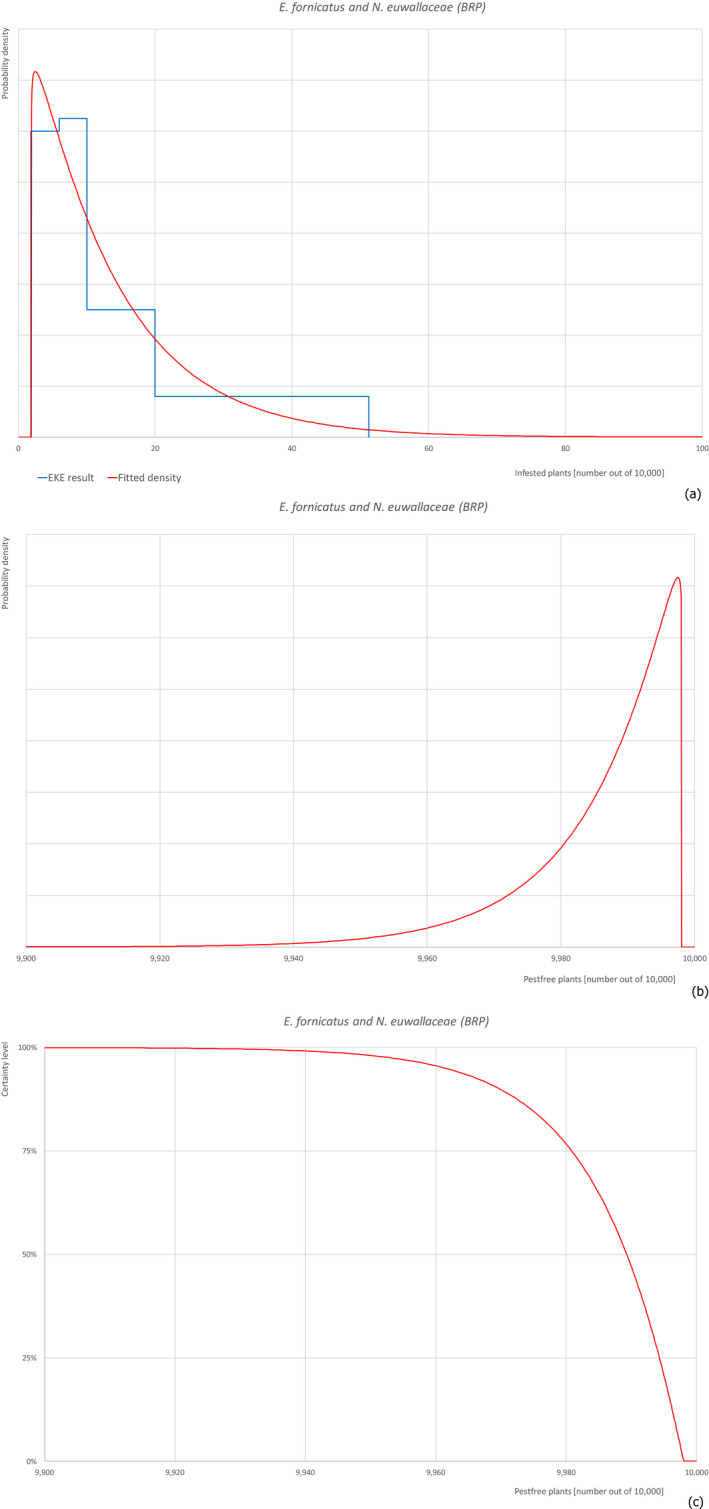
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.3.6. Overall likelihood of pest freedom for liners
A.3.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested liners
Although both E. fornicatus and N. euwallaceae are present in Israel, the scenario considers that, due to the small size of the liners, they are too small to be attractive for the beetles, and their diameters are below the threshold of 1.5 cm of suitability for colonisation. The Panel also assumes a low pest pressure from outside.
A.3.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested liners
Euwallacea fornicatus and N. euwallaceae are present in Israel. Euwallacea fornicatus has a very high biotic potential, and N. euwallaceae has been reported on Ficus in Israel. The scenario assumes a high pest pressure from outside so that the beetle is pushed to colonise small plants. However, diameters of liners are mainly lower than 1.5 cm, but in this scenario, it is assumed that some of them could be eventually larger than 1.5 cm. Pesticide treatments and inspections are expected not to be effective because of beetles are mainly inside the wood.
A.3.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested liners (Median)
Even when there is a high uncertainty regarding the pest pressure from outside, the Panel considers that the pest could be present in the surrounding and could also enter the nursery, although it is not likely that liners are attractive for the beetle. In consequence, the Panel assumes a lower central scenario which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.3.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery it results in high level of uncertainties for infestation rates below the median. Otherwise liners are less attractive for the pest and the diameter is mainly below the threshold of suitability for colonisations, which gives less uncertainties for rates above the median.
A.3.6.5. Elicitation outcomes of the assessment of the pest freedom for Euwallacea fornicatus and Neocosmospora euwallaceae on liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.9) and pest freedom (Table A.10).
Table A.9.
Elicited and fitted values of the uncertainty distribution of pest infestation by Euwallacea fornicatus and Neocosmospora euwallaceae per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.00 | 1.50 | 3.00 | 10.0 | 30.0 | ||||||||||
| EKE | 0.02 | 0.05 | 0.13 | 0.33 | 0.66 | 1.19 | 1.85 | 3.69 | 6.67 | 8.98 | 12.5 | 17.2 | 24.2 | 31.6 | 42.0 |
The EKE results is the Weibull (0.77815, 5.9024) distribution fitted with @Risk version 7.6.
Table A.10.
The uncertainty distribution of plants free of Euwallacea fornicatus and Neocosmospora euwallaceae per 10,000 plants calculated by Table A.9
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,970 | 9,990 | 9,997 | 9,999 | 10,000 | ||||||||||
| EKE results | 9,958 | 9,968 | 9,976 | 9,983 | 9,988 | 9,991 | 9,993 | 9,996 | 9,998.2 | 9,998.8 | 9,999.3 | 9,999.7 | 9,999.9 | 9,999.9 | 10,000.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.10.
Figure A.6.
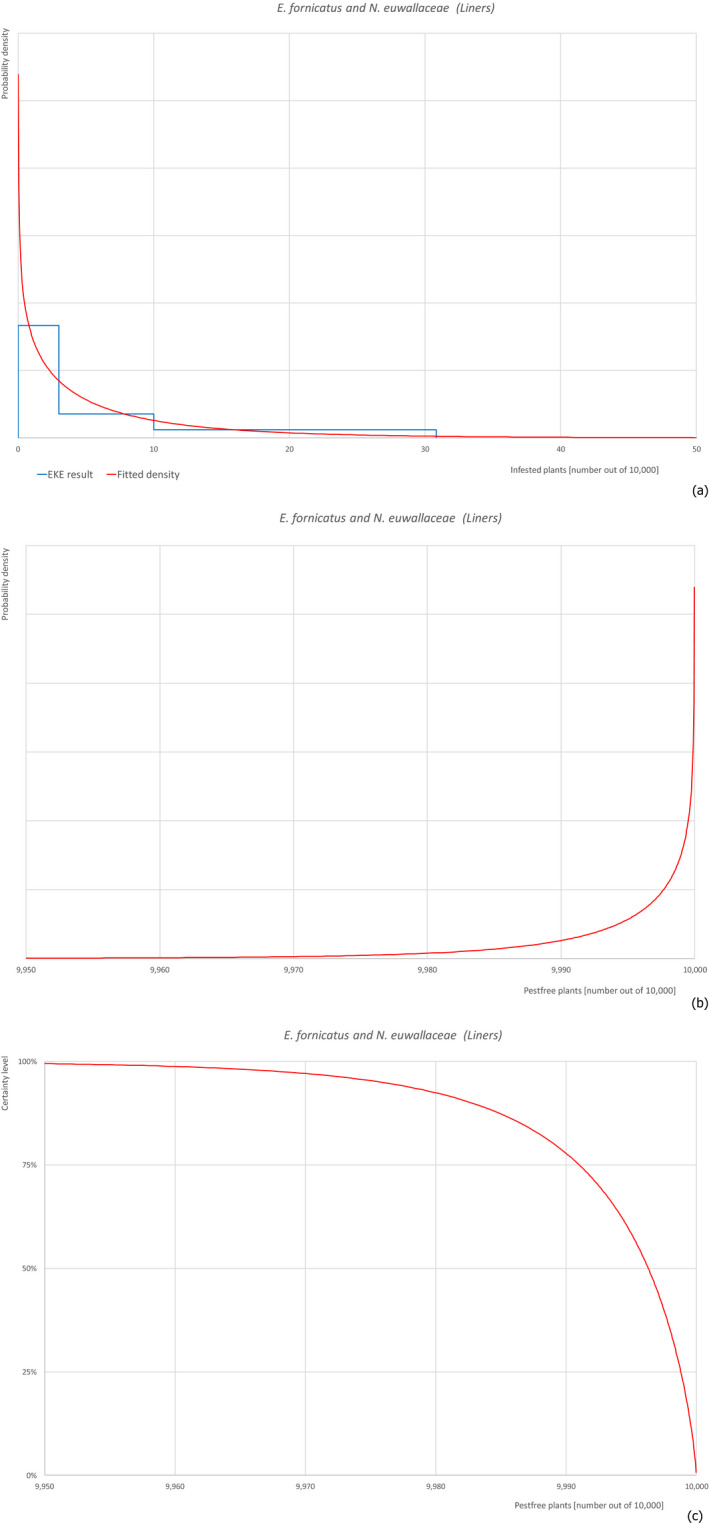
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
Figure A.7.
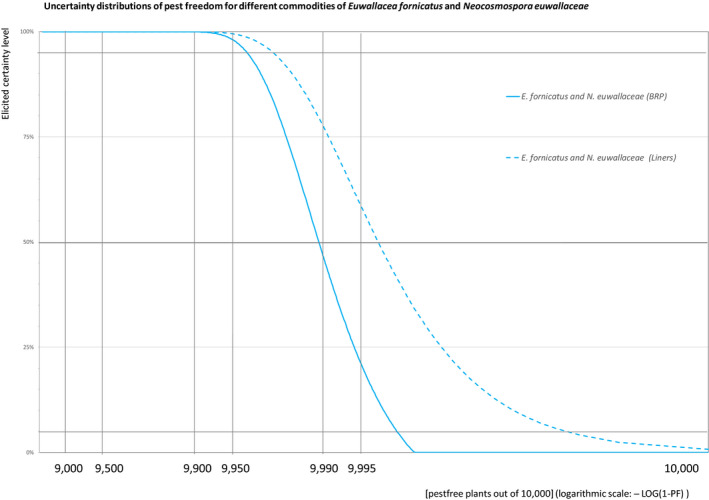
Elicited certainty (y‐axis) of the number of bare rooted plants or liners of Ficus carica pest free from Euwallacea fornicatus and Neocosmospora euwallaceae (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU introduced from Israel as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
A.3.7. Reference list
Browne FG, 1961. The biology of Malayan Scolytidae and Platypodidae. Malyan Forest Records, 22, 100–174.
CABI (Centre for Agriculture and Bioscience International), online. Euwallacea fornicatus (tea shot‐hole borer). Available online: https://www.cabi.org/isc/datasheet/57163 [Accessed: 11 July 2019].
California Department of Fish and Wildlife. Available online: http://www.southcoastsurvey.org/static_mapper/fieldguide/Kuroshio-and-Polyphagous-Shot-Hole-Borer-and-Associated-Host-Identification-Guide.pdf [Accessed: 6 November 2019].
Calnaido D, 1965. The flight and dispersal of shot‐hole borer of tea (Xyleborus fornicatus Eichh., Coleoptera: Scolytidae). Entomologia Experimentalis et Applicata, 8, 249–262. https://doi.org/10.1111/j.1570-7458.1965.tb00859.x
Coleman TW, Eskalen A and Stouthamer R, 2013. New Pest Complex in California: The Polyphagous Shot Hole Borer, Euwallacea sp., and Fusarium Dieback, Fusarium euwallaceae. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5441465.pdf
de Beer ZW and Paap T, 2019. The Polyphagous Shot Hole Borer (Euwallacea whitfordiodendrus) and Fusarium dieback (Fusarium euwallaceae). FABI (Forestry and Agricultural Biotechnology Institute). Available online: https://www.fabinet.up.ac.za/pdf/PSHB/1-PSHB_info_2019-03-22.pdf
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Chatzivassiliou E, Debode J, Manceau C, de la Peña E, Gardi C, Mosbach‐Schulz O, Preti S and Potting R, 2020_a. Scientific Opinion on the commodity risk assessment of Robinia pseudoacacia plants from Israel. EFSA Journal 2020;18(3):6039, 34 pp. https://doi.org/10.2903/j.efsa.2020.6039
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques MA, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Chatzivassiliou E, Debode J, Manceau C, de la Peña E, Gardi C, Mosbach‐Schulz O, Preti S and Potting R, 2020_b. Scientific Opinion on the commodity risk assessment of Albizia julibrissin plants from Israel. EFSA Journal 2020;18(1):5941, 49 pp. https://doi.org/10.2903/j.efsa.2020.5941
EPPO (European and Mediterranean Plant Protection Organization), 2016. Mini data sheet on Euwallacea sp. And its symbiotic fungus Fusarium euwallaceae. Available online: https://gd.eppo.int/taxon/FUSAEW/documents
EPPO (European and Mediterranean Plant Protection Organization), 2017. Report of a Pest Risk Analysis for Euwallacea fornicatus sensu lato and Fusarium euwallaceae. Available online: https://gd.eppo.int/taxon/FUSAEW/documents
EPPO (European and Mediterranean Plant Protection Organization), 2020. EPPO Technical Document No. 1081, EPPO Study on the risk of bark and ambrosia beetles associated with imported non‐coniferous wood, EPPO Paris. Available online: https://www.eppo.int/RESOURCES/eppo_publications
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO Global Database: Euwallacea fornicatus. Available online: https://gd.eppo.int/taxon/XYLBFO [Accessed: 11 July 2019].
EPPO (European and Mediterranean Plant Protection Organization), online_b. EPPO Global Database: Fusarium euwallaceae. Available online: https://gd.eppo.int/taxon/FUSAEW [Accessed: 16 July 2019].
Eskalen A, Stouthamer R, Lynch SC, Twizeyimana M, Gonzalez A and Thibault T, 2013. Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Disease, 97, 938–951. https://doi.org/10.1094/pdis-11-12-1026-re
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 15 January 2020].
Europhyt Oubreaks database, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 31 August 2020].
FERA (Food and Environment Research Agency), 2015. Rapid Pest Risk Analysis (PRA) for Polyphagous Shot Hole Borer (Euwallacea sp.) and Fusarium Dieback (Fusarium euwallaceae). Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/downloadExternalPra.cfm?id=4055
Freeman S, Sharon M, Maymon M, Mendel Z, Protasov A, Aoki T, Eskalen A and O'Donnell K, 2013. Fusarium euwallaceae sp. nov. — a symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia, 105(6), 1595–1606. https://doi.org/10.3852/13-066
Gomez DF, Skelton J, Steininger MS, Stouthamer R, Rugman‐Jones P, Sittichaya W, Rabaglia RJ and Hulcr J, 2018. Species delineation within the Euwallacea fornicatus (Coleoptera: Curculionidae) complex revealed by morphometric and phylogenetic analyses. Insect Systematics and Diversity, 2, 1–11. https://doi.org/10.1093/isd/ixy018
Greer K, Rice K and Lynch SC, 2018. Southern California Shot Hole Borers/Fusarium Dieback Management Strategy for Natural and Urban Landscapes. Available online: http://www.southcoastsurvey.org/static_mapper/fieldguide/Southern%20California%20Shot%20Hole%20Borers-Fusarium%20Dieback%20Management%20Strategy%20for%20Natural%20and%20Urban%20Landscapes%20-%20updated%20July%202018.pdf
Judenko E, 1956. Research work on the shot‐hole borer, October, 1955 to August, 1956. Tea Quarterly, 27, 103–105.
Kirkendall LR and Ødegaard F, 2007. Ongoing invasions of old‐growth tropical forests: establishment of three incestuous beetle species in southern Central America (Curculionidae: Scolytinae). Zootaxa, 1588, 53–62.
Kumar R, Rajkhowa G, Sankar M and Raja RK, 2011. A new host plant for the shoot‐hole borer, Euwallacea fornicatus (Eichhoff) (Coleoptera: Scolytidae) from India. Acta Entomologica Sinica, 54, 734–738.
Mendel Z, Protasov A, Sharon M, Zveibil A, Ben Yehuda S, O'Donnell K, Rabaglia R, Wysoki M and Freeman S, 2012. An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica, 40, 235–238. https://doi.org/10.1007/s12600-012-0223-7
Mendel Z, Protasov A, Maoz Y, Maymon M, Miller G, Elazar M and Freeman S, 2017. The role of Euwallacea nr. fornicatus (Coleoptera: Scolytinae) in the wilt syndrome of avocado trees in Israel. Phytoparasitica, 45, 341–359. https://doi.org/10.1007/s12600-017-0598-6
Ministerio De Agricultura, Alimentacion Y Medio Ambiente, 2015. Express Pest Risk Analysis For The Ambrosia* beetle Euwallacea sp. including all the species within the genus Euwallacea that are morphologically similar to E. fornicatus. Reino De España, Dirección General de Sanidad de la Producción Agraria Subdirección General de Sanidad e Higiene Vegetal y Forestal. Available online: https://gd.eppo.int/download/doc/1267_pra_exp_XYLBFO.pdf
Owens D, Seo M, Montgomery WS, Rivera MJ, Stelinski LL and Kendra PE, 2019. Dispersal behaviour of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae) in avocado groves and estimation of lure sampling range. Agricultural and Forest Entomology, 21, 199–208. https://doi.org/10.1111/afe.12321
Paap T, de Beer ZW, Migliorini D, Nel WJ and Wingfield MJ, 2018. The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: a new invasion in South Africa. Australasian Plant Pathology, 47, 23–237. https://doi.org/10.1007/s13313-018-0545-0
Sandoval‐Denis M, Lombard L and Crous PW, 2019. Back to the roots: a reappraisal of Neocosmospora. Persoonia‐Molecular Phylogeny and Evolution of Fungi, 43, 90–185.
Smith SM, Gomez DF, Beaver RA, Hulcr J and Cognato AI, 2019. Reassessment of the species in the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) complex after the rediscovery of the ‘lost’ type specimen. Insects, 10, 261. https://doi.org/10.3390/insects10090261
Walgama RS, 2012. Ecology and integrated pest management of Xyleborus fornicatus (Coleoptera: Scolytidae) in Sri Lanka. Journal of Integrated Pest Management, 3, A1‐A8. https://doi.org/10.1603/ipm11031
A.4. Hypothenemus leprieuri
A.4.1. Organism information
| Taxonomic information | Current valid scientific name: Hypothenemus leprieuriSynonyms: Dryocoetes leprieuri, Stephanoderes albipilis, Hypothenemus kraussei, Adiaeretus albipilis, Adiaeretus kraussei, Adiaeretus leprieuri, Archeophalus albipilis, Archeophalus kraussei, Archeophalus leprieuri, Chondronoderes albipilis, Chondronoderes kraussei, Chondronoderes leprieuri, Epsips albipilis, Epsips kraussei, Epsips leprieuri, Ernophloeus albipilis, Ernophloeus kraussei, Ernophloeus leprieuri, Homoeocryphalus albipilis, Homoeocryphalus kraussei, Homoeocryphalus leprieuri, Hypothenemus albipilis, Hypothenemus kraussei, Lepiceroides albipilis, Lepiceroides kraussei, Lepiceroides leprieuri, Macrocryphalus albipilis, Macrocryphalus kraussei, Macrocryphalus leprieuri, Pachynoderes albipilis, Pachynoderes kraussei, Pachynoderes leprieuri, Stephanoderes albipilis, Stephanoderes kraussei, Stephanoderes leprieuri, Stylotentus albipilis, Stylotentus kraussei, Stylotentus leprieuri, Triarmocerus albipilis, Triarmocerus kraussei, Triarmocerus leprieuri (de Jong et al., online)Name used in the EU legislation: Listed as EU‐quarantine pest as Scolytidae spp. (non‐European) [1SCOLF]Order: ColeopteraFamily: CurculionidaeSubfamily: ScolytinaeCommon name:–Name used in the Dossier: – | |
|---|---|---|
| Group | Insects | |
| EPPO code | HYOTLE | |
| Regulated status | The pest is listed in Part A of Annex II of Regulation (EU) 2019/2072, under the family Scolytidae spp. (non‐European) [1SCOLF].Hypothenemus leprieuri is not regulated anywhere else in the world neither listed by EPPO. | |
| Pest status in Israel | Hypothenemus leprieuri is present in Israel (Mifsud and Knizek, 2009). | |
| Pest status in the EU | Hypothenemus leprieuri is present in Cyprus, Malta and Sardinia (de Jong et al., online; Mifsud and Knizek, 2009). | |
| Host status on Ficus carica | Ficus carica is reported as a host of H. leprieuri (Mifsud and Knizek, 2009). | |
| PRA information | No Pest Risk Assessment is currently available. | |
| Other relevant information for the assessmentAccording to Dossier Section 9.0, bare rooted plants are 20–100 cm tall, with base diameter of up to 2 cm. Liners are about 10 cm high and with ~ 1 cm base diameter. | ||
| Biology | No information | |
| Symptoms | Main type of symptoms | There is no information in the literature. According to what is known for the congeneric Hypothenemus eruditus, it should develop under the bark of small branches and twigs. Body length of H. leprieuri (1.5 mm) (Faccoli et al., 2016) is higher than that of H. eruditus (1 mm) (EPPO 2020). Frass coming out from small entrance holes. |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pests | Hypothenemus leprieuri can be confused with Hypocryphalus scabricollis, a pest of Ficus in the Mediterranean. The two species have similar size and they can be distinguished from each other through the antennae (Faccoli et al., 2016). | |
| Host plant range | Ficus carica is the only known host plant of H. leprieuri (Mifsud et al., 2012) | |
| Pathways | The main pathways for entry of the non‐European Scolytinae are: plants for planting (including seeds), with or without soil, cut branches, fruits, round wood with bark, round wood without bark, sawn wood without bark, sawn wood with bark, wood packaging material, bark, manufactured wood items and wood chips (EFSA PLH Panel, 2020). | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.4.2. Possibility of pest presence in the nursery
A.4.2.1. Possibility of entry from the surrounding environment
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above, none of them are known to be hosts of H. leprieuri.
According to Dossier Section 9.0, bare rooted plants are 20–100 cm tall, with base diameter of up to 2 cm. Liners are about 10 cm high and with ~ 1 cm base diameter.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery because fig trees can occur around the nursery for local market or private gardens.
A.4.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the insect and the pathogen could enter the nursery with new plants/seeds or soil growing media.
A.4.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
Lagerstroemia indica and Morus alba are grown in the nursery (Dossier Section 9.0) but are not hosts of the pest.
Uncertainties
-
–
There is no information on the presence or population pressure of the pests in the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible.
A.4.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of H. leprieuri between the years 1995 and November 2019 (EUROPHYT, online).
A.4.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently proposed in Israel are summarised and an indication of their effectiveness on H. leprieuri is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | Beetles may immigrate the production fields from the surrounding environment and attack plants grown in open fields. Plants grown in net house are not protected by the net.Uncertainties:– No uncertainties | Plants grown in net house are not protected by the net.Uncertainties:– No uncertainties |
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Residual efficacy of the applied insecticides may not protect the plants for the full period covered by treatments.Uncertainties:– It is not certain whether the beetle can be affected by the use of pesticides. | Residual efficacy of the applied insecticides may not protect the plants for the full period covered by treatments.Uncertainties:– It is not certain whether the beetle can be affected by the use of pesticides. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | Yes, for bare rooted plants | Rinsing the plants may remove the frass which is an evidence of the beetle present under the bark.Uncertainties:– There is no information whether the plants carrying frass are removed from the lot before rinsing. | Not applicable |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Given the inspection frequency, it is likely that the frass caused by the beetle is detected. However, newly infested trees may be difficult to detect.Uncertainties:– Considering the small size of the plants, it is difficult to find frass.– It is not known whether the pesticide application is reliable for controlling the pest under the bark. | Given the inspection frequency, it is likely that the frass caused by the beetle is detected. However, newly infested trees may be difficult to detect.Uncertainties:– Considering the small size of the plants, it is difficult to find frass.– It is not known whether the pesticide application is reliable for controlling the pest under the bark. |
| 11 | Inspections before export | Yes | It is likely that the beetle is detected based on frass. However, newly infested trees may be difficult to detect.Uncertainties:– There is no information whether the plants carrying frass are removed from the lot before rinsing. | It is likely that the beetle is detected based on frass. However, newly infested trees may be difficult to detect.Uncertainties:– Considering the even smaller size of the plants, it is unlikely to find frass. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, H. leprieuri is common in Israel.Uncertainties:– There is no information on the density of H. leprieuri in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, H. leprieuri is common in Israel.Uncertainties:– There is no information on the density of H. leprieuri in the surrounding areas. |
A.4.5. Overall likelihood of pest freedom for bare rooted plants and liners
A.4.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants and liners
Although H. leprieuri is reported as specialised on F. carica and present in Israel, the scenario assumes a very low pest pressure from outside and limited transfer from the surrounding due to human activity and active flight. Inspections are expected to be effective as symptoms and frass can be easily detected. The nursery plants are expected to be poorly attractive for the beetle because they are vigorous. Mother plants are kept healthy by using treatments and a correct management.
A.4.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants and liners
Hypothenemus leprieuri is specialised on F. carica and is present in Israel. The scenario assumes that an introduction of the beetle from the surroundings may occur because the presence of wild and semi‐wild F. carica in the surrounding of the nursery. However, the introduction is very unlikely because the beetle is rare. Mother plants could act as reservoir, if they are stressed for any reason, e.g. phytosanitary, cultural.
A.4.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants and liners (Median)
Regarding the lack of information on the pest, the Panel assumes a central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants expressing the highest uncertainty.
A.4.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The lack of information on the pest results in the highest level of uncertainties for infestation rates below and above the median.
A.4.5.5. Elicitation outcomes of the assessment of the pest freedom for Hypothenemus leprieuri on bare rooted plants and liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.11) and pest freedom (Table A.12).
Table A.11.
Elicited and fitted values of the uncertainty distribution of pest infestation by Hypothenemus leprieuri per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.00 | 1.25 | 2.50 | 3.75 | 5.00 | ||||||||||
| EKE | 0.05 | 0.13 | 0.25 | 0.50 | 0.84 | 1.25 | 1.66 | 2.50 | 3.33 | 3.75 | 4.18 | 4.52 | 4.78 | 4.91 | 4.99 |
The EKE results is the BetaGeneral (1.0097, 1.0261, 0, 5.05) distribution fitted with @Risk version 7.6.
Table A.12.
The uncertainty distribution of plants free of Hypothenemus leprieuri per 10,000 plants calculated by Table A.11
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,995 | 9,996 | 9,998 | 9,999 | 10,000 | ||||||||||
| EKE results | 9,995 | 9,995 | 9,995 | 9,995 | 9,996 | 9,996 | 9,997 | 9,998 | 9,998.3 | 9,998.7 | 9,999.2 | 9,999.5 | 9,999.7 | 9,999.9 | 9,999.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.12.
Figure A.8.
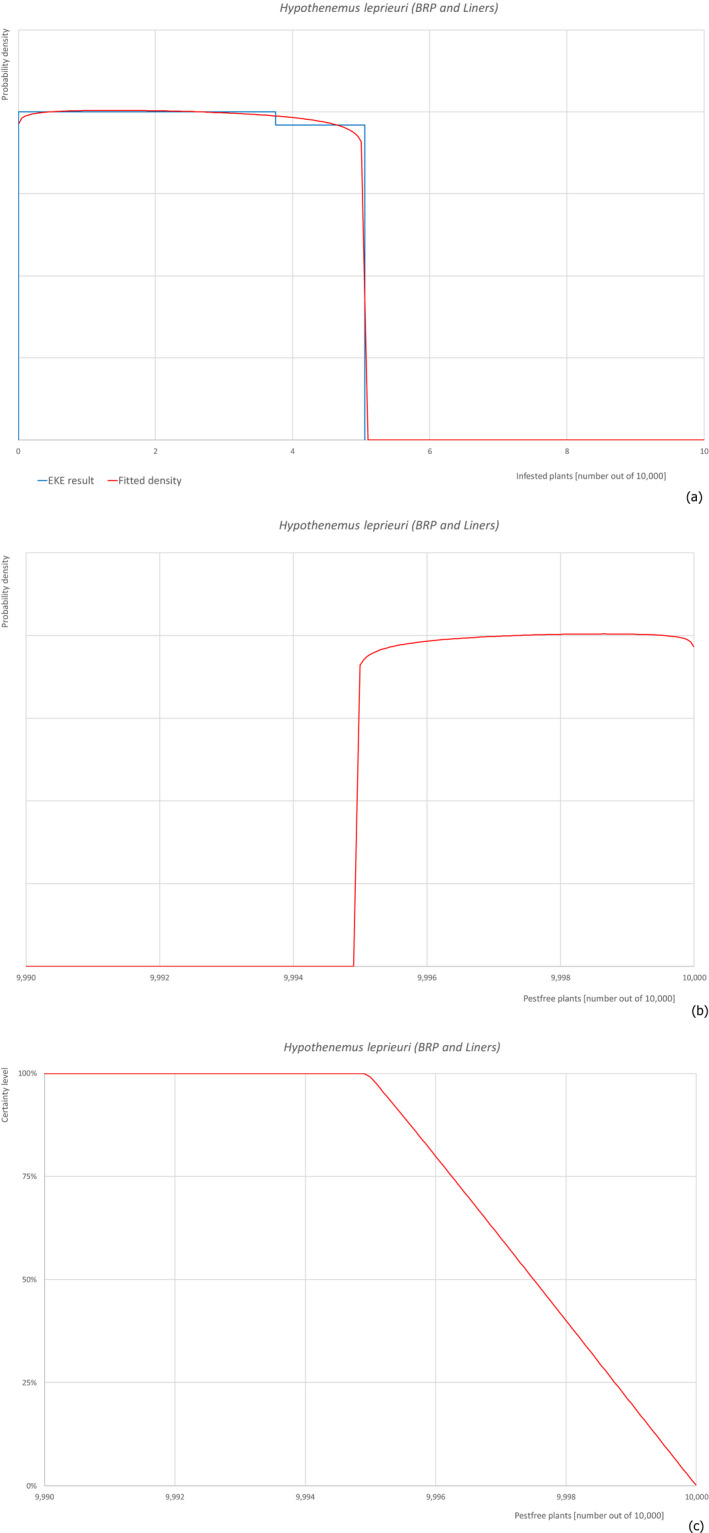
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.4.6. Reference list
de Jong Y, Verbeek M, Michelsen V, de Place Bjørn P, Los W, Steeman F, Bailly N, Basire C, Chylarecki P, Stloukal E, Hagedorn G, Wetzel FT, Glöckler F, Kroupa A, Korb G, Hoffmann A, Häuser C, Kohlbecker A, Müller A, Güntsch A, Stoev P, Penev L, online. Fauna Europaea, All European animal species online. Hypothenemus leprieuri (Perris, 1866). Available online: https://fauna-eu.org/cdm_dataportal/taxon/b829c9d3-1499-4a89-a7dd-ea5de17d7ac7 [Accessed: 30 April 2020].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Gregoire J‐C, Kertesz V, Streissl F and Milonas P, 2020. Scientific Opinion on the pest categorisation of non‐EU Scolytinae of coniferous hosts. EFSA Journal 2020;18(1):5934, 39 pp. https://doi.org/10.2903/j.efsa.2020.5934
EPPO (European and Mediterranean Plant Protection Organization), 2020. EPPO Technical Document No. 1081, EPPO Study on the risk of bark and ambrosia beetles associated with imported non‐coniferous wood, EPPO Paris. Available online: https://www.eppo.int/RESOURCES/eppo_publications
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 30 April 2020].
Faccoli M, Campo G, Perrotta G and Rassati D, 2016. Two newly introduced tropical bark and ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) damaging figs (Ficus carica) in southern Italy. Zootaxa, 4138, 189–194. https://doi.org/10.11646/zootaxa.4138.1.10
Mifsud D and Knizek M, 2009. The Bark Beetles (Coleoptera: Scolytidae) of the Maltese Islands (Central Mediterranean). Bulletin of the Entomological Society of Malta, 2, 25–52.
Mifsud D, Falzon A, Malumphy C, Lillo ED, Vovlas N and Porcelli F, 2012. On some arthropods associated with Ficus species (Moraceae) in the Maltese Islands. Bulletin of the Entomological Society of Malta, 5, 5–34.
A.5. Icerya aegyptiaca
A.5.1. Organism information
| Taxonomic information |
Current valid scientific name: Icerya aegyptiaca Synonyms: Crossotosoma aegyptiacum, Icerya aegyptiacum, Icerya tangalla Name used in the EU legislation: – Order: Hemiptera Family: Monophlebidae Common name: breadfruit mealybug, Egypt Icerya, Egyptian cushion scale, Egyptian fluted scale, Egyptian mealybug, Egyptian cottony cushion scale Name used in the Dossier: – |
|
|---|---|---|
| Group | Insects | |
| EPPO code | ICERAE | |
| Regulated status |
Icerya aegyptiaca is not regulated in the EU neither listed by EPPO. The pest is quarantine in Mexico and United States of America (EPPO, online_a). |
|
| Pest status in Israel | Present, widespread (Ben‐Dov, 2012; CABI, online; EPPO, online_b; García Morales et al., online). | |
| Pest status in the EU | Absent (CABI, online; EPPO, online_b; García Morales et al., online). | |
| Host status on Ficus carica | Ficus carica is a host of Icerya aegyptiaca (García Morales et al., online). | |
| PRA information |
Only Pest Risk Assessment currently available is from New Zealand: – Import risk analysis: Fresh Coconut (Cocos nucifera) from Tuvalu (Hardy, 2009) and Import Risk Analysis: Pears (Pyrus bretschneideri, Pyrus pyrifolia and Pyrus sp. nr. communis) fresh fruit from China (Tyson et al., 2009). |
|
| Other relevant information for the assessment | ||
| Biology |
Icerya aegyptiaca is either Australasian or Indo‐Malayan species (Unruh and Gullan, 2008). Icerya aegyptiaca is parthenogenic and it goes through five life stages: an egg, three larval instars and an adult. So far males have never been found. In Egypt there can be two or partially three generations per year. Depending on temperature, the duration of the life cycle ranges from 87.2 (28.7°C) to 105.4 days (26.4°C). The peak of adults can be observed in summer (Waterhouse, 1993). Female can lay from 70 to up to 200 eggs, which have yellow orange colour. They are laid into a waxy egg sac, attached to the abdomen. The egg sac is ruptured by the first‐instar larvae. They are bright orange crawlers, which settle within a day and become covered by a wax. The second and third instar larvae are yellow orange covered with white mealy secretion. Adults are deep orange with blackish legs and antennae. They are covered with white mealy secretion, mingled with granular wax. Through this waxy covering, the body appears salmon pink (Waterhouse, 1993). The main economic impact is reported on breadfruit trees, but also on avocado, banana, citrus, taro and young coconut palms (Waterhouse, 1993). In Egypt, I. aegyptiaca was reported as a serious pest of citrus, figs and shade trees (Waterhouse, 1993). |
|
| Symptoms | Main type of symptoms |
Main symptoms are white wax on leaves, leaf drop and dieback of branches (Uesato et al., 2011). Heavy infestations of mealybugs reduce yield and may cause death of plants (Waterhouse, 1993). On breadfruit trees, I. aegyptiaca can be usually found along the midribs and larger veins on the undersides of the leaves, and on fruits (Waterhouse, 1993). Icerya aegyptiaca produce honeydew, which is colonised by sooty mould that covers leaves and interferes with photosynthesis. The honeydew may be gathered by ants that hamper pest control by its many natural enemies (Gerson and Aplebaum, online). According to Uesata et al. (2011) in Japan, I. aegyptiaca produces little or no honeydew and it is rarely associated with sooty mould. |
| Presence of asymptomatic plants | Plant damage might not be obvious in early infestation, but the presence of scales on the plants could be observed because of white wax cover. During the crawler stage, infestation is difficult to be noted. | |
| Confusion with other pathogens/pests | Icerya aegyptiaca is very similar to Icerya imperatae. They can be distinguished from each other by specific morphological features (Miller et al., online; Unruh and Gullan 2008). | |
| Host plant range | Icerya aegyptiaca is highly polyphagous pest of 113 hosts at genus level (García Morales et al., online). The hosts of I. aegyptiaca are apple (Malus domestica), avocado (Persea americana), banana (Musa ap.), black pepper (Piper nigrum), breadfruit tree (Artocarpus altilis), citrus (Citrus sp.), coconut (Coccos nucifera), coffee (Coffea ap.), European pear (Pyrus communis), fig (Ficus sp.), maize (Zea mays), mora (Morus alba), roses (Rosa ap.), shoeblackplant (Hibiscus rosa‐sinensis), thuja (Thuja sp.), tomato (Solanum lycopersicum), vine (Vitis vinifera) and many more (CABI, online; García Morales et al., online). | |
| Pathways | Leaves, stems and whole plant are affected at flowering, fruiting and vegetative growing stages. Leaves, young stems or fruits are attacked (Tyson et al., 2009). Possible pathways of entry for I. aegyptiaca are Ficus plants without leaves (on the bark of stems). | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.5.2. Possibility of pest presence in the nursery
A.5.2.1. Possibility of entry from the surrounding environment
Icerya aegyptiaca is present in Israel (CABI, online; Gerson and Aplebaum, online). Possible pathways of spreading throughout the area and into the nursery can be by movement of infested plants, wind, human and animal dispersal. So far there is no information of males being present. Females do not fly (Waterhouse, 1993).
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Diospyros vera, Punica granatum, Acacia, Acacia decurrens are hosts of I. aegyptiaca (García Morales et al., online).
Uncertainties:
-
–
No information about the density of the population of I. aegyptiaca in the area surrounding the nursery is available.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by wind and human accidental transportation.
A.5.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media. Plants are produced inside the nursery and the scale insects are not associated with soil growing media.
A.5.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
According to Dossier Section 9.0, following plants are grown in the fig liner export nursery: Lagerstroemia indica and Morus alba, with a distance of a few dozens of metres between them and the fig liners. Morus alba is a host plant to Icerya aegyptiaca.
Therefore, it is possible for I. aegyptiaca to reproduce within the nursery on F. carica and on other hosts, which are present.
Possible pathways of spreading within the nursery can be by movement of infested plants, wind, human and animal dispersal. The first nymph instars (crawlers) can disperse by walking and by wind (Mani and Shivaraju, 2016).
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible either by wind or accidental transfer within the nursery.
A.5.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of I. aegyptiaca between the years 1995 and November 2019 (EUROPHYT, online).
According to Unruh and Gullan (2008), I. aegyptiaca was intercepted in England. This species was intercepted eight times at U.S. ports‐of‐entry on a variety of hosts (probably fruits, including Ficus from Egypt) between 1995 and 2012, with specimens originating from Egypt, Israel, Malaysia, Nigeria, The Philippines, Singapore, Syrian Arab Republic and Thailand (Miller et al., online).
A.5.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on Icerya aegyptiaca is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties |
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Pesticide sprays are generally effective against crawlers but have limited effectiveness against I. aegyptiaca when hidden in crevices, or protected by the waxy covering of its body.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty whether the pesticide can effectively reach all the bark parts where the scales are located because of the barrier effect of the leaves. | Pesticide sprays are generally effective against crawlers but have limited effectiveness against I. aegyptiaca when hidden in crevices, or protected by the waxy covering of its body.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty whether the pesticide can effectively reach all the bark parts where the scales are located because of the barrier effect of the leaves. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | No | Not applicable | Not applicable |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Scales could go undetected because of the small size of the pest and difficulty in the search, although white wax cover should make them obvious. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for scales is not known. | Scales could go undetected because of the small size of the pest and difficulty in the search, although white wax cover should make them obvious. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for scales is not known. |
| 11 | Inspections before export | Yes | Scales could go undetected because of the small size of the pest and difficulty in the search. In early stages of infestation and during dormancy symptoms may not be clear, although white wax cover should make them obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for scales is not known. | Scales could go undetected because of the small size of the pest and difficulty in the search. In early stages of infestation and during dormancy symptoms may not be clear, although white wax cover should make them obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for scales is not known. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, I. aegyptiaca is common in Israel.Uncertainties:– There is no information on the density of I. aegyptiaca in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, I. aegyptiaca is common in Israel.Uncertainties:– There is no information on the density of I. aegyptiaca in the surrounding areas. |
A.5.5. Overall likelihood of pest freedom for bare rooted plants and liners
A.5.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants and liners
Although I. aegyptiaca is common in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to wind and human activity. Inspections are expected to be effective because sessile stages of the insect are visible and honey dew is produced. Ficus carica is considered to be a minor host. Insecticide treatments are expected to be conducted at the right timing to target unprotected life stages of the insect. Mother plants are kept healthy as well by using treatments.
A.5.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants and liners
Icerya aegyptiaca is common in Israel; the scenario assumes a high pest pressure from outside and strong transfer from the surrounding due to wind and intensive human activity. Although honeydew is produced, inspections are expected to be ineffective because of the presence of hidden stages. Ficus carica is considered to be a major host. Insecticide treatments are expected to be conducted at timing when the insect is protected by wax. Mother plants are infested despite treatments and may contribute spreading the pest within the nursery.
A.5.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants and liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by wind and human activity, the weak information on the degree of susceptibility of F. carica, the internal spread and the absence of reported problems within the nursery, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.5.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, and unclear host suitability of F. carica, it results in high level of uncertainties for infestation rates below the median. Otherwise, detection of the pest especially before the export is likely, which gives less uncertainties for rates above the median.
A.5.5.5. Elicitation outcomes of the assessment of the pest freedom for Icerya aegyptiaca on bare rooted plants and liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.13) and pest freedom (Table A.14).
Table A.13.
Elicited and fitted values of the uncertainty distribution of pest infestation by Icerya aegyptiaca per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1.00 | 15.0 | 30.0 | 70.0 | 200 | ||||||||||
| EKE | 0.46 | 1.17 | 2.39 | 4.93 | 8.60 | 13.6 | 19.2 | 33.0 | 52.6 | 66.5 | 86.0 | 111 | 145 | 178 | 223 |
The EKE results is the Weibull (0.99094, 47.793) distribution fitted with @Risk version 7.6.
Table A.14.
The uncertainty distribution of plants free of Icerya aegyptiaca per 10,000 plants calculated by Table A.13
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,800 | 9,930 | 9,970 | 9,985 | 9,999 | ||||||||||
| EKE results | 9,777 | 9,822 | 9,855 | 9,889 | 9,914 | 9,934 | 9,947 | 9,967 | 9,981 | 9,986 | 9,991.4 | 9,995.1 | 9,997.6 | 9,998.8 | 9,999.5 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.14.
Figure A.9.
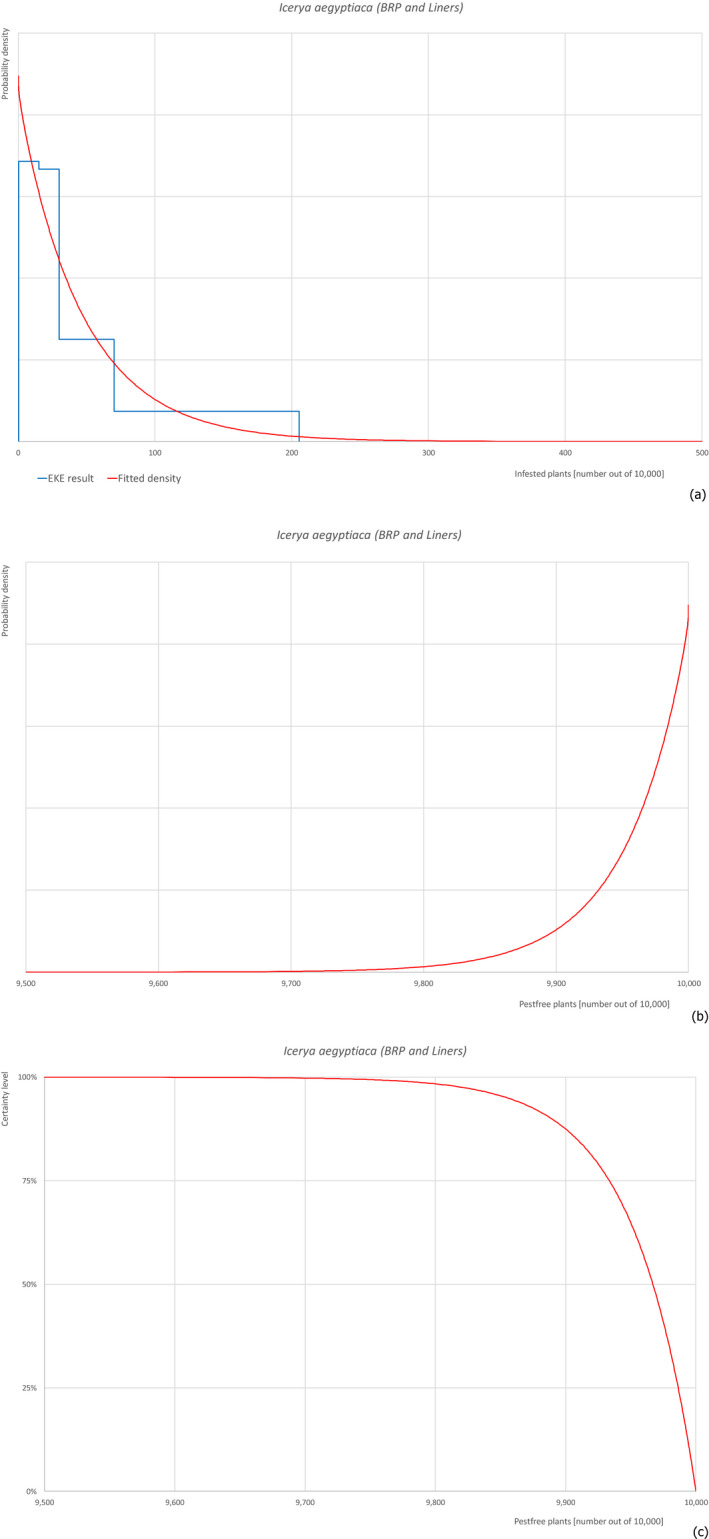
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.5.6. Reference list
Ben‐Dov Y, 2012. The scale insects (Hemiptera: Coccoidea) of Israel‐checklist, host plants, zoogeographical considerations and annotations on species. Israel Journal of Entomology, 41, 21–48.
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Icerya aegyptiaca (breadfruit mealybug). Available online: https://www.cabi.org/cpc/datasheet/28426 [Accessed: 17 January 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_a. Icerya aegyptiaca (ICERAE), Categorization. Available online: https://gd.eppo.int/taxon/ICERAE/categorization [Accessed: 17 January 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Icerya aegyptiaca (ICERAE), Distribution details in Israel. Available online: https://gd.eppo.int/taxon/ICERAE/distribution/IL [Accessed: 17 January 2020].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 January 2020].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: A literature‐based model of scale insect biology and systematics, Lecanodiaspis africana. Available online: http://scalenet.info/catalogue/Ferrisia%20virgata/ [Accessed: 22 January 2020].
Gerson U and Aplebaum S, online. Plant Pests of the Middle East. Icerya aegyptiaca (Douglas). Available online: http://www.agri.huji.ac.il/mepests/pest/Icerya_aegyptiaca/ [Accessed: 25 February 2020].
Hardy C, 2009. Import risk analysis: Fresh Coconut (Cocos nucifera) from Tuvalu. MAF (Ministry of Agriculture and Forest) Biosecurity New Zealand, 131 pp.
Mani M and Shivaraju C, 2016. Mealybugs and their management in agricultural and horticultural crops. Berlin, Germany, Springer, 655 pp.
Miller D, Rung A, Parikh G, Venable G, Redford AJ, Evans GA and Gill RJ, online. Scale insects, Icerya aegyptiaca. Available online: http://www.idtools.org/id/scales/factsheet.php?name=6952 [Accessed: 25 February 2020].
Tyson J, Rainey S, Breach J and Toy S, 2009. Import Risk Analysis: Pears (Pyrus bretschneideri, Pyrus pyrifolia, and Pyrus sp. nr. communis) fresh fruit from China. MAF (Ministry of Agriculture and Forest) Biosecurity New Zealand, 454 pp.
Uesato T, Kondo T, Unruh C and Williams DJ, 2011. Establishment and host records of Icerya aegyptiaca (Douglas) (Hemiptera: Coccoidea: Monophlebidae) in the Sakishima Islands of the Ryukyu Archipelago, Japan, with notes on its worldwide distribution. Entomological science, 14, 49–55. https://doi.org/10.1111/j.1479-8298.2010.00411.x
Unruh CM and Gullan PJ, 2008. Identification guide to species in the scale insect tribe Iceryini (Coccoidea: Monophlebidae). Zootaxa, 1803, 1–106. https://doi.org/10.11646/zootaxa.1803.1.1
Waterhouse DF, 1993. Biological Control Pacific Prospects‐Supplement 2 (No. 435‐2016‐33743). Australian Centre for International Agricultural Research, Canberra, 138 pp.
A.6. Neoscytalidium dimidiatum
A.6.1. Organism information
| Taxonomic information | Current valid scientific name: Neoscytalidium dimidiatumSynonyms: Fusicoccum dimidiatum, Hendersonula toruloidea, Neoscytalidium dimidiatum var. hyalinum, Neoscytalidium hyalinum, Scytalidium dimidiatum, Scytalidium hyalinum, Torula dimidiataName used in the EU legislation: –Order: BotryosphaerialesFamily: BotryosphaeriaceaeCommon name: sooty canker and branch wilt, internal black rotName used in the Dossier: – | |
|---|---|---|
| Group | Fungi | |
| EPPO code | HENLTO | |
| Regulated status | Neoscytalidium dimidiatum is not regulated in the EU.The pest is quarantine in Mexico and it is on A2 list in Egypt (EPPO, online). | |
| Pest status in Israel | Neoscytalidium dimidiatum is present in Israel (Ezra et al., 2013; Ezra et al., 2015; Farr and Rossman, online). | |
| Pest status in the EU | The pest is present in Cyprus (Georghiou and Papadopoulos, 1957), Greece (Tsahouridou and Thanassoulopoulos, 2000) and Italy (Polizzi et al., 2009). | |
| Host status on Ficus carica | Ficus carica is a host of N. dimidiatum (Elshafie and Ba‐Omar, 2002; Farr and Rossman, online; Ray et al., 2010). | |
| PRA information | No Pest Risk Assessment is currently available. | |
| Other relevant information for the assessment | ||
| Biology | Species belonging to Botryosphaeriaceae generally infect through wounds or natural openings (Slippers and Wingfield, 2007). N. dimidiatum has also been reported to infect juvenile dragon fruit cladodes via appressorium formation and direct penetration (Fullerton et al., 2018). The fungus is also reported to live as endophyte in the plant tissues (Ezra et al., 2015).Neoscytalidium spp. can grow between 15 and 40°C. Optimum temperature for mycelial growth is 30–35°C (Mayorquin et al., 2016).Pycniospores are the most important means of dispersal and infection. They are released from pycnidia during wet weather and spread by rain splash and wind (Adesemoye et al., 2014; Fullerton et al., 2018).The sources of inoculum can be infested stems and debris. From these infested substrates, the pathogens can spread to healthy plants and cause infection (Mohd et al., 2013). Therefore, the Panel assumes that the fungus may overwinter in twigs or plant debris in the soil.Neoscytalidium dimidiatum has also been reported as a human pathogen causing skin and nail infections (Elewski, 1996). | |
| Symptoms | Main type of symptoms | Neoscytalidium spp. are reported to cause branch wilt, dieback, canker, leaf blight, gummosis, tree death and fruit rot. In F. carica, N. dimidiatum has been reported to cause a dieback, root rot, canker and tree decline (Ray et al., 2010). On young fruit plants in nurseries symptoms of N. dimidiatum were seen as secretion of gummosis at the grafting area (Ezra et al., 2015).Symptoms are usually visible, but in young plants it may be difficult because of the presence of latent infections causing symptoms later in the growing cycle (Ezra et al., 2015). |
| Presence of asymptomatic plants | Botryosphaeriaceae species are known to be able to live in the host as endophytes (Slippers and Wingfield, 2007). Disease expression is almost exclusively associated with some form of stress or non‐optimal growth conditions of trees (Slippers and Wingfield, 2007).For Prunus spp., it has been reported that development of the disease caused by N. dimidiatum may be delayed and expressed later e.g. when plants are transferred from nurseries to orchards (Ezra et al., 2015). | |
| Confusion with other pathogens/pests | Several other fungi belonging to Botryosphaeriaceae may cause the same symptoms. | |
| Host plant range | Primarily reported from woody plants such as Prunus spp. (California, Hajlaoui et al., 2018; Turkey, Oksal et al., 2019; Israel, Ezra et al., 2015), Citrus spp. (Italy, Polizzi 2009, California, Adesemoye et al., 2014), Ficus spp. (Egypt, Al‐Bedak et al., 2018), walnut (Juglans regia) (Turkey, Derviş et al., 2019), mango (Mangifera indica) (Austalia, Ray et al., 2010), grapevine Vitis vinifera (Turkey, Oksal et al., 2019), Pinus spp. (Turkey, Türkölmez et al., 2019a), but also from tomato (Solanum lycopersicum) (Turkey, Türkölmez et al., 2019) and potato (Solanum tuberosum) (Turkey, Derviş et al., 2020). On F. carica, N. dimidiatum has been reported from the US (Farr and Rossman, online), Australia (Ray et al., 2010) and Oman (Elshafie and Ba‐Omar, 2002).According to answers provided by Israel to the questions raised by the working group dealing with the Dossier on Persea americanum from Israel, the pathogen is occasionally appearing in avocado orchards in Israel (Elad, 2020). | |
| Pathways | Possible pathways of entry for N. dimidiatum are via spores released from infected plants and plant material in the soil; via pruning and grafting tools contaminated by pathogen inoculum; and via latently infected plants, including grafting material e.g. cuttings and scions. | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.6.2. Possibility of pest presence in the nursery
A.6.2.1. Possibility of entry from the surrounding environment
N. dimidiatum has a wide host range.
The major source of inoculum is from infected plant material, which can be leaves, twigs, fruits and cankers on larger branches of the affected plant species. Dispersal of conidia can take place by rain and wind. Therefore, the presence of host species in the environment of the nursery is an important factor for the possible migration of inoculum into the nursery.
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Punica granatum, Populus alba, Populus fremontii, Populus nigra, Quercus brantii, Acacia auriculaeformis, Acacia auriculiformis, Acacia melanoxylon and Persea americana are hosts of N. dimidiatum (Elad, 2020; Farr and Rossman, online).
Uncertainties:
-
–
There are uncertainties about the presence and population pressure of the pest in the areas surrounding the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by rain and wind and possibly by human accidental transportation.
A.6.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pathogen could enter the nursery with new plants/seeds or soil growing media.
A.6.2.3. Possibility of spread within the nursery
If N. dimidiatum is present in mother plants either endophytically or not, it can spread within the nursery when cuttings are taken from mother plants to be planted. Conidia can spread by wind and rain. The fungus may overwinter in the twigs or in plant debris in the soil. If other potential host plants are present within the nursery, N. dimidiatum may spread from these. According to Dossier Section 9.0, following plants are grown in the fig liner export nursery: Lagerstroemia indica and Morus alba, with a distance of a few dozens of metres between them and the fig liners. Morus alba is a host of N. dimidiatum (Farr and Rossman, online).
Therefore, it is possible for N. dimidiatum to reproduce within the nursery on F. carica and other host plants, which are present.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nursery is possible.
A.6.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of N. dimidiatum between the years 1995 and November 2019 (EUROPHYT, online).
A.6.4. Evaluation of the risk mitigation measures
In the table below, all mitigation measures proposed in Israel are summarised and an indication of their effectiveness on N. dimidiatum is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The characteristics of the production field should not affect significantly the pathogen in open field.The use of commercial media always new in sack containers should prevent the entry of pathogen inoculum with the growing medium. However, the medium may become contaminated during production as a result of inoculum dispersal and incorporation of infected plant tissues.The net of the net house is designed for shading and it is not expected to prevent or reduce the entry of pathogen inoculum.Uncertainties:– There is uncertainty whether fallen leaves are periodically removed.– There is uncertainty on the level of viability of the inoculum in the soil (both the persistence in the soil and the ability to fruit). | The use of commercial media always new should prevent the entry of pathogen inoculum with the growing medium. However, the medium may become contaminated during production as a result of inoculum dispersal and incorporation of infected plant tissues.Irrigation by sprinklers may enhance splash dispersal of inoculum leading to new infections.The net of the net house is designed for shading and it is not expected to prevent or reduce the entry of pathogen inoculum.Uncertainties:– There is uncertainty whether fallen leaves are periodically removed.– There is uncertainty on the level of viability of the inoculum in the soil (both the persistence in the soil and the ability to fruit). |
| 2 | Soil treatment | Yes, for bare rooted plants | Soil solarisation may reduce the inoculum of N. dimidiatum present in plant debris in the soil.Uncertainties:– The level of reduction of inoculum in the soil as a result of solarisation is unknown. | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | No | Not applicable | Not applicable |
| 5 | Fungicide treatment | Yes | The Panel assumes that fungicide treatment with Myclobutanil or other appropriate fungicides occur in the case of any early signs of infection. Therefore, if plants are symptomless fungicide treatment will not be carried out.Post‐harvest treatment is targeting infection from root pathogens and is not expected to eradicate or to kill the pathogen if present in the plant.Uncertainties:– The level of effectiveness of the fungicides against the pathogen is unknown. | The Panel assumes that fungicide treatment with Myclobutanil or other appropriate fungicides occur in the case of any early signs of infection. Therefore, if plants are symptomless, fungicide treatment will not be carried out.Uncertainties:– The level of effectiveness of the fungicides against the pathogen is unknown. |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | Yes | Leaves removal could reduce the probability of carrying the pathogen. However, the pathogen may be mostly associated with other plant tissues than leaves.Uncertainties:– The level of the association of the pathogen with other plant tissues.– There is uncertainty regarding the association of the pathogen with leaves in F. carica. | Leaves removal and cleaning of the plant debris could reduce the probability of carrying the pathogen. However, the pathogen may be mostly associated with other plant tissues than leaves.Uncertainties:– The level of the association of the pathogen with other plant tissues.– There is uncertainty regarding the association of the pathogen with leaves in F. carica. |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | The inspections during the production should allow a prompt detection of any visible symptoms. However, low level of infections might be overlooked. Asymptomatic plants will go undetected.Uncertainties:– No uncertainties | The inspections during the production should allow a prompt detection of any visible symptoms. However, low level of infections might be overlooked. Asymptomatic plants will go undetected.Uncertainties:– No uncertainties |
| 11 | Inspections before export | Yes | The inspections before export should allow a prompt detection of any visible symptoms of any disease. However, low level of infections might be overlooked. Asymptomatic plants will go undetected.Uncertainties:– No uncertainties | The inspections before export should allow a prompt detection of any visible symptoms of any disease. However, low level of infections might be overlooked. Symptoms of root rot in roots cannot be observed. Asymptomatic plants will go undetected.Uncertainties:– No uncertainties |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, N. dimidiatum is present in Israel.Uncertainties:– There is no information on the presence and density of N. dimidiatum in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, N. dimidiatum is present in Israel.Uncertainties:– There is no information on the presence and density of N. dimidiatum in the surrounding areas. |
A.6.5. Overall likelihood of pest freedom for bare rooted plants
A.6.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants
There is low pest pressure from the surroundings and F. carica is poorly susceptible. Infections are mostly symptomatic with a very limited endophytic stage. Contaminations of the soil by infected wood debris including roots of the previous rotation rarely occur and are effectively controlled by soil solarisation. Inspections and control measures with fungicides are mostly effective.
A.6.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants
There is high pest pressure from the surroundings and F. carica is a suitable host. The fungus mainly infects host tissues asymptomatically as an endophyte. Contaminations of the soil by infected wood debris including roots of the previous rotation frequently occur and soil solarisation is poorly effective. Because the pathogen is poorly symptomatic, it is difficult to detect during inspections. There are no fungicide treatments without observation of symptoms.
A.6.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants (Median)
The median is closer to lower values because there is little evidence that F. carica is a main host of the pathogen. Although several hosts are reported to be present in the surroundings, entry into the nursery may be limited because of the way the conidia disperse, mostly locally through rain splash and wind.
A.6.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There are uncertainties about the susceptibility of the F. carica, the efficacy of the inspections, because of the endophytic (latent) stage of the pathogen, as well as on the efficacy of fungicide treatments.
A.6.5.5. Elicitation outcomes of the assessment of the pest freedom for Neoscytalidium dimidiatum on bare rooted plants
The following tables show the elicited and fitted values for pest infestation/infection (Table A.15) and pest freedom (Table A.16).
Table A.15.
Elicited and fitted values of the uncertainty distribution of pest infestation by Neoscytalidium dimidiatum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5.00 | 25.0 | 40.0 | 85.0 | 150 | ||||||||||
| EKE | 4.42 | 5.19 | 6.64 | 9.90 | 14.8 | 21.5 | 28.9 | 46.2 | 67.6 | 80.7 | 96.5 | 113 | 129 | 141 | 152 |
The EKE results is the BetaGeneral (0.87411, 2.0083, 4, 170) distribution fitted with @Risk version 7.6.
Table A.16.
The uncertainty distribution of plants free of Neoscytalidium dimidiatum per 10,000 plants calculated by Table A.15
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,915 | 9,960 | 9,975 | 9,995.0 | ||||||||||
| EKE results | 9,848 | 9,859 | 9,871 | 9,887 | 9,904 | 9,919 | 9,932 | 9,954 | 9,971 | 9,979 | 9,985 | 9,990.1 | 9,993.4 | 9,994.8 | 9,995.6 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.16.
Figure A.10.
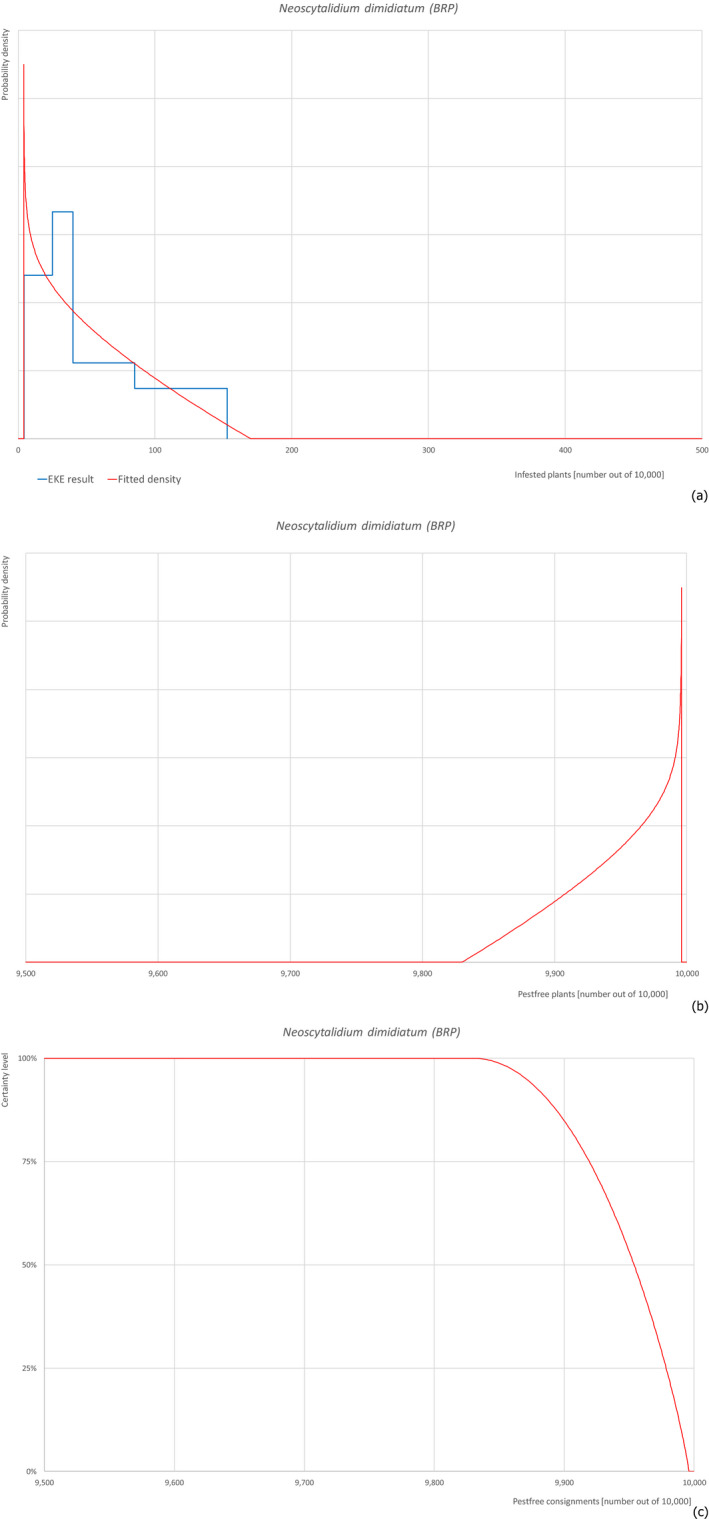
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.6.6. Overall likelihood of pest freedom for liners
A.6.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested liners
There is low pest pressure from the surroundings and F. carica is poorly susceptible. Infections are mostly symptomatic with a very limited endophytic stage. Irrigation using the sprinkling system is not expected to favour significantly the spread of the disease. Contaminations of the soil by infected wood debris are assumed to be rare. As a consequence, infections leading to root rots are also expected to be rare. Inspections and control measures with fungicides are mostly effective.
A.6.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested liners
There is high pest pressure from the surroundings and F. carica is a suitable host. The fungus mainly infects host tissues asymptomatically as an endophyte. Contaminations of the soil by infected wood debris may occur. Consequently, infections leading to root rots are also expected and these are not detectable during inspections before export. Fungicide treatment is not performed because plants remain asymptomatic. When treatments are applied these have little effects on the pathogen, which is mostly present in woody tissues.
A.6.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested liners (Median)
The median is closer to lower values because there is little evidence that F. carica is a main host of the pathogen. Although several hosts are reported to be present in the surroundings, entry into the nursery may be limited because of the way the conidia disperse, mostly locally through rain splash and wind. Woody plant debris in the soil potentially harbouring the pathogen is scanty.
A.6.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
There are uncertainties on the level of susceptibility of the F. carica, the efficacy of the inspections, because of the endophytic (latent) stage of the pathogen, as well as on the efficacy of fungicide treatments. There are uncertainties on whether the fungus could be associated with leaves of F. carica.
A.6.6.5. Elicitation outcomes of the assessment of the pest freedom for Neoscytalidium dimidiatum on liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.17) and pest freedom (Table A.18).
Table A.17.
Elicited and fitted values of the uncertainty distribution of pest infestation by Neoscytalidium dimidiatum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5.00 | 35.0 | 65.0 | 120 | 180 | ||||||||||
| EKE | 4.68 | 5.94 | 8.33 | 13.7 | 21.6 | 32.3 | 43.9 | 69.8 | 100 | 116 | 135 | 152 | 167 | 176 | 183 |
The EKE results are the BetaGeneral (0.86841, 1.3523, 4, 190) distribution fitted with @Risk version 7.6.
Table A.18.
The uncertainty distribution of plants free of Neoscytalidium dimidiatum per 10,000 plants calculated by Table A.17
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,820 | 9,880 | 9,935 | 9,965 | 9,995.0 | ||||||||||
| EKE results | 9,817 | 9,824 | 9,833 | 9,848 | 9,865 | 9,884 | 9,900 | 9,930 | 9,956 | 9,968 | 9,978 | 9,986 | 9,991.7 | 9,994.1 | 9,995.3 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.18.
Figure A.11.
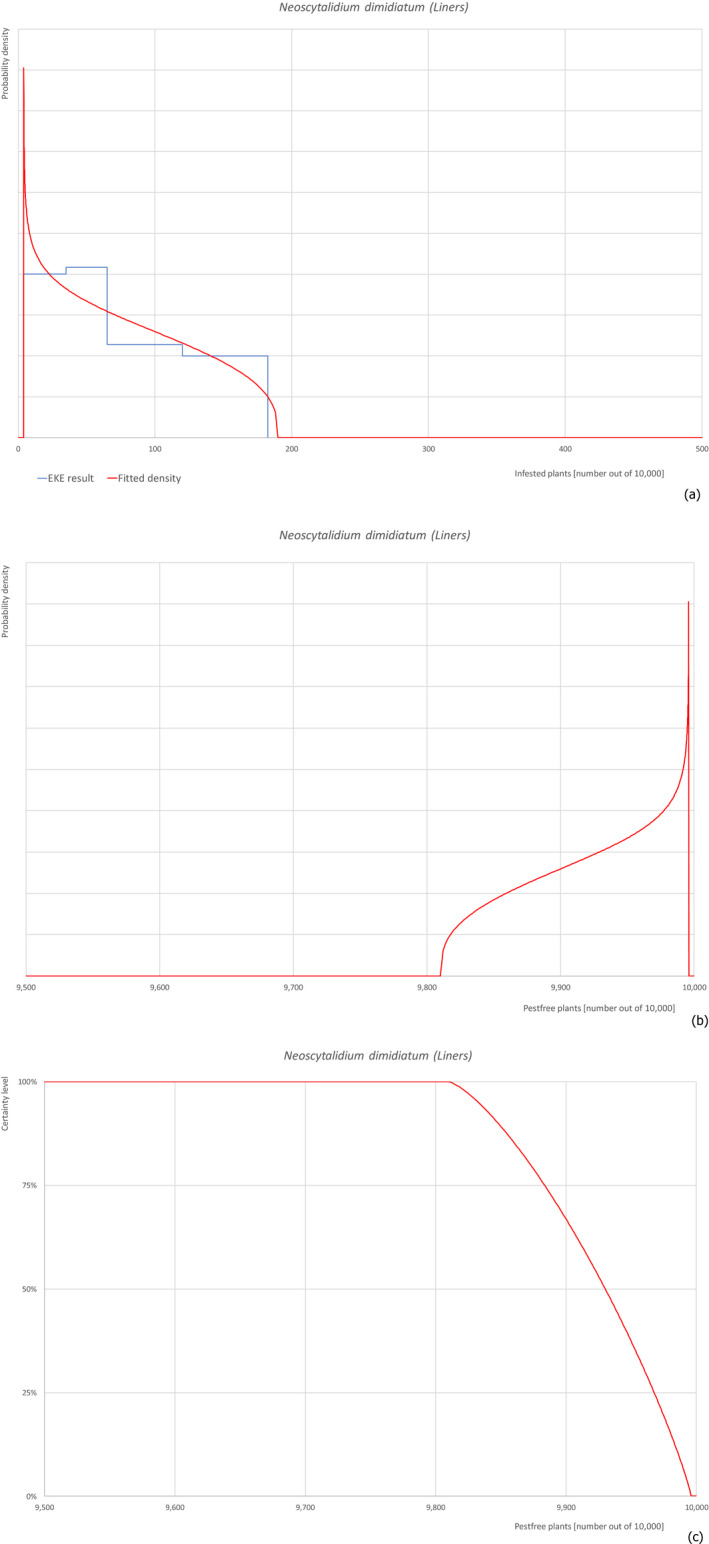
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
Figure A.12.
Elicited certainty (y‐axis) of the number of bare rooted plants or liners of Ficus carica pest free from Neoscytalidium dimidiatum (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU introduced from Israel as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
A.6.7. Reference list
Adesemoye AO, Mayorquin JS, Wang DH, Twizeyimana M, Lynch SC and Eskalen A, 2014. Identification of species of Botryosphaeriaceae causing bot gummosis in citrus in California. Plant Disease, 98, 55–61. https://doi.org/10.1094/pdis-05-13-0492-re
Al‐Bedak OA, Mohamed RA and Seddek NH, 2018. First detection of Neoscytalidium dimidiatum associated with canker disease in Egyptian Ficus trees. Forest Pathology, 48, 1–7. https://doi.org/10.1111/efp.12411
Derviş S, Türkölmez Ş, Çiftçi O, Ulubaş Serçe Ç and Dikilitas M, 2019. First report of Neoscytalidium dimidiatum causing black canker and root rot of walnut in Turkey. Plant Disease, 103, 1–3. https://doi.org/10.1094/pdis-02-19-0306-pdn
Derviş S, Özer G. and Türkölmez Ş, 2020. First report of Neoscytalidium dimidiatum causing tuber rot of potato in Turkey. Journal of Plant Pathology. https://doi.org/10.1007/s42161-020-00575-6
Elad Y, 2020. Available online: https://phytopathology.org.il/wp-content/uploads/2019/12/Avocado-1-2020-Elad.pdf [Accessed: 13 October 2020].
Elewski BE, 1996. Onychomycosis caused by Scytalidium dimidiatum. Journal of the American Academy of Dermatology; 35(2 Pt 2), 336–338. https://doi.org/10.1016/S0190-9622(96)90664-7
Elshafie AE and Ba‐Omar T, 2002. First report of Albizia lebbeck caused by Scytalidium dimidiatum in Oman. Mycopathologia, 154, 37–40. https://doi.org/10.1023/A:1015200707971
EPPO (European and Mediterranean Plant Protection Organization), online. Neoscytalidium dimidiatum (HENLTO), Categorization. Available online: https://gd.eppo.int/taxon/ICERAE/categorization [Accessed: 13 October 2020].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 13 October 2020].
Ezra D, Liarzi O, Gat T, Hershcovich M and Dudai M, 2013. First report of internal black rot caused by Neoscytalidium dimidiatum on Hylocereus undatus (Pitahaya) fruit in Israel. Plant Disease, 97, 1‐3. https://doi.org/10.1094/pdis-05-13-0535-pdn
Ezra D, Simanski E, Antman S, Shulhani R, Borenstein M, Golani M, Hershcovich M, Liarzi O and Shtienberg D, 2015. Botryosphaeria in deciduous trees: determination of the causal agent and disease development in young trees. Abstracts of presentations at the 36th Congress of the Israeli Phytopathological Society. Phytoparasitica 43, 369–381.
Farr DF and Rossman AY, online. Fungal Databases, US National Fungus Collections, ARS, USDA, Neoscytalidium dimidiatum. Available online: https://nt.ars-grin.gov/fungaldatabases/fungushost/new_frameFungusHostReport.cfm [Accessed: 12 October 2020].
Fullerton RA, Sutherland PA, Rebstock RS, Hieu NT, Thu NNA, Linh DT, Thanh NTK and Van Hoa N, 2018. The life cycle of dragon fruit canker caused by Neoscytalidium dimidiatum and implications for control. Management, 258.
Georghiou GP and Papadopoulos C, 1957. A second list of Cyprus fungi. Government of Cyprus, Department of Agriculture. 38 pp.
Hajlaoui MR, Nouri MT, Hamrouni N, Trouillas FP, Yahmed NB, Eddouzi J and Mnari‐Hattab M, 2018. First record of dieback and decline of plum caused by Neoscytalidium dimidiatum in Tunisia. New Disease Reports, 38, 20.
Mayorquin JS, Wang DH, Twizeyimana M and Eskalen A, 2016. Idenfication, distribution and pahtogenicity of Diatrypaceae and Botrysphaeriaceae associated with Citrus Branch Canker in the Southern California Desert. Plant Disease, 100, 2402–2413. https://doi.org/10.1094/pdis-03-16-0362-re
Mohd MH, Salleh B and Zakaria L, 2013. Identification and molecular characterizations of Neoscytalidium dimidiatum causing stem canker of red‐fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. Journal of Phytopathology, 161, 841–849.
Oksal E, Çelik Y and Özer G, 2019. Neoscytalidium dimidiatum causes canker and dieback on grapevine in Turkey. Australasian Plant Disease Notes, 14, 33. https://doi.org/10.1007/s13314-019-0363-4
Polizzi G, Aiello D, Vitale A, Giuffrida F, Groenewald JZ and Crous PW, 2009. First Report of Shoot Blight, Canker, and Gummosis Caused by Neoscytalidium dimidiatum on Citrus in Italy. Plant Disease, 93, 1215. https://doi.org/10.1094/pdis-93-11-1215a
Ray JD, Burgess T and Lanoiselet VM, 2010. First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Australasian Plant Disease Notes, 5, 48–50.
Slippers B and Wingfield MJ, 2007. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal biology reviews, 21, 90–106. https://doi.org/10.1016/j.fbr.2007.06.002
Tsahouridou PC and Thanassoulopoulos CC, 2000. First report of Hendersonula toruloidea as a foliar pathogen of strawberry‐tree (Arbutus unedo) in Europe. Plant disease, 84, 487.
Türkölmez S, Dervis S, Ciftci O and Dikilitas M, 2019. First report of Neoscytalidium dimidiatum causing shoot and needle blight of pines (Pinus spp.) in Turkey. Plant Disease, 103, 2960–2961. https://doi.org/10.1094/pdis-05-19-0964-pdn
A.7. Nipaecoccus viridis
A.7.1. Organism information
| Taxonomic information | Current valid scientific name: Nipaecoccus viridisSynonyms: Dactylopius perniciosus, Dactylopius vastator, Dactylopius viridis, Nipaecoccus vastator, Pseudococcus filamentosus var. corymbatus, Pseudococcus perniciosus, Pseudococcus solitarius, Pseudococcus vastator, Pseudococcus viridis, Ripersia theae, Trionymus sericeusName used in the EU legislation: –Order: HemipteraFamily: PseudococcidaeCommon name: spherical mealybug, coffee mealybug, cotton mealybug, globular mealybug, hibiscus mealybug, karoo thorn mealybug, lebbeck mealybugName used in the Dossier: Nipaecoccus viridis | |
|---|---|---|
| Group | Insects | |
| EPPO code | NIPAVI | |
| Regulated status | Nipaecoccus viridis is not regulated in the EU, neither is listed by EPPO (EPPO, online_a).It is categorised in Turkey (A1 list since 2016) and in countries of Asia and America (EPPO, online_a). | |
| Pest status in Israel | Nipaecoccus viridis is present in Israel (Ben‐Dov, 1994; CABI, online; EPPO, online_b; García Morales et al., online). | |
| Pest status in the EU | Nipaecoccus viridis is absent in the EU (CABI, online; EPPO, online_b; García Morales et al., online). | |
| Host status on Ficus carica | Ficus carica is host of N. viridis (Ben‐Dov, 1994; García Morales et al., online). | |
| PRA information | Pest Risk Assessment available from New Zealand:– Import Risk Analysis: Pears (Pyrus bretschneideri, Pyrus pyrifolia, and Pyrus sp. nr. communis) fresh fruit from China to New Zealand (Tyson et al., 2009). | |
| Other relevant information for the assessment | ||
| Biology | Nipaecoccus viridis is probably indigenous to the warm tropical areas of the Indian subcontinent (Franco et al., 2004) and is spread in many parts of the world, mainly in tropics and subtropics (Thomas and Leppla, 2008).Nipaecoccus viridis reproduce both sexually and parthenogenically. Eggs are laid in a large hemispherical ovisac, which usually hide the female (Sharaf and Meyerdirk, 1987). Females lay about 300–500 eggs in their lifetime (Mani and and Shivaraju, 2016) and sometimes more than 1,100 eggs (Bartlett, 1978). The mealybug prefers to feed and reproduce on fast growing tissues like new branches and fruits (Diepenbrock and Burrow, 2020).The development stages of N. viridis are egg, three nymphal instars (for females) and four nymphal instars (for males), and adult (Mani and Shivaraju, 2016). According to Sharaf and Meyerdirk (1987), the number of instars is four for females and five for males. The first‐instar nymph (crawler) can be carried away by wind. The development time lasts between 19 and 20 days at 25°C and 15–19 days at 32°C (Gerson and Aplebaum, online).Males have forewings and live up to 3 days. Females are wingless and live up to 50 days (Gerson and Aplebaum, online).The mealybug can have several overlapping generations per year (Sharaf and Meyerdirk, 1987). Six to seven generations occur annually in the Jordan Valley (Gerson and Aplebaum, online).In the Middle East mealybug overwinters as adult in cracks and crevices of the stems and branches (Gerson and Aplebaum, online). In Iraq, N. viridis overwinters as egg, nymph and adult (Jarjes et al., 1989). | |
| Symptoms | Main type of symptoms | Nipaecoccus viridis adults and larvae can damage all plant parts, such as leaves, fruits, twigs, flowers and even roots (Abdul‐Rassoul, 1970; Sharaf and Meyerdirk, 1987).Main symptoms are:– curling and dwarfing of the terminal growth,– abortion of flowers,– yellowing of leaves,– yellowing of fruits,– corky scars on fruits,– watery green spots on ripen fruits,– fruit size deformation,– dropping of fruits,– white or pale‐yellow waxy secretion,– honeydew,– sooty mould,– distortion and rosetting of plants,– wilting,– dieback,– defoliation (CABI, online; Gerson and Aplebaum, online; Sharaf and Meyerdirk, 1987) |
| Presence of asymptomatic plants | Plant damage might not be obvious in early infestation or during dormancy (due to the absence of leaves), but the presence of mealybugs on the plants could be observed. During the crawler stage, infestation is difficult to be noted. | |
| Confusion with other pests | Nipaecoccus viridis can be confused with several other mealybugs. | |
| Host plant range | Nipaecoccus viridis attacks 53 plant families and 140 genera (García Morales et al., online). Main hosts are avocado (Persea americana), citrus (Citrus spp.), coffee (Coffea spp.), cotton (Gossypium spp.), grapevine (Vitis vinifera), mango (Mangifera indica), pomegranate (Punica granatum) and tamarind (Tamarindus spp.) (CABI, online; Gerson and Aplebaum, online).Other host plants are fig (Ficus carica), Indian siris (Albizia lebbeck), jack fruit (Artocarpus heterophyllus), crape myrtle (Lagerstroemia indica), white mulberry (Morus alba), oleander (Nerium oleander), potato (Solanum tuberosum), rosemallows (Hibiscus spp.) and soybean (Glycine max) (CABI, online; García Morales et al., online).Nipaecoccus viridis is an agricultural pest in Asia that attacks food, forage, ornamental and fibre crops (Sharaf and Meyerdirk, 1987). It has economic impact on ber, citrus, custard apple, grapes, guava, jackfruit, mango, pomegranate and pummelo (Mani and Shivaraju, 2016). | |
| Pathways | Plants for planting (presence on roots is controversial) and fruits are the main pathways for introduction and spread of N. viridis (Grousset et al., 2016; Wistermann et al., 2016).Possible pathways of entry for mealybugs are plant materials of any kind (hiding in a protected site – on the bark, roots, stems, leaves), human transportation, irrigation water, wind, animals and ants (Mani and Shivaraju, 2016). | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.7.2. Possibility of pest presence in the nursery
A.7.2.1. Possibility of entry from the surrounding environment
Nipaecoccus viridis is present in Israel (Ben‐Dov, 1994; CABI, online; EPPO, online_b; García Morales et al., online). Possible pathways of entry into the nursery can be by movement of infested plants, wind, human and animal dispersal and irrigation water (Mani and Shivaraju, 2016). Males can fly but live only 3 days (Gerson and Aplebaum, online). The first nymph instars (crawlers) can disperse by walking and by wind (Mani and Shivaraju, 2016).
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation include cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Gossypium, Gossypium herbaceum, Gossypium hirsutum, Diospyros, Punica granatum, Acacia, Acacia modesta, Acacia nilotica, Ricinus communis and Persea americana are hosts of N. viridis (García Morales et al., online).
Uncertainties:
-
–
No information about the density of the population of N. viridis in the area surrounding the nursery is available.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by wind and human accidental transportation.
A.7.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media. Plants are produced inside the nursery and the mealybugs are not associated with soil growing media.
A.7.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
According to Dossier Section 9.0, following plants known to be hosts of the pest are grown in the fig liner export nursery: Lagerstroemia indica and Morus alba, with a distance of a few dozens of metres between them and the fig liners.
Therefore, it is possible for N. viridis to reproduce within the nursery on F. carica and on other hosts, which are present.
Possible pathways of spreading within the nursery can be by movement of infested plants, wind, human and animal dispersal and irrigation water (Mani and Shivaraju, 2016). Males can fly but live only 3 days (Gerson and Aplebaum, online). The first nymph instars (crawlers) can disperse by walking and by wind (Mani and Shivaraju, 2016).
According to Dossier Section 9.0, the growing medium is peat substrate (EU‐made). Liners are rooted directly in pots, in the same growing medium as used for the bare rooted plants. Soil solarisation is performed by covering the soil with transparent polyethylene for 2 months – July and August (normally the time of highest radiation). The polyethylene sheet is spread after the soil has been cleaned from the previous crop and has been processed for the next cycle. The polyethylene in the sheets is supplemented with ‘antidrip’ or ‘antifog’ substances which prevents water condensation and accumulation on the sheet, so improving treatment efficacy by raising the under‐sheet temperature by 4–5°C compared with regular polyethylene sheets. The max temperature in the top 20 cm of the soil is 44–48°C daily, for the duration of 2 months. The sheets are maintained clean and intact through the treatment duration, and the soil moisture is maintained to the field capacity level, by weekly irrigation with a water volume that parallels 1 m3 water/dunam per day.
According to Dossier Section 9.0, the water that is used for irrigation is regular tap water, that goes through a 120‐mesh filter to remove rough dirt like sand and stones. Liners are irrigated by sprinklers, and bare rooted plants receive drip irrigation.
Uncertainties:
-
–
There is uncertainty on whether plants are transplanted within the nursery thereby moving soil.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible either by wind or accidental transfer within the nursery.
A.7.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of N. viridis between the years 1995 and November 2019 (EUROPHYT, online).
Intercepted in the USA and Republic of Korea on Citrus (Grousset et al., 2016; Wistermann et al., 2016).
A.7.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on N. viridis is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties |
| 2 | Soil treatment | Yes, for bare rooted plants | Solarisation is sufficient to suppress any mealybugs eventually associated with old roots.Uncertainties:– No uncertainties | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Pesticide sprays are generally effective against crawlers but have limited effectiveness against N. viridis when hidden in crevices, or protected by the waxy covering of its body.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty whether the pesticide can effectively reach all the bark/root parts where the mealybugs are located because of the barrier effect of the leaves and soil. | Pesticide sprays are generally effective against crawlers but have limited effectiveness against N. viridis when hidden in crevices, or protected by the waxy covering of its body.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty whether the pesticide can effectively reach all the bark/root parts where the mealybugs are located because of the barrier effect of the leaves and soil. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | Yes | As weeds can host mealybugs, the treatment should reduce the pressure on the crop.Uncertainties:– There is uncertainty about the efficacy of the treatment and the species of weeds and whether they are host to N. viridis. | As weeds can host mealybugs, the treatment should reduce the pressure on the crop.Uncertainties:– There is uncertainty about the efficacy of the treatment and the species of weeds and whether they are host to N. viridis. |
| 8 | Plant treatment before export | Yes | Partly effective because it is not clear whether the washing may remove the adults possibly hidden in crevices and holes.Mealybugs can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers with the naked eye. | Mealybugs can be easily found during inspection with magnifying glasses even if symptoms are not obvious which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers with the naked eye. |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Mealybugs could go undetected because of the small size of the pest and difficulty in the search. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for mealybugs is not known. | Mealybugs could go undetected because of the small size of the pest and difficulty in the search. In addition, roots are not inspected. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties for the stem inspection:– There is unclear detection limit.– The effectiveness of the inspection for the mealybugs is not known. |
| 11 | Inspections before export | Yes | Mealybugs could go undetected because of the small size of the pest and difficulty in the search, including roots. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for mealybugs is not known. | Mealybugs could go undetected because of the small size of the pest and difficulty in the search, in addition roots are not inspected. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties for the stem inspection:– There is unclear detection limit.– The effectiveness of the inspection for the mealybugs is not known. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, N. viridis is common in Israel.Uncertainties:– There is no information on the density of N. viridis in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, N. viridis is common in Israel.Uncertainties:– There is no information on the density of N. viridis in the surrounding areas. |
A.7.5. Overall likelihood of pest freedom for bare rooted plants
A.7.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants
Although N. viridis is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to wind and human activity. Inspections are expected to be effective because waxy stages of the insect are visible. Insecticide treatments are expected to be conducted at the right timing to target unprotected life stages of the insect. Mother plants are kept healthy as well by using treatments.
A.7.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants
Nipaecoccus viridis is widespread in Israel; the scenario assumes a high pest pressure from outside and strong transfer from the surrounding due to wind and intensive human activity. Inspections are expected to be ineffective because of the presence of hidden stages. Insecticide treatments are expected to be conducted at timing when the insect is hidden or protected by wax. Mother plants are infested despite treatments and may contribute spreading the pest within the nursery.
A.7.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by wind and human activity, the internal spread and the absence of reported problems within the nursery and at EU borders, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.7.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, it results in high level of uncertainties for infestation rates below the median. Otherwise, detection of the pest especially before the export is likely, which gives less uncertainties for rates above the median.
A.7.5.5. Elicitation outcomes of the assessment of the pest freedom for Nipaecoccus viridis on bare rooted plants
The following tables show the elicited and fitted values for pest infestation/infection (Table A.19) and pest freedom (Table A.20).
Table A.19.
Elicited and fitted values of the uncertainty distribution of pest infestation by Nipaecoccus viridis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2.00 | 20.0 | 40.0 | 80.0 | 200 | ||||||||||
| EKE | 1.19 | 2.54 | 4.56 | 8.30 | 13.2 | 19.4 | 26.1 | 41.7 | 62.8 | 77.2 | 97.3 | 122 | 156 | 189 | 233 |
The EKE results are the Gamma (1.2287, 45.52) distribution fitted with @Risk version 7.6.
Table A.20.
The uncertainty distribution of plants free of Nipaecoccus viridis per 10,000 plants calculated by Table A.19
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,800 | 9,920 | 9,960 | 9,980 | 9,998 | ||||||||||
| EKE results | 9,767 | 9,811 | 9,844 | 9,878 | 9,903 | 9,923 | 9,937 | 9,958 | 9,974 | 9,981 | 9,987 | 9,991.7 | 9,995.4 | 9,997.5 | 9,998.8 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.20.
Figure A.13.
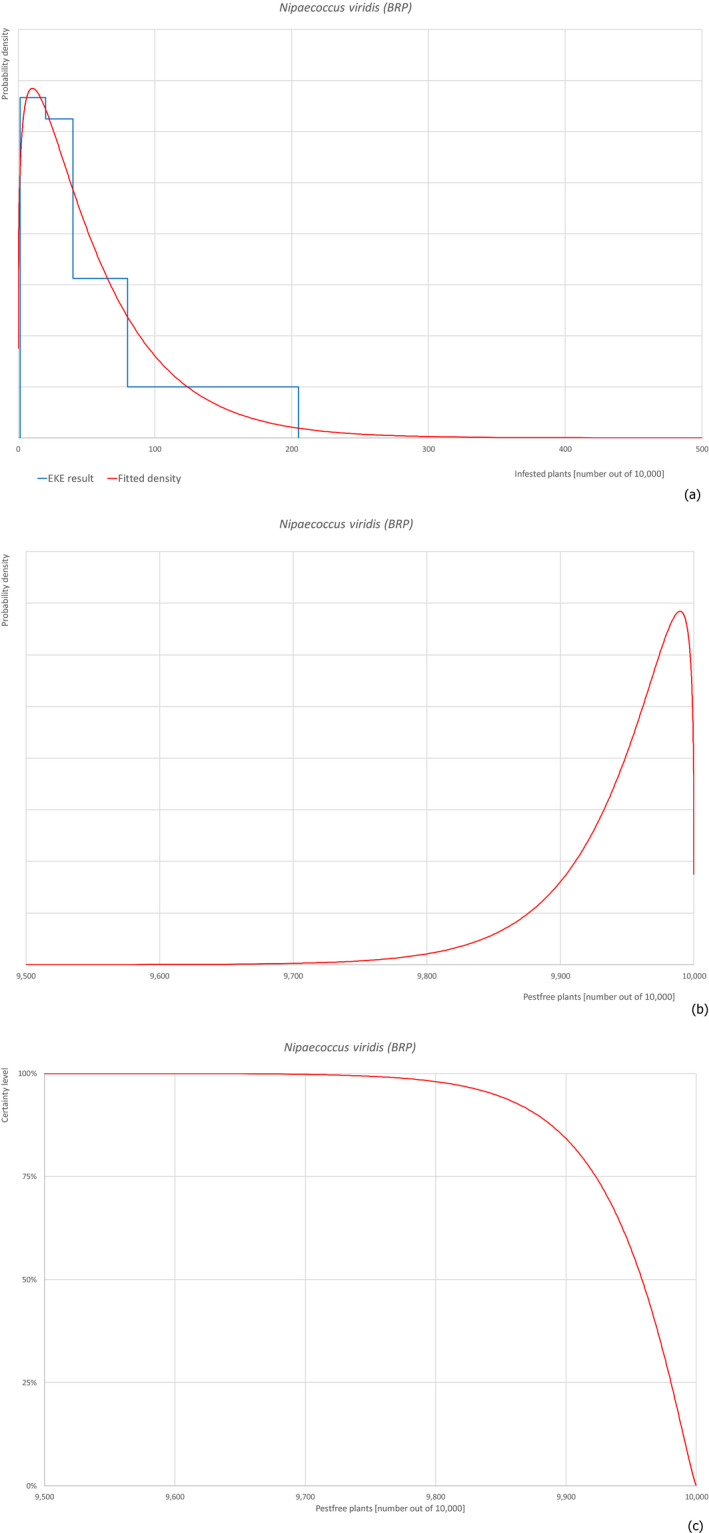
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.7.6. Overall likelihood of pest freedom for liners
A.7.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested liners
Although N. viridis is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to wind and human activity. Inspections are expected to be effective because waxy stages of the insect are visible, unless they are in the soil. Insecticide treatments are expected to be conducted at the right timing to target unprotected life stages of the insect. Mother plants are kept healthy as well by using treatments.
A.7.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested liners
Nipaecoccus viridis is widespread in Israel; the scenario assumes a high pest pressure from outside and strong transfer from the surrounding due to wind and intensive human activity. Inspections are expected to be ineffective because of the presence of hidden stages or when the pest is in the soil. Insecticide treatments are expected to be conducted at timing when the insect is hidden or protected by wax. Mother plants are infested despite treatments and may contribute spreading the pest within the nursery.
A.7.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by wind and human activity, the internal spread and the absence of reported problems within the nursery and at EU borders, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.7.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, it results in high level of uncertainties for infestation rates below the median. Otherwise, detection of the pest especially before the export is likely, which gives less uncertainties for rates above the median.
A.7.6.5. Elicitation outcomes of the assessment of the pest freedom for Nipaecoccus viridis on liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.21) and pest freedom (Table A.22).
Table A.21.
Elicited and fitted values of the uncertainty distribution of pest infestation by Nipaecoccus viridis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 8.00 | 40.0 | 70.0 | 160 | 300 | ||||||||||
| EKE | 7.44 | 8.41 | 10.4 | 15.3 | 23.2 | 34.7 | 48.0 | 80.5 | 123 | 151 | 185 | 221 | 260 | 290 | 318 |
The EKE results are the BetaGeneral (0.78642, 2.2897, 7, 375) distribution fitted with @Risk version 7.6.
Table A.22.
The uncertainty distribution of plants free of Nipaecoccus viridis per 10,000 plants calculated by Table A.21
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,700 | 9,840 | 9,930 | 9,960 | 9,992 | ||||||||||
| EKE results | 9,682 | 9,710 | 9,740 | 9,779 | 9,815 | 9,849 | 9,877 | 9,919 | 9,952 | 9,965 | 9,977 | 9,985 | 9,989.6 | 9,991.6 | 9,992.6 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.22.
Figure A.14.
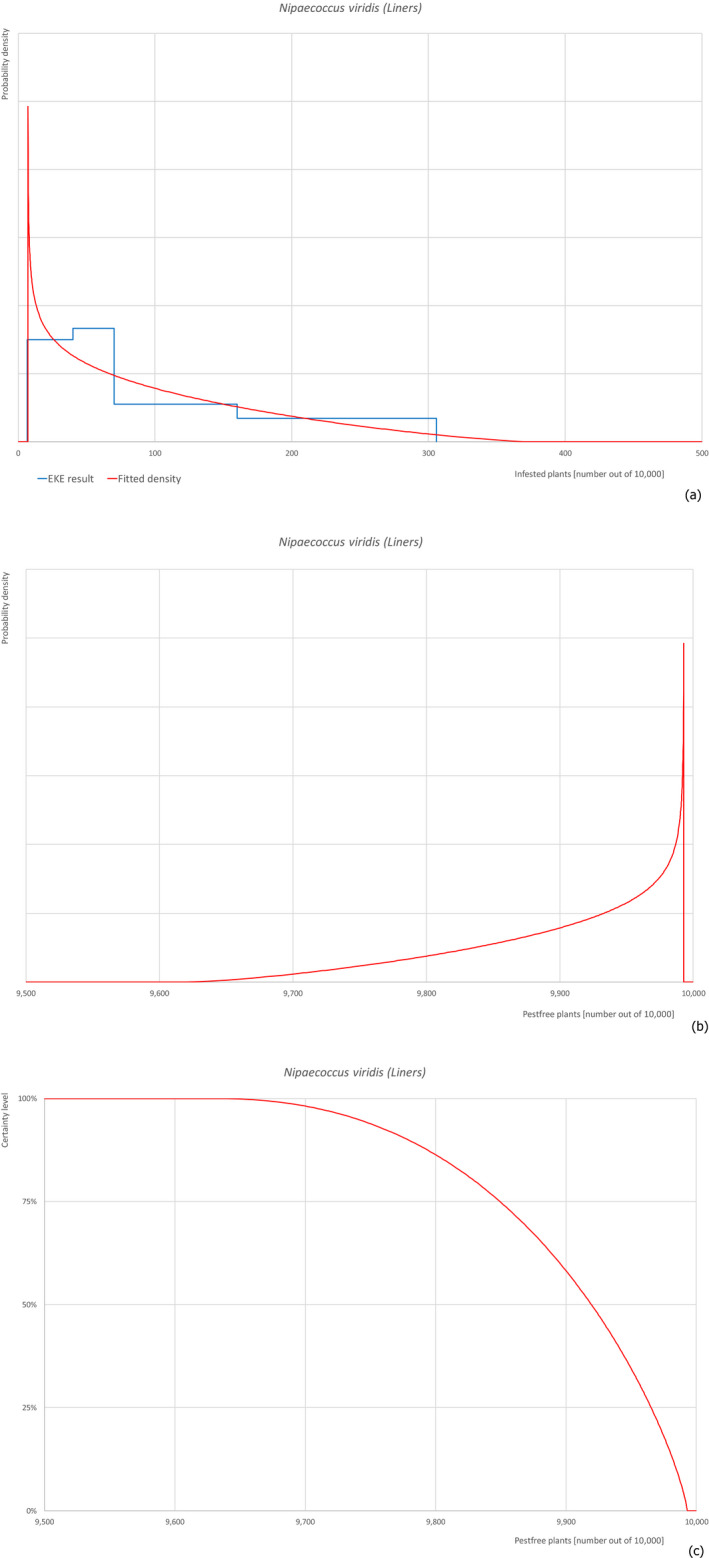
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
Figure A.15.
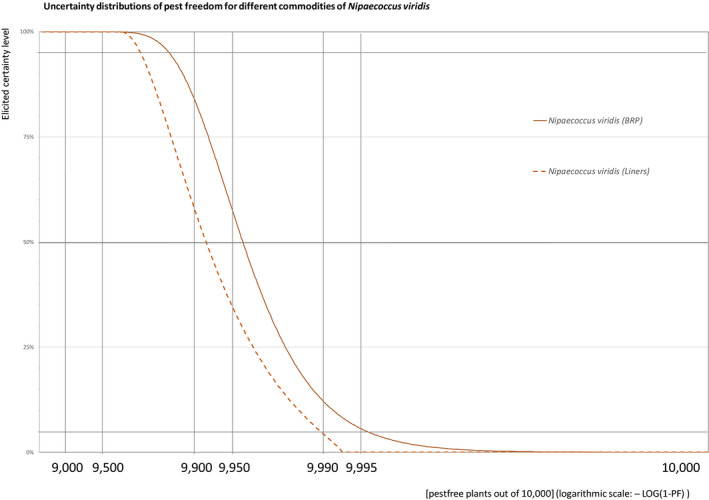
Elicited certainty (y‐axis) of the number of bare rooted plants or liners of Ficus carica pest free from Nipaecoccus viridis (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU introduced from Israel as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
A.7.7. Reference list
Abdul‐Rassoul MS, 1970. Notes on Nipaecoccus vastator (Maskell) (Coccidae: Homoptera). A serious pest of citrus trees and various plants – first record from Iraq. Bulletin Iraq Natural History Museum, 4, 105–108.
Bartlett BR, 1978. Pseudococcidae. In Clausen CP1 (ed.). Introduced parasites and predators of arthropod Epests and weeds: A world review. Agricultural Handbook, USDA, Washington, DC. p. 137–170. 480 pp.
Ben‐Dov Y, 1994. A systematic catalogue of the mealybugs of the world (Insecta: Homoptera: Coccoidea: Pseudococcidae and Putoidae) with data on geographical distribution, host plants, biology and economic importance. 100th Intercept Limited Andover, UK. 686 pp.
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Nipaecoccus viridis (spherical mealybug). Available online: https://www.cabi.org/cpc/datasheet/36335 [Accessed: 17 January 2020].
Diepenbrock LM and Burrow JD, 2020. Citrus Pest Quick Guide: Lebbeck mealybug Nipaecoccus viridis (Newstead). Entomology and Nematology Department, UF/IFAS Extension. https://doi.org/10.32473/edis-in1280-2020
EPPO (European and Mediterranean Plant Protection Organization), online_a. Nipaecoccus viridis (NIPAVI), Categorization. Available online: https://gd.eppo.int/taxon/NIPAVI/categorization [Accessed: 17 January 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Nipaecoccus viridis (NIPAVI), Distribution. Available online: https://gd.eppo.int/taxon/NIPAVI/distribution [Accessed: 17 January 2020]
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 17 January 2020].
Franco JC, Suma P, Da Silva EB, Blumberg D and Mendel Z, 2004. Management strategies of mealybug pests of citrus in Mediterranean countries. Phytoparasitica, 30, 507. https://doi.org/10.1007/bf02980445
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: A literature‐based model of scale insect biology and systematics, Nipaecoccus viridis. Available online: http://scalenet.info/catalogue/Nipaecoccus%20viridis/ [Accessed: 1 April 2020].
Gerson U and Aplebaum S, online. Plant Pests of the Middle East, Nipaecoccus viridis (Newstead). Available online: http://www.agri.huji.ac.il/mepests/pest/Nipaecoccus_viridis/ [Accessed: 1 April 2020].
Grousset F, Wistermann A, Steffen K, Petter F, Schrader G and Suffert M, 2016. DROPSA Deliverable 1.3 Part 7 ‐ Report for Oranges and Mandarins – Fruit pathway and Alert List. Available online: https://www.researchgate.net/publication/322314633_Work_package_1_Pathways_of_introduction_of_fruit_pests_and_pathogens_Deliverable_13_PART_7-REPORT_on_Oranges_and_Mandarins-Fruit_pathway_and_Alert_List_Dropsa_EU_project_number_613678
Jarjes SJ, Al‐Mallah NM and Abdulla SI, 1989. Insects and mites pests survey on rose‐bay shrubs in Mosul region with some ecological and biological aspects of Nipaecoccus viridis New. and Parlatoria crypta M on rose‐bay shrubs. Mesopotamia Journal of Agriculture, 21, 29.
Mani M and Shivaraju C, 2016. Mealybugs and their management in agricultural and horticultural crops. Berlin, Germany, Springer. 655 pp.
Sharaf NW and Meyerdirk DE, 1987. A review on the biology, ecology and control of Nipaecoccus viridis (Homoptera: Pseudococcidae). Miscellaneous Publications of the Entomological Society of America, 66, 1–18.
Thomas DD and Leppla NC, 2008. The likelihood and consequences of introduction of the spherical mealybug, Nipaecoccus viridis (newstead), into Florida, and its potential effect on citrus production. Proceedings of the Florida State Horticultural Society. Florida. Department of Agriculture. 121, 152–154.
Tyson J, Rainey S, Breach J and Toy S, 2009. Import Risk Analysis: Pears (Pyrus bretschneideri, Pyrus pyrifolia, and Pyrus sp. nr. communis) fresh fruit from China. MAF Biosecurity New Zealand, Wellington.
Wistermann A, Grousset F, Petter F, Schrader G and Suffert M, 2016. DROPSA Deliverable 1.3 Part 6 ‐ Report on Table grapes – Fruit pathway and Alert List. Available online: https://www.researchgate.net/publication/322314744_Work_package_1_Pathways_of_introduction_of_fruit_pests_and_pathogens_Deliverable_13_PART_6-REPORT_on_TABLE_GRAPES-Fruit_pathway_and_Alert_List_Dropsa_EU_project_number_613678
A.8. Oligonychus mangiferus
A.8.1. Organism information
| Taxonomic information | Current valid scientific name: Oligonychus mangiferusSynonyms: Oligonychus terminalis, Paratetranychus mangiferus, Paratetranychus terminalis, Paratetranychus insularisName used in the EU legislation: –Order: AcaridaFamily: TetranychidaeCommon name: mango red spider mite, mango spider miteName used in the Dossier: Oligonychus mangiferus | |
|---|---|---|
| Group | Mites | |
| EPPO code | OLIGMA | |
| Regulated status | Oligonychus mangiferus is not regulated in the EU neither is listed by EPPO. | |
| Pest status in Israel | Present in Israel (Ben‐David et al., 2013; CABI CPC, online; Migeon and Dorkeld, online). | |
| Pest status in the EU | Absent in the EU (CABI CPC, online; Migeon and Dorkeld, online). | |
| Host status on Ficus carica | Ficus carica is reported as a host of O. mangiferus (Gupta and Gupta, 1994; Migeon and Dorkeld, online). | |
| PRA information | No Pest Risk Assessment is currently available. | |
| Other relevant information for the assessment | ||
| Biology | Oligonychus mangiferus is a polyphagous pest and feeds mostly on the upper surfaces of the leaves of its hosts. Infestations reach a peak during late summer since the mite population is favoured by dry season (Beard, online).There are five development stages of O. mangiferus, which consist of egg, larva, protonymph, deutonymph and adult. Three immature stages are each followed by a quiescent stage. Females can deposit between 11.63 and 46.43 eggs (Abou‐Awad et al., 2011).Favourable temperature for fecundity, based on laboratory study, is from 25 up to 31°C. The female life cycle averaged from 10.78 to 12.18 days at temperatures of 31 and 25°C. The developmental time of males was shorter. Female longevity averaged from 18.86 to 22.78 days at temperatures of 31 and 25°C (Abu‐shosha et al., 2017).Oligonychus mangiferus can have annually up to 21 generations in Egypt (Ben‐David et al., 2013). According to Lin (2013) in Taiwan O. mangiferus may produce up to 26 generations per year.Assuming that the life history is similar to that of other spider mites, such as Oligonychus perditus (EFSA 2017), the overwintering may happen on bark as egg or adult. | |
| Symptoms | Main type of symptoms | On mango, leaves become yellow/pale, initially forming pale patches followed by premature leaf drop. It mostly lives and feeds on the upper leaf surface where it spins silk thread. It is more frequent to affect leaves at the upper level of trees than those closer to the ground (Gerson and Applebaum, online).Oligonychus mangiferus damages leaves and reduce fruit quality and quantity (Abu‐shosha et al., 2017; Lin, 2013). |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pests | Oligonychus coffeae and O. perseae are morphologically similar to O. mangiferus (Abu‐shosha et al., 2017; Lin, 2013). | |
| Host plant range | Oligonychus mangiferus is an important pest of mango, grape vines and occasionally of litchi, in India (Gupta and Gupta, 1994). It is serious pest of cotton and pomegranate in Egypt. In Israel, O. mangiferus was found on mango (Gerson and Applebaum, online).The main host plants are Alexandrian laurel (Calophyllum inophyllum), Annona sp., Arecaceae, avocado (Persea americana), banana (Musa sp.), bead tree (Melia azedarach), blackberry (Rubus allegheniensis), Brazilian guava (Psidium guajava), castor bean (Ricinus communis), champedak (Artocarpus integer), Combretum sp., Cotoneaster sp., crepe myrtle (Lagerstroemia indica), Cydonia sp., Delonix sp., dwarf white bauhinia (Bauhinia acuminate), Eugenia sp., Ficus sp., fig (Ficus carica), gardenia (Gardenia jasminoides), golden shower (Cassia fistula), grapevine (Vitis vinifera), indian laurel (Litsea chinensis), Italian cypress (Cupressus sempervirens), Japanese medlar (Eriobotrya japonica), Lagerstroemia thorelii, litchi (Litchi chinensis), longan (Dimocarpus longan), mango (Mangifera indica), Natal mahogany (Trichilia emetica), oriental thuja (Platycladus orientalis), palmiste rouge (Acanthophoenix sp.), peach (Prunus persica), pear (Pyrus communis), pine (Pinus sp.), pomegranate (Punica granatum), red frangipani (Plumeria rubra), red river gum (Eucalyptus camaldulensis), rose (Rosa sp.), Syzygium sp., Terminalia sp. and tree of heaven (Ailanthus altissima) (Migeon and Dorkeld, online). | |
| Pathways | According to the EFSA PLH Panel (2017) categorisation of O. perditus, the main pathways of entry are: plants for planting and ornamental branches. | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.8.2. Possibility of pest presence in the nursery
A.8.2.1. Possibility of entry from the surrounding environment
Oligonychus mangiferus is present in Israel (Ben‐David et al., 2013; CABI CPC, online; Migeon and Dorkeld, online). There is no information available about dispersal capacity of O. mangiferus. Possible pathways of spreading can be observed within other related species. It was reported that they are able to spread between plants, to the further places by wind, animal and human dispersal (EFSA PLH Panel, 2017).
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Punica granatum, Eucalyptus camaldulensis, Ricinus communis and Persea americana are hosts of O. mangiferus (Migeon and Dorkeld, online).
Uncertainties:
-
–
There is no surveillance information on the presence or population pressure of the pests in the area where nursery is located.
-
–
No information available on the distance of the nursery to sources of pests in the surrounding environment.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the insect to enter the nursery from the surrounding area. It is possible because suitable hosts are present in the surrounding and F. carica plants may be attacked.
A.8.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media.
A.8.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
Lagerstroemia indica is grown in the nursery (Dossier Section 9.0) and it is a host of the pest. Morus alba is grown in the nursery (Dossier Section 9.0), but it is not a host of the pest.
Uncertainties
-
–
There is no information on the presence or population pressure of the pests in the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible.
A.8.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of O. mangiferus between the years 1995 and November 2019 (EUROPHYT, online).
A.8.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on O. mangiferus is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | Mites may immigrate to the production fields through wind or carried by humans from the surrounding environment and attack plants grown in open fields.Uncertainties:– No uncertainties | Mites may immigrate to the production fields through wind or carried by humans from the surrounding environment and attack plants grown in open fields.Uncertainties:– No uncertainties |
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Acaricide treatments should be enough to keep the mite density under control.Uncertainties:– Development of resistance to the acaricides. | Acaricide treatments should be enough to keep the mite density under control.Uncertainties:– Development of resistance to the acaricides. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | No | Not applicable | Not applicable |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Mite symptoms on leaves are easily detectable at high density.Uncertainties:– Mite presence can go undetected at the very low density. | Mite symptoms on leaves are easily detectable at high density.Uncertainties:– Mite presence can go undetected at the very low density. |
| 11 | Inspections before export | Yes | Presence of overwintering stages is hardly detectable without a careful inspection with appropriate magnification.Uncertainties:– Mites can go undetected at low density. | Presence of overwintering stages is hardly detectable without a careful inspection with appropriate magnification.Uncertainties:– Mites can go undetected at low density. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, O. mangiferus is common in Israel.Uncertainties:– There is no information on the density of O. mangiferus in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, O. mangiferus is common in Israel.Uncertainties:– There is no information on the density of O. mangiferus in the surrounding areas. |
A.8.5. Overall likelihood of pest freedom for bare rooted plants and liners
A.8.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants and liners
Although O. mangiferus is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to wind and human activity. Inspections are expected to be effective because of the high density of mites and presence of silk. Ficus carica is not considered a preferential host. Insecticide/acaricide treatments are expected to be effective because mites feed on the upper side of leaves and hence they are directly exposed to the treatment.
A.8.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants and liners
Oligonychus mangiferus is widespread in Israel; the scenario assumes a high pest pressure from the surrounding because suitable hosts are present and significant transfer from the surrounding due to wind and human activity. Inspections are expected to be not completely effective due to low density of mites. Ficus carica is considered a preferential host. Insecticide/acaricide treatments are not expected to be fully effective because the individuals on the bark may not be reached. This scenario also assumes that the overwintering takes place on the bark as it is known for other mites.
A.8.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants and liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by wind and human activity, the weak information on the degree of susceptibility of F. carica, the internal spread and the absence of reported problems within the nursery and at EU borders, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.8.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, and unclear host suitability of F. carica, result in high level of uncertainties for infestation rates below the median. Otherwise, insecticide/acaricide treatments are expected to be effective, which gives less uncertainties for rates above the median.
A.8.5.5. Elicitation outcomes of the assessment of the pest freedom for Oligonychus mangiferus on bare rooted plants and liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.23) and pest freedom (Table A.24).
Table A.23.
Elicited and fitted values of the uncertainty distribution of pest infestation by Oligonychus mangiferus per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5.00 | 50.0 | 90.0 | 190 | 300 | ||||||||||
| EKE | 3.64 | 5.07 | 8.03 | 15.2 | 26.6 | 42.9 | 61.1 | 103 | 153 | 182 | 213 | 243 | 268 | 283 | 294 |
The EKE results are the BetaGeneral (0.78479, 1.2987, 3, 305) distribution fitted with @Risk version 7.6.
Table A.24.
The uncertainty distribution of plants free of Oligonychus mangiferus per 10,000 plants calculated by Table A.23
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,700 | 9,810 | 9,910 | 9,950 | 9,995 | ||||||||||
| EKE results | 9,706 | 9,717 | 9,732 | 9,757 | 9,787 | 9,818 | 9,847 | 9,897 | 9,939 | 9,957 | 9,973 | 9,985 | 9,992.0 | 9,994.9 | 9,996.4 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.24.
Figure A.16.
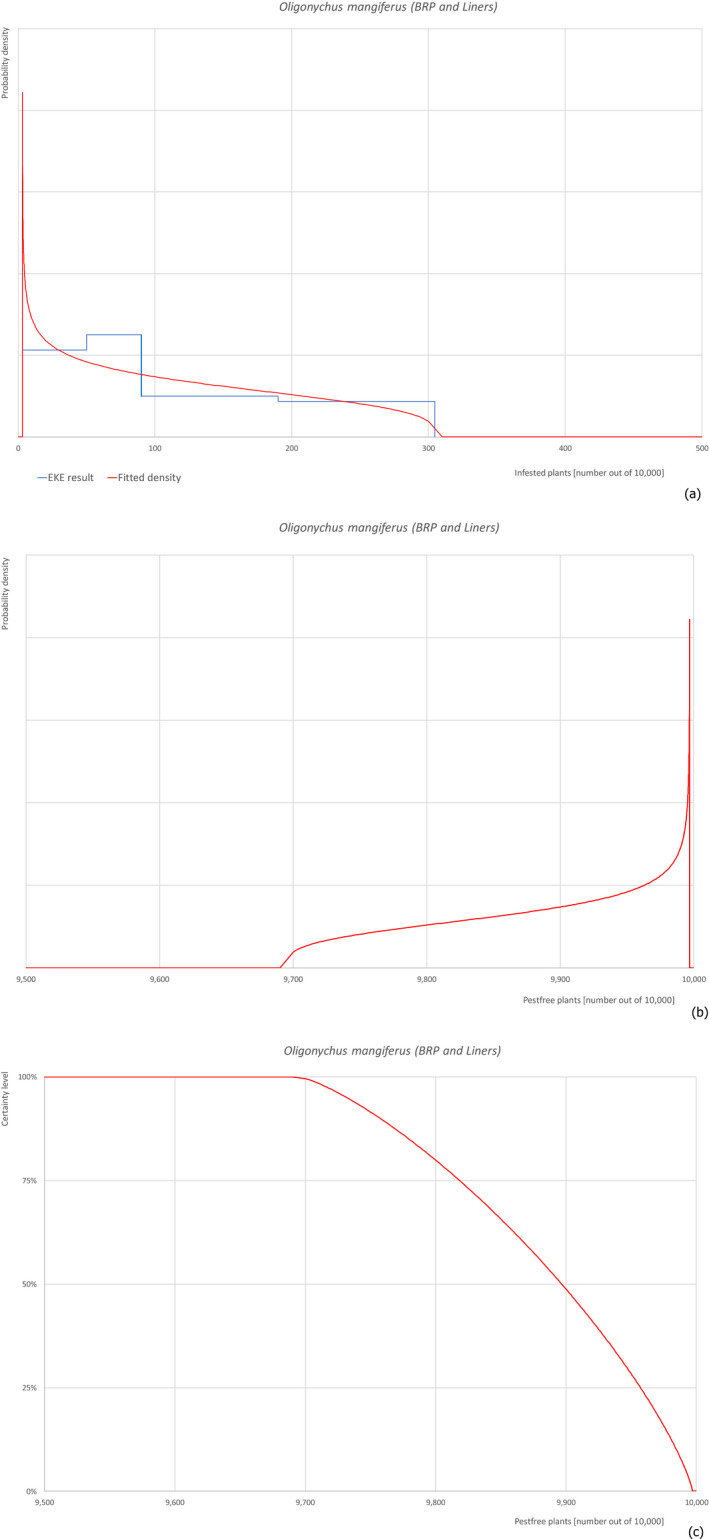
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.8.6. Reference list
Abou‐Awad BA, Al‐Azzazy MM and Afia SI, 2011. Effect of temperature and relative humidity on the rate of development, fecundity and life table parameters of the red spider mite Oligonychus mangiferus (Rahman and Sapra) (Acari: Tetranychidae). Archives of phytopathology and plant protection, 44, 1862–1866. https://doi.org/10.21608/jppp.2010.86938
Abu‐shosha MA, Abdallah AA, Abdel‐Aziz NM and Mahmoud AS, 2017. Effect of Temperature on Biology of Oligonychus mangiferus (Rahman and Sapra) (Acari: Tetranychidae). Journal of Plant Protection and Pathology, 8, 389–392. https://doi.org/10.21608/jppp.2017.46348
Beard JJ, online. Spider mite species of Australia (including key exotic southeast Asian pest species), Oligonychus mangiferus (Rahman & Sapra, 1940). Available online: https://keys.lucidcentral.org/keys/v3/spider_mites_australia/key/spider_mites_of_australia/Media/Html/entities/Oligonychus_mangiferus_Rahman__Sapra_1940.htm [Accessed: 22 April 2020].
Ben‐David T, Ueckermann EA and Gerson U, 2013. An annotated list of the spider mites (Acari: Prostigmata: Tetranychidae) of Israel. Israel Journal of Entomology, 43, 125–148.
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Oligonychus mangiferus (mango red spider mite). Available online: https://www.cabi.org/cpc/datasheet/37279 [Accessed: 22 April 2020].
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Kertesz V, Aukhojee M and Gregoire J‐C, 2017. Scientific Opinion on the pest categorisation of Oligonychus perditus. EFSA Journal 2017;15(11):5075, 20 pp. https://doi.org/10.2903/j.efsa.2017.5075
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 April 2020].
Gerson U and Appelebaum S, online. Plant Pests of the Middle East, Oligonychus mangiferus (Rahman and Sapra). Available online: http://www.agri.huji.ac.il/mepests/pest/Oligonychus_mangiferus/[Accessed: 22 April 2020].
Gupta SK and Gupta YN, 1994. A taxonomic review of Indian Tetranychidae (Acari: Prostigmata) with description of new species, re‐descriptions of known species and keys to genera and species. Memoirs of the Zoological Survey of India, 18, 1–196.
Lin MY, 2013. Temperature‐dependent life history of Oligonychus mangiferus (Acari: Tetranychidae) on Mangifera indica. Experimental and Applied Acarology, 61, 403–413. https://doi.org/10.1007/s10493-013-9716-4
Migeon A and Dorkeld F, online. Spider Mites Web, Oligonychus mangiferus. Available online: https://www1.montpellier.inra.fr/CBGP/spmweb/notespecies.php?id=561 [Accessed: 22 April 2020].
A.9. Phenacoccus solenopsis
A.9.1. Organism information
| Taxonomic information | Current valid scientific name: Phenacoccus solenopsisSynonyms: Phenacoccus cevalliae, Phenacoccus gossypiphilousName used in the EU legislation: –Order: HemipteraFamily: PseudococcidaeCommon name: cotton mealybug, solenopsis mealybugName used in the Dossier: Phenacoccus solenopsis | |
|---|---|---|
| Group | Insects | |
| EPPO code | PHENSO | |
| Regulated status | Phenacoccus solenopsis is not regulated in the EU, neither listed by EPPO.It is a quarantine pest in Bangladesh (Islam et al., 2017). | |
| Pest status in Israel | Present, widespread in Israel (EPPO, online; García Morales et al., online; Spodek et al., 2018).It was first reported in the Jordan Valley in 2008 on basil (Ocimum basilicum) and bell pepper (Capsicum annuum) (Spodek et al., 2018). | |
| Pest status in the EU | Restricted, present in Cyprus and the Netherlands (CABI, online; García Morales et al., online). In the Netherlands, the pest was observed only in greenhouses (CABI, online). According to Personal communication of Milonas (2020), the pest was recently found on tomato plants on Crete island. | |
| Host status on Ficus carica | Ficus carica was reported as host of P. solenopsis with incidental infestation level (Arif et al., 2009; Fallahzadeh et al., 2014). | |
| PRA information | The Pest Risk Assessments available for are Phenacoccus solenopsis:– Rapid pest risk analysis for Phenacoccus solenopsis (Cotton mealybug) and the closely related P. defectus and P. solani (Malumphy et al., 2013).– Pest risk analysis (PRA) of Mealybug Spp. in Bangladesh (Islam et al., 2017). | |
| Other relevant information for the assessment | ||
| Biology | Phenacoccus solenopsis originates from southern California and Nevada (Spodek et al., 2018). The life cycle of P. solenopsis ranges between 28 and 35 days. The pest can complete about 8–12 generations in a year (Fand and Suroshe, 2015).Female of P. solenopsis develops through an egg, three nymphal instars to an adult. The male has additional nymphal stage, the last two are called prepupa and pupa. Males have wings and females are wingless. Reproduction is sexual and ovoviviparous. Adult females are pale yellow to orange covered by powdery, wax secretion (Hodgson et al., 2008). They mate only once and lay ~ 150–600 eggs in a white, waxy ovisac (Fand and Suroshe, 2015). Facultative parthenogenesis was observed under laboratory conditions of mealybugs collected from Nagpur, India (Vennila et al., 2010).The first nymphs are crawlers, which disperse to other parts of the same plant or get carried by the wind or other means (machinery, workers, animals) to other areas (Hodgson et al., 2008).The adult males live from few hours up to 3 days, depending on the temperature (Hodgson et al., 2008). Adult females can live for up to 3 months (Gerson and Aplebaum, online).In Israel, the pest was observed on roots and root collars of weeds. In winter, P. solenopsis populations were found on the stems, branches and root collar of hibiscus plants (Spodek et al., 2018).It overwinters as an adult female, on the bark, the stem and branches of woody plants. It seems that it may develop in the ground on roots of non‐woody plants (Spodek et al., 2018). This mealybug has been reported to be capable of surviving temperatures ranging from 0 to 45°C, throughout the year (CABI, online).The crawlers of P. solenopsis have been reported be commonly dispersed by wind for distances ranging from a few meters to several kilometres (Islam et al., 2017). | |
| Symptoms | Main type of symptoms | Phenacoccus solenopsis prefers the upper parts of the plants, young shoots or branches carrying fruitlets (Spodek et al., 2018). Large populations of mealybugs cause general weakening, distortion, defoliation, dieback and death of susceptible plants (Malumphy et al., 2013). Plants become covered in sooty moulds that grow on the honeydew produced by mealybugs. The honeydew also attracts ants that protect the mealybugs from natural enemies (Hodgson et al., 2008).The infested plants of cotton become stunted, growth appears to stop, and most plants look dehydrated. In severe outbreaks, the bolls fail to open, and defoliation occurs (including the loss of flower buds, flowers and immature bolls) (Hodgson et al., 2008).On tomatoes the pest causes foliar yellowing, leaf wrinkling, puckering and severe damage, resulting in death (Ibrahim et al., 2015). |
| Presence of asymptomatic plants | Plant damage might not be obvious in early infestation or during dormancy (due to absence of leaves), but the presence of mealybugs on the plants could be observed. During the crawler stage, infestation is difficult to be noted (Ben‐Dov, 1994). | |
| Confusion with other pathogens/pests | Phenacoccus solenopsis is very similar to other species of Phenacoccus. A microscope observation with the morphological key are needed for identification of the pest (Hodgson et al., 2008). | |
| Host plant range | Phenacoccus solenopsis is highly invasive and polyphagous pest, and it is reported from more than 200 plant species (Fand and Suroshe, 2015).The host plants of economic importance are okra (Abelmoschus esculentus), sapota (Achras zapota), cashew (Anacardium occidentale), pigeon pea (Cajanus cajan), chilli (Capsicum annuum), papaya (Carica papaya), watermelon (Citrullus lanatus), round melon (Citrullus vulgaris), musk melon (Cucumis melo), pumpkin (Cucurbita moschata), cluster bean (Cyamopsis tetragonoloba), fig (Ficus carica), cotton (Gossypium hirsutum), sunflower (Helianthus annuus), mesta (Hibiscus cannabinus), ambadi (Hibiscus sabdariffa), bottle gourd (Lagenaria siceraria), crape myrtle (Lagerstroemia indica), ridged gourd (Luffa acutangula), sponge gourd (Luffa aegyptiaca), mango (Mangifera indica), bitter guard (Momordica charantia), white mulberry (Morus alba), guava (Psidium guajava), pomegranate (Punica granatum), sesame (Sesamum indicum), tomato (Solanum lycopersicum), brinjal (Solanum melongena), potato (Solanum tuberosum), jowari (Sorghum bicolor), green gram (Vigna radiata), common grape vine (Vitis vinifera), ber (Ziziphus mauritiana) and many other plants (Arif et al., 2009; Fallahzadeh et al., 2014; Fand and Suroshe, 2015; García Morales et al., online). Weed species are also suitable host plants to P. solenopsis (Vennila et al., 2013).The main economic impact was reported on cotton, causing 30–60% yield losses in India and Pakistan (Fand and Suroshe, 2015). In Israel, it is a serious pest in greenhouses (on bell pepper, tomato, eggplant) and on cotton fields (Spodek et al., 2018). | |
| Pathways | Possible pathways of entry for mealybugs are plant materials of any kind (hiding in a protected site – on the bark, roots, stems, leaves), human transportation, irrigation water, wind, animals and ants (Mani and Shivaraju, 2016). | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.9.2. Possibility of pest presence in the nursery
A.9.2.1. Possibility of entry from the surrounding environment
Phenacoccus solenopsis is widespread in Israel (EPPO, online; García Morales et al., online; Spodek et al., 2018). It is a serious pest in greenhouses (on bell pepper, tomato, eggplant) and on cotton fields in Israel (Spodek et al., 2018). If cotton is produced in the neighbourhood of the export nursery transfer of the insect may be more likely.
Possible pathways of entry into the nursery can be by movement of infested plants, wind, human and animal dispersal and irrigation water (Mani and Shivaraju, 2016). The first nymph instars (crawlers) can disperse by walking and by wind (Mani and Shivaraju, 2016).
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Diospyros kaki, Punica granatum, Citrullus lanatus, Eucalyptus camaldulensis, Acacia leucophloea, Acacia modesta, Acacia nilotica and Ricinus communis are hosts of P. solenopsis (García Morales et al., online).
Uncertainties:
-
–
No information about the density of the population of P. solenopsis in the area surrounding the nursery is available.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by wind and human accidental transportation.
A.9.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media. Plants are produced inside the nursery and the scale insects are not associated with soil growing media.
A.9.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
According to Dossier Section 9.0, following plants known to be hosts of the pest are grown in the fig liner export nursery: Lagerstroemia indica and Morus alba, with a distance of a few dozens of metres between them and the fig liners.
Therefore, it is possible for P. solenopsis to reproduce within the nursery on F. carica and other hosts, which are presents.
Possible pathways of spreading within the nursery can be by movement of infested plants, wind, human and animal dispersal and irrigation water. The first nymph instars (crawlers) can disperse by walking and by wind (Mani and Shivaraju, 2016).
If non woody plants (e.g. herbs, weeds) are present in the nursery, the spread of the pest would be more likely.
According to Dossier Section 9.0, the growing medium is peat substrate (EU‐made). Liners are rooted directly in pots, in the same growing medium as used for the bare rooted plants. Soil solarisation is performed by covering the soil with transparent polyethylene for 2 months – July and August (normally the time of highest radiation). The polyethylene sheet is spread after the soil has been cleaned from the previous crop and has been processed for the next cycle. The polyethylene in the sheets is supplemented with ‘antidrip’ or ‘antifog’ substances which prevents water condensation and accumulation on the sheet, so improving treatment efficacy by raising the under‐sheet temperature by 4–5°C compared with regular polyethylene sheets. The max temperature in the top 20 cm of the soil is 44–48°C daily, for the duration of 2 months. The sheets are maintained clean and intact through the treatment duration, and the soil moisture is maintained to the field capacity level, by weekly irrigation with a water volume that parallels 1 m3 water/dunam per day.
According to Dossier Section 9.0, the water that is used for irrigation is regular tap water, that goes through a 120‐mesh filter to remove rough dirt like sand and stones. Liners are irrigated by sprinklers, and bare rooted plants receive drip irrigation.
Uncertainties:
-
–
No information is available for the isolation or proximity of the mother plant stock for cuttings collection to other host plant species in the nursery.
-
–
No information on whether non‐woody plants hosting the mealybug (e.g. herbs, weeds) are present in the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible either by wind or accidental transfer within the nursery.
A.9.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of P. solenopsis between the years 1995 and November 2019 (EUROPHYT, online).
There have been multiple interceptions of P. solenopsis in England on fresh vegetables from West Africa, and most recently on herbs (basil) from Israel and bell peppers from East Africa (Malumphy et al., 2013).
A.9.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on P. solenopsis is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties |
| 2 | Soil treatment | Yes, for bare rooted plants | Solarisation is sufficient to suppress any scales eventually associated with old roots.Uncertainties:– No uncertainties | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Pesticide sprays are generally effective against crawlers but have limited effectiveness against P. solenopsis when hidden in crevices, or protected by the waxy covering of its body.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty whether the pesticide can effectively reach all the bark/root parts where the mealybugs are located because of the barrier effect of the leaves and soil. | Pesticide sprays are generally effective against crawlers but have limited effectiveness against P. solenopsis when hidden in crevices, or protected by the waxy covering of its body.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty whether the pesticide can effectively reach all the bark/root parts where the mealybugs are located because of the barrier effect of the leaves and soil. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | Yes | As weeds can host mealybugs, the treatment should reduce the pressure on the crop.Uncertainties:– There is uncertainty about the efficacy of the treatment and the species of weeds and whether they are host to P. solenopsis. | As weeds can host mealybugs, the treatment should reduce the pressure on the crop.Uncertainties:– There is uncertainty about the efficacy of the treatment and the species of weeds and whether they are host to P. solenopsis. |
| 8 | Plant treatment before export | Yes | Partly effective because it is not clear whether the washing may remove the adults possibly hidden in crevices and holes. Mealybugs can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers with the naked eye. | Mealybugs can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers with the naked eye. |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Mealybugs could go undetected because of the small size of the pest and difficulty in the search. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for mealybugs is not known. | Mealybugs could go undetected because of the small size of the pest and difficulty in the search. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for mealybugs is not known. |
| 11 | Inspections before export | Yes | Mealybugs could go undetected because of the small size of the pest and difficulty in the search, including roots. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for mealybugs is not known. | Mealybugs could go undetected because of the small size of the pest and difficulty in the search, including roots. In early stages of infestation and during dormancy symptoms may not be obvious.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for mealybugs is not known. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented, however P. solenopsis is common in Israel.Uncertainties:– There is no information on the density of P. solenopsis in the surrounding areas. | Surveillance in the surrounding area is not implemented, however P. solenopsis is common in Israel.Uncertainties:– There is no information on the density of P. solenopsis in the surrounding areas. |
A.9.5. Overall likelihood of pest freedom for bare rooted plants
A.9.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants
Although P. solenopsis is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to wind and human activity. Inspections are expected to be effective because waxy stages of the insect are visible. Insecticide treatments are expected to be conducted at the right timing to target unprotected life stages of the insect. Mother plants are kept healthy as well by using treatments.
A.9.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants
Phenacoccus solenopsis is widespread in Israel, the scenario assumes a high pest pressure from outside and strong transfer from the surrounding due to wind and intensive human activity. Inspections are expected to be ineffective because of the presence of hidden stages. Insecticide treatments are expected to be conducted at timing when the insect is hidden or protected by wax. Mother plants are infested despite treatments and may contribute spreading the pest within the nursery.
A.9.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by wind and human activity, the internal spread and the absence of reported problems within the nursery and at EU borders, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.9.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery results in high level of uncertainties for infestation rates below the median. Otherwise, detection of the pest especially before the export is likely, which gives less uncertainties for rates above the median.
A.9.5.5. Elicitation outcomes of the assessment of the pest freedom for Phenacoccus solenopsis on bare rooted plants
The following tables show the elicited and fitted values for pest infestation/infection (Table A.25) and pest freedom (Table A.26).
Table A.25.
Elicited and fitted values of the uncertainty distribution of pest infestation by Phenacoccus solenopsis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 10.0 | 35.0 | 60.0 | 100 | 300 | ||||||||||
| EKE | 9.87 | 13.1 | 16.7 | 22.1 | 28.2 | 35.3 | 42.6 | 59.5 | 83.0 | 100 | 125 | 160 | 212 | 270 | 358 |
The EKE results are the Lognorm (80.135, 72.327) distribution fitted with @Risk version 7.6.
Table A.26.
The uncertainty distribution of plants free of Phenacoccus solenopsis per 10,000 plants calculated by Table A.25
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,700 | 9,900 | 9,940 | 9,965 | 9,990 | ||||||||||
| EKE results | 9,642 | 9,730 | 9,788 | 9,840 | 9,875 | 9,900 | 9,917 | 9,941 | 9,957 | 9,965 | 9,972 | 9,978 | 9,983 | 9,987 | 9,990 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.26.
Figure A.17.
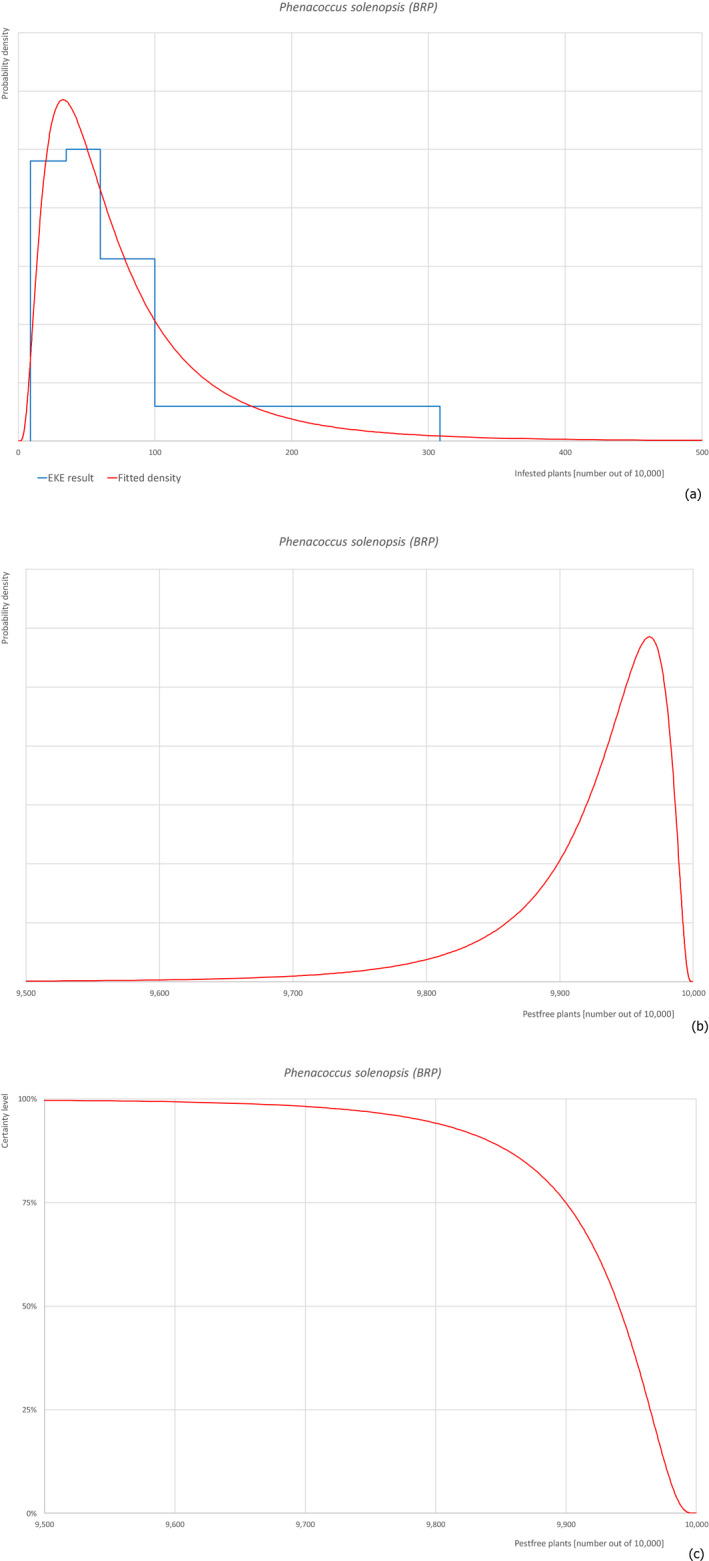
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.9.6. Overall likelihood of pest freedom for liners
A.9.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested liners
Although P. solenopsis is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to wind and human activity. Inspections are expected to be effective because waxy stages of the insect are visible, unless they are in the soil. Insecticide treatments are expected to be conducted at the right timing to target unprotected life stages of the insect. Mother plants are kept healthy as well by using treatments.
A.9.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested liners
Phenacoccus solenopsis is widespread in Israel; the scenario assumes a high pest pressure from outside and strong transfer from the surrounding due to wind and intensive human activity. Inspections are expected to be ineffective because of the presence of hidden stages or when the pest is in the soil. Insecticide treatments are expected to be conducted at timing when the insect is hidden or protected by wax. Mother plants are infested despite treatments and may contribute spreading the pest within the nursery.
A.9.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by wind and human activity, the internal spread and the absence of reported problems within the nursery and at EU borders, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.9.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery results in high level of uncertainties for infestation rates below the median. Otherwise, detection of the pest especially before the export is likely, which gives less uncertainties for rates above the median.
A.9.6.5. Elicitation outcomes of the assessment of the pest freedom for Phenacoccus solenopsis on liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.27) and pest freedom (Table A.28).
Table A.27.
Elicited and fitted values of the uncertainty distribution of pest infestation by Phenacoccus solenopsis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 20.0 | 60.0 | 100 | 250 | 500 | ||||||||||
| EKE | 18.3 | 19.1 | 21.1 | 26.6 | 36.5 | 52.0 | 70.8 | 120 | 188 | 232 | 288 | 349 | 414 | 463 | 509 |
The EKE results are the BetaGeneral (0.68547, 2.1937, 18, 600) distribution fitted with @Risk version 7.6.
Table A.28.
The uncertainty distribution of plants free of Phenacoccus solenopsis per 10,000 plants calculated by Table A.27
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,500 | 9,750 | 9,900 | 9,940 | 9,980 | ||||||||||
| EKE results | 9,491 | 9,537 | 9,586 | 9,651 | 9,712 | 9,768 | 9,812 | 9,880 | 9,929 | 9,948 | 9,964 | 9,973 | 9,979 | 9,981 | 9,982 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.28.
Figure A.18.
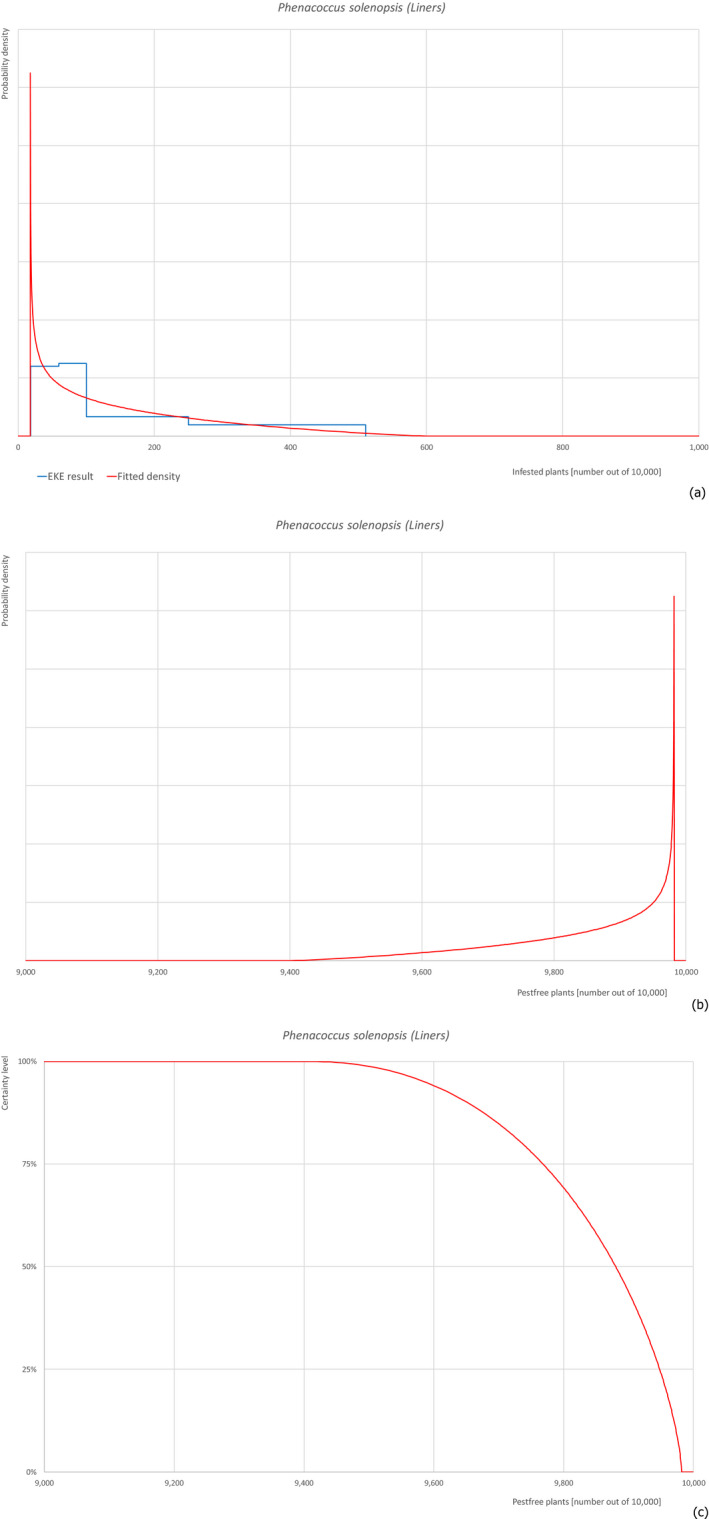
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
Figure A.19.
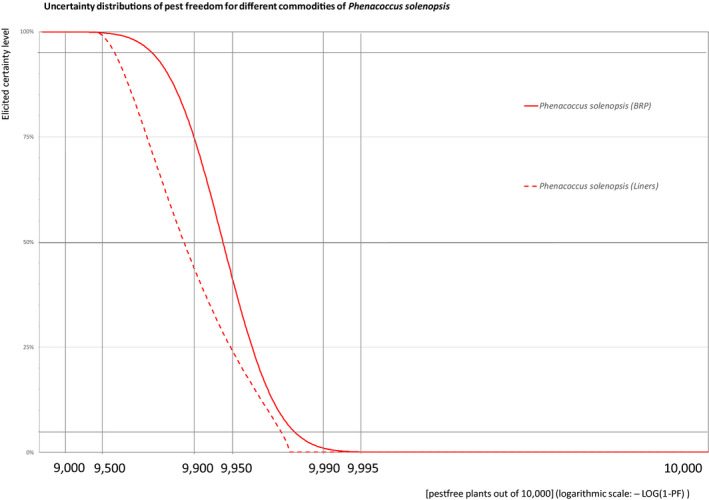
Elicited certainty (y‐axis) of the number of bare rooted plants or liners of Ficus carica pest free from Phenacoccus solenopsis (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU introduced from Israel as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
A.9.7. Reference list
Arif MI, Rafiq M and Ghaffar A, 2009. Host plants of cotton mealybug (Phenacoccus solenopsis): a new menace to cotton agroecosystem of Punjab, Pakistan. International Journal of Agriculture and Biology, 11, 163–167.
Ben‐Dov Y, 1994. A systematic catalogue of the mealybugs of the world (Insecta: Homoptera: Coccoidea: Pseudococcidae and Putoidae) with data on geographical distribution, host plants, biology and economic importance. 100th Intercept Limited Andover, UK. 686 pp.
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Phenacoccus solenopsis (cotton mealybug). Available online: https://www.cabi.org/isc/datasheet/109097 [Accessed: 27 February 2020].
EPPO (European and Mediterranean Plant Protection Organization), online. Phenacoccus solenopsis (PHENSO), Distribution. Available online: https://gd.eppo.int/taxon/PHENSO/distribution [Accessed: 27 February 2020].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 27 February 2020].
Fallahzadeh M, Abdimaleki R and Saghaei N, 2014. Host Plants of the Newly Invasive Mealybug Species, Phenacoccus solenopsis (Hemiptera: Pseudococcidae), in Hormozgan Province, Southern Iran. Zeitschrift fur Entomologie Entomofauna, 35, 169–176.
Fand B and Suroshe S, 2015. The invasive mealybug Phenacoccus solenopsis Tinsley, a threat to tropical and subtropical agricultural and horticultural production systems ‐ a review. Crop Protection, 69, 34–43. https://doi.org/10.1016/j.cropro.2014.12.001
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: A literature‐based model of scale insect biology and systematics, Phenacoccus solenopsis. Available online: http://scalenet.info/catalogue/Phenacoccus%20solenopsis/ [Accessed: 27 February 2020].
Gerson U and Aplebaum S, online. Plant Pests of the Middle East, Phenacoccus solenopsis Tinsley. Available online: http://www.agri.huji.ac.il/mepests/pest/Phenacoccus_solenopsis/ [Accessed: 28 February 2020].
Hodgson C, Abbas G, Arif MJ, Saeed S and Karar H, 2008. Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa, 1913, 1–35. https://doi.org/10.11646/zootaxa.1913.1.1
Ibrahim SS, Moharum FA and El‐Ghany NMA, 2015. The cotton mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) as a new insect pest on tomato plants in Egypt. Journal of plant protection research, 55, 48–51. https://doi.org/10.1515/jppr-2015-0007
Islam KS, Ali R, Hossain A, Aminuzzaman FM, Ullah J, Alam F, Saha S and Abdullah‐Al‐Mahamud KM, 2017. Pest Risk Analysis (PRA) of Mealybug Spp. in Bangladesh. Strengthening Phytosanitary Capacity in Bangladesh Project. Plant Quarantine Wing Department of Agricultural Extension Khamarbari, Farmgate, Dhaka‐1205. 128 pp.
Malumphy C, Baker R and Anderson H, 2013. Rapid pest risk analysis for Phenacoccus solenopsis (cotton mealybug) and the closely related P. defectus and P. solani. FERA (The Food and Environment Research Agency), UK. 8 pp.
Mani M and Shivaraju C, 2016. Mealybugs and their management in agricultural and horticultural crops. Berlin, Germany, Springer. 655 pp.
Spodek M, Ben‐Dov Y, Mondaca L, Protasov A, Erel E and Mendel Z, 2018. The cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) in Israel: pest status, host plants and natural enemies. Phytoparasitica, 46, 45–55. https://doi.org/10.1007/s12600-018-0642-1
Vennila S, Deshmukh AJ, Pinjarkar D, Agarwal M, Ramamurthy VV, Joshi S, Kranthi KR and Bambawale OM, 2010. Biology of the mealybug, Phenacoccus solenopsis on cotton in the laboratory. Journal of Insect Science, 10, 115.
Vennila S, Prasad YG, Prabhakar M, Agarwal M, Sreedevi G and Bambawale OM, 2013. Weed hosts of cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Journal of Environmental Biology, 34, 153–158.
A.10. Plicosepalus acaciae
A.10.1. Organism information
| Taxonomic information | Current valid scientific name: Plicosepalus acaciaeSynonyms: Loranthus acaciaeName used in the EU legislation: –Order: SantalalesFamily: LoranthaceaeCommon name: acacia strap flowerName used in the Dossier: – | |
|---|---|---|
| Group | Plants | |
| EPPO code | LOAAC | |
| Regulated status | Plicosepalus acaciae is not regulated anywhere in the world neither listed by EPPO. | |
| Pest status in Israel | Present in Israel, in the Jordan Valley and in the area between the north of Eilat and the Dead Sea (Veste et al., 2015). | |
| Pest status in the EU | Plicosepalus acaciae is absent in the EU. | |
| Host status on Ficus carica | Ficus carica is a host of P. acaciae (Qasem, 2009). | |
| PRA information | No Pest Risk Assessment is currently available. | |
| Other relevant information for the assessmentAccording to Dossier Section 9.0, bare rooted plants are 20–100 cm tall, with base diameter of up to 2 cm. Liners are about 10 cm high and with ~ 1 cm base diameter. | ||
| Biology | Plicosepalus acaciae is a perennial leafy hemiparasitic mistletoe with 6–7 years of lifespan (Qasem, 2009).Spectacled bulbul birds (Pycnonotus xanthopygos) are dispersing viable seeds of mistletoe, when consuming their fruits. Movement patterns of bulbuls were observed with a maximum distance of 267 m. Movements on a larger scale probably occur but were not observed and are presumed to be rare (Green et al., 2009).The fruits are red berries with sticky seeds (Veste et al., 2014). The fruiting occurs from June to April, with a peak in October and November (Green et al., 2009).At seed germination, the modified hypocotyl forms a pad that adheres to the host branch to form a haustorium (Qasem, 2009). Plicosepalus acaciae is connected with its host through the haustorium, which allows the transportation of water, inorganic and organic compounds from the host's transpiration stream directly into the parasite (Veste et al., 2014). This mistletoe has chlorophyll and so photosynthesise independently but takes water and nutrients from its host (Qasem, 2009)The parasitic plants must have lower water potentials than their hosts to ensure the flow of water and nutrients through the haustorial connection (Qasem, 2009).The rapid invasion of P. acaciae in Israel is caused by the increase in the population of bulbul birds (Ward et al., 2006). | |
| Symptoms | Main type of symptoms | The characteristic sign of mistletoe infection is the presence of the evergreen plant growing on branches or trunks of trees (Mathiasen et al., 2008).The effects of mistletoes on their hosts include dieback of branches, hypertrophy, reductions in growth, vigour, fruiting, and seed production. Severe infection by mistletoes is often associated with premature mortality of host trees (Mathiasen et al., 2008).Christ thorn jujube (Ziziphus spina‐christi) suffered high mortality and had significantly lower fruit production when infected by P. acaciae. Moreover, mistletoes on trees that were heavily infested produced more fruits (Ward et al., 2006).Acacia raddiana populations parasitised by the mistletoe P. acaciae are suffering high levels of mortality in the Negev Desert. However, the tree mortality seems not to be directly related to the mistletoe but caused by other mechanical damages of road building in close distance of the plants (Bowie and Ward, 2004). |
| Presence of asymptomatic plants | At early infestation, the shoots of mistletoes may be easily overlooked. | |
| Confusion with other pathogens/pests | Plicosepalus acaciae is similar to other Plicosepalus species, such as P. curviflorus, P. kalachariensis, P. meridianus, P. nummulariifolius, P. sagittifolius and P. undulates (Royal Botanic Gardens et al., online). | |
| Host plant range | Hosts of P. acaciae include acacia (Acacia spp.), Australian pine (Casuarina equisetifolia), bushwillow (Combretum spp.), Dobera spp., fig (Ficus carica), chinaberry (Melia azedarach), oleander (Nerium oleander), terebinth (Pistacia atlantica), poinciana (Poinciana gilliesii), pomegranate (Punica granatum), white weeping broom (Retama raetam), sumac (Rhus tripartita), Ozoroa spp., tamarisk (Tamarix pentandra), Terminalia spp., common jujube (Ziziphus jujube), African jujube (Ziziphus lotus), Christ thorn jujube (Ziziphus spina‐christi) and other species (Qasem, 2009).The mistletoe is rapidly expanding its host range (Qasem, 2009). | |
| Pathways | The main pathways of entry for dwarf mistletoes are plants for planting and cut branches (EFSA PLH Panel, 2018). The Panel considers that the same pathways could apply to P. acaciae. | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.10.2. Possibility of pest presence in the nursery
A.10.2.1. Possibility of entry from the surrounding environment
Plicosepalus acaciae is present in Israel (Veste et al., 2014) and it is rapidly expanding because the population of spectacled bulbul (Pycnonotus xanthopygos) is increasing (Ward et al., 2006). Possible pathways of spreading throughout the area and into the nursery can be by movement of the spectacled bulbul (Pycnonotus xanthopygos) (Green et al., 2009). According to Dossier Section 9.0, the bulbul birds (Pycnonotus xanthopygos) are present in the area surrounding production sites.
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
Of the above plant species, Acacia spp. are hosts of P. acaciae (Qasem, 2009).
The bare rooted plants are grown either in soil in open fields or in commercial growing medium in sack containers in net house. The liners are cultivated in the same commercial growing medium as above in pots in a net house (Dossier Section 1.0). According to Dossier Section 9.0, the net used is designed for shading and the net house is not entirely sealed; therefore, it is not intended to prevent the birds from entry.
According to Personal communication of Veste (2020), it is highly possible that the parasite can be found on thin branches of small trees as it was observed on Ochradenus baccatus, which is a small shrub with small branches, and on Calligonum comosum, which also has small branches. For infection to occur seed needs to stick on the branch.
According to Dossier Section 9.0, bare rooted plants are 20–100 cm tall, with base diameter of up to 2 cm. Liners are about 10 cm high and with ~ 1 cm base diameter.
Based on the above information, the Panel considers that the commodities can become infested with P. acaciae.
Uncertainties:
-
–
There are uncertainties about the abundance of the main host plants in the areas surrounding the nursery.
-
–
There are uncertainties about the level of attractiveness of the nursery plants and mother plants of F. carica to spectacled bulbul (Pycnonotus xanthopygos).
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The spectacled bulbul (Pycnonotus xanthopygos) is present in the surrounding areas, F. carica is a host of the mistletoe and the size of the nursery plants is not a limiting factor for an infection.
A.10.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height. The mistletoe is not associated with growing media.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media.
A.10.2.3. Possibility of spread within the nursery
The crops designated for export are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
In the nursery a net is used to protect plants for export. The net is designed for shading – 40% shade which can be adapted to be bird proof. The net is presently not entirely sealed (Dossier Section 9.0).
Lagerstroemia indica and Morus alba are grown in the nursery (Dossier Section 9.0) but are not host of the pest.
The spread of the mistletoe within the nursery requires reproduction. As the commodity plants are 1‐year‐old, the Panel assumes that reproduction may occur only on mother plants. Spread is also dependent on the presence of spectacled bulbul (Pycnonotus xanthopygos), which according to the Dossier Section 9.0 is present in the area surrounding production sites.
Spread of P. acaciae within the nursery through the movement of soil, water, equipment, tools and humans is irrelevant.
Uncertainties:
-
–
There is uncertainty on the presence and population density of the mistletoe within the nursery.
-
–
There is uncertainty on whether the mother plants which are trimmed may allow the reproduction of the mistletoe.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pest within the nursery is possible. The mistletoe can be present and can reproduce on the mother plants and be spread within the nursery by the spectacled bulbul (Pycnonotus xanthopygos).
A.10.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of P. acaciae between the years 1995 and November 2019 (EUROPHYT, online).
A.10.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on P. acaciae is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | Bare rooted plants are grown either in soil in open fields or in commercial growing medium in sack containers in net house.The net houses are not completely protecting against the birds.Uncertainties:– Presence of the pest in the surrounding areas. | The net houses are not completely protecting against the birds.Uncertainties:– Presence of the pest in the surrounding areas. |
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | No | Not applicable | Not applicable |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | No | Not applicable | Not applicable |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Plants parasitised by mistletoe can be identified by inspections. However, at early infestation, the shoots of mistletoes may be easily overlooked.Uncertainties:– There is an uncertainty about the efficacy of the visual inspections. | Plants parasitised by mistletoe can be identified by inspections. However, at early infestation, the shoots of mistletoes may be easily overlooked.Uncertainties:– There is an uncertainty about the efficacy of the visual inspections. |
| 11 | Inspections before export | Yes | Plants parasitised by mistletoe can be identified by inspections. However, at early infestation, the shoots of mistletoes may be easily overlooked.Uncertainties:– There is an uncertainty about the efficacy of the visual inspections. | Plants parasitised by mistletoe can be identified by inspections. However, at early infestation, the shoots of mistletoes may be easily overlooked.Uncertainties:– There is an uncertainty about the efficacy of the visual inspections. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, P. acaciae is present in Israel and in the surroundings of the nursery.Uncertainties:– Abundance of the pest and host plants in the surrounding areas of the nursery. | Surveillance in the surrounding area is not implemented; however, P. acaciae is present in Israel and in the surroundings of the nursery.Uncertainties:– Abundance of the pest and host plants in the surrounding areas of the nursery. |
A.10.5. Overall likelihood of pest freedom for bare rooted plants and liners
A.10.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants and liners
Although the parasitic plant is reported as present in Israel, the scenario assumes the pest is not present in the surroundings of the nursery, despite the presence of its bird vector and some host plants (Acacia). Inspections, including final inspections, are effective in finding infestations even at their early stages. Although the net of the net house is only intended for shading, it also reduces the presence of birds and may screen plants from seed droppings from birds. The scenario also assumes the nursery plants are not attractive for the bird and that the pest is unable to reproduce on mother plants.
A.10.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants and liners
The scenario assumes a relatively high pest pressure from the surroundings because suitable hosts (Acacia) are present. In addition, the bird vector is also present in the surrounding, which increases the likelihood of entry into the nursery from the surroundings. Inspections are ineffective at the early stages of infestation or for seeds of the mistletoe. The net of the net house is not effective in preventing infestation. The scenario also assumes the nursery plants are attractive for the bird and that the pest is able to reproduce on mother plants.
A.10.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants and liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the absence of interceptions at EU borders, the Panel assumes a central scenario skewed to the left, meaning that medium values are closer to the lower interval limit.
A.10.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the surroundings of the nursery results in high level of uncertainties for infestation rates both below and above the median.
A.10.5.5. Elicitation outcomes of the assessment of the pest freedom for Plicosepalus acaciae on bare rooted plants and liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.29) and pest freedom (Table A.30).
Table A.29.
Elicited and fitted values of the uncertainty distribution of pest infestation by Plicosepalus acaciae per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.0000 | 1.50 | 3.00 | 6.50 | 10.0 | ||||||||||
| EKE | 0.0153 | 0.0538 | 0.139 | 0.360 | 0.730 | 1.28 | 1.91 | 3.40 | 5.20 | 6.23 | 7.37 | 8.41 | 9.30 | 9.81 | 10.2 |
The EKE results are the BetaGeneral (0.73069, 1.2283, 0, 10.5) distribution fitted with @Risk version 7.6.
Table A.30.
The uncertainty distribution of plants free of Plicosepalus acaciae per 10,000 plants calculated by Table A.29
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,990 | 9,994 | 9,997 | 9,999 | 10,000 | ||||||||||
| EKE results | 9,990 | 9,990 | 9,991 | 9,992 | 9,993 | 9,994 | 9,995 | 9,997 | 9,998.1 | 9,998.7 | 9,999.3 | 9,999.6 | 9,999.9 | 9,999.9 | 10,000.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.30.
Figure A.20.
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.10.6. Reference list
Bowie M and Ward D, 2004. Water and nutrient status of the mistletoe Plicosepalus acaciae parasitic on isolated Negev Desert populations of Acacia raddiana differing in level of mortality. Journal of Arid Environments, 56, 487–508. https://doi.org/10.1016/s0140-1963(03)00067-3
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA,Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent A, Yuen J, Zappalà L, Boberg J, Pautasso M and Dehnen‐Schmutz K, 2018. Scientific Opinion on the pest categorisation of Arceuthobiumspp. (non‐EU). EFSA Journal 2018;16(7):5384, 23 pp. https://doi.org/10.2903/j.efsa.2018.5384
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 3 March 2020].
Green AK, Ward D and Griffiths ME, 2009. Directed dispersal of mistletoe (Plicosepalus acaciae) by Yellow‐vented Bulbuls (Pycnonotus xanthopygos). Journal of Ornithology, 150, 167–173. https://doi.org/10.1614/wt-08-079.1 https://doi.org/10.1007/s10336-008-0331-9
Mathiasen RL, Nickrent DL, Shaw DC and Watson DM, 2008. Mistletoes: pathology, systematics, ecology, and management. Plant disease, 92, 988–1006.
Qasem JR, 2009. An updated inventory of mistletoe (Plicosepalus acaciae and Viscum cruciatum) distribution in Jordan, hosts, and severity of infestation. Weed Technology, 23, 465–469. https://doi.org/10.1614/wt-08-079.1
Royal Botanic Gardens, Kew and Missouri Botanical Garden, online. The Plant List, Plicosepalus. Available online: http://www.theplantlist.org/browse/A/Loranthaceae/Plicosepalus/ [Accessed: 3 March 2020].
Veste M, Todt H and Breckle SW, 2015. Influence of halophytic hosts on their parasites—the case of Plicosepalus acaciae. AoB Plants, 7, 1–12. https://doi.org/10.1093/aobpla/plu084
Ward D, Shrestha MK and Musli I, 2006. Are invasive mistletoes killing Ziziphus spina‐christi? Israel Journal of Plant Sciences, 54, 113–117. https://doi.org/10.1560/ijps_54_2_113
A.11. Retithrips syriacus
A.11.1. Organism information
| Taxonomic information | Current valid scientific name: Retithrips syriacusSynonyms: Dictyothrips zanoniana, Dictyothrips aegyptiacus, Heliothrips syriacus, Retithrips aegyptiaca, Retithrips aegyptiacus, Stylothrips bondariName used in the EU legislation: –Order: ThysanopteraFamily: ThripidaeCommon name: black vine thrips, castor thrips, grape thripsName used in the Dossier: Retithrips syriacus | |
|---|---|---|
| Group | Insects | |
| EPPO code | RETTSY | |
| Regulated status | Retithrips syriacus is not regulated in the EU neither is listed by EPPO.The pest is quarantine in Mexico (EPPO, online). | |
| Pest status in Israel | Present (CABI, online; Hamon and Edwards, 1994), widespread in North and Centre of Israel (Dossier Section 6.0).According to Dossier Section 9.0 the nursery is not in a pest free area. | |
| Pest status in the EU | Absent in the EU (CABI, online). | |
| Host status on Ficus carica | Ficus carica is a host of R. syriacus (Avidov and Harpaz, 1969). | |
| PRA information | Available Pest Risk Assessments:– Final Import Policy: Fresh persimmon fruit from Japan, Korea and Israel (Australian Government Department of Agriculture, Fisheries and Forestry, 2004).– Final group pest risk analysis for thrips and orthotospoviruses on fresh fruit, vegetable, cut‐flower and foliage imports (Australian Government Department of Agriculture and Water Resources, 2017). | |
| Other relevant information for the assessment | ||
| Biology | Thrips R. syriacus probably originates from Central Africa (Elimem et al., 2011).Life stages of R. syriacus are eggs, two larval instars, pupae and adults (Sujatha et al., 2011). A complete life cycle can take between 15 to 30 days under open air conditions and less in greenhouses. Retithrips syriacus can produce several generations per year (Gerson and Aplebaum, online), up to seven (CABI, online). In India on castor (Ricinus communis), the generation cycle is completed in 15 to 20 days (Sujatha et al., 2011).Females lay eggs in the leaf tissue or less frequently on the leaf surface (Medina‐Gaud and Franqui, 2001). Each female lays around 40 to 60 eggs in 5 to 10 days. Eggs hatch in 4 to 5 days (Sujatha et al., 2011). Oviposition stops when temperatures drop below 17°C or rise above 37°C. Only males emerge from unfertilised eggs. Retithrips syriacus can be sometimes parthenogenic (CABI, online).Larvae and pupa have a bright red colour (Medina‐Gaud and Franqui, 2001). Larvae become fully grown in 7–9 days. Then they drop down, enter into the soil and pupate. The pupal stage lasts for 2–3 days (Sujatha et al., 2011).Adults usually mate on the day of emergence and females start laying eggs 3 days after. Females usually out‐number males, only in autumn the numbers of sexes are equal (CABI, online). Adults can fly and live for more than one month (Gerson and Aplebaum, online).During winter R. syriacus is very rarely on plants, the adults overwinter in the soil (Ben‐Yakir, 2012). | |
| Symptoms | Main type of symptoms | Retithrips syriacus adults and larvae damage foliage (especially the lower leaf surface), fruits and flower sepals. When infestation is heavy, the upper surfaces of leaves are also attacked and fruits fail to develop normally (CABI, online).The main symptoms are:– grey dots on leaves (from insertions of the stylets),– shiny black dots on leaves (excrements),– fruits turn grey (at feeding sites),– crinkling of the terminal leaves with a silvery appearance,– stunted growth of plants,– fruit discoloration,– fruit size deformation,– defoliation, (CABI, online; Hamon and Edwards, 1994; Sujatha et al., 2011). |
| Presence of asymptomatic plants | Plant damage might not be obvious in early infestation or during dormancy (due to absence of leaves). The presence of R. syriacus on the plants could hardly be observed. | |
| Confusion with other pests | The most precise identification of the pest is combination of molecular and morphological methods. | |
| Host plant range | Retithrips syriacus is polyphagous pest and has over 50 host species (Gerson and Aplebaum, online).Retithrips syriacus is a pest of avocado (Persea americana), Brazil pepper tree (Schinus molle), cotton (Gossypium hirsitum), grapevine (Vitis vinifera), kaki (Diospyros kaki), myrtle (Myrtus communis), peppervine (Ampelopsis orientale), rose (Rosa spp.), walnut (Juglans regia), wild apple (Malus sylvestris) (Doganlaw and Yigit, 2002), apple (Malus domestica), banana (Musa spp.), coconut (Cocos nucifera), coffee (Coffea spp.), European pear (Pyrus communis), Japanese plum (Prunus salicina), poplar (Populus spp.) and other plants (CABI, online).The economic damage of R. syriacus in Israel is mainly reported on persimmon and avocado plants. It commonly infests grapevine, myrtle, rose, and cotton (Ben‐Yakir, 2012). According to Avidov and Harpaz (1969), F. carica is a host to R. syriacus in Israel. | |
| Pathways | Fruits and plants for planting are the main pathways for introduction and spread of R. syriacus (Wistermann et al., 2016). As R. syriacus can be associated with soil (Ben‐Yakir, 2012), soil is also considered as pathway. | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.11.2. Possibility of pest presence in the nursery
A.11.2.1. Possibility of entry from the surrounding environment
Retithrips syriacus is widespread in centre and north of Israel (Dossier Section 6.0). Adults fly actively for short distances and passively on wind currents, which enables long‐distance spread.
It is very likely that the adults can spread over large distances by combination of active flight and wind dispersal.
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Gossypium, Diospyros kaki, Platanus, Populus, Eucalyptus, Acacia longifolia and Ricinus communis are hosts of R. syriacus (CABI, online).
Uncertainties:
-
–
No information about the density of the population of R. syriacus in the area surrounding the nursery is available.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by flight, wind and human accidental transportation.
A.11.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media. Plants are produced inside the nursery.
A.11.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
Lagerstroemia indica and Morus alba are grown in the nursery (Dossier Section 9.0) but are not host of the pest.
It is possible that R. syriacus can reproduce within the nursery on F. carica.
The insect is highly mobile by combination of active and passive dispersal, so there is no doubt it can spread within the nursery. Another pathway can be movement of plant material within the nursery.
Uncertainties:
-
–
No information is available for the isolation or proximity of the mother plant stock for cuttings collection to other host plant species in the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible either by wind, active flight or accidental transfer within the nursery.
A.11.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of R. syriacus between the years 1995 and November 2019 (EUROPHYT, online).
A.11.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on R. syriacus is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties |
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Pesticide sprays are generally effective against thrips. Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is uncertainty whether the pest can develop resistance to pesticides, or whether resistant strains spread into the nursery. | Pesticide sprays are generally effective against thrips. Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is uncertainty whether the pest can develop resistance to pesticides, or whether resistant strains spread into the nursery. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | Yes | Pupae and adults can stay in leaf litter/soil so the washing should remove them. However, inspection of soil does not allow to detect their presence.Uncertainties:– The degree of cleaning of roots from soil particles is not defined. | Pupae and adults can stay in leaf litter/soil or protected plant parts, so the cleaning should remove them only partly. However, inspection of soil or protected plant parts does not allow to detect their presence.Uncertainties:– The degree of cleaning of litter or plant debris is not defined as well as the thorough check of protected plant parts. |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Thrips could go undetected because of the small size of the pest and difficulty in the search in roots and soil particles.Uncertainties:– It is unclear how many samples are required to declare the production site to be free of the pest. | Thrips could go undetected because of the small size of the pest and difficulty in the search in litter.Uncertainties:– There is unclear detection limit– The effectiveness of the inspection for R. syriacus is not known because soil is not checked. |
| 11 | Inspections before export | Yes | Thrips could go undetected because of the small size of the pest and difficulty in the search in roots and soil particles.Uncertainties:– It is unclear how many samples are required to declare the pest freedom of the production site. | Thrips could go undetected because of the small size of the pest and difficulty in the search in litter.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for R. syriacus is not known. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, R. syriacus is common in Israel.Uncertainties:– There is no information on the density of R. syriacus in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, R. syriacus is common in Israel.Uncertainties:– There is no information on the density of R. syriacus in the surrounding areas. |
A.11.5. Overall likelihood of pest freedom for bare rooted plants
A.11.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants
Although R. syriacus is present in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to active flight, wind and human activity. Inspections are expected to be effective because symptoms are typical. Ficus carica is not considered a preferential host. Insecticide treatments are expected to be effective because thrips are not protected, although they could escape treatment when hidden in soil. Soil removal through root washing before export may contribute to reduced thrips density.
A.11.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants
Retithrips syriacus is present in Israel, the scenario assumes a high pest pressure from outside and enhanced colonisation of the nursery plants. Inspections may not be effective at initial low density. Ficus carica is considered a good host. Insecticide treatments are not expected to be effective against insect stages hidden in soil. Soil removal through root washing before export may not remove all the thrip population.
A.11.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by active flight, wind and human activity, the weak information on the degree of susceptibility of F. carica, the internal spread and the absence of reported problems within the nursery and at EU borders on plants for planting, the Panel assumes a central scenario skewed to the left, meaning that medium values are closer to the lower interval limit.
A.11.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, and unclear host suitability of F. carica, result in high level of uncertainties for infestation rates below the median. Detection of the pest especially before the export is unlikely, which gives very high uncertainties for rates above the median.
A.11.5.5. Elicitation outcomes of the assessment of the pest freedom for Retithrips syriacus on bare rooted plants
The following tables show the elicited and fitted values for pest infestation/infection (Table A.31) and pest freedom (Table A.32).
Table A.31.
Elicited and fitted values of the uncertainty distribution of pest infestation by Retithrips syriacus per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5.00 | 30.0 | 50.0 | 120 | 200 | ||||||||||
| EKE | 4.24 | 4.85 | 6.21 | 9.74 | 15.7 | 24.6 | 35.0 | 60.5 | 92.9 | 113 | 136 | 158 | 180 | 194 | 206 |
The EKE results are the BetaGeneral (0.72979, 1.5543, 4, 220) distribution fitted with @Risk version 7.6.
Table A.32.
The uncertainty distribution of plants free of Retithrips syriacus per 10,000 plants calculated by Table A.31
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,800 | 9,880 | 9,950 | 9,970 | 9,995 | ||||||||||
| EKE results | 9,794 | 9,806 | 9,820 | 9,842 | 9,864 | 9,887 | 9,907 | 9,940 | 9,965 | 9,975 | 9,984 | 9,990.3 | 9,993.8 | 9,995.1 | 9,995.8 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.32.
Figure A.21.
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.11.6. Overall likelihood of pest freedom for liners
A.11.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested liners
Although R. syriacus is present in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to active flight, wind and human activity. Inspections are expected to be effective because symptoms are typical, and measures should be taken accordingly. Based on that presence of thrips in soil should be limited. Ficus carica is not considered a preferential host. Insecticide treatments are expected to be effective because thrips are not protected, although they could escape treatment when hidden in soil.
A.11.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested liners
Retithrips syriacus is present in Israel; the scenario assumes a high pest pressure from outside and significant transfer from the surrounding due to active flight, wind and human activity. Inspections are expected to be less effective because symptoms are difficult to be detected at early stage. Based on that presence of thrips in soil should be enhanced. Ficus carica is considered a good host. Insecticide treatments are expected to be less effective when thrips are hidden in soil.
A.11.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by active flight, wind and human activity, the weak information on the degree of susceptibility of F. carica, the internal spread and the absence of reported problems within the nursery and at EU borders, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.11.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, and unclear host suitability of F. carica, together with the overwintering in soil, result in relevant levels of uncertainty for infestation rates both below and above the median.
A.11.6.5. Elicitation outcomes of the assessment of the pest freedom for Retithrips syriacus on liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.33) and pest freedom (Table A.34).
Table A.33.
Elicited and fitted values of the uncertainty distribution of pest infestation by Retithrips syriacus per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 20.0 | 75.0 | 140 | 260 | 400 | ||||||||||
| EKE | 18.9 | 20.7 | 24.6 | 34.1 | 49.1 | 70.4 | 94.4 | 150 | 215 | 253 | 294 | 333 | 366 | 386 | 401 |
The EKE results are the BetaGeneral (0.78573, 1.3014, 18, 415) distribution fitted with @Risk version 7.6.
Table A.34.
The uncertainty distribution of plants free of Retithrips syriacus per 10,000 plants calculated by Table A.33
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,600 | 9,740 | 9,860 | 9,925 | 9,980 | ||||||||||
| EKE results | 9,599 | 9,614 | 9,634 | 9,667 | 9,706 | 9,747 | 9,785 | 9,850 | 9,906 | 9,930 | 9,951 | 9,966 | 9,975 | 9,979 | 9,981 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.34.
Figure A.22.
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
Figure A.23.
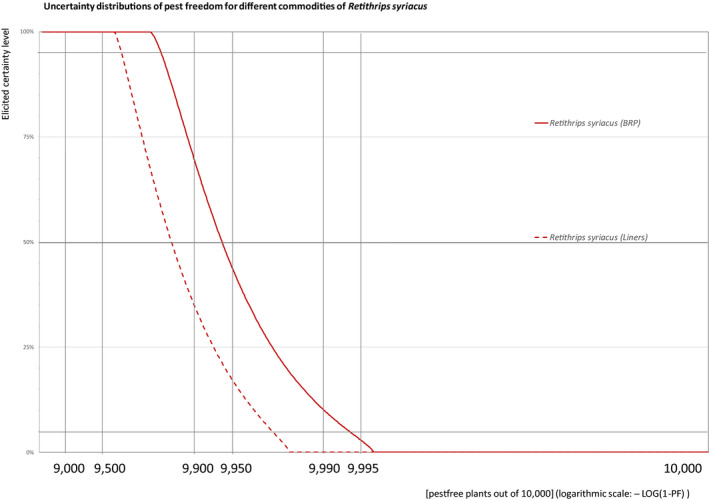
Elicited certainty (y‐axis) of the number of bare rooted plants or liners of Ficus carica pest free from Retithrips syriacus (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU introduced from Israel as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
A.11.7. Reference list
Australian Government Department of Agriculture, Fisheries and Forestry, 2004. Final Import Policy: Fresh persimmon fruit from Japan, Korea and Israel. Commonwealth of Australia. Available online: https://www.agriculture.gov.au/sites/default/files/sitecollectiondocuments/ba/plant/ungroupeddocs/persimmon_final.pdf
Australian Government Department of Agriculture and Water Resources, 2017. Final group pest risk analysis for thrips and orthotospoviruses on fresh fruit, vegetable, cut‐flower and foliage imports. Commonwealth of Australia. Available online: https://www.agriculture.gov.au/sites/default/files/sitecollectiondocuments/biosecurity/risk-analysis/plant-reviews/final-report-thrips-orthotospoviruses.pdf
Avidov Z and Harpaz I, 1969. Plant Pests of Israel. Israel Universities Press, Jerusalem. 549 pp.
Ben‐Yakir D, 2012. The black vine thrips, Retithrips syriacus (Mayet), as a pest of fruit trees and grape vine. ‘Alon HaNotea’, 66, 40–41.
CABI (Centre for Agriculture and Bioscience International), online. Retithrips syriacus (black vine thrips). Available online: https://www.cabi.org/cpc/datasheet/46972 [Accessed: 24 March 2020].
Doğanlar M and Yiğit A, 2002. Hatay'da yeni bir potansiyel meyve ve bağ zararlısı: Siyah bağ thripsi, Retithrips syriacus (Mayet) (Thysanoptera: Thripidae). Türkiye Entomoloji Dergisi, 26, 283–294.
Elimem M, Navarro‐Campos C and Chermiti B, 2011. First record of black vine thrips, Retithrips syriacus Mayet, in Tunisia. EPPO Bulletin, 41, 174–177. https://doi.org/10.1111/j.1365-2338.2011.02461.x
EPPO (European and Mediterranean Plant Protection Organization), online. Retithrips syriacus (RETTSY), Categorization. Available online: https://gd.eppo.int/taxon/RETTSY/categorization [Accessed: 24 March 2020].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 24 March 2020].
Gerson U and Aplebaum S, online. Plant Pests of the Middle East, Retithrips syriacus (Mayet). Available online: http://www.agri.huji.ac.il/mepests/pest/Retithrips_syriacus/[Accessed: 23 March 2020].
Hamon AB and Edwards GB, 1994. Thrips (Thysanoptera) new to Florida: 1. Thripidae: Panchaetothripinae. Entomology Circular (Gainesville), 365, 2 pp.
Medina‐Gaud S and Franqui RA, 2001. Retithrips syriacus (Mayet), the black vine thrips (Insecta: Thysanoptera: Thripidae) new to Puerto Rico. The Journal of Agriculture of the University of Puerto Rico, 85, 85–89.
Sujatha M, Devi PV and Reddy TP, 2011. Insect Pests of Castor (Ricinus communis L) and their Management Strategies. In: Reddy VD, Rao PN and Rao KV (eds.). Pests and Pathogens: Management Strategies. BS Publications. India. pp. 177–198.
Wistermann A, Grousset F, Petter F, Schrader G and Suffert M, 2016. DROPSA Deliverable 1.3 Part 6 ‐ Report on Table grapes – Fruit pathway and Alert List. Available online: https://www.researchgate.net/publication/322314744_Work_package_1_Pathways_of_introduction_of_fruit_pests_and_pathogens_Deliverable_13_PART_6-REPORT_on_TABLE_GRAPES-Fruit_pathway_and_Alert_List_Dropsa_EU_project_number_613678
A.12. Russellaspis pustulans
A.12.1. Organism information
| Taxonomic information | Current valid scientific name: Russellaspis pustulansSynonyms: Asterodiaspis pustulans, Asterolecanium pustulans, Planchonia pustulans, Asterolecanium pustulans sambuci, Asterolecanium pustulans seychellarum, Asterolecanium sambuci, Asterolecanium morini, Russellaspis pustulansSubspecies of Russellaspis pustulans: Russellaspis pustulans pustulans and Russellaspis pustulans principe (García Morales et al., online_a)Name used in the EU legislation: –Order: HemipteraFamily: AsterolecaniidaeCommon name: oleander pit scale, fig pustule scale, akee fringed scale Name used in the Dossier: Russellaspis pustulans | |
|---|---|---|
| Group | Insects | |
| EPPO code | ASTLPU | |
| Regulated status | Russellaspis pustulans pustulans is prohibited organism in Australia (Government of Western Australia, Department of Primary Industries and Regional Development, online). | |
| Pest status in Israel | Russellaspis pustulans is present in Israel (Ben‐Dov, 2012; García Morales et al., online_b; Russell, 1941). The subspecies R. pustulans principe has not been found in Israel.The Dossier Section 2.0 states that R. pustulans is present in Israel. | |
| Pest status in the EU | Russellaspis pustulans is present in Cyprus, Italy and possibly Malta (García Morales et al., online_a; García Morales et al., online_b; Mifsud et al., 2014). | |
| Host status on Ficus carica | Ficus carica is a host plant of R. pustulans (Ben‐Dov, 2012; García Morales et al., online_a) and R. pustulans pustulans (García Morales et al., online_b).Russellaspis pustulans is mentioned in the Dossier Section 2.0 as secondary pest of figs. | |
| PRA information | No Pest Risk Assessment is currently available. | |
| Other relevant information for the assessment | ||
| Biology | Russellaspis pustulans is present in tropical and subtropical areas all over the world (Malumphy, 2014).In the study from Egypt by El‐Minshawy et al. (1971) females went through two larval stages and no males were observed. The pest had two annual generations and only non‐gravid females were able to overwinter. The duration of the life cycle in summer was from 93 to 120 days, in winter from 240 to 275 days.In Egypt on fig trees, females laid on average between 90 to 195 eggs/female (Abd El‐Salam and Mangoud, 2001). The pest is present on pine needle trees from Mid‐April to late summer (Badr, 2014). Russellaspis pustulans is a major pests of fig trees in Burg El‐Arab although specific symptoms are not described (Hassan et al., 2012). | |
| Symptoms | Main type of symptoms | Main symptoms of infection are formation of pits (Çalişkan et al., 2015; Moursi et al., 2007; Russell, 1941), wilting of leaves and twigs, defoliation and dieback of branches, death of trees and yield loss (Abd El‐Salam and Mangoud, 2001).Infested plants by R. pustulans have usually symptoms of deep or shallow pits. On some plants, no pits can be observed, it all depends on the host susceptibility (Çalişkan et al., 2015; Moursi et al., 2007; Russell, 1941). Pits usually occur on stems and branches. On leaves and fruits generally, no pits can be seen (Çalişkan et al., 2015).The pest infests mainly branches and stems, but also new twigs, leaves and fruits (Moursi et al., 2007). |
| Presence of asymptomatic plants | Scales are generally obvious. | |
| Confusion with other pathogens/pests | Other scale insects. Require taxonomic identification. | |
| Host plant range | Russellaspis pustulans is a polyphagous pest and feeds on plants belonging to 67 families (García Morales et al., online_a; García Morales et al., online_b).Some of the many hosts of R. pustulans are fig trees (Ficus aurea, F. benjamina, F. carica, F. drupacea, F. elastica, F. lutea, F. minahassae, F. religiosa, F. sur, F. sycomorus, F. virens), guava (Psidium guajava), mango (Mangifera indica), olive trees (Olea europaea), peach (Prunus persica), pear (Pyrus communis), plum (Prunus domestica), other fruit trees and ornamental plants (García Morales et al., online_a; García Morales et al., online_b). | |
| Pathways | As the pest can be usually found on branches, stems, twigs, leaves and fruits (Moursi et al., 2007), the Panel considers that the possible pathways of entry are plants for planting, cut branches, cut foliage and fruits. | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.12.2. Possibility of pest presence in the nursery
A.12.2.1. Possibility of entry from the surrounding environment
Russellaspis pustulans is present in Israel (Ben‐Dov, 2012; García Morales et al., online_a; García Morales et al., online_b; Russell, 1941). Possible pathways of entry into the nursery can be movement of crawlers by wind or by humans.
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Gossypium, Diospyros, Brassica oleracea, Quercus, Eucalyptus, Acacia, Acacia decurrens, Acacia farnesiana, Acacia nilotica and Persea are hosts of both R. pustulans and R. pustulans pustulans.
Uncertainties:
-
–
No information about the density of the population of R. pustulans in the area surrounding the nursery is available.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by wind and human transportation.
A.12.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media. Plants are produced inside the nursery and the scale insects are not associated with soil growing media.
A.12.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
According to Dossier Section 9.0, following plants known to be hosts of the pest are grown in the fig liner export nursery: Lagerstroemia indica and Morus alba, with a distance of a few dozens of meters between them and the fig liners.
Therefore, it is possible for R. pustulans and R. pustulans pustulans to reproduce within the nursery on F. carica and on other hosts, which are present.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible.
A.12.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of R. pustulans between the years 1995 and November 2019 (EUROPHYT, online).
A.12.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on R. pustulans is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties |
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Pesticide sprays are generally effective against crawlers and less effective against the fixed stages of R. pustulans because of the wax covering of its body.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty whether the pesticide can effectively reach all the bark parts where the scales are located because of the barrier effect of the leaves. | Pesticide sprays are generally effective against crawlers and less effective against the fixed stages of R. pustulans because of the wax covering of its body.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is one uncertainty whether the pesticide can effectively reach all the bark parts where the scales are located because of the barrier effect of the leaves. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | No | Not applicable | Not applicable |
| 8 | Plant treatment before export | Yes | Rinsing of the plants is not removing the pest. Scales can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. | Cleaning of plant debris is not removing the pest. Scales can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | R. pustulans are generally detectable except at the crawler stage.Scales can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. | R. pustulans are generally detectable except at the crawler stage.Scales can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. |
| 11 | Inspections before export | Yes | Scales can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. | Scales can be easily found during inspection with magnifying glasses which is triggered by the observation of suspected symptoms.Uncertainties:– There is uncertainty on the capacity to detect crawlers on the bark with the naked eye. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented; however, R. pustulans is common in Israel.Uncertainties:– There is no information on the density of R. pustulans in the surrounding areas. | Surveillance in the surrounding area is not implemented; however, R. pustulans is common in Israel.Uncertainties:– There is no information on the density of R. pustulans in the surrounding areas. |
A.12.5. Overall likelihood of pest freedom for bare rooted plants and liners
A.12.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants and liners
Although R. pustulans is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to wind and human activity. Inspections are expected to be effective because sessile stages of the insect are visible. Ficus carica is deemed as a minor host. Insecticide treatments are expected to be conducted at the right timing to target unprotected life stages of the insect. Mother plants are kept healthy as well by using treatments.
A.12.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants and liners
Russellaspis pustulans is widespread in Israel; the scenario assumes a high pest pressure from outside and strong transfer from the surrounding due to wind and intensive human activity. Inspections are expected to be ineffective because of presence of hidden stages. Ficus carica is deemed as a major host. Insecticide treatments are expected to be conducted at timing when the insect is protected by wax. Mother plants are infested despite treatments and may contribute spreading the pest within the nursery.
A.12.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants and liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by wind and human activity, the weak information on the degree of susceptibility of F. carica, the internal spread and the absence of reported problems within the nursery and at EU borders, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.12.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, and unclear host suitability of F. carica result in high level of uncertainties for infestation rates below the median. Otherwise, detection of the pest especially before the export is likely, which gives less uncertainties for rates above the median.
A.12.5.5. Elicitation outcomes of the assessment of the pest freedom for Russellaspis pustulans on bare rooted plants and liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.35) and pest freedom (Table A.36).
Table A.35.
Elicited and fitted values of the uncertainty distribution of pest infestation by Russellaspis pustulans per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2.00 | 40.0 | 80.0 | 200 | 500 | ||||||||||
| EKE | 1.07 | 2.81 | 5.88 | 12.5 | 22.2 | 35.7 | 51.1 | 89.7 | 145 | 185 | 242 | 315 | 415 | 516 | 651 |
The EKE results are the Weibull (0.9556, 131.58) distribution fitted with @Risk version 7.6.
Table A.36.
The uncertainty distribution of plants free of Russellaspis pustulans per 10,000 plants calculated by Table A.35
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,500 | 9,800 | 9,920 | 9,960 | 9,998 | ||||||||||
| EKE results | 9,349 | 9,484 | 9,585 | 9,685 | 9,758 | 9,815 | 9,855 | 9,910 | 9,949 | 9,964 | 9,978 | 9,988 | 9,994 | 9,997 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.36.
Figure A.24.
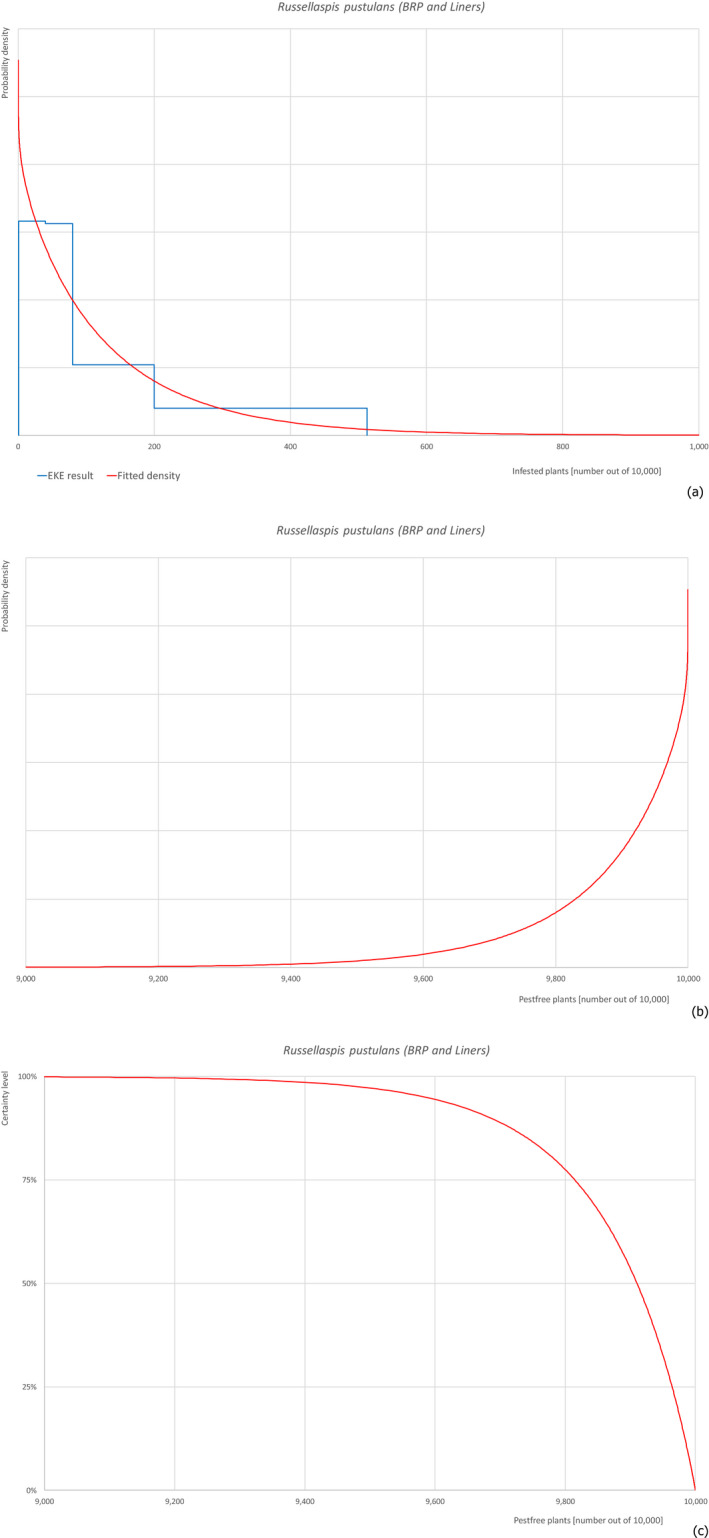
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.12.6. Reference list
Abd El‐Salam A and Mangoud H, 2001. Development and Implementation of Integrated Pest management to Programs of Apple Trees in Reclaimed Lands in Egypt: I‐The Fig Scale Insect (FSI), Russellaspis (Asterolecanium) pustulans (Cockerell). Journal of Agriculture in the Tropics and Subtropics, 102, 33–44.
Badr SA, 2014. Insects and non insects species associated with pine needle trees in Alexandria Egypt. Journal of Entomology, 11, 49–55. https://doi.org/10.3923/je.2014.49.55
Ben‐Dov Y, 2012. The scale insects (Hemiptera: Coccoidea) of Israel‐checklist, host plants, zoogeographical considerations and annotations on species. Israel Journal of Entomology, 41, 21–48.
Çalişkan AF, Kaydan MB, Satar S and Ulusoy MR, 2015. First record of Russellaspis pustulans (Cockerell) (Hemiptera: Asterolecaniidae) in Turkey. Turkish Journal of Zoology, 39, 715–716. https://doi.org/10.3906/zoo-1406-2
El‐Minshawy AM, El‐Sawaf SK, Hammad SM and Donia A, 1971. The biology of Asterolecanium pustulans Cockerell in Alexandria district [Hemiptera‐Homoptera: Asterolecaniidae]. Faculty of Agriculture, Alexandria University, Bulletin de la Société entomologique d’Égypte, LV, 441–446.
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_a. ScaleNet: A literature‐based model of scale insect biology and systematics, Russellaspis pustulans. Available online: http://scalenet.info/catalogue/Russellaspis%20pustulans/ [Accessed: 24 August 2020].
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online_b. ScaleNet: A literature‐based model of scale insect biology and systematics, Russellaspis pustulans pustulans. Available online: http://scalenet.info/catalogue/Russellaspis%20pustulans%20pustulans/ [Accessed: 24 August 2020].
Government of Western Australia, Department of Primary Industries and Regional Development, online. Russellaspis pustulans pustulans (Cockerell, 1892). Available online: https://www.agric.wa.gov.au/organisms/109862 [Accessed: 24 August 2020].
Hassan NA, Radwan SG and El‐Sahn OMN, 2012. Common scale insects (Hemiptera: coccoidea) in Egypt. Egyptian Academic Journal of Biological Sciences, 5, 153–160.
Malumphy C, 2014. An annotated checklist of scale insects (Hemiptera: Coccoidea) of Saint Lucia, Lesser Antilles. Zootaxa, 3846, 69–86. https://doi.org/10.11646/zootaxa.3846.1.3
Mifsud D, Mazzeo G, Russo A and Watson GW, 2014. The scale insects (Hemiptera: Coccoidea) of the Maltese archipelago. Zootaxa, 3866 (4), 499–525. https://doi.org/10.11646/zootaxa.3866.4.3
Moursi GA, Moussa SFM, Fatma AA and Basma A, 2007. Seasonal abundance of the fig pustule scale Insect, Rusalaspis pustulans Cockerell (Homoptera: Asterolecaniidae) and its parasitoids in Middle Egypt. Plant protection Research, Institute, Agriculture Research centre, Giza, Egypt. 19 pp.
Russell LM, 1941. A classification of the scale insect genus Asterolecanium (Vol. 424). US Department of Agriculture. 322 pp. https://doi.org/10.5962/bhl.title.65621
A.13. Scirtothrips dorsalis
A.13.1. Organism information
| Taxonomic information | Current valid scientific name: Scirtothrips dorsalisSynonyms: Anaphothrips andreae, Anaphothrips dorsalis, Anaphothrips fragariae, Heliothrips minutissimus, Neophysopus fragariae, Scirtothrips andreae, Scirtothrips dorsalis padmae, Scirtothrips fragariae, Scirtothrips minutissimus, Scirtothrips padmaeName used in the EU legislation: Scirtothrips dorsalis Hood [SCITDO]Order: ThysanopteraFamily: ThripidaeCommon name: Assam thrips, chilli thrips, flower thrips, strawberry thrips, yellow tea thrips, castor thripsName used in the Dossier: Scirtothrips dorsalis | |
|---|---|---|
| Group | Insects | |
| EPPO code | SCITDO | |
| Regulated status | The pest is listed in Part A of Annex II of Regulation (EU) 2019/2072 as Scirtothrips dorsalis Hood [SCITDO].Scirtothrips dorsalis is included in the EPPO A2 list (EPPO, online_a).The pest is quarantine in Israel, Mexico and Morocco (EPPO, online_b). | |
| Pest status in Israel | Present, widespread in Israel (EPPO, online_c). | |
| Pest status in the EU | Present in the Netherlands and Spain (EPPO, online_c).There was one outbreak in Netherlands and two in Spain, in mango greenhouses, they are under eradication (Europhyt Oubreaks database, online). | |
| Host status on Ficus carica | Scirtothrips dorsalis is a pest of F. carica (Cabrera‐Asencio and Ramírez, 2007; Hodges et al., 2005). | |
| PRA information | Available Pest Risk Assessments:– CSL pest risk analysis for Scirtothrips dorsalis (MacLeod and Collins, 2006),– Pest Risk Assessment Scirtothrips dorsalis (Vierbergen and van der Gaag, 2009),– Scientific Opinion on the pest categorisation of Scirtothrips dorsalis (EFSA PLH Panel, 2014),– Scientific opinion on the commodity risk assessment of Jasminum polyanthum plants from Israel (EFSA PLH Panel, 2020). | |
| Other relevant information for the assessment | ||
| Biology | Scirtothrips dorsalis is native to the Indian subcontinent. The pest can have annually up to 8 generations in temperate regions and up to 18 generations in warm subtropical and tropical areas (Kumar et al., 2013).The stages of the life cycle include egg, first and second instar larva, prepupa, pupa and adult (Kumar et al., 2013). They can be found on all the aboveground plant parts (Kumar et al., 2014). Temperature threshold for development is 9.7°C and 32°C, with 265 degree‐days required for development from egg to adult (Tatara, 1994). The adult can live up to 13–15 days (Kumar et al., 2013).Females can lay between 60 and 200 eggs per generation (Seal and Klassen, 2012). Females develop from fertilised and males from unfertilised eggs (Kumar et al., 2013). The eggs are inserted into soft plant tissues and hatch between 2 and 7 days (Kumar et al., 2014).Larvae and adults tend to gather near the mid‐vein or near the damaged part of leaf tissue. Pupae are found in the leaf litter, on the axils of the leaves, in curled leaves or under the calyx of flowers and fruits (Kumar et al., 2013; MacLeod and Collins, 2006). Adults can overwinter in soil or protected in plant parts (Holtz, 2006).The pest cannot overwinter, if the temperature remains below – 4°C for 5 or more days (Nietschke et al., 2008).Adults fly actively for short distances and passively on wind currents, which enables long‐distance spread (EFSA PLH Panel, 2014). They overwinter as adults (Okada and Kudo, 1982) in bark, litter and soil (Shibao, 1991).Scirtothrips dorsalis is a vector of plant viruses including chilli leaf curl virus (CLC), peanut necrosis virus (PBNV), peanut yellow spot virus (PYSV), tobacco streak virus (TSV), watermelon silver mottle virus (WsMoV), capsicum chlorosis virus (CaCV) and melon yellow spot virus (MYSV) (Kumar et al., 2013). | |
| Symptoms | Main type of symptoms | The pest damages young leaves, buds, tender stems and fruits by puncturing tender tissues with their stylets (Kumar et al., 2013).Main symptoms are:– ‘sandy paper lines’ on the epidermis of the leaves,– leaf crinkling and upwards leaf curling,– leaf size reduction,– discoloration of buds, flowers and young fruits,– silvering of the leaf surface,– linear thickenings of the leaf lamina,– brown frass markings on the leaves and fruits,– fruits develop corky tissues,– grey to black markings on fruits,– fruit distortion and early senescence of leaves,– defoliation, (Kumar et al., 2013; Kumar et al., 2014).When the population is high, thrips may feed on the upper surfaces of leaves and cause defoliation and yield loss (Kumar et al., 2013). |
| Presence of asymptomatic plants | Plant damage might not be obvious in early infestation or during dormancy (due to absence of leaves). The presence of S. dorsalis on the plants could hardly be observed. | |
| Confusion with other pathogens/pests | Plants infested by S. dorsalis appear similar to plants damaged by the feeding of broad mites (Kumar et al., 2013).Due to small size and morphological similarities within the genus, the identification of S. dorsalis, using traditional taxonomic keys, is difficult. The most precise identification of the pest is combination of molecular and morphological methods (Kumar et al., 2013). | |
| Host plant range | Scirtothrips dorsalis is a polyphagous pest with more than 100 reported hosts (Kumar et al., 2013). The pest can infect many more plant species, but they are not considered to be true hosts, since the pest cannot reproduce on all of them (EFSA PLH Panel, 2014).The hosts of the pest are kiwi (Actinidia deliciosa), peanut (Arachis hypogaea), tea (Camellia sinensis), pepper (Capsicum annuum), chilli pepper (Capsicum frutescens), citrus (Citrus spp.), muskmelon (Cucumis melo), cucumber (Cucumis sativus), pumpkin (Cucurbita pepo), fig (Ficus carica), Burgundy rubber tree (Ficus elastica ‘Burgundy’), strawberry (Fragaria spp.), cotton (Gossypium hirsutum), litchi (Litchi chinensis), mango (Mangifera indica), tobacco (Nicotiana tabacum), avocado (Persea americana), poplar (Populus deltoids), castor (Ricinus communis), rose (Rose spp.), eggplant (Solanum melongena), grapevine (Vitis vinifera), corn (Zea mays) and other plants (Hodges et al., 2005; Kumar et al., 2014).Scirtothrips dorsalis causes economic loses to chilli pepper, mango, grapevine, citrus, vegetables and tea (Kumar et al., 2013). | |
| Pathways | Plants for planting, cut flowers, fruits and vegetables, soil and growing media are pathways for introduction and spread of S. dorsalis (EFSA PLH Panel, 2014). | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.13.2. Possibility of pest presence in the nursery
A.13.2.1. Possibility of entry from the surrounding environment
Scirtothrips dorsalis is widespread in Israel (EPPO, online_c). Adults fly actively for short distances and passively on wind currents, which enables long‐distance spread (EFSA PLH Panel, 2014).
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Gossypium hirsutum, Diospyros kaki, Quercus glauca, Acacia arabica, Acacia spp., Ricinus communis and Persea americana are hosts of S. dorsalis (Hodges et al., 2005; Kumar et al., 2014).
Uncertainties:
-
–
No information about the density of the population of S. dorsalis in the area surrounding the nursery is available.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by flight, wind and human accidental transportation.
A.13.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media. Plants are produced inside the nursery.
A.13.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20–200 plants/m2, depending on the size/age of the plants.
Lagerstroemia indica and Morus alba are grown in the nursery (Dossier Section 9.0) but are not host of the pest.
It is possible that S. dorsalis can reproduce within the nursery on F. carica.
The insect is highly mobile by combination of active and passive dispersal so there is no doubt it can spread within the nursery. Another pathway can be movement of plant material within the nursery.
Uncertainties:
-
–
No information is available for the isolation or proximity of the mother plant stock for cuttings collection to other host plant species in the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible either by wind, active flight or accidental transfer within the nursery.
A.13.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of S. dorsalis between the years 1995 and November 2019 (EUROPHYT, online).
A.13.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on S. dorsalis is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on bare rooted plants | Evaluation and uncertainties on liners |
|---|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties |
| 2 | Soil treatment | No | Not applicable | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable | Not applicable |
| 4 | Insecticide treatment | Yes | Pesticide sprays are generally effective against thrips.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is uncertainty whether the pest can develop resistance to pesticides, or whether resistant strains spread into the nursery. | Pesticide sprays are generally effective against thrips.Issues with pesticides resistance should be avoided by rotation of the pesticides.Uncertainties:– There is uncertainty whether the pest can develop resistance to pesticides, or whether resistant strains spread into the nursery. |
| 5 | Fungicide treatment | No | Not applicable | Not applicable |
| 6 | Nematicide treatment | No | Not applicable | Not applicable |
| 7 | Treatment against weeds | Yes | Treatments are effective because the thrip can feed on weeds. | Treatments are effective because the thrip can feed on weeds. |
| 8 | Plant treatment before export | Yes | Pupae and adults can stay in leaf litter/soil or protected plant parts so the washing should remove them. However, inspection of soil or protected plant parts does not allow to detect their presence.Uncertainties:– The degree of cleaning of roots from soil particles is not defined as well as the thorough check of protected plant parts. | Pupae and adults can stay in leaf litter/soil or protected plant parts so the cleaning should remove them only partly. However, inspection of soil or protected plant parts does not allow to detect their presence.Uncertainties:– The degree of cleaning of litter or plant debris is not defined as well as the thorough check of protected plant parts. |
| 9 | Sampling and testing | No | Not applicable | Not applicable |
| 10 | Inspections during the production | Yes | Thrips could go undetected because of the small size of the pest and difficulty in the search.Uncertainties:– It is unclear how many samples are required to declare the production site to be free of the pest. | Thrips could go undetected because of the small size of the pest and difficulty in the search in litter.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for S. dorsalis is not known because soil is not checked. |
| 11 | Inspections before export | Yes | Thrips could go undetected because of the small size of the pest and difficulty in the search in roots and soil particles.Uncertainties:– It is unclear how many samples are required to declare the pest freedom of the production site. | Thrips could go undetected because of the small size of the pest and difficulty in the search in litter.Uncertainties:– There is unclear detection limit.– The effectiveness of the inspection for S. dorsalis is not known. |
| 12 | Surveillance and monitoring | Yes | Surveillance in the surrounding area is not implemented, however S. dorsalis is common in Israel.Uncertainties:– There is no information on the density of S. dorsalis in the surrounding areas. | Surveillance in the surrounding area is not implemented, however S. dorsalis is common in Israel.Uncertainties:– There is no information on the density of S. dorsalis in the surrounding areas. |
A.13.5. Overall likelihood of pest freedom for bare rooted plants
A.13.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare rooted plants
Although S. dorsalis is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to active flight, wind and human activity. Inspections are expected to be effective because symptoms are typical. Ficus carica is not considered a preferential host. Insecticide treatments are expected to be effective because thrips are not protected, although they could escape treatment when hidden in bark/soil. Soil removal through root washing before export may contribute to reduced thrips density.
A.13.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare rooted plants
Scirtothrips dorsalis is widespread in Israel; the scenario assumes a high pest pressure from outside and enhanced colonisation of the nursery plants. Inspections may not be effective at initial low density. Ficus carica is considered a good host. Insecticide treatments are not expected to be effective against insect stages hidden in bark and soil. Soil removal through root washing before export may not remove all the thrip population.
A.13.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare rooted plants (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by active flight, wind and human activity, the weak information on the degree of susceptibility of F. carica, the internal spread and the absence of reported problems within the nursery and at EU borders on plants for planting, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.13.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, and unclear host suitability of F. carica result in high level of uncertainties for infestation rates below the median. Detection of the pest especially before the export is unlikely, which gives relatively high uncertainties for rates above the median as well.
A.13.5.5. Elicitation outcomes of the assessment of the pest freedom for Scirtothrips dorsalis on bare rooted plants
The following tables show the elicited and fitted values for pest infestation/infection (Table A.37) and pest freedom (Table A.38).
Table A.37.
Elicited and fitted values of the uncertainty distribution of pest infestation by Scirtothrips dorsalis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 10.0 | 55.0 | 100 | 250 | 400 | ||||||||||
| EKE | 8.26 | 9.07 | 11.1 | 17.1 | 28.1 | 45.8 | 67.4 | 122 | 192 | 234 | 281 | 326 | 365 | 388 | 404 |
The EKE results are the BetaGeneral (0.64775, 1.2606, 8, 420) distribution fitted with @Risk version 7.6.
Table A.38.
The uncertainty distribution of plants free of Scirtothrips dorsalis per 10,000 plants calculated by Table A.37
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,600 | 9,750 | 9,900 | 9,945 | 9,990 | ||||||||||
| EKE results | 9,596 | 9,612 | 9,635 | 9,674 | 9,719 | 9,766 | 9,808 | 9,878 | 9,933 | 9,954 | 9,972 | 9,983 | 9,989 | 9,990.9 | 9,991.7 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.38.
Figure A.25.
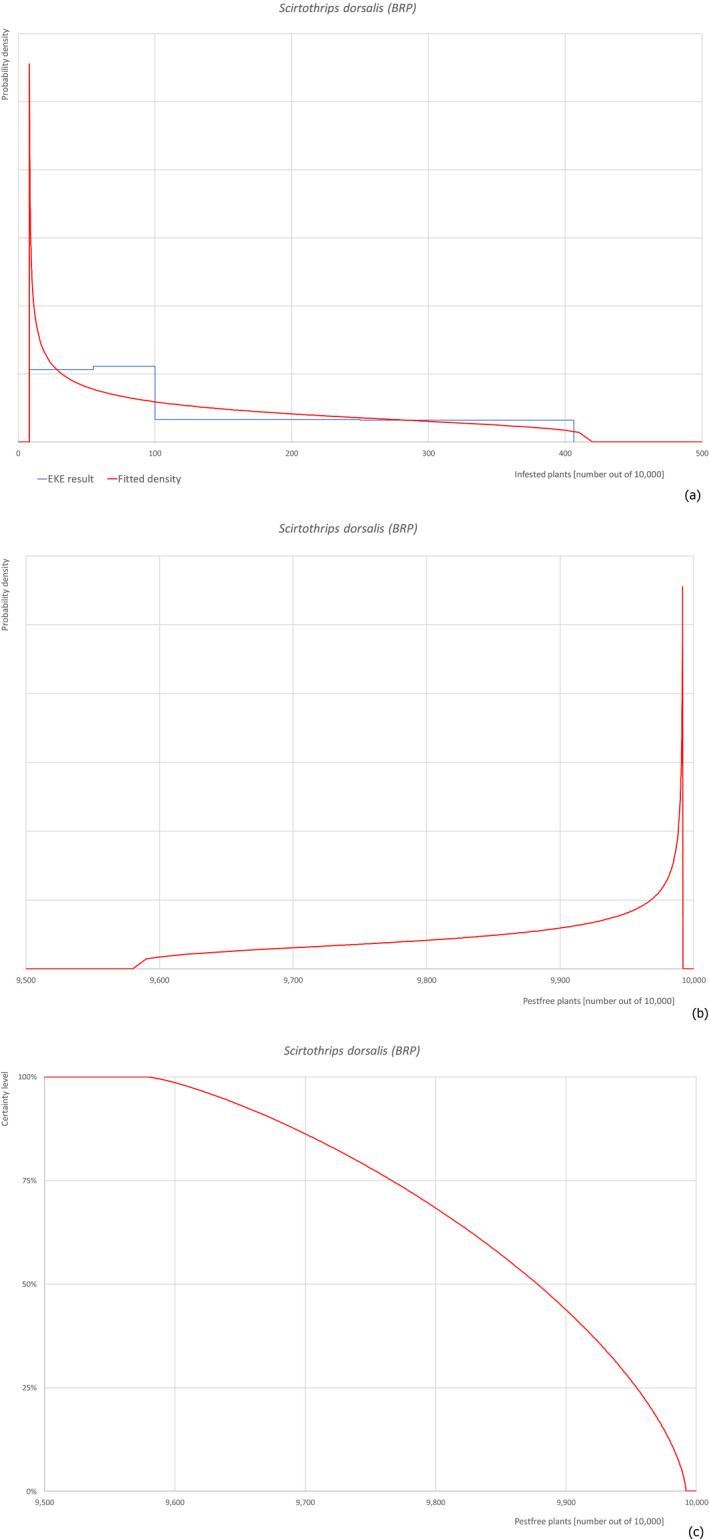
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.13.6. Overall likelihood of pest freedom for liners
A.13.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested liners
Although S. dorsalis is widespread in Israel, the scenario assumes a low pest pressure from outside and limited transfer from the surrounding due to active flight, wind and human activity. Inspections are expected to be effective because symptoms are typical, and measures should be taken accordingly. Based on that presence of thrips in soil should be limited. Ficus carica is not considered a preferential host. Insecticide treatments are expected to be effective because thrips are not protected, although they could escape treatment when hidden in bark/soil.
A.13.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested liners
Scirtothrips dorsalis is widespread in Israel; the scenario assumes a high pest pressure from outside and significant transfer from the surrounding due to active flight, wind and human activity. Inspections are expected to be less effective because symptoms are difficult to be detected at early stage. Based on that presence of thrips in soil should be enhanced. Ficus carica is considered a good host. Insecticide treatments are expected to be less effective when thrips are hidden in bark/soil.
A.13.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested liners (Median)
Regarding the uncertainties on the pest pressure outside the nursery and the likelihood of introduction into the nursery by active flight, wind and human activity, the weak information on the degree of susceptibility of F. carica, the internal spread and the absence of reported problems within the nursery and at EU borders on plants for planting, the Panel assumes a lower central scenario, which is equally likely to over‐ or underestimate the number of infested F. carica plants.
A.13.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing monitoring data in the environment of the nursery, and unclear host suitability of F. carica, together with the overwintering in bark/soil, result in some level of uncertainty for infestation rates either below or above the median.
A.13.6.5. Elicitation outcomes of the assessment of the pest freedom for Scirtothrips dorsalis on liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.39) and pest freedom (Table A.40).
Table A.39.
Elicited and fitted values of the uncertainty distribution of pest infestation by Scirtothrips dorsalis per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 45.0 | 150 | 250 | 400 | 600 | ||||||||||
| EKE | 45.1 | 51.1 | 61.2 | 81.6 | 109 | 145 | 181 | 259 | 346 | 394 | 448 | 498 | 544 | 572 | 594 |
The EKE results are the BetaGeneral (1.0083, 1.4815, 41, 620) distribution fitted with @Risk version 7.6.
Table A.40.
The uncertainty distribution of plants free of Scirtothrips dorsalis per 10,000 plants calculated by Table A.39
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,400 | 9,600 | 9,750 | 9,850 | 9,955 | ||||||||||
| EKE results | 9,406 | 9,428 | 9,456 | 9,502 | 9,552 | 9,606 | 9,654 | 9,741 | 9,819 | 9,855 | 9,891 | 9,918 | 9,939 | 9,949 | 9,955 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.40.
Figure A.26.
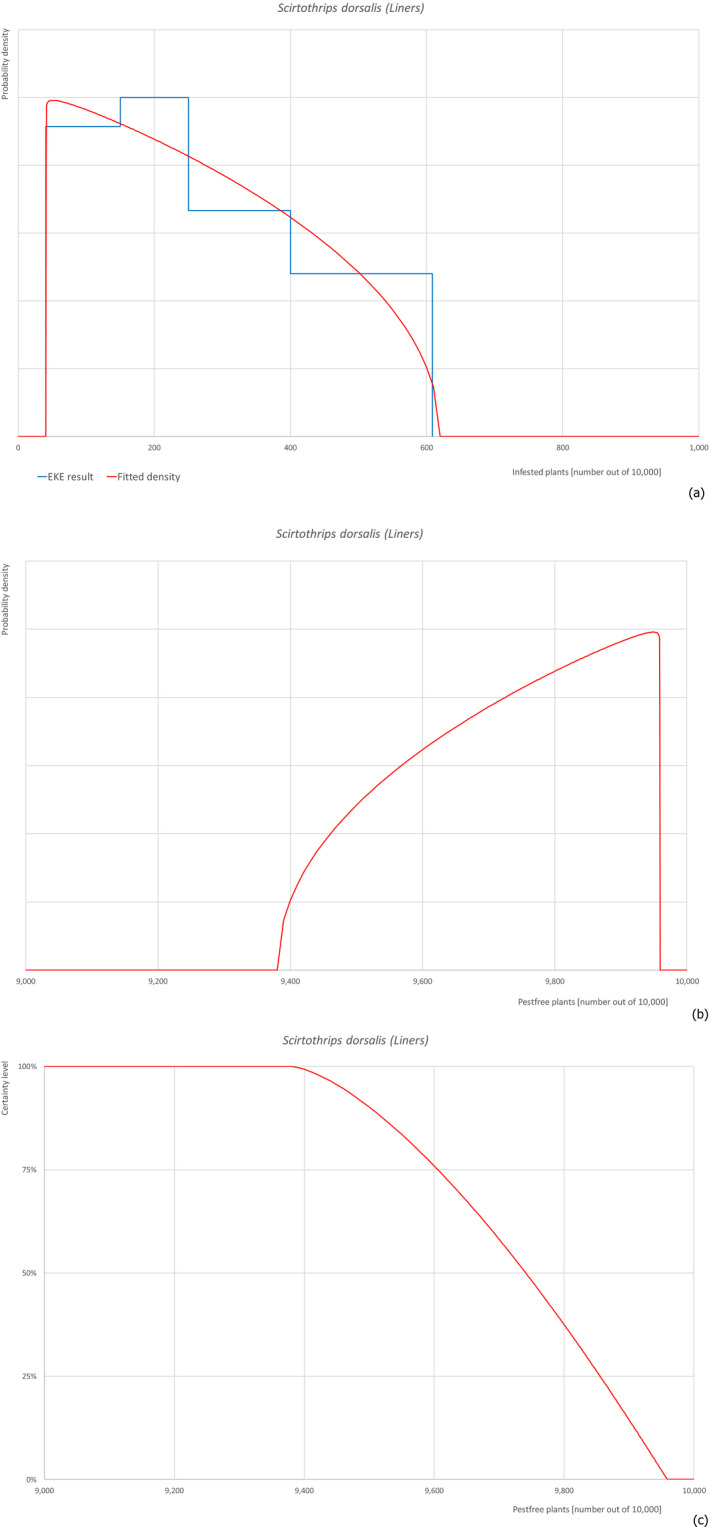
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
Figure A.27.
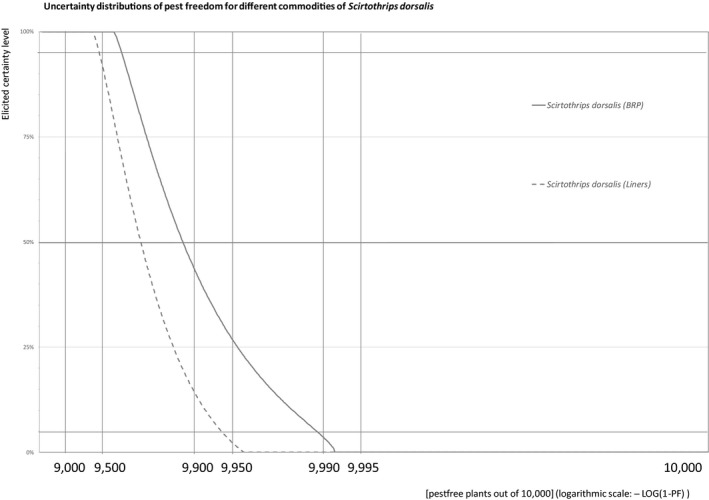
Elicited certainty (y‐axis) of the number of bare rooted plants or liners of Ficus carica pest free from Scirtothrips dorsalis (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU introduced from Israel as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
A.13.7. Reference list
Cabrera‐Asencio I and Ramírez A, 2007. Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) un nuevo record para Puerto Rico. The Journal of Agriculture of the University of Puerto Rico, 91, 49–52.
EFSA PLH Panel (EFSA Panel on Plant Health), 2014. Scientific Opinion on the pest categorisation of Scirtothrips dorsalis. EFSA Journal 2014;12(12):3915, 29 pp. https://doi.org/10.2903/j.efsa.2014.3915
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques MA, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Chatzivassiliou E, Debode J, Manceau C, Gardi C, Mosbach‐Schulz O and Potting R, 2020. Scientific Opinion on the commodity risk assessment of Jasminum polyanthum plants from Israel. EFSA Journal 2020;18(8):6225, 78 pp. https://doi.org/10.2903/j.efsa.2020.6225
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2019‐09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 9 March 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Scirtothrips dorsalis (SCITDO), Categorization. Available online: https://gd.eppo.int/taxon/SCITDO/categorization [Accessed: 9 March 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Scirtothrips dorsalis (SCITDO), Distribution. Available online: https://gd.eppo.int/taxon/SCITDO/distribution [Accessed: 9 March 2020].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 9 March 2020].
Europhyt Oubreaks database, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 31 August 2020].
Hodges G, Edwards GB and Dixon W, 2005. Chilli thrips Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) A new pest thrips for Florida. Florida Department of Agriculture and Consumer Service, Department of Primary Industries. Available on: http://www.doacs.state.fl.us/pi/enpp/ento/chillithrips.html
Holtz T, 2006. Scirtothrips dorsalis Hood: Chilli Thrips. New Pest Advisory Group (NPAG) Report. Plant Epidemiology and Risk Analysis Laboratory, Center for Plant Health Science and Technology, USDA‐APPHIS. USA: USDA‐APHIS. Available online: https://mrec.ifas.ufl.edu/lso/DOCUMENTS/Scirtothrips%20dorsalis%20NPAG%20et%20Report%20060310.pdf
Kumar V, Kakkar G, McKenzie CL, Seal DR and Osborne LS, 2013. An overview of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) biology, distribution and management. Weed and pest control‐Conventional and new challenges, 53–77. https://doi.org/10.5772/55045
Kumar V, Seal DR and Kakkar G, 2014. Chilli thrips Scirtothrips dorsalis Hood (Insecta: Thysanoptera: Thripidae). Journal of Entomology and Zoology Studies 2014, 2, 104–106. https://doi.org/10.1007/springerreference_85820
MacLeod A and Collins D, 2006. CSL pest risk analysis for Scirtothrips dorsalis. CSL (Central Science Laboratory), 8 pp.
Nietschke BS, Borchert DM, Magarey RD and Ciomperlik MA, 2008. Climatological potential for Scirtothrips dorsalis (Thysanoptera: Thripidae) establishment in the United States. Florida Entomologist, 91, 79–86. https://doi.org/10.1653/0015-4040(2008)091[0079:cpfsdt]2.0.co;2
Okada T and Kudo I, 1982. Overwintering sites and stages of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in Tea Fields. Japanese Journal of Applied Entomology and Zoology, 26, 177–182.
Seal DR and Klassen W, 2012. Chilli thrips (castor thrips, Assam thrips, yellow tea thrips, strawberry thrips), Scirtothrips dorsalis Hood, provisional management guidelines. University of Florida, Gainesville, FL, 4 pp.
Shibao M, 1991. Overwintering Sites and Stages of the Chillie Thrip Scirtothrips dorsalis HOOD (ThysanopteraThripidae) in Grapevine Fields. Japanese Journal of Applied Entomology and Zoology, 35, 161–163. https://doi.org/10.1303/jjaez.35.161
Tatara A, 1994. Effect of temperature and host plant on the development, fertility and longevity of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae). Applied Entomology and Zoology, 29, 31–37. https://doi.org/10.1303/aez.29.31
Vierbergen B and van der Gaag DJ, 2009. Pest Risk Assessment Scirtothrips dorsalis. Plant Protection Service, the Netherlands. pp. 9. Available online: https://pra.eppo.int/getfile/ddcf51cf-df6d-40f9-9d28-46f447652ed7
A.14. Spodoptera frugiperda
A.14.1. Organism information
| Taxonomic information | Current valid scientific name: Spodoptera frugiperdaSynonyms: Caradrina frugiperda, Laphygma frugiperda, Laphygma inepta, Laphygma macra, Noctua frugiperda, Phalaena frugiperda, Prodenia autumnalis, Prodenia plagiata, Prodenia signifera, Trigonophora frugiperdaName used in the EU legislation: Spodoptera frugiperda (Smith) [LAPHFR]Order: LepidopteraFamily: NoctuidaeCommon name: alfalfa worm; buck worm; budworm; corn budworm; corn leafworm; cotton leaf worm; daggy's corn worm; fall armyworm; grass caterpillar; grass worm; maize budworm; overflow worm; rice caterpillar; southern armyworm; southern grass worm; wheat cutworm; whorl wormName used in the Dossier: – | |
|---|---|---|
| Group | Insects | |
| EPPO code | LAPHFR | |
| Regulated status | The pest is listed in Part A of Annex II of Regulation (EU) 2019/2072 as Spodoptera frugiperda (Smith) [LAPHFR]. Spodoptera frugiperda is listed as a priority pest under Commission Delegated Regulation (EU) 2019/1702. Commission Implementing Decision (EU) 2018/638 and 2019/1598 lay down emergency measures to prevent the introduction and spread of S. frugiperda in the EU.Spodoptera frugiperda is included in the EPPO A1 list (EPPO, online_a).The pest is quarantine in Israel, Morocco and Tunisia (EPPO, online_b). | |
| Pest status in Israel | Spodoptera frugiperda is present in Israel. First record of the pest was reported in late July 2020, in Southern (in Western Negev) and Northern Israel (in Bet Shaan valley) and official control measures are being implemented (EPPO Reporting Service (2020/161), online). | |
| Pest status in the EU | Spodoptera frugiperda is absent in the EU (EPPO, online_c).In 1999, the pest was intercepted in Germany from the USA and was successfully eradicated (EPPO Reporting Service (2000/171), online). | |
| Host status on Ficus carica | Ficus carica is a host of S. frugiperda among hundreds of other host plants, mainly herbaceous (EPPO, online_d; Montezano et al., 2018). Schmidt‐Durán et al. (2014) consider it as a pest of F. carica in Costa Rica plantations. | |
| PRA information | Pest Risk Assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology | Spodoptera frugiperda is native to tropical and subtropical America (Sparks, 1979) and during summer migrates to temperate regions of North and South America (Johnson, 1987). The pest was introduced to Africa in 2016 (Goergen et al., 2016), Asia in 2018 (Ganiger et al., 2018) and Australia in 2020 (FAO, online). Since then it is rapidly spreading throughout all three continents (EPPO, online_e).It has two morphologically identical strains with different host preference, also called host forms. Corn strain prefers maize, sorghum and cotton. Rice strain prefers rice and wild grasses (Juárez et al., 2014). Life stage of S. frugiperda consists of egg, six larval instars, pupa and adult (Sparks, 1979). In some scientific papers it was reported that there can be five or even up to eight larval instars. Adult longevity is approximately between 12 to 18 days. Total generation time can last from 28 to 90 days depending on the temperature (Johnson, 1987). In laboratory conditions developmental time from egg to adult varied between 18 days at 35.0°C and 66 days at 15.6°C (Barfield et al., 1978).Adults are nocturnal and can fly long distances. Females produce sex pheromones to attract males to mate. The mating starts second day after the emergence of adults. Females can mate several times but only once per night (Sparks, 1979). Reproductive rate is between 900 to 1000 eggs per female (Johnson, 1987). Eggs are laid in clusters, on average between 100 to 200 eggs (dos Santos et al., 2004). They are usually on underside of leaves, but when the population is high the eggs are laid over the entire plant (Johnson, 1987).The first instars move to find suitable feeding sites on the plant where eggs were laid (Pannuti et al., 2015). First three instar larvae are quite small and eat around 2% of the total life consumption (Sparks, 1979). At high larval densities larvae can become cannibalistic (Andow et al., 2015). The sixth instar drops to the ground. The larva then pupates in the soil in depth of approximately between 2.5 and 7.5 cm (Sparks, 1979). If the population is high the pupation can occur also on plant parts (Johnson, 1987). The pupal stage lasts around 7 days at 29°C, while at 15°C, it takes ~ 37 days (Sparks, 1979).Spodoptera frugiperda lacks diapause mechanism and overwinters in mild climates in any developmental stage (Sparks, 1979).It is a migratory species with long‐distance migrations (up to several hundred km) possibly helped by wind. The number of generations per year depends on temperature. There are continuous generations all year around in Central and South America (Johnson, 1987) where there can be four to six generations annually (CABI, online). There is no information about the current population in Israel so it can be assumed that any developmental stage can be found in the winter period. | |
| Symptoms | Main type of symptoms | On leaves, larvae of S. frugiperda cause window‐pane damage, large holes, ragged edges and presence of frass. Holes can be observed also on fruits. When the infestation is high plants may look as if they have been hit by a severe hailstorm (CABI, online). |
| Presence of asymptomatic plants | No report was found on the presence of asymptomatic plants. | |
| Confusion with other pathogens/pests | Spodoptera frugiperda can be confused with other Spodoptera species, in the early larval stages with S. littoralis, S. litura and S. exigua (EPPO, 2015). Adults are very similar to S. exempta and S. littoralis (EPPO, 2020). | |
| Host plant range | Spodoptera frugiperda is a polyphagous pest of crops, ornamental plants and weeds. In America, it was reported to feed on plants belonging to 76 families, mainly on Poaceae, Asteraceae and Fabaceae (Montezano et al., 2018).Main economic damage of S. frugiperda is on maize (Zea mays), sorghum (Sorghum bicolor), cotton (Gossypium) and soybean (Glycine max) (Montezano et al., 2018). Spodoptera frugiperda is a pest of F. carica in Costa Rica (Schmidt‐Durán et al., 2014).Other host plants are onion (Allium cepa), garlic (Allium sativum), asparagus (Asparagus officinalis), aster (Aster sp.), sugar beet (Beta vulgaris), Brassica spp., pepper (Capsicum annuum), coconut (Cocos nucifera), melon (Cucumis melo), cucumber (Cucumis sativus), pumpkin (Cucurbita maxima), carrot (Daucus carota), fig (Ficus carica), sunflower (Helianthus annuus), sweet potato (Ipomoea batatas), lettuce (Lactuca sativa), mango (Mangifera indica), banana (Musa paradisiaca), rice (Oryza sativa), avocado (Persea americana), beans (Phaseolus), sugarcane (Saccharum officinarum), tomato (Solanum lycopersicum), eggplant (Solanum melongena), potato (Solanum tuberosum), vine grape (Vitis vinifera) and many more (Montezano et al., 2018). | |
| Pathways | Possible pathways of entry for S. frugiperda are plant products from where the pest is present, sweet corn, peppers, asparagus, eggplants, other vegetables, cut flowers of roses and cut flowers of other plants (EFSA PLH Panel, 2018). The Panel assumes that soil could be a pathway in case plants are traded with soil. | |
| Surveillance information | No surveillance information for this pest is currently available from PPIS. There is no information on whether the pest has ever been found in the nursery or their surrounding environment. | |
A.1.4.2. Possibility of pest presence in the nursery
A.14.2.1. Possibility of entry from the surrounding environment
First record of S. frugiperda in Israel was reported in late July 2020, in Southern (in Western Negev) and Northern Israel (in Bet Shaan valley) (EPPO Reporting Service (2020/161), online).
In Dossier Section 9.0, it is stated that ‘The fields of bare rooted fig plants are located in a distance of ~ 1 km from other plants’. And the minimum distance between fig trees cultivated for export and for the local market, is over 1 km.
According to Dossier Section 9.0, agricultural crops in a radius of 2 km from the fig cultivation includes cotton (Gossypium), tubers of various ornamental plants as well as persimmon (Diospyros), pomegranate (Punica granatum), Brassica spp., watermelon (Citrullus lanatus). In addition, Platanus spp., Populus spp. and Quercus spp. are grown in the area. Other woody species for export are cultivated in a minimal distance of ~ 500 m from the fig for export.
In addition, Dossier Section 9.0 states that the fig nursery is located in an urban area with thousands of private gardens with a large variety of plants, including woody species. There are no sites of natural vegetation, including forests, in a radius of 2 km from the nursery. There is sporadic growth of wild plants in the urban area. There are some man‐made bush parks with trees such as eucalyptus (Eucalyptus) and acacia (Acacia). Ricinus communis is also present in the wild and Persea americana may be present in private yards in the area within 2 km radius of the export nursery. The nearest natural areas are the beach and adjacent dunes, which are ~ 10 km from the nursery. The nearest natural forests are ~ 15 km from the nursery.
From these plant species mentioned above Gossypium, Diospyros, Brassica, Citrullus lanatus, Platanus occidentalis, Eucalyptus camaldulensis, Eucalyptus robusta, Eucalyptus urophylla, Acacia mearnsii and Ricinus communis are hosts of S. frugiperda (Montezano et al., 2018).
Uncertainties:
-
–
Population density around the nursery.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery from the surrounding area. The pest can be present in the surrounding areas and the transferring rate could be enhanced by proximity of outbreak area especially in herbaceous crops.
A.14.2.2. Possibility of entry with new plants/seeds
According to Dossier Section 9.0, all propagation material come from a single mother orchard located inside the nursery. Mother plants are continuously monitored for pests and undergo an annual spraying scheme, as well as annual trimming to 1 m height. Soil growing media used for liners is declared pest free.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers it is not possible that the pest could enter the nursery with new plants/seeds or soil growing media.
A.14.2.3. Possibility of spread within the nursery
The crops designated for export, are grown in different fields from the crops designated for the local market (Dossier Section 1.0). According to Dossier Section 9.0, the coverage in the export nursery is 20 – 200 plants/m2, depending on the size/age of the plants.
According to Dossier Section 9.0, following plants are grown in the fig liner export nursery: Lagerstroemia indica and Morus alba, with a distance of a few dozens of metres between them and the fig liners. According to Montezano et al. (2018), Lagerstroemia is a host of the pest.
Therefore, it is possible for S. frugiperda to reproduce within the nursery on F. carica and spread among host plants as it is a very mobile species at the adult stage, so it can move from ground plants/weeds to liners.
Uncertainties:
-
–
No uncertainties
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nursery is possible.
A.14.3. Information from interceptions
In the EUROPHYT database, there are no records of notification of F. carica plants for planting neither from Israel nor from other countries due to the presence of S. frugiperda between the years 1995 and November 2019 (EUROPHYT, online).
Spodoptera frugiperda was intercepted many times associated with plants for planting, cuttings, cut flowers and branches with foliage, fruit and vegetables (EUROPHYT, online).
A.14.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures proposed in Israel are summarised and an indication of their effectiveness on S. frugiperda is provided.
| Number | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties on liners |
|---|---|---|---|
| 1 | Characteristics of the production field | Yes | The production field condition does not allow isolation of the field used for growing plants for export.Uncertainties:– No uncertainties |
| 2 | Soil treatment | No | Not applicable |
| 3 | Rotation of the growing fields | No | Not applicable |
| 4 | Insecticide treatment | Yes | Pesticide sprays are generally effective against the fall armyworm caterpillars. Pesticides resistance should not occur because pesticides are rotated.Uncertainties:– There is uncertainty whether the pest can develop resistance to pesticides, or whether resistant strains spread into the nursery. |
| 5 | Fungicide treatment | No | Not applicable |
| 6 | Nematicide treatment | No | Not applicable |
| 7 | Treatment against weeds | Yes | Treatments are effective because the fall armyworm can feed on weeds. |
| 8 | Plant treatment before export | Yes | Pupae can stay in soil at some cm depth. Therefore, cleaning of litter of plants debris is not enough to detect pupae deep in the soil.Uncertainties:– No uncertainties |
| 9 | Sampling and testing | No | Not applicable |
| 10 | Inspections during the production | Yes | Eggs and caterpillars are easily found during inspections. The pupae of fall armyworm could go undetected because soil is not checked.Uncertainties:– The effectiveness of the inspection for the fall armyworm is not known because soil is not checked. |
| 11 | Inspections before export | Yes | The pupae of fall armyworm could go undetected because soil is not checked.Uncertainties:– The effectiveness of the inspection for the fall armyworm is not known because soil is not checked. |
| 12 | Surveillance and monitoring | Yes | No information is available on surveillance in the surrounding area. However, the fall armyworm is invading Israel from the south and it is likely to be deployed.Uncertainties:– There is no information on the density of the fall armyworm in the surrounding areas. |
A.14.5. Overall likelihood of pest freedom for liners
A.14.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested liners
Spodoptera frugiperda has been reported in Israel very recently and is under official control. Therefore, the scenario assumes a low pressure from outside. Inspections during production are expected to be effective because symptoms are obvious. Ficus carica is not considered a major host. Insecticide treatments are deemed effective, thereby reducing the number of individuals surviving until pupation.
A.14.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested liners
Spodoptera frugiperda has been reported in Israel recently, but it has demonstrated to be invasive everywhere in the world. Therefore, the scenario assumes a high pressure from outside also considering the high spreading potential of the moth. Inspections before export do not allow the detection of pupae in the soil. Ficus carica is considered a major host. Insecticide treatments are deemed not be fully effective, because some individuals could escape or be resistant to active ingredient.
A.14.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested liners (Median)
The Panel assumes a lower central scenario which is equally likely to over‐ or underestimate the number of infested F. carica liners. This scenario is considered more likely because distribution of the pest is poorly known, measures are taken, and pesticides are effective.
A.14.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Missing data on the distribution of the pest in the surrounding and on the suitability of nursery fig plants to the pest result in high level of uncertainties for infestation rates below the median. Detection of the pest especially before the export is unlikely, which gives relatively high uncertainties for rates above the median as well.
A.14.5.5. Elicitation outcomes of the assessment of the pest freedom for Spodoptera frugiperda on liners
The following tables show the elicited and fitted values for pest infestation/infection (Table A.41) and pest freedom (Table A.42).
Table A.41.
Elicited and fitted values of the uncertainty distribution of pest infestation by Spodoptera frugiperda per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1.00 | 10.0 | 20.0 | 40.0 | 100 | ||||||||||
| EKE | 0.595 | 1.27 | 2.28 | 4.15 | 6.60 | 9.72 | 13.1 | 20.8 | 31.4 | 38.6 | 48.6 | 61.2 | 78.0 | 94.5 | 116 |
The EKE results are the Gamma (1.2287, 22.76) distribution fitted with @Risk version 7.6.
Table A.42.
The uncertainty distribution of plants free of Spodoptera frugiperda per 10,000 plants calculated by Table A.41
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,900 | 9,960 | 9,980 | 9,990 | 9,999 | ||||||||||
| EKE results | 9,884 | 9,905 | 9,922 | 9,939 | 9,951 | 9,961 | 9,969 | 9,979 | 9,986.9 | 9,990.3 | 9,993.4 | 9,995.8 | 9,997.7 | 9,998.7 | 9,999.4 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants, the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.42.
Figure A.28.
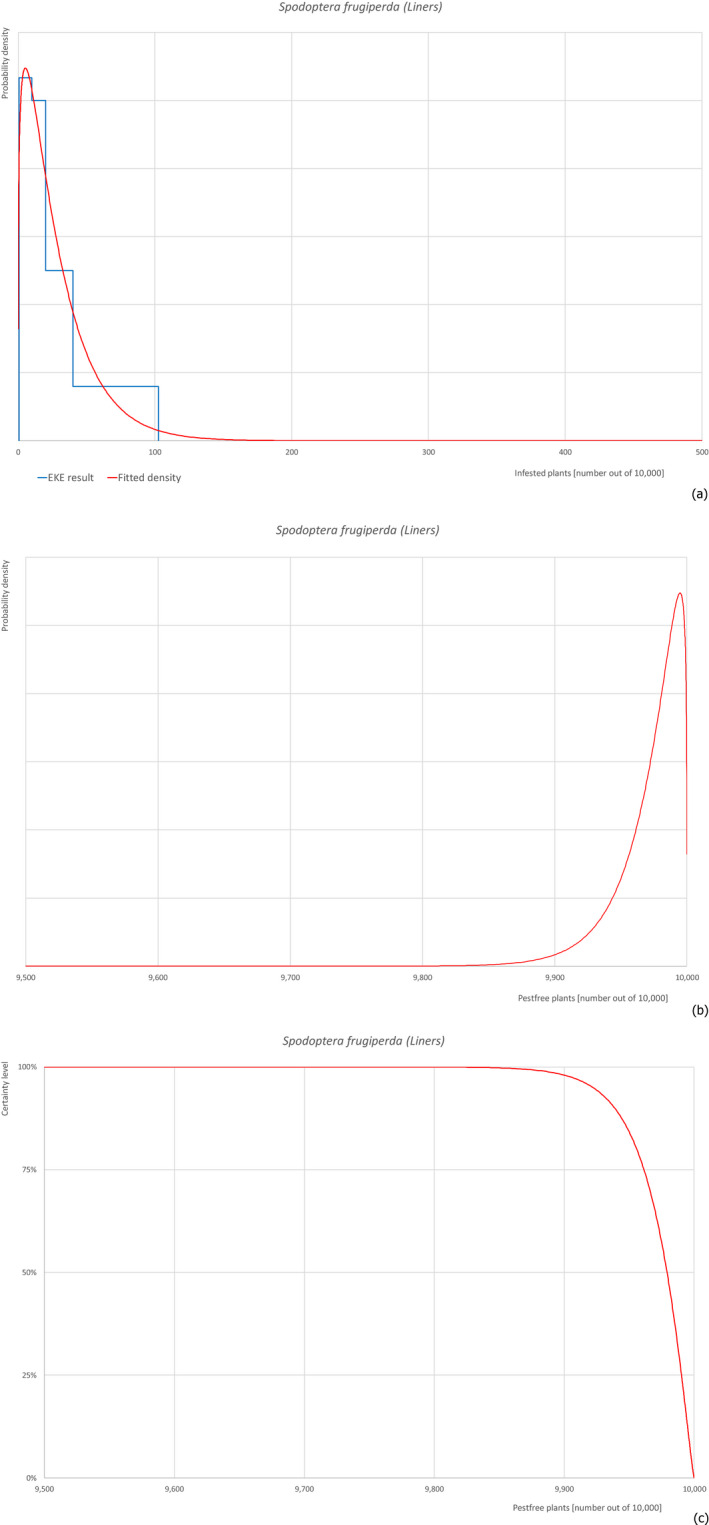
(a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 plants (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.14.6. Reference list
Andow DA, Farias JR, Horikoshi RJ, Bernardi D, Nascimento AR and Omoto C, 2015. Dynamics of cannibalism in equal‐aged cohorts of Spodoptera frugiperda. Ecological Entomology, 40, 229–236. https://doi.org/10.1111/een.12178
Barfield CS, Mitchell ER and Poeb SL, 1978. A temperature‐dependent model for fall armyworm development 1,2. Annals of the Entomological Society of America, 71, 70–74. https://doi.org/10.1093/aesa/71.1.70
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Spodoptera frugiperda (fall armyworm). Available online: https://www.cabi.org/cpc/datasheet/29810 [Accessed: 21 September 2020].
Commission Delegated Regulation (EU) 2019/1702 of 1 August 2019 supplementing Regulation (EU) 2016/2031 of the European Parliament and of the Council by establishing the list of priority pests. OJ L 260, 11.10.2019, p. 8–10.
Commission Implementing Decision (EU) 2018/638 of 23 April 2018 establishing emergency measures to prevent the introduction into and the spread within the Union of the harmful organism Spodoptera frugiperda (Smith) (notified under document C (2018) 2291). Official Journal of the European Union L 105, 25.4.2018, pp. 31–34.
Commission Implementing Decision (EU) 2019/1598 of 26 September 2019 amending Implementing Decision (EU) 2018/638 establishing emergency measures to prevent the introduction into and the spread within the Union of the harmful organism Spodoptera frugiperda (Smith) (notified under document C (2019) 6818). Official Journal of the European Union L 248, 27.9.2019, pp. 86–87.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019. OJ L 319, 10.12.2019, pp. 1–279.
dos Santos LMD, Redaelli LR, Diefenbach LMG and Efrom CFS, 2004. Fertilidade e longevidade de Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) em genótipos de milho. Ciência Rural, 34, 345–350.
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, Navarro MN, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Day R, Early R, Hruska A, Nagoshi R, Gardi C, Mosbach‐Schultz O and MacLeod A, 2018. Scientific Opinion on the pest risk assessment of Spodoptera frugiperda for the European Union. EFSA Journal 2018; 16(8):5351, 120 pp. https://doi.org/10.2903/j.efsa.2018.5351
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A1 List of pests recommended for regulation as quarantine pests, version 2019‐09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list [Accessed: 17 September 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Spodoptera frugiperda (LAPHFR), Categorization. Available online: https://gd.eppo.int/taxon/LAPHFR/categorization [Accessed: 17 September 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Spodoptera frugiperda (LAPHFR), Distribution. Available online: https://gd.eppo.int/taxon/LAPHFR/distribution [Accessed: 17 September 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_d Spodoptera frugiperda (LAPHFR), Hosts. Available online: https://gd.eppo.int/taxon/LAPHFR/hosts [Accessed: 17 September 2020].
EPPO (European and Mediterranean Plant Protection Organization), online_e Spodoptera frugiperda (LAPHFR), Reporting Service articles. Available online: https://gd.eppo.int/taxon/LAPHFR/reporting [Accessed: 24 September 2020].
EPPO (European and Mediterranean Plant Protection Organization), 2015. PM 7/124 (1) Spodoptera littoralis, Spodoptera litura, Spodoptera frugiperda, Spodoptera eridania. Bulletin OEPP/EPPO Bulletin, 45, 410–444.
EPPO (European and Mediterranean Plant Protection Organization), 2020. Spodoptera frugiperda. EPPO datasheets on pests recommended for regulation. Available online: https://gd.eppo.int
EPPO Reporting Service (2020/161), online. Spodoptera frugiperda (LAPHFR), Distribution details in Israel. Available online: https://gd.eppo.int/taxon/LAPHFR/distribution/IL [Accessed: 17 September 2020].
EPPO Reporting Service (2000/171), online. Spodoptera frugiperda (LAPHFR), Distribution details in Germany. Available online: https://gd.eppo.int/taxon/LAPHFR/distribution/DE [Accessed: 17 September 2020].
FAO (Food and Agriculture Organization), online. Detections of Spodoptera frugiperda (fall armyworm) on mainland Australia. IPPC (International Plant Protection Convention) Official Report, No. AUS‐97/2. FAO: Rome, Italy. Available online: https://www.ippc.int/en/countries/australia/pestreports/2020/03/detections-of-spodoptera-frugiperda-fall-armyworm-on-mainland-australia/ [Accessed: 24 September 2020].
Ganiger PC, Yeshwanth HM, Muralimohan K, Vinay N, Kumar ARV and Chandrashekara K, 2018. Occurrence of the new invasive pest, fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), in the maize fields of Karnataka, India. Current Science, 115, 621–623.
Goergen G, Kumar PL, Sankung SB, Togola A and Tamò M, 2016. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE, 11, 1–12. https://doi.org/10.1371/journal.pone.0165632
Heinrichs EA, Sidhu J, Muniappan R, Fayad A, Adiga A, Marath A, Mcnitt J and Venkatramanan S, 2018. Pest Risk Assessment of the Fall Armyworm, Spodoptera frugiperda in Egypt. Feed the Future. The US Government's Global Hunger and Food Security Initiative. 50 pp.
Johnson SJ, 1987. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the Western Hemisphere. Insect Science Application, 8, 543–549.
Juárez ML, Schöfl G, Vera MT, Vilardi JC, Murúa MG, Willink E, Hänniger S, Heckel DG and Groot AT, 2014. Population structure of Spodoptera frugiperda maize and rice host forms in South America: are they host strains? Entomologia Experimentalis et Applicata, 152, 182–199. https://doi.org/10.1111/eea.12215
Montezano DG, Specht A, Sosa‐Gómez DR, Roque‐Specht VF, Sousa‐Silva JC, de Paula‐Moraes SD, Peterson JA and Hunt T, 2018. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. African Entomology, 26, 286–300.
Pannuti LER, Baldin ELL, Hunt TE and Paula‐Moraes SV, 2015. On‐plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages. Environmental Entomology, 2015, 1–9. https://doi.org/10.1093/ee/nvv159
Schmidt‐Durán A, Villalba‐Velásquez V, Chacón‐Cerdas R, Martínez K and Flores‐Mora D, 2015. Larval stage prediction model of Spodoptera frugiperda collected in fig (Ficus carica) and discovery of Apantele ssp. as its parasitoid. Revista Tecnología en Marcha, 28, 47–58.
Sparks AN, 1979. A review of the biology of the fall armyworm. The Florida Entomologist, 62, 82–87.
van der Gaag DJ and van der Straten M, 2017. Assessment of the potential impact of American Spodoptera species for the European Union. Netherlands Food and Consumer Product Safety Authority (NVWA), Utrecht, the Netherlands. 42 pp.
Appendix B – Web of Science All Databases Search String
1.
In the table below, the search string used in Web of Science is reported. Totally, 408 papers were retrieved. Titles and abstracts were screened, and 241 pests were added to the list of pests (see Appendix E).
| Web of Science All databases |
TOPIC: (“Ficus carica” OR “F. carica”) AND TOPIC: (pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR “host” OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR “malaise” OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viroses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR “rot” OR “rots” OR “rotten” OR “damping off” OR “damping‐off” OR blister$ OR “smut” OR “mould” OR “mold” OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootknot OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding” OR “gall” OR “ambrosia beetle$” OR “gall$” OR “bark beetle$”)NOT TOPIC: (“winged seeds” OR metabolites OR *tannins OR climate OR “maple syrup” OR syrup OR mycorrhiz* OR “carbon loss” OR pollut* OR weather OR propert* OR probes OR spectr* OR antioxidant$ OR transformation OR RNA OR DNA OR “Secondary plant metabolite$” OR metabol* OR “Phenolic compounds” OR Quality OR Abiotic OR Storage OR Pollen* OR fertil* OR Mulching OR Nutrient* OR Pruning OR drought OR “human virus” OR “animal disease*” OR “plant extracts” OR immunological OR “purified fraction” OR “traditional medicine” OR medicine OR mammal* OR bird* OR “human disease*” OR biomarker$ OR “health education” OR bat$ OR “seedling$ survival” OR “anthropogenic disturbance” OR “cold resistance” OR “salt stress” OR salinity OR “aCER method” OR “adaptive cognitive emotion regulation” OR nitrogen OR hygien* OR “cognitive function$” OR fossil$ OR *toxicity OR Miocene OR postglacial OR “weed control” OR landscape) NOT TOPIC: (“Anastrepha ludens” OR “Aonidiella citrina” OR “Diaphorina citri” OR “Eotetranychus lewisi” OR “Eutetranychus orientalis” OR “Ralstonia solanacearum sensu lato” OR “Apriona germari” OR “Batocera rubus” OR “Greenidea ficicola” OR “Psacothea hilaris” OR “Xylosandrus crassiusculus” OR “Zaprionus indianus” OR “Anoplophora chinensis” OR “Bactrocera zonata” OR “Ceratitis capitata” OR “Ceratitis rosa” OR “Lopholeucaspis japonica” OR “Meloidogyne enterolobii” OR “Meloidogyne mali” OR “Opogona sacchari” OR “Parabemisia myricae” OR “Parasaissetia nigra” OR “Phytophthora cinnamomi” OR “Ripersiella hibisci” OR “Singhiella simplex” OR “Thrips palmi” OR “Xylella fastidiosa” OR “Xylosandrus compactus” OR “Anoplophora chinensis” OR “Apriona cinerea” OR “Apriona rugicollis” OR “Ceratitis quilicii” OR “Ceroplastes ceriferus” OR “Ceroplastes floridensis” OR “Drosophila suzukii” OR “Fiorinia phantasma” OR “Hop stunt viroid” OR “Lycorma delicatula” OR “Oemona hirta” OR “Pseudococcus comstocki” OR “Rotylenchus buxophilus” OR “Xylella fastidiosa subsp. multiplex” OR “Zaprionus tuberculatus” OR “Aceria ficus” OR “Armillaria mellea” OR “Asota spp.” OR “Batocera rufomaculata” OR “Brevipalpus phoenicis” OR “Cadra cautella” OR “Carpophilus hemipterus” OR “Ceratitis capitata” OR “Ceratocystis fimbriata” OR “Ceroplastes rusci” OR “Ceroplastes sinensis” OR “Choanephora cucurbitarum” OR “Corticium microsclerotia” OR “Diaporthe cinerescens” OR “Drosophila suzukii” OR “Eutetranychus orientalis” OR “Fig mosaic virus” OR “Frankliniella occidentalis” OR “Gibberella fujikuroi” OR “Gynaikothrips ficorum” OR “Homotoma ficus” OR “Lepidosaphes ulmi” OR “Longidorus” OR “Nattrassia mangiferae” OR “Nipaecoccus nipae” OR “Olenecamptus bilobus” OR “Parthenolecanium persicae” OR “Phymatotrichopsis omnivora” OR “Pinnaspis strachani” OR “Pratylenchus coffeae” OR “Pratylenchus vulnus” OR “Pseudococcus comstocki” OR “Pythium ultimum” OR “Rosellinia necatrix” OR “Silba adipata” OR “Spodoptera littoralis” OR “Tetranychus urticae” OR “Trichodorus” OR “Xanthomonas campestris pv. fici” OR “Xiphinema” OR “Xiphinema americanum” OR “Xiphinema index” OR “Adoretus versutus” OR “Alternaria alternata” OR “Amphitetranychus viennensis” OR “Amritodus atkinsoni” OR “Anastrepha fraterculus” OR “Anastrepha suspensa” OR “Anoplophora chinensis” OR “Aonidiella aurantii” OR “Aonidiella orientalis” OR “Aphelenchoides fragariae” OR “Aspergillus flavus” OR “Aspergillus niger” OR “Aspidiotus destructor” OR “Bactrocera cucurbitae” OR “Bactrocera zonata” OR “Botryotinia fuckeliana” OR “Candidatus Phytoplasma solani” OR “Ceratitis rosa” OR “Ceroplastes floridensis” OR “Ceroplastes rubens” OR “Choreutis nemorana” OR “Chrysodeixis chalcites” OR “Cladosporium ficii‐caricaen” OR “Conogethes punctiferalis” OR “Corcyra cephalonica” OR “Eudocima fullonia” OR “Eutypa lata” OR “Glomerella cingulata” OR “Haematonectria haematococca” OR “Halyomorpha halys” OR “Helicotylenchus multicinctus” OR “Hemiberlesia lataniae” OR “Hop stunt viroid” OR “Hyphantria cunea” OR “Imperata cylindrica” OR “Indarbela quadrinotata” OR “Lasiodiplodia theobromae” OR “Leveillula taurica” OR “Longidorus elongatus” OR “Maconellicoccus hirsutus” OR “Monilinia fructigena” OR “Orgyia leucostigma” OR “Parabemisia myricae” OR “Paratrichodorus porosus” OR “Parthenolecanium corni” OR “Phycodes radiata” OR “Phyllophaga” OR “Phytophthora palmivora” OR “Pratylenchus brachyurus” OR “Pseudaonidia duplex” OR “Pseudococcus viburni” OR “Rhizobium radiobacter” OR “Rhizobium rhizogenes” OR “Rosellinia bunodes” OR “Rotylenchulus reniformis” OR “Sowbane mosaic virus” OR “Sybra alternans” OR “Tetranychus pacificus” OR “Uncinula aspera var. aspera” OR “Zaprionus indianus” OR “Zeuzera pyrina” OR “Phenacoccus solenopsis” OR “Aspergillus parasiticus;” OR “Aspergillus tamarii” OR “Ceratocystis ficicola” OR “Cerotelium fici” OR “Dysmicoccus grassii” OR “Enneadesmus obtusedentatus” OR “Haptoncus luteolus” OR “Lepidosaphes conchiformis” OR” Lonchaea aristella” OR “Metcalfa pruinosa” OR “Nipaecoccus filamentosus” OR “Nipaecoccus viridis” OR “Oxycarenus hyalinipennis” OR “Planococcus ficus” OR “Psacothea hilaris” OR “Pythium paroecandrum” OR “Rhizopus stolonifer” OR “Rhyncaphytoptus ficifoliae” OR “Tetranychus arabicus” OR “Udumbaria nainiensis” OR “Aphis aurantii” OR “Aphis citricidus” OR “Aphis eugeniae” OR “Aphis fabae” OR “Aphis gossypii” OR “Aphis kachkoulii” OR “Aphis spiraecola” OR “Greenidea ficicola” OR “Greenidea guangzhouensis” OR “Myzus persicae” OR “Reticulaphis distylii” OR “Sitobion africanum” OR “Eriophyes fici” OR “Carpophilus hemipterus” OR “Carpophilus mutilatus” OR “Asterodiaspis pustulans” OR “Cerostegia rusci” OR “Coccus hesperidum” OR “Hemiberlesia lataniae” OR “Lepidosaphes conchyformis” OR “Quadraspidiotus ostreaeformis” OR “Quadraspidiotus pyri” OR “Homotoma ficus” OR “Acanthococcus lagerstroemiae” OR “Anomalococcus crematogastri” OR “Antecerococcus ovoides” OR “Aonidiella aurantii” OR “Aonidiella orientalis” OR “Asiacornococcus kaki” OR “Aspidiotus nerii” OR “Aulacaspis litzeae” OR “Aulacaspis yunnanensis” OR “Ceronema africanum” OR “Ceroplastes actiniformis” OR “Ceroplastes floridensis” OR “Ceroplastes japonicus” OR “Ceroplastes rubens” OR “Ceroplastes rusci” OR “Ceroplastes sinensis” OR “Chrysomphalus aonidum” OR “Chrysomphalus dictyospermi” OR “Chrysomphalus pinnulifer” OR “Clavaspis herculeana” OR “Coccus hesperidum hesperidum” OR “Coccus longulus” OR “Dactylaspis crotonis” OR “Diaspidiotus africanus” OR “Diaspidiotus braunschvigi” OR “Diaspidiotus lenticularis” OR “Diaspidiotus ostreaeformis” OR “Diaspidiotus pyri” OR “Diaspidiotus zonatus” OR “Didesmococcus unifasciatus” OR “Drosicha corpulenta” OR “Dysmicoccus debregeasiae” OR “Ericerus farsicus” OR “Eucalymnatus tessellatus” OR “Eulecanium ficiphilum” OR “Eulecanium tiliae” OR “Ferrisia virgata” OR “Fiorinia fioriniae” OR “Geococcus coffeae” OR “Hemiberlesia lataniae” OR “Hemiberlesia loranthi” OR “Hemiberlesia rapax” OR “Howardia biclavis” OR “Icerya aegyptiaca” OR “Icerya purchasi” OR “Kerria fici fici” OR “Kerria lacca lacca” OR “Lecanodiaspis africana” OR “Lecanodiaspis dendrobii” OR “Lecanodiaspis rugosa” OR “Lepidosaphes beckii” OR “Lepidosaphes conchiformis” OR “Lepidosaphes granati” OR “Lepidosaphes ulmi” OR “Leucaspis riccae” OR “Lopholeucaspis japonica” OR “Maconellicoccus hirsutus” OR “Morganella longispina” OR “Neopinnaspis harperi” OR “Nipaecoccus nipae” OR “Nipaecoccus viridis” OR “Nipponpulvinaria horii” OR “Oceanaspidiotus spinosus” OR “Paracoccus ficus” OR “Parasaissetia nigra” OR “Parlatoreopsis longispina” OR “Parlatoria fluggeae” OR “Parthenolecanium persicae” OR “Phenacoccus aceris” OR “Phenacoccus pergandei” OR “Phenacoccus solenopsis” OR “Philephedra tuberculosa” OR “Pinnaspis strachani” OR “Planococcus citri” OR “Planococcus ficus” OR “Planococcus kraunhiae” OR “Planococcus minor” OR “Pollinia pollini” OR “Pseudaonidia duplex” OR “Pseudaulacaspis cockerelli” OR “Pseudococcus comstocki” OR “Pseudococcus kikuyuensis” OR “Pseudococcus longispinus” OR “Pseudococcus viburni” OR “Pulvinaria vitis” OR “Russellaspis pustulans pustulans” OR “Saharaspis ceardi” OR “Saissetia coffeae” OR “Saissetia ficinum” OR “Saissetia miranda” OR “Saissetia oleae oleae” OR “Saissetia privigna” OR “Sphaerolecanium prunastri” OR “Suturaspis davatchi” OR “Waxiella mimosae mimosae” OR “Meloidogyne hapla” OR “Hemicycliophora poranga” OR “Hemicycliophora ripa” OR “Paralongidorus halepensis” OR “Meloidogyne sp.” OR “Pratylenchus thornei” OR “Xiphinema index” OR “Heterodera mediterranea” OR “Rotylenchulus macrodoratus” OR “Pratylenchus neglectus” OR “Meloidogyne hispanica” OR “Tylenchorhynchus sp.” OR “Pratylenchus vulnus” OR “Schistonchus caprifici” OR “Paratylenchus hamatus” OR “Paratylenchus nainianus” OR “Paratylenchus neoamblycephalus” OR “Xiphinema italiae” OR “Xiphinema pachtaicum” OR “Heterodera sp.” OR “Heterodera fici” OR “Paratylenchus sp.” OR “Xiphinema vuittenezi” OR “Rotylenchulus reniformis” OR “Meloidogyne enterolobii” OR “Trichodorus lusitanicus” OR “Trichodorus porosus” OR “Xiphinema americanum” OR “Merlinius brevidens” OR “Meloidogyne javanica” OR “Longidorus euonymus” OR “Longidorus pauli” OR “Pratylenchus pratensis” OR “Meloidogyne incognita” OR “Tylenchorhynchus capitatus” OR “Meloidogyne arenaria” OR “Ammalo helops” OR “Delphyre rufiventris” OR “Hyphantria cunea” OR “Ischnocampa lugubris” OR “Lymire albipennis” OR “Trilocha varians” OR “Phycodes minor” OR “Phycodes radiata” OR “Choreutis nemorana” OR “Tortyra fulgens” OR “Paropta paradoxus” OR “Drepanodes ephyrata” OR “Melanocercops ficuvorella” OR “Parasa lepida” OR “Emesis mandana” OR “Orgyia leucostigma” OR “Perina nuda” OR “Achaea janata” OR “Ascalapha odorata” OR “Asota caricae” OR “Asota ficus” OR “Asota plaginota” OR “Dysgonia algira” OR “Scoliopteryx libatrix” OR “Spodoptera litura” OR “Cyrestis thyodamas” OR “Lycorea cleobaea” OR “Lycorea ilione” OR “Marpesia eleuchea” OR “Marpesia petreus” OR “Stathmopoda sycastis” OR “Opostega” OR “Amyelois transitella” OR “Azochis gripusalis” OR “Cadra calidella” OR “Cadra cautella” OR “Cadra figulilella” OR “Cirrhochrista spissalis” OR “Conogethes punctiferalis” OR “Cryptoblabes gnidiella” OR “Ectomyelois ceratoniae” OR “Elasmopalpus” OR “Glyphodes pyloalis” OR “Glyphodes sibillalis” OR “Glyphodes stolalis” OR “Microphestia” OR “Plodia interpunctella” OR “Vitula serratilineella” OR “Ficivora leucoteles” OR “Pachylia ficus” OR “Pachylioides resumens” OR “Erechthias minuscula” OR “Potyvirus” OR “Carlavirus” OR “Amphitetranychus viennensis” OR “Bryobia caricae” OR “Bryobia praetiosa” OR “Bryobia rubrioculus” OR “Eotetranychus fremonti” OR “Eotetranychus hirsti” OR “Eotetranychus irregularensis” OR “Eotetranychus lewisi” OR “Eutetranychus africanus” OR “Eutetranychus banksi” OR “Eutetranychus caricae” OR “Eutetranychus orientalis” OR “Oligonychus mangiferus” OR “Oligonychus sayedi” OR “Panonychus caricae” OR “Panonychus citri” OR “Panonychus hadzhibejliae” OR “Panonychus ulmi” OR “Petrobia” OR “Schizonobia megaperitremata” OR “Schizotetranychus sayedi” OR “Sonotetranychus kermanensis” OR “Tetranychus desertorum” OR “Tetranychus lombardinii” OR “Tetranychus ludeni” OR “Tetranychus neocaledonicus” OR “Tetranychus pacificus” OR “Tetranychus turkestani” OR “Tetranychus urticae” OR “Acrostalagmus sp.” OR “Alternaria alternata” OR “Alternaria fici” OR “Alternaria longissima” OR “Alternaria sp.” OR “Alternaria tenuis” OR “Armillaria mellea” OR “Ascochyta caricae” OR “Aspergillus echinulatus” OR “Aspergillus niger” OR “Aspergillus sp.” OR “Botryodiplodia sp.” OR “Botryodiplodia theobromae” OR “Botryosphaeria berengeriana” OR “Botryosphaeria disrupta” OR “Botryosphaeria dothidea” OR “Botryosphaeria ficus” OR “Botryosphaeria quercuum” OR “Botryosphaeria ribis” OR “Botryosphaeria sp.” OR “Botryotinia fuckeliana” OR “Botrytis cinerea” OR “Botrytis depraedans” OR “Botrytis sp.” OR “Calonectria colhounii” OR “Calosphaeria fici” OR “Camarosporium ficus” OR “Capnodium batistae” OR “Capnodium footii” OR “Capnodium salicinum” OR “Capnodium sp.” OR “Cephalosporium acremonium” OR “Cephalosporium fici” OR “Ceratocystis ficicola” OR “Ceratocystis fimbriata” OR “Ceratocystis fimbriata f. sp. caricae” OR “Ceratocystis sp.” OR “Ceratostomella hystricina” OR “Cercospora annulata” OR “Cercospora bolleana” OR “Cercospora elasticae” OR “Cercospora fici” OR “Cercospora fici‐caricae” OR “Cercospora ficina” OR “Cercospora sp.” OR “Cercospora sycina” OR “Cerotelium fici” OR “Cerotelium sp.” OR “Chaetomium globosum” OR “Chaetomium megalocarpum” OR “Choanephora cucurbitarum” OR “Cladosporium coralloides” OR “Cladosporium funiculosum” OR “Cladosporium herbarum” OR “Cladosporium sp.” OR “Cladosporium sphaerospermum” OR “Cladosporium sycophilum” OR “Clitocybe tabescens” OR “Colletotrichum acutatum” OR “Colletotrichum caricae” OR “Colletotrichum crassipes” OR “Colletotrichum elasticae” OR “Colletotrichum ficus” OR “Colletotrichum gloeosporioides” OR “Colletotrichum gloeosporioides var. minor” OR “Colletotrichum siamense” OR “Colletotrichum sp.” OR “Collybia velutipes” OR “Coniothyrium anserinum” OR “Coniothyrium syconophilum” OR “Corticium centrifugum” OR “Corticium laetum” OR “Corticium microsclerotium” OR “Corticium salmonicolor” OR “Cryptophaeella trematosphaeriicola” OR “Curvularia sp.” OR “Cylindrocladium colhounii var. colhounii” OR “Cylindrocladium scoparium” OR “Cytospora chrysosperma” OR “Cytospora sp.” OR “Cytospora syconophilum” OR “Cytosporella sycina” OR “Cytosporium sp.” OR “Daldinia caldariorum” OR “Dematophora necatrix” OR “Dendrocorticium polygonioides” OR “Diaporthe cinerascens” OR “Diaporthe eres” OR “Diatrype macowaniana” OR “Diatrypella favacea” OR “Diplodia macrostoma” OR “Diplodia natalensis” OR “Diplodia sp.” OR “Diplodia sycina” OR “Diplodia sycina var. syconophila” OR “Diplodia sycina var. syconophyla” OR “Diplodiella caricae” OR “Elsinoe fici‐caricae” OR “Elsinoe sp.” OR “Erysiphe pirottiana” OR “Erythricium salmonicolor” OR “Eutypa lata” OR “Eutypa leptoplaca” OR “Eutypa sp.” OR “Eutypella caricae” OR “Eutypella cryptovalsoidea” OR “Eutypella fici” OR “Flammulina velutipes” OR “Fumago vagans” OR “Fusarium avenaceum” OR “Fusarium chlamydosporum” OR “Fusarium coccidicola” OR “Fusarium decemcellulare” OR “Fusarium euwallaceae” OR “Fusarium ficicrescens” OR “Fusarium lactis” OR “Fusarium lateritium” OR “Fusarium moniliforme” OR “Fusarium proliferatum” OR “Fusarium ramigenum” OR “Fusarium roseum” OR “Fusarium semitectum” OR “Fusarium sp.” OR “Fusarium verticillioides” OR “Ganoderma lucidum” OR “Gibberella baccata” OR “Gibberella fujikuroi” OR “Gibberella moricola” OR “Gibberella saubinetii” OR “Gloeosporium elasticae” OR “Gloeosporium intermedium” OR “Gloeosporium sp.” OR “Glomerella cingulata” OR “Glomerella cingulata var. minor” OR “Gloniella sampaioi” OR “Gonatophragmium mori” OR “Guignardia itizikucola” OR “Helicobasidium mompa” OR “Hendersonula sp.” OR “Hendersonula toruloidea” OR “Hormodendrum sp.” OR “Hymenopsis decipiens” OR “Hyphodermella corrugata” OR “Hyphodontia sambuci” OR “Inonotus cuticularis” OR “Inonotus rickii” OR “Kuehneola fici” OR “Lasiodiplodia theobromae” OR “Lentinus lepideus” OR “Leptosphaeria medicaginum” OR “Leveillula sp.” OR “Libertella ulcerata” OR “Macrophoma fici” OR “Macrophoma fici‐caricae” OR “Macrophoma sp.” OR “Macrophoma sycophila var. corticola” OR “Macrosporium commune” OR “Macrosporium sp.” OR “Macrosporium torulosum” OR “Malupa fici” OR “Melanomma pulvis‐pyrius” OR “Meruliopsis corium” OR “Microdiplodia fici” OR “Mycosphaerella bolleana” OR “Mycosphaerella sp.” OR “Nectria cinnabarina” OR “Nectria mauritiicola” OR “Nectria sp.” OR “Nectriella pironii” OR “Neocosmospora solani” OR “Neoscytalidium dimidiatum” OR “Neoscytalidium hyalinum” OR “Neoscytalidium novaehollandiae” OR “Oidium sp.” OR “Oospora sp.” OR “Ormathodium fici” OR “Passalora bolleana” OR “Pellicularia koleroga” OR “Pellicularia rolfsii” OR “Penicillium expansum” OR “Penicillium sp.” OR “Peniophora boidinii” OR “Peniophora lycii” OR “Peniophora nuda” OR “Pestalotiopsis fici” OR “Phaeoacremonium alvesii” OR “Phaeoacremonium australiense” OR “Phaeoacremonium inflatipes” OR “Phaeoacremonium italicum” OR “Phaeoacremonium parasiticum” OR “Phaeoacremonium sicilianum” OR “Phakopsora fici” OR “Phakopsora fici‐erectae” OR “Phakopsora nishidana” OR “Phanerochaete deflectens” OR “Phellinus robustus” OR “Phoma caricae” OR “Phoma cinerescens” OR “Phoma fici” OR “Phoma sp.” OR “Phomopsis cinerascens” OR “Phomopsis cinerescens” OR “Phomopsis sp.” OR “Phragmidium sp.” OR “Phyllachora abyssinica” OR “Phyllachora brittoniana” OR “Phyllachora ficuum” OR “Phyllachora howardiana” OR “Phyllachora pseudes” OR “Phyllachora sp.” OR “Phyllosticta caricae” OR “Phyllosticta fici‐caricae” OR “Phyllosticta ficicola” OR “Phyllosticta physopellae” OR “Phyllosticta robertii” OR “Phyllosticta sp.” OR “Phyllosticta sycophila” OR “Phymatotrichum omnivorum” OR “Physalospora abdita” OR “Physalospora fusca” OR “Physalospora obtusa” OR “Physalospora rhodina” OR “Physopella fici” OR “Phytophthora cambivora” OR “Phytophthora capsici” OR “Phytophthora carica” OR “Phytophthora cinnamomi” OR “Phytophthora citrophthora” OR “Phytophthora melonis” OR “Phytophthora nicotianae” OR “Phytophthora nicotianae var. nicotianae” OR “Phytophthora niederhauseri” OR “Phytophthora palmivora” OR “Phytophthora palmivora var. palmivora” OR “Phytophthora parsiana” OR “Phytophthora sp.” OR “Plenodomus sp.” OR “Pleospora coloradensis” OR “Pleurotus cystidiosus” OR “Pleurotus ostreatus” OR “Pleurotus sp.” OR “Pseudocercospora fici” OR “Pseudocercospora fici‐caricae” OR “Pseudocercospora sp.” OR “Pseudoidium sp.” OR “Punctularia subhepatica” OR “Pythium aphanidermatum” OR “Ramularia glennii” OR “Ramularia sp.” OR “Rhabdospora tenuis” OR “Rhinotrichum macrosporum” OR “Rhizoctonia microsclerotia” OR “Rhizoctonia solani” OR “Rhizoctonia sp.” OR “Rhizoctonia violacea” OR “Rhizopus nigricans” OR “Rhizopus sp.” OR “Rhizopus stolonifer” OR “Rhizopus stolonifer var. stolonifer” OR “Rhynchostoma sp.” OR “Rosellinia bunodes” OR “Rosellinia echinata” OR “Rosellinia necatrix” OR “Rosellinia sp.” OR “Saccharomyces sp.” OR “Sarocladium kiliense” OR “Schizophyllum commune” OR “Schizothyrium pomi” OR “Sclerotinia sclerotiorum” OR “Sclerotium rolfsii” OR “Scytinostroma mediterraneense” OR “Septobasidium pseudopedicellatum” OR “Septobasidium sp.” OR “Septoria oculata” OR “Sphaceloma caricae” OR “Sphaceloma sp.” OR “Sphaeropsis malorum” OR “Sphaeropsis sp.” OR “Sphaerulina caricae” OR “Sphinctrina tubiformis” OR “Stegonsporium ficinum” OR “Thanatephorus cucumeris” OR “Trabutia evansii” OR “Trabutia sp.” OR “Trematosphaeria communis” OR “Trichaptum biforme” OR “Trichosporum fici” OR “Trichothecium roseum” OR “Tryblidiella rufula” OR “Tubercularia ailanthi” OR “Tubercularia fici” OR “Tubercularia sp.” OR “Tubercularia vulgaris” OR “Uncinula aspera var. aspera” OR “Uncinula pirottiana” OR “Uredo fici” OR “Uredo ficina” OR “Uredo sawadae” OR “Venturia sp.” OR “Verticillium sp.” OR “Zygophiala jamaicensis” OR “Cerotelium fici” OR “Mycosphaerella bolleana” OR “Ctenopseustis obliquana” OR “Dettopsomyia nigrovittata” OR “Liothula sp.” OR “Mycopsylla fici” OR “Navomorpha lineata” OR “Oemona hirta” OR “Badnavirus Fig badnavirus”) |
Appendix C – List of potential pests not further assessed
1.
Table C.1.
List of potential pests not further assessed
| Pest name | EPPO code | Group | Pest present in ISRAEL | Pest present in the EU | Ficus carica confirmed as a host (reference) | Pest can be associated with both commodities | Impact | Justification for inclusion in this list |
|---|---|---|---|---|---|---|---|---|
| Eotetranychus hirsti | – | Mites | Yes | No | Yes (Migeon and Dorkeld, online) | Yes | NoData | There is no information about the impact. |
| Lecanodiaspis africana | – | Insects | Yes | No | Yes (García Morales et al., online) | Yes | NoData | There is no information about the impact. |
| Saissetia privigna | – | Insects | Yes | Restricted | Yes (García Morales et al., online) | Yes | NoData | In the EU, present only in Greece. There is no information about the impact. |
Appendix D – Personal communications
1.
Maik Veste, 2020
In May 2020, the Panel contacted Dr rer. nat. Maik Veste (Academy Researcher at Institute of Environmental Sciences ‐ Department Soil protection and recultivation ‐ of the Brandenburg University of Technology, Konrad‐Wachsmann‐Allee 6, 03046 Cottbus, Germany) in order to obtain information regarding a possible association of Plicosepalus acaciae with 1‐year‐old F. carica plants for planting (in dormant stage and without leaves).
The information provided was as follows: ‘Actually my research activity on Plicosepalus acaciae dated back to the early 1992–1996 long‐term research visits in Israel. While being interested in ecophysiology I was interested in the host–mistletoes relation of this specific species in Israel. I never saw the parasite on Ficus in the Arava/Jordan valley. In my publication, I added the Ficus after some observations which are done on the other side in the Jordan. It is highly possible that the parasite will be found also on Ficus, even that I have no personal observations. And we did some years of searching for it. If you see my list of planted non‐indigenous trees, you see that the parasite has a much larger range of hosts as it was published before my two papers.
It is also highly possible that the parasite can be found on thin branches of smaller trees! Ochradenus baccatus is a smaller shrub with small branches!! Therefore, I see that possibility that the parasite might be found on Ficus which are smaller. The important thing is that the seeds are falling down and stick on the branch. From my experiences with Ochradenus, it might be possible, it was rare, but we found several cases. Also, Calligonum comosum has small branches, and here, the parasite is also on. The area of Yotavata was a kind of hotspot of parasite in the entire Arava valley.’
Maik Veste provided his consent with the way his contribution has been presented in this opinion.
The Panel wishes to acknowledge Maik Veste for his contribution.
Enrico de Lillo, 2020
In August 2020, the Panel contacted Enrico de Lillo (Professor, Università di Bari, Dipartimento di Scienze del Suolo, della Pianta e degli Alimenti, Bari, Italy) in order to obtain information regarding the presence or absence of Aceria benghalensis in Israel.
The information provided is as follows: ‘Aceria benghalensis is an eriophyoid mite not reported for Israel. It has been reported on Ficus benghalensis in Egypt (type locality) (Soliman and Abou‐Awad, 1977) and on F. carica in Saudi Arabia (Al‐Atawi and Halawa, 2011).’
The information provided by Enrico de Lillo has been used when evaluating the pest list of F. carica specified in Appendix E.
Enrico de Lillo provided his consent with the way his contribution has been presented in this opinion.
The Panel wishes to acknowledge Enrico de Lillo for his contribution.
Panagiotis Milonas, 2020
In November 2020, Dr Panagiotis Milonas (Head of the Department of Entomology and Agricultural Zoology, Benaki, Phytopathological Institute, Kifisia, Greece) was asked, as member of the EFSA Scientific Panel on Plant Health, to comment a draft version of this opinion. By this occasion, Panagiotis Milonas provided following information: ‘Phenacoccus solenopsis was found this summer on Crete island on tomato plants.’
Panagiotis Milonas provided his consent with the way his contribution has been presented in this opinion.
The Panel wishes to acknowledge Panagiotis Milonas for his contribution.
Appendix E – Excel file with the pest list of Ficus carica
1.
Appendix E can be found in the online version of this output (in the ‘Supporting information’ section):
https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2021.6353#support-information-section
Supporting information
Excel file with the pest list of Ficus carica
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, van der Werf W, Civera AV, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Mosbach‐Schulz O and Gonthier P, 2021. Scientific Opinion on the commodity risk assessment of Ficus carica plants from Israel. EFSA Journal 2021;19(1):6353, 249 pp. 10.2903/j.efsa.2021.6353
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00655
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent, Jonathan Yuen and Lucia Zappalà.
Acknowledgements: EFSA Panel on Plant Health wishes to thank Světla Kozelská for the support during the whole process of the opinion development and to acknowledge the important contribution of the trainee Alžběta Mikulová, who provided an essential contribution to the literature search, the compilation of the pest list and the pest datasheets and drafting and reviewing the opinion.
Adopted: 26 November 2020
Notes
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) 228/2013, (EU) 652/2014 and (EU) 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. OJ L 317, 23.11.2016, pp. 4–104.
Commission Implementing Regulation (EU) 2018/2019 of 18 December 2018 establishing a provisional list of high risk plants, plant products or other objects, within the meaning of Article 42 of Regulation (EU) 2016/2031 and a list of plants for which phytosanitary certificates are not required for introduction into the Union, within the meaning of Article 73 of that Regulation C/2018/8877. OJ L 323, 19.12.2018, pp. 10–15.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019, OJ L 319, 10.12.2019, p. 1–279.
Commission Implementing Decision (EU) 2018/638 of 23 April 2018 establishing emergency measures to prevent the introduction into and the spread within the Union of the harmful organism Spodoptera frugiperda (Smith). OJ L 105, 25.4.2018, p. 31–34.
Commission Implementing Regulation (EU) 2020/1201 of 14 August 2020 as regards measures to prevent the introduction into and the spread within the Union of Xylella fastidiosa (Wells et al.). OJ L 269, 17.8.2020, p. 2–39.
References
- Al‐Atawi FJ and Halawa AM, 2011. New Records of Eriophyoid Mites (Acari: Prostigmata: Eriophyoidea) from Saudi Arabia. Pakistan Journal of Biological Sciences, 14, 112–117. [DOI] [PubMed] [Google Scholar]
- CABI (Centre for Agriculture and Bioscience International), online. CABI Crop Protection Compendium. Available online: https://www.cabi.org/cpc/ [Accessed: 4 November 2019].
- Cooperband MF, Stouthamer R, Carrillo D, Eskalen A, Thibault T, Cossé AA, Castrillo LA, Vandenberg JD and Rugman‐Jones PF, 2016. Biology of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae), recently invasive in the USA, reared on an ambrosia beetle artificial diet. Agricultural and Forest Entomology, 18, 223–237. 10.1111/afe.12155 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2018. Guidance on quantitative Pest Risk Assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2019. Guidance on commodity risk assessment for the evaluation of high risk plants dossiers. EFSA Journal 2019;17(4):5668, 20 pp. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, van der Werf W, Civera AV, Yuen J, Zappalà L and Grègoire J‐C, Streissl F, Kertész V and Milonas P, 2020. Scientific Opinion on the list of non‐EU Scolytinae of coniferous hosts. EFSA Journal 2020;18(1):5933, 56 pp. 10.2903/j.efsa.2020.5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122ISSN:1831-4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://www.eppo.int/ [Accessed: 26 October 2019]
- EUROPHYT , online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 14 October 2019].
- Faccoli M, Campo GI, Perrotta G and Rassati DA, 2016. Two newly introduced tropical bark and ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) damaging figs (Ficus carica) in southern Italy. Zootaxa, 4138, 189–194. 10.11646/zootaxa.4138.1.10 [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations), 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations), 2017. ISPM (International standards for phytosanitary measures) No. 5. Glossary of phytosanitary terms. FAO, Rome. Available online: https://www.ippc.int/en/publications/622/
- Ferris H, online. Nemaplex (The Nematode‐Plant Expert Information System). Available online: http://nemaplex.ucdavis.edu/
- Gaaliche B, Ben Abdelaali N, Mouttet R, Ben Halima‐Kamel M and Hajlaoui MR, 2018. Nouveau signalement de Hypocryphalus scabricollis (Eichhoff, 1878) en Tunisie, un ravageur émergent sur figuier (Ficus carica L.). EPPO Bulletin, 48, 164–166. 10.1111/epp.12459 [DOI] [Google Scholar]
- García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: a literature‐based model of scale insect biology and systematics. Available online: http://scalenet.info/associates/ [Accessed: 4 November 2019]. [DOI] [PMC free article] [PubMed]
- Kajii C, Morita T, Jikumaru S, Kajimura H, Yamaoka Y and Kuroda K, 2013. Xylem dysfunction in Ficus carica infected with wilt fungus Ceratocystis ficicola and the role of the vector beetle Euwallacea interjectus . IAWA Journal, 34, 301–312. 10.1163/22941932-00000025 [DOI] [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- MacLeod A and Korycinska A, 2019. Detailing Koppen‐Geiger climate zones at a country and regional level: a resource for pest risk analysis. EPPO Bulletin, 49, 73–82. [Google Scholar]
- Mifsud D, Falzon A, Malumphy C, Lillo ED, Vovlas N and Porcelli F, 2012. On some arthropods associated with Ficus species (Moraceae) in the Maltese Islands. Bulletin of the Entomological Society of Malta, 5, 5–34. [Google Scholar]
- Migeon A and Dorkeld F, online. Spider Mites Web, Eotetranychus hirsti. Available online: https://www1.montpellier.inrae.fr/CBGP/spmweb/notespecies.php?id=247 [Accessed: 3 September 2020].
- Robinson GS, Ackery PR, Kitching IJ, Beccaloni GW and Hernández LM, online. HOSTS ‐ A Database of the World's Lepidopteran Hostplants. Natural History Museum, London. Available online: https://www.nhm.ac.uk/our-science/data/hostplants/search/index.dsml [Accessed: 30 November 2019].
- Schmidt‐Durán A, Villalba‐Velásquez V, Chacón‐Cerdas R, Martínez K and Flores‐Mora D, 2015. Larval stage prediction model of Spodoptera frugiperda collected in fig (Ficus carica) and discovery of Apantele ssp. as its parasitoid. Revista Tecnología en Marcha, 28, 47–58. 10.18845/tm.v28i1.2191 [DOI] [Google Scholar]
- Soliman ZR and Abou‐Awad BA, 1977. Five new species of the genus Eriophyes in the A. R. E. (Acarina: Eriophyoidea: Eriophyidae). Acarologia, 19, 668–677. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel file with the pest list of Ficus carica


