Abstract
Cognitive impairment is a common clinical manifestation of multiple sclerosis, but its pathophysiology is not completely understood. White and grey matter injury together with synaptic dysfunction do play a role. The measurement of biomarkers in the cerebrospinal fluid and the study of their association with cognitive impairment may provide interesting in vivo evidence of the biological mechanisms underlying multiple sclerosis-related cognitive impairment. So far, only a few studies on this topic have been published, giving interesting results that deserve further investigation. Cerebrospinal fluid biomarkers of different pathophysiological mechanisms seem to reflect different neuropsychological patterns of cognitive deficits in multiple sclerosis. The aim of this review is to discuss the studies that have correlated cerebrospinal fluid markers of immune, glial and neuronal pathology with cognitive impairment in multiple sclerosis. Although preliminary, these findings suggest that cerebrospinal fluid biomarkers show some correlation with cognitive performance in multiple sclerosis, thus providing interesting insights into the mechanisms underlying the involvement of specific cognitive domains.
Key Words: biomarkers, cerebrospinal fluid, cognitive impairment, information processing speed, memory, multiple sclerosis
Introduction
Cognitive impairment (CI) is an important determinant of disability in patients with multiple sclerosis (MS) (Chiaravalloti and DeLuca, 2008). Since cognitive deficits due to MS have a subtle extent, for a long time CI has not been considered as part of the clinical picture of the disease (Grzegorski and Losy, 2017; Messinis et al., 2018). Conversely, CI is now recognized as a core feature of MS, involving multiple cognitive domains, especially learning, episodic memory and processing speed (Chiaravalloti and DeLuca, 2008; Benedict et al., 2017; Artemiadis et al., 2018; Matías-Guiu et al., 2018; Sumowski et al., 2018). More than a half of individuals with MS manifests some degree of neuropsychological impairment along the disease course, even at its earliest and preclinical stages (Langdon, 2011; Amato et al., 2012). Studies on the prevalence of CI in MS have shown heterogeneous results, largely due to the choice of different inclusion criteria and neuropsychological tests (Artemiadis et al., 2018; Messinis et al., 2018). Indeed, prevalence of CI has been reported to range between 40% and 70% in MS (Chiaravalloti et al., 2013; Benedict et al., 2017; Grzegorski and Losy, 2017; Artemiadis et al., 2018). On the other side, the prevalence of dementia is quite low, reaching no more than 10–25% of patients with MS (Grzegorski and Losy, 2017).
The precise mechanisms responsible for CI in MS are not completely known, and this hampers a correct evaluation and outcome estimation of CI, as well as the identification of possible management strategies. MS is an immune-mediated disease of the central nervous system (CNS), usually starting in the early adulthood, and it is characterized by the appearance of focal inflammatory demyelination and neuronal injury in both the white and grey matter, due to recurrent infiltration of activated lymphocytes from the periphery to the CNS (Dendrou et al., 2015). Along the disease course, the frequency of externally driven focal inflammation decreases, and progressive axonal and neuronal loss, together with a diffuse glial dysfunction and organization of lymphocytes within ectopic follicle-like structures, prevail (Stadelmann, 2011). Because of the involvement of both immunological and neuronal players in the disease pathophysiology, the biological mechanisms underlying MS-related CI are complex and intertwined (Di Filippo et al., 2018). In a simplistic view, CI has been variably found to be associated with both structural injury and functional impairment of neuronal networks in MS brain.
Structural neuroimaging studies have provided clear evidence of the correlation between CI and focal lesions/atrophy due to MS in strategic brain areas involved in cognition, such as cortical areas (namely medial temporal lobe), deep grey matter nuclei, and cerebellum (Bergsland et al., 2016; Cocozza et al., 2017; Planche et al., 2018). Networks disconnection due to subcortical white matter lesions may also play a significant role in MS-related CI (Dineen et al., 2009). White matter lesion volume was indeed found to predict deficits in memory and processing speed, probably due to cortico-cortical and cortico-subcortical disconnections (Artemiadis et al., 2018; Matías-Guiu et al., 2018).
However, structural lesions do not sufficiently explain CI, since frequently a dissociation between lesion load, atrophy burden and cognitive functioning can be found (Mollison et al., 2017). One possible explanation able to fill the gap between neuroimaging findings and cognition lies in the dysfunction of neuronal circuits, which can occur in MS even in the normally appearing white and grey matter. Indeed, immunological mediators may impair long-term forms of synaptic plasticity, the cellular mechanisms responsible for shaping connectivity between neurons (Di Filippo et al., 2016; Rizzo et al., 2018). These results have led to the hypothesis that, at least in part, CI in MS could be driven by a synaptopathy favored by the CNS inflammatory milieu (Di Filippo et al., 2015; Mandolesi et al., 2015).
A spared functional connectivity, modulated by the cognitive reserve, can mitigate the impact of structural damage on cognitive functioning (Fuchs et al., 2019). This means that, despite impaired grey and white matter structural integrity, brain is resilient to lesion burden and can tolerate structural damage by means of mechanisms of neural efficiency, which are in part mediated by cognitive reserve. From a neural network perspective, a loss of structural connectivity and a maladaptive increase in functional connectivity may both be associated with worse cognitive performances in MS, even in patients with very mild disability (Has Silemek et al., 2020).
A better understanding of the pathophysiology of MS-related CI might potentially rely on fluid biomarkers studies. A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention (Biomarkers Definitions Working Group, 2001). As indicators of pathogenic processes, biomarkers reflecting the undergoing MS pathology and the study of their correlation with CI, may provide precious details on the possible biological determinants of neuropsychological impairment in MS. Due to its close proximity to CNS, cerebrospinal fluid (CSF) represents the ideal matrix where to investigate pathophysiological biomarkers and their association with CI. So far, a few studies have tried to correlate CSF biomarkers and neuropsychological performance in MS patients. Here, we review these studies by focusing on those biomarkers with the strongest pathophysiological rationale in MS (Table 1). Finally, we will discuss the limitations of current studies, thus trying to identify possible strategies to improve clinical research in this specific context.
Table 1.
Overview of the studies performed on CSF biomarkers and cognitive performance in MS patients
| CSF biomarker | Studies | Patients | Neuropsychological evaluation | Association with overall CI | Association with specific cognitive domains |
|---|---|---|---|---|---|
| NfL | Modvig et al. (2015) | 86 ON | PASAT | N/A | Trend towards negative correlation with follow-up IPS |
| Quintana et al. (2018) | 51 MS | BRBN and TMT-A | N/A | Negative correlation with verbal fluency scores | |
| Kalatha et al. (2019) | 27 RRMS | BICAMS | Negative correlation with BICAMS score in progressive MS | Negative correlation with BVMT scores (visuospatial memory) | |
| 2 SPMS | |||||
| 5 PPMS | |||||
| Gaetani et al. (2019b) | 28 RRMS | BRBN | Higher in patients with overall CI | Higher in patients with IPS and verbal fluency impairment | |
| Aβ42 | Mori et al. (2011) | 4 CIS | BRBN | Lower in patients with overall CI | Positive correlation with SDMT and PASAT scores (IPS) |
| 17 RRMS | |||||
| OCB | Anagnostouli et al. (2015) | 59 RRMS | TMT-A and TMT-B | No significant differences between OCB+ and OCB– | OCB+ worst performance on RCFT (visual memory) |
| 35 SPMS | Stroop test, WAIS | ||||
| 13 PPMS | RAVLT, BSRT | ||||
| RCFT copy | |||||
| COWAT, WCST | |||||
| CHI3L1 | Modvig et al. (2015) | 86 ON | PASAT | N/A | Negative correlation with follow-up IPS |
| Quintana et al. (2018) | 51 MS | BRBN and TMT-A | N/A | Negative correlation with IPS scores | |
| CHI3L2 | Møllgaard et al. (2016) | 73 ON | PASAT | N/A | Negative correlation with follow-up IPS |
Aβ42: Amyloid β peptide 42; BICAMS: Brief International Cognitive Assessment for Multiple Sclerosis; BRBN: Brief Repeatable Battery of Neuropsychological Tests; BSRT: Babcock Story Recall Test; BVMT: Brief Visuospatial Memory Test; CHI3L1: chitinase 3-like protein 1; CHI3L2: chitinase 3-like protein 2; CI: cognitive impairment; CIS: clinically isolated syndrome suggestive of multiple sclerosis; COWAT: Controlled Oral Word Association Test; CSF: cerebrospinal fluid; IPS: information processing speed; MS: multiple sclerosis; NfL: neurofilament light chain; N/A: not assessed; OCB: oligoclonal bands; ON: optic neuritis; PASAT: Paced Auditory Serial Addition Test; PPMS: primary progressive multiple sclerosis; RAVLT: Rey Auditory Verbal Learning Test; RCFT: Rey’s Complex Figure Test; RRMS: relapsing remitting multiple sclerosis; SDMT: Symbol Digit Modalities Test; SPMS: secondary progressive multiple sclerosis; TMT-A: Trail Making Test A; TMT-B: Trail Making Test B; WAIS: Wechsler Adult Intelligence Scale; WCST: Wisconsin Card Sorting Test.
Search Strategy and Selection Criteria
We searched on PubMed for the literature published between January 1, 2000, and January 31, 2020, and references from relevant articles. The search terms “biomarkers”, “multiple sclerosis”, “cerebrospinal fluid”, “cognition”, “cognitive impairment”, “memory”, “information processing speed”, “attention”, “verbal fluency” were used. If one of these papers referred to another relevant study, published after January 1, 2000, we also included the cited study. Only articles written in English were considered. The final reference list was generated on the basis of relevance to the topics covered in this Review.
The Profile of Cognitive Impairment in Multiple Sclerosis
Classically, as a consequence of diffuse subcortical damage, clinically relevant CI in MS has been classified as “subcortical dementia”, which is mainly characterized by reduced information processing speed and increased attention deficits (Filley et al., 1989). However, given the contribution of both white and grey matter involvement in MS, it is now well known that both cortical and subcortical cognitive domains can be affected along the disease course (Matías-Guiu et al., 2018). Although in the past several research papers focused on global CI, recent literature explored the correlations between specific cognitive domains, neuropsychological measures and neural correlates (Rocca et al., 2015; Matías-Guiu et al., 2018). In a recent paper, almost all of the neuropsychological measures showed an association with white matter lesion burden and with regional cortical and subcortical grey matter volumes. Of interest, the strongest correlation with cognitive scores was found for the volumes of thalamus, cerebellum, caudate, insula, inferior and middle frontal gyrus, parietal and para-hippocampal cortices (Matías-Guiu et al., 2018). This evidence, largely confirmed by other studies, highlights the complexity of the pathophysiology of CI in MS and it may explain the heterogeneity of cognitive profiles in MS (Chiaravalloti and DeLuca, 2008).
Rao et al. (1991) defined a typical neuropsychological pattern in MS. By using an extensive battery of neuropsychological tests, the Rao’s Brief Repeatable Battery of Neuropsychological tests (BRBN), they demonstrated that the majority of MS patients showed deficits in different domains, including verbal and visuo-spatial memory, attention and information processing speed, with a relative sparing of language.
Nevertheless, by using a different neuropsychological battery, a prevailing cortical pattern of CI was observed in MS, with visual memory, followed by information processing speed, as the most frequently impaired domain (Benedict et al., 2006). However, recent studies highlighted a main contribution of information processing speed impairment for learning and memory deficits. Indeed, learning new information, encoding and final retrieval are strongly dependent from attention, working memory, and processing speed.
Therefore, it is worth noting that many tasks are built as multicomponent measures, assessing different processes simultaneously. Additionally, some findings support the evidence that working memory, learning and episodic memory, executive functions and speed processing are interdependent and influence each other (Sumowski et al., 2018). For this reason, it is difficult to disentangle specific contributions of these distinct cognitive domains on overall CI in MS.
Cerebrospinal Fluid Biomarkers of Axonal Damage
Although CI in MS is not exclusively linked to subcortical damage, the involvement of white matter in the disease course plays a prominent role (Di Filippo et al., 2018). One of the most studied CSF biomarkers reflecting the entity of subcortical axonal damage in a variety of neurological diseases, including MS, is represented by the light chain of neurofilaments (NfL) (Gaetani et al., 2019a). The great applicability of this biomarker to MS relies on the fact that its concentration increases in the CSF proportionally to the damage of large calibre axons (Yuan et al., 2017). Therefore, NfL changes in CSF accurately reflect the ongoing focal white matter pathology and inflammation-driven axonal injury in MS (Gaetani et al., 2019a). Recently, the possibility to reliably measure it in blood, has made this biomarker a good candidate for accurately quantifying and monitoring axonal injury over time (Disanto et al., 2017). This feature also makes blood NfL measurement a reliable tool for checking the efficacy of disease-modifying drugs (Piehl et al., 2017; Akgün et al., 2019; Sejbaek et al., 2019).
For its properties, the study of CSF NfL and CI in MS may provide interesting insights on the contribution of axonal damage to disease-related cognitive deficits. Of interest, in a cohort of newly diagnosed MS patients, CSF NfL was found to be significantly higher in individuals with global CI (defined by impaired score at the BRBN), compared to cognitively preserved MS subjects (Gaetani et al., 2019b). This finding supports a role of NfL in tracking the overall impairment in cognitive functioning, even at the earliest stages of the disease. A similar result was obtained in an independent group of progressive MS patients assessed with the Brief International Cognitive Assessment for MS (BICAMS), where BICAMS scores were found to negatively correlate with CSF NfL values (Kalatha et al., 2019).
When analyzing the single cognitive domains, it was found that patients with reduced information processing speed had higher CSF NfL compared to those with normal performance (Gaetani et al., 2019b). In another study performed on MS patients with a median follow-up of 13 years, baseline CSF NfL showed a trend towards a negative correlation with information processing speed measures (Modvig et al., 2015). The association between CSF NfL and information processing speed is supported also by neuroimaging-based studies. Indeed, in a cohort of early MS, it was demonstrated that patients with higher CSF NfL had lower activity of the putamen at the higher attentional load task on functional brain MRI (Tortorella et al., 2015). Information processing speed is functionally overlapped with attentive functions, so BRBN tests exploring information processing speed also measure attention and working memory (Roth et al., 2015). As such, information processing speed relies on the normal functionality of multiple integrated neuronal networks (Tombaugh, 2006). Processing speed is a basic component of cognitive functioning that influences other cognitive domains. A sensitive measure for assessing speed processing is the Symbol Digit Modalities Test (SDMT). This measure is included in all cognitive batteries for MS, including the BICAMS. The SDMT, in fact, demonstrated better psychometric properties and more specific construct validity compared to other measures assessing speed processing, such as the Paced Auditory Serial Addition Test (PASAT; Strober et al., 2019). Performance on the PASAT reflects the combination of speed processing, sustained attention, working memory and executive functions. Therefore, the association between reduced information processing speed and increased CSF NfL in MS patients supports the idea that a mechanism of cortico-subcortical disconnection due to MS might be responsible for information processing speed impairment.
CSF NfL also tended to be high in MS patients showing impairment in the verbal fluency domain, assessed by means of the Word List Generation (WLG) based on semantic input (Gaetani et al., 2019b). This same association was obtained on a similar, independent cohort of newly diagnosed MS patients (Quintana et al., 2018). WLG based on semantic input is a sort of ‘whole brain test’, since it assesses the functionality of retrosplenial (parieto-temporal-occipital) and sensorimotor cortices and since it has been associated with whole grey matter brain volume (Lazeron et al., 2005; Biesbroek et al., 2016). All these findings make it conceivable that injury to large myelinated axons may be a determinant of CI in MS, mostly due to a “disconnection syndrome”, whose degree of severity can be measured with CSF NfL.
Cerebrospinal Fluid Biomarkers of Amyloid Metabolism
Brain amyloid pathology is a well-known histopathological hallmark of AD, and it is also present in other neurodegenerative diseases (Jellinger, 2008). As a reflection of brain amyloidosis, a reduced concentration of CSF amyloid β42 (Aβ42) is found in AD patients (Blennow and Hampel, 2003). Low level of CSF Aβ42 represents an AD change, and it is a mandatory first step for the biological definition of AD (Jack et al., 2018). Low levels of CSF Aβ42 in other neurodegenerative diseases, such as PD, correlate with the presence of CI and with the risk of developing it along the disease course (Parnetti et al., 2014).
Of interest, it has been demonstrated that in MS lesions, axonal accumulation of the amyloid precursor protein (APP) occurs, an evidence that opens to the possibility that amyloid dysmetabolism could play a role in the irreversible axonal damage taking place in MS (Ferguson et al., 1997). A few studies have investigated CSF Aβ42 as a biomarker in MS, and they have found that CSF soluble APP (s-APP) and Aβ42 are reduced in MS patients compared to controls, with higher values after the start of high efficacy disease-modifying drugs (Augutis et al., 2013). Since disease-modifying drugs are effective in counteracting the abnormal peripheral immune activation, their effect on CSF Aβ42 levels might suggest that amyloid dysmetabolism in MS could be an after-effect of CNS inflammation. Based on the evidence that APP accumulates in axons of MS lesions, it is therefore possible that acute axonal injury due to MS inflammatory lesions leads to the abnormal accumulation of APP and amyloid peptides.
CSF Aβ42 has been demonstrated to be significantly lower in cognitively impaired than in cognitively preserved MS patients, as defined by the BRBN score. Interestingly, CSF Aβ42 significantly correlated with the scores of BRBN tests exploring information processing speed, and not with tests exploring verbal and visuospatial learning (Mori et al., 2011). This is in agreement with what observed for the axonal damage marker NfL (Gaetani et al., 2019b), suggesting that CSF Aβ42 levels might be associated with cognitive performance because they reflect axonal pathology. Another evidence supporting the idea of Aβ42 as an indicator of acute axonal damage comes from the association between low levels of CSF Aβ42 and the presence of gadolinium enhancing (Gd+) lesions on brain MRI of MS patients (Mori et al., 2011). Also, in other neurological diseases not primarily related to brain amyloidosis, such as Creutzfeldt Jakob disease, CSF Aβ42 levels might be low even in the absence of extracellular Aβ brain deposits (Lattanzio et al., 2017), thus confirming that decreased CSF Aβ42 might generally reflect axonal injury.
In degenerative CNS diseases, extracellular amyloid pathology can be considered a driver for neurodegenerative processes and it can interact with other important pathophysiological mechanisms, such as tauopathy and synucleinopathy (Jellinger, 2008). Consequently, CSF Aβ42 has not only a clear diagnostic value in AD, but it may also have a significant prognostic value in different neurodegenerative diseases. On the contrary, in MS, the aggregation of APP and Aβ42 may occur in the intraneuronal space as a consequence of acute inflammatory-driven axonal damage, and it may be responsible for axonal swelling at the site of axonal transection (Ferguson et al., 1997; Trapp et al., 1998). This might explain why, in MS, CSF Aβ42 behaves as a marker of cortico-subcortical disconnection similarly to NfL and does not correlate with other cortical cognitive domains, such as verbal and visuospatial memory.
On the other hand, it is worth noting that Aβ42 has shown to impair synaptic plasticity, i.e. the activity-dependent long-term synaptic changes that are responsible for learning and memory, in animal models (Oddo et al., 2003; Klyubin et al., 2005; Shankar et al., 2008; Townsend et al., 2010). Moreover, in cognitively impaired MS patients, an abnormal plasticity of the cerebral cortex, as assessed by means of transcranial magnetic stimulation, has been demonstrated. A positive correlation has been reported between CSF Aβ42 and the magnitude of long-term potentiation-like effects induced by transcranial magnetic stimulation (Mori et al., 2011). This evidence hampers against the idea of CSF Aβ42 as a simple marker of axonal damage, opening the possibility that amyloid pathology might actively participate in driving synaptic dysfunction in MS. As such, the study of amyloid dysmetabolism and CI through CSF biomarkers deservers further investigations.
Finally, like CSF NfL, CSF Aβ42 is not function-specific. Indeed, CSF Aβ42 reduction at baseline seems to predict the overall physical disability at 3- and 5-year follow-up in MS patients (Pietroboni et al., 2017, 2019). Therefore, CSF Aβ42 reduction can be considered as an overall marker of disease-severity in MS, not specifically linked to CI. Further studies are needed in order to assess the relationship between CSF Aβ42 and cognitive performance along time in MS.
Cerebrospinal Fluid Biomarkers of Intrathecal Immunoglobulins Synthesis
To date, the only clinically useful CSF biomarker for MS is represented by immunoglobulin G (IgG) oligoclonal bands (OCBs) (McNicholas et al., 2018). OCBs reflect the intrathecal synthesis of IgG, which in turn is a general marker of adaptive immunity activation within the CNS (Bankoti et al., 2014). OCBs are not MS-specific, since they can be found in a variety of inflammatory neurological diseases (Petzold, 2013). Also, in about 5% of MS cases, CSF OCBs cannot be found according to standard measurements (Link and Kostulas, 1983; Freedman et al., 2005). The presence of CSF OCBs may support the diagnosis of MS. Although their absence does not necessarily rule out a diagnosis of MS, it should prompt neurologists to carefully consider the possibility of alternative diagnoses (Thompson et al., 2017). Apart from their supportive diagnostic role, OCBs are considered a prognostic tool, able to predict subsequent disease activity after the first clinical manifestation of the disease (Dobson et al., 2013). As such, OCBs primarily reflect disease severity, and they could correlate with worse cognitive performance in MS patients. Recently, it has been demonstrated that OCBs positive compared to OCBs negative MS patients, more frequently show CI (Farina et al., 2017). Of interest, when evaluating a single cognitive domain, OCBs were found to specifically correlate with memory impairment. Indeed, a study performed on a cohort of MS patients who were assessed by means of a comprehensive battery of neuropsychological tests, showed that OCBs positive MS patients had a worse performance in different cognitive domains (i.e., memory, attention, information processing speed, perception, constructive abilities, reasoning and executive functions). However, a statistically significant difference was found only for Rey’s Complex Figure Test-recall form, a test exploring visual-spatial memory (Anagnostouli et al., 2015). These results are in line with those from an independent study, where the presence of CSF OCBs was associated with a higher cortical lesion load on brain MRI, as opposed to no association with white matter lesion load (Farina et al., 2017). Therefore, the presence of OCBs may identify a subgroup of patients with a marked involvement of cortical grey matter, which in turns may be responsible for impaired cortical cognitive functions, such as memory.
The pathophysiological link between OCBs and CI still needs to be unraveled. Intrathecal IgG synthesis might be the quantifiable epiphenomenon of a more severe pattern of immune activation within the CNS, and other immune players could be involved in impairing cognitive performance. For instance, in the CSF of OCBs positive patients it was possible to cluster a panel of increased cytokines and chemokines. As expected, most of these cytokines are associated with B cell activation, such as the chemokine ligand 13 and 12, the B cell activating factor, and osteopontin (Farina et al., 2017). The potential contribution on cognition of these B cell markers must be elucidated.
In MS, B cells also play a significant role in the organization of meningeal follicle-like structures in the CNS and they correlated with the presence of cortical demyelinating lesions (Howell et al., 2011). Therefore, intrathecal IgG synthesis, as measured by means of OCBs, might reflect a more intense compartmentalization of immune activity into tertiary lymphoid structures releasing inflammatory mediators that are detrimental for cortical functionality and neuronal survival.
Unfortunately, as a biomarker of B cells activity, OCBs are limited by the fact that they do not provide a quantitative measure of immune activation, rather representing only a qualitative measure (Freedman et al., 2005). To overcome this limitation, other better quantifiable IgG intrathecal synthesis markers, such as Ig free light chains, are under investigation (Gaetani et al., 2020). Further studies with quantitative markers of intrathecal B cell activation are needed to better characterize the pathophysiological role of B cells and their markers on CI in MS.
Cerebrospinal Fluid Biomarkers of Neuroinflammation
The inflammatory milieu within the CNS in MS could influence the cellular mechanisms responsible for cognitive functioning. Indeed, during experimental autoimmune encephalomyelitis, the animal model of MS, synaptic plasticity is impaired in the hippocampus, the key structure for learning and memory (Di Filippo et al., 2013). Long-term potentiation is the most studied form of synaptic plasticity, and its impairment in experimental autoimmune encephalomyelitis has been variably associated with the over-expression of pro-inflammatory mediators, such as interleukin-1β (Kim et al., 2012; Di Filippo et al., 2013), and with microglial activation (Di Filippo et al., 2016).
Studies performed on the correlation between CSF immune mediators and cognitive performance in MS patients seem to confirm this preclinical evidence, as it is the case for CSF concentration of chitinase 3-like protein 1 (CHI3L1), a protein also known as YKL-40. This protein, which is involved in innate and acquired immunity (Lee et al., 2011), accumulates at sites of chronic inflammation (Kzhyshkowska et al., 2007) and correlates with worse performance on attention and information processing speed (Quintana et al., 2018). In CSF, this biomarker, and its homologous CHI3L2, were able to predict future impairment on information processing speed in MS patients (Modvig et al., 2015; Møllgaard et al., 2016). Both of them are released by astrocytes; CHI3L1 is also secreted by microglial cells (Hinsinger et al., 2015). Therefore, the association between CSF concentration of chitinase like proteins and information processing speed performance seems to suggest that glial activation may negatively influence this cognitive domain.
Discussion and Conclusions
The impact of CI in the overall disability of MS patients is nowadays well recognized (Chiaravalloti and DeLuca, 2008). The mechanisms leading to abnormal cognition could be different and interconnected to each other (Di Filippo et al., 2018). The study of CSF biomarkers and their association with CI is providing interesting clues on the pathophysiological determinants of neuropsychological deficits in MS patients (Figure 1). Evidence from the studies discussed in this review seems to associate biomarkers of axonal injury to abnormal information processing speed. For instance, CSF NfL has been found to negatively correlate with performances in this domain, suggesting that white matter lesion load and cortico-subcortical disconnection might play a role in impairing information processing speed. A similar behavior has been demonstrated for CSF Aβ42, whose reduced levels in MS patients might reflect axonal injury and the subsequent morphological changes of transected axons. As such, CSF Aβ42 changes in MS should be interpreted differently from those observed in neurodegenerative diseases related to brain amyloidosis. Indeed, its CSF reduction in MS might be a downstream effect of axonal injury more than a driving mechanism of neuronal loss. Interestingly, the so-called neuroinflammation, i.e. the abnormal activation of non-neuronal cells within the CNS, might have a similar effect on attention and on information processing speed, as demonstrated by studies performed on CSF CHI3L1 and CHI3L2, two astrocytic and microglial markers. On the contrary, markers of peripherally activated lymphocytes – i.e., CSF OCBs - seem to be associated with impairment in higher cortical functions such as memory, and not with information processing speed and attention. However, the association between a given biomarker and a specific cognitive domain should be carefully considered, since it is not always possible to distinguish between cognitive domains by means of neuropsychological tests, nor to tie a cognitive domain to a specific brain area or neuronal network.
Figure 1.
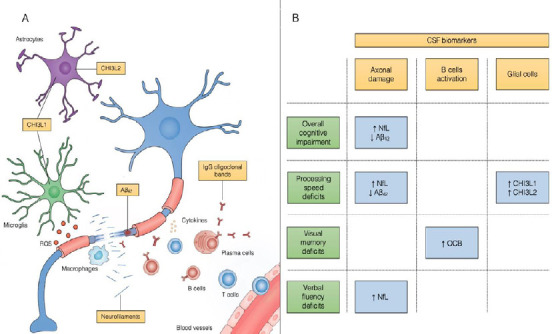
CSF biomarkers in MS: pathophysiological rationale and association with cognitive impairment.
(A) Pathophysiological mechanisms in MS and the corresponding CSF biomarkers that have been investigated in cognitive impairment. (B) Association between CSF biomarkers and impairment in different cognitive domains in MS patients. Aβ42: Amyloid β42; CHI3L1: chitinase 3-like protein 1; CHI3L2: chitinase 3-like protein 2; CSF: cerebrospinal fluid; MS: multiple sclerosis; NfL: neurofilament light chain; OCB: oligoclonal bands; ROS: reactive oxygen species.
When interpreting these preliminary findings, several sources of bias should be considered. First of all, the studies performed on this topic are limited by a small sample size, not exceeding a total of around 90 patients per study. Therefore, investigations on larger cohorts are needed to confirm or confute these results. Additionally, findings from different studies are not easily comparable to each-other, due to different patients’ characteristics and to different neuropsychological tests used for cognitive evaluation (Table 1). Finally, in most of these studies, the correlation between CSF biomarkers and cognitive functions was not assessed using multivariate models taking into account other possible disease-related factors potentially influencing cognition. Despite these limitations, however, the preliminary findings from studies correlating CSF biomarkers to CI seem interesting, and certainly open up the possibility of insisting on this field of research by trying to overcome the limitations of the current studies.
In the future, it will be necessary to carry out further studies by correcting the association between CSF biomarkers and CI by age, disability level, T2 lesion load, atrophy burden, and cortical lesion load on brain MRI. Only in this way, it will be possible to understand whether or not CSF biomarkers can be considered valid tracers of CI, regardless of other disease-severity measures.
Additional file: Open peer review report 1. (81.7KB, pdf)
Footnotes
Conflicts of interest: LGa participated on advisory boards for, and received writing honoraria and travel grants from Almirall, Biogen, Merck, Mylan, Novartis, Roche, Sanofi Genzyme and Teva. MDF participated on advisory boards for and received speaker or writing honoraria and funding for travelling from Bayer, Biogen Idec, Genzyme, Merck, Mylan, Novartis, Roche and Teva. Others report no conflict of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Tetsuya Akaishi, Tohoku University Graduate School of Medicine, Japan.
P-Reviewer: Akaishi T; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Akgün K, Kretschmann N, Haase R, Proschmann U, Kitzler HH, Reichmann H, Ziemssen T. Profiling individual clinical responses by high-frequency serum neurofilament assessment in MS. Neurol Neuroimmunol Neuroinflamm. 2019;6:e555. doi: 10.1212/NXI.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amato MP, Hakiki B, Goretti B, Rossi F, Stromillo ML, Giorgio A, Roscio M, Ghezzi A, Guidi L, Bartolozzi ML, Portaccio E, De Stefano N Italian RIS/MS Study Group. Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology. 2012;78:309–314. doi: 10.1212/WNL.0b013e31824528c9. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostouli M, Christidi F, Zalonis I, Nikolaou C, Lyrakos D, Triantafyllou N, Evdokimidis I, Kilidireas C. Clinical and cognitive implications of cerebrospinal fluid oligoclonal bands in multiple sclerosis patients. Neurol Sci. 2015;36:2053–2060. doi: 10.1007/s10072-015-2303-1. [DOI] [PubMed] [Google Scholar]
- 4.Artemiadis A, Anagnostouli M, Zalonis I, Chairopoulos K, Triantafyllou N. Structural MRI correlates of cognitive function in multiple sclerosis. Mult Scler Relat Disord. 2018;21:1–8. doi: 10.1016/j.msard.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Augutis K, Axelsson M, Portelius E, Brinkmalm G, Andreasson U, Gustavsson MK, Malmeström C, Lycke J, Blennow K, Zetterberg H, Mattsson N. Cerebrospinal fluid biomarkers of β-amyloid metabolism in multiple sclerosis. Mult Scler. 2013;19:543–552. doi: 10.1177/1352458512460603. [DOI] [PubMed] [Google Scholar]
- 6.Bankoti J, Apeltsin L, Hauser SL, Allen S, Albertolle ME, Witkowska HE, von Büdingen HC. In multiple sclerosis, oligoclonal bands connect to peripheral B-cell responses. Ann Neurol. 2014;75:266–276. doi: 10.1002/ana.24088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedict RH, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N, Weinstock-Guttman B. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) J Int Neuropsychol Soc. 2006;12:549–558. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- 8.Benedict RHB, DeLuca J, Enzinger C, Geurts JJG, Krupp LB, Rao SM. Neuropsychology of multiple aclerosis: looking back and moving forward. J Int Neuropsychol Soc. 2017;23:832–842. doi: 10.1017/S1355617717000959. [DOI] [PubMed] [Google Scholar]
- 9.Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R Multiple Sclerosis Outcome Assessments Consortium. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:721–733. doi: 10.1177/1352458517690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B, Benedict RH. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler. 2016;22:1327–1336. doi: 10.1177/1352458515616204. [DOI] [PubMed] [Google Scholar]
- 11.Biesbroek JM, van Zandvoort MJ, Kappelle LJ, Velthuis BK, Biessels GJ, Postma A. Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion-symptom mapping in patients with ischemic stroke. Brain Struct Funct. 2016;221:2123–2134. doi: 10.1007/s00429-015-1033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 13.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 14.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 15.Chiaravalloti ND, Stojanovic-Radic J, DeLuca J. The role of speed versus working memory in predicting learning new information in multiple sclerosis. J Clin Exp Neuropsychol. 2013;35:180–191. doi: 10.1080/13803395.2012.760537. [DOI] [PubMed] [Google Scholar]
- 16.Cocozza S, Petracca M, Mormina E, Buyukturkoglu K, Podranski K, Heinig MM, Pontillo G, Russo C, Tedeschi E, Russo CV, Costabile T, Lanzillo R, Harel A, Klineova S, Miller A, Brunetti A, Morra VB, Lublin F, Inglese M. Cerebellar lobule atrophy and disability in progressive MS. J Neurol Neurosurg Psychiatry. 2017;88:1065–1072. doi: 10.1136/jnnp-2017-316448. [DOI] [PubMed] [Google Scholar]
- 17.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 18.Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampà C, Costa C, Tantucci M, Zianni E, Boraso M, Siliquini S, de Iure A, Ghiglieri V, Colcelli E, Baker D, Sarchielli P, Fusco FR, Di Luca M, Calabresi P. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis. 2013;52:229–236. doi: 10.1016/j.nbd.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Di Filippo M, de Iure A, Durante V, Gaetani L, Mancini A, Sarchielli P, Calabresi P. Synaptic plasticity and experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Brain Res. 2015;1621:205–213. doi: 10.1016/j.brainres.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Di Filippo M, de Iure A, Giampà C, Chiasserini D, Tozzi A, Orvietani PL, Ghiglieri V, Tantucci M, Durante V, Quiroga-Varela A, Mancini A, Costa C, Sarchielli P, Fusco FR, Calabresi P. Persistent activation of microglia and NADPH oxidase [corrected] drive hippocampal dysfunction in experimental multiple sclerosis. Sci Rep. 2016;6:20926. doi: 10.1038/srep20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Filippo M, Portaccio E, Mancini A, Calabresi P. Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci. 2018;19:599–609. doi: 10.1038/s41583-018-0053-9. [DOI] [PubMed] [Google Scholar]
- 22.Dineen RA, Vilisaar J, Hlinka J, Bradshaw CM, Morgan PS, Constantinescu CS, Auer DP. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132:239–249. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- 23.Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, Zecca C, Blennow K, Zetterberg H, Leppert D, Kappos L, Gobbi C, Kuhle J Swiss Multiple Sclerosis Cohort Study Group. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81:857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobson R, Ramagopalan S, Davis A, Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry. 2013;84:909–914. doi: 10.1136/jnnp-2012-304695. [DOI] [PubMed] [Google Scholar]
- 25.Farina G, Magliozzi R, Pitteri M, Reynolds R, Rossi S, Gajofatto A, Benedetti MD, Facchiano F, Monaco S, Calabrese M. Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: a combined CSF and MRI study. J Neuroinflammation. 2017;14:40. doi: 10.1186/s12974-017-0812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 27.Filley CM, Heaton RK, Nelson LM, Burks JS, Franklin GM. A comparison of dementia in Alzheimer’s disease and multiple sclerosis. Arch Neurol. 1989;46:157–161. doi: 10.1001/archneur.1989.00520380061013. [DOI] [PubMed] [Google Scholar]
- 28.Freedman MS, Thompson EJ, Deisenhammer F, Giovannoni G, Grimsley G, Keir G, Ohman S, Racke MK, Sharief M, Sindic CJ, Sellebjerg F, Tourtellotte WW. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol. 2005;62:865–870. doi: 10.1001/archneur.62.6.865. [DOI] [PubMed] [Google Scholar]
- 29.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019a;90:870–881. doi: 10.1136/jnnp-2018-320106. [DOI] [PubMed] [Google Scholar]
- 30.Gaetani L, Di Carlo M, Brachelente G, Valletta F, Eusebi P, Mancini A, Gentili L, Borrelli A, Calabresi P, Sarchielli P, Ferri C, Villa A, Di Filippo M. Cerebrospinal fluid free light chains compared to oligoclonal bands as biomarkers in multiple sclerosis. J Neuroimmunol. 2020;339:577108. doi: 10.1016/j.jneuroim.2019.577108. [DOI] [PubMed] [Google Scholar]
- 31.Gaetani L, Salvadori N, Lisetti V, Eusebi P, Mancini A, Gentili L, Borrelli A, Portaccio E, Sarchielli P, Blennow K, Zetterberg H, Parnetti L, Calabresi P, Di Filippo M. Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J Neurol. 2019b;266:2157–2163. doi: 10.1007/s00415-019-09398-7. [DOI] [PubMed] [Google Scholar]
- 32.Grzegorski T, Losy J. Cognitive impairment in multiple sclerosis - a review of current knowledge and recent research. Rev Neurosci. 2017;28:845–860. doi: 10.1515/revneuro-2017-0011. [DOI] [PubMed] [Google Scholar]
- 33.Has Silemek AC, Fischer L, Pöttgen J, Penner IK, Engel AK, Heesen C, Gold SM, Stellmann JP. Functional and structural connectivity substrates of cognitive performance in relapsing remitting multiple sclerosis with mild disability. Neuroimage Clin. 2020;25:102177. doi: 10.1016/j.nicl.2020.102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinsinger G, Galéotti N, Nabholz N, Urbach S, Rigau V, Demattei C, Lehmann S, Camu W, Labauge P, Castelnovo G, Brassat D, Loussouarn D, Salou M, Laplaud D, Casez O, Bockaert J, Marin P, Thouvenot E. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Mult Scler. 2015;21:1251–1261. doi: 10.1177/1352458514561906. [DOI] [PubMed] [Google Scholar]
- 35.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jellinger KA. Neuropathological aspects of Alzheimer disease, Parkinson disease and frontotemporal dementia. Neurodegener Dis. 2008;5:118–121. doi: 10.1159/000113679. [DOI] [PubMed] [Google Scholar]
- 37.Kalatha T, Arnaoutoglou M, Koukoulidis T, Hatzifilippou E, Bouras E, Baloyannis S, Koutsouraki E. Does cognitive dysfunction correlate with neurofilament light polypeptide levels in the CSF of patients with multiple sclerosis. J Int Med Res. 2019;47:2187–2198. doi: 10.1177/0300060519840550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One. 2012;7:e35476. doi: 10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 40.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 41.Langdon DW. Cognition in multiple sclerosis. Curr Opin Neurol. 2011;24:244–249. doi: 10.1097/WCO.0b013e328346a43b. [DOI] [PubMed] [Google Scholar]
- 42.Lattanzio F, Abu-Rumeileh S, Franceschini A1, Kai H, Amore G, Poggiolini I, Rossi M, Baiardi S, McGuire L, Ladogana A, Pocchiari M5, Green A, Capellari S, Parchi P. Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol. 2017;133:559–578. doi: 10.1007/s00401-017-1683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazeron RH, Boringa JB, Schouten M, Uitdehaag BM, Bergers E, Lindeboom J, Eikelenboom MI, Scheltens PH, Barkhof F, Polman CH. Brain atrophy and lesion load as explaining parameters for cognitive impairment in multiple sclerosis. Mult Scler. 2005;11:524–531. doi: 10.1191/1352458505ms1201oa. [DOI] [PubMed] [Google Scholar]
- 44.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Link H, Kostulas V. Utility of isoelectric focusing of cerebrospinal fluid and serum on agarose evaluated for neurological patients. Clin Chem. 1983;29:810–815. [PubMed] [Google Scholar]
- 46.Mandolesi G, Gentile A, Musella A, Fresegna D, De Vito F, Bullitta S, Sepman H, Marfia GA, Centonze D. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat Rev Neurol. 2015;11:711–724. doi: 10.1038/nrneurol.2015.222. [DOI] [PubMed] [Google Scholar]
- 47.Matías-Guiu JA, Cortés-Martínez A, Montero P, Pytel V, Moreno-Ramos T, Jorquera M, Yus M, Arrazola J, Matías-Guiu J. Identification of cortical and subcortical correlates of cognitive performance in multiple sclerosis using voxel-based morphometry. Front Neurol. 2018;9:920. doi: 10.3389/fneur.2018.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNicholas N, Lockhart A, Yap SM, O’Connell K, Tubridy N, Hutchinson M, McGuigan C. New versus old: Implications of evolving diagnostic criteria for relapsing-remitting multiple sclerosis. Mult Scler. 2018;25:867–870. doi: 10.1177/1352458518770088. [DOI] [PubMed] [Google Scholar]
- 49.Messinis L, Papathanasopoulos P, Kosmidis MH, Nasios G, Kambanaros M. Neuropsychological features of multiple sclerosis: impact and rehabilitation. Behav Neurol. 2018;2018:4831647. doi: 10.1155/2018/4831647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Modvig S, Degn M, Roed H, Sørensen TL, Larsson HB, Langkilde AR, Frederiksen JL, Sellebjerg F. Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis. Mult Scler. 2015;21:1761–1770. doi: 10.1177/1352458515574148. [DOI] [PubMed] [Google Scholar]
- 51.Møllgaard M, Degn M, Sellebjerg F, Frederiksen JL, Modvig S. Cerebrospinal fluid chitinase-3-like 2 and chitotriosidase are potential prognostic biomarkers in early multiple sclerosis. Eur J Neurol. 2016;23:898–905. doi: 10.1111/ene.12960. [DOI] [PubMed] [Google Scholar]
- 52.Mollison D, Sellar R, Bastin M, Mollison D, Chandran S, Wardlaw J, Connick P. The clinico-radiological paradox of cognitive function and MRI burden of white matter lesions in people with multiple sclerosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0177727. doi: 10.1371/journal.pone.0177727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mori F, Rossi S, Sancesario G, Codecà C, Mataluni G, Monteleone F, Buttari F, Kusayanagi H, Castelli M, Motta C, Studer V, Bernardi G, Koch G, Bernardini S, Centonze D. Cognitive and cortical plasticity deficits correlate with altered amyloid-β CSF levels in multiple sclerosis. Neuropsychopharmacology. 2011;36:559–568. doi: 10.1038/npp.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 55.Pagani E, Rocca MA, De Meo E, Horsfield MA, Colombo B, Rodegher M, Comi G, Filippi M. Structural connectivity in multiple sclerosis and modeling of disconnection. Mult Scler. 2019;26:220–232. doi: 10.1177/1352458518820759. [DOI] [PubMed] [Google Scholar]
- 56.Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, Giannandrea D, Salvadori N, Lisetti V, Tambasco N, Rossi A, Majbour NK, El-Agnaf O, Calabresi P. Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson’s Disease. Front Aging Neurosci. 2014;6:53. doi: 10.3389/fnagi.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petzold A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J Neuroimmunol. 2013;262:1–10. doi: 10.1016/j.jneuroim.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Piehl F, Kockum I, Khademi M, Blennow K, Lycke J, Zetterberg H, Olsson T. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler. 2017;24:1046–1054. doi: 10.1177/1352458517715132. [DOI] [PubMed] [Google Scholar]
- 59.Pietroboni AM, Caprioli M, Carandini T, Scarioni M, Ghezzi L, Arighi A, Cioffi S, Cinnante C, Fenoglio C, Oldoni E, De Riz MA, Basilico P, Fumagalli GG, Colombi A, Giulietti G, Serra L, Triulzi F, Bozzali M, Scarpini E, Galimberti D. CSF β-amyloid predicts prognosis in patients with multiple sclerosis. Mult Scler. 2019;25:1223–1231. doi: 10.1177/1352458518791709. [DOI] [PubMed] [Google Scholar]
- 60.Pietroboni AM, Schiano di Cola F, Scarioni M, Fenoglio C, Spanò B, Arighi A, Cioffi SM, Oldoni E, De Riz MA, Basilico P, Calvi A, Fumagalli GG, Triulzi F, Galimberti D, Bozzali M, Scarpini E. CSF β-amyloid as a putative biomarker of disease progression in multiple sclerosis. Mult Scler. 2017;23:1085–1091. doi: 10.1177/1352458516674566. [DOI] [PubMed] [Google Scholar]
- 61.Planche V, Koubiyr I, Romero JE, Manjon JV, Coupé P, Deloire M, Dousset V, Brochet B, Ruet A, Tourdias T. Regional hippocampal vulnerability in early multiple sclerosis: Dynamic pathological spreading from dentate gyrus to CA1. Hum Brain Mapp. 2018;39:1814–1824. doi: 10.1002/hbm.23970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pytel V, Matias-Guiu JA, Matías-Guiu J, Cortés-Martínez A, Montero P, Moreno-Ramos T, Arrazola J, Carreras JL, Cabrera-Martín MN. Amyloid PET findings in multiple sclerosis are associated with cognitive decline at 18 months. Mult Scler Relat Disord. 2020;39:101926. doi: 10.1016/j.msard.2020.101926. [DOI] [PubMed] [Google Scholar]
- 63.Quintana E, Coll C, Salavedra-Pont J, Muñoz-San Martín M, Robles-Cedeño R, Tomàs-Roig J, Buxó M, Matute-Blanch C, Villar LM, Montalban X, Comabella M, Perkal H, Gich J, Ramió-Torrentà L. Cognitive impairment in early stages of multiple sclerosis is associated with high cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain. Eur J Neurol. 2018;25:1189–1191. doi: 10.1111/ene.13687. [DOI] [PubMed] [Google Scholar]
- 64.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I.Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 65.Rizzo FR, Musella A, De Vito F, Fresegna D, Bullitta S, Vanni V, Guadalupi L, Stampanoni Bassi M, Buttari F, Mandolesi G, Centonze D, Gentile A. Tumor mecrosis factor and interleukin-1 β modulate synaptic plasticity during neuroinflammation. Neural Plast. 2018;2018:8430123. doi: 10.1155/2018/8430123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, Rovira A, Sumowski JF, Valsasina P, Filippi M MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14:302–317. doi: 10.1016/S1474-4422(14)70250-9. [DOI] [PubMed] [Google Scholar]
- 67.Roth AK, Denney DR, Lynch SG. Information processing speed and attention in multiple sclerosis: Reconsidering the Attention Network Test (ANT) J Clin Exp Neuropsychol. 2015;37:518–529. doi: 10.1080/13803395.2015.1037252. [DOI] [PubMed] [Google Scholar]
- 68.Sejbaek T, Nielsen HH, Penner N, Plavina T, Mendoza JP, Martin NA, Elkjaer ML, Ravnborg MH, Illes Z. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J Neurol Neurosurg Psychiatry. 2019;90:1324–1330. doi: 10.1136/jnnp-2019-321321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol. 2011;24:224–229. doi: 10.1097/WCO.0b013e328346056f. [DOI] [PubMed] [Google Scholar]
- 71.Strober L, DeLuca J, Benedict RH, Jacobs A, Cohen JA, Chiaravalloti N, Hudson LD, Rudick RA, LaRocca NG. Multiple Sclerosis Outcome Assessments Consortium (MSOAC) (2019) Symbol Digit Modalities Test: A valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult Scler. 25:1781–1790. doi: 10.1177/1352458518808204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sumowski JF, Benedict R, Enzinger C, Filippi M, Geurts JJ, Hamalainen P, Hulst H, Inglese M, Leavitt VM, Rocca MA, Rosti-Otajarvi EM, Rao S. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology. 2018;90:278–288. doi: 10.1212/WNL.0000000000004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2017;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 74.Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT) Arch Clin Neuropsychol. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Tortorella C, Direnzo V, Taurisano P, Romano R, Ruggieri M, Zoccolella S, Mastrapasqua M, Popolizio T, Blasi G, Bertolino A, Trojano M. Cerebrospinal fluid neurofilament tracks fMRI correlates of attention at the first attack of multiple sclerosis. Mult Scler. 2015;21:396–401. doi: 10.1177/1352458514546789. [DOI] [PubMed] [Google Scholar]
- 76.Townsend M, Qu Y, Gray A, Wu Z, Seto T, Hutton M, Shearman MS, Middleton RE. Oral treatment with a gamma-secretase inhibitor improves long-term potentiation in a mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2010;333:110–119. doi: 10.1124/jpet.109.163691. [DOI] [PubMed] [Google Scholar]
- 77.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 78.Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a018309. doi: 101101/cshperspecta018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


