Abstract
Stroke is a main cause of death and disability worldwide. The ability of the brain to self-repair in the acute and chronic phases after stroke is minimal; however, promising stem cell-based interventions are emerging that may give substantial and possibly complete recovery of brain function after stroke. Many animal models and clinical trials have demonstrated that neural stem cells (NSCs) in the central nervous system can orchestrate neurological repair through nerve regeneration, neuron polarization, axon pruning, neurite outgrowth, repair of myelin, and remodeling of the microenvironment and brain networks. Compared with other types of stem cells, NSCs have unique advantages in cell replacement, paracrine action, inflammatory regulation and neuroprotection. Our review summarizes NSC origins, characteristics, therapeutic mechanisms and repair processes, then highlights current research findings and clinical evidence for NSC therapy. These results may be helpful to inform the direction of future stroke research and to guide clinical decision-making.
Key Words: brain, central nervous system, cognitive impairment, neurological function, plasticity, recovery, regeneration, stem cells, stroke
Introduction
Stroke is a main cause of death and disability worldwide, with ischemic stroke accounting for more than 80% of strokes (Lackland et al., 2014; Benjamin et al., 2017). In the United States, it is estimated that about 7 million adults have a history of stroke, which is about 2.5% of the population (Benjamin et al., 2019). Current effective treatments include reperfusion therapies, such as recombinant tissue plasminogen activator; but this must be administered in a limited time window (within 4.5 hours of stroke onset); therefore, only 2–4% patients benefit from it (Hankey, 2017). Disability after stroke brings serious social and economic burdens; few people spontaneously recover and many are permanently disabled (Koh and Park, 2017). Traditional rehabilitation often has little effect (Feigin et al., 2017).
The ability of a damaged central nervous system (CNS) to self-repair is limited, so finding alternative ways to promote recovery will revolutionize stroke treatment (Barker et al., 2018). Stem cell therapy is a novel treatment that has promise for replacing acute phase thrombolysis (Liao et al., 2019) and chronic phase rehabilitation (Kalladka et al., 2016; Boese et al., 2018). Neural stem cells (NSCs) and neural stem/progenitor cells (NSPCs) are considered ideal candidates for the establishment of a stem cell pool that can provide a continuous supply of neurons, astrocytes, and oligodendrocytes for repairing neural networks and vascular remodeling (Trounson and McDonald, 2015; Annese et al., 2017).
The use of NSCs for transplantation has four widely accepted advantages: (1) Higher brain-like similarity than embryonic stem cells (ESCs); NSCs can divide and differentiate into cell types corresponding to cells in their surrounding microenvironment and their morphologies and functions are very similar to those of nearby host cells (Golas, 2018). (2) Easier delivery than mesenchymal stem cells; the CNS has a special structure, the blood-brain barrier (BBB), that makes it difficult for exogenous “seed cells” to enter, while NSCs routinely transplanted into the subependymal zone or dentate gyrus have a “placeholder” effect (Gao et al., 2017). (3) Lower rejection than neuronal analogs; immune rejection is extremely rare even for transplanted inter-individual or inter-species NSCs (Morizane et al., 2017). (4) Strong chemotaxis and migration abilities (Addington et al., 2015); NSCs have the ability to migrate to the damaged site (Xu and Heilshorn, 2013; Addington et al., 2017).
NSCs have achieved functional recovery of injured brain by (1) homing and cell replacement (Baker et al., 2019), (2) a nutritional and growth factor paracrine effect (Barker et al., 2018) [including exosome release (Zhang et al., 2019b)], (3) regulation of inflammation and a neuro-protective effect (Stonesifer et al., 2017), as in preclinical studies (Stroemer et al., 2009; Pendharkar et al., 2010; Vu et al., 2014; Nucci et al., 2015; Azad et al., 2016; Chen et al., 2016; Baker et al., 2019) and clinical studies (Trounson and McDonald, 2015; Kalladka et al., 2016). Ischemic stroke is caused by a variety of situations that result in insufficient blood supply to the brain. This causes a complex cascade of reactions, including the slowing down of cell energy metabolism and depolarization of the cell membrane. These responses destroy tissue and the surrounding circulatory system, leading to further necrosis of the BBB and neurons, loss of neurovascular unit (NVU) function, and disruption of the brain network (Ahmad et al., 2014; Yew et al., 2019). Some NSC mechanisms have been explained (Rosado-de-Castro et al., 2013; Trounson and McDonald, 2015; Sandvig et al., 2017; Baker et al., 2019; Zhang et al., 2019b). Liao et al. (2019) described the mechanism of neuroplasticity after stroke with reference to recovery stages: (1) neuron polarization and synapse pruning, (2) neurite growth and neuroregeneration, (3) myelin repair, (4) synaptic rewiring and remodeling of brain networks, and (5) vascular regeneration. However, few reviews have examined every stage of the recovery process after NSC therapy or discussed clinical trial results and potential clinical applications. This review summarizes the results of recently published studies, focusing on NSC resources, the processes and mechanisms of functional repair, and clinical trials, with the aim of providing a decision-making basis for therapeutic interventions and to provide suggestions for future research directions.
The references referred to in this review were retrieved by an electronic search of the Medline database for animal models and clinical trials of stroke from 1946 to 2020 using the following conditions: (Stroke[MeSH Terms]) OR Stroke, Lacunar[MeSH Terms]) OR (National Institute of Neurological Disorders and Stroke[MeSH Terms]) OR MELAS Syndrome[MeSH Terms]) OR Brain infarction[MeSH Terms]) OR Hemorrhagic cerebrovascular disease[MeSH Terms]) OR Ischemic cerebrovascular disease[MeSH Terms]) AND (Cell- and Tissue-based therapy[MeSH Terms]) OR Tissue therapy, Historical[MeSH Terms]) OR Cellular immunotherapy, adoptive[MeSH Terms]) AND (Neural stem cell) OR Neural progenitor cell). In addition, an electronic search of the Medline database for methods to induce plasticity was performed. This included publications prior to March, 2020, with the following search criteria: (Plasticity[MeSH Terms]) OR Neuronal plasticity[MeSH Terms]) OR Synaptic plasticity[MeSH Terms]) and Stroke (MeSH Terms) AND (Models, Animal (MeSH Terms) OR Behavior, Animal/Physiology (MeSH Terms) OR Animal experimentation (MeSH Terms). Non-NSC experiments and review articles were excluded.
Type and Source of Neural Stem Cells
ESC-derived NSC lines (embryonic donors)
These cell lines are NSCs derived from an early embryo or embryonic neural tissue. Early ESCs are undifferentiated cells in the blastocyst at 5–7 days after fertilization, before implantation into the uterus. They are omnipotent stem cells and have the ability to develop into any cell type. Part of the ectoderm will differentiate into the cells of the nervous system. ESC-NSCs are pluripotent stem cells contained in the embryonic ectoderm that theoretically lose their ability to differentiate into any cell type. Fetal-derived NSCs can be generated by dissociating human fetal cortex, mesencephalon, or spinal cord tissues between 7 and 21 days post-conception. The fetal brain contains stem cells that can produce every type of cell in neural tissue (i.e., neurons, astrocytes, oligodendrocytes) (McGrath et al., 2018). These two kinds of stem cells are often used in research; however, ethical concerns and tumorigenicity limit their clinical use.
Adult brain NSC lines (adult donor)
There are two major neurogenic niches in the human brain, the subventricular zone (SVZ) and sub-granular zone, from which adult NSCs can be acquired. The SVZ is the largest neural germination area in the adult brain. The adult SVZ ultrastructure has been observed by electron microscopy, which shows that neuroblasts are adjacent to the ependymal layer, arranged in a chain-like pattern. They constantly migrate, eventually gathering into the rostral migratory stream toward the olfactory bulb. The neuroblasts are surrounded by astrocytes. The SVZ provides a special microenvironment. Many factors affect cell proliferation, adhesion, migration and differentiation. Epidermal growth factor and basic fibroblast growth factor enhance neurogenesis and increase the number of migrating cells (Cheng et al., 2020). Ciliary neurotrophic factor and leukocyte inhibitory factor are involved in determining the direction of self-renewal or differentiation, and they are regulated by the Notch pathway (Müller et al., 2009). In addition, some NSCs are produced in the lower layer of the dentate gyrus of the adult hippocampus. These cells migrate transiently to the granular layer, their dendrites extend to the molecular layer, and their axons extend to the Cornu Ammonis 3 region of the hippocampus. Differentiated hippocampal neurons may be associated with learning and memory abilities and their number gradually decreases with age. However, from a practical application perspective, many scholars question whether the neuroplasticity of these stem cells results from fusion with ESCs. In addition, in vitro culture after harvest may cause the cell state to be altered and immune rejection after passage cultivation (Qin et al., 2017).
Induced pluripotent stem cell-derived NSCs
Takahashi et al. (2017) successfully reprogrammed human cells into induced pluripotent stem cells (iPSCs), and these cells have since become an ideal autologous transplant cell resource. The advantages of transplanted iPSC-NSCs in the treatment of stroke are numerous, such as enhancing motor sensory function, reducing infarct size, promoting axonal development, promoting angiogenesis, and regulating inflammatory factors (Eckert et al., 2015). A patient’s own iPSC-NSCs are amenable to ex vivo gene therapy, and have been validated to improve brain pathology in a reversible homologous mouse model (Griffin et al., 2015). iPSC-NSCs may have similar plasticity to ESCs and they can differentiate into NSCs for the effective treatment of ischemic stroke in rodent models (Takahashi and Yamanaka, 2006). The combination of iPSC-NSCs and tissue engineering techniques has great promise in the treatment of multiple sclerosis and spinal cord injury (Zhang et al., 2016a; Zhou et al., 2018). However, tumorigenic and immunogenic problems need to be further considered (Stonesifer et al., 2017).
Clinical grade NSC products
Transgenic stem cell lines have been explored in clinical trials (Kalladka et al., 2016). Modified cell lines can be propagated asexually at a steady rate in vitro to form an immortalized cell line. Immortalized cell lines have the advantages of strong proliferative ability, good adaptability to the environment, easy control of cell identity and assimilation into human tissues. However, theoretically, tumorigenesis, genetic stability remain concerns, as do ethical considerations.
CTX0E03
CTX0E03 is a human NSC line that has been genetically modified with a conditional immortalized c-MYC gene fused with a mutated estrogen receptor (c-MYCERTAM) (Pollock et al., 2006). Serum-free-produced CTX0E03 is considered the most promising therapeutic-grade human NSC line (Thomas et al., 2009). The review by Sinden et al. (2017) detailed the whole process of CTX cell therapy from its laboratory origin to clinical progress. The transplantation of human NSCs (CTX0E03) promoted significant behavioral recovery in stroke patients (Kalladka et al., 2016). The degree of recovery was positively correlated with cell dose and a paracrine mechanism (Stroemer et al., 2009; Table 1).
Table 1.
Clinical grade neural stem cell products
| Name | Initial source | Processing method | Cell source | Preclinical studies evidence | Clinical trials | Disease | Limitations | References |
|---|---|---|---|---|---|---|---|---|
| CTX0E3 | Allogeneic | Transfection with c-mycER | Immortalizing human neural stem cell line | Neurogenesis and angiogenesis (exosome) | NCT01151124 | Chronic ischemic stroke | Long period recovery after enrollment | Stroemer et al., 2009; Kalladka et al., 2016 |
| NT2N | Allogeneic | Neuronal phenotype in vitro following retinoic acid treatment | Clonal human teratocarcinoma cell line | Dopaminergic phenotypes | Nelson et al. (2002) | PD, HD, trauma | Tumor formation experienced; apoptotic-like cell death; negligible therapeutic influence for stroke | Borlongan et al., 1998; Hurlbert et al., 1999; Baker et al., 2000; Nelson et al., 2002 |
| SB623 | Allogeneic | Transfection expression vector containing Notch-1 intracellular domain | Bone marrow- derived mesenchymal stem cells | Neurotrophic, angiogenic, and neuroprotective effects | NCT01287936 | Chronic stable stroke & trauma | Less studies and trials; nonrandomized design | Dezawa et al., 2004; Steinberg et al., 2016 |
| NSI-566 | Allogeneic | A single fetal spinal cord without genetic modification | Primary adherent human neural stem cell line | Integrate with host tissues; Immunosuppressive effects | NCT03296618 | Amyotrophic lateral sclerosis, spinal cord injury | Less clinical trials | Glud et al., 2016; Curtis et al., 2018; Zhang et al., 2019a |
c-mycER: c-myc gene fused with a mutated estrogen receptor; HD: Huntington’s disease; PD: Parkinson’s disease.
NT2N
NT2N (hNT) cells are neurons derived from a clonal human teratocarcinoma cell line (NTera-2 or NT2) that acquires a permanent postmitotic neuronal phenotype after retinoic acid treatment in vitro and has been shown to form operational synapsis and to secrete neurotransmitters in vivo (Andrews, 1984). NURR1-transfected NT2N cells showed a more stable neurophenotype and higher levels of glial cell-derived neurotrophic factor secretion. NURR1 is a transcription factor that induces tyrosine hydroxylase expression and promotes the differentiation of NT2N cells into a dopaminergic neuro-phenotype (Hara et al., 2008). However, Preclinical studies should further verify the risk of NT2N implantation, especially the way in which the regional microenvironment may affect abnormal proliferation (Miyazono et al., 1995). Also, dopaminergic cells differentiated by NURR1 have only a negligible therapeutic effect on stroke patients (Stonesifer et al., 2017). A 71-year-old stroke patient received 2 × 106 hNT cells by stereotactic injection into the brain. After 34 months, he died of myocardial infarction. The patient’s brain pathology indicated that there were neurons in the medial part of the infarction that were consistent with the transplanted region, but the patient’s symptoms before death were not significantly improved (Nelson et al., 2002) (Table 1).
SB623
SB623 cells were developed by transient transfection of mesenchymal stem cells with an expression vector encoding the intracellular domain of human NOTCH1 and have been shown to reduce lesion volume and promote functional recovery after delivery to the rodent brain after experimental focal ischemia (Dezawa et al., 2004). Animal studies confirmed the potential of SB623 cells for neuro-protection in Parkinson’s disease and brain trauma models (Tajiri et al., 2016), and the “biobridge” paradigm of SB623 cells is expected to construct a traumatic brain injury repair loop from the cortical injection site to the SVZ neurogenesis site (Tajiri et al., 2014). NCT01287936 is a single-arm open-label study of an SB623 intra-cerebral implant in 18 patients with chronic stroke. Although all scales suggested a significant improvement in motor function after treatment during the 12-month follow-up period, it should be noted that the study lacked a control study and a standardized definition of chronic stroke, and that six patients had severe adverse reactions that might be unrelated to the transplanted cells that affected the outcome (Steinberg et al., 2016) (Table 1).
NSI-566
NSI-566 is a stable primary adherent human-NSC line from a single fetal spinal cord without genetic modification and is U.S. Food and Drug Administration-authorized for clinical trials. It has shown good safety and tolerability in completed phase II amyotrophic lateral sclerosis and phase I spinal cord injury trials (Glass et al., 2016; Curtis et al., 2018). Various animal models have demonstrated that NSI-566 cells can differentiate into neural tissue and may have the ability to emit/receive long-range projection and integrate with host tissues (Usvald et al., 2010; Lu et al., 2012), and to have only transient immunosuppressive effects (Tadesse et al., 2014). A small single-arm feasibility clinical study was conducted in nine chronic stroke patients. The preliminary results are encouraging, but the nature of “extraneous tissue” in the infarcted cavity needs further monitoring, and the conclusions need to be verified by double-blind controlled studies (Zhang et al., 2019a; Table 1).
Non-neuronal direct lineage
Non-neuronal direct lineage reprogramming is a strategy that utilizes the plasticity of differentiated non-neuronal cells to transform lineages into ideal neuronal cell types (induced neurons) for disease modeling and tissue repair (Karow et al., 2014; Heinrich et al., 2015). Cells from the cerebral cortex that express pericyte hallmarks can be reprogrammed into neuronal cells by retrovirus-mediated co-expression of the transcription factors, SOX2 and MASH1 (Karow et al., 2012). The single factor Ascl1 can efficiently reprogram mouse astrocytes into induced neurons (Karow et al., 2018). Compared with using iPSCs, direct lineage reprogramming of somatic cells to generate induced neurons for stroke without experiencing pluripotent states has many advantages, such as short induction cycle, high transdifferentiation efficiency, no ethical concerns, and no risk of neoplasia (An et al., 2018; Table 1).
The direct reprogramming approach may provide new alternative sources of cells for regenerative medicine, but the future use of induced neurons may be limited by the genetic manipulation involved. Chemically-induced neurons are thought to have neuron-specific expression patterns and these cells generate action potentials and form functional synapses. Small molecules were able to convert mouse fibroblasts directly into neurons, and the TUJ1-positive yield was up to 90% 16 days after induction (Li et al., 2015). Zhang et al. (2016c) also reported the efficient conversion of mouse fibroblasts into induced neural stem-like cells using a mixture of nine components (M9; Table 1). It is expected that it will be possible to use this small molecule chemical reprogramming strategy to manipulate the fate of human somatic cells for the treatment of stroke patients because transplanting functionally mature neurons induced from fibroblasts with chemicals alone is a relatively simple and safe procedure.
Therapeutic Mechanism of Neural Stem Cells
Homing and cell replacement
Homing is the process by which neural progenitor cells (NPCs)/NSCs utilize chemotaxis to move to an injury site. A niche provides a special microenvironment for the growth of NSCs (Otsuki and Brand, 2017). Stromal derived factor-1/C-X-C chemokine receptor type 4, monocyte chemotactic pro-tein-3/cinnamoyl-coenzyme A reductase, and other signaling pathways promote the homeostasis, self-renewal and differentiation of NSCs (Belmadani et al., 2015; Nunes et al., 2016). Stromal cell-derived factor 1 receptor is expressed at injured sites after infarction, and NSCs expressing C-X-C chemokine receptor type 4 receptor migrated to the injured site within 24 hours, and activated integrin β1 was responsible for adhesion of the transplanted cells (Gójska-Grymajło et al., 2018). The transplantation of in vitro-labeled cells into the brain is the best way to demonstrate their homing mechanism for determining the efficacy of NPC treatment. The literature on in vitro superparamagnetic iron oxide nanoparticle labeling and magnetic resonance imaging (MRI) is abundant in the field of stroke and glioma, and has been used in multiple phase I clinical trials. In rodent models of ischemic stroke, exogenous NPCs labeled with iron oxide particles and magnetic particles were observed to have successfully migrated to the stroke site by MRI (Hoehn et al., 2002; Jiang et al., 2005; Zhang et al., 2016b). Furthermore, compared with the control group, the lesion range of the magnetic labeled group was reduced and no secondary injury appeared (Obenaus et al., 2011). Angiogenesis (Jiang et al., 2005) and glucose utilization (Daadi et al., 2013) were also increased in the impaired areas of the brain. However, after cell transplantation and homing, the basic experimental and clinical evidence for how cells differentiate is still insufficient. In addition to intra-ventricular injection, cells can be introduced by arterial or venous injection; however, the exact homing path taken and the number and location of NPCs/NSCs after these routes of administration need to be further studied using labeled-cell assays. In theory, arterial injection of cells will result in greater numbers and a more widespread distribution of cells than the venous route, but there may also be more adverse reactions. Transnasal implantation may also be a new, convenient and safe implantation method (Mao et al., 2018).
Paracrine action
As studies progressed, replacement of lost neurons with stem cells was shown to not be the primary neural remodeling mechanism (Li et al., 2002) because stem cell replacement cannot fully explain the phenomena observed in in vivo experiments.
Paracrine evidence
Firstly, tracer methods indicated that the number of homing stem cells was far smaller than the number of transplanted stem cells, and cells injected via the arteriovenous route could still exert effects. In addition, it was thought that stem cells in the vascular pathway rarely pass through the BBB (Pendharkar et al., 2010). Secondly, cell replacement mechanisms cannot explain the efficacy of NSC transplantation at an early stage; the differentiation of nerve cells, the reconstruction of synapses and the rearrangement of neural networks are difficult to accomplish in a short period of time (Avena-Koenigsberger et al., 2017).
Paracrine vesicles
Extracellular vesicles are believed to be key in mediating recovery of damaged brain tissue (Doeppner et al., 2018). The discovery of exosomes and microRNAs (miRs) provided a basis for the brain remodeling process and cell repair mechanisms. For example, miR-9, which regulates axon regeneration in the peri-infarct region (Buller et al., 2012), miR-200b, which mediates myelin expression in the white matter (Buller et al., 2012), miR-17-92, which activates neuronal growth signals (Zhang et al., 2013), and miR-15a, which regulates angiogenesis in brain tissue (Yin et al., 2015), are detected in exosomes isolated from cerebrospinal fluid (Frühbeis et al., 2013). Cerebrospinal fluid and NSC-exosomes can regulate stem cell proliferation and immune function by intercellular pathways. For example, ESC-derived NSCs activate the insulin-like growth factor/mechanistic target of rapamycin complex-1 pathway to promote self-proliferation (Feliciano et al., 2014). Exosomal-associated interferon-γ/interferon-γ receptor 1 regulates the immune response through signal transducer and activator of transcription-1 signaling (Cossetti et al., 2014). Differentiated neurons and glial-derived exosomes co-ordinate and participate in axonal growth and myelin repair processes. Exosomes from neurons treated with a retinoic acid receptor β2 agonist enhance neurite outgrowth by inactivating phosphatase and tensin homolog signaling (Goncalves et al., 2015). miR-124 in cortical neuron exosomes can be transferred to astrocytes, thereby increasing the expression of the excitatory amino acid transporter glutamate transporter 1 and regulating the excessive proliferation of astrocytes (Morel et al., 2013). In addition, exosomes also contain alpha-amino-3-hydroxy-5-methyl-4-isooxazolpropionic acid receptors, which, together with microtubule-related protein 1b, which is abundant in depolarized neurite exosomes, activate astrocytes and promote myelin regeneration and axon germination in rodent stroke models (Dajas-Bailador et al., 2012; Morel et al., 2013; Goldie et al., 2014).
Inflammatory effects
Microglial cells differentiated from NSCs are a heterogeneous cell population involved in the immune response in the CNS. Primary microglia release aminopeptidase CD13 and the lactate transporter, monocarboxylate transporter 1. CD13 is transported to injured neurons by the exosome pathway, causing neuropeptide degradation. Microglial exosomes are also involved in internalization and reuptake by oligodendrocyte precursor cells, a process that is critical for synaptic pruning. In addition, microglia resident in the hippocampus activate oligodendrocytes and promote neurogenesis after activation by interleukin 4 and interferon-γ. The immune microenvironment under physiological conditions assumes a steady state. Secondary inflammation exacerbates oligodendrocyte death after stroke, and stem cells containing endogenous T-regulatory cells can regulate fibroblast growth factor and interleukin 6 levels and inhibit white matter injury (Zarriello et al., 2019). A key step in modulating the inflammatory response is to inhibit the activation of microglia. miR-126 exosomes inhibit microglial activation and inflammatory factor expression in vivo and in vitro (Geng et al., 2019). The regulation of inflammation in various types of stem cells is similar, and the effect of stem cells from a single source is limited. Mixed component transplants or stem cell additives may have better inflammatory effects. A joint delivery of interferon-γ (50 ng) combined with NSCs can increase neurogenesis and the number of 5′-bromodeoxyuridine/doublecortin double-positive cells in the ischemic area of stroke rats (Zhang et al., 2018). Yew et al. (2019) advocated that antibiotic pretreatment may improve prognosis.
Repair Processes of Transplanted Neural Stem Cells after Stroke Injury
Establishment of neuron polarity and weak synapse pruning (within 7 days after stroke)
Without clinical intervention, 120 million neurons, 83 billion synaptic connections, and 714 km of myelinated fibers are lost per hour after stroke (Saver, 2006). Polarity is the result of asymmetric spatial arrangement and distribution of cell components and subcellular structures during NSPC division, marking the beginning of neuronal regeneration (Fietz and Huttner, 2011). NSPCs undergo symmetric and asymmetric cell division, the former being the splitting into two similar neural progenitor cells in neurogenesis, and the latter being division and differentiation into a neuron and a progenitor cell (Miyata et al., 2001). Asymmetric cell division occurs mainly at a splitting angle of 0–30° (Zhao et al., 2011). Polar NSCs produce different types of functional nerve cells. The occurrence of polarity contributes to the diversity of cell types and to the integrity of functional networks (Wilsch-Bräuninger et al., 2016). During neurogenesis, neuroepithelial cells differentiate into radial glial cells (Taverna et al., 2014; Arai and Taverna, 2017; Hansen et al., 2017). Radial glial cells are stem/progenitor cells polarized along the apical basal axis that support networks and scaffolds of neural tissue. At the same time, radial glial cells undergo division and amplification just like neuroepithelial cells through symmetry on the one hand, and generate neurons through asymmetric division on the other hand, which leads to the continuous process of neuron polarization, migration and the acquisition of layer-type specific phenotypes. There are many factors that influence the regulation of NPC polarization, such as key regulatory genes (e.g. PAX6) and human-specific genes (e.g. ARHGAP11B) (Wilsch-Bräuninger et al., 2016), complement C5a (a cell polarity regulator) (Coulthard et al., 2017), direct current, and daughter cells. A small direct current electric field is thought to guide neurite growth and migration of rodent NPCs, while human NPCs may respond to a small electric field by directional migration. The application of an electric field may further help to guide human NPCs to a damaged area (Feng et al., 2012). Different types of NSCs do not have the same rate of neuron polarization. The transcription factor, ZEB1, may also be involved in regulating the direction of cleavage in dividing progenitor cells, neuron polarity, and migration (Liu et al., 2019). These interventional polarization measures may help shorten the differentiation time of stem cells after transplantation and improve survival rate.
Neurite growth and neuroregeneration (within 1 month after stroke)
Impaired synaptic regeneration involves two main physiological stages: early synaptic pruning and synaptic rewiring (Cohen et al., 2017). Detailed mechanisms of neurite pruning are reviewed by Schuldiner and Yaron (2015). Neurite growth is an important step for the establishment of a neural network in transplanted neurons. In a mouse model, vascular endothelial growth factor (VEGF), and thrombospondin 1 and 2 released by NSCs improve neonatal axonal transport function. The continuous growth of axons requires good nutrient and metabolite transport capabilities (Andres et al., 2011). Among the substances transported, various trophic factors and chemokines synergistically promote neurogenesis of NSCs. Sherman and Bang (2018) used high-throughput screening to identify 108 human iPSC-derived neuronal compounds that promote neurite growth, including 37 previously identified signaling pathway factors. Many of these regulatory factors that persist throughout the repair process are secreted by astrocytes. Basic fibroblast growth factor coupled with epidermal growth factor increased the length and number of new neurites (Zhao et al., 2019). Brain-derived neurotrophic factor secreted by glial cells derived from differentiated NSCs can maintain a stable secretion for 20 days, continuously promoting dendrite sprouting and redevelopment of myelin (Shi et al., 2016; Bierlein De la Rosa et al., 2017). The combination of biodegradable hydrogels with NSCs improved the environment for graft cell survival and integration. Functionalized fibrin hydrogels with synthetic peptide engaging integrin α6β1 (HYD1) enhanced the affinity, migration and extension of newborn axons (Silva et al., 2017). The properties of neural tissue engineering materials in the hydrogel system, such as peptide affinity and scaffold stiffness, have been continuously improved for use with transplanted NSCs to promote efficient differentiation of stem cells (Stukel and Willits, 2018). This is the basis of functional neuronal relay circuit formation after NSPC transplantation, and also informs in vitro axon growth models of nerve injury.
Myelin repair (within 3 months after stroke)
After stroke injury, myelin repair is critical for establishing proper neural network function and activity-dependent reorganization (Jia et al., 2019). The mechanics of myelin repair after brain injury were reviewed by Kondiles and Horner (2018). Remyelination may include three forms: myelination of previously bare axons, remodeling of existing sheaths, and removal of the sheath by new internode replacement (Kondiles and Horner, 2018). Myelin repair may occur through the recruitment and differentiation of oligodendrocyte precursor cells. The presence of oligodendrocytes with mature functions is essential. In adult mice, a sudden loss of oligodendrocytes led to axonal deterioration and an autoimmune myelin attack (Traka et al., 2016). In an ischemic injury model in aged mice, oligodendrocyte precursor cells could differentiate into astrocytes instead of mature oligodendrocytes in the area of complete demyelinating injury, possibly because of blocked Nogo receptor (Sozmen et al., 2016). In addition to mature oligodendrocytes, mature neurons in zebrafish models directly promote the maturation of myelin repair (Hines et al., 2015; Mensch et al., 2015).
Synaptic rewiring and remodeling of brain networks (6 months after stroke)
The restoration of function after brain injury is marked by completed reconstruction the of brain network (Navlakha et al., 2018). The anatomical basis of reconstruction of the brain network is the functional differentiation of neurons, the growth of neurites and myelin regeneration (Park and Friston, 2013; Avena-Koenigsberger et al., 2017). Topological network analysis of neural circuits shows that modular and hierarchical networks are particularly suitable for functional integration of local neuron operations (functional specialty) as a basis for cognition (Park and Friston, 2013). The essence of stroke injury is that the structural network damage limits the functional network. The research focus of stroke has shifted from the study of direct ischemic injury to the impact of lesions on the whole brain. stem cell transplantation may be advantageous for repair of the whole brain network after injury (Green et al., 2018).
Synaptic rewiring occurs in the repairing brain (Stepanyants et al., 2002). Dendritic spines and varicose axonal veins tightly bind to form “potential synapses” within the cortical column (Stepanyants et al., 2002). Dendritic spines can form new synapses with axons in pre- and postsynaptic neurons (Letellier et al., 2019). Tornero et al. (2017) demonstrated that transplanted neurons can accept direct synaptic inputs from neurons in different regions of the brain. The projection pattern was similar to that of endogenous cortical neurons of the same function. At the same time, the host stroke-injured brain cells could also gain signals from transplanted neurons. These results indicate that transplanted human iPSC-derived cortical neurons can be incorporated into damaged cortical circuits (Tornero et al., 2017). Theoretically, NSCs can potentially repair structural networks and are also the practical basis for in vitro human brain organoids (Bengoa-Vergniory and Kypta, 2015; Quadrato et al., 2017). Great progress has been made in the construction of neural circuits in primary nerve centers. The characteristic selective excitation of newborn hippocampal neurons is the dendritic integration of inputs from multiple brain regions (Bloss et al., 2018). Autonomous rewiring of damaged neural networks is the ideal goal for the recovery of behavioral functions (Atwal et al., 2008). The establishment of a neural network requires neuronal proliferation, functional differentiation, neurite growth and myelin sheath repair, to enable information transmission and electrical activity in an appropriate neural microenvironment. However, the process of neural network remodeling in the adult CNS may be complex and lengthy. The myelin-associated protein, Nogo receptor, and paired immunoglobulin-like receptor B (Atwal et al., 2008), are potential therapeutic targets for post-stroke enhancement of axonal regeneration and synaptic plasticity, while myelin-associated glycoprotein and oligodendrocyte myelin glycoprotein are widely thought to inhibit synaptic rewiring (Gou et al., 2014; Deng et al., 2018). At the very least, in the short term, exogenous cell transplantation may be a way to construct a network among damaged nerves (Moritz, 2018). The stem cells may provide a “wake-up” function to functionally integrate host neural networks (Wei et al., 2016).
Vascular regeneration and stabilization of the microenvironment (from 4 to 7 days after stroke)
Reestablishing vascular function as early as possible is the most important treatment in the acute phase of stroke to rescue live cells. After stroke, NVUs grow around the surviving tissue and begin repair. NVUs are not only coupled by pericytes to the BBB, but are also an important part of neural network construction and microenvironment regulation (Oztop-Cakmak et al., 2017). NVUs incorporate endothelial cells, pericytes, basal lamina, astrocytes, pericapillary microglia, and neurons (Fernández-Klett et al., 2013; Cai et al., 2017), and are fundamental structural and functional units of the CNS that regulate blood flow and homeostasis of the internal environment (Muoio et al., 2014). In preclinical stroke models and human stroke patients, NSC treatment enhances NVU formation and promotes tissue and functional recovery of stoke by producing neurotrophic and regenerative growth factors to promote angiogenesis between NSCs and vascular compartments (Baker et al., 2019). Angiogenesis can repair the network to improve blood flow in the brain and ameliorate brain function. Neurogenesis and angiogenesis have synergic effects to improve functional recovery outcome (Ruan et al., 2015).
Neovascular regeneration occurs in the ischemic core and marginal sites within 4–7 days after cerebral ischemia. Regenerating blood vessels may promote neuronal repair in two ways. One is to induce the sprouting of damaged axons of neurons with cell bodies; the other is to promote the proliferation and differentiation of NSCs and to give perfusion nutrition (Hatakeyama et al., 2020). NSC-mediated angiogenesis is largely modulated by VEGF signaling. VEGF secreted by NSCs is a potent angiogenic factor with direct neurotrophic signaling that stimulates adult neurogenesis and angiogenesis. Exogenous administration of VEGF reduces infarction size and improves functional outcome. Intravenous infusion of human NSCs reduces behavioral deficits and focal lesions in stroke rats by inducing angiogenesis (Ryu et al., 2016). The vascular remodeling effect of the modified NSCs was significant. The transplantation of superoxide dismutase-1-overexpressing NSCs enhanced angiogenesis in the ischemic border zone by up-regulating VEGF expression, which can reduce infarct size and improve behavioral performance, compared with non-modified NSCs (Sakata et al., 2012). Similar results were confirmed after transplantation of TAT-Hsp70-transduced NPCs (Doeppner et al., 2012), CTX0E03 cells, and iPSC-NSCs (Stroemer et al., 2009; Oki et al., 2012; Hicks et al., 2013). Hicks et al. (2013) assessed the angiogenic activity of CTX0E03 in vitro and in vivo. Their results indicated that micro-vessels were significantly increased at the implantation site in both naive and middle cerebral artery occlusion rats after CTX0E03 cell transplant (Figure 1).
Figure 1.
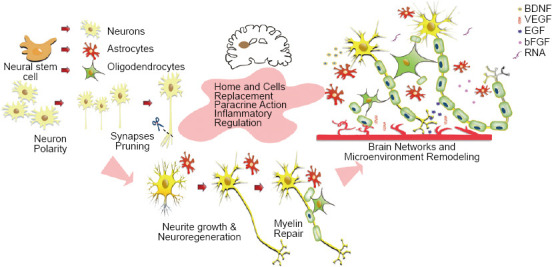
Scheme for transplanting neural stem cells to repair brain injury.
Neural stem cell-mediated angiogenesis is modulated with VEGF signaling. BDNF: Brain-derived neurotrophic factor; bFGF: basic fibroblast growth factor; EGF: epidermal growth factor; VEGF: vascular endothelial growth factor
Outcomes and Outlook
Animal models for NSC stroke therapy
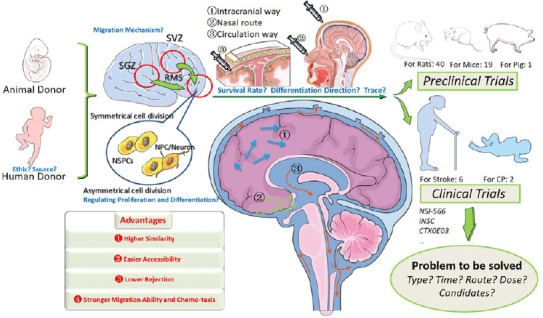
The rodent stroke model is the most commonly used animal stroke model. Among published studies, rat model experiments are about twice as common as mouse model experiments (40:19). Because of relatively easy surgery, the rat model is favored for the study of cognitive and sensorimotor functions after transient cerebral artery occlusion (Huang et al., 2018). In addition, interventional operations, such as transcranial electromagnetic stimulation, acupuncture, live small animal imaging, rat treadmills, and other technologies such as brain slice or brain tissue pathology experiments have matured (Tan et al., 2018; Peng et al., 2019; Yu et al., 2019). Mouse models are more widely used in large-scale behavioral experiments, and multiple interventions can be used to reduce costs. In particular, clinical-grade NSC lines are often modeled in mice prior to clinical application (Tuazon et al., 2019).
Although the number of pig stroke model studies is small, their conclusions are valuable. First, pigs are of similar body size to humans but their brain volume is 7.5 times smaller than that of human (Lind et al., 2007). Secondly, the structure of the pig brain is gyrencephalic and white matter accounts for 60%, which is closer to the human brain compared with lissencephalic and < 10% white matter rodent brains (Kuluz et al., 2007; Nakamura et al., 2009). Therefore, Webb et al. (2018) used a pig stroke model to demonstrate that novel NSC-extracellular vesicles can significantly improve the preservation and function of nerve tissue after post-middle cerebral artery occlusion, indicating that NSC-extracellular vesi-cles may be therapeutic for stroke. Induction of NPC-NSCs also allows stroke pigs to restore appetite and posture more quickly (Lau et al., 2018). In a beagle stroke model, Lu et al. (2013) tracked super-paramagnetic iron oxide-labeled transplanted cells through the flow of the ipsilateral middle cerebral artery into the brain and demonstrated the phenomenon of homing. Madelaine et al. (2017) used a zebrafish model to reveal that miR-9 is linked to neurogenesis and angiogenesis through the formation of VEGF-A-expressing neurons, reflecting the advantage of zebrafish to directly observe neovasculari-zation. In short, the choice of animal model should consider experimental design and statistical meth-ods.
Overview of preclinical trials
The efficacy and safety of NSCs in the treatment of stroke-related diseases have always been a focus for scholars. The evidence and reference basis for meta-analysis of preclinical studies using animal models have been relatively systematic. Huang et al. (2018) included 62 controlled animal studies pub-lished between 2004 and 2018. Of these, 28 used human NSPC donors and 34 used rat or mouse donors. The stroke models used were 40 rats, 19 mice, two Mongolian gerbils, and one pig. In 28 trials the transplanted NSPCs were derived from pluripotent stem cells, and in 21 studies the animals received immunosuppressant drugs before the experiment. The average quality score of the included trials was 5.1, ranging from 2 to 8, and the randomized double-blind controlled studies were less than 43.5%. From summary results of modified neurological severity score, rotarod performance test, cylinder test and infarct volume detection, Huang et al. (2018) confirmed that NSPC transplantation is beneficial to the recovery of neurobehavioral and histological outcomes. Reductions in apoptosis and axonal function are noticeable according to higher effect size. Interestingly, lower cell injection doses were more likely to produce better cylinder test results, with the most effective cell dose less than 1 × 106 cells/kg. Heterogeneity of modified neurological severity score, cylinder test results, and infarct volume was high among studies, but infarct volume was still greater than 75% after sensitivity analysis. A similar situation existed for human cell donor studies (Huang et al., 2018). Chen et al. (2016) performed a meta-analysis on 37 studies and 54 independent therapeutic interventions and reached similar conclusions. They suggested that trials should be conducted with as many homologous cells as possible. In addition, when these cells were given within the first 24 hours after a stroke, infarct volume improved significantly. Lees et al. (2012) suggested that autologous stem cells were more effective for structural outcomes than allogeneic stem cells, but the reverse was true for functional outcomes. The meta-analysis by Nucci et al. (2015) included 22 studies on super-paramagnetic iron oxide nanoparticle-labeled stem cells in preclinical stroke models published between January 2000 and October 2014 that provided evidence of transplanted cells in and around the core of the injury site, and suggested that stem cell therapy in the early stages of infarction was promising.
Overview of clinical trials and problems at hand
Clinical trials are far more difficult to advance than animal model experiments. Eighty-eight clinical trials for stroke have been registered with clinicalTrials.gov since April 20, 2020. Among six studies involving NSCs, three have posted their results. There are also two NSC clinical trials for children with hypoxic ischemic encephalopathy and ischemia-related cerebral palsy (Table 2).
Table 2.
Clinical trials using neural stem cells for the treatment of stroke (active and recruiting)
| NCT number | Tittle | Status | Interventions | Study type | Outcome measure | Population | Sponsor | Process observed |
| NCT03296618 | Intracerebral transplantation of neural stem cells for the treatment of ischemic stroke | Active (completed) | NSI-566: A group: 1.2 × 107; B group: 2.4 × 107; C group: 7.2 × 107 | Interventional (one-time intra-cerebral injections) | AE & NIHSS & mRS, MMSE, FMMS | Enrollment: 9; age: 33–65 yr (150–743 d post-stroke) | Suzhou Neuralstem Biopharmaceuticals Neuralstem Inc. | NP; NG; MR; VR |
| NCT03725865 | A clinical study of iNSC intervent cerebral hemorrhagic stroke | Not yet recruiting | iNSCs | Interventional | Emergent AE | Enrollment:12; age: 30–65 yr; sex: both | Allife Medical Science and Technology Co. Ltd. | NP; NG; MR; VR |
| NCT01151124 | Pilot investigation of stem cells in stroke | Active (completed) | CTX0E03 (NSCs), single doses of 2 million, 5 million, 10 million, or 20 million cells | Intervention (stereotactic ipsilateral putamen injection) | AE & BI & MMSE, mRS, EQ-5D | Enrollment: 12; age: 60–85 yr (29 mon post-stroke); sex: male | ReNeuron Limited (Division of Clinical Neurosciences, Glasgow) | NP; NG; MR; VR |
| NCT03629275 | Investigation of neural stem cells in ischemic stroke | Recruiting | Combination product: CTX0E03 drug product and delivery device; Drug: placebo | Intervention | mRS at 6 mon BI; basic mobility changing and TUG, CAHAI & SDMT & COWAT & MNT & MCA | Enrollment: 110; Age: 35–75 yr (adult, older adult); aex: both | ReNeuron Limited (University of California, Irvine, CA, USA) | NP; NG; MR; BN; VR |
| NCT02854579 | Neural progenitor cell and paracrine factors to treat hypoxic ischemic encephalopathy | Active (completed) | Biological: NPC; Biological: paracrine factors; Biological: progenitor cell and paracrine factors | Interventional; intra-cerebral injections | Neonatal behavioral assessment; adverse events; Bayley score; Peabody development measure scale; MRI or CT | Enrollment:120; age: up to 14 d (child); sex: both | Navy General Hospital, Beijing, China | NP; NG; MR; BN; VR |
| NCT02117635 | Pilot investigation of stem cells in stroke phase II efficacy | Complete | Biological: CTX DP | Interventional | ARAT and after CTX; NIHSS; RFA & BI after CTX; Safety/ Tolerability; FMMS after CTX | Enrollment: 23; age: 40 yr older (adult, older adult); sex: both | ReNeuron Limited (Queen Elizabeth Hospital, Birmingham, UK) | NP; NG; MR; VR; |
| NCT04047563 | Efficacy of sovateltide (PMZ-1620) in patients of acute ischemic stroke | Recruiting | Drug: PMZ-1620 (sovateltide) along with standard treatment | Interventional, phase 3 | NIHSS/mRS; change in quality-of-life; change in Stroke-Specific Quality of Life | Enrollment: 110; age: 18–78 yr (adult, older adult); sex: both | Pharmazz, Inc. | NP; NG; MR; VR |
| NCT03005249 | Neural stem cells therapy for cerebral palsy | Recruiting | Biological: NSC | Interventional, intranasal administration | GMFM-88 score; Fine Motor Function Measure score; modified Ashworth Scale score; AEEG/ MRI/EMG | Enrollment: 20; age: 1–12 yr (child); cerebral palsy | The First Affiliated Hospital of Dalian Medical University, Dalian, China | NP; NG; MR; VR |
Information obtained from ClinicalTrials.gov on April 20, 2020. AE: Adverse events; AEEG: amplitude-integrated electroencephalography; ARAT: Action Research Arm Test; BI: Barthel Index; BN: brain networks; CAHAI: Chedoke Arm and Hand Activity Inventory; COWAT: Controlled Oral Word Association tasks (Language skills); CTX: CTX0E03, a human NSC line; DP: drug product; EMG: electromyography; EQ-5D: EuroQol-5D; FMMS: Fugl-Meyer Motor Score; GMFM-88: Gross Motor Function Measure-88; iNSC: induced NSC; MCA: Montreal Cognitive Assessment (Language skills); MMSE: Mini-mental State Examination; MNT: Multilingual Naming Test (Language skills); MR: myelin repair; mRS: modified Rankin Scale; NG: neurite growth; NIHSS: National Institutes of Health Stroke Scale; NP: neuron polarity; NPC: neural progenitor cell; NSCs: neural stem cells; RFA: Rankin Focused Assessment; SDMT: Symbol Digit Modalities Test (Language skills); TUG: Timed Up and Go Test; VR: vascular regeneration.
NCT01151124 is one of the most influential clinical studies on the treatment of stroke by stem cells in recent years. The favorable efficacy and safety of CTX0E03 cells have provided sufficient confidence for human NSCs projects to proceed (Kalladka et al., 2016). The NCT03629275 study, hosted by the company ReNeuron (PISCES-III), will include more participants than the NCT01151124 trial (12 centers, 110 patients expected) to further demonstrate the clinical effects of CTX0E03 cells. However, the proangiogenic properties of CTX0E03 cells, especially for microvascular construction, need further exploration in animal models (Hicks et al., 2013) and tumorigenesis should still be given attention (Stonesifer et al., 2017).
It should be noted that ReNeuron has also used immortalized human fetal NSC grafts for stroke patients, and no cell-related or immunological adverse events were found in phase I and phase II studies (11 and 42 patients, respectively) (Trounson and McDonald, 2015). However, from the positive results of the SB623 transplantation trial in which Steinberg et al. (2016) recruited 18 stable patients with chronic stroke, it should also be recognized that despite long-term efficacy, the safety of the transplantation cannot be ignored.
The NCT03296618 study using NSI-566 cells, which were previously clinically trialed for spinal cord injury (Curtis et al., 2018), recently published encouraging conclusions. Three cohorts were transplanted with one-time intracerebral injections of 1.2 × 107, 2.4 × 107, or 7.2 × 107 NSI-566 cells for the treatment of hemiparesis resulting from chronic motor stroke to determine the maximum tolerated dose for future trials. Immunosuppression therapy with tacrolimus was maintained for 28 days. After 12 months, the mean Fugl-Meyer Motor Score of nine participants showed 16 points of improvement (P = 0.0078), the mean modified Rankin Scale showed 0.8 points of improvement (P = 0.031), and the mean National Institutes of Health Stroke Scale showed 3.1 points of improvement (P = 0.020). The changes remained stable at the 24-month follow-up. Longitudinal MRI studies showed cavity-filling by new neural tissue formation in all nine patients (Zhang et al., 2019a). However, further animal experiments or autopsy needs to verify the association of neonatal tissue properties, cellular components and correlation between new tissues and recovered function.
Clinical trials to reprogram human peripheral blood mononuclear cells to induced NSCs have not been conducted. Previous animal experiments have demonstrated that peripheral blood mononuclear cell-derived induced NSCs have similar characteristics to fetal NSCs. They have high neuroplasticity and can become dopaminergic, cholinergic, glutamatergic, and γ-aminobutyric acidergic neurons (Yuan et al., 2018). However, the higher plasticity poses a higher risk of tumorigenicity and the accumulation of harmful mutations during reprogramming and expansion (Lynch, 2010). Each haplotype genome acquires 3–30 mutations per mitotic somatic cell resulting in genome replication not being 100% accurate (Yuan et al., 2018). Therefore, the intracerebral transplantation of human peripheral blood-derived NSCs (NCT03725865) will be difficult, but the results are worth looking forward to.
Despite the positive results, the clinical application of NSCs is difficult. One of the problems encoun-tered in NSC clinical trials is the source of cells. Rejection of autologous cells is relatively uncommon, but compared with fetal NSCs, there is very limited data for adult human NSCs with a lack of systematic characterization and differences in extraction sites (Hermann et al., 2004). However, only about 20% of freshly isolated cells from subcortical white matter and the hippocampus express oligodendrocyte progenitor markers, and 1 of 694 cells from white matter or 1 of 1331 hippocampal cells was able to generate neurospheres (Lojewski et al., 2014). Allogeneic fetal-derived human NSCs show good safety, but require immunosuppression after transplantation, and donors are very limited. In this case, obtaining iPSC-derived NSCs with reliable “NSC characteristics” may be a solution (Meneghini et al., 2017).
Another problem is the ethical limitation of using fluorescently-labeled transplanted cells in human trials. Currently, transplantation of labeled cells into rodents and using live small animal imaging techniques provide more evidence of the location and fate of transplanted cells than human trials (Li et al., 2019).
Time
Whether the time for transplantation should be selected in the acute phase of stroke (within 24–72 hours) is controversial. The state of the recipient during this time period is difficult to estimate. NSC therapy may be advocated during the stroke recovery period and in the chronic stroke period (90 days after stroke). Boese et al. (2018) also believed that NSC treatment may be more beneficial for sub-acute strokes and the NCT03296618 trial selected the transplant time as 150–743 days after stroke (Zhang et al., 2019a). Gutierrez-Fernandez et al. (2011) suggested that 14 days after theoretical implantation of NSCs is the best time for functional recovery. In other words, the transplant time is most likely to be most beneficial around a hundred days after injury. All phase I clinical trials currently performed assume that the longer the span of the treatment period, the less risk the cells pose for personal safety (Kalladka et al., 2016).
Evaluation of delivery potential (route and dose)
The effective dose varies significantly with different administration routes: intravenous > intra-arterial > intrathecal > intracerebral (Vu et al., 2014). For stroke, NSPCs are mostly studied using the intracere-bral route. Intracerebrally-injected neurons can reconstitute long axon projections and synaptic connections impaired in the host brain. Autopsy pathology confirmed that new tissue formed from the injection site (Zhang et al., 2019a, 2020). Chronic stroke patients receiving treatment also gained significant improvements in motor functions compared to the rehabilitation control group (Kalladka et al., 2016; Detante et al., 2017). Intracerebral and intrathecal administrations are rapid and direct, but both are invasive treatments with high risks (Rodríguez-Frutos et al., 2016), such as secondary cerebral edema and intracranial hypertension. Basic research has shown that cell replacement is not the main therapeutic mechanism of stem cells, which have the ability to migrate; therefore, the risk of delivering the cells to the site in this way needs to be reassessed (Detante et al., 2017). Intra-arterial and intravascular injections are less invasive and easier to perform than surgical implantation, but serious consequences, such as vascular occlusion and pulmonary embolism may occur (Bosi and Bartolozzi, 2010; Smith et al., 2011). Vascular delivery may have a significant impact on cell survival, and it is difficult to ensure that an effective number of cells pass through the BBB (Boese et al., 2018). Therefore, there is not enough evidence to show that vascular pathways of NSC delivery have therapeutic potential (Kondziolka et al., 2005; Savitz et al., 2005). Notably, some of the more noninvasive and acceptable delivery routes are being evaluated. For example, after intranasal delivery, stem cells and neurotrophic factors can migrate into the CNS through the cribiform plate in the rostral migration stream (Danielyan et al., 2009; Du et al., 2018; Mao et al., 2018; Aly et al., 2019). The NCT03005249 trial is registered for NSC intranasal administration. Convincing assessment of NSC delivery may have to rely on techniques that trace stem cells in humans. It is also important to track the behavior of NSCs in vivo, including proliferation, migration, viability, and functional reconstruction. Non-invasive MRI techniques, such as magnetic particle imaging, is one such technique with great clinical potential (Zheng et al., 2017).
In the NCT01151124 trial, CTX0E03 cells were transplanted at different doses (2 million, 5 million, 10 million or 20 million) by stereotactic ipsilateral putamen injection (Kalladka et al., 2016). To date, no graft-related adverse reactions have been observed; therefore, a dose of at least 20 million cells can be applied in the short term, and there is no conclusive evidence that the injection dose is positively correlated with the chance of tumorigenesis. The dose of human fetal-derived NPSCs for the treatment of late-infantile ceroid lipofuscinosis (Batten’s disease) is 1 billion, indicating that cell delivery to the brain is well tolerated (Trounson and McDonald, 2015).
Special candidates
Newborns
Ischemic-hypoxic brain injury in neonates may have serious consequences. It is now thought that this injury can result in chronic degeneration and damage caused by disruption to developing neurons and neural networks, resulting in complete stagnation of development, which may lead to physical disability and intellectual delay (cerebral palsy and neonatal stroke) (Stone et al., 2008). White matter changes are frequently observed in the brains of newborns who have experienced clinical ischemia and hypoxia, and are different from the pathological manifestations of brain damage in adults. The process of perinatal asphyxiation is unknown but a significant reduction in the number of oligodendrocyte precursor cells in an oligodendrocyte maturation disorder has been recognized (Fernández-López et al., 2014; Back, 2017). A recent study showed that endogenous progenitor cells in the neonatal brain may spontaneously participate in compensation after hypoxia injury (Ziemka-Nalecz et al., 2018). This result may help us to understand and apply endogenous NPCs/NSCs to neonatal stroke patients. Lian’s clinical trial results show that NSCs are very active and effective in treating cerebral palsy. NCT03005249 trial results will soon be published and our basic research has confirmed that human NSCs have good directional differentiation ability, especially toward glial cells (Lian et al., 2012).
Neuropathological aspects of stroke with hemorrhage and their impact on NSC transplantation
Intracerebral hemorrhage (ICH) is one of the deadliest types of stroke. The first stage after ICH is re-ferred to as the primary injury. Damaged tissue is usually surrounded by edema, inflammation, and necrosis. Mechanical pressure in the bleeding zone leads to extensive cell death with mitochondrial dysfunction (Adeoye and Broderick, 2010). After 3–12 hours of hematoma dilation, 73% of patients show cascades of coagulation activation and thrombus and hemoglobin decomposition products, which further activate the inflammatory response dominated by glial cells, leading to secondary injury (Qureshi et al., 2003). The mechanism involved in the repair by stem cells is mainly related to nutritional effects and anti-inflammatory and immunomodulatory activities (Takeuchi et al., 2013). Tang et al. (2004) discovered a large number of nestin-positive cells around ICH-injured tissue. Jeong et al. (2003) injected exogenous human NSCs intravenously into ICH model rats to show that surviving NSCs are helpful for functional recovery and new neurons and astrocytes were found in the brain 7 days after transplantation. These results were confirmed using superparamagnetic iron oxide-labeled human NSCs (Chang et al., 2008). However, few clinical trials have been designed for hemorrhagic strokes (Shimamura et al., 2017) and the effectiveness of cell therapy for ICH and subarachnoid hemorrhage has yet to be determined in clinical trials, possibly because cerebral hemorrhage surgical trials (STICH trials I and II) failed to provide convincing evidence that cell therapy could reduce mortality or reduce neurological deficits (Mendelow et al., 2005, 2013). Furthermore, nimodipine, a calcium channel blocker, provides beneficial results for subarachnoid hemorrhage.
Strategies to increase the success rate of stem cell transplantation
Without clinical intervention, stroke-damaged brain tissue can lose 120 million neurons, 83 billion synaptic connections and 714 km of myelinated fibers per hour (Saver, 2006). The internal environment of the damaged site should be modified as much as possible so that transplanted cells can survive in a more suitable environment. Meanwhile, transplanted cells should adapt to the post-injury ischemic and hypoxic environment (Sandvig et al., 2017). Although human NSCs show great tissue-specific regeneration potential in vitro, the efficacy of clinical applications is limited due to the lack of standardized in vitro cell production methods and insufficient simulation of the injured microenvironment. NPSCs need to be generated in vitro on a large scale and then differentiated into neurons. Mature and scalable protocols have allowed the cost-effective generation of robust NPSCs from iPSCs as a source (Beevers et al., 2013). Following culture in neurobasal medium supplemented with B27 and brain-derived neurotrophic factor, NPSCs mainly differentiate into vesicular glutamate transporter 1-positive neurons displaying features of layer 3 pyramidal cells (D’Aiuto et al., 2014). These iPSC-derived neurons can express functional ligand-gated channels and other synaptic proteins (D’Aiuto et al., 2012, 2014; Zhao et al., 2016). The cost to culture iPSCs into neurons (more than 90% TUJ1-positive cells) that are evenly distributed in a 384-well plate is about $190 and takes approximately 4 weeks (D’Aiuto et al., 2014).
Three-dimensional culture systems for in vitro culture of neural tissues include hydrogels, solid porous polymers, fibrous materials and acellular tissues, and microfluidic devices, which provide powerful conditions for standardized in vitro experiments (Murphy et al., 2017). The proliferation and differenti-ation capabilities of stem cells are constantly being explored, for example by two-dimensional and three-dimensional cultures in normoxic (21% O2) and hypoxic conditions (3% O2), with and without epidermal growth factor and fibroblast growth factor 2 (Han et al., 2012; Ghourichaee et al., 2017). In the presence of growth factors, the size of the culture (three-dimensional) and low oxygen concentra-tions enhance the survival and proliferation of human NSCs (Ghourichaee et al., 2017). Extracellular matrix perfusion can promote the proliferation and differentiation of NSCs in three-dimensional culture (Wang et al., 2017). Comparing NSCs with stem cells from other sources, after transplantation into hypoxic brain tissue (O2 concentrations < 5%) (Sandvig et al., 2017) there is no significant difference in differentiation ability (Lee et al., 2015; Teixeira et al., 2015; Burian et al., 2017), but the survival rate of NSCs may be higher (Madelaine et al., 2017). Teixeira et al. (2015) showed that many functional proteins and factors from NSC-exosomes, most of which are membrane proteins, are up-regulated in hypoxic environments.
Potential of combining neural stem cell therapy with other therapeutic strategies
The continuous development of new biomaterials and microsurgery technology for their delivery has enabled stem cells to be combined with minimally invasive repair technology. The in vivo tracking and precision sensing capabilities of minimally invasive and regenerative therapeutics will become more cost-effective and the risks will reduce with the help of nano-biomaterials and soft bioelectronic devices (Ashammakhi et al., 2019). Engineered neuroplasticity technology has already been demonstrated to affect long-term changes in spinal cord injury, a tractable model of CNS repair (Ievins and Moritz, 2017). However, drug and surgical intervention, brain electrical stimulation technology, and even artificial intelligence robot technology are unlikely to completely obviate the advantages of NSCs (Moritz, 2018). NSC therapy aims to promote the regeneration of myelin sheath (Kondiles and Horner, 2018a), restore the conduction of damaged axons (Anderson et al., 2017) and improve the internal environment of the damaged nervous system (Schmidt-Hieber et al., 2004). At present, it is believed that only stem cell therapy combined with engineering devices can shape this neuroplastic repair into specific, functioning neural circuits (Mondello et al., 2014).
Conclusion
NSCs are beneficial for the repair of brain structure and function after injury and are strongly validated in preclinical and clinical research. It is expected that, NSCs will achieve significant results in the treatment of stroke. However, there are still several pressing issues that need to be addressed for clinical application of NSCs. (1) Thorough and detailed explanation of the functional recovery process and the mechanism of structural repair of the network microenvironment; (2) in-depth study of the therapeutic mechanism of NSCs; (3) selection of the most reasonable route of administration, dosage and timing; and (4) improvement of the evaluation system and ethical standards. Our review preliminarily summarizes the current mechanisms of NSCs neuroregeneration and functional recovery after stroke injury. We also present the current progress in basic and clinical research to provide a reference for future research (Figure 2).
Figure 2.
Summary of the review showing the steps and challenges for basic NSC research and for clinical trials of stroke treatment.
NSCs have unique mechanisms for cell replacement, paracrine action, inflammatory regulation and neuroprotection. Basic and clinical research into NSC therapy promises to achieve functional recovery after stroke. CP: Cerebral palsy; NPC: neural progenitor cell; NSC: neural stem cell; NSPC: neural stem/progenitor cell; RMS: rostral migratory stream; SGZ: subgranular zone; SVZ: subventricular zone.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81471308 (to JL); Program of China National Health Commission and National Medical Products Administration (NMPA), No. CMR-20161129-1003 (to JL); Liaoning Province Excellent Talent Program Project, No. XLYC1902031 (to JL); Dalian Innovation Fund, No. 2018J11CY025 (to JL).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81471308 (to JL); Program of China National Health Commission and National Medical Products Administration (NMPA), No. CMR-20161129-1003 (to JL); Liaoning Province Excellent Talent Program Project, No. XLYC1902031 (to JL); Dalian Innovation Fund, No. 2018J11CY025 (to JL).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song CP; T-Editor: Jia Y
References
- 1.Addington CP, Dharmawaj S, Heffernan JM, Sirianni RW, Stabenfeldt SE. Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1α. Matrix Biol. 2017;60-61:206–216. doi: 10.1016/j.matbio.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addington CP, Heffernan JM, Millar-Haskell CS, Tucker EW, Sirianni RW, Stabenfeldt SE. Enhancing neural stem cell response to SDF-1α gradients through hyaluronic acid-laminin hydrogels. Biomaterials. 2015;72:11–19. doi: 10.1016/j.biomaterials.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat Rev Neurol. 2010;6:593–601. doi: 10.1038/nrneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad M, Dar NJ, Bhat ZS, Hussain A, Shah A, Liu H, Graham SH. Inflammation in ischemic stroke: mechanisms, consequences and possible drug targets. CNS Neurol Disord Drug Targets. 2014;13:1378–1396. doi: 10.2174/1871527313666141023094720. [DOI] [PubMed] [Google Scholar]
- 5.Aly AE, Harmon B, Padegimas L, Sesenoglu-Laird O, Cooper MJ, Yurek DM, Waszczak BL. Intranasal delivery of hGDNF plasmid DNA nanoparticles results in long-term and widespread transfection of perivascular cells in rat brain. Nanomedicine. 2019;16:20–33. doi: 10.1016/j.nano.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 6.An N, Xu H, Gao WQ, Yang H. Direct Conversion of Somatic Cells into Induced Neurons. Mol Neurobiol. 2018;55:642–651. doi: 10.1007/s12035-016-0350-0. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf-Lavi E, Widerström-Noga E, Wood P, Levi AD. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma. 2017;34:2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 8.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- 10.Annese V, Navarro-Guerrero E, Rodríguez-Prieto I, Pardal R. Physiological plasticity of neural-crest-derived stem cells in the adult mammalian carotid body. Cell Rep. 2017;19:471–478. doi: 10.1016/j.celrep.2017.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai Y, Taverna E. Neural progenitor cell polarity and cortical development. Front Cell Neurosci. 2017;11:384. doi: 10.3389/fncel.2017.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashammakhi N, Ahadian S, Darabi MA, El Tahchi M, Lee J, Suthiwanich K, Sheikhi A, Dokmeci MR, Oklu R, Khademhosseini A. Minimally invasive and regenerative therapeutics. Adv Mater. 2019;31:e1804041. doi: 10.1002/adma.201804041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 14.Avena-Koenigsberger A, Misic B, Sporns O. Communication dynamics in complex brain networks. Nat Rev Neurosci. 2017;19:17–33. doi: 10.1038/nrn.2017.149. [DOI] [PubMed] [Google Scholar]
- 15.Azad TD, Veeravagu A, Steinberg GK. Neurorestoration after stroke. Neurosurg Focus. 2016;40:E2. doi: 10.3171/2016.2.FOCUS15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134:331–349. doi: 10.1007/s00401-017-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker EW, Kinder HA, West FD. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019;9:e01214. doi: 10.1002/brb3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker KA, Hong M, Sadi D, Mendez I. Intrastriatal and intranigral grafting of hNT neurons in the 6-OHDA rat model of Parkinson’s disease. Exp Neurol. 2000;162:350–360. doi: 10.1006/exnr.1999.7337. [DOI] [PubMed] [Google Scholar]
- 19.Barker RA, Götz M, Parmar M. New approaches for brain repair-from rescue to reprogramming. Nature. 2018;557:329–334. doi: 10.1038/s41586-018-0087-1. [DOI] [PubMed] [Google Scholar]
- 20.Beevers JE, Caffrey TM, Wade-Martins R. Induced pluripotent stem cell (iPSC)-derived dopaminergic models of Parkinson’s disease. Biochem Soc Trans. 2013;41:1503–1508. doi: 10.1042/BST20130194. [DOI] [PubMed] [Google Scholar]
- 21.Belmadani A, Ren D, Bhattacharyya BJ, Rothwangl KB, Hope TJ, Perlman H, Miller RJ. Identification of a sustained neurogenic zone at the dorsal surface of the adult mouse hippocampus and its regulation by the chemokine SDF-1. Hippocampus. 2015;25:1224–1241. doi: 10.1002/hipo.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bengoa-Vergniory N, Kypta RM. Canonical and noncanonical Wnt signaling in neural stem/progenitor cells. Cell Mol Life Sci. 2015;72:4157–4172. doi: 10.1007/s00018-015-2028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 25.Bierlein De la Rosa M, Sharma AD, Mallapragada SK, Sakaguchi DS. Transdifferentiation of brain-derived neurotrophic factor (BDNF)-secreting mesenchymal stem cells significantly enhance BDNF secretion and Schwann cell marker proteins. J Biosci Bioeng. 2017;124:572–582. doi: 10.1016/j.jbiosc.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Bloss EB, Cembrowski MS, Karsh B, Colonell J, Fetter RD, Spruston N. Single excitatory axons form clustered synapses onto CA1 pyramidal cell dendrites. Nat Neurosci. 2018;21:353–363. doi: 10.1038/s41593-018-0084-6. [DOI] [PubMed] [Google Scholar]
- 27.Boese AC, Le QE, Pham D, Hamblin MH, Lee JP. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res Ther. 2018;9:154. doi: 10.1186/s13287-018-0913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 29.Bosi A, Bartolozzi B. Safety of bone marrow stem cell donation: a review. Transplant Proc. 2010;42:2192–2194. doi: 10.1016/j.transproceed.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Buller B, Chopp M, Ueno Y, Zhang L, Zhang RL, Morris D, Zhang Y, Zhang ZG. Regulation of serum response factor by miRNA-200 and miRNA-9 modulates oligodendrocyte progenitor cell differentiation. Glia. 2012;60:1906–1914. doi: 10.1002/glia.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burian E, Probst F, Palla B, Riedel C, Saller MM, Cornelsen M, König F, Schieker M, Otto S. Effect of hypoxia on the proliferation of porcine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells in 2- and 3-dimensional culture. J Craniomaxillofac Surg. 2017;45:414–419. doi: 10.1016/j.jcms.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Cai W, Liu H, Zhao J, Chen LY, Chen J, Lu Z, Hu X. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res. 2017;8:107–121. doi: 10.1007/s12975-016-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang NK, Jeong YY, Park JS, Jeong HS, Jang S, Jang MJ, Lee JH, Shin SS, Yoon W, Chung TW, Kang HK. Tracking of neural stem cells in rats with intracerebral hemorrhage by the use of 3T MRI. Korean J Radiol. 2008;9:196–204. doi: 10.3348/kjr.2008.9.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Zhang G, Gu Y, Guo X. Meta-analysis and systematic review of neural stem cells therapy for experimental ischemia stroke in preclinical studies. Sci Rep. 2016;6:32291. doi: 10.1038/srep32291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng XQ, Liang XZ, Wei S, Ding X, Han GH, Liu P, Sun X, Quan Q, Tang H, Zhao Q, Shang AJ, Peng J. Protein microarray analysis of cytokine expression changes in distal stumps after sciatic nerve transection. Neural Regen Res. 2020;15:503–511. doi: 10.4103/1673-5374.266062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen EJ, Quarta E, Bravi R, Granato A, Minciacchi D. Neural plasticity and network remodeling: From concepts to pathology. Neuroscience. 2017;344:326–345. doi: 10.1016/j.neuroscience.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 37.Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini HK, Davis MP, Schaeffer J, Vega B, Stefanini M, Zhao C, Muller W, Garcia-Verdugo JM, Mathivanan S, Bachi A, Enright AJ, Mattick JS, Pluchino S. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56:193–204. doi: 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coulthard LG, Hawksworth OA, Li R, Balachandran A, Lee JD, Sepehrband F, Kurniawan N, Jeanes A, Simmons DG, Wolvetang E, Woodruff TM. Complement C5aR1 signaling promotes polarization and proliferation of embryonic neural progenitor cells through PKCζ. J Neurosci. 2017;37:5395–5407. doi: 10.1523/JNEUROSCI.0525-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T, Marsala S, Jamieson C, Marsala M, Ciacci JD. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950.e946. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 40.D’Aiuto L, Zhi Y, Kumar Das D, Wilcox MR, Johnson JW, McClain L, MacDonald ML, Di Maio R, Schurdak ME, Piazza P, Viggiano L, Sweet R, Kinchington PR, Bhattacharjee AG, Yolken R, Nimgaonkar VL. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. Organogenesis. 2014;10:365–377. doi: 10.1080/15476278.2015.1011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Aiuto L, Di Maio R, Heath B, Raimondi G, Milosevic J, Watson AM, Bamne M, Parks WT, Yang L, Lin B, Miki T, Mich-Basso JD, Arav-Boger R, Sibille E, Sabunciyan S, Yolken R, Nimgaonkar V. Human induced pluripotent stem cell-derived models to investigate human cytomegalovirus infection in neural cells. PLoS One. 2012;7:e49700. doi: 10.1371/journal.pone.0049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daadi MM, Hu S, Klausner J, Li Z, Sofilos M, Sun G, Wu JC, Steinberg GK. Imaging neural stem cell graft-induced structural repair in stroke. Cell Transplant. 2013;22:881–892. doi: 10.3727/096368912X656144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, Papalopulu N. microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci. 2012;15:697–699. doi: 10.1038/nn.3082. [DOI] [PubMed] [Google Scholar]
- 44.Danielyan L, Schäfer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, Burkhardt U, Proksch B, Verleysdonk S, Ayturan M, Buniatian GH, Gleiter CH, Frey WH., 2nd Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315–324. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Deng B, Li L, Gou X, Xu H, Zhao Z, Wang Q, Xu L. TAT-PEP enhanced neurobehavioral functional recovery by facilitating axonal regeneration and corticospinal tract projection after stroke. Mol Neurobiol. 2018;55:652–667. doi: 10.1007/s12035-016-0301-9. [DOI] [PubMed] [Google Scholar]
- 46.Detante O, Rome C, Papassin J. How to use stem cells for repair in stroke patients. Rev Neurol (Paris) 2017;173:572–576. doi: 10.1016/j.neurol.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doeppner TR, Bähr M, Giebel B, Hermann DM. Immunological and non-immunological effects of stem cell-derived extracellular vesicles on the ischaemic brain. Ther Adv Neurol Disord. 2018;11:1756286418789326. doi: 10.1177/1756286418789326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doeppner TR, Ewert TA, Tönges L, Herz J, Zechariah A, ElAli A, Ludwig AK, Giebel B, Nagel F, Dietz GP, Weise J, Hermann DM, Bähr M. Transduction of neural precursor cells with TAT-heat shock protein 70 chaperone: therapeutic potential against ischemic stroke after intrastriatal and systemic transplantation. Stem Cells. 2012;30:1297–1310. doi: 10.1002/stem.1098. [DOI] [PubMed] [Google Scholar]
- 50.Du Z, Zhang H, Chen Q, Gao Y, Sun B. Intranasal calcitonin gene-related peptide protects against focal cerebral ischemic injury in rats through the Wnt/β-catenin pathway. Med Sci Monit. 2018;24:8860–8869. doi: 10.12659/MSM.913777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckert A, Huang L, Gonzalez R, Kim HS, Hamblin MH, Lee JP. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cells Transl Med. 2015;4:841–851. doi: 10.5966/sctm.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res. 2017;120:439–448. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 53.Feliciano DM, Zhang S, Nasrallah CM, Lisgo SN, Bordey A. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PLoS One. 2014;9:e88810. doi: 10.1371/journal.pone.0088810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, Zhao M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells. 2012;30:349–355. doi: 10.1002/stem.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernández-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J, Engel O, Stenzel W, Genové G, Priller J. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab. 2013;33:428–439. doi: 10.1038/jcbfm.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernández-López D, Natarajan N, Ashwal S, Vexler ZS. Mechanisms of perinatal arterial ischemic stroke. J Cereb Blood Flow Metab. 2014;34:921–932. doi: 10.1038/jcbfm.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fietz SA, Huttner WB. Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr Opin Neurobiol. 2011;21:23–35. doi: 10.1016/j.conb.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182. doi: 10.3389/fncel.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao M, Yao H, Dong Q, Zhang Y, Yang Y, Zhang Y, Yang Z, Xu M, Xu R. Neurotrophy and immunomodulation of induced neural stem cell grafts in a mouse model of closed head injury. Stem Cell Res. 2017;23:132–142. doi: 10.1016/j.scr.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 60.Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, Hong W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res. 2019;11:780–792. [PMC free article] [PubMed] [Google Scholar]
- 61.Ghourichaee SS, Powell EM, Leach JB. Enhancement of human neural stem cell self-renewal in 3D hypoxic culture. Biotechnol Bioeng. 2017;114:1096–1106. doi: 10.1002/bit.26224. [DOI] [PubMed] [Google Scholar]
- 62.Glass JD, Hertzberg VS, Boulis NM, Riley J, Federici T, Polak M, Bordeau J, Fournier C, Johe K, Hazel T, Cudkowicz M, Atassi N, Borges LF, Rutkove SB, Duell J, Patil PG, Goutman SA, Feldman EL. Transplantation of spinal cord-derived neural stem cells for ALS: Analysis of phase 1 and 2 trials. Neurology. 2016;87:392–400. doi: 10.1212/WNL.0000000000002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glud AN, Bjarkam CR, Azimi N, Johe K, Sorensen JC, Cunningham M. Feasibility of three-dimensional placement of human therapeutic stem cells using the intracerebral microinjection instrument. Neuromodulation. 2016;19:708–716. doi: 10.1111/ner.12484. [DOI] [PubMed] [Google Scholar]
- 64.Gójska-Grymajło A, Zieliński M, Wardowska A, Gąsecki D, Pikuła M, Karaszewski B. CXCR7+ and CXCR4+ stem cells and neuron specific enolase in acute ischemic stroke patients. Neurochem Int. 2018;120:134–139. doi: 10.1016/j.neuint.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Golas MM. Human cellular models of medium spiny neuron development and Huntington disease. Life Sci. 2018;209:179–196. doi: 10.1016/j.lfs.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 66.Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV, Cairns MJ. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014;42:9195–9208. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goncalves MB, Malmqvist T, Clarke E, Hubens CJ, Grist J, Hobbs C, Trigo D, Risling M, Angeria M, Damberg P, Carlstedt TP, Corcoran JP. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35:15731–15745. doi: 10.1523/JNEUROSCI.1339-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gou Z, Mi Y, Jiang F, Deng B, Yang J, Gou X. PirB is a novel potential therapeutic target for enhancing axonal regeneration and synaptic plasticity following CNS injury in mammals. J Drug Target. 2014;22:365–371. doi: 10.3109/1061186X.2013.878939. [DOI] [PubMed] [Google Scholar]
- 69.Green C, Minassian A, Vogel S, Diedenhofen M, Beyrau A, Wiedermann D, Hoehn M. Sensorimotor functional and structural networks after intracerebral stem cell grafts in the ischemic mouse brain. J Neurosci. 2018;38:1648–1661. doi: 10.1523/JNEUROSCI.2715-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Griffin TA, Anderson HC, Wolfe JH. Ex vivo gene therapy using patient iPSC-derived NSCs reverses pathology in the brain of a homologous mouse model. Stem Cell Reports. 2015;4:835–846. doi: 10.1016/j.stemcr.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gutiérrez-Fernández M, Rodríguez-Frutos B, Alvarez-Grech J, Vallejo-Cremades MT, Expósito-Alcaide M, Merino J, Roda JM, Díez-Tejedor E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. doi: 10.1016/j.neuroscience.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 72.Han S, Yang K, Shin Y, Lee JS, Kamm RD, Chung S, Cho SW. Three-dimensional extracellular matrix-mediated neural stem cell differentiation in a microfluidic device. Lab Chip. 2012;12:2305–2308. doi: 10.1039/c2lc21285d. [DOI] [PubMed] [Google Scholar]
- 73.Hankey GJ. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 74.Hansen AH, Duellberg C, Mieck C, Loose M, Hippenmeyer S. Cell polarity in cerebral cortex development-cellular architecture shaped by biochemical networks. Front Cell Neurosci. 2017;11:176. doi: 10.3389/fncel.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, Ali M, Yu G, Xu L, Kim SU, Hess DC, Borlongan CV. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol. 2008;85:318–334. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Hatakeyama M, Ninomiya I, Kanazawa M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen Res. 2020;15:16–19. doi: 10.4103/1673-5374.264442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heinrich C, Spagnoli FM, Berninger B. In vivo reprogramming for tissue repair. Nat Cell Biol. 2015;17:204–211. doi: 10.1038/ncb3108. [DOI] [PubMed] [Google Scholar]
- 78.Hermann A, Gerlach M, Schwarz J, Storch A. Neurorestoration in Parkinson’s disease by cell replacement and endogenous regeneration. Expert Opin Biol Ther. 2004;4:131–143. doi: 10.1517/14712598.4.2.131. [DOI] [PubMed] [Google Scholar]
- 79.Hicks C, Stevanato L, Stroemer RP, Tang E, Richardson S, Sinden JD. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 2013;22:1541–1552. doi: 10.3727/096368912X657936. [DOI] [PubMed] [Google Scholar]
- 80.Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoehn M, Küstermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Föcking M, Arnold H, Hescheler J, Fleischmann BK, Schwindt W, Bührle C. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang H, Qian K, Han X, Li X, Zheng Y, Chen Z, Huang X, Chen H. Intraparenchymal neural stem/progenitor cell transplantation for ischemic stroke animals: a meta-analysis and systematic review. Stem Cells Int. 2018;2018:4826407. doi: 10.1155/2018/4826407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hurlbert MS, Gianani RI, Hutt C, Freed CR, Kaddis FG. Neural transplantation of hNT neurons for Huntington’s disease. Cell Transplant. 1999;8:143–151. doi: 10.1177/096368979900800106. [DOI] [PubMed] [Google Scholar]
- 84.Ievins A, Moritz CT. Therapeutic stimulation for restoration of function after spinal cord injury. Physiology (Bethesda) 2017;32:391–398. doi: 10.1152/physiol.00010.2017. [DOI] [PubMed] [Google Scholar]
- 85.Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–2263. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- 86.Jia W, Kamen Y, Pivonkova H, Káradóttir RT. Neuronal activity-dependent myelin repair after stroke. Neurosci Lett. 2019;703:139–144. doi: 10.1016/j.neulet.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Jiang Q, Zhang ZG, Ding GL, Zhang L, Ewing JR, Wang L, Zhang R, Li L, Lu M, Meng H, Arbab AS, Hu J, Li QJ, Pourabdollah Nejad DS, Athiraman H, Chopp M. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. Neuroimage. 2005;28:698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 88.Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 89.Karow M, Schichor C, Beckervordersandforth R, Berninger B. Lineage-reprogramming of pericyte-derived cells of the adult human brain into induced neurons. J Vis Exp. 2014:51433. doi: 10.3791/51433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karow M, Sánchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascón S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Götz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Karow M, Camp JG, Falk S, Gerber T, Pataskar A, Gac-Santel M, Kageyama J, Brazovskaja A, Garding A, Fan W, Riedemann T, Casamassa A, Smiyakin A, Schichor C, Götz M, Tiwari VK, Treutlein B, Berninger B. Direct pericyte-to-neuron reprogramming via unfolding of a neural stem cell-like program. Nat Neurosci. 2018;21:932–940. doi: 10.1038/s41593-018-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koh SH, Park HH. Neurogenesis in stroke recovery. Transl Stroke Res. 2017;8:3–13. doi: 10.1007/s12975-016-0460-z. [DOI] [PubMed] [Google Scholar]
- 93.Kondiles BR, Horner PJ. Myelin plasticity, neural activity, and traumatic neural injury. Dev Neurobiol. 2018;78:108–122. doi: 10.1002/dneu.22540. [DOI] [PubMed] [Google Scholar]
- 94.Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonte R, Lebowitz J, Flickinger JC, Tong D, Marks MP, Jamieson C, Luu D, Bell-Stephens T, Teraoka J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 95.Kuluz JW, Prado R, He D, Zhao W, Dietrich WD, Watson B. New pediatric model of ischemic stroke in infant piglets by photothrombosis: acute changes in cerebral blood flow, microvasculature, and early histopathology. Stroke. 2007;38:1932–1937. doi: 10.1161/STROKEAHA.106.475244. [DOI] [PubMed] [Google Scholar]
- 96.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; Council on Functional Genomics and Translational Biology. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lau VW, Platt SR, Grace HE, Baker EW, West FD. Human iNPC therapy leads to improvement in functional neurologic outcomes in a pig ischemic stroke model. Brain Behav. 2018;8:e00972. doi: 10.1002/brb3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JS, Park JC, Kim TW, Jung BJ, Lee Y, Shim EK, Park S, Choi EY, Cho KS, Kim CS. Human bone marrow stem cells cultured under hypoxic conditions present altered characteristics and enhanced in vivo tissue regeneration. Bone. 2015;78:34–45. doi: 10.1016/j.bone.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 99.Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke. 2012;7:582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- 100.Letellier M, Levet F, Thoumine O, Goda Y. Differential role of pre- and postsynaptic neurons in the activity-dependent control of synaptic strengths across dendrites. PLoS Biol. 2019;17:e2006223. doi: 10.1371/journal.pbio.2006223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, Zhu J, Du X, Xiong L, Du Y, Xu J, Xiao X, Wang J, Chai Z, Zhao Y, Deng H. Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell. 2015;17:195–203. doi: 10.1016/j.stem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 103.Li Z, Liu C, Yu C, Chen Y, Jia P, Zhu H, Zhang X, Yu Y, Zhu B, Sheng W. A highly selective and sensitive red-emitting fluorescent probe for visualization of endogenous peroxynitrite in living cells and zebrafish. Analyst. 2019;144:3442–3449. doi: 10.1039/c9an00347a. [DOI] [PubMed] [Google Scholar]
- 104.Liao LY, Lau BW, Sánchez-Vidaña DI, Gao Q. Exogenous neural stem cell transplantation for cerebral ischemia. Neural Regen Res. 2019;14:1129–1137. doi: 10.4103/1673-5374.251188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Liu Y, Shao J, Li Y, Qin L, Shen H, Xie Y, Xia W, Gao WQ. Zeb1 is important for proper cleavage plane orientation of dividing progenitors and neuronal migration in the mouse neocortex. Cell Death Differ. 2019;26:2479–2492. doi: 10.1038/s41418-019-0314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lojewski X, Hermann A, Wegner F, Araúzo-Bravo MJ, Hallmeyer-Elgner S, Kirsch M, Schwarz J, Schöler HR, Storch A. Human adult white matter progenitor cells are multipotent neuroprogenitors similar to adult hippocampal progenitors. Stem Cells Transl Med. 2014;3:458–469. doi: 10.5966/sctm.2013-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu SS, Liu S, Zu QQ, Xu XQ, Yu J, Wang JW, Zhang Y, Shi HB. In vivo MR imaging of intraarterially delivered magnetically labeled mesenchymal stem cells in a canine stroke model. PLoS One. 2013;8:e54963. doi: 10.1371/journal.pone.0054963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luan Z, Liu W, Qu S, Du K, He S, Wang Z, Yang Y, Wang C, Gong X. Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell Transplant. 2012;21(Suppl 1):S91–98. doi: 10.3727/096368912X633806. [DOI] [PubMed] [Google Scholar]
- 111.Lynch M. Evolution of the mutation rate. Trends Genet. 2010;26:345–352. doi: 10.1016/j.tig.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Madelaine R, Sloan SA, Huber N, Notwell JH, Leung LC, Skariah G, Halluin C, Paşca SP, Bejerano G, Krasnow MA, Barres BA, Mourrain P. MicroRNA-9 couples brain neurogenesis and angiogenesis. Cell Rep. 2017;20:1533–1542. doi: 10.1016/j.celrep.2017.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mao G, Wang Y, Guo X, Liu J, Zheng Z, Chen L. Neurorestorative effect of olfactory ensheathing cells and Schwann cells by intranasal delivery for patients with ischemic stroke: design of a multicenter randomized double-blinded placebo-controlled clinical study. J Neurorestoratol. 2018;6:74–80. [Google Scholar]
- 114.McGrath EL, Gao J, Wu P. Proliferation and differentiation of human fetal brain neural stem cells in vitro. J Neurorestoratol. 2018;6:19–27. [Google Scholar]
- 115.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 117.Meneghini V, Frati G, Sala D, De Cicco S, Luciani M, Cavazzin C, Paulis M, Mentzen W, Morena F, Giannelli S, Sanvito F, Villa A, Bulfone A, Broccoli V, Martino S, Gritti A. Generation of human induced pluripotent stem cell-derived bona fide neural stem cells for ex vivo gene therapy of metachromatic leukodystrophy. Stem Cells Transl Med. 2017;6:352–368. doi: 10.5966/sctm.2015-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18:628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 120.Miyazono M, Lee VM, Trojanowski JQ. Proliferation, cell death, and neuronal differentiation in transplanted human embryonal carcinoma (NTera2) cells depend on the graft site in nude and severe combined immunodeficient mice. Lab Invest. 1995;73:273–283. [PubMed] [Google Scholar]
- 121.Mondello SE, Kasten MR, Horner PJ, Moritz CT. Therapeutic intraspinal stimulation to generate activity and promote long-term recovery. Front Neurosci. 2014;8:21. doi: 10.3389/fnins.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang Y. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288:7105–7116. doi: 10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moritz CT. Now is the critical time for engineered neuroplasticity. Neurotherapeutics. 2018;15:628–634. doi: 10.1007/s13311-018-0637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morizane A, Kikuchi T, Hayashi T, Mizuma H, Takara S, Doi H, Mawatari A, Glasser MF, Shiina T, Ishigaki H, Itoh Y, Okita K, Yamasaki E, Doi D, Onoe H, Ogasawara K, Yamanaka S, Takahashi J. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 2017;8:385. doi: 10.1038/s41467-017-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Müller S, Chakrapani BP, Schwegler H, Hofmann HD, Kirsch M. Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells. 2009;27:431–441. doi: 10.1634/stemcells.2008-0234. [DOI] [PubMed] [Google Scholar]
- 126.Muoio V, Persson PB, Sendeski MM. The neurovascular unit - concept review. Acta Physiol (Oxf) 2014;210:790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 127.Murphy AR, Laslett A, O’Brien CM, Cameron NR. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 2017;54:1–20. doi: 10.1016/j.actbio.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 128.Nakamura M, Imai H, Konno K, Kubota C, Seki K, Puentes S, Faried A, Yokoo H, Hata H, Yoshimoto Y, Saito N. Experimental investigation of encephalomyosynangiosis using gyrencephalic brain of the miniature pig: histopathological evaluation of dynamic reconstruction of vessels for functional anastomosis. Laboratory investigation. J Neurosurg Pediatr. 2009;3:488–495. doi: 10.3171/2008.6.PEDS0834. [DOI] [PubMed] [Google Scholar]
- 129.Navlakha S, Bar-Joseph Z, Barth AL. Network design and the brain. Trends Cogn Sci. 2018;22:64–78. doi: 10.1016/j.tics.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 130.Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, Lee VM, Trojanowski JQ. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nucci LP, Silva HR, Giampaoli V, Mamani JB, Nucci MP, Gamarra LF. Stem cells labeled with superparamagnetic iron oxide nanoparticles in a preclinical model of cerebral ischemia: a systematic review with meta-analysis. Stem Cell Res Ther. 2015;6:27. doi: 10.1186/s13287-015-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nunes AK, Rapôso C, de Oliveira WH, Thomé R, Verinaud L, Tovar-Moll F, Peixoto CA. Phosphodiesterase-5 inhibition promotes remyelination by MCP-1/CCR-2 and MMP-9 regulation in a cuprizone-induced demyelination model. Exp Neurol 275 Pt. 2016;1:143–153. doi: 10.1016/j.expneurol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 133.Obenaus A, Dilmac N, Tone B, Tian HR, Hartman R, Digicaylioglu M, Snyder EY, Ashwal S. Long-term magnetic resonance imaging of stem cells in neonatal ischemic injury. Ann Neurol. 2011;69:282–291. doi: 10.1002/ana.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, Monni E, Tornero D, Ahlenius H, Ladewig J, Brüstle O, Lindvall O, Kokaia Z. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 135.Otsuki L, Brand AH. The vasculature as a neural stem cell niche. Neurobiol Dis. 2017;107:4–14. doi: 10.1016/j.nbd.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 136.Oztop-Cakmak O, Solaroglu I, Gursoy-Ozdemir Y. The role of pericytes in neurovascular unit: emphasis on stroke. Curr Drug Targets. 2017;18:1386–1391. doi: 10.2174/1389450117666160613104523. [DOI] [PubMed] [Google Scholar]
- 137.Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- 138.Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, De A, Choi R, Chen S, Rutt BK, Gambhir SS, Guzman R. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke. 2010;41:2064–2070. doi: 10.1161/STROKEAHA.109.575993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peng JJ, Sha R, Li MX, Chen LT, Han XH, Guo F, Chen H, Huang XL. Repetitive transcranial magnetic stimulation promotes functional recovery and differentiation of human neural stem cells in rats after ischemic stroke. Exp Neurol. 2019;313:1–9. doi: 10.1016/j.expneurol.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 140.Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 141.Qin H, Zhao A, Fu X. Small molecules for reprogramming and transdifferentiation. Cell Mol Life Sci. 2017;74:3553–3575. doi: 10.1007/s00018-017-2586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K, Guterman LR, Hopkins LN. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31:1482–1489. doi: 10.1097/01.CCM.0000063047.63862.99. [DOI] [PubMed] [Google Scholar]
- 144.Rodríguez-Frutos B, Otero-Ortega L, Gutiérrez-Fernández M, Fuentes B, Ramos-Cejudo J, Díez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016;7:378–387. doi: 10.1007/s12975-016-0482-6. [DOI] [PubMed] [Google Scholar]
- 145.Rosado-de-Castro PH, Pimentel-Coelho PM, da Fonseca LM, de Freitas GR, Mendez-Otero R. The rise of cell therapy trials for stroke: review of published and registered studies. Stem Cells Dev. 2013;22:2095–2111. doi: 10.1089/scd.2013.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ruan L, Wang B, ZhuGe Q, Jin K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015;1623:166–173. doi: 10.1016/j.brainres.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ryu S, Lee SH, Kim SU, Yoon BW. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res. 2016;11:298–304. doi: 10.4103/1673-5374.177739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sakata H, Niizuma K, Wakai T, Narasimhan P, Maier CM, Chan PH. Neural stem cells genetically modified to overexpress Cu/Zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke. 2012;43:2423–2429. doi: 10.1161/STROKEAHA.112.656900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sandvig I, Gadjanski I, Vlaski-Lafarge M, Buzanska L, Loncaric D, Sarnowska A, Rodriguez L, Sandvig A, Ivanovic Z. Strategies to enhance implantation and survival of stem cells after their injection in ischemic neural tissue. Stem Cells Dev. 2017;26:554–565. doi: 10.1089/scd.2016.0268. [DOI] [PubMed] [Google Scholar]
- 150.Saver JL. Time is brain--quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 151.Savitz SI, Dinsmore J, Wu J, Henderson GV, Stieg P, Caplan LR. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc Dis. 2005;20:101–107. doi: 10.1159/000086518. [DOI] [PubMed] [Google Scholar]
- 152.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 153.Schuldiner O, Yaron A. Mechanisms of developmental neurite pruning. Cell Mol Life Sci. 2015;72:101–119. doi: 10.1007/s00018-014-1729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sherman SP, Bang AG. High-throughput screen for compounds that modulate neurite growth of human induced pluripotent stem cell-derived neurons. Dis Model Mech. 2018;11:dmm031906. doi: 10.1242/dmm.031906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shi W, Huang CJ, Xu XD, Jin GH, Huang RQ, Huang JF, Chen YN, Ju SQ, Wang Y, Shi YW, Qin JB, Zhang YQ, Liu QQ, Wang XB, Zhang XH, Chen J. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016;45:247–261. doi: 10.1016/j.actbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 156.Shimamura N, Kakuta K, Wang L, Naraoka M, Uchida H, Wakao S, Dezawa M, Ohkuma H. Neuro-regeneration therapy using human Muse cells is highly effective in a mouse intracerebral hemorrhage model. Exp Brain Res. 2017;235:565–572. doi: 10.1007/s00221-016-4818-y. [DOI] [PubMed] [Google Scholar]
- 157.Silva J, Bento AR, Barros D, Laundos TL, Sousa SR, Quelhas P, Sousa MM, Pêgo AP, Amaral IF. Fibrin functionalization with synthetic adhesive ligands interacting with α6β1 integrin receptor enhance neurite outgrowth of embryonic stem cell-derived neural stem/progenitors. Acta Biomater. 2017;59:243–256. doi: 10.1016/j.actbio.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 158.Sinden JD, Hicks C, Stroemer P, Vishnubhatla I, Corteling R. Human neural stem cell therapy for chronic ischemic stroke: charting progress from laboratory to patients. Stem Cells Dev. 2017;26:933–947. doi: 10.1089/scd.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Smith AR, Gulbahce E, Burke MJ, Cao Q, Macmillan ML, Tolar J, Orchard PJ, Blazar BR, Baker KS, Wagner JE, Verneris MR. Lower leukemia relapse in pediatric patients with pulmonary cytolytic thrombi following allogeneic transplant. Bone Marrow Transplant. 2011;46:368–371. doi: 10.1038/bmt.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sozmen EG, Rosenzweig S, Llorente IL, DiTullio DJ, Machnicki M, Vinters HV, Havton LA, Giger RJ, Hinman JD, Carmichael ST. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proc Natl Acad Sci U S A. 2016;113:E8453–E8462. doi: 10.1073/pnas.1615322113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47:1817–1824. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stepanyants A, Hof PR, Chklovskii DB. Geometry and structural plasticity of synaptic connectivity. Neuron. 2002;34:275–288. doi: 10.1016/s0896-6273(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 163.Stone BS, Zhang J, Mack DW, Mori S, Martin LJ, Northington FJ. Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann Neurol. 2008;64:535–546. doi: 10.1002/ana.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94–131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair. 2009;23:895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- 166.Stukel JM, Willits RK. The interplay of peptide affinity and scaffold stiffness on neuronal differentiation of neural stem cells. Biomed Mater. 2018;13:024102. doi: 10.1088/1748-605X/aa9a4b. [DOI] [PubMed] [Google Scholar]
- 167.Tadesse T, Gearing M, Senitzer D, Saxe D, Brat DJ, Bray R, Gebel H, Hill C, Boulis N, Riley J, Feldman E, Johe K, Hazel T, Polak M, Bordeau J, Federici T, Glass JD. Analysis of graft survival in a trial of stem cell transplant in ALS. Ann Clin Transl Neurol. 2014;1:900–908. doi: 10.1002/acn3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tajiri N, Borlongan CV, Kaneko Y. Cyclosporine A treatment abrogates ischemia-induced neuronal cell death by preserving mitochondrial integrity through upregulation of the Parkinson’s disease-associated protein DJ-1. CNS Neurosci Ther. 2016;22:602–610. doi: 10.1111/cns.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tajiri N, Duncan K, Antoine A, Pabon M, Acosta SA, de la Pena I, Hernadez-Ontiveros DG, Shinozuka K, Ishikawa H, Kaneko Y, Yankee E, McGrogan M, Case C, Borlongan CV. Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Front Syst Neurosci. 2014;8:116. doi: 10.3389/fnsys.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 171.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 172.Takeuchi S, Wada K, Nagatani K, Otani N, Mori K. Decompressive hemicraniectomy for spontaneous intracerebral hemorrhage. Neurosurg Focus. 2013;34:E5. doi: 10.3171/2013.2.FOCUS12424. [DOI] [PubMed] [Google Scholar]
- 173.Tan F, Wang J, Liu JX, Wang C, Li M, Gu Y. Electroacupuncture stimulates the proliferation and differentiation of endogenous neural stem cells in a rat model of ischemic stroke. Exp Ther Med. 2018;16:4943–4950. doi: 10.3892/etm.2018.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tang T, Li XQ, Wu H, Luo JK, Zhang HX, Luo TL. Activation of endogenous neural stem cells in experimental intracerebral hemorrhagic rat brains. Chin Med J (Engl) 2004;117:1342–1347. [PubMed] [Google Scholar]
- 175.Taverna E, Götz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- 176.Teixeira FG, Panchalingam KM, Anjo SI, Manadas B, Pereira R, Sousa N, Salgado AJ, Behie LA. Do hypoxia/normoxia culturing conditions change the neuroregulatory profile of Wharton Jelly mesenchymal stem cell secretome. Stem Cell Res Ther. 2015;6:133. doi: 10.1186/s13287-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thomas RJ, Hope AD, Hourd P, Baradez M, Miljan EA, Sinden JD, Williams DJ. Automated, serum-free production of CTX0E03: a therapeutic clinical grade human neural stem cell line. Biotechnol Lett. 2009;31:1167–1172. doi: 10.1007/s10529-009-9989-1. [DOI] [PubMed] [Google Scholar]
- 178.Tornero D, Tsupykov O, Granmo M, Rodriguez C, Gronning-Hansen M, Thelin J, Smozhanik E, Laterza C, Wattananit S, Ge R, Tatarishvili J, Grealish S, Brustle O, Skibo G, Parmar M, Schouenborg J, Lindvall O, Kokaia Z. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017;140:692–706. doi: 10.1093/brain/aww347. [DOI] [PubMed] [Google Scholar]
- 179.Traka M, Podojil JR, McCarthy DP, Miller SD, Popko B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat Neurosci. 2016;19:65–74. doi: 10.1038/nn.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 181.Tuazon JP, Castelli V, Lee JY, Desideri GB, Stuppia L, Cimini AM, Borlongan CV. Neural stem cells. Adv Exp Med Biol. 2019;1201:79–91. doi: 10.1007/978-3-030-31206-0_4. [DOI] [PubMed] [Google Scholar]
- 182.Usvald D, Vodicka P, Hlucilova J, Prochazka R, Motlik J, Kuchorova K, Johe K, Marsala S, Scadeng M, Kakinohana O, Navarro R, Santa M, Hefferan MP, Yaksh TL, Marsala M. Analysis of dosing regimen and reproducibility of intraspinal grafting of human spinal stem cells in immunosuppressed minipigs. Cell Transplant. 2010;19:1103–1122. doi: 10.3727/096368910X503406. [DOI] [PubMed] [Google Scholar]
- 183.Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang Y, Ma J, Li N, Wang L, Shen L, Sun Y, Wang Y, Zhao J, Wei W, Ren Y, Liu J. Microfluidic engineering of neural stem cell niches for fate determination. Biomicrofluidics. 2017;11:014106. doi: 10.1063/1.4974902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West FD, Stice SL. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49:1248–1256. doi: 10.1161/STROKEAHA.117.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wei JK, Wang WC, Zhai RW, Zhang YH, Yang SC, Rizak J, Li L, Xu LQ, Liu L, Pan MK, Hu YZ, Ghanemi A, Wu J, Yang LC, Li H, Lv LB, Li JL, Yao YG, Xu L, Feng XL, et al. (2016) Neurons differentiated from transplanted stem cells respond functionally to acoustic stimuli in the awake monkey brain. Cell Rep. 16:1016–1025. doi: 10.1016/j.celrep.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 187.Wilsch-Bräuninger M, Florio M, Huttner WB. Neocortex expansion in development and evolution - from cell biology to single genes. Curr Opin Neurobiol. 2016;39:122–132. doi: 10.1016/j.conb.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 188.Xu H, Heilshorn SC. Microfluidic investigation of BDNF-enhanced neural stem cell chemotaxis in CXCL12 gradients. Small. 2013;9:585–595. doi: 10.1002/smll.201202208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yew WP, Djukic ND, Jayaseelan JSP, Walker FR, Roos KAA, Chataway TK, Muyderman H, Sims NR. Early treatment with minocycline following stroke in rats improves functional recovery and differentially modifies responses of peri-infarct microglia and astrocytes. J Neuroinflammation. 2019;16:6. doi: 10.1186/s12974-018-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yin KJ, Hamblin M, Chen YE. Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc Pharmacol. 2015;13:352–365. doi: 10.2174/15701611113119990016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu D, Cheng Z, Ali AI, Wang J, Le K, Chibaatar E, Guo Y. Down-expressed GLT-1 in PSD astrocytes inhibits synaptic formation of NSC-derived neurons in vitro. Cell Cycle. 2019;18:105–114. doi: 10.1080/15384101.2018.1560201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yuan Y, Tang X, Bai YF, Wang S, An J, Wu Y, Xu ZD, Zhang YA, Chen Z. Dopaminergic precursors differentiated from human blood-derived induced neural stem cells improve symptoms of a mouse Parkinson’s disease model. Theranostics. 2018;8:4679–4694. doi: 10.7150/thno.26643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zarriello S, Neal EG, Kaneko Y, Borlongan CV. T-regulatory cells confer increased myelination and stem cell activity after stroke-induced white matter injury. J Clin Med. 2019;8:537. doi: 10.3390/jcm8040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang C, Cao J, Li X, Xu H, Wang W, Wang L, Zhao X, Li W, Jiao J, Hu B, Zhou Q, Zhao T. Treatment of multiple sclerosis by transplantation of neural stem cells derived from induced pluripotent stem cells. Sci China Life Sci. 2016a;59:950–957. doi: 10.1007/s11427-016-0114-9. [DOI] [PubMed] [Google Scholar]
- 195.Zhang F, Duan X, Lu L, Zhang X, Zhong X, Mao J, Chen M, Shen J. In vivo targeted MR imaging of endogenous neural stem cells in ischemic stroke. Molecules. 2016b;21:1143. doi: 10.3390/molecules21091143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang G, Guo X, Chen L, Li B, Gu B, Wang H, Wu G, Kong J, Chen W, Yu Y. Interferon-γ promotes neuronal repair by transplanted neural stem cells in ischemic rats. Stem Cells Dev. 2018;27:355–366. doi: 10.1089/scd.2017.0225. [DOI] [PubMed] [Google Scholar]
- 197.Zhang G, Cunningham M, Zhang H, Dai Y, Zhang P, Ge G, Wang B, Bai M, Hazel T, Johe K, Xu R. First human trial of stem cell transplantation in complex arrays for stroke patients using the intracerebral microinjection instrument. Oper Neurosurg (Hagerstown) 2020;18:503–510. doi: 10.1093/ons/opz204. [DOI] [PubMed] [Google Scholar]
- 198.Zhang G, Li Y, Reuss JL, Liu N, Wu C, Li J, Xu S, Wang F, Hazel TG, Cunningham M, Zhang H, Dai Y, Hong P, Zhang P, He J, Feng H, Lu X, Ulmer JL, Johe KK, Xu R. Stable intracerebral transplantation of neural stem cells for the treatment of paralysis due to ischemic stroke. Stem Cells Transl Med. 2019a;8:999–1007. doi: 10.1002/sctm.18-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang M, Lin YH, Sun YJ, Zhu S, Zheng J, Liu K, Cao N, Li K, Huang Y, Ding S. Pharmacological reprogramming of fibroblasts into neural stem cells by signaling-directed transcriptional activation. Cell Stem Cell. 2016c;18:653–667. doi: 10.1016/j.stem.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, Zhang ZG. The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci. 2013;33:6885–6894. doi: 10.1523/JNEUROSCI.5180-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019b;15:193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 202.Zhao H, Zuo X, Ren L, Li Y, Tai H, Du J, Xie X, Zhang X, Han Y, Wu Y, Yang C, Xu Z, Hong H, Li S, Su B. Combined use of bFGF/EGF and all-trans-retinoic acid cooperatively promotes neuronal differentiation and neurite outgrowth in neural stem cells. Neurosci Lett. 2019;690:61–68. doi: 10.1016/j.neulet.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 203.Zhao J, Yao Y, Xu C, Cheng B, Xu Q. Expression of GAP-43 in fibroblast cell lines influences the orientation of cell division. Int J Dev Neurosci. 2011;29:469–474. doi: 10.1016/j.ijdevneu.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 204.Zhao Y, Liu Q, Sun H, Chen D, Li Z, Fan B, George J, Xue C, Cui Z, Wang J, Chen J. Electrical property characterization of neural stem cells in differentiation. PLoS One. 2016;11:e0158044. doi: 10.1371/journal.pone.0158044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zheng Y, Huang J, Zhu T, Li R, Wang Z, Ma F, Zhu J. Stem cell tracking technologies for neurological regenerative medicine purposes. Stem Cells Int. 2017;2017:2934149. doi: 10.1155/2017/2934149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhou X, Shi G, Fan B, Cheng X, Zhang X, Wang X, Liu S, Hao Y, Wei Z, Wang L, Feng S. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int J Nanomedicine. 2018;13:6265–6277. doi: 10.2147/IJN.S175914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ziemka-Nalecz M, Janowska J, Strojek L, Jaworska J, Zalewska T, Frontczak-Baniewicz M, Sypecka J. Impact of neonatal hypoxia-ischaemia on oligodendrocyte survival, maturation and myelinating potential. J Cell Mol Med. 2018;22:207–222. doi: 10.1111/jcmm.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]


