Abstract
Peripheral nerve injury (PNI) is common and, unlike damage to the central nervous system injured nerves can effectively regenerate depending on the location and severity of injury. Peripheral myelinating glia, Schwann cells (SCs), interact with various cells in and around the injury site and are important for debris elimination, repair, and nerve regeneration. Following PNI, Wallerian degeneration of the distal stump is rapidly initiated by degeneration of damaged axons followed by morphologic changes in SCs and the recruitment of circulating macrophages. Interaction with fibroblasts from the injured nerve microenvironment also plays a role in nerve repair. The replication and migration of injury-induced dedifferentiated SCs are also important in repairing the nerve. In particular, SC migration stimulates axonal regeneration and subsequent myelination of regenerated nerve fibers. This mobility increases SC interactions with other cells in the nerve and the exogenous environment, which influence SC behavior post-injury. Following PNI, SCs directly and indirectly interact with other SCs, fibroblasts, and macrophages. In addition, the inter- and intracellular mechanisms that underlie morphological and functional changes in SCs following PNI still require further research to explain known phenomena and less understood cell-specific roles in the repair of the injured peripheral nerve. This review provides a basic assessment of SC function post-PNI, as well as a more comprehensive evaluation of the literature concerning the SC interactions with macrophages and fibroblasts that can influence SC behavior and, ultimately, repair of the injured nerve.
Key Words: axon regeneration, cell-cell interactions, nerve injury, nerve repair, peripheral nerve, recovery, regeneration, repair, Schwann cell migration
Introduction
As many as 3% of trauma patients, including those with combat-related injuries, experience nerve injury alone or in polytrauma to the extremities and body (Noble et al., 1998; Yegiyants et al., 2010). Peripheral nerve injury (PNI), as a medical condition, encompasses numerous forms of nerve injury in highly variable peripheral tissue microenvironments (Namgung, 2014; Bombeiro et al., 2020). After PNI, the microenvironment of the damaged nerve and the Schwann cells (SCs) associated with the nerve are exposed to cells in the surrounding tissue to varying degrees. Such contact can be direct, whereby cells physically interact with other cells, or can be indirect, whereby SC responses to biochemical cues are communicated by nerve-associated cells (e.g. fibroblasts and infiltrating macrophages) and the surrounding tissue (Namgung, 2014; Wood and Mackinnon, 2015). Many cells, including SCs, are immediately killed at the site of primary injury. However, surviving SCs adjacent to the injured nerve stump take part in a massive systemic and local initiative to repair the nerve depending on the type and severity of injury. Such repairs can range from mild trauma management with minimal affected tissue to complete regeneration of a nerve following transection. In the latter form of injury, a major multicellular effort is waged by SCs, macrophages, lymphocytes, fibroblasts, and even astrocytes if the injury is close to the spinal cord root exit or to the spinal cord itself. This effort requires extensive SC contact with different cells involved in the process. These interactions, as well as the chaotic nature of the nerve environment and associated cleanup of debris after injury, are extremely complex and not fully understood. This review attempts to establish a more complete understanding of SCs and their cellular responses and intercellular interactions involved in migration for repair following PNI and myelination of regenerating damaged axons. We provide an overview of the SC response to injury and describe the responses and interactions of macrophages and fibroblasts with SCs in the repair and regeneration processes post-PNI. These cells were selected as they are either involved in PNI or have well-documented interactions and effects on SCs, including repair post-nerve injury and SC migration.
Literature Search Strategy
To obtain relevant literature related to SC involvement and its intercellular interactions in nerve repair and regeneration for this review, we searched the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) for work that had been published between 1950 to 2020. Search term combinations included “Schwann cells” (MeSH terms) AND “nerve repair” OR “macrophages” OR “fibroblasts” OR “repair Schwann cells” OR “axon regeneration”. We further narrowed down the obtained search results using publication titles and abstracts retrieved using the stated search terms. We excluded literature that did not address PNI, nerve-associated cells, or neural regeneration.
Overview of Schwann Cell Responses to Nerve Injury
Following PNI, the cellular and structural components of the nerve experience a rapid shift in environmental cues and physical interactions that shape the behavior of the cell constituents in the repair and regeneration process. Wallerian degeneration (WD) involves many components of the nerve, as well as exogenous participants induced by injury, and is initiated by the degradation of damaged axons. Axonal breakdown triggers physical SC dissociation from the axons, which induces a dedifferentiation of SCs toward an immature repair phenotype and an influx and activation of macrophages for debris cleanup (Waller, 1851; Lunn et al., 1989; Gaudet et al., 2011; Rotshenker, 2011; Chen et al., 2015). These series of events are necessary for successful axonal regeneration and repair of the damaged peripheral nerve, which is a beneficial characteristic that differentiates the peripheral and central nervous systems. In a normal, uninjured peripheral nerve, mature SCs serve to myelinate larger diameter fibers, while non-myelinating SCs, also referred to as Remak SCs, wrap themselves around small sensory and autonomic axons (Jessen and Mirsky, 2019). Myelinating SCs are larger than Remak cells, owing to the extensive area of myelin that wraps around axons (Jessen and Mirsky, 2016, 2019). Despite the differences in these two SC types, both play important roles in the protection and repair of the injured nerve and their neuronal components. Perineurium and epineurium connective tissue encapsulate SCs and their associated nerve fibers, and are composed of fibroblasts. In peripheral nerve crush injuries, the surrounding epineurium of the nerve remains intact, and is thus easier to regenerate post-injury, while injuries that physically disrupt the integrity of the nerve and its epineurium (e.g., nerve transection) cause more severe nerve damage and require a more extensive repair response. As such, functional recovery is relatively fast in crush PNI. For example, considerable functional improvement can be observed after only several days to 2 weeks in rat facial nerve crush injury (Lal et al., 2008; Rivera et al., 2017).
For more common nerve transection injuries, the surrounding peri- and epineurium, blood vessels, and myelinated and unmyelinated axons are severed, which leaves a proximal and distal nerve stump that require reconnection to allow for repair and regeneration of damaged axons and associated cells within the nerve. Although nerves can undergo repair and regeneration following surgical reconnection of the nerve endings, functional recovery is slow and nerve function is often sub-optimal compared with pre-injury functional ability (Brown et al., 2019). Owing to the various cell types involved in the injury, as well as the influx of cells that are not intrinsic to the nerve (i.e., circulating macrophages), the SC functional interplay between these cells is important during the repair and regeneration process. Indeed, SCs can recruit such cells to the injury site through cytokine secretion, and infiltrating macrophages and other cells release factors that, in turn, influence SC responses. For example, increased nuclear factor-kappa beta in SCs and localized T-cells in the injured nerve can promote macrophage recruitment, potentially through elevated tumor necrosis factor-alpha-mediated activation of nuclear factor-kappa beta (Shubayev and Myers, 2000; Smith et al., 2009).
Much of the behavior of SCs following PNI is guided by intrinsic physiological changes in the SC in response to environmental cues produced by SCs, infiltrating immune cells, axons, and other cells in the surrounding region. Debris cleanup and WD occur rapidly after nerve injury. Shortly following PNI, dedifferentiated SCs begin to migrate from either the proximal or distal nerve stump, or both, to begin forming a connective tissue cable between the remaining nerve endings that will bridge a nerve gap and support axonal regeneration in the post-injury nerve (Torigoe et al., 1996, 1999). This also supports neovascularization of the regenerating nerve (Torigoe et al., 1999), which promotes a change in SCs toward a phenotype known as repair SCs (Jessen and Mirsky, 2016).
Repair Schwann Cells in Nerve Injury and Regeneration
Following PNI, the interaction of myelinating and non-myelinating SCs with axons is disrupted, which alters the environmental cues that modulate SC differentiation status. As such, the SCs at and near the site of injury dedifferentiate toward a precursor cell phenotype that is similar to that observed during development (Jessen and Mirsky, 2008), while also exhibiting marked transcription changes that are unique to SCs responding to acute injury (Jessen and Arthur-Farraj, 2019). At this stage, the response of SCs to various environmental cues initiated by injury facilitates their transition toward a repair phenotype, called repair SCs. Specific modulations of SC transcription in response to PNI include alterations in c-Jun expression, epigenetic histone modifications, increased expression of signal transducer and activator of transcription 3, and increased cytokine/chemokine secretion for the recruitment of macrophages and other immune cells to the injury site (Gaudet et al., 2011; Arthur-Farraj et al., 2012; Hung et al., 2015; Ma et al., 2016; Stratton and Shah, 2016), which facilitates a multi-cellular framework for cleanup and repair.
Acutely following injury, reprogrammed SCs release cytokines that assist in the infiltration of numerous macrophages to the injury site, where they begin secreting various neurotrophic factors. These neurotrophic factors include brain-derived neurotrophic factor, glial cell-line neurotrophic factor, and fibroblast growth factor (FGF), all of which have potential a pro-regenerative influence on damaged and regenerating axons (Namgung, 2014). Immature SCs affected by injury upregulate c-Jun, part of the activator protein-1 transcription factor, which induces the expression of trophic factors and promotes establishment of the repair SCs (Arthur-Farraj et al., 2012; Fontana et al., 2012; Huang et al., 2015). This injury-induced upregulation of c-Jun expression has been shown to be mediated through mitogen-activated kinase signaling, and in particular, extracellular-related kinase 1/2 (Blom et al., 2014). Upregulation of activating transcription factor 3 (ATF3) is also triggered acutely in SCs near and along the extent of the nerve following transection injury (Blom et al., 2014), and is considered a key marker of PNI. ATF3 is a transcription factor that has been reported to be upregulated by both repair SCs and neurons post-PNI, and is important for proper axonal re-generation and subsequent nerve repair (Stenberg et al., 2012; Blom et al., 2014; Gey et al., 2016; Wahane et al., 2019). Aside from axonal regeneration, a recent electromyography study reported that ATF3 plays a role in improving the functional outcome, whereby target musculature is functionally preserved or reinnervated (Holland et al., 2019). Much like the increase in c-Jun observed in SCs following PNI, ATF3 upregulation has also been shown to be dependent on mitogen-activated kinase signaling/extracellular-related kinase 1/2 signaling, and may be involved in acute SC proliferation in the injured nerve (Blom et al., 2014). However, because the SC and the neuron affected by PNI are both influenced by ATF3 expression and function, their overall or combined mechanism in promoting nerve regeneration remains unclear.
Repair SCs migrate toward the site of injury to construct a bridge across the injury gap in support of axon regeneration into the distal portion of the nerve; simultaneously, EphrinB2/EphB2 signaling via the sex determining region Y-box 2 promotes presentation of the cell adhesion molecule N-cadherin on the membrane surface of the SC (Parrinello et al., 2010; Torres-Mejía et al., 2020). The expression of N-cadherin allows for an SC-SC interaction that is necessary for SC organization into the nerve bridge and appropriate axon regeneration (Parrinello et al., 2010; Torres-Mejía et al., 2020). Nerve fibroblast secretion of EphrinB and its interaction with the EphB receptor on the SC surface have also been shown to play a role in promoting SC-induced nerve bridge formation and axonal regeneration (Dun and Parkinson, 2020).
Molecular interactions between SCs and axons are also integral for axon regeneration, as well as for maturation of the SC toward a myelinating phenotype and the targeting of regenerating axons (Chen et al., 2007; Chang et al., 2012; Glenn and Talbot, 2013). To support axonal growth during the repair process, proteins on the axon and SC promote their interaction and trigger SC maturation and the myelination program (Lemons and Condic, 2008). Integrins are a major protein involved in this interaction. Studies have indicated that the expression of one particular integrin, β1 integrin, increases in SCs following nerve injury and stimulates the regeneration of damaged axons (Chang et al., 2012). In addition, α6 and β1 integrin subunits produced by SCs distal to the injury site in the injured nerve have been shown to be important for axon growth through and beyond the site of nerve damage (Chang et al., 2018). Axon-SC interaction has long been a topic of interest, and it is clear that SCs are required to dedifferentiate and migrate toward the injury site, build a bridge for axonal regeneration, and myelinate the regenerating nerve fibers during the repair process. Meanwhile, many molecular processes, including epigenetic modifications, serve key functions in peripheral axons and their cell bodies; changes in these processes influence their interaction of peripheral axons and cell bodies with SCs and their regenerative potential (Shin and Cho, 2017; Tedeschi and Bradke, 2017; Hahn et al., 2019; Wahane et al., 2019; Shen et al., 2020). Many cell types play a role in the response to PNI via their interaction with SCs, which involves their activation, migration, and organization for nerve injury repair.
Macrophages
Macrophages rapidly enter the damaged nerve site in large numbers following insult due to nerve vascular disruption, but they are also recruited to aid in clearing debris following the onset of WD of the distal nerve segment. SCs play a role in the recruitment of macrophages to the injury site through the expression of monocyte chemoattractant protein-1 (Stratton and Shah, 2016) and leukemia inhibitory factor (Tofaris et al., 2002), among other factors and cytokines (Martini et al., 2008; Rotshenker, 2011). Modulation of macrophage polarity to that of an anti-inflammatory M2 macrophage has also been linked to monocyte chemoattractant protein-1 expression and secretion by SCs in an animal model of facial nerve injury (Stratton and Shah, 2016; Kano et al., 2017). In turn, the recruited M2 polarized macro-phages promote the development of an SC bridge rather than having a fibrotic contribution to nerve reparation, and ultimately improve regeneration and functional recovery.
FGF9 is expressed in SCs. Recent research has shown that FGF9 is downregulated in SCs of the injured nerve for as long as 1 month post-injury, and that this FGF9 dowregulation in SCs affects the macrophage response to injury via increased proinflammatory cytokine expression at the injury site (Lv et al., 2019). FGF9 was also recently demonstrated to play an integral role in SC maturation, myelination, and inflammation following nerve injury (Deng et al., 2018). In that study, knock-out of Fgf9 limited SC morphological changes and decreased inflammatory cytokine secretion, and subsequently delayed macrophage infiltration. Specifically, FGF9 appears to be important for the proliferative and differentiation capacity of SCs and the corresponding effects on macrophages; in one study, more repair SCs accumulated and debris cleanup was reduced with a loss of FGF9 (Lv et al., 2019). Part of this macrophage influence may also be due to improved stability of the blood-nerve barrier under these conditions, which possibly results from SC physiological modifications (Lv et al., 2019). Nevertheless, providing exogenous FGF9 reverses these alterations to the nerve injury responses, and this effect may be mediated by a mechanism involving FGF9-mediated stimulation of extracellular related kinase 1/2 activation in SCs (Deng et al., 2018; Lv et al., 2019).
If the immune response affecting macrophages and SCs is suppressed, regeneration is sub-optimal (Scheib and Höke, 2016). Age plays a role in the influence of macrophage and SC functions in debris clearance and WD, with more remaining debris observed in the distal portion of the nerve due to an age-related reduction in SC- and macrophage-mediated cleanup, which hinders axon regeneration (Tanaka et al., 1992; Kang and Lichtman, 2013). Such studies have demonstrated that both the infiltration of macrophages and the migration and dedifferentiation of SCs are essential for effective axon regeneration after nerve injury, although our understanding of the influence of macrophages on the migration of SCs post-injury has only improved in recent years.
Intercellular communication between the two cell types has been shown to influence SC behavior post-nerve injury. In 2015, one study reported that macrophage-derived microvesicles, specifically M2 macrophage microvesicles, promoted SC proliferation and migration in vitro and in a rat model of sciatic nerve transection (Zhan et al., 2015). The physiological response of SCs to these microvesicles was shown to include upregulated nerve growth factor (NGF) and laminin, which has therapeutic implications for the promotion of axonal regeneration. That study indicated that anti-inflammatory M2 macrophages secrete microvesicles that carry a specific microRNA, miR-223, and that this was associated with modified proliferative and migratory capabilities of SCs. This evidence was supported by transfection of M2 microvesicles with miR-223 inhibitor and subsequent incubation with SCs, whereby downregulation of the SC functions was observed (Zhan et al., 2015). As such, the activation and inflammatory status of present macrophages can influence SC effects, and, potentially, axon regeneration and nerve repair. However, experimental evidence is needed to confirm a connection between miR-223 and modulation of SC physiology, such as the increase in c-Jun expression or function.
As described earlier, c-Jun activation promotes the expression of trophic factors that are important for axon growth, including glial cell-line neurotrophic factor and its related family member, artemin (Fontana et al., 2012). Injury itself induces upregulation of c-Jun (Shy et al., 1996) and the production of these trophic factors, as well as NGF (Heumann et al., 1987) and brain-derived neurotrophic factor (Heumann et al., 1987). Given that injury causes an upregulation of the NGF receptor in SCs (Taniuchi et al., 1986), NGF could be supportive for SCs as well as axons. Glial cell-line neurotrophic factor administration has been found to enhance SC dedifferentiation, migration, and myelination in the injured peripheral nerve (Höke et al., 2003). NGF has also been shown to significantly enhance SC migration in vitro and ex vivo (Anton et al., 1994). Neurotrophin-3 has also been shown to promote SC migration (Yamauchi et al., 2004) and peripheral axon regeneration (Carvalho et al., 2019), and evidence indicates that SC migratory behavior is mediated via the c-Jun pathway in SCs (Yamauchi et al., 2003). Interestingly, the loss of c-Jun in SCs also decreases macrophage density in the injured peripheral nerve, which indicates that there is a close intercellular communication network between SCs and macrophages for promoting debris cleanup and WD progression.
Macrophage-derived slit guidance ligand 3 (Slit-3) also has an influence on fibroblasts through its interaction with roundabout guidance receptor 1 (Robo-1). Robo-1 is a cell surface receptor for Slit family proteins and a known modulator of axonal guidance in the peripheral nervous system (Blockus and Chédotal, 2016). It has also been recently shown to influence SC migration and function in repairing nerve damage and promoting regeneration (Dun et al., 2019). Expression of Slit and Robo proteins are known to be upregulated in the injured nerve (Carr et al., 2017; Chen et al., 2020), and the production and secretion of Slit-3 by macrophages in the injured nerve signals to migrating SCs via binding to the SC-expressed receptor Robo-1 (Dun et al., 2019). This effect is believed to be mediated via SC-expressed sex determining region Y-box 2, as a loss of this transcription factor has been found to reduce SC migratory ability and reparation of the damaged nerve. Likewise, the loss of Slit-3 and Robo-1 can lead to abnormal fibroblast and SC migration and aberrant axon regeneration (Dun et al., 2019), which strongly supports the idea that there is a critical connection between macrophage Slit-3 and SC influence in the repair of the damaged peripheral nerve. Along with communication and interaction, SCs and macrophages have been shown to cooperate in the process of myelin debris clearance through SC-mediated upregulation of TAM (Tyro3, Axl, Mer) receptor-mediated phagocytosis and autophagy post-nerve injury (Brosius Lutz et al., 2017). A recent study also reported that other immune cells, such as neutrophils, play a critical role in myelin debris clearance after PNI; this further highlights the complexity of this multicellular process and the role of immune and inflammatory processes in peripheral nerve repair (Lindborg et al., 2017). Despite such developments in the field, the relationship between nervous system cells and immune cells remains complicated, and additional research will be required to shed light on the interactions between macrophages and SCs in PNI.
Fibroblasts
Fibroblasts are a key cell type within the peripheral nerve microenvironment. However, their role in nerve injury and regeneration, as well as in SC function, has not been extensively studied. Upon transection injury, fibroblasts are not only damaged, but, along with SCs, also respond to the damage for an initial clearing of tissue debris. As SCs rapidly dedifferentiate and migrate to begin the repair process and form Bands of Büngner to initiate the regenerative response, fibroblasts contribute to this process both by interacting with SCs and producing and secreting extracellular matrix molecules (ECMs) (Li and Wang, 2011; Zhang et al., 2016). The use of fibroblast-derived ECMs has even been shown to support the production of a decellularized nerve graft (Harris et al., 2017). The involvement of fibroblasts in the nerve repair process is particularly important in transection injures in which nerve sheaths are damaged, which allows fibroblasts to infiltrate the site of damage (Bobarnac Dogaru et al., 2018).
One method that fibroblasts use to respond and communicate with local cells following injury is their release of ECMs called tenascins (Zhang et al., 2016). Tenascins are large ECM glycoproteins in vertebrates, one of which, tenascin-C (TNC), plays a role in the modulation of neurite extension and directionality (Chiquet-Ehrismann et al., 1988; Meiners et al., 1999). It has been shown that TNC is increased in the proximal nerve segment post-injury, and that fibroblasts overexpressed TNC after PNI (Zhang et al., 2016). This upregulation of TNC by fibroblasts was shown to promote SC migration, but not proliferation, in response to nerve injury, and the influence of TNC on SCs was achieved via direct binding of TNC to SC-expressed β1 integrin (Zhang et al., 2016). The study’s evidence further suggests that fibroblast-mediated SC migration via a TNC-β1 integrin interaction enhanced axon regeneration. Much like SC-SC interactions, fibroblasts and SCs appear to require a physical interaction if they are to promote the necessary migration of SCs for bridging the injury site and enhance axon regeneration after PNI. Fibroblasts have also been shown to promote vascularization of tissue through trophic factor and cytokine expression and secretion (Sorrell and Caplan, 2009; Dun and Parkinson, 2020). Given that SC bridge formation and vacularization are important in the repair and survival of the damaged nerve and associated cells, these processes likely involve complex fibroblast-mediated cell migration within and into the injured nerve tissue (Figure 1). Additional research will hopefully reveal more about the role of fibroblasts in SC behavior and nerve repair.
Figure 1.
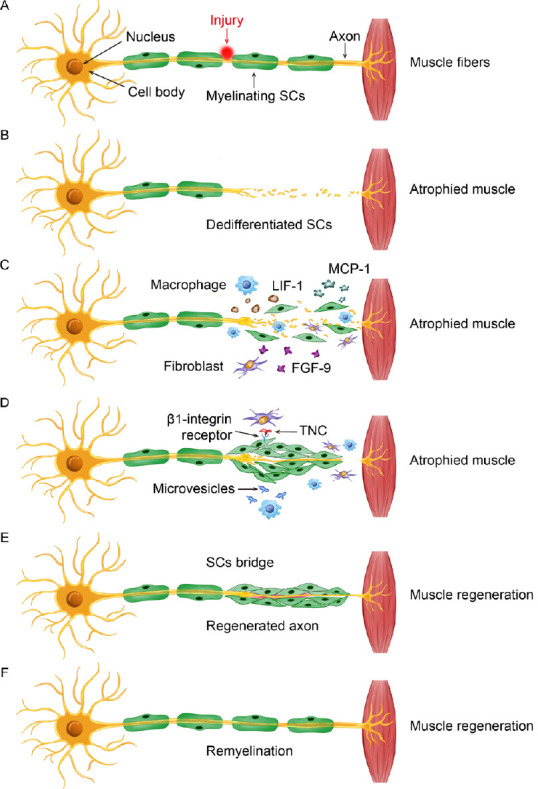
Schematic showing the general sequence of events following PNI involving fibroblasts, macrophages, and SCs to promote SC migration and nerve repair.
(A) Example of injury to a motor nerve projecting from the ventral horn of the spinal cord to target muscle tissue in the periphery. The cell body is located in the spinal cord, while the SC myelinated axon projects toward a target, where it synapses and initiates its action. (B) Nerve injury damages the integrity of the peripheral axon and induces Wallerian degeneration. (C) In response to injury, debris clearance and repair is initiated by activated SCs, recruited macrophages, and disrupted fibroblasts. Paracrine signaling involving MCP-1, LIF-1, and FGF-9 that is secreted from these cells facilitates a multicellular response to the injured nerve. (D) There is a fibroblast influence on SC behavior, which is partly mediated through direct binding of TNC to SC-expressed β1 integrin. Likewise, microvesicles released by activated macrophages also affect SC function in the repair process and depend on the inflammatory status of macrophages recruited to the injury site. (E) These cellular interactions contribute to the promotion of SC establishment of a bridge across the injury site for supporting axon regeneration and eventual myelination. (F) A repaired and regenerated peripheral nerve. FGF-9: Fibroblast growth factor 9; LIF-1: leukemia inhibitory factor-1; MCP-1: monocyte chemoattractant protein-1; PNI: peripheral nerve injury; SC: Schwann cell; TNC: tenascin-C.
Clinical Relevance
From a clinical perspective, SCs are well known to be critical players in repairing damaged nerves via the mechanisms described in this review. As such, SCs have been utilized and manipulated in a variety of ways to improve nerve repair and regeneration, with hopes of promoting better functional outcomes. The timing of nerve repair post-injury is a major consideration in the prognosis of outcome, and influences SC responses and other integral nerve components that are involved in the nerve repair process (Höke, 2011; Ronchi et al., 2017). Delayed nerve repair has been demonstrated to negatively affect SC function that are associated with modified expression of characteristic SC markers (Ronchi et al., 2017). In addition, when peripheral nerve repair is delayed, axon regeneration is diminished and functional outcomes are poor. Downregulated expression of ATF3 in both SCs and neurons following delayed PNI repair is correlated with reduced regeneration and functional recovery (Saito and Dahlin, 2008); however, further research is necessary to establish a causative connection between these events. In addition, various factors concerning the type of repair need to be taken into consideration. Direct reconnection of the two severed ends of a nerve are not usually amenable to direct reconnection, so nerve grafts are used to bridge the gap between the proximal and distal stumps. Which of these grafts are used, how they have been modified, and the patient’s response to them can potentially affect regenerative and functional outcomes that involve SCs and other cellular functions.
Recent research has focused on the modifications of different nerve grafts and scaffolds to improve axonal regeneration and related benefits to nerve repair. The promotion of axon regeneration and nerve repair by neurotrophic factors has long been studied, and we have performed studies integrating stem cells, SCs, and engineered versions of these to either overexpress trophic factors or other intracellular proteins to improve trophic factor production and secretion (Wang et al., 2017; Li et al., 2019). Krüppel-like factor 7, a zinc-finger DNA-binding protein family member, promotes axonal regeneration following nerve injury (Wang et al., 2017). When Krüppel-like factor 7 was virally transfected into SCs that were then seeded into an acellular nerve allograph and engrafted in a rodent sciatic nerve gap, trophic factor NGF, as well as growth-associated protein-43 and trophic factor receptors, were upregulated in the transplanted SCs. Alongside these changes, axon regeneration and myelination into and through the graft were enhanced (Wang et al., 2017). In a similar animal model, bone marrow-derived stem cells were transfected with Krüppel-like factor 7 and seeded into an acellular autologous nerve allograft in rats; motor axon regeneration and neuromuscular innervation were also enhanced (Li et al., 2019). Preclinical studies such as these demonstrate that a patient’s own SCs could be harvested, expanded, and engineered to potentially improve outcomes following PNI.
Although nerve grafts are not new in clinical practice, the modification of grafts with engineered cells to promote regeneration and function has been limited. However, one recent study utilized a canine ansa cervicalis to culture autologous SCs, and seeded them into a collagen scaffold (Chitose et al., 2017). Transplantation of this SC-seeded scaffold into a 20-mm recurrent laryngeal nerve lesion in canines promoted axon regeneration, with evidence of myelination in the graft. In addition, partial vocal fold function was observed (Chitose et al., 2017). Alignment of the SCs in the scaffold was important, and use of this technique is not consistent in animal models of nerve grafts. While this was not a human study, it presents promising findings for the application of this nerve grafting approach in the repair of injured human peripheral nerves, and highlights its potential to enhance axonal growth, myelination, and functional recovery.
Conclusion
In this review, we have discussed several key cell types that play a role in mediating or modulating SC migration following PNI. We have also highlighted interesting related findings to provide some context and contrast with our focus on SC migration in PNI. Concerning a macrophage- and fibroblast-mediated influence on SC migration following PNI, there is crosstalk, chemically and/or physically, between these cell types that are ultimately beneficial for nerve repair and regeneration. This is most evident in the acute stages following PNI. Despite the complexities of the cellular makeup and reaction to injury in peripheral nerves, the multitude of ways SCs interact with macrophages and fibroblasts supports our knowledge of the pro-regenerative aspect of the peripheral nervous system versus the central nervous system. Further research is required to better understand all the cellular interactions discussed in this paper. Nevertheless, the current review of the literature serves as a valuable overview and assessment of the studies to date on intercellular interactions that affect SC migration in PNI.
Footnotes
Conflicts of interest: None declared.
Financial support: This work was also supported by the National Natural Science Foundation of China, No. 81901365 (to WRQ); Jilin Science and Technology Agency Funds in China, Nos. 20180101118JC (to RL), 20180520115JH (to BPC) and 20190103076JH (to WRQ).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was also supported by the National Natural Science Foundation of China, No. 81901365 (to WRQ); Jilin Science and Technology Agency Funds in China, Nos. 20180101118JC (to RL), 20180520115JH (to BPC) and 20190103076JH (to WRQ).
P-Reviewer: Calliari A; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Cason N, Yu J, Song LP; T-Editor: Jia Y
Open peer reviewer: Aldo Calliari, Universidad de la República, Uruguay.
References
- 1.Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci U S A. 1994;91:2795–2799. doi: 10.1073/pnas.91.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blockus H, Chédotal A. Slit-Robo signaling. Development. 2016;143:3037–3044. doi: 10.1242/dev.132829. [DOI] [PubMed] [Google Scholar]
- 4.Blom CL, Mårtensson LB, Dahlin LB. Nerve injury-induced c-Jun activation in Schwann cells is JNK independent. Biomed Res Int. 2014;2014:392971. doi: 10.1155/2014/392971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobarnac Dogaru GL, Juneja SC, Shokrani A, Hui RY, Chai Y, Pepper JP. The role of Hedgehog-responsive fibroblasts in facial nerve regeneration. Exp Neurol. 2018;303:72–79. doi: 10.1016/j.expneurol.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Bombeiro AL, Lima BHM, Bonfanti AP, Oliveira ALR. Improved mouse sciatic nerve regeneration following lymphocyte cell therapy. Mol Immunol. 2020;121:81–91. doi: 10.1016/j.molimm.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Brosius Lutz A, Chung WS, Sloan SA, Carson GA, Zhou L, Lovelett E, Posada S, Zuchero JB, Barres BA. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc Natl Acad Sci U S A. 2017;114:E8072–8080. doi: 10.1073/pnas.1710566114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown BL, Asante T, Welch HR, Sandelski MM, Drejet SM, Shah K, Runge EM, Shipchandler TZ, Jones KJ, Walker CL. Functional and anatomical outcomes of facial nerve injury with application of polyethylene glycol in a rat model. JAMA Facial Plast Surg. 2019;21:61–68. doi: 10.1001/jamafacial.2018.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr L, Parkinson DB, Dun XP. Expression patterns of Slit and Robo family members in adult mouse spinal cord and peripheral nervous system. PLoS One. 2017;12:e0172736. doi: 10.1371/journal.pone.0172736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho CR, Oliveira JM, Reis RL. Modern trends for peripheral nerve repair and regeneration: beyond the hollow nerve guidance conduit. Front Bioeng Biotechnol. 2019;7:337. doi: 10.3389/fbioe.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang IA, Kim KJ, Namgung U. α6 and β1 integrin heterodimer mediates schwann cell interactions with axons and facilitates axonal regeneration after peripheral nerve injury. Neuroscience. 2018;371:49–59. doi: 10.1016/j.neuroscience.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 12.Chang IA, Oh MJ, Kim MH, Park SK, Kim BG, Namgung U. Vimentin phosphorylation by Cdc2 in Schwann cell controls axon growth via β1-integrin activation. FASEB J. 2012;26:2401–2413. doi: 10.1096/fj.11-199018. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Carr L, Dun XP. Dynamic expression of Slit1-3 and Robo1-2 in the mouse peripheral nervous system after injury. Neural Regen Res. 2020;15:948–958. doi: 10.4103/1673-5374.268930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130:605–618. doi: 10.1007/s00401-015-1482-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 16.Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988;53:383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- 17.Chitose SI, Sato K, Fukahori M, Sueyoshi S, Kurita T, Umeno H. Recurrent laryngeal nerve regeneration using an oriented collagen scaffold containing Schwann cells. Laryngoscope. 2017;127:1622–1627. doi: 10.1002/lary.26389. [DOI] [PubMed] [Google Scholar]
- 18.Deng B, Lv W, Duan W, Liu Y, Li Z, Song X, Cui C, Qi X, Wang X, Li C. FGF9 modulates Schwann cell myelination in developing nerves and induces a pro-inflammatory environment during injury. J Cell Biochem. 2018;119:8643–8658. doi: 10.1002/jcb.27105. [DOI] [PubMed] [Google Scholar]
- 19.Dun XP, Parkinson DB. Classic axon guidance molecules control correct nerve bridge tissue formation and precise axon regeneration. Neural Regen Res. 2020;15:6–9. doi: 10.4103/1673-5374.264441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dun XP, Carr L, Woodley PK, Barry RW, Drake LK, Mindos T, Roberts SL, Lloyd AC, Parkinson DB. Macrophage-derived Slit3 controls cell migration and axon pathfinding in the peripheral nerve bridge. Cell Rep. 2019;26:1458–1472e1454. doi: 10.1016/j.celrep.2018.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol. 2012;198:127–141. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gey M, Wanner R, Schilling C, Pedro MT, Sinske D, Knöll B. Atf3 mutant mice show reduced axon regeneration and impaired regeneration-associated gene induction after peripheral nerve injury. Open Biol. 2016;6:160091. doi: 10.1098/rsob.160091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glenn TD, Talbot WS. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr Opin Neurobiol. 2013;23:1041–1048. doi: 10.1016/j.conb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn I, Voelzmann A, Liew YT, Costa-Gomes B, Prokop A. The model of local axon homeostasis - explaining the role and regulation of microtubule bundles in axon maintenance and pathology. Neural Dev. 2019;14:11. doi: 10.1186/s13064-019-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris GM, Madigan NN, Lancaster KZ, Enquist LW, Windebank AJ, Schwartz J, Schwarzbauer JE. Nerve guidance by a decellularized fibroblast extracellular matrix. Matrix Biol. 2017;60-61:176–189. doi: 10.1016/j.matbio.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Höke A. A (heat) shock to the system promotes peripheral nerve regeneration. J Clin Invest. 2011;121:4231–4234. doi: 10.1172/JCI59320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Höke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci. 2003;23:561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland SD, Ramer LM, McMahon SB, Denk F, Ramer MS. An ATF3-CreERT2 knock-in mouse for axotomy-induced genetic editing: proof of principle. eNeuro. 2019;6:ENEURO0025–00192019. doi: 10.1523/ENEURO.0025-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Quan X, Liu Z, Ma T, Wu Y, Ge J, Zhu S, Yang Y, Liu L, Sun Z, Huang J, Luo Z. c-Jun gene-modified Schwann cells: upregulating multiple neurotrophic factors and promoting neurite outgrowth. Tissue Eng Part A. 2015;21:1409–1421. doi: 10.1089/ten.tea.2014.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung HA, Sun G, Keles S, Svaren J. Dynamic regulation of Schwann cell enhancers after peripheral nerve injury. J Biol Chem. 2015;290:6937–6950. doi: 10.1074/jbc.M114.622878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 34.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jessen KR, Mirsky R. The success and failure of the Schwann cell response to nerve injury. Front Cell Neurosci. 2019;13:33. doi: 10.3389/fncel.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jessen KR, Arthur-Farraj P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia. 2019;67:421–437. doi: 10.1002/glia.23532. [DOI] [PubMed] [Google Scholar]
- 37.Kang H, Lichtman JW. Motor axon regeneration and muscle reinnervation in young adult and aged animals. J Neurosci. 2013;33:19480–19491. doi: 10.1523/JNEUROSCI.4067-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kano F, Matsubara K, Ueda M, Hibi H, Yamamoto A. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 synergistically regenerate transected rat peripheral nerves by altering macrophage polarity. Stem Cells. 2017;35:641–653. doi: 10.1002/stem.2534. [DOI] [PubMed] [Google Scholar]
- 39.Lal D, Hetzler LT, Sharma N, Wurster RD, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation facilitates rat facial nerve recovery from a crush injury. Otolaryngol Head Neck Surg. 2008;139:68–73. doi: 10.1016/j.otohns.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Lemons ML, Condic ML. Integrin signaling is integral to regeneration. Exp Neurol. 2008;209:343–352. doi: 10.1016/j.expneurol.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability. 2011;20:108–120. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li WY, Zhu GY, Yue WJ, Sun GD, Zhu XF, Wang Y. KLF7 overexpression in bone marrow stromal stem cells graft transplantation promotes sciatic nerve regeneration. J Neural Eng. 2019;16:056011. doi: 10.1088/1741-2552/ab3188. [DOI] [PubMed] [Google Scholar]
- 43.Lindborg JA, Mack M, Zigmond RE. Neutrophils are critical for myelin removal in a peripheral nerve injury model of wallerian degeneration. J Neurosci. 2017;37:10258–10277. doi: 10.1523/JNEUROSCI.2085-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of wallerian degeneration does not hinder regeneration in peripheral nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 45.Lv W, Deng B, Duan W, Li Y, Song X, Ji Y, Li Z, Liu Y, Wang X, Li C. FGF9 alters the Wallerian degeneration process by inhibiting Schwann cell transformation and accelerating macrophage infiltration. Brain Res Bull. 2019;152:285–296. doi: 10.1016/j.brainresbull.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Ma KH, Hung HA, Svaren J. Epigenomic regulation of Schwann cell reprogramming in peripheral nerve injury. J Neurosci. 2016;36:9135–9147. doi: 10.1523/JNEUROSCI.1370-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martini R, Fischer S, López-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008;56:1566–1577. doi: 10.1002/glia.20766. [DOI] [PubMed] [Google Scholar]
- 48.Meiners S, Mercado ML, Nur-e-Kamal MS, Geller HM. Tenascin-C contains domains that independently regulate neurite outgrowth and neurite guidance. J Neurosci. 1999;19:8443–8453. doi: 10.1523/JNEUROSCI.19-19-08443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Namgung U. The role of Schwann cell-axon interaction in peripheral nerve regeneration. Cells Tissues Organs. 2014;200:6–12. doi: 10.1159/000370324. [DOI] [PubMed] [Google Scholar]
- 50.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 51.Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivera A, Raymond M, Grobman A, Abouyared M, Angeli SI. The effect of n-acetyl-cysteine on recovery of the facial nerve after crush injury. Laryngoscope Investig Otolaryngol. 2017;2:109–112. doi: 10.1002/lio2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ronchi G, Cillino M, Gambarotta G, Fornasari BE, Raimondo S, Pugliese P, Tos P, Cordova A, Moschella F, Geuna S. Irreversible changes occurring in long-term denervated Schwann cells affect delayed nerve repair. J Neurosurg. 2017;127:843–856. doi: 10.3171/2016.9.JNS16140. [DOI] [PubMed] [Google Scholar]
- 54.Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saito H, Dahlin LB. Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci. 2008;9:88. doi: 10.1186/1471-2202-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheib JL, Höke A. An attenuated immune response by Schwann cells and macrophages inhibits nerve regeneration in aged rats. Neurobiol Aging. 2016;45:1–9. doi: 10.1016/j.neurobiolaging.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Shen YY, Gu XK, Zhang RR, Qian TM, Li SY, Yi S. Biological characteristics of dynamic expression of nerve regeneration related growth factors in dorsal root ganglia after peripheral nerve injury. Neural Regen Res. 2020;15:1502–1509. doi: 10.4103/1673-5374.274343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin JE, Cho Y. Epigenetic regulation of axon regeneration after neural injury. Mol Cells. 2017;40:10–16. doi: 10.14348/molcells.2017.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83–89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- 60.Shy ME, Shi Y, Wrabetz L, Kamholz J, Scherer SS. Axon-Schwann cell interactions regulate the expression of c-jun in Schwann cells. J Neurosci Res. 1996;43:511–525. doi: 10.1002/(SICI)1097-4547(19960301)43:5<511::AID-JNR1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 61.Smith D, Tweed C, Fernyhough P, Glazner GW. Nuclear factor-kappaB activation in axons and Schwann cells in experimental sciatic nerve injury and its role in modulating axon regeneration: studies with etanercept. J Neuropathol Exp Neurol. 2009;68:691–700. doi: 10.1097/NEN.0b013e3181a7c14e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorrell JM, Caplan AI. Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161–214. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 63.Stenberg L, Kanje M, Dolezal K, Dahlin LB. Expression of activating transcription factor 3 (ATF 3) and caspase 3 in Schwann cells and axonal outgrowth after sciatic nerve repair in diabetic BB rats. Neurosci Lett. 2012;515:34–38. doi: 10.1016/j.neulet.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Stratton JA, Shah PT. Macrophage polarization in nerve injury: do Schwann cells play a role. Neural Regen Res. 2016;11:53–57. doi: 10.4103/1673-5374.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka K, Zhang QL, Webster HD. Myelinated fiber regeneration after sciatic nerve crush: morphometric observations in young adult and aging mice and the effects of macrophage suppression and conditioning lesions. Exp Neurol. 1992;118:53–61. doi: 10.1016/0014-4886(92)90022-i. [DOI] [PubMed] [Google Scholar]
- 66.Taniuchi M, Clark HB, Johnson EM., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tedeschi A, Bradke F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr Opin Neurobiol. 2017;42:118–127. doi: 10.1016/j.conb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torigoe K, Hashimoto K, Lundborg G. A role of migratory Schwann cells in a conditioning effect of peripheral nerve regeneration. Exp Neurol. 1999;160:99–108. doi: 10.1006/exnr.1999.7202. [DOI] [PubMed] [Google Scholar]
- 70.Torigoe K, Tanaka HF, Takahashi A, Awaya A, Hashimoto K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp Neurol. 1996;137:301–308. doi: 10.1006/exnr.1996.0030. [DOI] [PubMed] [Google Scholar]
- 71.Torres-Mejía E, Trümbach D, Kleeberger C, Dornseifer U, Orschmann T, Bäcker T, Brenke JK, Hadian K, Wurst W, López-Schier H, Desbordes SC. Sox2 controls Schwann cell self-organization through fibronectin fibrillogenesis. Sci Rep. 2020;10:1984. doi: 10.1038/s41598-019-56877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wahane S, Halawani D, Zhou X, Zou H. Epigenetic regulation of axon regeneration and glial activation in injury responses. Front Genet. 2019;10:640. doi: 10.3389/fgene.2019.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waller A. Experiments on the section of the glosso-pharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Edinb Med Surg J. 1851;76:369–376. [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Li WY, Jia H, Zhai FG, Qu WR, Cheng YX, Liu YC, Deng LX, Guo SF, Jin ZS. KLF7-transfected Schwann cell graft transplantation promotes sciatic nerve regeneration. Neuroscience. 2017;340:319–332. doi: 10.1016/j.neuroscience.2016.10.069. [DOI] [PubMed] [Google Scholar]
- 75.Wood MD, Mackinnon SE. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp Neurol. 2015;265:171–175. doi: 10.1016/j.expneurol.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamauchi J, Chan JR, Shooter EM. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci U S A. 2003;100:14421–14426. doi: 10.1073/pnas.2336152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamauchi J, Chan JR, Shooter EM. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc Natl Acad Sci U S A. 2004;101:8774–8779. doi: 10.1073/pnas.0402795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yegiyants S, Dayicioglu D, Kardashian G, Panthaki ZJ. Traumatic peripheral nerve injury: a wartime review. J Craniofac Surg. 2010;21:998–1001. doi: 10.1097/SCS.0b013e3181e17aef. [DOI] [PubMed] [Google Scholar]
- 79.Zhan C, Ma CB, Yuan HM, Cao BY, Zhu JJ. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem Biophys Res Commun. 2015;468:343–348. doi: 10.1016/j.bbrc.2015.10.097. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Z, Yu B, Gu Y, Zhou S, Qian T, Wang Y, Ding G, Ding F, Gu X. Fibroblast-derived tenascin-C promotes Schwann cell migration through beta1-integrin dependent pathway during peripheral nerve regeneration. Glia. 2016;64:374–385. doi: 10.1002/glia.22934. [DOI] [PubMed] [Google Scholar]


