Abstract
Background
Cesarean delivery reduces the risk of infant and maternal morbidity and mortality when medically indicated, however, the cesarean delivery rate is estimated to be two to three times higher than medically necessary. The World Health Organization and American College of Obstetricians and Gynecologists have expressed concern over the high rates of cesarean delivery, citing evidence that cesarean delivery has negative short- and long-term consequences for the health of the infant, mother, and for future pregnancies. Infants delivered by cesarean are at an increased risk of metabolic disease and immune dysfunction throughout the lifespan. Preliminary research suggests that the hypothalamic pituitary adrenal (HPA) axis is a plausible pathway linking cesarean delivery to poor health later in life. The present study examines the relation between mode of delivery and HPA axis function in six-month-old infants. We also examine whether the cesarean delivery was elective or indicated altered to the relation between mode of delivery and infant cortisol profiles.
Methods
The sample included 136 mother/infant pairs. Thirty-nine women delivered by cesarean and 97 delivered vaginally. Maternal and infant medical records were reviewed for prenatal medical history and birth outcomes. Infant saliva was collected for cortisol analysis at a 6-month well-baby checkup. Samples were collected upon arrival to the appointment (baseline) and 20 minutes after exposure to a painful stressor, the inoculation procedure (response). A mixed model ANCOVA was conducted to determine whether salivary cortisol concentrations differed between the two delivery groups. To examine whether complications related to having an indicated cesarean delivery contributed to any association between mode of delivery and cortisol production, cortisol concentrations were compared between the subgroup of infants whose cesarean deliveries were elective (e.g. maternal request or previous cesarean delivery) to infants delivered vaginally.
Results
Infants delivered by cesarean had lower cortisol concentrations at baseline and after the inoculation procedure compared to those delivered vaginally. Further, the relation between mode of delivery and cortisol levels persisted even when the analyses were restricted to compare only the elective cesarean deliveries (e.g. maternal request or previous cesarean delivery) to those delivered vaginally.
Discussion
This study provides evidence for an association between cesarean delivery and infant HPA axis function in infancy. Findings are consistent with the hypothesis that the HPA axis is a plausible pathway that links cesarean delivery with long-term health outcomes.
Keywords: cesarean delivery, cortisol, HPA axis, delivery mode, stress
1.0. Introduction
Cesarean delivery is a life-saving treatment that reduces risk of maternal and infant morbidity and mortality when medically indicated (Molina et al., 2015). The World Health Organization estimates that intervention with cesarean delivery prevents these risks in 10–15% of births (World Health Organization Human Reproduction Programme, 2015). However, the cesarean delivery rate in the United States is 32% (Martin et al., 2017), and cesarean delivery rates are as high as 40.5% in some countries (Betrán et al., 2016). This high rate is in part due to cesarean procedures that are not medically necessary (i.e. elective) (Barber et al., 2011; Meikle et al., 2005; Osterman and Martin, 2014; Zhang et al., 2010). Although cesarean delivery has clear benefits when medically indicated, it also is associated with long-term adverse health consequences for the infant and mother that are particularly important considerations for elective procedures (Sandall et al., 2018). Empirical and meta-analytic evidence indicates that offspring delivered by cesarean are at increased risk for metabolic disease and immune dysfunction later in life, including obesity, diabetes, asthma, allergies, and celiac disease (Cardwell et al., 2008; Cho and Norman, 2013; Darmasseelane et al., 2014; Sevelsted et al., 2015; Thavagnanam et al., 2008). A recent study utilizing a within-family design to partially account for genetic and environmental effects concluded that individuals delivered by cesarean were 64% more likely to develop obesity compared to their siblings delivered vaginally (Yuan et al., 2016). The American College of Obstetricians and Gynecologists and the World Health Organization have expressed concern over the high rate of cesarean delivery given evidence of short and long-term health consequences for the infant, mother, and her potential future pregnancies, prompting efforts to reduce rates of non-medically indicated cesarean delivery (American College of Obstetricians and Gynecologists, 2019; Curtin et al., 2013; National Institutes of Health, 2006; U.S. Department of Health and Human Services, 2010; World Health Organization, 2018).
The majority of research investigating pathways leading cesarean delivery to poor health outcomes has focused on colonization of the infant microbiome during delivery (Houghteling and Walker, 2015; Neu and Rushing, 2012; Penders et al., 2014). Infants delivered vaginally are exposed to the host of microflora present in the birth canal, which then colonize the infant gut (Mueller et al., 2015). This colonization promotes development of the infant immune system, intestinal tract, and metabolism (Mueller et al., 2017). Thus disruptions to this colonization, such as cesarean delivery, may lead to long-term poor immune and metabolic health outcomes (Penders et al., 2013; Sevelsted et al., 2015). The infant microbiome is not the only system affected by cesarean delivery, however, and it is likely that additional mechanisms contribute to health outcomes in offspring.
A plausible, yet understudied, pathway by which cesarean delivery might impact later health is alterations to the hypothalamic pituitary adrenal (HPA) axis following cesarean delivery. Cortisol is a glucocorticoid and an end-product of the HPA axis, one of the primary stress regulatory systems (Chrousos, 2009; Engel and Gunnar, 2019; Maniam et al., 2014; McEwen et al., 1997). Dysregulation of cortisol production increases risk for metabolic disease and immune dysfunction throughout the lifespan (Adam et al., 2017; Bellavance and Rivest, 2014; Bose et al., 2009; Incollingo Rodriguez et al., 2015; Müller and Quinkler, 2018). Several studies that have examined cortisol concentrations in infants born by vaginal and cesarean delivery indicate that cesarean delivery disrupts cortisol production. For example, venous and mixed cord blood concentrations of cortisol are lower in cesarean deliveries compared with vaginal deliveries (Bird et al., 1996; Gitau et al., 2001; Goldkrand et al., 1976; Mears et al., 2004; Nejad, 2016; Vogl et al., 2006; Warren and Goland, 1995). These differences are observed 72 hours after birth (Schuller et al., 2012), and evidence suggests that the association persists to two months of age (Miller et al., 2005; Taylor et al., 2000). It is unknown whether these alterations persist beyond early infancy. If reduced production continues beyond the neonatal period, such hypocortisolism may confer long-term health risks (Maripuu et al., 2016; McEwen, 2008; Varghese et al., 2016). Evaluating the impact of cesarean delivery on infant cortisol production may help explain the link between cesarean delivery and poor health outcomes.
1.2. Objective
The current study examines the relation between mode of delivery and infant cortisol response to a routine inoculation at six months of age. We test the hypothesis that cesarean delivery is associated with infant cortisol levels. Further, to address the possibility that the reason for having an indicated cesarean delivery contributed to the relation between cesarean delivery and infant cortisol, we compare cortisol values in the subgroup of infants whose cesarean deliveries were elective (e.g. maternal request or previous cesarean delivery) to those delivered vaginally.
2.0. Materials and Methods
2.1. Participants
The study sample included 136 mothers and their full-term infants (72 male, 64 female). Women were recruited prenatally into a longitudinal study of maternal and child development. Participants provided written informed consent approved by the Institutional Review Board for Protection of Human Subjects. Initial recruitment criteria included singleton pregnancy, English-speaking, and over the age of 18. Exclusion criteria were fetal chromosomal or congenital anomalies, maternal endocrine problems, prenatal corticosteroid treatment, and substance use during pregnancy (smoking, recreational drugs, alcohol). Ninety-seven women delivered vaginally and 39 by cesarean.
2.2. Measures
2.2.1. Demographic and Clinical Data
Demographic data were collected by semi-structured interview (Table 1). Household income and years of education were used a calculate a socioeconomic status composite score (Cohen et al., 2006). Maternal and infant medical records were reviewed to assess prenatal medical history and birth outcomes.
Table 1.
Descriptive information for infants and mothers.
| Infant Characteristics | Vaginal (n=97) | Delivery Cesarean Delivery (n=39) | p |
|---|---|---|---|
| Gestational age at birth (weeks)b | 39.5 (1.1) | 39.3 (1.1) | 0.427 |
| Birth weight (grams)b | 3384.3 (414.7) | 3349.9 (518.5) | 0.440 |
| Race/Ethnicity a | 0.139 | ||
| Non-Hispanic White | 49 (50.5) | 23 (59) | |
| Hispanic | 18 (18.6) | 10 (25.6) | |
| Other | 30 (30.9) | 6 (15.4) | |
| Sex (% Male)a | 48 (49.5) | 24 (61.5) | 0.203 |
| Apgar score at 5-minb | 9 (0.3) | 9.1 (0.4) | 0.061 |
| Age at assessment (weeks)b | 27 (2.1) | 26.7 (1.8) | 0.433 |
| Maternal Demographics | |||
| Maternal age at delivery (years)b | 29.9 (5) | 31.9(5) | 0.031 |
| Married a | 72 (73.2) | 33 (84.6) | 0.192 |
| Cohabitating with baby’s father a | 88 (90.7) | 36 (92.3) | 0.768 |
| Body Mass Indexb1 | 24.9 (6.6) | 26.7 (7.2) | 0.427 |
| Highest level of education a2 | 0.821 | ||
| High school or less | 12 (12.3) | 3 (7.7) | |
| Some college or certificate | 38 (39.2) | 19 (48.8) | |
| Bachelor degree or higher | 47 (48.4) | 17 (43.6) | |
| Annual household income a2 | 0.469 | ||
| $0–50,000 | 36 (37.1) | 12 (30.8) | |
| $50,001–100,000 | 38 (39.2) | 17 (43.6) | |
| Over $100,000 | 21 (21.6) | 10 (25.6) | |
Chi-square; N(%)
t-test; Mean (SD)
Maternal body mass index (BMI) was calculated using height and weight [weight (kg)/height2 (m2)] at 15 weeks gestation.
Household income and level of education were used to calculate a socioeconomic status composite score.
2.2.2. Elective Cesarean Delivery
A dichotomous variable was used to indicate whether cesarean deliveries were considered elective or medically necessary. Cesarean deliveries were considered elective if the only indication for cesarean was previous cesarean delivery (n=17). Five of the women in the elective condition experienced labor.
2.2.3. Indicated Cesarean Delivery
Twenty-two women had medical indications for cesarean delivery other than previous cesarean. Indications included abnormal fetal heart rate patterns(American College of Obstetricians and Gynecologists, 2009) (n=4), breech presentation (n=5), maternal medication condition (n=3), macrosomia (n=4), protracted active phase (n=1), arrest of active phase (n=7), protracted descent (n=1), severe intrauterine growth restriction (n=1), cord prolapse (n=1), failed vacuum (n=1), and polyhydramnios (n=1). Fourteen of the women in the indicated condition experienced labor.
2.2.4. Infant Salivary Cortisol Response to Stress
Infant saliva was collected at a 6-month well-baby checkup and assayed for cortisol. Salivary cortisol reflects the unbound or active fraction of cortisol and is highly correlated with plasma cortisol in infants and adults (Calixto et al., 2002; Kirschbaum and Hellhammer, 1989). Saliva was collected upon arrival at the appointment and a second sample was collected 20 minutes after inoculation to capture peak response to the painful stressor (Gunnar et al., 1991). Saliva was obtained by placing a swab in the infant’s mouth for up to one minute. Samples were spun and stored at −20 degrees Celsius until assayed. At the time of assay, samples were thawed then centrifuged at 3,000 rpm for 15 minutes.
Cortisol levels were determined by a competitive luminescence immunoassay (LIA; IBL-America, Minneapolis, MN) with a detection limit of .005 μg/dL. The intra- and inter-assay coefficients of variance were 5.5% and 7.6% respectively. Both samples each infant were included in the same assay batch to eliminate subject inter-assay variance. Samples were assayed in duplicate and averaged.
Four infants (three born vaginally and one by cesarean) had cortisol concentrations that were four standard deviations above the mean, thus were considered outliers and excluded final analyses. However, the inclusion of outliers in analyses did not alter the significant of study findings. Infant salivary cortisol values at baseline and response were not normally distributed and were therefore log-transformed.
2.3. Analyses
Preliminary analyses were conducted using Pearson correlations and t-tests to identify maternal and infant characteristics that were associated with mode of delivery (Table 1). Only maternal age and five-minute Apgar scores differed between the two delivery groups at the p<.10 level and thus were included as covariates in all analyses. Time of cortisol assessment did not significantly differ between the two groups (vaginal delivery, M = 11:56, SD = 2:33; cesarean delivery M = 12:15, SD = 2:29; t(−.672), p = .50).
First, we examined whether infant cortisol concentrations increased in response to the inoculation stressor. Next, we examined whether mode of delivery was associated with cortisol profiles using a mixed model ANCOVA with mode of delivery (vaginal vs. cesarean) as the between groups factor and cortisol response to the stressor (cortisol at baseline and response) as the within-groups factor.
To examine whether indicated cesarean deliveries contributed to the association between mode of delivery and cortisol levels, we repeated the mixed model ANCOVA including only the subgroup of infants whose cesarean deliveries were elective (e.g. maternal request and previous cesarean delivery) in the cesarean group. To test whether the experience of labor contributed to findings, we compared cortisol between infants in the cesarean group that experienced labor (n=19) and did not experience labor (n=20) prior to cesarean delivery using a mixed model ANCOVA to determine whether the presence or absence of labor contributed to differences in cortisol levels.
3.0. Results
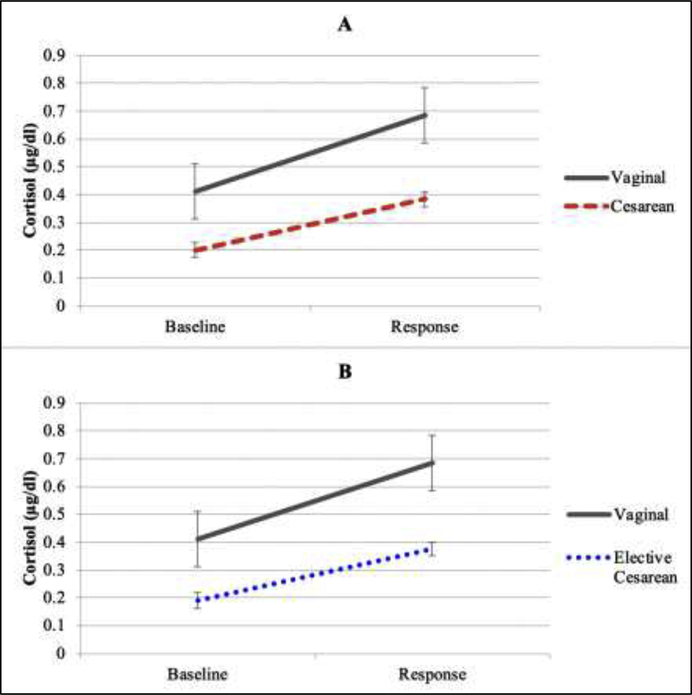
The manipulation, the inoculation stressor, elicited an increase in infant salivary cortisol 0.35 μg/dL (SD=0.84) to 0.6 μg/dL (SD=0.84) [F(1, 132) = 72.71, p<.001] in the full sample. There was a main effect of group such that salivary cortisol concentration was significantly lower in infants delivered by cesarean as compared to those delivered vaginally [F(1, 130) = 6.07, p=0.02] (Figure 1a). The interaction between cortisol response to the stressor and delivery mode showed that infants delivered vaginally tended to exhibit a greater increase in cortisol baseline to response compared to those born by cesarean (mean=.18 μg/dL, SD=.23), but this did not reach standard statistical significance [F (1, 130) = 2.94, p=0.09] (Figure 1a).
Figure 1.
Infant cortisol concentrations at baseline and 20 minutes after the inoculation procedure by (A) delivery mode, and (B) among infants whose cesarean deliveries were elective compared to those delivered vaginally.
When the cesarean group was restricted to only those infants who had been delivered by elective cesarean, salivary cortisol levels were lower in the cesarean group as compared to the vaginal group [F(1, 110) = 3.72, p=.06] (Figure 1b). Finally, cortisol levels did not differ between infants that experienced labor and did not experience labor in the cesarean delivery group [F (1, 36) = .001, p=.98].
4.0. Discussion
Our prospective research reveals that six-month-old infants delivered by cesarean at term exhibited lower levels of cortisol at baseline and after an inoculation procedure compared to those delivered vaginally. Notably, neither whether the caesarean delivery was elective versus medically indicated nor the experience of labor accounted for this association. These results are consistent with prior studies showing that cesarean delivery is associated with lower cortisol birth to eight weeks of age (Miller et al., 2005; Taylor et al., 2000). Our study expands this literature by showing that suppressed cortisol production persists through at least six months of age. These findings provide support to the hypotheses that delivery mode impacts development of the HPA axis, and that altered infant HPA axis functioning following cesarean delivery may be a pathway by which cesarean delivery impacts subsequent health.
There are three important contributions of our study. First, we examined cortisol measured directly the infant, whereas most previous research has examined cord blood (Bird et al., 1996; Gitau et al., 2001; Goldkrand et al., 1976; Mears et al., 2004; Nejad, 2016; Vogl et al., 2006; Warren and Goland, 1995). Second, our findings indicate that the hypocortisolemic pattern observed in neonates delivered by cesarean persists through six months of age. Finally, our consideration of elective cesarean deliveries suggest that medically indicated deliveries do not account for the cortisol differences observed between infants delivered vaginally or by cesarean delivery.
Vaginal labor and delivery are normative experiences that serve a critical function for offspring development including stimulation of the respiratory and metabolic systems (Houghteling and Walker, 2015; Nejad, 2016). During labor and delivery, infants endure the physical stress of contractions, periods with reduced oxygen, and significant increases in maternal glucocorticoids, often for several hours (Lagercrantz and Slotkin, 1986). By comparison, cesarean delivery is a relatively calm experience for the infant. The physical stress of vaginal birth and exposure to maternal glucocorticoids during labor and delivery are important for infant development of vital organs and may facilitate programming of the HPA axis (Lagercrantz, 1996; Taylor et al., 2000). Infants delivered by cesarean are deprived of this normative experience, which may lead to reduced cortisol levels and alterations to the development of the HPA axis in infancy. This study is not powered to test differences in amount of labor experienced in the cesarean group, we did not find that the experience of labor accounted for links with cortisol in the cesarean group. Future research should compare infant HPA axis function by labor experiences and delivery mode.
The pattern of hypocortisolism observed in infants delivered by cesarean may have implications for long-term health. Cortisol is necessary for development of vital organs including the brain, lungs, liver, and pancreas, that are implicated in health and disease (Bellavance and Rivest, 2014; Davis et al., 2017, 2004; Turner-Cobb et al., 2011; Xiong and Zhang, 2013). Glucocorticoids modulate gastrointestinal and metabolic functions, and have anti-inflammatory properties that help regulate the immune system (Chrousos, 2009; Coutinho and Chapman, 2011). Hypocortisolism is associated with immune dysfunction, including allergies and atopic dermatitis, in childhood through adulthood (Adam et al., 2017; Buske-Kirschbaum, 2009; Priftis et al., 2009; Ruttle et al., 2014; Varghese et al., 2016). Further, hypocortisolism is linked to obesity, higher BMI, dysregulated cholesterol, and metabolic syndrome (Adam et al., 2017; Daniel et al., 2006; Maripuu et al., 2016; Ruttle et al., 2013). Thus, it is plausible that the hypocortisolism observed during infancy may be associated with long term health consequences.
Future research that examines the relations among delivery mode, cortisol, and health outcomes is warranted to better understand the mechanisms by which cesarean delivery is associated with subsequent poor health. The primary focus of research on the pathway linking cesarean delivery to subsequent health has been on the gut microbiome (Mueller et al., 2017; Neu and Rushing, 2012). The present study illustrates that cesarean delivery is associated with alterations to HPA axis regulation as well. Future studies should examine the joint and independent contributions of these two systems as potential mechanisms by which cesarean delivery may be associated with subsequent health.
4.1. Strenghts and Limitations
Strengths of the present investigation include the prospective and longitudinal design with direct biological assessments of the infants. This study, however, was observational in nature and thus, causality cannot be determined. Further, due to sample size, this study cannot test the impact of specific obstetric complications that may have contributed to links between cesarean delivery and infant cortisol outcomes. We instead performed secondary analyses examining only the subgroup of infants whose cesarean deliveries were elective. That cortisol levels were lower even in the elective group partially addresses concerns related to the confounding impact of obstetric complications.
4.2. Conclusions
The benefits of cesarean delivery are clear when medically indicated. The present finding suggests that cesarean delivery has associations with the development of infant HPA axis regulation. Thus, the potential short- and long-term consequences of cesarean delivery support the call of WHO and ACOG to reduce the number of non-medically indicated cesarean deliveries (American College of Obstetricians and Gynecologists, 2019; National Institutes of Health, 2010; World Health Organization Human Reproduction Programme, 2015).
Highlights.
Cesarean delivery is linked to subsequent metabolic disease and immune dysfunction.
Hypothalamic pituitary adrenocortical axis dysregulation is one potential mechanism.
Cesarean delivery predicts hypocortisolism through 6 months of age.
Cortisol dysregulation may contribute to health consequences of cesarean delivery.
Acknowledgement
This research was funded by NIH awards HD065823, HD051852, MH109662.
Role of the funding source
The funding source played no role in study design, interpretation of results or manuscript preparation.
Footnotes
Conflict of interest
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE, 2017. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 83, 25–41. 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2009. Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet. Gynecol. 114, 192–202. 10.1097/AOG.0b013e3181aef106 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2019. Cesarean delivery on maternal request. Obstet. Gynecol. 133, 73–77. [Google Scholar]
- Barber EL, Lundsberg L, Belanger K, Pettker CM, Funai EF, Illuzzi JL, 2011. Indications contributing to the increasing cesarean delivery rate. Obstet. Gynecol. 118, 29–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance MA, Rivest S, 2014. The HPA - immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 5, 1–13. 10.3389/fimmu.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betrán AP, Ye J, Moller A-B, Zhang J, Gülmezoglu AM, Torloni MR, 2016. The increasing trend in caesarean section rates: Global, regional and national estimates: 1990–2014 . PLoS One 11, e0148343 10.1371/journal.pone.0148343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J, Spencer J, Mould T, 1996. Endocrine and metabolic adaptation following caesarean section or vaginal delivery. Arch. Dis. 132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M, Oliván B, Laferrére B, 2009. The effect of remote ischemic conditioning for kidney transplantation. Curr Opin Endocrinol Diabetes Obes. 16, 340–346. 10.1097/MED.0b013e32832fa137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, 2009. Cortisol responses to stress in allergic children: Interaction with the immune response. Neuroimmunomodulation 16, 325–332. 10.1159/000216190 [DOI] [PubMed] [Google Scholar]
- Calixto C, Martinez FE, Jorge SM, Moreira AC, Martinelli CE, 2002. Correlation between plasma and salivary cortisol levels in preterm infants. J. Pediatr. 140, 116–8. 10.1067/mpd.2002.120765 [DOI] [PubMed] [Google Scholar]
- Cardwell CR, Stene LC, Joner G, Cinek O, Svensson J, Goldacre MJ, Parslow RC, Pozzilli P, Brigis G, Stoyanov D, Urbonaite B, Šipetić S, Schober E, Ionescu-Tirgoviste C, Devoti G, De Beaufort CE, Buschard K, Patterson CC, 2008. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: A meta-analysis of observational studies. Diabetologia 51, 726–735. 10.1007/s00125-008-0941-z [DOI] [PubMed] [Google Scholar]
- Cho CE, Norman M, 2013. Cesarean section and development of the immune system in the offspring. Am. J. Obstet. Gynecol. 208, 249–254. 10.1016/j.ajog.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Chrousos GP, 2009. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381. 10.1038/nrendo.2009.106 [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle W, Baum A, 2006. Socioeconomic status is associated with stress hormones. Psychosom Med 68, 414–420. 10.1097/01.psy.0000221236.37158.b9 [DOI] [PubMed] [Google Scholar]
- Coutinho AE, Chapman KE, 2011. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 335, 2–13. 10.1016/j.mce.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin SC, Gregory KD, Korst LM, 2013. Maternal morbidity for vaginal and cesarean deliveries, according to previous cesarean history: New data the birth certificate, 2013. Natl. Vital Stat. Reports 64. [PubMed] [Google Scholar]
- Daniel M, Moore DS, Decker S, Belton L, DeVellis B, Doolen A, Campbell MK, 2006. Associations among education, cortisol rhythm, and BMI in blue-collar women. Obesity 14, 327–335. 10.1038/oby.2006.42 [DOI] [PubMed] [Google Scholar]
- Darmasseelane K, Hyde MJ, Santhakumaran S, Gale C, Modi N, 2014. Mode of delivery and offspring body mass index, overweight and obesity in adult life: A systematic review and meta-analysis. PLoS One 9 10.1371/journal.pone.0087896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Head K, Buss C, Sandman CA, 2017. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology 75, 56–63. 10.1016/j.psyneuen.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Townsend EL, Gunnar MR, Georgieff MK, Guiang SF, Ciffuentes RF, Lussky RC, 2004. Effects of prenatal betamethasone exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrinology 1028–1036. 10.1016/j.psyneuen.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Engel ML, Gunnar MR, 2019. The development of stress reactivity and regulation during human development. Int. Rev. Neurobiol. 150, 41–76. 10.1016/BS.IRN.2019.11.003 [DOI] [PubMed] [Google Scholar]
- Gitau R, Menson E, Pickles V, Fisk NM, Glover V, MacLachlan N, 2001. Umbilical cortisol levels as an indicator of the fetal stress response to assisted vaginal delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 98, 14–17. 10.1016/S0301-2115(01)00298-6 [DOI] [PubMed] [Google Scholar]
- Goldkrand JW, Schulte RL, Messer RH, 1976. Maternal and fetal plasma cortisol levels and parturition. Obstet. Gynecol. 47, 41–45. [PubMed] [Google Scholar]
- Gunnar MR, Hertsgaard L, Larson M, Rigatuso J, 1991. Cortisol and behavioral responses to repeated stressors in the human newborn. Dev. Psychobiol. 24, 487–505. [DOI] [PubMed] [Google Scholar]
- Houghteling PD, Walker WA, 2015. Why is initial bacterial colonization of the intestine important to infants’ and children’s health? J. Pediatr. Gastroenterol. Nutr. 60, 294–307. 10.1097/MPG.0000000000000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ, 2015. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology 62, 301–318. 10.1016/j.psyneuen.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH, 1989. Salivary cortisol in psychobiological research: An overview. Biol. Psychol. 22, 150–169. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, 1996. Stress, arousal, and gene activation at birth. Physiology 11, 214–218. [Google Scholar]
- Lagercrantz H, Slotkin TA, 1986. The “stress” of being born can be important to the neonate’s survival outside the womb. Sci. Am. 254, 100–107. [DOI] [PubMed] [Google Scholar]
- Maniam J, Antoniadis C, Morris MJ, 2014. Early-life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Front. Endocrinol. (Lausanne). 5, 73 10.3389/fendo.2014.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback KF, 2016. Relative hypocortisolism is associated with obesity and the metabolic syndrome in recurrent affective disorders. J. Affect. Disord. 204, 187–196. 10.1016/j.jad.2016.06.024 [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Mathews TJ, 2017. Births: Final data for 2015. Natl. Vital Stat. Rep. 66, 1–104. [PubMed] [Google Scholar]
- McEwen BS, 2008. Stress mediators. Eur. J. Pharmacol. 583, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM, 1997. The role of adrenocorticoids as modulators of immune function in health and disease: Neural, endocrine and immune interactions. Brain Res. Brain Res. Rev. 23, 79–133. [DOI] [PubMed] [Google Scholar]
- Mears K, Mcauliffe F, Grimes H, Morrison JJ, 2004. Fetal cortisol in relation to labour, intrapartum events and mode of delivery. J. Obstet. Gynaecol. (Lahore). 24, 129–132. 10.1080/01443610410001645389 [DOI] [PubMed] [Google Scholar]
- Meikle SF, Steiner CA, Zhang J, Lawrence WL, 2005. A national estimate of the elective primary cesarean delivery rate. Obstet. Gynecol. 105, 751–756. 10.1097/01.AOG.0000157435.67138.78 [DOI] [PubMed] [Google Scholar]
- Miller NM, Fisk NM, Modi N, Glover V, 2005. Stress responses at birth: Determinants of cord arterial cortisol and links with cortisol response in infancy. BJOG An Int. J. Obstet. Gynaecol. 112, 921–926. 10.1111/j.1471-0528.2005.00620.x [DOI] [PubMed] [Google Scholar]
- Molina G, Weiser TG, Lipsitz SR, Esquivel MM, Uribe-Leitz T, Azad T, Shah N, Semrau K, Berry WR, Gawande AA, Haynes AB, 2015. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA - J. Am. Med. Assoc. 314, 2263–2270. 10.1001/jama.2015.15553 [DOI] [PubMed] [Google Scholar]
- Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Maria G, 2015. The infant microbiome development: Mom matters. Trends Mol Med 21, 109–117. 10.1016/j.molmed.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NT, Shin H, Pizoni A, Werlang IC, Matte U, Goldani MZ, Goldani HAS, Dominguez-Bello MG, 2017. Delivery mode and the transition of pioneering gut-microbiota structure, composition and predicted metabolic function. Genes (Basel). 8 10.3390/genes8120364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L, Quinkler M, 2018. Adrenal disease: Imitating the cortisol profile improves the immune system. Nat. Rev. Endocrinol. 14, 137–139. 10.1038/nrendo.2018.5 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, 2010. Statement on vaginal birth after cesarean: New insights. NIH Consens. State. Sci. Statements 27, 1–41. [PubMed] [Google Scholar]
- National Institutes of Health, 2006. NIH State-of-the-Science Conference Statement on Cesarean Delivery on Maternal Request, NIH Consensus and State-of-the-Science Statements. [PubMed] [Google Scholar]
- Nejad RK, 2016. Comparison of oxidative stress markers and serum cortisol between normal labor and selective cesarean section born neonates. J. Clin. Diagnostic Res. 38–40. 10.7860/JCDR/2016/16935.7974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J, Rushing J, 2012. Cesarean versus vaginal delivery: Long term infant outcomes and the hygiene hypothesis. Clin. Perinatol. 38, 321–331. 10.1016/j.clp.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman MJK, Martin JA, 2014. Trends in Low-risk Cesarean Delivery in the United States, 1990–2013. Natl. Vital Stat. Reports 63, 1–16. [PubMed] [Google Scholar]
- Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, Hamelmann E, 2013. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 132, 601–607.e8. 10.1016/j.jaci.2013.05.043 [DOI] [PubMed] [Google Scholar]
- Penders J, Gerhold K, Thijs C, Zimmermann K, Wahn U, Lau S, Hamelmann E, 2014. New insights into the hygiene hypothesis in allergic diseases: Mediation of sibling and birth mode effects by the gut microbiota. Gut Microbes 5, 239–244. 10.4161/gmic.27905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priftis KN, Papadimitriou A, Anthracopoulos MB, Fretzayas A, Chrousos GP, 2009. Endocrine-immune interactions in adrenal function of asthmatic children on inhaled corticosteroids. Neuroimmunomodulation 16, 333–339. 10.1159/000216191 [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Javaras KN, Klein MH, Armstrong JM, Burk LR, Essex MJ, 2013. Concurrent and longitudinal associations between diurnal cortisol and body mass index across adolescence. J. Adolesc. Heal. 52, 731–737. 10.1016/j.jadohealth.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Serbin LA, Martin-Storey A, Stack DM, Schwartzman AE, 2014. Longitudinal associations between infections and atopic disorders across childhood and dysregulated adrenocortical functioning in early adolescence. Dev. Psychobiol. 56, 897–907. 10.1002/dev.21163 [DOI] [PubMed] [Google Scholar]
- Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, Gibbons D, Kelly NM, Kennedy HP, Kidanto H, Taylor P, Temmerman M, 2018. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 392, 1349–1357. 10.1016/S0140-6736(18)31930-5 [DOI] [PubMed] [Google Scholar]
- Schuller C, Känel N, Müller O, Kind AB, Tinner EM, Hösli I, Zimmermann R, Surbek D, 2012. Stress and pain response of neonates after spontaneous birth and vacuum-assisted and cesarean delivery. Am. J. Obstet. Gynecol. 207, 416.e1–416.e6. 10.1016/j.ajog.2012.08.024 [DOI] [PubMed] [Google Scholar]
- Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H, 2015. Cesarean section and chronic immune disorders. Obstet. Gynecol. Surv. 70, 303–305. 10.1097/01.ogx.0000466336.81671.9f [DOI] [PubMed] [Google Scholar]
- Taylor A, Fisk NM, Glover V, 2000. Mode of delivery and subsequent stress response. Lancet 355, 120 10.1016/S0140-6736(99)02549-0 [DOI] [PubMed] [Google Scholar]
- Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR, 2008. A meta-analysis of the association between Caesarean section and childhood asthma. Clin. Exp. Allergy 38, 629–633. 10.1111/j.1365-2222.2007.02780.x [DOI] [PubMed] [Google Scholar]
- Turner-Cobb JM, Rixon L, Jessop DS, 2011. Hypothalamic-pituitary-adrenal axis activity and upper respiratory tract infection in young children transitioning to primary school. Psychopharmacology (Berl). 214, 309–317. 10.1007/s00213-010-1965-x [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, O. of Di.P. and H.P., 2010. Healthy people 2010: Understanding and improving health. Washington, D.C. [Google Scholar]
- Varghese R, Rajappa M, Chandrashekar L, Kattimani S, Archana M, Munisamy M, Revathy G, Thappa DM, 2016. Association among stress, hypocortisolism, systemic inflammation, and disease severity in chronic urticaria. Ann. Allergy, Asthma Immunol. 116, 344–348.e1. 10.1016/j.anai.2016.01.016 [DOI] [PubMed] [Google Scholar]
- Vogl SE, Worda C, Egarter C, Bieglmayer C, Szekeres T, Huber J, Husslein P, 2006. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG An Int. J. Obstet. Gynaecol. 113, 441–445. 10.1111/j.1471-0528.2006.00865.x [DOI] [PubMed] [Google Scholar]
- Warren WB, Goland RS, 1995. Effects of parturition on corticotropin releasing hormone and products of the pituitary and adrenal in term fetuses at delivery. J. Perinat. Med. 23, 453–458. 10.1515/jpme.1995.23.6.453 [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2018. WHO recommendations: Intrapartum care for a positive childbirth experience. [PubMed] [Google Scholar]
- World Health Organization Human Reproduction Programme, 10 April 2015, 2015. WHO Statement on caesarean section rates. Reprod. Health Matters 23, 149–50. 10.1016/j.rhm.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Xiong F, Zhang L, 2013. Role of the hypothalamic – pituitary – adrenal axis in developmental programming of health and disease. Front. Neuroendocr. 34, 27–46. 10.1016/j.yfrne.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Gaskins AJ, Blaine AI, Zhang C, Gillman MW, Missmer SA, Field AE, Chavarro JE, 2016. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 170 10.1001/jamapediatrics.2016.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, Landy HJ, Hibbard JU, Haberman S, Ramirez MM, Bailit JL, Hoffman MK, Gregory KD, Gonzalez-Quintero VH, Kominiarek M, Learman LA, Hatjis CG, Van Veldhuisen P, 2010. Contemporary cesarean delivery practice in the United States. Am. J. Obstet. Gynecol. 203, 326.e1–326.e10. 10.1016/j.ajog.2010.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]