Abstract
The intraocular pressure inside the human eye maintains 10–21 mmHg above the atmospheric pressure. Elevation of intraocular pressure is highly correlated with the retinopathy in glaucoma, and changes in the exterior pressure during mountain hiking, air traveling, and diving may also induce vision decline and retinopathy. The pathophysiological mechanism of these pressure-induced retinal disorders has not been completely clear. Retinal neurons express pressure-sensitive channels intrinsically sensitive to pressure and membrane stretch, such as the transient receptor potential channel (TRP) family permeable to Ca2+ and Na+ and the two-pore domain K channel family. Recent data have shown that pressure excites the primate retinal bipolar cell by opening TRP vanilloid 4 to mediate transient depolarizing currents, and TRP vanilloid 4 agonists enhance the membrane excitability of primate retinal ganglion cells. The eyeball wall is constructed primarily by the sclera and cornea of low elasticity, and the flow rate of the aqueous humor and intraocular pressure both fluctuate, but the mathematical relationship between the ocular elasticity, aqueous humor volume, and intraocular pressure has not been established. This review will briefly review recent literature on the pressure-related retinal pathophysiology in glaucoma and other pressure-induced retinal disorders, the elasticity of ocular tissues, and pressure-sensitive cation channels in retinal neurons. Emerging data support the global volume and the elasticity and thickness of the sclera and cornea as variables to affect the intraocular pressure level like the volume of the aqueous humor. Recent results also suggest some potential routes for TRPs to mediate retinal ganglion cell dysfunction: TRP opening upon intraocular pressure elevation and membrane stretch, enhancing glutamate release from bipolar cells, increasing intracellular Na+, Ca2+ concentration in retinal ganglion cells and extracellular glutamate concentration, inactivating voltage-gated Na+ channels, and causing excitotoxicity and dysfunction of retinal ganglion cells. Further studies on these routes likely identify novel targets and therapeutic strategies for the treatment of pressure-induced retinal disorders.
Key Words: glaucoma, ocular elasticity, patch-clamp, pressure-sensitive ion channel, retina, retinal bipolar cell, retinal ganglion cell, TRP
Introduction
Elevated intraocular pressure (IOP) is highly correlated with the retinopathy in glaucoma, and changes in the exterior pressure (EP) during mountain hiking, air traveling, and diving may also induce vision decline. The pathophysiological mechanism of these pressure-induced retinal disorders (PIRDs) has not been completely clear. Retinal neurons express pressure-sensitive ion channels intrinsically sensitive to pressure and membrane stretch, such as the transient receptor potential channels (TRPs) and the two-pore domain K channels. This article will briefly review the literature on the pathophysiology of glaucoma and other PIRDs, the elasticity of ocular tissues, and pressure-sensitive cation channels in retinal neurons in recent decades, especially focusing on those closely related to retinal ganglion cell (RGC) dysfunction, for better understanding the pathophysiological mechanism of acute and chronic PIRDs. Due to the space limitation, glaucoma therapy and the circulation of aqueous humor are not intensively discussed.
The strategy for searching the PubMed database: [neuron] (OR [retina] OR [retinal]) AND [pressure-sensitive] (OR [mechanosensitive]), [glaucoma] AND [retina] (OR [retinal] OR [aqueous humor]), [ocular pressure] AND [diving] (OR [hiking] OR [mountaineer] OR [flight] OR [astronaut]), [vision] (OR [visual]) AND [pressure] (OR [diving] OR [hiking] OR [mountaineer] OR [flight] OR [astronaut]), etc. Google Scholar was used as an addition. Searches were performed mostly in and before 2019, and no date limit was applied. Reports more relevant to the topic and more comprehensive were preferentially selected.
Pressure-Induced Retinal Disorders
Glaucoma is a blinding disease characterized by the axon and soma loss of RGCs, involving multiple risk factors, such as age, race, genetic defects, glutamate excitotoxicity, etc. The elevation of the IOP, either the mean level or the fluctuation (Asrani et al., 2000; Caprioli and Coleman, 2008), has been accepted as the most significant risk factor (Quigley, 2011). Axons of RGCs form the optic nerve to carry visual signals to the visual cortex. Vision loss and RGC death in glaucoma are generally thought to follow IOP-induced optic nerve damage: IOP elevation → optic nerve injury → vision loss and RGC death. Studies from animal models have demonstrated that crushing the optic nerve can indeed retrogradely damage RGC somas. On the other hand, in human patients, the optical coherence tomography and immunobiological studies often show concurrent damage in the nerve fiber layer and RGC somas (Lei et al., 2008; Weinreb et al., 2014). In glaucoma animal models, recent anatomical and functional data support pathological changes in RGC dendrites (Berry et al., 2015; El-Danaf and Huberman, 2015) and synapses of bipolar cells (BCs) (Vrabec and Levin, 2007; Agostinone and Di, 2015; Ou et al., 2016), as well as the loss of highly sensitive BC inputs (Pang et al., 2015) in A2 amacrine cells and ON RGCs before RGC axon loss. These studies do not fully support that RGC axonal loss is the only early event in glaucoma. Instead, they appear to suggest that the early stage of IOP elevation can concurrently affect RGC axons, RGC dendrites, and BC synapses.
Glutamate excitotoxicity is a common mechanism for neurodegeneration, including glaucoma (Hare and Wheeler, 2009). However, whether BCs release more glutamate under elevated IOP to mediate RGC dendritic damage is unclear. Pressure-sensitive transient receptor potential channel vanilloid 4 (TRPV4) has been recently reported in mammalian retinal neurons. TRPV4 immunoreactivity was found in somas and axons of RGCs (Ryskamp et al., 2011; Jo et al., 2015; Gao et al., 2019) and the plexiform layers (Sappington et al., 2015; Taylor et al., 2016; Gao et al., 2019). We have recently observed TRPV4 expression in primate BCs (Gao et al., 2019), where the pressure change/membrane expansion opens TRPV4 and induces transient cation currents with a reversal potential of ~ –10 mV. The half-maximum effect appears at ~20 mmHg, in line with the clinical standard of elevated IOP. Others’ and our data (Ryskamp et al., 2011; Sappington et al., 2015; Taylor et al., 2016; Gao et al., 2019) have also revealed the functional TRPV4 in mammalian RGCs, and activating TRPV4 increases RGC spontaneous firing rate and the excitability. TRP-mediated membrane depolarization and Ca2+ influxes are likely to mediate glutamate release in BCs and excitotoxicity in RGCs, which, as a novel mechanism for glaucoma RGC damage, deserves further investigations. Glutamate excitotoxicity is critically mediated by N-methyl-D-aspartate receptor and Ca2+, and N-methyl-D-aspartate receptor antagonists and Mg2+ have shown a neuroprotective role in RGCs. Mammalian Müller cells also express TRPV4, and opening TRPV4 depolarizes Müller cells (Jo et al., 2015; Netti et al., 2017), which is likely to reduce glutamate intake, increasing the extracellular glutamate concentration ([Glu]o) and triggering the excitotoxicity. Besides TRPV4, retinal neurons also express other types of pressure-sensitive channels, which will be further discussed in the last section.
Congenital glaucoma in children exhibits IOP elevation and the dramatic expansion of the eyeball and cornea (Ho and Walton, 2004; Papadopoulos et al., 2007). Stress and strain like IOP and tissue stretch are a pair of inseparable physical parameters capable of activating mechanical-sensitive ion channels. D2 mice develop congenital glaucoma and are widely used as a glaucoma model. D2 mice may exhibit either IOP elevation (Jakobs et al., 2005), IOP elevation plus eyeball expansion, or normal IOP with eyeball expansion (Pang and Wu, 2014), and RGC loss was observed under these conditions. It has been unclear whether the ocular expansion occurs in normal-tension glaucoma to mediate retinopathy like IOP dose in high-tension glaucoma. Normal-tension glaucoma consists of typical glaucomatous disc and field changes, an open angle, and IOP within the statistically normal range. It has been related to changes in the blood pressure and intracranial pressure, translaminar pressure gradient (Killer and Pircher, 2018), migraine, shock, blood loss, optic disc hemorrhages, etc. (Anderson, 2011). Reducing IOP is also beneficial for some normal-tension glaucoma patients, but the mechanism is unclear.
Changes in the external pressure (EP) for the eye may also affect IOP and vision (Van de Veire et al., 2008). The elevation-caused acute drop in the atmospheric pressure (101 kPa, at the sea level) has been reported to cause low visual acuity, blur vision, visual field defect, and vision loss (McFadden et al., 1981; Hexdall and Butler, 2012) in mountain hikers at 84–75 kPa (Tingay et al., 2003; Horng et al., 2008; Ho et al., 2011), as well as flight passengers (Chang et al., 2004) and pilots (Vecchi et al., 2014) at 93–88 kPa. The capability of teenagers (Karakucuk et al., 2004) to differentiate colors reduces at 91 kPa. Under enhanced EP during diving, it is very common to see retinal damage, too. The standard diving depths for creational and technical divers are below 18 and 50 m (300 and 600 kPa), respectively. About 50% of professional divers show various degrees of retinal lesions (Zhou et al., 2014), and 45% show blue-yellow color vision defect (Macarez et al., 2005). Transient vision loss occurs in some individuals during or after diving (Hexdall and Butler, 2012; Mowatt and Foster, 2013), which sometimes may completely and immediately recover upon surfacing (Hexdall and Butler, 2012). While some structural retinal damage has been documented under these pathological conditions, brief RGC dysfunction or the direct disturbance of pressure on visual signals has not been ruled out. Pressure stress is the primary cause of PIRDs, but the roles of retinal pressure-sensitive ion channels in PIRDs have not been clear.
Chronic ophthalmic changes occur after spaceflight, including the reduction of near visual acuity, changes in the choroid and retina tissues, optic disk edema (Taibbi et al., 2013), a hyperopic shift of refraction, an increase of IOP, and an increase of intracranial pressure (Makarov et al., 2013). These disorders are usually attributed to the loss of the gravity or microgravity, posture changes, and the change in the translaminar pressure (Nelson et al., 1985; Berdahl et al., 2012; Taibbi et al., 2013). Except for the gravity change, EP for the eye in space suits is reduced for 71% for the space shuttle era (to 30 kPa) and 43% for space station era (to 57 kPa) per the data in the space educators’ handbook from NASA. Whether the lowering of EP may contribute to the ophthalmic changes in astronauts has been unknown.
The Elasticity of the Spherical Shell of the Eye
IOP in human eyeballs is normally 10–21 mmHg (1.3–2.8 kPa) higher than the atmospheric pressure. IOP is primarily determined by the volume of aqueous humor and expressed by Pi (IOP) = Pe+ (Fin– Fu)/Ctrab (Kaufman, 2011), where Pe is the episcleral venous pressure, Fin is the inflow/formation rate, Fu is the outflow rate via uveoscleral pathway, and Ctrab is the facility of the outflow through the trabecular pathway. Aqueous humor formation involves active transportation/secretion, ultrafiltration, and diffusion, while a majority of aqueous humor is produced by active transportation and not pressure-dependent. The circulation of the aqueous humor acts to stabilize IOP, but IOP still fluctuates with the heartbeat, breath, and exercise. It shows 2–3 pulses/s in primates (Downs et al., 2011), and the amplitude is up to 10 mmHg and larger under higher IOP levels. IOP also fluctuates 2–6 mmHg with circadian rhythm (Liu et al., 1999; Sugimoto et al., 2006; Li and Liu, 2008). The normal flow rate of the aqueous humor is 2– 2.5 μL/min, which falls by ~60% during the nighttime (Sit et al., 2008; Fan et al., 2011). Fu is measured 1.64 μL/min in normal subjects of 20–30 years, 1.16 μL/min in normal subjects of 60 years, and 0.3 μL/min in glaucoma patients (Toris et al., 1999; Kaufman, 2011). Water has a bulk modulus (K) of 2.2 × 109 Pa. Assuming that the eyeball shell is not expandable, all eyeball contents are composed of water, and the eyeball inner volume (V) is 4.96 mL, every 1 μL of an extra amount of aqueous humor (ΔV) would elevate IOP for 500 mmHg (ΔP = K × ΔV/V). However, such hypothetic IOP fluctuation does not occur, and clinical IOP elevation is usually much less intensive. This is probably due to the elasticity of the eyeball shell.
IOP is a physical parameter of the eyeball. To better understand the harmful effect of the accumulation of aqueous humor in glaucoma, we still need to learn other physical properties of the eyeball. The elasticity or stiffness is defined by the ratio of the stress to the strain/deformation in the elasticity regime. E (Young’s), G (shear), and K (bulk) modulus can be calculated simply by: E = (F × l)/(A × Δl), G = (F × l)/(A × Δx), and K = (F × V)/(A × ΔV), where F/A or P is the linear, shear, and volumetric stress for E, G and K, respectively, and l/Δl, l/Δx, and V/ΔV are the linear, shear, and volumetric strain, respectively. In the human, E was measured 2.45 × 104 Pa in the cornea in vivo (Sjontoft and Edmund, 1987), 6.0 × 105 Pa in strips of choroidal complex, and 1.8–2.9 × 106 Pa in sclera strips (Friberg and Lace, 1988; Eilaghi et al., 2010). In freshly isolated pig eyeballs, it was 0.5–2.4 × 105 Pa for the cornea and 1.5–8.3 × 105 Pa for the sclera (Asejczyk-Widlicka and Pierscionek, 2008). In mouse eyeballs, scleral stiffness was measured 0.36–3.2 × 105 Pa. E may change with age and the younger sclera is significantly more compliant than older sclera in both mouse (Myers et al., 2010) and human (Friberg and Lace, 1988) eyes.
Human eyeball was reported to be ~6.5 mL for the total volume, ~1 mm for the sclera thickness (h), and 310 μL (Va) for the aqueous volume, which gives an eyeball inner volume (V) of 4.96 mL and a radius (R) of 10.58 mm. Taking E of the ocular tissue as 0.6–3 × 106 Pa and the initial length l as 10.58 mm, every 10 mmHg increase of pressure (ΔP) would theoretically linearly stretch the tissue for ~155–777 μm (Δl = (σ × l)/E , σ = (ΔP × R)/2 × h , where σ is the circumferential stress). Interestingly, this theoretical value is well comparable with the calculated stretch on RGC axons induced by the optic disc cupping in chronic glaucoma [~70–646 μm, derived from (Shin et al., 1989; Sigal et al., 2004)]. Eyeball volumetric expansion has been observed in childhood glaucoma (also known as buphthalmias (Mark, 2011)) and inherited glaucoma in D2 mice (Pang and Wu, 2014), which predicts general retinal expansion. Besides V and E, h may also change, and glaucoma animals and patients show thinner sclera (Norman et al., 2010; Nguyen et al., 2013). Thus, it appears that Va, E, V, and h are all variables critically affecting IOP levels. A mathematical relationship between them that is important for better understanding ocular physiology and PIRDs is still missing.
Pressure-Sensitive Ion Channels in Retinal Neurons
Pressure and membrane stretch are suitable stimuli for some ion channels expressed in the plasma membrane of cells, and these channels can be directly opened by force (Liu and Montell, 2015), known as mechano-gated or mechanical sensitive channels. TRPs are variably modulated by temperature, membrane tension, osmolality, phorbol esters, and G-protein-mediated regulation (Clapham, 2007; Liu and Montell, 2015). TRPs include seven subfamilies, namely TRPC (canonical), TRPV, TRPM (melastatin), TRPN (NOMPC), TRPA (ANKTM1), TRPP (polycystin), and TRPML (mucolipin) (Montell, 2005; Nilius and Szallasi, 2014). The retina expresses multiple types of TRPs, and TRPV4 (Tan et al., 2006; Krizaj, 2016) and TRPV1 (Sappington et al., 2015) have been suggested to contribute to glaucoma. TRPV4 opens by pressure (Suzuki et al., 2003), membrane stretch (Liedtke et al., 2000), warm temperature and specific pharmacological agonists like GSK1016790A (GSK) and 4 alpha-phorbol 12,13-didecanoate (4α PDD) (Nilius and Szallasi, 2014). TRPV4 is expressed in mammalian RGCs, BCs, Müller cells, and the plexiform layers. In our recent data, TRPV4 immunoreactivity in the primate retina exhibited a low-intensity component in Müller cells and amacrine cells and a high-intensity component in plexiform layers, and RGCs and BCs showed both the components. TRPV4 agonists increase the spontaneous firing rate of mouse RGCs and mediate apoptosis of cultured RGCs (Ryskamp et al., 2011). TRPV4 antagonists enhance RGCs survival rate in retinal explants (Taylor et al., 2016). Large RGC somas are more vulnerable than smaller ones in glaucoma patients and animal models (Glovinsky et al., 1991; Quigley, 1999; Shou et al., 2003; Filippopoulos et al., 2006), and the large somas in the primate retina possess heavier TRPV4 immunoreactivity in our results (Gao et al., 2019). These data support the idea that retinal TRPV4 could probably contribute to glaucoma RGC death. Glaucoma animal models showed a decrease in retinal TRPV4 expression (Sappington et al., 2015) and RGC firing rate (Della et al., 2013), consistent with the loss of RGC somas and BC synapses.
TRPV1 was found in rat inner nuclear layer (Leonelli et al., 2013), RGCs in rat, mouse, and primate retinas (Sappington et al., 2015), and photoreceptor ribbons in goldfish and zebrafish retinas (Zimov and Yazulla, 2004). Glaucoma retinas from animal models express a higher level of TRPV1 (Sappington et al., 2015), and knocking out TRPV1 reduces RGC loss induced by 70 mmHg hydrostatic pressure in retinal explants in vitro. TRPC6 was observed in RGCs, amacrine cells, and the outer plexiform layer in the rat retina (Wang et al., 2010). TRPC6 agonists significantly reduce the reperfusion-induced RGC death (Wang et al., 2010). TRPC6, TRPV1, and TRPV4 have a similar PCa/PNa ratio, but those located in BCs and amacrine cells presumably have opposite effects on RGCs. To better understand the roles of TRPs in PIRDs, we need to better differentiate the roles of presynaptic and postsynaptic TRPs in RGC physiology, acute pressure responses, and the chronic pathologies in PIVDs.
Intraocular injection of TRPV4 antagonists has been found to lower IOP in glaucomatous mouse eyes and protect retinal neurons from IOP-induced cell death (Ryskamp et al., 2016). The latter is consistent with the related results obtained from retinal neurons, while the former likely involves TRPV4 located in the trabecular meshwork (TM) (Ryskamp et al., 2016). Although these works support a neuronal protective effect of TRP antagonists in glaucoma, pharmacological modulators of TRPs have not been tested in clinical trials for treating PIRDs (Nilius and Szallasi, 2014). Besides TRPs, retinal neurons also express mechanosensitive channels permeable to K+, including the large calcium-activated potassium channel (BK) previously found in goldfish and salamander rod terminals and mouse A17 amacrine cells, as well as the two-pole domain potassium channel (K2P, such as TASK1, TASK2, TREK-1, and TREK-2) observed in mouse RGCs, the inner nuclear layer, amacrine cells, or/and the inner plexiform layer. K2Ps are gated primarily by the membrane tension, and their opening is facilitated by the convex membrane deformation (Enyedi and Czirjak, 2010), giving rise to the leak (also called background) K+ current to stabilize the negative resting membrane potential and counterbalance depolarization. The variable cation permeability of pressure-sentivie cation channels appears to allow them to differentialy modulate the membrane potential of retinal neurons, but their roles in glaucoma have been unclear. Retinal neurons have also been found to express Na+ and Ca2+ permeable P2X receptors, which can be activated by ATP released to the extracellular space, involving the sensing of tissue-damaging and inflammatory stimuli.
Potential Routes for Retinal Transient Receptor Potential Channels to Mediate Retinal Ganglion Cell Pathophysiology in Glaucoma
The role of retinal TRPs in glaucoma has not been certain. Opening TRPs can increase [Ca2+]i and [Na+]i and reduce Na+ electrochemical gradients (Na]g. Increased [Ca2+]i has been known to mediate neuronal degeneration, while the energy stored in the normal [Na]g is critically required for the generation of action potentials and postsynaptic potentials mediated by GluRs, glutamate transportation by the excitatory amino acid transporters, and the extrusion of Ca2+ by Na-Ca exchanger (Attwell and Laughlin, 2001; Howarth et al., 2012). Na-Ca exchanger imports 3 Na+ to extrude 1 Ca2+, transportation of each glutamate molecule is coupled with the co-transportation of 3 Na+ (Bouvier et al., 1992; Arriza et al., 1994; Wadiche et al., 1995), and the opening of GluRs also induces Ca2+ and Na+ influxes. Cells rely on Na, K-ATP pump to maintain a normal [Na]g, which is powered by mitochondria and consumes 1 ATP to extrude 3 Na+. While the membrane is depolarized above –40 mV (Armstrong, 2006; Maier et al., 2002), the voltage-gated Na+ channel is largely inactivated, and the activation and inactivation of voltage-gated Na+ channel also can be modulated by membrane stretch (Morris and Juranka, 2007) and TRPV4 activation (Gao et al., 2019). Based on these classic concepts and current studies on TRPs, I propose three potential routes for retinal TRPs to mediate RGC pathophysiology in glaucoma, which are to be participated by BCs, RGCs, and Müller cells and primarily involved by the glutamate release from BCs and Ca2+ and glutamate-mediated excitotoxicity, mitochondria damage, and the inactivation of voltage-gated Na channels in RGCs (Figure 1).
Figure 1.
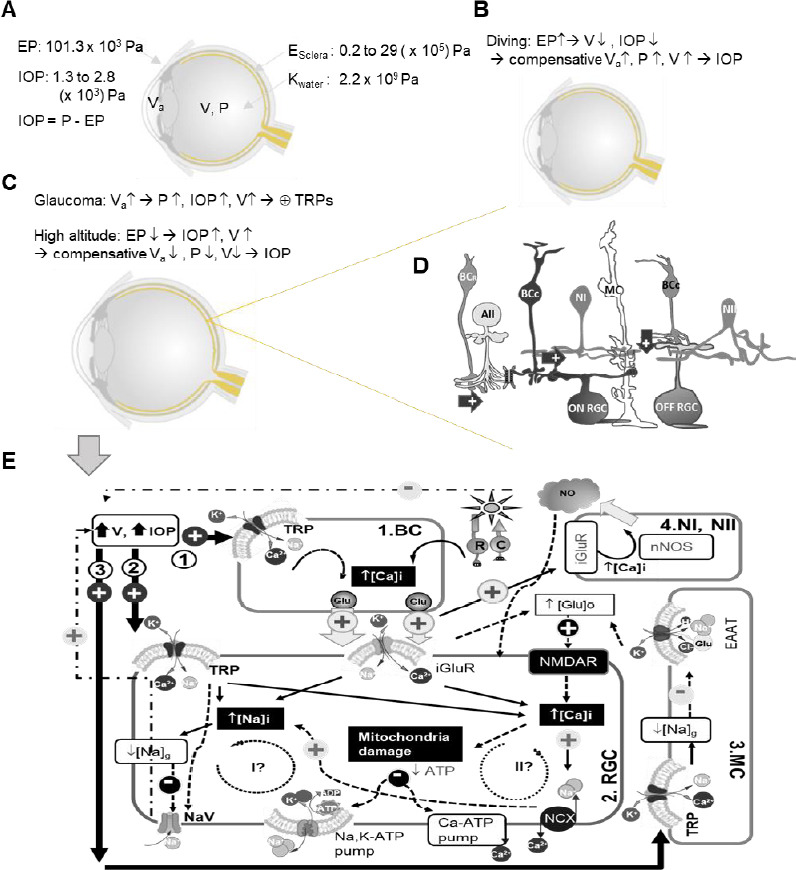
Diagram of potential routes for TRPs to mediate RGC pathophysiology in glaucoma.
Ocular physical parameters are depicted in A–C. E: Young’s modulus; EP: external pressure; IOP: intraocular pressure; K: bulk modulus; P: pressure; V: eyeball volume; Va: volume of aqueous humor. In D, arrow: excitatory glutamate synapse; AII: AII amacrine cell; and butterfly shape: gap junction. In D and E, BCR: rod bipolar cell (BC); BCC: cone BC; MC: Müller cell; Type I and II nitroxidergic amacrine cell (NI and NII) and RGC: retinal ganglion cell. E displays the possible (arrow) and presumable (dashed arrow) effect of TRP activation upon increasing V and IOP, and three potential pathophysiological routes are illustrated involved by BC (1), RGC (2), and MC (3). TRP activation is known to cause influxes of Na+ and Ca2+, higher [Ca2+]i, and the reduction of Na+ electrochemical gradien ([Na]g)/membrane depolarization, which are to be superimposed on light-evoked activities driven by the photoreceptor rod (R) and cone (C) and mediated by iGluRs in RGCs. Higher [Ca2+]i presumably increases glutamate (Glu) release in BCs, and in Müller cell (MC) TRP-mediated decrease of [Na]g would reduce Glu transportation by the excitatory amino acid transporter (EAAT) to increase [Glu]o. The increased [Glu]o can activate N-methyl-D-aspartate receptor (NMDAR) in RGCs, damaging RGC mitochondria via Ca2+ and other excitotoxic mechanisms. The increase of [Ca2+]i and [Na+]i needs to consume more ATPs to restore their normal electrochemical gradients. Two potential pathophysiological cycles (I and II) are further proposed for RGCs. Cycle I anticipates that an extra amount of Na+ influx would consume more ATPs to cause or enhance ATP shortage, and a dramatic reduction of [Na]g could lead to the deactivation of the voltage-gated Na+ channel (NaV) and the loss of RGC function. Cycle II proposes that an extra amount of Ca2+ influx via TRPs would increase [Ca2+]i to further damage the mitochondria, causing or enhancing ATP shortage to further increase [Ca2+]i and exacerbate the cellular damage.
Retinal neurons are not just the victim of elevated IOP, and they have shown some potential to influence IOP. First, vision is known to critically mediate the pupillary and accommodation reflex, whose effectors are the ciliary and iris muscles, respectively. The accommodation has been found to raise IOP in some patients (Yan et al., 2014; Aggarwala, 2020). Ciliary muscle contraction leads to distension of the TM with a subsequent reduction in outflow (an increase of IOP), while the contraction of iris muscles and TM leads to the opposite effect (Wiederholt et al., 2000). TM expresses mechanosensitive TRPV4, TREK1, and BK(Ca) channels (Dismuke and Ellis, 2009; Ryskamp et al., 2016; Yarishkin et al., 2018). Besides, Multiple types of neurotransmitter receptors have been observed in the ciliary and iris muscles, such as muscarinic cholinergic receptors, dopaminergic receptors, and adrenergic receptors. Thus, IOP and the circulation of aqueous humor can be affected by retinal light signaling, mechanical sensitive ion channels, and the circulation and nervous systems. Whether the dysfunction of RGCs in the early glaucoma stage facilitates IOP elevation as anticipated requires further investigation in the future.
Second, nitric oxide (NO) donor has been shown to decrease IOP by increasing aqueous outflow facility in TM and Schlemm’s canal (Dismuke et al., 2008). NO may be released by retinal nitroxidergic amacrine cells (NOACs), which express the neuronal nitric oxide synthase. Two subtypes of NOACs (NI and NII) are nearly evenly distributed in the retina and receive excitatory glutamatergic synapses from BCs (Pang et al., 2010). Given the anatomical proximity, I anticipate that the presumable TRP-mediated glutamate release from BCs could enhance NO release from NOACs, serving as a compensative mechanism to suppress IOP elevation. NO is a free-radical gas, involving multiple retinal neuronal activities and glaucoma retinal pathology (Pang et al., 2010; Aliancy et al., 2017). The significance of retinal TRPs and NOACs in glaucoma needs further investigation.
Pressure-sensitive ion channels in retinal neurons can open upon pressure and membrane stretch, but their roles in retinal physiology and pathology have not been clear. To facilitate the channel therapy to be used in clinical treatment and promote vision health, we still need to better determine the normal role of these channels in retinal light signaling, the interaction between different types of the channels, and the effective channel modification capable of reducing IOP and the vulnerability of RGCs to IOP elevation. Future studies in this direction are likely to bring out novel mechanisms for PIVDs and novel cellular and molecular targets for clinical treatment of RIVDs.
Footnotes
Conflicts of interest: The author declares no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Aggarwala KRG. Ocular accommodation, intraocular pressure, development of myopia and glaucoma: role of ciliary muscle, choroid and, metabolism. Med Hypothesis Discov Innov Ophthalmol. 2020;9:66–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Agostinone J, Di PA. Retinal ganglion cell dendrite pathology and synapse loss: Implications for glaucoma. Prog Brain Res. 2015;220:199–216. doi: 10.1016/bs.pbr.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Aliancy J, Stamer WD, Wirostko B. A review of nitric oxide for the treatment of glaucomatous disease. Ophthalmol Ther. 2017;6:221–232. doi: 10.1007/s40123-017-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson DR. Normal-tension glaucoma (Low-tension glaucoma) Indian J Ophthalmol. 2011;59:S97–101. doi: 10.4103/0301-4738.73695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong CM. Na channel inactivation from open and closed states. Proc Natl Acad Sci U S A. 2006;103:17991–17996. doi: 10.1073/pnas.0607603103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asejczyk-Widlicka M, Pierscionek BK. The elasticity and rigidity of the outer coats of the eye. Br J Ophthalmol. 2008;92:1415–1418. doi: 10.1136/bjo.2008.140178. [DOI] [PubMed] [Google Scholar]
- 8.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–142. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Berdahl JP, Yu DY, Morgan WH. The translaminar pressure gradient in sustained zero gravity, idiopathic intracranial hypertension, and glaucoma. Med Hypotheses. 2012;79:719–724. doi: 10.1016/j.mehy.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Berry RH, Qu J, John SW, Howell GR, Jakobs TC. Synapse loss, and dendrite remodeling in a mouse model of glaucoma. PLoS One. 2015;10:e0144341. doi: 10.1371/journal.pone.0144341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouvier M, Szatkowski M, Amato A, Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992;360:471–474. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- 13.Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115:1123–1129. doi: 10.1016/j.ophtha.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Chang B, Nolan H, Mooney D. High-altitude flight retinopathy. Eye (Lond) 2004;18:653–656. doi: 10.1038/sj.eye.6700730. [DOI] [PubMed] [Google Scholar]
- 15.Clapham DE. SnapShot: mammalian TRP channels. Cell. 2007;129:220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Della SL, Inman DM, Lupien CB, Horner PJ, Wong RO. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci. 2013;33:17444–17457. doi: 10.1523/JNEUROSCI.5461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dismuke WM, Ellis DZ. Activation of the BK(Ca) channel increases outflow facility and decreases trabecular meshwork cell volume. J Ocul Pharmacol Ther. 2009;25:309–314. doi: 10.1089/jop.2008.0133. [DOI] [PubMed] [Google Scholar]
- 18.Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008;294:C1378–1386. doi: 10.1152/ajpcell.00363.2007. [DOI] [PubMed] [Google Scholar]
- 19.Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;52:7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eilaghi A, Flanagan JG, Tertinegg I, Simmons CA, Wayne BG, Ross EC. Biaxial mechanical testing of human sclera. J Biomech. 2010;43:1696–1701. doi: 10.1016/j.jbiomech.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 21.El-Danaf RN, Huberman AD. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J Neurosci. 2015;35:2329–2343. doi: 10.1523/JNEUROSCI.1419-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 23.Fan S, Hejkal JJ, Gulati V, Galata S, Camras CB, Toris CB. Aqueous humor dynamics during the day and night in volunteers with ocular hypertension. Arch Ophthalmol. 2011;129:1162–1166. doi: 10.1001/archophthalmol.2011.226. [DOI] [PubMed] [Google Scholar]
- 24.Filippopoulos T, Danias J, Chen B, Podos SM, Mittag TW. Topographic and morphologic analyses of retinal ganglion cell loss in old DBA/2NNia mice. Invest Ophthalmol Vis Sci. 2006;47:1968–1974. doi: 10.1167/iovs.05-0955. [DOI] [PubMed] [Google Scholar]
- 25.Friberg TR, Lace JW. A comparison of the elastic properties of human choroid and sclera. Exp Eye Res. 1988;47:429–436. doi: 10.1016/0014-4835(88)90053-x. [DOI] [PubMed] [Google Scholar]
- 26.Gao F, Yang Z, Jacoby RA, Wu SM, Pang JJ. The expression and function of TRPV4 channels in primate retinal ganglion cells and bipolar cells. Cell Death Dis. 2019;10:364. doi: 10.1038/s41419-019-1576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991;32:484–491. [PubMed] [Google Scholar]
- 28.Hare WA, Wheeler L. Experimental glutamatergic excitotoxicity in rabbit retinal ganglion cells: block by memantine. Invest OphthalmolVis Sci. 2009;50:2940–2948. doi: 10.1167/iovs.08-2103. [DOI] [PubMed] [Google Scholar]
- 29.Hexdall EJ, Butler FK. Transient vision loss at depth due to presumed barotraumatic optic neuropathy. Undersea Hyperb Med. 2012;39:911–914. [PubMed] [Google Scholar]
- 30.Ho CL, Walton DS. Primary megalocornea: clinical features for differentiation from infantile glaucoma. J Pediatr Ophthalmol Strabismus. 2004;41:11–17. doi: 10.3928/0191-3913-20040101-05. [DOI] [PubMed] [Google Scholar]
- 31.Ho TY, Kao WF, Lee SM, Lin PK, Chen JJ, Liu JH. High-altitude retinopathy after climbing Mount Aconcagua in a group of experienced climbers. Retina. 2011;21:1650–1655. doi: 10.1097/IAE.0b013e318207ceab. [DOI] [PubMed] [Google Scholar]
- 32.Horng CT, Liu CC, Wu DM, Wu YC, Chen JT, Chang CJ, Tsai ML. Visual fields during acute exposure to a simulated altitude of 7620 m. Aviat Space Environ Med. 2008;79:666–669. doi: 10.3357/asem.2160.2008. [DOI] [PubMed] [Google Scholar]
- 33.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jo AO, Ryskamp DA, Phuong TT, Verkman AS, Yarishkin O, MacAulay N, Krizaj D. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal muller glia. J Neurosci. 2015;35:13525–13537. doi: 10.1523/JNEUROSCI.1987-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karakucuk S, Oner AO, Goktas S, Siki E, Kose O. Color vision changes in young subjects acutely exposed to 3,000 m altitude. Aviat Space Environ Med. 2004;75:364–366. [PubMed] [Google Scholar]
- 37.Kaufman P. Adler’s Physiology of the Eye. 11th ed. Philadelphia, USA: Saunders; 2011. [Google Scholar]
- 38.Killer HE, Pircher A. Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye (Lond) 2018;32:924–930. doi: 10.1038/s41433-018-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krizaj D. Polymodal sensory integration in retinal ganglion cells. Adv Exp Med Biol. 2016;854:693–698. doi: 10.1007/978-3-319-17121-0_92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei Y, Garrahan N, Hermann B, Becker DL, Hernandez MR, Boulton ME, Morgan JE. Quantification of retinal transneuronal degeneration in human glaucoma: a novel multiphoton-DAPI approach. Invest Ophthalmol Vis Sci. 2008;49:1940–1945. doi: 10.1167/iovs.07-0735. [DOI] [PubMed] [Google Scholar]
- 41.Leonelli M, Martins DO, Britto LR. Retinal cell death induced by TRPV1 activation involves NMDA signaling and upregulation of nitric oxide synthases. Cell Mol Neurobiol. 2013;33:379–392. doi: 10.1007/s10571-012-9904-5. [DOI] [PubMed] [Google Scholar]
- 42.Li R, Liu JH. Telemetric monitoring of 24 h intraocular pressure in conscious and freely moving C57BL/6J and CBA/CaJ mice. Mol Vis. 2008;14:745–749. [PMC free article] [PubMed] [Google Scholar]
- 43.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Montell C. Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem Biophys Res Commun. 2015;460:22–25. doi: 10.1016/j.bbrc.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu JH, Kripke DF, Twa MD, Hoffman RE, Mansberger SL, Rex KM, Girkin CA, Weinreb RN. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- 46.Macarez R, Dordain Y, Hugon M, Kovalski JL, Guigon B, Bazin S, May F, Colin J. Long-term effects of iterative diving on visual field, color vision and contrast sensitivity in professional divers. J Fr Ophtalmol. 2005;28:825–831. doi: 10.1016/s0181-5512(05)81000-9. [DOI] [PubMed] [Google Scholar]
- 47.Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci U S A. 2002;99:4073–4078. doi: 10.1073/pnas.261705699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makarov IA, Voronkov YI, Aslanjan MG. Ophthalmic changes associated with long-term exposure to microgravity. Fiziol Cheloveka. 2017;43:111–120. [PubMed] [Google Scholar]
- 49.Mark HH. Buphthalmos: early glaucoma history. Acta Ophthalmol. 2011;89:591–594. doi: 10.1111/j.1755-3768.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 50.McFadden DM, Houston CS, Sutton JR, Powles AC, Gray GW, Roberts RS. High-altitude retinopathy. JAMA. 1981;245:581–586. [PubMed] [Google Scholar]
- 51.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 52.Morris CE PF. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys J. 2007;93:822–833. doi: 10.1529/biophysj.106.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mowatt L, Foster T. Sphenoidal sinus mucocele presenting with acute visual loss in a scuba diver. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-010309. doi: 101136/bcr-2013-010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myers KM, Cone FE, Quigley HA, Gelman S, Pease ME, Nguyen TD. The in vitro inflation response of mouse sclera. Exp Eye Res. 2010;91:866–875. doi: 10.1016/j.exer.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson ES, Mulugeta L, Feola A, Raykin J, Myers JG, Samuels BC, Ethier CR. The impact of ocular hemodynamics and intracranial pressure on intraocular pressure during acute gravitational changes. J Appl Physiol. 1985;123:352–363. doi: 10.1152/japplphysiol.00102.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Netti V, Fernandez J, Kalstein M, Pizzoni A, Di GG, Rivarola V, Ford P, Capurro C. TRPV4 Contributes to Resting Membrane Potential in Retinal Muller Cells: Implications in Cell Volume Regulation. J Cell Biochem. 2017;118:2302–2313. doi: 10.1002/jcb.25884. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen C, Cone FE, Nguyen TD, Coudrillier B, Pease ME, Steinhart MR, Oglesby EN, Jefferys JL, Quigley HA. Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Invest Ophthalmol Vis Sci. 2013;54:1767–1780. doi: 10.1167/iovs.12-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev. 2014;66:676–814. doi: 10.1124/pr.113.008268. [DOI] [PubMed] [Google Scholar]
- 59.Norman RE, Flanagan JG, Rausch SM, Sigal IA, Tertinegg I, Eilaghi A, Portnoy S, Sled JG, Ethier CR. Dimensions of the human sclera: Thickness measurement and regional changes with axial length. Exp Eye Res. 2010;90:277–284. doi: 10.1016/j.exer.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Ou Y, Jo RE, Ullian EM, Wong RO, Della SL. Selective vulnerability of specific retinal ganglion cell types and synapses after transient ocular hypertension. J Neurosci. 2016;36:9240–9252. doi: 10.1523/JNEUROSCI.0940-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pang JJ, Frankfort BJ, Gross RL, Wu SM. Elevated intraocular pressure decreases response sensitivity of inner retinal neurons in experimental glaucoma mice. Proc Natl Acad Sci U S A. 2015;112:2593–2598. doi: 10.1073/pnas.1419921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pang JJ, Gao F, Wu SM. Light responses and morphology of bNOS-immunoreactive neurons in the mouse retina. J Comp Neurol. 2010;518:2456–2474. doi: 10.1002/cne.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pang JJ, Wu SM. Retinal ganglion cell death is correlated with eyeball expansion in mammals. J Ophthalmol. 2014;1:1–5. [Google Scholar]
- 64.Papadopoulos M, Cable N, Rahi J, Khaw PT. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci. 2007;48:4100–4106. doi: 10.1167/iovs.06-1350. [DOI] [PubMed] [Google Scholar]
- 65.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 66.Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 67.Ryskamp DA, Frye AM, Phuong TT, Yarishkin O, Jo AO, Xu Y, Lakk M, Iuso A, Redmon SN, Ambati B, Hageman G, Prestwich GD, Torrejon KY, Križaj D. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci Rep. 2016;6:30583. doi: 10.1038/srep30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Rentería RC, Liedtke W, Krizaj D. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci. 2011;31:7089–7101. doi: 10.1523/JNEUROSCI.0359-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sappington RM, Sidorova T, Ward NJ, Chakravarthy R, Ho KW, Calkins DJ. Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels (Austin) 2015;9:102–113. doi: 10.1080/19336950.2015.1009272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin DH, Bielik M, Hong YJ, Briggs KS, Shi DX. Reversal of glaucomatous optic disc cupping in adult patients. Arch Ophthalmol. 1989;107:1599–1603. doi: 10.1001/archopht.1989.01070020677026. [DOI] [PubMed] [Google Scholar]
- 71.Shou T, Liu J, Wang W, Zhou Y, Zhao K. Differential dendritic shrinkage of alpha and beta retinal ganglion cells in cats with chronic glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3005–3010. doi: 10.1167/iovs.02-0620. [DOI] [PubMed] [Google Scholar]
- 72.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Finite element modeling of optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2004;45:4378–4387. doi: 10.1167/iovs.04-0133. [DOI] [PubMed] [Google Scholar]
- 73.Sit AJ, Nau CB, McLaren JW, Johnson DH, Hodge D. Circadian variation of aqueous dynamics in young healthy adults. Invest Ophthalmol Vis Sci. 2008;49:1473–1479. doi: 10.1167/iovs.07-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sjontoft E, Edmund C. In vivo determination of Young’s modulus for the human cornea. Bull Math Biol. 1987;49:217–232. doi: 10.1007/BF02459699. [DOI] [PubMed] [Google Scholar]
- 75.Sugimoto E, Aihara M, Ota T, Araie M. Effect of light cycle on 24-hour pattern of mouse intraocular pressure. J Glaucoma. 2006;15:505–511. doi: 10.1097/01.ijg.0000212275.57853.c2. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 77.Taibbi G, Cromwell RL, Kapoor KG, Godley BF, Vizzeri G. The effect of microgravity on ocular structures and visual function: a review. Surv Ophthalmol. 2013;58:155–163. doi: 10.1016/j.survophthal.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Tan JC, Kalapesi FB, Coroneo MT. Mechanosensitivity and the eye: cells coping with the pressure. Br J Ophthalmol. 2006;90:383–388. doi: 10.1136/bjo.2005.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor L, Arner K, Ghosh F. Specific inhibition of TRPV4 enhances retinal ganglion cell survival in adult porcine retinal explants. Exp Eye Res. 2016;154:10–21. doi: 10.1016/j.exer.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Tingay DG, Tsimnadis P, Basnyat B. A blurred view from Everest. Lancet. 2003;362:1978. doi: 10.1016/s0140-6736(03)15017-9. [DOI] [PubMed] [Google Scholar]
- 81.Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999;127:407–412. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- 82.Van de Veire S, Germonpre P, Renier C, Stalmans I, Zeyen T. Influences of atmospheric pressure and temperature on intraocular pressure. Invest Ophthalmol Vis Sci. 2008;49:5392–5396. doi: 10.1167/iovs.07-1578. [DOI] [PubMed] [Google Scholar]
- 83.Vecchi D, Morgagni F, Guadagno AG, Lucertini M. Visual function at altitude under night vision assisted conditions. Aviat Space Environ Med. 2014;85:60–65. doi: 10.3357/asem.3587.2014. [DOI] [PubMed] [Google Scholar]
- 84.Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye. 2007;2:S11–S14. doi: 10.1038/sj.eye.6702880. [DOI] [PubMed] [Google Scholar]
- 85.Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Teng L, Li A, Ge J, Laties AM, Zhang X. TRPC6 channel protects retinal ganglion cells in a rat model of retinal ischemia/reperfusion-induced cell death. Invest Ophthalmol Vis Sci. 2010;51:5751–5758. doi: 10.1167/iovs.10-5451. [DOI] [PubMed] [Google Scholar]
- 87.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19:271–295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 89.Yan L, Huibin L, Xuemin L. Accommodation-induced intraocular pressure changes in progressing myopes and emmetropes. Eye (Lond) 2014;28:1334–1340. doi: 10.1038/eye.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yarishkin O, Phuong TTT, Bretz CA, Olsen KW, Baumann JM, Lakk M, Crandall A, Heurteaux C, Hartnett ME, Krizaj D. TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. J Gen Physiol. 2018;150:1660–1675. doi: 10.1085/jgp.201812179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou D, Wei W, Tian B, Wang C, Shi X, Jiao X. Observation and management of retinal changes related to diving in professional divers. Chin Med J (Engl) 2014;127:729–733. [PubMed] [Google Scholar]
- 92.Zimov S, Yazulla S. Localization of vanilloid receptor 1 (TRPV1/VR1)-like immunoreactivity in goldfish and zebrafish retinas: restriction to photoreceptor synaptic ribbons. J Neurocytol. 2004;33:441–452. doi: 10.1023/B:NEUR.0000046574.72380.e8. [DOI] [PubMed] [Google Scholar]


