Abstract
Perceptions of emotional facial expressions and trustworthiness of others guides behavior and has considerable implications for individuals who work in fields that require rapid decision making, such as law enforcement. This is particularly complicated for more ambiguous expressions, such as ‘neutral’ faces. We examined behavioral and electrocortical responses to facial expressions in 22 student police officers (18 males; 23.2 ± 3.63 years). Participants completed an emotional face appraisal task that involved viewing three expressions (fearful, neutral, happy) and were asked to identify the emotion and rate the trustworthiness of each face. The late positive potential (LPP), an event-related potential that tracks emotional intensity and/or salience of a stimulus, was measured during the task. Overall, participants rated neutral faces similarly to fearful faces and responded fastest to these expressions. Neutral faces also elicited a robust late LPP response that did not differ from LPP to fearful or happy faces, and there was substantial individual variation in trustworthiness ratings for neutral faces. Together, ‘neutral’ facial expressions elicited similar trustworthiness ratings to negatively-valenced stimuli. Brain and behavioral responses to neutral faces also varied across student officers; thus, encounters with ambiguous faces in the field may promote increased perceived threat in some officers, which may have real-world consequences (e.g., decision to shoot, risk of psychopathology).
Keywords: emotion, late positive potential, trustworthiness, police officers
1. Introduction
The perception of emotional facial expressions can be used to discern an individual’s emotional state and disposition (Knutson, 1996; Oosterhof and Todorov, 2008), the latter of which has implications for decision making and resultant behaviors. The interpretation of transient emotional expressions as significant representations of a person’s more enduring attributes, such as personality or trustworthiness, is referred to as emotion overgeneralization (Zebrowitz et al., 2010; Zebrowitz and Montepare, 2008). The ability to not only identify the facial emotional expression, but also to judge whether or not another individual is trustworthy, is critical for guiding social interactions. The trustworthiness of a face is judged spontaneously and rapidly (Willis and Todorov, 2006), and these perceptions have been shown to predict real-world outcomes, such as court rulings or investment decisions (Olivola et al., 2014; Olivola and Todorov, 2010). Previous studies have found that individuals attribute apparent personality traits in a stereotypical way to briefly encountered faces with a high degree of consistency (see review Cuddy, Fiske, & Glick, 2008). Moreover, these attributions are particularly frequent for faces with easily identifiable emotional categories, such as angry or happy faces. For example, Blasi et al. (2009), showed that happy faces are rated as more trustworthy and less hostile than angry faces, even when controlling for confounding variables (i.e., ‘baby face’, attractiveness). Similar to these findings, several studies have reported that happy faces are interpreted as significantly more approachable than angry faces (Oosterhof and Todorov, 2008; Willis et al., 2011; Zebrowitz et al., 2010). Perceptions of emotional facial expressions and the resulting actions have considerable implications for individuals who work in fields that require rapid decision making, such as in law enforcement.
The interpretation of facial displays becomes more complicated with more ambiguous expressions, such as ‘neutral’ faces (Blasi et al., 2009). The common-sense assumption that neutral faces lack perceived emotional content has been challenged by studies reporting that these faces elicit negative emotion (Carrera-Levillain and Fernandez-Dols, 1994). Indeed, ‘neutral’ faces are often perceived as emotionally negative, and rated similar to sad and angry faces (Kesler/West et al., 2001; Lee, Kang, Park, Kim, & An, 2007). There are individual differences in the tendency to interpret ‘neutral’ facial expressions as threatening or untrustworthy, and these individual differences have been shown to relate to important outcomes in clinical populations. For example, major cognitive models of emotion-related psychopathology (e.g., Beck’s model; Beck & Alford, 2009) emphasize the role of a ‘negativity bias’ in the etiology and maintenance of emotion-related disorders. In addition to biased interpretation of ambiguous or negative expressions, prior studies also suggest that biases against positive stimuli may be involved in the pathology of emotion-related disorders. For example, severity of combat-related posttraumatic stress disorder (PTSD) has been associated with a bias for avoiding positive affective stimuli, e.g., happy faces (Clausen et al., 2016). Further, individual differences in trait anger have been shown to predict automatic approach behavior to angry faces (Veenstra et al., 2017). Together, a large body of literature implicates biased interpretations of facial expressions in risk of several mental disorders, including depression, anxiety, and substance use disorders (Loijen et al., 2020). Individual differences in attributions of positively- or negatively-valenced stimuli may also reflect trauma exposure, genetic predisposition, or learning history (Loijen et al., 2020).
Several functional magnetic resonance imaging (fMRI) studies suggest that processing of ambiguous stimuli, such as neutral faces, can elicit activation of brain regions involved in the automatic detection of threat or ambiguity (e.g., amygdala; see Kim, Somerville, Johnstone, Alexander, Andrew, & Whalen, 2003). For example, Blasi et al. (2009) report amygdala activation in response to unfamiliar, neutral facial expressions and a meta-analysis of 20 fMRI studies suggests that amygdala activation to faces positively correlates with facial trustworthiness ratings (Santos et al., 2016). These studies suggest that neutral faces can elicit similar brain activation as compared to when viewing negatively-valenced facial expressions, and brain activation can correlate with social evaluation of faces, such as trustworthiness judgements. Further, the available neuroimaging and behavioral data indicate that there are individual differences in the processing and evaluation of neutral faces.
As a solution to facing ambiguous stimuli in the field, police officers use threat appraisal to assess potential dangers and involve thinking through worst case scenarios (Anshel, 2000). Experienced police officers display automatic search processes and a threat superiority effect that places greater attentional processing on threatening facial cues (Damjanovic, Pinkham, Clarke, & Phillips, 2014). Although this framework is necessary in fields such as police work, it is also highly stressful and associated with chronic increase in vigilance and cautiousness (Alkus and Padesky, 1983; Cantelon et al., 2018). The shared neurocircuitry between processing of ambiguous facial expressions and threat-related anxiety disorders (e.g., posttraumatic stress disorder; PTSD) raises concerns for emotional outcomes of officers. For instance, a study by Mumford, Taylor, & Kubu (2015) found that individuals in law enforcement are twice as likely to develop posttraumatic stress disorder (PTSD; 8.8%) as compared to the general population (3.5%; Kessler et al., 2015). Heightened risk of PTSD, the experience of trauma itself, learning history, and/or genetic factors may contribute to individual differences in threat and trustworthiness biases (Loijen et al., 2020). A greater bias to interpret threat from stimuli has been linked to specific brain and behavioral outcomes, such as increased heart rate, increased startle amplitudes, and higher amygdala activation in response to fear-related cues (Williams et al., 2009). These perceptual biases and their underlying neural signatures are important to study in student police officers, who are at heightened environmental risk of PTSD and other negative outcomes. Although most trauma-exposed individuals do not develop PTSD (Kearns et al., 2012), identification of risk factors (e.g., lack of trust) in student officers before they enter the field may identify targets for early, preventive interventions.
Event-related potentials (ERPs) can be utilized to understand the modulation of biases in appraisal on electrocortical processing of ambiguous stimuli. The late positive potential (LPP) is a sustained positive ERP component that tracks the emotional intensity and/or salience of a stimulus and is higher for more arousing as compared to less arousing stimuli (Cuthbert et al., 2000; Dunning et al., 2011; Hajcak et al., 2010; Pastor et al., 2008). The LPP is thought to reflect facilitated attention to emotional stimuli, and index downstream processes of amygdala activity (see review Hajcak et al., 2010). The LPP can be divided into two independent components: the early and late LPP (Dennis and Hajcak, 2009; Hajcak et al., 2010; Hajcak and Foti, 2020). Early LPP is thought to reflect the emotional significance of a stimulus, whereas late LPP may reflect a deeper evaluation of a stimulus and can be manipulated by factors external to the stimulus itself (e.g., preceding descriptions; Foti and Hajcak, 2008). Previous studies have examined the LPP to test for individual differences in the perception of emotional stimuli, and appraisals of threat and negativity (Dong, Zhou, Zhao, & Lu, 2011; Ho, Sun, Ting, Chan, & Lee, 2015). Recent studies suggest that the LPP is also sensitive to ambiguous information, such as ambiguous facial expressions (e.g., neutral faces; Calvo, Marrero, & Beltrán, 2013) and may be a more general neural signature of perceptual ambiguity (Sun et al., 2017a). Further, compared to healthy individuals, individuals with higher anxiety symptoms have been found to have greater LPPs for fearful (MacNamara et al., 2019), as well as, neutral faces (Weinberg and Sandre, 2018). Interestingly, a treatment study on attention bias modification for social anxiety found that greater reductions in social anxiety symptoms was associated with greater reductions in LPP response to neutral faces, in particular (Pan et al., 2020). The authors interpreted the reduction in LPP response to neutral faces as reflecting a reduced bias towards threat and negativity following treatment (Pan et al., 2020), given that anxious individuals may be more likely to interpret neutral social signals as threatening. Electrocortical responses have also been correlated with trauma exposure and PTSD symptoms among police officers (Covey et al., 2013).
The present study evaluated behavioral and electrocortical responses to facial expressions, including ambiguous (neutral) faces, among student police officers who have not yet entered the field. We are particularly interested in this population because given the high prevalence of situational uncertainty in police work, individual differences in threat perception may predict subsequent on-the-job performance, as well as emotional outcomes. Given that the LPP is responsive to emotionally-laden stimuli, we hypothesized that the LPP elicited by both threatening (fear) and ambiguous (neutral) faces will be higher than by non-threatening (happy) faces or a non-face control condition (shapes). Further, we also hypothesized that neutral faces would be rated similar in trustworthiness to the negatively-valanced stimuli (fearful faces), as compared to the positively-valanced stimuli (happy faces). We also examined accuracy and reaction time for each condition to further test potential brain-behavior correlations and individual differences therein.
2. Methods
2.1. Participants
Twenty-six adult volunteers were recruited as part of an ongoing longitudinal study designed to detect neural, psychophysiological, and behavioral antecedents of PTSD among police officers. Student police officers were recruited prior to field deployment from the Detroit Police Academy, an accredited police training institution by the Michigan Commission on Law Enforcement Standards. Potential participants were informed of the study via direct contact with research personnel during academy classes and distribution of printed flyers. Participants were considered eligible for the study if they were 1) an in-service police officer, 2) currently attending a police academy in Michigan, and 3) between the ages of 18 and 50. Since the purpose of the larger study was to identify biomarkers that precede the development of PTSD following on-the-job trauma exposure, eligible participants 4) scored < 20 on the Clinician-Administered PTSD Scale for DSM 5 (CAPS-5; Cronbach’s alpha = .72; Weathers et al., 2015). The cutoff score of 20 on the CAPS-5 was determined based on the minimum score if a participant were to endorse mild/subthreshold symptoms (i.e., score of 1) in every single symptom category, which still would not meet criteria for PTSD. The maximum CAPS-5 score in the study sample was 10 and no participants were excluded due to high CAPS-5 scores. Exclusion criteria consisted of previous employment in the military, emergency services, or law enforcement, or history or signs of neurological, psychiatric (including substance and alcohol abuse/dependence), or medical illness as confirmed by an interview regarding medical and psychiatric history, as well as, a modified Structured Clinical Interview for DSM-IV (SCID-NP) (First et al., 1995). These exclusion criteria were defined given the overall aim of the study in examining the subsequent development of psychopathology, and current psychopathology likely interferes with behavioral and electrocortical responses. Four participants from the initial sample were excluded due to incomplete study visits and/or poor task performance (accuracy for shapes < 50%), which resulted in a final sample size N = 22 (see Table 1 for sample demographics). An a priori power analysis was performed for sample size estimation, based on data from Smith, Weinberg, Moran, & Hajcak (2013; N = 23) comparing LPP response during fear, happy and neutral faces. The effect sizes in this study were .86 (fear vs. happy) and .86 (fear vs. neutral), considered to be large using Cohen’s (1988) criteria. With an alpha = .05 and power = .80, the projected sample size needed with these effect sizes (G*Power 3.1; Faul, Erdfelder, Lang, & Buchner, 2007) is N = 13–22.
Table 1.
Sample Demographics
| M | SD | |
|---|---|---|
| Age (years) | 23.20 | 3.63 |
| BDI-II | 4.64 | 5.92 |
| STAI | ||
| State | 31.64 | 9.19 |
| Trait | 33.91 | 8.31 |
| LEC-5 | 4.45 | 2.52 |
| Experienced | 2.05 | 1.29 |
| Witnessed | 2.41 | 1.92 |
| CAPS-5 (total) | 1.59 | 2.68 |
| n (%) | ||
| Gender (male) | 18 (82%) | |
| Race | ||
| African American/Black | 10 (45%) | |
| Caucasian | 12 (55%) | |
| Ethnicity (Hispanic or Latinx) | 2 (9%) |
Note. BDI = Beck Depression Inventory; STAI = State-Trait Anxiety Inventory; LEC-5 = Life Events Checklist for DSM 5; CAPS-5 = Clinician Administered PTSD Scale for DSM 5. Values depict mean (and standard deviation) except where noted.
All participants had negative urine toxicology and alcohol breathalyzer screens at the time of the study. Participants were paid up to $100 ($50 for questionnaires and $50 for the EEG session). All participants provided informed consent as approved by the Wayne State University Institutional Review Board (IRB), and all study procedures were approved by the IRB. A Certificate of Confidentiality (CC-MH-15-068) from the National Institutes of Health was obtained to further protect the identity of the student officers.
2.2. Emotional face appraisal task (EFAT)
The Emotional Face Appraisal Task (EFAT; adapted from Winston, Strange, & Dolan, 2002) was used to evaluate participant ratings of trustworthiness in facial expressions (see Figure 1). The task consisted of three emotional face stimuli (fear, happy, neutral) and one non-face (shapes) stimulus, for a total of 120 trials (30 trials/condition). The task was modified for feasibility in an ERP environment; i.e., to not make the task prohibitively long for student officers. Fearful faces were selected as the ‘negative’ valence rather than angry faces, as fearful expressions are a more ambiguous threat than angry faces, given that the source of the threat is unclear (Davis and Whalen, 2001). Full-color adult Caucasian face stimuli (30 of each valence, 14 males and 16 females) were derived from a standardized database of 3-dimensional images of emotional facial expressions where all faces were tested to ensure the digital facial expressions conveyed the intended emotion. (Gur et al., 2002), and each face only appeared once throughout the task. Shape stimuli consisted of 30 white shapes (circles, triangles, rectangles). Face and shape stimuli were presented on a black background using Presentation software (Neurobehavioral Systems, Inc., Albany, CA) and participants were seated approximately 60 cm from the screen.
Figure 1.
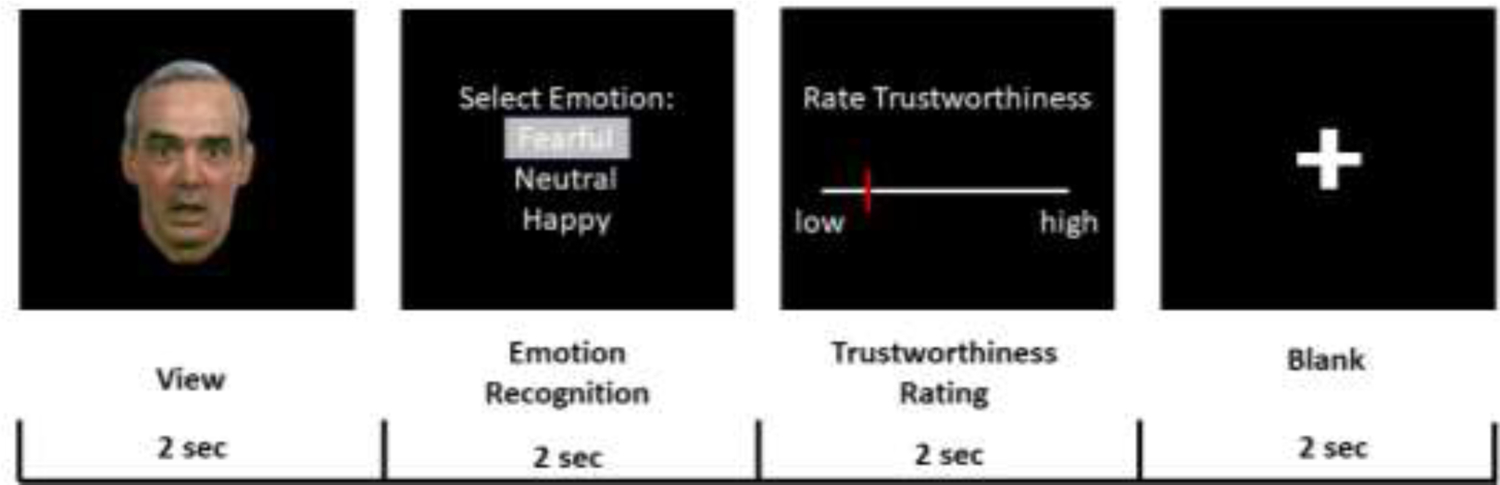
Exemplar EFAT trial for a fearful facial expression. During the trustworthiness rating period, participants selected how trustworthy they perceived the face on a scale from 0–100 (0 = low trustworthiness, 100 = high trustworthiness). Of note, face stimuli shown are from a standardized database of 3-dimensional images of emotional facial expressions (Gur et al., 2002) and reprinted here with permission.
Face trials were 6 s long and consisted of three phases: 1) 2 s face viewing; 2) 2 s emotion recognition response; and 3) 2 s trustworthiness rating. During the emotion recognition period, all participants used their right hand to press “1” on the number pad of the computer keyboard with their index finger to indicate “fearful”, “2” with their middle finger to indicate “neutral”, or “3” with their ring finger to indicate “happy”. During the trustworthiness rating period, participants selected, by sliding a mouse with their left hand, the perceived trustworthiness of the face on a continuous scale from 0–100 (0 = low trustworthiness, 100 = high trustworthiness). Assignment of the left hand to the mouse and right hand to the number pad of the computer keyboard was arbitrary. Non-face trials were 4 s long and consisted of two phases: 1) 2 s shape viewing; and 2) 2 s shape recognition response. During the shape recognition period, all participants used their right hand to press “1” (index finger) to indicate “circle”, “2” (middle finger) to indicate “rectangle”, or “3” (ring finger) to indicated triangle. Each trial was separated by a 2–4 s fixation period, in which a white cross was presented on a black background. Trials were pseudorandomized, such that three faces trials, one of each valence (fear, neutral, happy), occurred in a row then 1 shape trial was presented. The order of valence for each set of three face trials was randomized. Of note, we found no differences in the distribution of trials between the three stimuli (χ2 = .33, p = .86), and no evidence that the sequence of the trials was predictable or made an impact on the behavioral outcomes (ps > .60). Before beginning the experiment, participants practiced the task with a separate set of stimuli derived from the same face database.
2.3. EEG collection and data analysis
Continuous EEG data were collected during the EFAT, using the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands) and a custom 34-electrode cap based on the 10/20 system. Electrodes were placed on the right and left mastoids, and the electrooculogram (EOG) was recorded using four facial electrodes to capture artifacts from eye movement (horizontal and vertical) and eye blinks. Electrical signals were pre-amplified at the electrode to improve the signal-to-noise ratio, using a gain of 16x. The EEG trace was then digitized at 64-bit resolution using a sampling rate of 512-Hz, a low-pass fifth-order sinc filter with a half-power cutoff of 104-Hz. Off-line analyses were performed using Brain Vision Analyzer 2.0 software (Brain Products; Gilching, Germany) following standardized preprocessing steps. In brief, all data were referenced to the average of the left and right mastoids, and band-pass-filtered from .1 and 30 Hz. A semi-automatic procedure was employed to detect and reject artifacts. The criteria applied were a voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, and a maximum voltage difference of less than .50 μV within 100-ms intervals. These intervals were rejected from individual channels in each trial. Visual inspection of the data was then conducted to detect and reject any remaining artifacts. Data from an entire trial was removed if all channels for that trial contained an artifact. No more than 2% of the total trials per condition were excluded from each participant. Overall (out of 120 trials total), a maximum of 18 trials (15%) were removed from a given channel, and each channel had a minimum of 23 trials (77%) for each condition, across subjects. At the electrodes used in the analyses (see below), the average number of artifact-free trials included per electrode per condition was 29 (range: 23 to 30 for fearful faces; 27 to 30 for happy faces; 25 to 30 for neutral faces; and 26 to 30 for shapes). Eye-blink and ocular corrections were conducted using independent components analysis (ICA; Jung et al., 1998; Makeig, J. Bell., Jung, & Sejnowski, 1996). On average, we removed 6 (± 2.02; range: 3–9) components per participant. No subjects were excluded from the analyses. The EEG trace was segmented for each trial beginning 500 ms before stimulus onset during the ‘viewing’ period and continuing for 2000 ms, and a 500 ms window from −500 to 0 ms prior to stimulus onset served as the baseline.
Consistent with previous studies, LPP was estimated using mean activity averaged across four electrode sites (CP2, Pz, P4, PO3), for early (400–1000 ms post-stimulus onset) and late (1000–2000 ms) time windows separately. These four electrode sites were selected based on previous studies and showed the top 15% magnitude of response in our data (Dunning et al., 2011; Foti, Olvet, Klein, & Hajcak, 2010; Moser, Hajcak, Bukay, & Simons, 2006). Of note, follow-up analyses pooling across bilateral electrodes (CP2, CP1, Pz, P3, P4, PO3, PO4) were conducted, and the results reported did not change.
2.4. Statistical analyses
Accuracy and reaction time (all trials) were analyzed using separate repeated-measures ANOVAs and follow-up t-tests in SPSS software v.24 (IBM Corp.). Condition (fearful, neutral, happy, shapes) was entered as a within subject factor. To examine changes in LPP between early and late, we performed an ANOVA with condition and time as within subject factors and follow-up t-tests. Pearson Bivariate Correlation was used to examine associations between behavioral measures and ERP and behavioral data. We also calculated and report δRM, an index of effect size, for within-group/repeated measures effects (Equation 8 from Morris & DeShon, 2002), as well as 95% confidence intervals. Nonparametric tests were used for trustworthiness ratings (Friedman’s test, Wilcoxon Signed-Rank test), and r values were calculated as a measure of effect size. Outlier detection was performed for all variables using |Z| > 3. Pairwise comparisons were considered significant at a Bonferroni-adjusted alpha level of ≤ .008 (.05/6) for accuracy and reaction time; ≤ .02 (.05/3) for trustworthiness ratings, and ≤ .003 (.05/16) for all LPP analyses.
3. Results
3.1. Behavioral data
3.1.1. Trustworthiness.
Friedman’s test showed a statistically significant difference in trustworthiness ratings between conditions (χ2(2) = 22.45, p < .001; Figure 2a). Post hoc analysis with Wilcoxon Signed-Rank tests revealed that happy faces were rated as more trustworthy than neutral and fearful faces (all ps < .001; Table 2). Although neutral faces were rated as less trustworthy than fearful faces, this difference did not reach significance (p = .03, corrected; Table 2; Figure 2a). Of note, there were substantial individual differences in trustworthiness ratings for neutral faces, ranging from 21 to 95 (out of a 0–100 scale where 0 = least trustworthy and 100 = most trustworthy). Further, there was one outlier (|Z| > 3) in trustworthiness ratings to neutral faces. Results remained significant when this outlier was removed.
Figure 2.
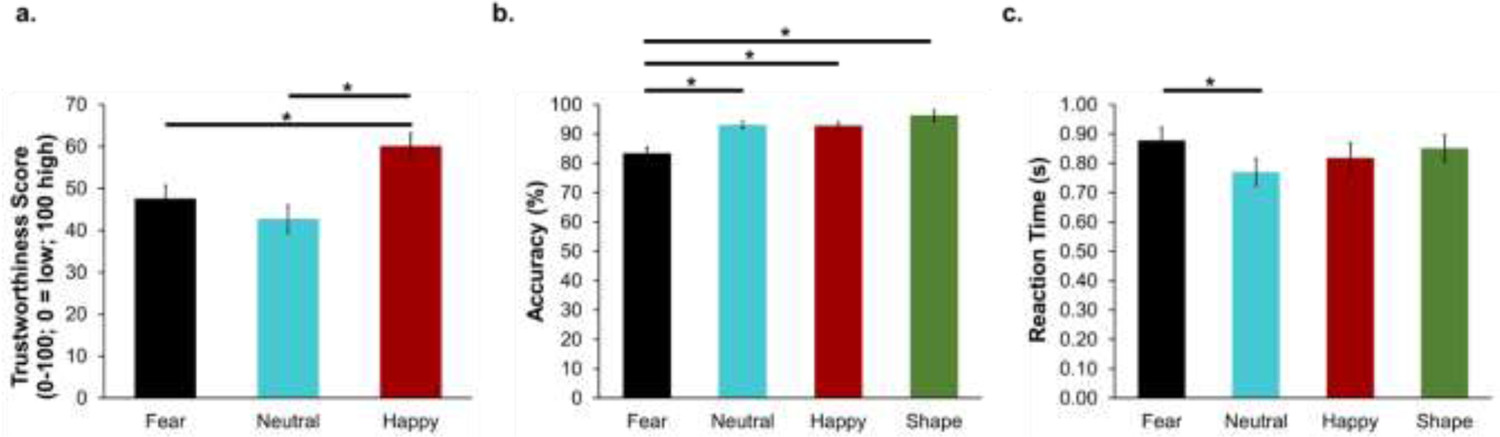
Mean trustworthiness rating (a), accuracy (b), and reaction time (c) for each condition. During the emotion recognition period, participants selected the emotion that best represented the affect displayed by the face (fear, happy, neutral) for which accuracy and reaction time were recorded. Error bars represent standard error of the mean. *denotes significant p-value after correcting for multiple comparisons (Trustworthiness: p ≤ .02; Accuracy, Reaction Time: p ≤ .008).
Table 2.
Behavioral Differences for Emotional Facial Expressions and Shapes
| t(21) | p-value | δRM | 95% CI |
||
|---|---|---|---|---|---|
| LL | UL | ||||
| Accuracy | |||||
| Fear vs. Neutral | −3.73* | .001 | .82 | −14.96 | −4.26 |
| Fear vs. Happy | −4.65* | < .001 | 1.08 | −13.63 | −5.21 |
| Neutral vs. Happy | .12 | .90 | .03 | −3.09 | 3.47 |
| Fear vs. Shapes | −5.61* | < .001 | 1.21 | −17.68 | −8.12 |
| Neutral vs. Shapes | −2.17 | .04 | .49 | −6.44 | −.14 |
| Happy vs. Shapes | −1.84 | .08 | .40 | −7.42 | .46 |
| Reaction Time | |||||
| Fear vs. Neutral | 3.79* | .001 | .81 | .05 | .17 |
| Fear vs. Happy | 2.51 | .02 | .56 | .01 | .11 |
| Neutral vs. Happy | −2.01 | .06 | .44 | −.10 | .00 |
| Fear vs. Shapes | .64 | .53 | .14 | −.06 | .11 |
| Neutral vs. Shapes | −1.87 | .08 | .40 | −.17 | .01 |
| Happy vs. Shapes | −.87 | .40 | .19 | −.11 | .05 |
| Trustworthiness$ | Z | r | |||
| Fear vs. Neutral | −2.16 | .03 | −.46 | ||
| Fear vs. Happy | −3.52* | < .001 | −.75 | ||
| Neutral vs. Happy | −4.04* | < .001 | −.86 | ||
Note. LPP = late positive potential; CI = confidence interval; LL = lower limit, UL = upper limit.
denotes significant p-value after correcting for multiple comparisons (Accuracy, Reaction Time: p ≤ .008; Trustworthiness: p ≤ .02).
Wilcoxon Signed-Rank Test.
3.1.2. Accuracy.
Repeated-measures ANOVA showed a significant main effect of condition on accuracy (F(3,63) = 15.20, p < .001, ηp2 = .42; Figure 2b), such that accuracy was highest for the shapes and lowest for fearful faces. Follow-up t-tests revealed that accuracy was significantly lower when identifying fearful faces compared to neutral, happy, and shapes (ps ≤ .001; Table 2). Accuracy between happy, neutral, and shapes did not significantly differ (ps > .008, corrected; Table 2).
3.1.3. Reaction time.
Repeated-measures ANOVA showed a significant main effect of condition on reaction time (F(3,63) = 3.73, p = .016, ηp2 = .15), such that reaction time was fastest when identifying neutral faces followed by happy faces, shapes, and then fear (Figure 2c). Follow-up t-tests revealed that reaction time was slower to fearful faces compared to neutral faces (p = .001; Table 2). No other comparisons between conditions reached statistical significance (ps > .008, corrected; Table 2).
3.1.4. Correlations between behavioral outcomes.
There were no significant differences in behavioral measures for each condition (i.e., accuracy, reaction time, trustworthiness ratings; ps > .05, corrected).
3.2. ERPs
Repeated-measures ANOVA showed a significant main effect of condition (F(3,63) = 21.03, p < .001, ηp2 = .50), main effect of time (F(1,21) = 55.42, p < .001, ηp2 = .73), and a condition × time interaction (F(3,63) = 8.88, p < .001, ηp2 = .30) for the LPP (Figure 3).
Figure 3.
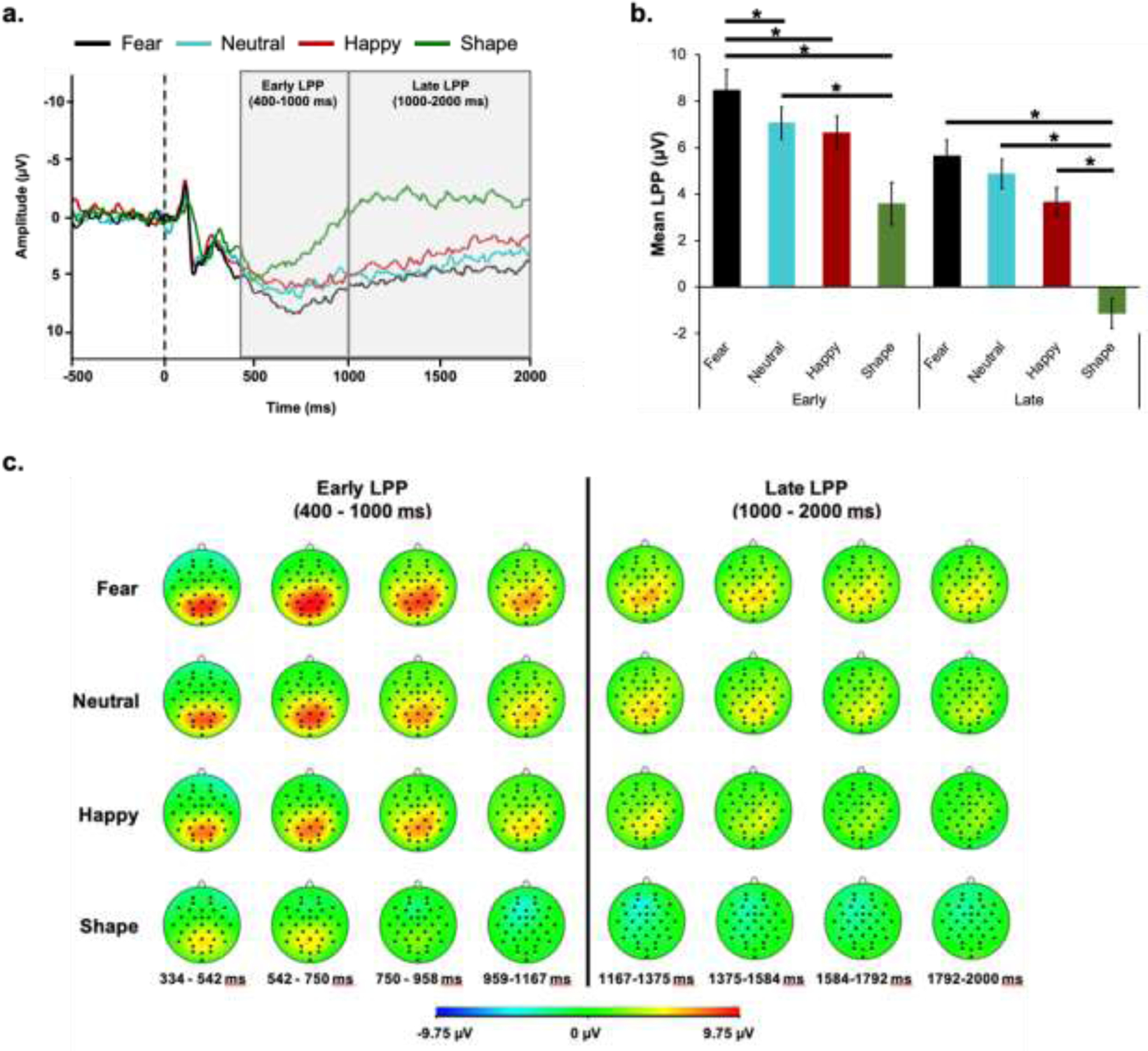
Late positive potential (LPP) grand-average waveforms for each condition in the emotional faces task (EFAT) (a), mean activity of early and late LPP (b), and head maps depicting the spatial distribution of voltage differences for each condition (c). Of note, the LPP response shown in panel (a) was pooled across four parieto-occipital electrodes, where LPP was shown to be maximal. In addition, positive amplitude is plotted downwards on the y-axis, for panel (a).
3.2.1. Early LPP.
Early LPP was largest for fearful faces as compared to neutral faces, happy faces, and shapes (ps < .003; Table 3). Early LPP was larger for neutral faces as compared to shapes (p = .003) but was not significantly different from happy faces (p = .41). Early LPP did not significantly differ for happy faces as compared to shapes (p = .01, corrected).
Table 3.
Differences in Brain Measures for Emotional Facial Expressions and Shapes
| t(21) | p-value | δRM | 95% CI |
||
|---|---|---|---|---|---|
| LL | UL | ||||
| Early LPP | |||||
| Fear vs. Neutral | 4.19* | < .001 | .99 | .73 | 2.16 |
| Fear vs. Happy | 3.67* | .001 | .80 | .80 | 2.91 |
| Neutral vs. Happy | .85 | .41 | .18 | −.60 | 1.43 |
| Fear vs. Shapes | 4.58* | < .001 | .98 | 2.68 | 7.14 |
| Neutral vs. Shapes | 3.40* | .003 | .74 | 1.35 | 5.59 |
| Happy vs. Shapes | 2.84 | .01 | .61 | .82 | 5.29 |
| Late LPP | |||||
| Fear vs. Neutral | 1.38 | .18 | .29 | −.40 | 1.98 |
| Fear vs. Happy | 3.02 | .006 | .65 | .62 | 3.38 |
| Neutral vs. Happy | 2.11 | .05 | .45 | .02 | 2.40 |
| Fear vs. Shapes | 6.50* | < .001 | 1.39 | 4.64 | 9.01 |
| Neutral vs. Shapes | 5.78* | < .001 | 1.23 | 3.86 | 8.21 |
| Happy vs. Shapes | 4.82* | < .001 | 1.03 | 2.74 | 6.91 |
| Early vs. Late LPP | |||||
| Fear | 5.12* | < .001 | 1.15 | 1.68 | 3.99 |
| Neutral | 6.55* | < .001 | 1.04 | 2.03 | 3.93 |
| Happy | 4.83* | < .001 | 1.43 | 1.24 | 3.12 |
| Shapes | 7.29* | < .001 | 1.69 | 3.39 | 6.11 |
Note. LPP = late positive potential; CI = confidence interval; LL = lower limit, UL = upper limit;
denotes significant p-value after correcting for multiple comparisons (p ≤ .003).
3.2.2. Late LPP.
Late LPP was largest for fearful, neutral, and happy faces, followed by shapes (ps < .001; Table 3). Both fearful and happy LPPs did not significantly differ from neutral (ps > .003, corrected). Late LPP did not significantly differ between fearful and happy faces (p = .18).
3.2.3. Early vs. late ERP.
LPP response to each condition significantly decreased from the early to the late LPP time window (ps < .001; Table 3).
3.3. Brain-behavior correlations
There were no significant brain-behavior correlations for early or late LPP with behavioral measures (i.e., accuracy, reaction time, trustworthiness ratings; ps > .05, corrected).
4. Discussion
This study investigated individual differences in behavioral and electrocortical responses to facial expressions in student police officers. We found that (1) overall, student police officers rated neutral facial expressions similarly to fearful faces and responded fastest to these expressions (as compared to fearful and happy faces). (2) Our results also revealed that neutral faces elicited a robust late LPP response that did not differ from the response elicited by fearful or happy faces. (3) Further, we found that there was substantial individual variation in trustworthiness ratings for neutral faces (ranging from 21 to 95 out of a 0–100 scale). Taken together, our study suggests that ‘neutral’ facial expressions elicit similar trustworthiness ratings to negatively-valenced stimuli. Further, brain and behavioral responses to neutral faces varied across student police officers; thus, encounters with neutral faces in the field may promote an increased perceived threat in some police officers, which may have real-world consequences (e.g., decision to shoot; Correll et al., 2006).
Our behavioral data support the hypothesis that neutral faces are rated as negative among student police officers. Indeed, we found neutral faces were rated as less trustworthy than happy faces, and similar to fearful faces. The trustworthiness ratings for fearful and happy faces are consistent with previous studies that find that negative expressions are rated as less trustworthy when compared to happy faces (Blasi et al., 2009). Further, these findings support the notion of emotion overgeneralization such that perceptions of negative emotional facial expressions are interpreted as less trustworthy. Interestingly, although fearful and neutral faces were rated similar in terms of trustworthiness, there were differences in accuracy and reaction time for facial expression identification. In particular, facial expression ratings were slower and less accurate for fearful as compared to neutral faces. This could reflect more time examining fearful faces to identify the potential source of the threat, which may be potentially useful for police officers in the field. Alternatively, faces that are more emotionally expressive (e.g., fearful) may require additional processing time than neutral faces, which may be more quickly appraised as untrustworthy or ambiguous. Together, we found that neutral faces were rated similar to fearful faces in terms of level of trustworthiness in this sample of student police officers, but the accuracy and speed of emotion ratings differed between fearful and neutral expressions.
Although our main analyses examined overall effects between conditions, it is important to note that we observed substantial individual variation in trustworthiness ratings in our sample of student police officers. This individual variability is consistent with the notion that individual differences in life history and personal experience may bias processing of ambiguous cues, and future studies should evaluate the potential relevance of these individual differences for future real-world outcomes (e.g., on-the-job performance, development of psychopathology). These data are also consistent with the notion that student police officers are not explicitly trained in how to react when facing ambiguous situation but are rather trained to develop common sense knowledge. Common sense knowledge is a form of informal learning wherein knowledge of who to trust and what to expect is developed based on everyday routines and experiences. This approach is commonly taken in police training academies given the high prevalence of situational uncertainty in police work (McNulty, 1994). Heightened risk of threat-related anxiety disorders, such as PTSD, may be linked to individual differences in appraisal of face stimuli, such as trustworthiness ratings (Saraiya et al., 2019). Such biases may be important to study in student police officers, who are at heightened risk of PTSD and other negative outcomes (Habersaat et al., 2015; Mumford et al., 2015). Individual differences in trustworthiness ratings or in perceptions of emotional stimuli in general may also reflect genetic factors, learning history, or exposure to trauma (e.g., childhood trauma; Fleurkens et al., 2018). Nonetheless, trust is considered central to every social interaction and the appraisal of trust – which occurs rapidly and automatically (Todorov et al., 2008; Winston et al., 2002) – shapes subsequent decisions and behaviors. Future studies should explore whether individual differences in trustworthiness judgments among student police officers predict on-the-job behavioral outcomes and mental health outcomes.
In addition to the observed behavioral effects on trustworthiness, the ERP data showed that neutral faces elicited a robust late LPP component response that did not differ from fearful faces. The LPP for neutral and fearful faces did not differ from happy faces either; however, this is consistent with the notion that LPP does not distinguish between valence, rather differentiating between emotional and non-emotional stimuli (see Table 3; Ashley et al., 2004; Liu et al., 2012). Interestingly, exploring the time course and topography of the LPP may allow us to examine differences in electrocortical processing. In particular, the early component of the LPP may reflect initial reactivity to emotional stimuli whereas the late component may reflect more regulated stages of affective processing (Hajcak et al., 2009). Here, we found that amplitude of the early component of the LPP was higher for fearful as compared to neutral and happy faces. This suggests that fearful faces may initially elicit greater initial emotional reactivity as compared to neutral or happy faces. During late component of the LPP, in contrast, amplitude was similarly high across fearful, neutral, and happy faces. This is interesting because prior studies suggest that the late component of the LPP reflects evaluations of stimulus meaning (Hajcak et al., 2010). In terms of social evaluation, happy faces may signal approach (i.e., higher trustworthiness) whereas neutral and fearful faces may trigger suspicions of threat among student police officers (i.e., low of trustworthiness). Indeed, trustworthiness ratings are typically inversely related to threat perceptions (Oosterhof and Todorov, 2008; Todorov and Engell, 2008). Further, a strong LPP response has also been reported for decisions related to ambiguity and motivational salience (Sun et al., 2017b); therefore, appraisal judgements, such as deciding whether or not someone is trustworthy, may be reflected in the high late LPP observed for both fearful and neutral faces. Interestingly, a prior study demonstrated that amplitude LPP in response to neutral faces can be modulated by preceding contextual information, such as secondhand information about the target person (Wieser et al., 2014). This contextual modulation may be relevant for police officers in the field, who often obtain verbal information prior to the social interaction which may impact subsequent neural processing of social cues. Another prior study reported no difference in ERP amplitudes (N100 and N650) between angry and neutral faces among individuals with PTSD, which the authors interpreted as a reduced ability to discriminate between non-threat and threat stimuli in PTSD (Felmingham et al., 2003).
One limitation of our study is the relatively limited sample size. Given that this study focused on neural and behavioral correlates of facial emotion processing in a relatively selective population of student police officers, we performed an a priori power analysis and the observed effect sizes were in the medium-to-large range. Another limitation was that our study sample was predominantly male. This distribution is similar to our population of interest (i.e., police and other first-responders), but precludes examination of potential effects of sex and/or generalization to female populations. For example, prior studies indicate sex differences in behavioral and electrocortical responses to facial expressions (Guillem and Mograss, 2005; Hofmann et al., 2006). Future studies with larger sample sizes are needed to better examine individual differences, and to explore interactive effects of sex or other important variables. Further, we did not detect significant correlations between the LPP responses and behavioral measures. This is consistent with prior studies that have also found that LPP was not significantly correlated with self-report ratings (e.g., valence, arousal, threat) (Wheaton et al., 2013), or with behavioral measures (e.g., reaction time) (Codispoti et al., 2016). This may relate to differences in timing of measures. In particular, self-report ratings (e.g., trustworthiness) were submitted following the initial face presentation (and LPP onset), which may diverge from the brain’s rapid judgements of facial stimuli that occurs without conscious awareness (Freeman et al., 2014). Moreover, behavioral measures include the duration of several processes other than initial perception of stimuli associated with the LPP (Brown et al., 2012). Additionally, we did not examine specific features of the faces that may have contributed to the trustworthiness ratings (e.g., perception of threat, shallow cheekbones; Todorov, Baron, & Oosterhof, 2008). Moreover, our study is unable to determine if neutral faces have an inherent emotionality (i.e., similar to emotionality in happy or fearful faces), or if they include features that resemble negative facial expressions and thus elicit a perceptual similarity response. Further, this study included only one type of ambiguous facial expression (i.e., neutral faces) and one type of threatening facial expression (i.e., fearful faces). Thus, it is unclear whether similar results would be observed for other ambiguous facial expressions (e.g., surprised faces), or for other negative facial expressions (e.g., anger). Future studies should also evaluate approachability and threat perception in faces, and to evaluate whether these patterns translate to the real-world (i.e., outside of a laboratory setting). Future studies should also compare the observed patterns to a civilian control group, or a group of experienced police officers.
Taken together, these data suggest that student police officers rate neutral and fearful faces as similar in trustworthiness, and theses expressions elicit similar late LPP responses. Further, we observed substantial individual differences in brain and behavioral responses to ambiguous (neutral) faces. Encounters with ambiguous cues in the field may elicit threat responses and emotion overgeneralizations that could impact decisions for action, especially given the often-limited time frames for which officers must make a decision. Individual differences in threat biases and trustworthiness have also been linked to emotion-related outcomes and neural responses in cortico-limbic circuitry implicated in PTSD and other stress-related disorders. Neurobehavioral functioning may therefore be relevant for predicting on-the-job performance or psychological adjustment following trauma exposure. Future longitudinal research is needed to link variation in threat detection with outcomes among officers.
Highlights.
Threat perception by law enforcement is critical for effective job performance
Ambiguous situations and potential threat can interfere with decision-making
Neutral and fearful faces were rated as less trustworthy than happy faces
Neutral and fearful faces elicited a similar neural response
Encounters with neutral faces in the field may promote increased perceived threat
Acknowledgements
This research reported was supported in part by the Wayne State University Department of Pharmacy Practice, the Wayne State University Undergraduate Opportunities Research Program, and the National Institutes of Health under award numbers GM118981, GM118982 and GM118983. Special thanks to Jennifer Mahn, MPH, Narcis Marshall, and Brian Silverstein for assistance in data collection and analysis, as well as, the student police officers who generously shared their time to participate in this study and for their service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest to declare with the work submitted.
References
- Alkus S, Padesky C, 1983. Special Problems of Police Officers: Stress-Related Issues and Interventions. Couns. Psychol 11, 55–64. [Google Scholar]
- Anshel MH, 2000. A Conceptual Model and Implications for Coping with Stressful Events in Police Work. Crim. Justice Behav 27, 375–400. [Google Scholar]
- Ashley V, Vuilleumier P, Swick D, 2004. Time course and specificity of event-related potentials to emotional expressions. Neuroreport. 10.1097/00001756-200401190-00041 [DOI] [PubMed]
- Beck A, Alford B, 2009. Part III: Theoretical Aspects of Depression, in: Depression: Causes and Treatment. University of Pennsylvania Press, Philadelphia, pp. 212–223. [Google Scholar]
- Blasi G, Hariri AR, Alce G, Taurisano P, Sambataro F, Das S, Bertolino A, Weinberger DR, Mattay VS, 2009. Preferential Amygdala Reactivity to the Negative Assessment of Neutral Faces. Biol. Psychiatry 66, 847–853. 10.1016/j.biopsych.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SBRE, van Steenbergen H, Band GPH, de Rover M, Nieuwenhuis S, 2012. Functional significance of the emotion-related late positive potential. Front. Hum. Neurosci 10.3389/fnhum.2012.00033 [DOI] [PMC free article] [PubMed]
- Calvo MG, Marrero H, Beltrán D, 2013. When does the brain distinguish between genuine and ambiguous smiles? An ERP study. Brain Cogn 81, 237–246. 10.1016/j.bandc.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Cantelon JA, Giles GE, Eddy MD, Haga Z, Mahoney CR, Taylor HA, Davis FC, 2018. Exerting Cognitive Control Under Threat: Interactive Effects of Physical and Emotional Stress. Emotion 1–8. 10.1037/emo0000509 [DOI] [PubMed]
- Carrera-Levillain P, Fernandez-Dols JM, 1994. Neutral Faces in Context: Their Emotional Meaning and Their Function. J. Nonverbal Behav 10.1007/BF02172290 [DOI]
- Clausen AN, Youngren W, Sisante JFV, Billinger SA, Taylor C, Aupperle RL, 2016. Combat PTSD and implicit behavioral tendencies for positive affective stimuli: A brief report. Front. Psychol 10.3389/fpsyg.2016.00758 [DOI] [PMC free article] [PubMed]
- Codispoti M, De Cesarei A, Biondi S, Ferrari V, 2016. The fate of unattended stimuli and emotional habituation: Behavioral interference and cortical changes. Cogn. Affect. Behav. Neurosci 10.3758/s13415-016-0453-0 [DOI] [PubMed]
- Correll J, Urland GR, Ito TA, 2006. Event-related potentials and the decision to shoot: The role of threat perception and cognitive control. J. Exp. Soc. Psychol 10.1016/j.jesp.2005.02.006 [DOI]
- Covey TJ, Shucard JL, Violanti JM, Lee J, Shucard DW, 2013. The effects of exposure to traumatic stressors on inhibitory control in police officers: A dense electrode array study using a Go/NoGo continuous performance task. Int. J. Psychophysiol 87, 363–375. 10.1016/j.ijpsycho.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Cuddy AJC, Fiske ST, Glick P, 2008. Warmth and Competence as Universal Dimensions of Social Perception: The Stereotype Content Model and the BIAS Map, in: Zanna M (Ed.), Advances in Experimental Psychology, Volume 40 pp. 61–137. 10.1016/S0065-2601(07)00002-0 [DOI] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ, 2000. Brain Potentials in Affective Picture Processing: Covariation with Autonomic Arousal and Affective Report. Biol. Psychol 52, 95–111. 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Damjanovic L, Pinkham AE, Clarke P, Phillips J, 2014. Enhanced Threat Detection in Experienced Riot Police Officers: Cognitive Evidence from the Face-in-the-Crows Effect. Q. J. Exp. Psychol 67, 1004–1018. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ, 2001. The amygdala: Vigilance and emotion. Mol. Psychiatry 10.1038/sj.mp.4000812 [DOI] [PubMed]
- Dennis TA, Hajcak G, 2009. The late positive potential: A neurophysiological marker for emotion regulation in children. J. Child Psychol. Psychiatry Allied Discip 50, 1373–1383. 10.1111/j.1469-7610.2009.02168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Zhou H, Zhao X, Lu Q, 2011. Early Negativity Bias Occurring Prior to Experiencing of Emotion: An ERP Study. J. Psychophysiol 25, 9–17. 10.1027/0269-8803/a000027 [DOI] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ, 2011. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users - an ERP study. Eur. J. Neurosci 33, 1716–1723. 10.1111/j.1460-9568.2011.07663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham KL, Bryant RA, Gordon E, 2003. Processing angry and neutral faces in posttraumatic stress disorder: An event-related potentials study. Neuroreport 14, 777–780. 10.1097/00001756-200304150-00024 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1995. Structured Clinical Interview for DSM-IV Axis I Disorders - Nonpatient Edition (SCID-I/NP, Version 2.0). New York. [Google Scholar]
- Fleurkens P, Van Minnen A, Becker ES, Van Oostrom I, Speckens A, Rinck M, Vrijsen JN, 2018. Automatic approach-avoidance tendencies as a candidate intermediate phenotype for depression: Associations with childhood trauma and the 5-HTTLPR transporter polymorphism. PLoS One. 10.1371/journal.pone.0193787 [DOI] [PMC free article] [PubMed]
- Foti D, Hajcak G, 2008. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. J. Cogn. Neurosci 20, 977–988. 10.1162/jocn.2008.20066 [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G, 2010. Reduced electrocortical response to threatening faces in major depressive disorder. Depress. Anxiety 27, 813–820. 10.1002/da.20712 [DOI] [PubMed] [Google Scholar]
- Freeman JB, Stolier RM, Ingbretsen ZA, Hehman EA, 2014. Amygdala Responsivity to High-Level Social Information from Unseen Faces. J. Neurosci 34, 10573–10581. 10.1523/JNEUROSCI.5063-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem F, Mograss M, 2005. Gender differences in memory processing: Evidence from event-related potentials to faces. Brain Cogn 10.1016/j.bandc.2004.08.026 [DOI] [PubMed]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE, 2002. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods 115, 137–43. [DOI] [PubMed] [Google Scholar]
- Habersaat SA, Geiger AM, Abdellaoui S, Wolf JM, 2015. Health in police officers: Role of risk factor clusters and police divisions HHS Public Access. Soc Sci Med 143, 213–222. 10.1016/j.socscimed.2015.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D, 2009. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clin. Neurophysiol 10.1016/j.clinph.2008.11.028 [DOI] [PubMed]
- Hajcak G, Foti D, 2020. Significance?& Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology. 10.1111/psyp.13570 [DOI] [PubMed]
- Hajcak G, Macnamara A, Olvet DM, 2010. Event-related potentials, emotion, and emotion regulation: An integrative review. Dev. Neuropsychol 35, 129–155. 10.1080/87565640903526504 [DOI] [PubMed] [Google Scholar]
- Ho NSP, Sun D, Ting KH, Chan CCH, Lee TMC, 2015. Mindfulness Trait Predicts Neurophysiological Reactivity Associated with Negativity Bias: An ERP Study. Evidence-based Complement. Altern. Med 2015, 1–15. 10.1155/2015/212368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Suvak M, Litz BT, 2006. Sex differences in face recognition and influence of facial affect. Pers. Individ. Dif 10.1016/j.paid.2005.12.014 [DOI]
- Jung T-P, Humphries C, Lee T-W, Makeig S, McKeown MJ, Iragui V, Sejnowski TJ, 1998. Extended ICA removes artifacts from electroencephalographic recordings. Adv. Neural Inf. Process. Syst 10, 894–900. [Google Scholar]
- Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO, 2012. Early Interventions for PTSD: A Review. Depress. Anxiety 29, 833–842. 10.1002/da.21997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, Blonder LX, 2001. Neural substrates of facial emotion processing using fMRI. Cogn. Brain Res 11, 213–226. 10.1016/S0926-6410(00)00073-2 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE, Cummings CM, Caporino NE, Kendall PC, 2015. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 140, 816–845. 10.1037/a0034733.Comorbidity [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander, Andrew L, Whalen PJ, 2003. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 14, 2317–2322. 10.1097/01.wnr.0000101520.44335.20 [DOI] [PubMed] [Google Scholar]
- Knutson B, 1996. Facial expressions of emotion influence interpersonal trait inferences. J. Nonverbal Behav 20, 165–182. [Google Scholar]
- Lee E, Kang JI, Park IH, Kim J-J, An SK, 2007. Is a neutral face really evaluated as being emotionally neutral? Psychiatry Res 157, 77–85. 10.1016/j.psychres.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M, 2012. Neural Substrate of the Late Positive Potential in Emotional Processing. J. Neurosci 32, 14563–14572. 10.1523/JNEUROSCI.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loijen A, Vrijsen JN, Egger JIM, Becker ES, Rinck M, 2020. Biased approach-avoidance tendencies in psychopathology: A systematic review of their assessment and modification. Clin. Psychol. Rev 10.1016/j.cpr.2020.101825 [DOI] [PubMed]
- MacNamara A, Jackson TB, Fitzgerald JM, Hajcak G, Phan KL, 2019. Working Memory Load and Negative Picture Processing: Neural and Behavioral Associations With Panic, Social Anxiety, and Positive Affect. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 151–159. 10.1016/j.bpsc.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, J. Bell. A, Jung T-P, Sejnowski TJ, 1996. Independent Component Analysis of Electroencephalographic Data. Adv. Neural Inf. Process. Syst 8, 145–151. 10.1109/ICOSP.2002.1180091 [DOI] [Google Scholar]
- McNulty EW, 1994. Generating Common Sense Knowledge Among Police Officers. Symb. Interact 17, 281–294. 10.1525/si.1994.17.3.281 [DOI] [Google Scholar]
- Morris SB, DeShon RP, 2002. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 7, 105–125. 10.1037/1082-989X.7.1.105 [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF, 2006. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology 43, 292–296. 10.1111/j.1469-8986.2006.00402.x [DOI] [PubMed] [Google Scholar]
- Mumford EA, Taylor BG, Kubu B, 2015. Law Enforcement Officer Safety and Wellness. Police Q 18, 111–133. 10.1177/1098611114559037 [DOI] [Google Scholar]
- Olivola CY, Funk F, Todorov A, 2014. Social attributions from faces bias human choices. Trends Cogn. Sci 10.1016/j.tics.2014.09.007 [DOI] [PubMed]
- Olivola CY, Todorov A, 2010. Fooled by first impressions? Reexamining the diagnostic value of appearance-based inferences. J. Exp. Soc. Psychol 46, 315–324. 10.1016/j.jesp.2009.12.002 [DOI] [Google Scholar]
- Oosterhof NN, Todorov A, 2008. The functional basis of face evaluation, Proceedings of National Academy of Sciences. PNAS August [DOI] [PMC free article] [PubMed]
- Pan DN, Wang Yi, Lei Z, Wang Yang, Li X, 2020. The altered early components and the decisive later process underlying attention bias modification in social anxiety: evidence from event-related potentials. Soc. Cogn. Affect. Neurosci 10.1093/scan/nsz098 [DOI] [PMC free article] [PubMed]
- Pastor MC, Bradley MM, Löw A, Versace F, Moltó J, Lang PJ, 2008. Affective picture perception: Emotion, context, and the late positive potential. Brain Res 10.1016/j.brainres.2007.10.072 [DOI] [PMC free article] [PubMed]
- Santos S, Almeida I, Oliveiros B, Castelo-Branco M, 2016. The role of the amygdala in facial trustworthiness processing: A systematic review and meta-analyses of fMRI studies. PLoS One 10.1371/journal.pone.0167276 [DOI] [PMC free article] [PubMed]
- Saraiya T, Fareri D, López-Castro T, Hien D, Fertuck E, Melara R, 2019. The social cognitive appraisal of trustworthiness in individuals with dimensional levels of posttraumatic stress symptoms: a translational study. Eur. J. Psychotraumatol 10.1080/20008198.2019.1697582 [DOI] [PMC free article] [PubMed]
- Sun S, Yu R, Wang S, 2017a. A Neural Signature Encoding Decisions under Perceptual Ambiguity. eneuro 4, ENEURO.0235-17.2017. 10.1523/ENEURO.0235-17.2017 [DOI] [PMC free article] [PubMed]
- Sun S, Zhen S, Fu Z, Wu D, Shimojo S, 2017b. Decision ambiguity is mediated by a late positive potential originating from cingulate cortex. Neuroimage 157, 400–414. 10.1016/j.neuroimage.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Baron S, Oosterhof N, 2008. Evaluating face trustworthiness: a model based approach. SCAN 3, 119–127. 10.1093/scan/nsn00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Engell AD, 2008. The role of the amygdala in implicit evaluation of emotionally neutral faces. Soc. Cogn. Affect. Neurosci 10.1093/scan/nsn033 [DOI] [PMC free article] [PubMed]
- Veenstra L, Schneider IK, Bushman BJ, Koole SL, 2017. Drawn to danger: trait anger predicts automatic approach behaviour to angry faces. Cogn. Emot 10.1080/02699931.2016.1150256 [DOI] [PubMed]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx B, Keane TM, 2015. Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). PTSD Natl. Cent. PTSD 1–18. 10.1037/t00072-000 [DOI]
- Weinberg A, Sandre A, 2018. Distinct Associations Between Low Positive Affect, Panic, and Neural Responses to Reward and Threat During Late Stages of Affective Picture Processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 59–68. 10.1016/j.bpsc.2017.09.013 [DOI] [PubMed] [Google Scholar]
- Wheaton MG, Holman A, Rabinak CA, MacNamara A, Proudfit GH, Phan KL, 2013. Danger and disease: Electrocortical responses to threat- and disgust-eliciting images. Int. J. Psychophysiol 90, 235–239. 10.1016/j.ijpsycho.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Gerdes ABM, Büngel I, Schwarz KA, Mühlberger A, Pauli P, 2014. Not so harmless anymore: How context impacts the perception and electrocortical processing of neutral faces. Neuroimage 10.1016/j.neuroimage.2014.01.022 [DOI] [PubMed]
- Williams LM, Gatt JM, Schofield PR, Olivieri G, Peduto A, Gordon E, 2009. “Negativity bias” in risk for depression and anxiety: Brain-body fear circuitry correlates, 5-HTT-LPR and early life stress. 10.1016/j.neuroimage.2009.05.009 [DOI] [PubMed]
- Willis J, Todorov A, 2006. First impressions: Making up your mind after a 100-ms exposure to a face. Psychol. Sci 10.1111/j.1467-9280.2006.01750.x [DOI] [PubMed]
- Willis ML, Palermo R, Burke D, 2011. Judging Approachability on the Face of It: The Influence of Face and Body Expressions on the Perception of Approachability. Emotion 11, 514–523. 10.1037/a0022571 [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, Dolan RJ, 2002. Automatic and Intentional Brain Responses During Evaluation of Trustworthiness of Faces. Nat. Neurosci 5, 277–283. 10.1038/nn816 [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Kikuchi M, Fellous J-M, 2010. Facial Resemblance to Emotions: Group Differences, Impression Effects, and Race Stereotypes. J. Pers. Soc. Psychol 98, 175–89. 10.1037/a0017990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz LA, Montepare JM, 2008. Social Psychological Face Perception: Why Appearance Matters. Soc. Personal. Psychol. Compass 2, 1497–1517. 10.1111/j.1751-9004.2008.00109.x [DOI] [PMC free article] [PubMed] [Google Scholar]


