Key Words: in vivo, injury, motor, neurological function, peripheral nerve injury, rat, recovery, regeneration, repair
Abstract
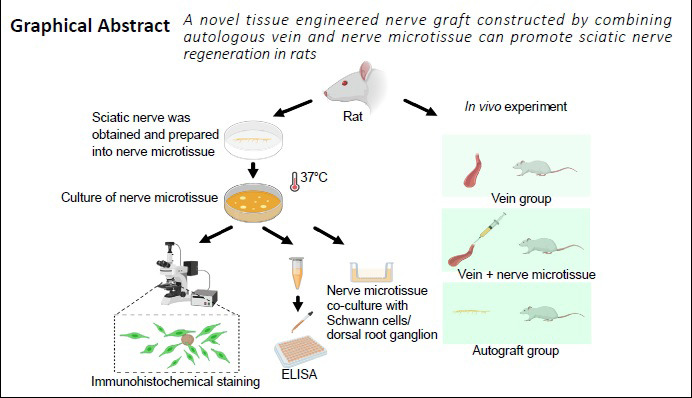
Veins are easy to obtain, have low immunogenicity, and induce a relatively weak inflammatory response. Therefore, veins have the potential to be used as conduits for nerve regeneration. However, because of the presence of venous valves and the great elasticity of the venous wall, the vein is not conducive to nerve regeneration. In this study, a novel tissue engineered nerve graft was constructed by combining normal dissected nerve microtissue with an autologous vein graft for repairing 10-mm peripheral nerve defects in rats. Compared with rats given the vein graft alone, rats given the tissue engineered nerve graft had an improved sciatic static index, and a higher amplitude and shorter latency of compound muscle action potentials. Furthermore, rats implanted with the microtissue graft had a higher density and thickness of myelinated nerve fibers and reduced gastrocnemius muscle atrophy compared with rats implanted with the vein alone. However, the tissue engineered nerve graft had a lower ability to repair the defect than autogenous nerve transplantation. In summary, although the tissue engineered nerve graft constructed with autologous vein and nerve microtissue is not as effective as autologous nerve transplantation for repairing long-segment sciatic nerve defects, it may nonetheless have therapeutic potential for the clinical repair of long sciatic nerve defects. This study was approved by the Experimental Animal Ethics Committee of Chinese PLA General Hospital (approval No. 2016-x9-07) on September 7, 2016.
Chinese Library Classification No. R456; R363; R741
Introduction
Peripheral nerve injury repair is challenging in reconstructive surgery. Autogenous nerve grafts are the gold standard for repairing peripheral nerve defects; however, there are some associated problems, such as loss of function and sensation in the donor area and mismatched nerve tube diameter (Chiu et al., 1982; Rinkel et al., 2013; Wang et al., 2018). Artificial nerve guide conduits can also be used to repair nerve defects (Griffin et al., 2013; Lin et al., 2017; Sun et al., 2018; Quan et al., 2019). Although the conduit eliminates donor-side morbidity, the degradation of the conduit in the body is slow and may result in a rejection reaction (Pabari et al., 2014).
Compared with nerve guide conduits, veins are easy to obtain, have low immunogenicity, and cause less pronounced inflammatory reactions (Braga Silva et al., 2017). Moreover, the outer membrane of veins is rich in collagen, and the middle membrane is rich in laminin, which can promote nerve regeneration (Wang et al., 1993; Tseng et al., 2003). Chiu et al. (1982) showed that autogenous vein grafts can be used as effective conduits for nerve regeneration. Since then, a series of clinical studies have demonstrated that autologous vein grafts can be used to repair peripheral nerves, with satisfactory results (Chiu and Strauch, 1990; Tang et al., 1993). Although vein grafts can be used for nerve regeneration, they are prone to collapse. Therefore, many researchers have sought to prevent collapse by filling the vein with various cells and tissues, such as muscles, tendons and stem cells (Risitano et al., 1989; Raimondo et al., 2005; Wang et al., 2017). Nerve microtissues, which contain Schwann cells (SCs) and neurotrophic factors and can promote nerve growth, are obtained from normal nerves by microscopic sectioning (Terzis and Kostas, 2007; Sahin et al., 2014; Xia and Lv, 2018). In this study, a new type of nerve guide conduit composed of vein and nerve microtissue was designed and used to repair a 10-mm sciatic nerve defect in rats. Regeneration was evaluated by functional, electrophysiological and histopathological examination.
Materials and Methods
Animals and ethics statement
Sprague-Dawley (SD) rats were provided by the Laboratory Animal Research Center of Chinese PLA General Hospital (license No. SCXK (Jing) 2016-002). This study was approved by the Experimental Animal Ethics Committee of Chinese PLA General Hospital (approval No. 2016-x9-07) on September 7, 2016. Five 3-day-old rats were used for nerve microtissue preparation, six 3-day-old rats were used for SC preparation, two 12-hour-old rats were used for dorsal root ganglion (DRG) culture, and 24 adult female rats (8 weeks of age and weighing 220 ± 10 g) were used for in vivo experiments.
Preparation of nerve microtissue
Five 3-day-old SD rats were intraperitoneally injected with 2% sodium pentobarbital solution before operation. The sciatic nerve was removed and cut into 1-mm3 pieces using microscissors (Jinzhong, Shanghai, China). Nerve microtissue was cultured in Dulbecco’s modified Eagle medium/F12 (Corning, Christiansburg, VA, USA) supplemented with 10% fetal bovine serum (Corning), 10 ng/mL heregulin-β1 (Sigma-Aldrich, St. Louis, MO, USA) and 2 mM forskolin (Sigma-Aldrich) in six-well plates in an incubator (Heal Force, Shanghai, China) containing 5% CO2 at 37°C. Then, after 1, 3, 5 or 7 days in culture, enzyme-linked immunosorbent assay (ELISA) and immunofluorescence staining were performed (Figure 1).
Figure 1.
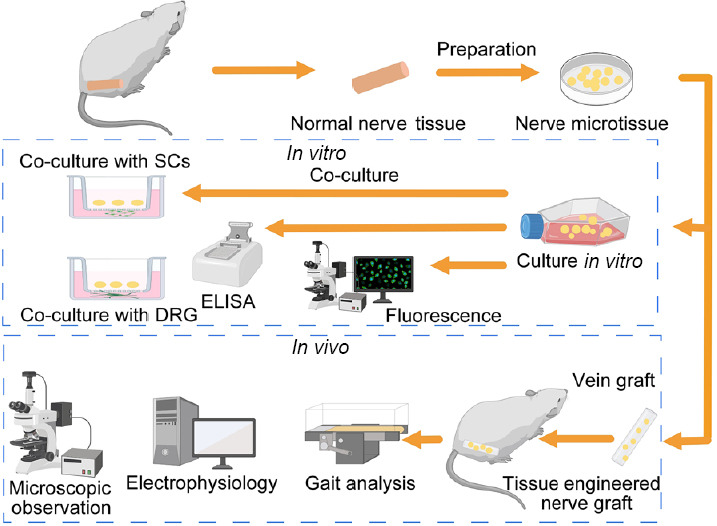
Schematic illustration of the overall study design.
DRG: Dorsal root ganglion; ELISA: enzyme-linked immunosorbent assay; SCs: Schwann cells.
SC or DRG co-culture with nerve microtissue
SCs were harvested from six 3-day-old SD rats as described previously (Gu et al., 2012; Forciniti et al., 2014). Briefly, sciatic nerve was obtained from SD rats and digested with 0.2% NB4 enzyme (SERVA, Baden-Wurttemberg, Germany). SCs were cultured in Dulbecco’s modified Eagle medium/F12 supplemented with 10% fetal bovine serum, 10 ng/mL heregulin-β1 and 2 mM forskolin. After 3 days of culture, the medium was replaced. When the cells reached 80–90% confluency, they were digested with NB4 enzyme and plated in the lower chamber of a 12-transwell plate at 8 × 104 cells/well, and nerve microtissue was placed in the upper chamber of the transwell plate. After co-culture for 1, 3, 5 or 7 days, cell counting kit-8 proliferation assay and immunofluorescence staining were performed. The cell counting kit-8 assay was used to assess Schwann cell proliferation. Briefly, after co-culture for 1, 3, 5 or 7 days, the upper chamber of the transwell plate was removed, and 300 μL phosphate-buffered saline and 30 μL cell counting kit-8 solution (Dojindo, Beijing, China) were added to each lower chamber. After incubation for 2 hours, the absorbance at 450 nm was measured using a trace spectrophotometer (Bio Tek, Winooski, VT, USA). All measurements were independently repeated three times.
For DRG culture, the spinal cords from two 12-hour-old SD rats were collected, and the DRG were carefully excised (Boyer et al. 2015, Quan et al. 2019). Transwell plates were used to co-culture the neural microtissue with dorsal root ganglia for 7 days, and the axon lengths of the dorsal root ganglion were observed. The DRG were placed in the lower chamber of a 12-well transwell plate, and the nerve microtissue was placed in the upper chamber. After 7 days, the insert was carefully removed, fixed with 4% paraformaldehyde, and used for immunofluorescence staining.
ELISA
On days 1, 3, 5 and 7, the media from the cultured nerve microtissues were collected, and the proteins were extracted. Rat nerve growth factor, vascular endothelial growth factor and glial cell line-derived neurotrophic factor were detected using ELISA kits (Boster, Wuhan, China) according to the manufacturer’s protocols, and read on an ELISA plate reader (Bio Tek) at a wavelength of 450 nm.
Immunohistochemistry
Nerve microtissues were fixed with 4% paraformaldehyde for 30 minutes and immunostained with rabbit anti-S100 antibody (1:200; Sigma-Aldrich) overnight at 4°C, followed by incubation with Alexa Fluor-488-conjugated goat anti-rabbit IgG (1:200; Abcam, Cambridge, UK) at room temperature for 2 hours. Finally, samples were mounted with 4′,6-diamidino-2-phenylindole (DAPI) and coverslipped. Images were taken on a fluorescence microscope (Olympus, Tokyo, Japan).
SCs were fixed with 4% paraformaldehyde for 30 minutes and immunostained with rabbit anti-S100 antibody (1:200) overnight at 4°C, followed by incubation with Alexa Fluor-594-conjugated goat anti-rabbit IgG (1:200; Abcam) at room temperature for 2 hours. Finally, samples were mounted with DAPI, coverslipped, and imaged by fluorescence microscopy.
DRGs were fixed with 4% paraformaldehyde for 30 minutes and immunostained with rabbit anti-S100 antibody (1:200) or mouse anti-neurofilament 200 antibody (1:400; Sigma-Aldrich) overnight at 4°C, followed by incubation with Alexa Fluor-488-conjugated goat anti-mouse IgG (1:200) or Alexa Fluor-594-conjugated goat anti-rabbit IgG (1:200) at room temperature for 2 hours. Finally, samples were mounted with DAPI and coverslipped. Images were obtained by fluorescence microscopy. Axon length was measured using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA).
Animal surgery
A total of 24 adult female SD rats underwent surgery at the same time. The surgical procedure has been described previously (Sahin et al., 2014). Briefly, the rats were intraperitoneally injected with 2% sodium pentobarbital solution (30 mg/kg). The right hind area served as the experimental region for surgical procedures and was cleaned with iodine solution. Then, a 10-mm segment of the sciatic nerve was transected and removed distal to the posterior femoral cutaneous nerve. The rats were randomly divided into three groups (n = 8 per group). In the vein group (autologous vein graft), an autologous femoral vein graft, 12 mm in length, was harvested and rinsed with saline solution. The distal and proximal stumps of the femoral vein were reversed and sutured to the ends of the sciatic nerve (the nerve stumps were inserted 1 mm into the autologous vein graft). In the microtissue group (designed tissue engineered nerve graft containing nerve microtissue and vein), a femoral vein graft, 12 mm in length, was harvested and rinsed in saline solution. Then, a 10-mm segment of the sciatic nerve was transected and removed. A 3-mm-long sciatic nerve segment was cut into microtissues with microscissors and re-suspended in 0.1 mL saline solution. Then, the suspension was aspirated into a 1-mL syringe. The proximal stump of the vein graft was sutured to the distal stump of the sciatic nerve with 10-0 nylon monofilament threads, and the nerve microtissue suspension was evenly injected into the vein conduit from the other end. The other stump of the vein graft was sutured to the proximal side of the sciatic nerve with 10-0 nylon monofilament threads. Each of the nerve stumps was inserted 1 mm into the vein graft. In the autograft group, (autologous nerve graft), inverted autogenous nerve was sutured to the stumps of the nerves with four 10-0 nylon monofilament threads, and the wound was sutured with 3-0 nylon threads. The rats were given food and water after waking from anesthesia.
Ultrasound examination
Twelve weeks after surgery, the surface projection area of the sciatic nerve on the side of the operation was fully exposed, and the size, echo and continuity of the nerve graft were observed using a Toshiba Apolio 500 color ultrasonic diagnostic instrument (Toshiba, Tokyo, Japan). The sciatic nerve was examined with an ultrasound diagnostic instrument, and the proximal and distal ends of the nerve grafts were identified, and the inner diameter of the middle segment of the nerve grafts in each group were measured (n = 8 rats in each group). Then, the mean inner diameter was calculated for analysis.
Functional evaluation
At 4, 8 and 12 weeks (n = 8 rats in each group) following nerve reconstruction, functional motor recovery was assessed with the CatWalk gait analysis system (Noldus, Wageningen, Netherlands). The rat was placed on the catwalk plate and allowed to walk forward. Real-time movie images and 3D footprints were displayed when the toes touched the stepping surface. The length and width of the toes and the static sciatic index (SFI) were measured from the footprint in each group. SFI = 0 indicates normal motor function, and SFI = –100 indicates complete loss of motor function.
Electrophysiological assessment
At 12 weeks after surgery, the function of the sciatic nerve was evaluated using electrophysiology as previously de-scribed (Sanli et al., 2019). In brief, the sciatic nerve (n = 8 rats per group) on the injured and uninjured sides was re-exposed under anesthesia with sodium phenobarbital. An electromyography machine (Medtronic, Minneapolis, MN, USA) was used for the evaluation. Stimulation electrodes were placed on the proximal and distal ends of the regenerated nerve, and recording electrodes were placed on the ventral side of the same gastrocnemius muscle to record the combined muscle action potentials (CMAPs) after electrical stimulation. The peak amplitude and latency of CMAPs were recorded to assess the recovery of nerve conduction function. Meanwhile, the peak amplitude and latency of CMAPs of the contralateral normal sciatic nerve were recorded for the standardization of the data.
Immunohistochemical staining
At 12 weeks after surgery, five rats in each group were killed, and the nerve graft was removed and cut into 7-µm-thick transverse sections. The sections were incubated with rabbit anti-S100 antibody (1:200) or mouse anti-neurofilament 200 antibody (1:400) at 4°C overnight, followed by incubation with goat anti-rabbit antibody (1:200; Abcam) and goat anti-mouse antibody (1:200; Abcam) for 2 hours at room temperature. Finally, the samples were incubated with DAPI and observed under a fluorescence microscope (Olympus). Immunohistochemistry with anti-S100 and anti-neurofilament 200 was performed to evaluate Schwann cell and axon regrowth, respectively. Quantitative analysis of regenerated myelinated axons in the intermediate and distal region of the graft was measured using Image-Pro Plus by selecting eight random images in each group.
Electron microscopy analysis
The intermediate nerve segment samples were combined for nerve histology after 12 weeks (n = 3 rats per group). Tissues were subjected to an initial fixation with 3% glutaraldehyde for 3 hours, followed by incubation in 1% osmium tetroxide solution for 1.5 hours. After dehydration, 1-µm transverse sections were cut and stained with toluidine blue. Eight randomly selected fields for each group were captured to measure the density of myelinated nerve fibers using Image-Pro Plus. The diameter of myelinated fibers and the thickness of the myelin sheath were examined under a transmission electron microscope (HT7700; Hitachi, Tokyo, Japan) and calculated using Image-Pro Plus by selecting eight random images in each group.
Muscle wet-weight analysis and histological evaluation
The rats were sacrificed after 12 weeks, and the gastrocnemius muscles of both lower limbs were excised, washed with saline, and weighed to calculate the muscle weight ratio (injured side vs. uninjured side) (n = 8 rats per group). The gastrocnemius muscle belly was fixed with 4% paraformaldehyde for 2 days, and then cut into 7-µm-thick sections. After dewaxing, the sections were stained with a modified Masson staining kit (Solarbio, Beijing, China), followed by dehydration, clearing and mounting. Eight randomly selected fields for each group were captured to measure the average cross-sectional area of the gastrocnemius muscle with Image-Pro Plus.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). SPSS Statistics ver. 18.0 (SPSS, Chicago, IL, USA) was used for data analysis. Comparisons of different groups were performed by Student’s t-test or one-way analysis of variance followed by Tukey’s post hoc test or Dunnett’s T3 post hoc test. P-values < 0.05 were considered statistically significant.
Results
Morphology and identification of nerve microtissue
When the nerve microtissue was cultured in vitro for 1, 3, 5 and 7 days, a large number of cells were observed proliferating around the nerve microtissue, and the microtissue itself gradually dissipated. As shown in Figure 2A, immunofluorescence showed that the proliferated cells were positive for S100, confirming that they were Schwann cells. ELISA for nerve growth factor, vascular endothelial growth factor and glial cell line-derived neurotrophic factor in the media of nerve microtissue showed a gradual increase after 3 days (Figure 2B).
Figure 2.
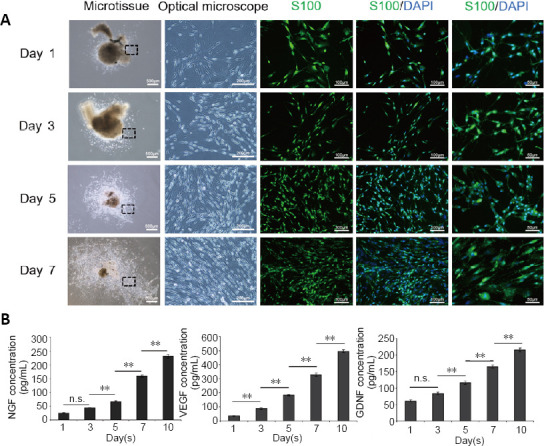
Preparation and detection of nerve microtissue.
(A) The cells proliferating from the microtissues were stained with S100 and DAPI, which confirmed that they were Schwann cells. The optical microscope images show enlarged views of the area in the black rectangle. With increasing time in culture, the proliferation of cells around the nerve microtissue increased gradually, and the morphology was still long and narrow. Scale bars: 500 μm in the microtissue column, 200 μm in the optical microscope column, 100 μm in other columns. (B) The enzyme-linked immunosorbent assay results showed that nerve microtissues can secrete large amounts of NGF, VEGF and GDNF (n = 3 independently repeated assay for each group). Data are expressed as the mean ± SD. **P < 0.01 (one-way analysis of variance followed by Tukey's post hoc test). DAPI: 4',6-Diamidino-2-phenylindole; GDNF: glial cell line-derived neurotrophic factor; NF200: neurofilament 200; NGF: nerve growth factor; n.s.: not significant; VEGF: vascular endothelial growth factor.
The proliferation of SCs and DRGs after co-culture with nerve microtissue
SCs and nerve microtissues were cultured together for different durations. The proliferation rate of SCs was higher when co-cultured with nerve microtissues compared with SC monocultures (Figure 3A). These results were corroborated by the cell counting kit-8 assay (global P < 0.05; Figure 3B). Similarly, after 7 days of DRG and nerve microtissue co-culture, DRG axon length was longer compared with monoculture (P < 0.01; Figure 3C and D).
Figure 3.
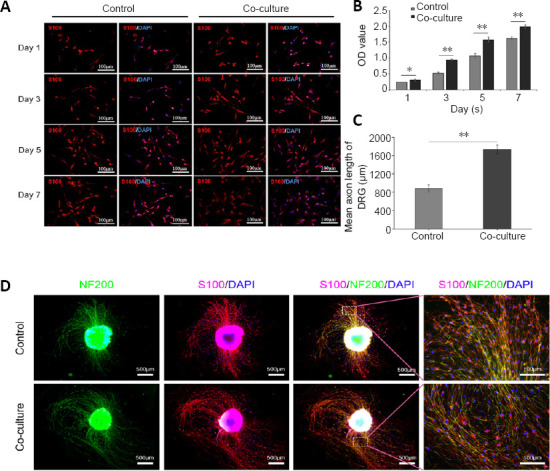
The proliferation of SCs and DRGs after co-culture with nerve microtissue.
(A) SCs and nerve microtissues were cultured together for different periods of time. The proliferation rate of SCs was greater in co-culture than in single culture. S100 expression is in red (Alexa Fluor-594). (B) Cell counting kit-8 proliferation assay also showed that the SC proliferation rate increased when co-cultured with nerve microtissue (global P < 0.05; n = 3 independently repeated assays per group). (C, D) After 7 days of co-culture of DRGs and nerve microtissue, the axon lengths of the nerve microtissue was greater compared with single culture (P < 0.01; n = 3 independently repeated assays per group). S100 expression is in red (Alexa Fluor-594), and NF200 expression is in green (Alexa Fluor-488). Scale bars: 100 μm in A and D right column, 500 μm in D other columns. Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 (Student’s t-test). DAPI: 4′,6-Diamidino-2-phenylindole; DRG: dorsal root ganglion; NF200: neurofilament 200; OD: optical density; SCs: Schwann cells.
Ultrasound structure of nerve grafts in the sciatic nerve
At 12 weeks after surgery, ultrasound was used to examine the continuity and shape of nerve grafts. The nerve grafts of each group were linearly parallel with a strong echo. The transverse section showed a circular medium echo structure with a spot-shaped strong echo in the center. The position of the nerve graft was relatively constant, without movement of muscles, tendons or ligaments, and no surrounding edema. The average internal diameter in the vein group was 0.30 ± 0.06 mm, which was significantly smaller compared with the microtissue group (P < 0.01), while there was no significant difference between the average internal diameter in the microtissue and autograft groups (P > 0.05; Figure 4). Ultrasound revealed that the continuity of nerve grafts was good in each group. However, compared with the vein group, the microtissue group and autograft group had a larger inner nerve diameter.
Figure 4.
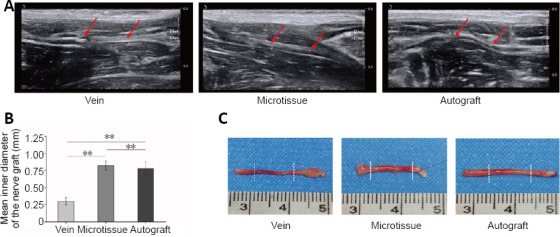
Ultrasound examination and gross appearance of the sciatic nerve at 12 weeks after surgery.
(A) Using ultrasonic examination of nerve graft, it was found that nerve continuity was good in all three groups, and no edema was observed around the nerve. However, it was smaller in diameter in the vein group compared with the microtissue and autograft groups. The junction (red arrows) at both ends of the graft can be seen under ultrasonic examination. (B) The mean inner diameter of the nerve graft in each group (n = 8 rats per group). Data are expressed as the mean ± SD. **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test). (C) The gross morphology of the sciatic nerve in each group. Similar to the ultrasound results, the diameter of nerve grafts in the vein group was smaller than that in the microtissue group and the autograft group. The two stumps of the nerve graft, indicated by the white dashed lines, are shown in the general view.
Recovery of sciatic nerve function in rats with vein/nerve microtissue hybrid scaffold graft
Behavioral evaluation was performed using the CatWalk system. Twelve weeks after surgery, compared with the vein group, the microtissue group showed an improved degree of toe separation, comparable to the autograft group. The 3D footprints showed that the contact area between the toe and the ground on the operative side (right side) was decreased in each group compared with the normal side (left side). However, the length and width of the toe prints on the operative side in the microtissue group were improved compared with the vein group, and were similar to those in the autograft group. Sciatic nerve functional recovery in rats was evaluated using the SFI. The walking track analysis results are presented in Figure 5. All groups displayed a significant decline in SFI before 4 weeks after surgery, indicating a gradual deterioration of nerve function after surgery. After 4 weeks, the SFI began to increase as nerve regeneration proceeded. After 4 weeks, the microtissue group began to show better neurological functional recovery than the other groups, with an SFI that was significantly better than that in the vein group (P < 0.01), which was similar to that in the autograft group.
Figure 5.
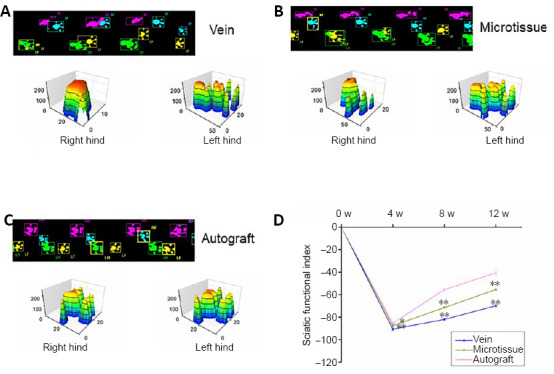
Recovery of sciatic nerve function in rats given vein/nerve microtissue hybrid scaffold graft.
(A–C) Real-time movie images and 3D footprints in the vein, microtissue, and autograft groups at 12 weeks after transplantation. In the 2D figures, blue represents the right forelimb, yellow represents the left forelimb, pink represents the right hind limb (operative side), and green represents the left hind limb (normal side). The 3D footprints show that the contact area between the toe and the ground on the operative side (right side) was decreased in each group compared with the normal side (left side). However, the length and width of the toe prints on the operative side in the microtissue group were improved compared with the vein group and similar to the autograft group. (D) The static sciatic index values at different time points after surgery (n = 8 for each group per time point). Data are expressed as the mean ± SD. **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test).
Electrophysiological recovery in rats with vein/nerve microtissue hybrid scaffold graft
At 12 weeks after surgery, electromyography was performed on the various transplant groups. Following stimulation at the proximal or distal end of the injured nerve, the CMAP amplitude ratio was significantly higher in the microtissue and the autograft groups compared with the vein group (P < 0.01). The CMAP latency ratio showed the same trend (P < 0.01), with the CMAP latency ratio in the autograft and the microtissue groups significantly smaller than that in the vein group (P < 0. 01), reflecting the role of nerve microtissue in promoting the recovery of nerve conduction function (Figure 6).
Figure 6.
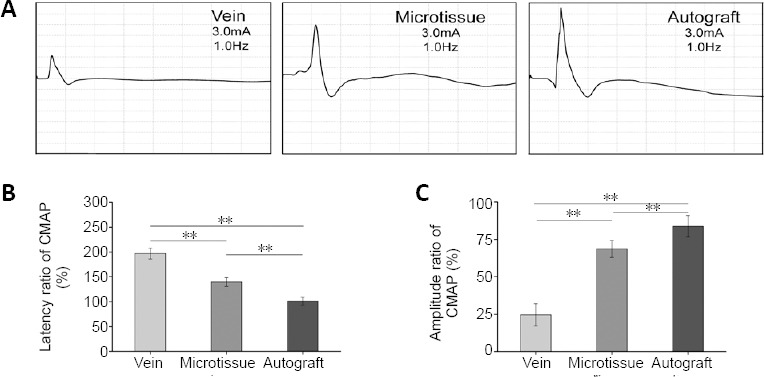
Electrophysiological recovery in rats with vein/nerve microtissue hybrid scaffold transplant at 12 weeks post-surgery.
(A) Representative CMAP recordings for the vein, microtissue and autograft groups, respectively. (B) Latency ratio of CMAP (injury side/normal side) (n = 8 rats per group). (C) Amplitude ratio of CMAP (injury side/normal side) (n = 8 rats per group). Data are expressed as the mean ± SD. **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test). CMAP: Compound muscle action potential.
Morphology of regenerated nerves in rats with vein/nerve microtissue hybrid scaffold transplant 12 weeks post-surgery
Immunohistochemistry showed that the regenerated axons grew from the proximal end to the distal end, and the regenerated nerve grew in the wall of the vein (Figure 7A). In each group, there was no evidence that the venous valve hindered the growth of the nerve. The middle and distal segments of the graft in each group were selected for sectioning (Figure 7B). The density of middle and distal regenerated nerve fibers was greater in the microtissue and autograft groups compared with the vein group (P < 0.05; Figure 7C).
Figure 7.
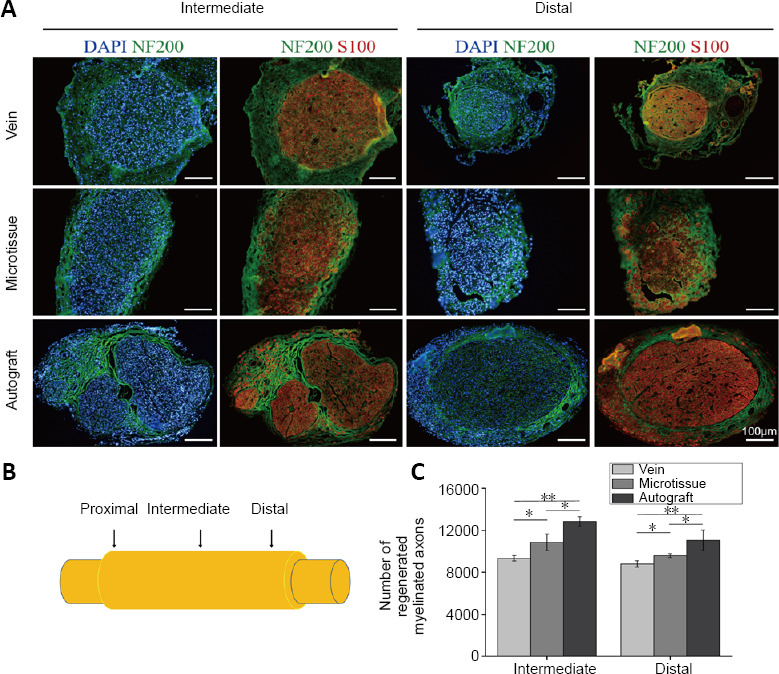
Immunofluorescence staining of nerve grafts in rats with vein/nerve microtissue hybrid scaffold transplant at 12 weeks post-surgery.
(A) Labeling of histological cross-sections for S100 (red, Alexa Fluor-594), NF200 (green, Alexa Fluor-488) and DAPI (blue) in rats implanted with each nerve guide conduit. Scale bars: 100 μm. (B) Schematic diagram of the nerve graft. (C) Total number of regenerated myelinated axons in the intermediate and distal region of a graft in the three groups at 12 weeks post-surgery (n = 8 randomly selected fields for each group). Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test). DAPI: 4′,6-Diamidino-2-phenylindole; NF200: neurofilament 200.
Effect of vein/nerve microtissue hybrid scaffold graft on the ultrastructure of the injured sciatic nerve
Toluidine blue staining and microscopic observation of myelinated fibers in the different groups at 12 weeks post-surgery are shown in Figure 8A. The mean density of myelinated nerve fibers in the microtissue group was significantly higher than that in the vein group (P < 0.01). Similarly, the average diameter and average thickness of myelinated nerve fibers in the middle of the nerve graft were significantly greater in the microtissue group than in the vein group, but slightly lower than in the autograft group (Figure 8B–D).
Figure 8.
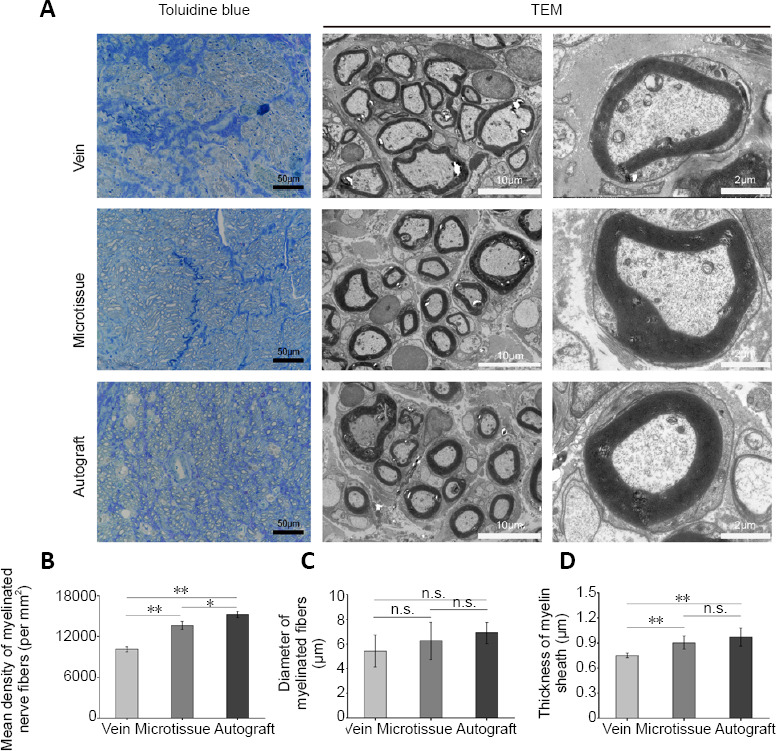
Morphometric analyses of the middle sciatic nerve in rats with vein/nerve microtissue hybrid scaffold transplant at 12 weeks post-surgery.
(A) Toluidine blue staining and transmission electron microscope (TEM) images of the regenerated nerves in the middle segment of the nerve. Scale bars: 50 μm (left), 10 μm (middle), and 2 μm (right). (B) The mean density of myelinated nerve fibers (n = 8 randomly selected fields per group). (C) The diameter of myelinated fibers (n = 8 randomly selected fields per group). (D) The thickness of the myelin sheath (n = 8 randomly selected fields per group). Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test).
Effects of vein/nerve microtissue hybrid scaffold graft on the morphology and structure of the gastrocnemius muscle
Twelve weeks after transplant, atrophy of the gastrocnemius muscles was more severe in the vein group than in the other groups. Statistical analysis of the recovery rate of the wet weight of the gastrocnemius muscle demonstrated muscle atrophy in all groups (Figure 9B). The microtissue group had a significantly higher wet-weight recovery rate than the vein group (P < 0.01), but it was still significantly lower than in the autograft group (P < 0.01). Masson’s trichrome staining revealed fewer blue-dyed collagen fibers in the microtissue group, and the cross-sectional area of the muscle fibers was significantly larger compared with the vein group (P < 0.01; Figure 9A and C).
Figure 9.
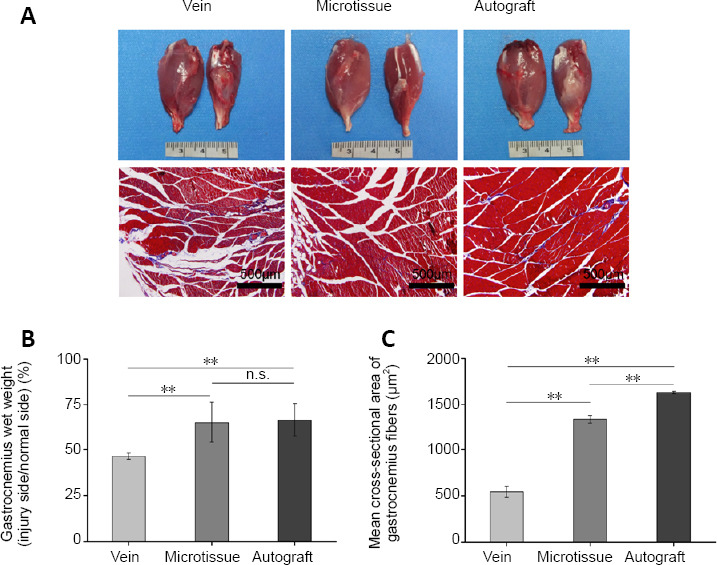
Gastrocnemius muscle weight ratio in rats with vein/nerve microtissue hybrid scaffold graft at 12 weeks post-surgery.
(A) The general view and Masson staining of the gastrocnemius muscle. The left gastrocnemius muscle represents the healthy side and the right gastrocnemius muscle represents the operative side. The atrophy of the gastrocnemius muscles on the operative side in the vein group was more severe than in the other groups. Masson staining showed that the muscle fiber area of the Vein group was also smaller than that of the other groups. Scale bars: 500 μm. (B) The gastrocnemius wet weight (n = 8 randomly selected fields per group). (C) The mean cross-sectional area of gastrocnemius fibers (n = 8 randomly selected fields per group). Data are expressed as the mean ± SD. **P < 0.01 (one-way analysis of variance followed by Tukey’s post hoc test).
Discussion
Vein tubes have been widely used as nerve conduits for nerve regeneration for many years (Chiu et al., 1982; Manoli et al., 2014; Eren et al., 2018). Veins are nonimmunogenic, with less pronounced inflammatory responses, and can be obtained in a variety of sizes. They can also provide mechanical support for regenerating axon growth cones and a protected microenvironment for nerve regeneration (Meng et al., 2017). Nerve microtissue, which contains large numbers of Schwann cells and high levels of nerve growth factor, can effectively promote nerve regeneration.
Both vein graft and nerve microtissue have been shown to promote nerve regeneration. However, the combination of vein graft and nerve microtissue as a new tissue engineered nerve graft has been rarely reported. Therefore, in this study, we compared the effectiveness of single and combined vein graft and nerve microtissue to repair the sciatic nerve in rats. The combined strategy yielded better repair. It avoided vein graft collapse, ensured the vein graft had a larger inner diameter, improved SFI, reduced the latency ratio of the CMAP, increased the amplitude ratio of the CMAP, increased the number of myelinated fibers, axon diameter and thickness of myelinated fibers, and reduced atrophy of the gastrocnemius muscle.
Nerve regeneration requires a conducive microenvironment, including sufficient Schwann cells, neurotrophic factors and extracellular matrix. Sahin et al. (2014) used nerve microtissue to fill the nerve guide tube to repair a 10-mm nerve defect in rats, and showed that the microtissues could increase the number of myelinated fibers, increase the nerve function index, and promote the repair of peripheral nerves, similar to autologous nerve transplantation. D’Arpa et al. (2018) sutured autologous nerve microtissues to the distal stump of the sciatic nerve, which enhanced the ability of autologous nerve grafts to repair sciatic nerve defects. Terzis and Kostas (2007) reported two clinical cases of brachial plexus nerve injury repair in children with nerve microtissues. Postoperatively, shoulder joint flexion and extension were good in both cases, and nerve function recovered similarly to normal. We conjectured that microtissues consisting of SCs would provide a microenvironment containing a variety of nerve growth factors and exhibit a strong ability to support nerve regeneration. In future experiments, we will analyze the secretion of nerve growth factors by the microtissues and the migration of Schwann cells, and we will use this combined strategy to repair an 80-mm nerve defect in dogs.
Vein grafts can be obtained from many parts of the body. They are plentiful, inexpensive, and relatively less invasive than nerve grafts (Sabongi et al., 2015; Stößel et al., 2018). Furthermore, venous walls are elastic enough to support axon growth (Ramli et al., 2019). When using a vein to bridge a nerve, the wall of the vein and the outer membrane of the nerve are stitched. Then, the wall of the vein is clamped with two microscopic pinchers, which are gently pulled outward to expand the diameter of the tube. At this time, the broken end of the nerve can be fully inserted in the venous cavity so that it can grow completely in the vein, which we believe is key to nerve regeneration. Although the femoral vein was removed here, we found that within 2 days after removal of the femoral vein, the lower extremities developed mild congestion over time. Perhaps because of the compensatory effect of superficial veins, this symptom gradually alleviates.
There are several risks with veins as nerve conduits. One problem is the presence of venous valves, and another is the possibility of lumen collapse, which may hinder nerve growth (Sabongi et al., 2015). The vein conduit can be pulled inside-out to invert the normal orientation, placing the adventitial layer within the lumen (Wang et al., 1993, 1995). Another solution is to fill the veins to prevent collapse and inhibit the blocking effect of the valves (Sabongi et al., 2014, 2015). Attempts have been made to maintain conduit patency using muscle, extracellular matrix proteins, and minced nerve tissue inside the lumen (Glasby et al., 1986; Gravvanis et al., 2004; Terzis and Kostas, 2007; Tos et al., 2007; Sahin et al., 2014). We inverted the distal end of the vein to allow the axon to grow along the valve, thereby preventing the valve from obstructing the process. We found that there was no graft collapse in either group, and the microtissue group had a greater diameter and more new myelin than the vein group. These neuroactive microtissues contain many Schwann cells, which can proliferate and provide a guide for axons. We found that the microtissue decomposed slowly over time without hindering the growth of regenerating axons in the nerve tubes.
A limitation of this study is that the repaired nerve defect was only 10 mm long, and the feasibility of using this approach to repair long-distance defects remains to be studied. Terzis and Kostas (2007) used this technique to repair a 22-cm brachial plexus defect; however, a venous graft exceeding 30 mm was prone to collapse, thereby preventing nerve regeneration. Therefore, further experiments are needed to assess effectiveness. Compared with other nerve guide conduits, there are abundant sources of nerve microtissues and vein grafts, and the microtissues can promote nerve regeneration. Therefore, our method is a potential new strategy for treating nerve defects in the clinic. The expression of neurotrophic factors in the sections of the graft/sciatic nerve and the role of inhibitory factors will be studied and discussed in future study. In summary, in this study, a new type of tissue engineered nerve graft was constructed using bioactive microtissues dissected from normal nerves combined with vein as the nerve conduit. In in vitro experiments, we found that nerve microtissues induced the proliferation of Schwann cells and the secretion of a large number of neurotrophic factors. The nerve graft was then used to repair sciatic nerve defects in rats. Ultrasound, gait analysis, electrophysiology and histochemical staining showed that the autologous vein graft containing nerve microtissues was more effective than the vein graft alone for nerve repair. Our findings demonstrate that the combination of nerve microtissues and vein in a hybrid scaffold is a novel design that supports peripheral nerve regeneration.
Footnotes
Conflicts of interest: The authors declare that there is no conflict of interests.
Financial support: This work was supported by the National Natural Science Foundation of China, Nos. 31771052 (to YW), 81671684 (to YXW), 81871788 (to CZ); National Key Research and Development Program of China, Nos. 2017YFA0104702, 2017YFA0104703the Natural Science Foundation of Beijing of China, No. 7172202 (to YW); PLA Youth Training Project for Medical Science of China, No. 16QNP144 (to YW), the Project for Science and Technology Leader of Anhui Province of China, No. 2018H177 (to CZ); Funding of “Panfeng” Innovation Team Project for Scientifc Research of Yijishan Hospital, Wannan Medical College, China, No. PF2019007 (to HGX); Funding of “Peak” Training Program for Scientifc Research of Yijishan Hospital, Wannan Medical College, China, No. GF2019T02 (to HGX). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Institutional review board statement: This study was approved by the Experimental Animal Ethics Committee of Chinese PLA General Hospital (approval No. 2016-x9-07) on September 7, 2016.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 31771052 (to YW), 81671684 (to YXW), 81871788 (to CZ); National Key Research and Development Program of China, Nos. 2017YFA0104702, 2017YFA0104703; the Natural Science Foundation of Beijing of China, No. 7172202 (to YW); PLA Youth Training Project for Medical Science of China, No. 16QNP144 (to YW), the Project for Science and Technology Leader of Anhui Province of China, No. 2018H177 (to CZ); Funding of “Panfeng” Innovation Team Project for Scientifc Research of Yijishan Hospital, Wannan Medical College, China, No. PF2019007 (to HGX); Funding of “Peak” Training Program for Scientifc Research of Yijishan Hospital, Wannan Medical College, China, No. GF2019T02 (to HGX).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Patel D, Yu J, Song CP; T-Editor: Jia Y
References
- 1.Braga Silva J, Marchese GM, Cauduro CG, Debiasi M. Nerve conduits for treating peripheral nerve injuries: A systematic literature review. Hand Surg Rehabil. 2017;36:71–85. doi: 10.1016/j.hansur.2016.10.212. [DOI] [PubMed] [Google Scholar]
- 2.Chiu DT, Strauch B. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast Reconstr Surg. 1990;86:928–934. doi: 10.1097/00006534-199011000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Chiu DT, Janecka I, Krizek TJ, Wolff M, Lovelace RE. Autogenous vein graft as a conduit for nerve regeneration. Surgery. 1982;91:226–233. [PubMed] [Google Scholar]
- 4.D’Arpa S, Zabbia G, Cannizzaro C, Salimbeni G, Plescia F, Mariolo AV, Cassata G, Cicero L, Puleio R, Martorana A, Moschella F, Cordova A. Seeding nerve sutures with minced nerve-graft (MINE-G): a simple method to improve nerve regeneration in rats. Acta Chir Belg. 2018;118:27–35. doi: 10.1080/00015458.2017.1353237. [DOI] [PubMed] [Google Scholar]
- 5.Eren A, Atalar H, Seymen CM, Alpaslan Pınarlı F, Take Kaplanoglu G, Turanlı S. Sutureless approach with vein grafts and mesen-chymal stem cells in primary nerve repair: Functional and immunohistological results. Microsurgery. 2018;38:780–789. doi: 10.1002/micr.30315. [DOI] [PubMed] [Google Scholar]
- 6.Forciniti L, Ybarra J, 3rd, Zaman MH, Schmidt CE. Schwann cell response on polypyrrole substrates upon electrical stimulation. Acta Biomater. 2014;10:2423–2433. doi: 10.1016/j.actbio.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Glasby MA, Gschmeissner SE, Huang CL, De Souza BA. Degenerated muscle grafts used for peripheral nerve repair in primates. J Hand Surg Br. 1986;11:347–351. doi: 10.1016/0266-7681(86)90155-5. [DOI] [PubMed] [Google Scholar]
- 8.Gravvanis AI, Tsoutsos DA, Tagaris GA, Papalois AE, Patralexis CG, Iconomou TG, Panayotou PN, Ioannovich JD. Beneficial effect of nerve growth factor-7S on peripheral nerve regeneration through inside-out vein grafts: an experimental study. Microsurgery. 2004;24:408–415. doi: 10.1002/micr.20055. [DOI] [PubMed] [Google Scholar]
- 9.Griffin JW, Hogan MV, Chhabra AB, Deal DN. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 10.Gu Y, Ji Y, Zhao Y, Liu Y, Ding F, Gu X, Yang Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials. 2012;33:6672–6681. doi: 10.1016/j.biomaterials.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Lin SC, Wang Y, Wertheim DF, Coombes AGA. Production and in vitro evaluation of macroporous, cell-encapsulating alginate fibres for nerve repair. Mater Sci Eng C Mater Biol Appl. 2017;73:653–664. doi: 10.1016/j.msec.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Manoli T, Schulz L, Stahl S, Jaminet P, Schaller HE. Evaluation of sensory recovery after reconstruction of digital nerves of the hand using muscle-in-vein conduits in comparison to nerve suture or nerve autografting. Microsurgery. 2014;34:608–615. doi: 10.1002/micr.22302. [DOI] [PubMed] [Google Scholar]
- 13.Meng WK, Huang Z, Tan Z, Li L, Guo Q, Zhang Z, Wu CX, Liu L, Huang FG, Wang GL. Vein nerve conduit supported by vascular stent in the regeneration of peripheral nerve in rabbits. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:687–692. [PubMed] [Google Scholar]
- 14.Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A. Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg. 2014;133:1420–1430. doi: 10.1097/PRS.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 15.Quan Q, Meng H, Chang B, Hong L, Li R, Liu G, Cheng X, Tang H, Liu P, Sun Y, Peng J, Zhao Q, Wang Y, Lu S. Novel 3-D he-lix-flexible nerve guide conduits repair nerve defects. Biomaterials. 2019;207:49–60. doi: 10.1016/j.biomaterials.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Raimondo S, Nicolino S, Tos P, Battiston B, Giacobini-Robecchi MG, Perroteau I, Geuna S. Schwann cell behavior after nerve repair by means of tissue-engineered muscle-vein combined guides. J Comp Neurol. 2005;489:249–259. doi: 10.1002/cne.20625. [DOI] [PubMed] [Google Scholar]
- 17.Ramli K, Gasim AI, Ahmad AA, Htwe O, Mohamed Haflah NH, Law ZK, Hasan S, Naicker AS, Mokhtar SA, Muhamad Ariffin MH, Baharudin A, Tan GC, Haji Idrus R, Abdullah S, Ng MH. Efficacy of human cell-seeded muscle-stuffed vein conduit in rat sciatic nerve repair. Tissue Eng Part A. 2019;25:1438–1455. doi: 10.1089/ten.TEA.2018.0279. [DOI] [PubMed] [Google Scholar]
- 18.Rinkel WD, Huisstede BM, van der Avoort DJ, Coert JH, Hovius SE. What is evidence based in the reconstruction of digital nerves. A systematic review? A systematic review J Plast Reconstr Aesthet Surg. 2013;66:151–164. doi: 10.1016/j.bjps.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Risitano G, Cavallaro G, Lentini M. Autogenous vein and nerve grafts: a comparative study of nerve regeneration in the rat. J Hand Surg Br. 1989;14:102–104. doi: 10.1016/0266-7681(89)90027-2. [DOI] [PubMed] [Google Scholar]
- 20.Sabongi RG, Fernandes M, Dos Santos JB. Peripheral nerve regeneration with conduits: use of vein tubes. Neural Regen Res. 2015;10:529–533. doi: 10.4103/1673-5374.155428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabongi RG, De Rizzo LA, Fernandes M, Valente SG, Gomes dos Santos JB, Faloppa F, Leite VM. Nerve regeneration: is there an alternative to nervous graft. J Reconstr Microsurg. 2014;30:607–616. doi: 10.1055/s-0034-1372477. [DOI] [PubMed] [Google Scholar]
- 22.Sahin C, Karagoz H, Kulahci Y, Sever C, Akakin D, Kolbasi B, Ulkur E, Peker F. Minced nerve tissue in vein grafts used as conduits in rat tibial nerves. Ann Plast Surg. 2014;73:540–546. doi: 10.1097/SAP.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 23.Sanli E, Dincel GC, Umay E. Effect of local and systemic dimethylsulfoxide on peripheral nerve repair: a controlled randomized experimental study. J Invest Surg. 2019;1:12. doi: 10.1080/08941939.2019.1644403. [DOI] [PubMed] [Google Scholar]
- 24.Stößel M, Wildhagen VM, Helmecke O, Metzen J, Pfund CB, Freier T, Haastert-Talini K. Comparative evaluation of chitosan nerve guides with regular or increased bendability for acute and delayed peripheral nerve repair: a comprehensive comparison with autologous nerve grafts and muscle-in-vein grafts. Anat Rec (Hoboken) 2018;301:1697–1713. doi: 10.1002/ar.23847. [DOI] [PubMed] [Google Scholar]
- 25.Sun X, Wang Y, Guo Z, Xiao B, Sun Z, Yin H, Meng H, Sui X, Zhao Q, Guo Q, Wang A, Xu W, Liu S, Li Y, Lu S, Peng J. Acellular cauda equina allograft as main material combined with biodegradable chitin conduit for regeneration of long-distance sciatic nerve defect in rats. Adv Healthc Mater. 2018;7:e1800276. doi: 10.1002/adhm.201800276. [DOI] [PubMed] [Google Scholar]
- 26.Tang JB, Gu YQ, Song YS. Repair of digital nerve defect with autogenous vein graft during flexor tendon surgery in zone 2. J Hand Surg Br. 1993;18:449–453. doi: 10.1016/0266-7681(93)90144-5. [DOI] [PubMed] [Google Scholar]
- 27.Terzis JK, Kostas I. Vein grafts used as nerve conduits for obstetrical brachial plexus palsy reconstruction. Plast Reconstr Surg. 2007;120:1930–1941. doi: 10.1097/01.prs.0000287391.12943.00. [DOI] [PubMed] [Google Scholar]
- 28.Tos P, Battiston B, Nicolino S, Raimondo S, Fornaro M, Lee JM, Chirila L, Geuna S, Perroteau I. Comparison of fresh and pre-degenerated muscle-vein-combined guides for the repair of rat median nerve. Microsurgery. 2007;27:48–55. doi: 10.1002/micr.20306. [DOI] [PubMed] [Google Scholar]
- 29.Tseng CY, Hu G, Ambron RT, Chiu DT. Histologic analysis of Schwann cell migration and peripheral nerve regeneration in the autogenous venous nerve conduit (AVNC) J Reconstr Microsurg. 2003;19:331–340. doi: 10.1055/s-2003-42502. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Lu CF, Peng J, Hu CD, Wang Y. Roles of neural stem cells in the repair of peripheral nerve injury. Neural Regen Res. 2017;12:2106–2112. doi: 10.4103/1673-5374.221171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Ding X, Zhang J, Song X, Wu Y, Svensson P, Wang K. Somatosensory changes at forearm donor sites following three different surgical flap techniques. Int J Surg. 2018;53:326–332. doi: 10.1016/j.ijsu.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Wang KK, Costas PD, Bryan DJ, Jones DS, Seckel BR. Inside-out vein graft promotes improved nerve regeneration in rats. Microsurgery. 1993;14:608–618. doi: 10.1002/micr.1920140914. [DOI] [PubMed] [Google Scholar]
- 33.Wang KK, Costas PD, Bryan DJ, Eby PL, Seckel BR. Inside-out vein graft repair compared with nerve grafting for nerve regenera-tion in rats. Microsurgery. 1995;16:65–70. doi: 10.1002/micr.1920160205. [DOI] [PubMed] [Google Scholar]
- 34.Xia B, Lv Y. Dual-delivery of VEGF and NGF by emulsion electrospun nanofibrous scaffold for peripheral nerve regeneration. Mater Sci Eng C Mater Biol Appl. 2018;82:253–264. doi: 10.1016/j.msec.2017.08.030. [DOI] [PubMed] [Google Scholar]


