Abstract
Objectives
We retrospectively analyzed our experience of mitral valve repair for native mitral valve endocarditis in a single institution.
Methods
From January 1991 to October 2011, 171 consecutive patients underwent surgery for infective endocarditis. Of these, 147 (86%) had mitral valve repair. At the time of surgery, 98 patients had healed (group A) and 49 had active infective endocarditis (group B). Repair procedures included resection of all infected tissue and thick restricted post-infection tissue, leaflet and annulus reconstruction with treated autologous pericardium, chordal reconstruction with polytetrafluoroethylene sutures, and ring annuloplasty if necessary. Fifty-two (35%) patients required concomitant procedures. The study endpoints were overall survival, freedom from reoperation, and freedom from valve-related events. The median follow-up was 78 months.
Results
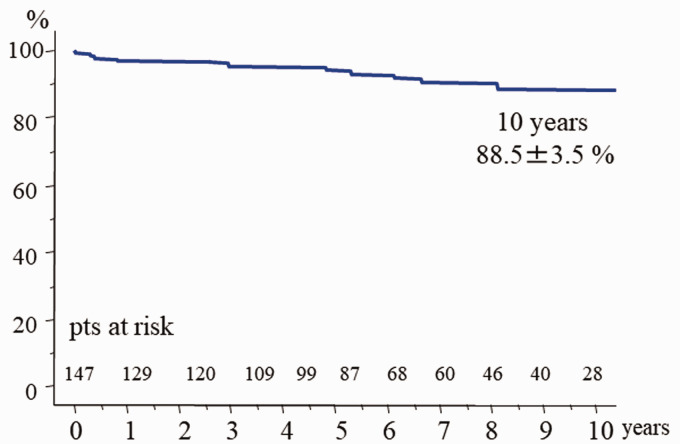
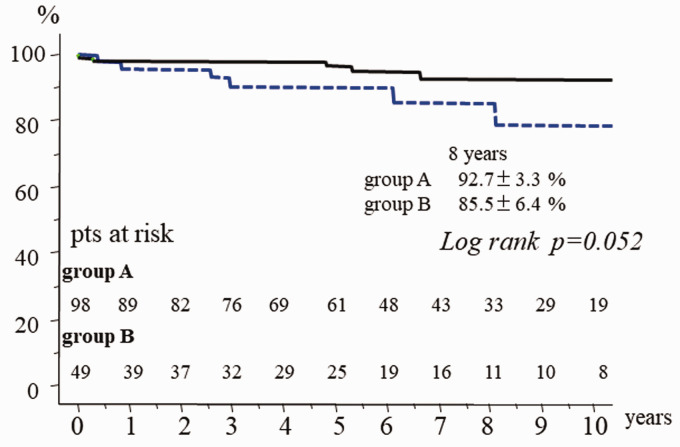
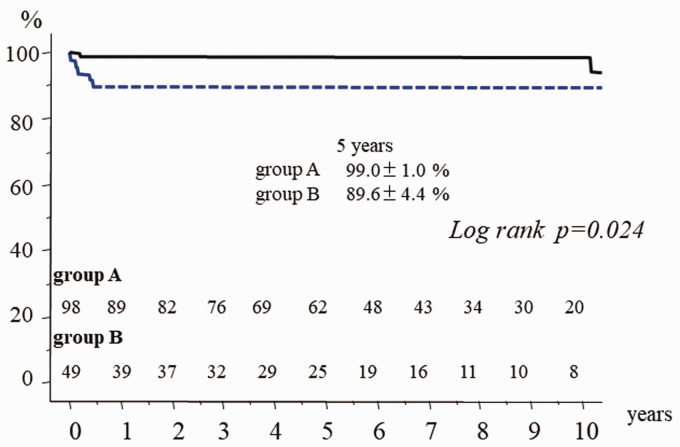
There was one hospital death (hospital mortality 0.7%). Survival at 10 years was 88.5% ± 3.5% with no significant difference between the two groups (p = 0.052). Early reoperation was required in 4 patients in group B due to persistent infection or procedure failure. Freedom from reoperation at 5 years was 99% ± 1.0% in group A and 89.6 ± 4.0% in group B (p = 0.024). Event-free survival at 10 years was 79.3% ± 4.8% (group A: 83.4% ± 5.9%, group B: 72.6% ± 6.9%, p = 0.010).
Conclusions
Mitral valve repair was highly successful using autologous pericardium, chordal reconstruction, and ring annuloplasty if required. Long-term results were acceptable in terms survival, freedom from reoperation, and event-free survival. Mitral valve repair is recommended for mitral infective endocarditis in most patients.
Keywords: Endocarditis, bacterial, mitral valve insufficiency, pericardium, prostheses and implants, transplantation, autologous, treatment outcome
Introduction
Surgery has been required in patients with infective endocarditis (IE), with mitral valve replacement as the standard procedure until the reports from Dreyfus and colleagues1 in 1990 and Hendren and colleagues2 in 1992. They concluded that mitral valve repair was possible without recurrent infection or valve-related morbidity. Mitral valve repair is currently the choice of procedure for degenerative mitral regurgitation (MR) with simple posterior prolapse. However, bileaflet prolapse requiring multiple reparative procedures is still challenging. Mitral lesions due to IE vary from simple posterior leaflet prolapse to bileaflet damage including the mitral annulus. Several reparative techniques are required to make a mitral valve with IE functionally normal without regurgitation or stenosis. We introduced standard ring annuloplasty, chordal reconstruction with expanded polytetrafluoroethylene (ePTFE) sutures, and autologous pericardium to increase the coaptation surface area from 1991.3,4 Repair was used in 1988 for patients with mitral IE with a simple posterior lesion, and a small number of mitral valve repairs for mitral IE was reported in 1995.5 We retrospectively analyzed our clinical results of mitral valve repair for mitral IE.
Patients and methods
This was a retrospective single-center study including patients who required mitral valve repair for mitral IE from January 1991 to October 2011. The study was approved by our institutional review board. There were 171 patients who required mitral surgery for IE during this period. Of these, 147 (86%) underwent mitral valve repair with or without concomitant procedures. The diagnosis of mitral IE was based on the Duke criteria. Active IE was confirmed according to findings in specimens resected at surgery. The definition of active IE was microorganisms and/or white blood cells in the specimen or a positive culture from the surgical specimen. There were 98 healed IE patients (group A) and 49 active IE patients (group B). The patient characteristics are listed in Table 1. The mean age of the patients was significantly younger than those who required mitral valve repair for degenerative MR.3 As we reported previously, our basic concept has changed from conventional treatment to early surgical intervention for active IE patients.6 There were 48 (33%) patients who demonstrated stroke by brain computed tomography before surgery.
Table 1.
Characteristics of patients undergoing mitral valve repair for infective endocarditis.
| Variable | No. of patients |
|---|---|
| Age (years) | 49.6 ± 17.5 |
| Male | 85 (58%) |
| Active infective endocarditis | 49 (33%) |
| Cardiac rhythm | |
| Sinus rhythm | 138 (94%) |
| Atrial fibrillation | 9 (6%) |
| NYHA class III/IV | 64 (44%) |
| Stroke | 48 (33%) |
NYHA: New York Heart Association.
After a median sternotomy, autologous pericardium was harvested mainly from the anterior part of the pericardium and immersed in 0.625% glutaraldehyde solution for 10–15 min in selected cases before establishment of cardiopulmonary bypass (CPB). All operations were carried out with mild to moderate hypothermic CPB with ascending aorta and bicaval cannulation. Myocardial protection was achieved with antegrade and/or retrograde cold blood cardioplegia. The mitral valve was exposed through a right-sided atriotomy. In 29 patients who required simultaneous aortic valve surgery, careful examination of the aortic valve by aortotomy was performed before mitral exposure. In patients who required aortic valve replacement, aortic valvectomy and careful examination of the left ventricular side of the anterior mitral leaflet was mandatory. Mitral valve assessment was carefully performed after a left atriotomy. Mitral exposure was supported with annular stitches before assessment. In the first step, all IE lesions were carefully resected, leaving intact leaflets and chordae. In patients with healed IE, the thick and immobile posterior leaflet (usually the P3 area) was resected to obtain leaflet motion. IE lesions extended to the anterior and posterior leaflets in 60% of both active and healed patients. Treated autologous pericardium was very useful for reconstructing leaflet deficiency after resection of the leaflets (Figure 1). To reduce the suture tension, we did not hesitate to use autologous pericardium to repair the mitral leaflet, especially in cases of active IE. In these cases, the size of the autologous pericardium should be larger enough, like the sail of a boat during systole. Reparative procedures, except resection of infected segments, are listed in Table 2. Chordal reconstruction with ePTFE sutures (CV-5) and ring annuloplasty were applied if required.
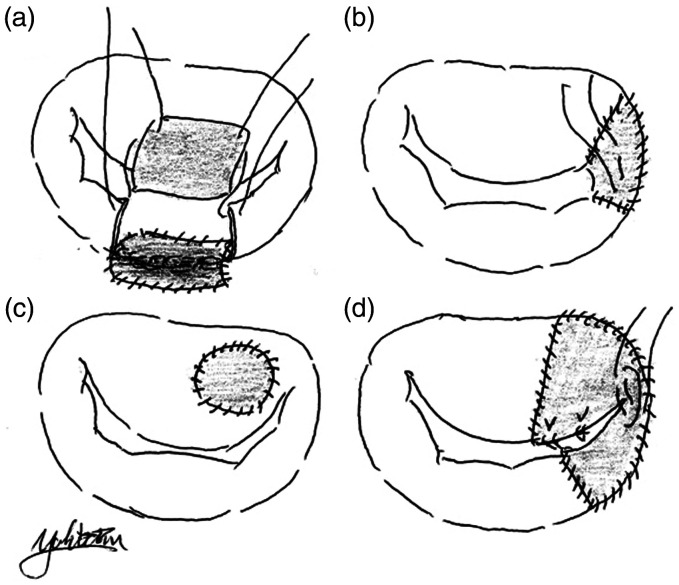
Figure 1.
Several patterns of pericardial patch grafting for leaflet and annulus. (a) Annulus and leaflet reconstruction. (b) Leaflet reconstruction around the commissure. (c) Patch grafting at a leaflet perforation. (d) Leaflet reconstruction of P3, PC, and A3 with new expanded polytetrafluoroethylene chordae.
Table 2.
Reparative procedures in addition to resection of infected tissue.
| Variable | No. of patients |
|---|---|
| Autologous pericardium | 51 (35%) |
| Leaflet reconstruction | 48 (33%) |
| Annulus reconstruction | 3 (2%) |
| New chordae | 71 (48%) |
| Annuloplasty | 131 (89%) |
| Prosthetic ring | 104 (71%) |
| Pericardium (partial) | 24 (16%) |
| Suture annuloplasty | 3 (2%) |
| Cardiopulmonary bypass time (min) | 156 ± 58 |
| Aortic crossclamp time (min) | 115 ± 44 |
Concomitant procedures were required in 52 (35%) patients (Table 3). Before weaning from CPB, intraoperative transesophageal echocardiography was carried out to assess the repair. Residual regurgitation that required a second pump run was detected 13 (8.8%) patients who were all repaired successfully during the second pump run. CPB time was 156 ± 58 min and aortic crossclamp time was 115 ± 44 min. Antibiotic therapy was started preoperatively and continued postoperatively for 3–4 weeks. Anticoagulation for 3 months was indicated in patients receiving prosthetic ring annuloplasty.
Table 3.
Concomitant procedures in 52 patients.
| Variable | No. of patients |
|---|---|
| Aortic valve replacement | 22 |
| Aortic valve repair | 5 |
| Aortic root replacement | 1 |
| Tricuspid annuloplasty | 17 |
| Maze procedure | 7 |
| ASD/PFO closure | 5 |
| Ventricular septal defect closure | 4 |
| Coronary artery bypass | 2 |
| Resection of papillary fibroelastoma | 1 |
ASD: atrial septal defect; PFO: patent foramen ovale.
Statistical analyses were performed with JMP15 software (SAS Institute, Inc., Cary, NC, USA). Continuous data are summarized as mean ± standard deviation. Survival, freedom from reoperation, and event-free survival were assessed by Kaplan-Meier analysis, and differences between the two groups were examined with the log-rank test. A p value < 0.05 was considered to be statistically significant.
Results
There was one hospital death (hospital mortality 0.7%) due to uncontrollable hemorrhage. There were 12 late deaths including 6 non-cardiac deaths (cancer 4, hepatitis 1, pneumonia 1). Other causes of late death were congestive heart failure in 2 patients, and unknown in 4 patients. Survival at 10 years was 88.5% ± 3.5% (Figure 2). There was no significant difference in late survival between the groups (Figure 3). Reoperation required in 7 patients (group A: 2, group B: 5). In group A, 2 patients required mitral valve replacement long after repair because of mitral stenosis due to leaflet thickening. In group B, 2 patients had persistent infection. In one of these, Candida albicans infection persisted on the anterior leaflet after resection of the infected posterior P2 leaflet. This patient required valve replacement with permanent medication for Candida. The other patient was an emergency case of methicillin-resistant Staphylococcus aureus infection.7 Posterior mitral valve repair with posterior left atrial wall and annulus reconstruction with xenopericardium was tried at the initial surgery. Reoperation was required after 3 months because of a persistent annular abscess. Left atrial repeat reconstruction, annular reconstruction, and mitral valve replacement with a mechanical valve were successfully performed. Technical failure developed early after repairs in 3 patients. A tissue tear developed soon after patch grafting of the anterior mitral leaflet. Repeat repair by suturing autologous pericardium was required. In the patient who underwent simultaneous aortic valve replacement and resection of the strut chordae and rough zone chordae from the left ventricular side of the anterior mitral leaflet due to infection, only chordal reconstructions using ePTFE at the A2 and A3 free margins developed tissue tears at the suture sites of the leaflet and ePTFE sutures. Excess tension on the suture line might develop without strut chordae. Mitral valve replacement was selected at redo surgery. Recurrent MR developed in the patient who underwent chordal reconstruction for anterior leaflet prolapse due to new chordal rupture; another chordal reconstruction was selected. All 7 patients underwent redo surgery successfully. Redo surgery was required in the active IE patients because of instability of the reparative technique within one year. Freedom from reoperation was significantly inferior in the active IE patients (Figure 4). A thromboembolic event developed in 2 patients, and a bleeding event due to anticoagulant therapy occurred in 5 who underwent simultaneous aortic valve replacement with a mechanical valve. Reinfection was detected in only one patient. As a result, freedom from valve-related events was 79.3% ± 4.8% (group A: 94.4% ± 2.4%, group B: 72.6% ± 6.9%, p = 0.010).
Figure 2.
Total survival after mitral valve repair for mitral infective endocarditis.
Figure 3.
Total survival in group A and group B.
Figure 4.
Freedom from reoperation in group A and group B.
Discussion
In this study, 86% of patients who required a surgical intervention for mitral IE underwent mitral valve repair using a wide armamentarium of repair techniques including glutaraldehyde-treated autologous pericardium as leaflet material, ePTFE sutures as chordae tendineae, and annulus remodeling using a prosthetic ring, if required. The surgical treatment of mitral valve IE is primarily determined by valvular and annular destruction. Advanced valvular and annular disease historically required complete excision and valve replacement with/without annular reconstruction. In our consecutive series of mitral IE cases, annular destruction was found only 3 patients, and IE lesions extended to both the anterior and posterior leaflets in 60%. In this setting, mitral valve replacement might be selected without leaflet augmentation. The reoperation rate will be higher in patients undergoing repair for mitral IE due to loss of tissue available for repair and inadequate debridement of the infected tissue. After radical excision of infected mitral valve lesions, some material is theoretically required to reconstruct the leaflets and/or annulus. Glutaraldehyde-treated autologous pericardium is one of the materials used to repair mitral leaflets and it allows us to extend the reparability of mitral IE. Because glutaraldehyde-treated autologous pericardium is very helpful to reduce suture tension on the leaflet or annulus suture line, we aggressively applied it in active IE. Chauvaud and colleagues8 reported valve extension with glutaraldehyde-preserved autologous pericardium in 1991. Freedom from reoperation at 6 years was 79%, and 17% of patients had mitral IE. They concluded that leaflet extension is a simple and safe technique of valve reconstruction, allowing repair of mitral valves that otherwise would need to be replaced. We started to use glutaraldehyde-treated autologous pericardium for several types of mitral pathology including mitral IE in 1991.4 Freedom from reoperation at 10 years was 82% ± 7%. Glutaraldehyde-treated autologous pericardium is a reliable material for mitral valve repair.
In this series, only one patient required early redo surgery due to a tear in the center of the autologous pericardium. Size mismatching and/or mechanical injury of the pericardium at the time of harvesting might be the cause of a tear. Simple closure of the tear was the choice of procedure at redo surgery. Solari and colleagues9 also reported mitral valve repair for active mitral IE. They compared the clinical results of mitral valve repair between 90 patients with a patch and 65 without a patch. Freedom from reoperation at 15 years was 75.6% ± 8.6% in the patch group and 92% ± 4.5% in the no-patch group (p = 0.33). They concluded that an early repair-oriented surgical approach can achieve higher repair rates with good long-term durability of the repair and a very low rate of recurrence of endocarditis. Quinn and colleagues10 recently reported the long-term performance of fresh autologous pericardium for mitral leaflet repair, including 69% of patients with IE. Fresh autologous pericardium is an excellent material for complex mitral leaflet patch repairs, which can be used with the expectation of durable long-term function. There are, however, no comparative studies between treated and fresh autologous pericardium for mitral valve repair. Mitral valve repair is currently recommended for mitral IE “when technically feasible”. It is possible to increase the repair rate in surgery for mitral IE by using autologous pericardium.
Because mitral valve repair in patients with active IE requires complex reparative techniques, the incidence of a second pump run for residual MR may increase. Our incidence of a second pump runs in mitral valve repair for degenerative MR was 6%. In this study, a second pump run was required in 13 (8.8%) patients. All of them fortunately underwent repeat repair successfully after careful analysis of intraoperative transesophageal echocardiography. Quality control of mitral valve repair for IE is necessary to avoid early redo surgery. Our 5 active IE cases who required early redo surgery did not demonstrate residual MR at the first repair. Intravascular hemolysis due to recurrent mild to moderate MR developed in 2 cases, requiring early redo surgery.
Gammie and colleagues11 reported surgical treatment of mitral valve endocarditis in North America. Among 6627 patients requiring mitral surgery for IE between 1994 and 2003, the overall frequency of mitral valve repair for IE was 29.7% (active IE: 15.9%, treated IE: 40.9%) and was associated with a lower risk of mortality. Their results provide support for performing mitral valve repair for IE when technically feasible. As mentioned before, this group expanded the use of fresh autologous pericardium for mitral valve repair.10 Feringa and colleagues12 reviewed 24 reports that included 1194 patients undergoing mitral valve surgery for IE; the repair rate was 39% and mitral valve repair was associated with better clinical outcomes including mortality, reoperation, recurrent endocarditis, and cerebrovascular events. Byrne and colleagues13 reported the surgical management of endocarditis, the Society of Thoracic Surgeons Clinical Practice Guideline, in 2011. In the case of mitral valve endocarditis, mitral valve repair when technically feasible is recommended to treat native mitral valve endocarditis in terms of in-hospital and long-term survival, freedom from recurrent endocarditis, and freedom from reoperation. A systemic review and meta-analysis of mitral valve repair or replacement in 8978 patients with mitral IE demonstrated that mitral valve repair had good clinical outcomes both in-hospital and after 1 and 5 years of follow-up.14
Toyoda and colleagues15 reported real-world outcomes of surgery for native mitral valve endocarditis. They analyzed the clinical outcomes of 1970 patients undergoing isolated primary mitral valve repair (19%) or replacement (81%) for active IE between 1998 and 2010 in New York and California states. Mitral valve repair rates increased from 10.7% to 19.4% over the study period. They also concluded that mitral valve repair is associated with better survival and a low risk of recurrent infection, and should be the surgery of choice when feasible. Lee and colleagues16 reported a nationwide cohort study of mitral valve repair versus replacement for IE. Although the repair rate for mitral IE was 21.2%, statistical analysis by propensity-score matching of 352 patients in each group showed better perioperative and late outcomes in the repair group.
From these systematic reviews and meta-analyses, we recognize that mitral valve repair has the possibility to improve clinical outcomes in mitral surgery for IE. The mitral valve repair strategy, including repair methods for complex valve destruction due to IE, may be improved by continuous educational programs at academic conferences. In the field of mitral valve repair for degenerative MR, progress in materials and procedures, such as ePTFE sutures and intraoperative monitoring by transesophageal echocardiography, has allowed us to standardize the procedures and significantly increase the repair rate. The incidence of mitral valve repair for IE was 15% of the total mitral repair cases between 1991 and 2011 in our institute. In our limited experience of mitral surgery for IE, mitral valve repair using a wide armamentarium of repair techniques was not associated recurrence of infection or recurrence of MR. Hu and Wan17 stated in their review that it is noteworthy that repair strategies involving artificial materials, including ePTFE chordae and prosthetic bands or rings, did not carry an increased reinfection risk even when used in acute IE. Complete resection of infected tissue and complete repair of the function of the mitral complex without residual MR are essential.
This was a retrospective study covering almost 20 years of mitral valve repair for mitral IE at a single institution. Mitral valve repair for mitral IE is a technically demanding procedure and depends on the surgeon’s experience. Most of our patients underwent mitral valve repair by one surgeon. Other surgeons preferred mitral valve replacement in cases requiring emergency surgery due to congestive heart failure during this period. The number of patients in this series was very small, and the pathoanatomical lesions of mitral IE were very heterogenous. The etiology and organisms of mitral IE might be different from those in other countries. In our series, the mean age of the patients was significantly younger than those requiring mitral valve repair for degenerative MR. Younger age encouraged an aggressive approach to increase mitral repair using a wide range of repair techniques. We concluded that mitral valve repair was highly successful using autologous pericardium, ePTFE sutures, and ring annuloplasty if required. Long-term results were acceptable in terms survival, freedom from reoperation, and valve-related event-free survival. Mitral valve repair is recommended for mitral IE in most patients.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Yukikatsu Okada https://orcid.org/0000-0002-2805-8410
References
- 1.Dreyfus G, Serraf A, Jebara VA, et al. Valve repair in acute endocarditis. Ann Thorac Surg 1990; 49: 706–713. [DOI] [PubMed] [Google Scholar]
- 2.Hendren WG, Morris AS, Lytle BW, et al. Mitral valve repair for bacterial endocarditis. J Thorac Cardiovasc Surg 1992; 103: 124–128. [PubMed] [Google Scholar]
- 3.Okada Y, Nasu M, Takahashi Y, et al. Late results of mitral valve repair for mitral regurgitation. Jpn J Thorac Cardiovasc Surg 2003; 51: 282–288. [DOI] [PubMed] [Google Scholar]
- 4.Shomura Y, Okada Y, Nasu M, et al. Late results of mitral valve repair with glutaraldehyde-treated autologous pericardium Ann Thorac Surg 2013; 95: 2000–2005. [DOI] [PubMed] [Google Scholar]
- 5.Okada Y, Nasu M, Shomura T, Yamaura Y, Yoshida K, Yoshikawa J. Mitral valve repair for infectious endocarditis. J Cardiol 1995; 25: 243–246. [PubMed] [Google Scholar]
- 6.Funakoshi S, Kaji S, Okada Y, et al. Impact of early surgery in the active phase on long-term outcomes in left-sided native valve infective endocarditis. J Thorac Cardiovasc Surg 2011; 142: 836–842.e1. [DOI] [PubMed] [Google Scholar]
- 7.Tani T, Okada Y, Kita T, Furukawa Y. Destructive acute infective endocarditis and purulent pericarditis. J Echocardiography 2013; 11: 164–166. [DOI] [PubMed] [Google Scholar]
- 8.Chauvaud S, Jebara V, Perier P, et al. Valve extension with glutaraldehyde-preserved autologous pericardium. Results in mitral valve repair. J Thorac Cardiovasc Surg 1991; 102: 171–177. [PubMed] [Google Scholar]
- 9.Solari S, De Kerchove L, Tamer S, et al. Active infective mitral valve endocarditis: is a repair-oriented surgery safe and durable? Eur J Cardiothorac Surg 2019; 55: 256–262. [DOI] [PubMed] [Google Scholar]
- 10.Quinn RW, Wang L, Foster N, et al. Long-term performance of fresh autologous pericardium for mitral valve leaflet repair. Ann Thorac Surg 2010; 109: 36–41. [DOI] [PubMed] [Google Scholar]
- 11.Gammie JS, O’Brien SM, Griffith BP, Peterson ED. Surgical treatment of mitral endocarditis in North America. Ann Thorac Surg 2005; 80: 2199–2204. [DOI] [PubMed] [Google Scholar]
- 12.Feringa HH, Shaw LJ, Poldermans D, et al. Mitral valve repair and replacement in endocarditis: a systemic review of literature. Ann Thorac Surg 2007; 83: 564–570. [DOI] [PubMed] [Google Scholar]
- 13.Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the Society of Thoracic Surgeons Clinical Practice Guideline. Ann Thorac Surg 2011; 91: 2012–2019. [DOI] [PubMed] [Google Scholar]
- 14.Harky A, Hof A, Garner M, Froghi S, Bashir M. Mitral valve repair or replacement in native valve endocarditis? Systemic review and meta-analysis. J Card Surg 2018; 33: 364–371. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda N, Itagaki S, Anyanwu A, et al. Real-world outcomes of surgery for native mitral valve endocarditis. J Thorac Cardiovasc Surg 2017; 154: 1906–1912.e9. [DOI] [PubMed] [Google Scholar]
- 16.Lee HA, Cheng YT, Wu VC, et al. Nationwide cohort of mitral valve repair versus replacement for infective endocarditis. J Thorac Cardiovasc Surg 2018; 156: 14473–14483.e2. [DOI] [PubMed] [Google Scholar]
- 17.Hu YN, Wan S. Repair of infected mitral valves: what have we learned? Surg Today 2018; 48: 899–908. [DOI] [PubMed] [Google Scholar]