Abstract
Background:
Hypotension is common under anesthesia and can cause organ underperfusion and ischemia, especially during induction. This could be because of the cardiovascular depressant and vasodilatory effects of anesthetic agents, as well as lack of surgical stimulation.
Aim of Study:
The aim was to evaluate the utility of preinduction inferior vena cava (IVC) measurement to predict significant hypotension after induction of anesthesia with propofol.
Settings and Design:
This was a prospective, open-label study conducted in a tertiary care institute.
Subjects and Methods:
This study was conducted on 50 patients undergoing general anesthesia. Ultrasound machine (Mindray® M7) was used to visualize IVC. The measurements taken were maximum diameter of IVC (IVCmax) and minimum diameter of IVC (IVCmin). IVC collapsibility index (IVC-CI) was calculated as (IVCmax − IVCmin)/IVCmax and was expressed as a ratio.
Statistical Tests Used:
Receiver operating characteristic (ROC) curve analysis and Chi-square test were used for statistical tests.
Results:
The relation between significant hypotension and IVC-CI was evaluated using ROC curve analysis. We found the area under curve to be 0.959 and a cutoff of 0.43 (43% collapsibility). The association of significant hypotension with IVC-CI of >43% was calculated and found to be statistically significant (P < 0.001). The sensitivity of IVC CI of >43% in predicting development of significant hypotension was 86.67% and the specificity was 94.29%. It had very high negative and positive predictive values (94.29% and 86.67%, respectively) with an accuracy of 92%.
Conclusion:
Patients with an IVC collapsibility of more than 43%, as assessed by ultrasonography, are more likely to develop significant hypotension after induction with propofol.
Keywords: General anesthesia, hypotension, inferior vena cava, propofol, ultrasound
INTRODUCTION
Hypotension is common during the perioperative period. This can lead on to organ underperfusion and ischemia. Hypotension is frequently seen in the interim between induction of general anesthesia and skin incision. Reich et al.[1] identified predictors of significant hypotension after induction of general anesthesia. Propofol is a common cause of hypotension.[2] There are many studies which have explained the effect of hypotension on various organ systems and their functioning; it can present as stroke,[3] myocardial injury, or acute kidney injury in patients undergoing general[3] and cardiac[4] surgeries. Postinduction hypotension can lead to increased postoperative morbidity and mortality, as well as postoperative stay.[1]
The role of preinduction volume status in the development of postinduction hypotension has not been evaluated fully.[5] However, propofol is known to cause hypotension.[1,2,5,6] Hypotension following propofol is due to myocardial suppression and decreased systemic vascular resistance.[6] A multimodal approach consisting of nonpharmacological and pharmacological interventions has been advocated to reduce postinduction hypotension, and assessment of inferior vena cava (IVC) diameter is one of them. Ultrasound measurement of IVC can be used to predict hypotension[5,6] and assess the volume status of the patient. The maximum and the minimum diameters of the IVC (IVCmax and IVCmin, respectively) are measured to calculate the collapsibility index of IVC (IVC CI) using the formula, IVC-CI = (IVCmax–IVCmin)/IVCmax.
The present study was undertaken with the need to evolve a suitable method to predict hypotension after induction with propofol. In this study, the efficacy and usefulness of measurement of different diameters of IVC and calculating the collapsibility were evaluated.
SUBJECTS AND METHODS
This study was conducted after getting approval from the ethics committee and the institutional review board. This diagnostic accuracy study was conducted between July 2018 and June 2019. The study was conducted in 50 consenting patients aged more than18 years, with the American Society of Anesthesiologists physical status classes I and II, who required general anesthesia (induction with propofol) followed by endotracheal intubation. Exclusion criteria included patients with cardiac valvular diseases, anticipated difficult airway, and emergency surgery. Based on earlier[6] results (sensitivity and specificity) of IVC-CI of <50% in predicting the development of significant hypotension, and with 95% confidence and 20% allowable error, the minimum sample size was 50 cases.
The aim of our diagnostic accuracy study was to evaluate the utility of preinduction IVC measurement to predict significant hypotension after induction of general anesthesia with propofol. Preinduction monitors such as electrocardiogram, noninvasive blood pressure, and pulse oximeter were attached to the patient. Heart rate (HR), mean arterial pressure (MAP), and oxygen saturation (SpO2) were noted.
All patients were made to lie down while breathing spontaneously for 5 min. For visualizing IVC, ultrasound machine Mindray ® M7 was used. The same anesthesiologist (RK) performed the scan. The IVC was visualized through subxiphoid transabdominal long-axis view 2–3 cm caudal to the right atrial junction.[7,8,9] Readings noted were IVCmax and IVCmin. IVC collapsibility index was calculated as mentioned earlier and expressed as a percentage.
The patients were then induced as per a standard protocol. They were given glycopyrrolate 5 to10 μg.kg − 1, midazolam 0.05–0.1 mg.kg − 1, and fentanyl 2–3 μg.kg − 1 before induction. Patients were induced with propofol 1–2 mg.kg − 1, till there was loss of response to verbal commands. They were then mask ventilated with 100% oxygen and isoflurane 1.0%–1.2%. Atracurium (0.5 mg.kg − 1) was given for muscle relaxation. Three minutes after atracurium, patients were intubated by performing a gentle and quick laryngoscopy lasting not more than 15 s. In males, 8 mm or 8.5 mm internal diameter endotracheal tube was used. In females, 7 mm or 7.5 mm internal diameter endotracheal tube was used.
Data collected were systolic blood pressure, diastolic blood pressure, MAP, and HR at baseline, followed by 3 min (after induction), 6 min (after laryngoscopy), 10 min, and 15 min, respectively. The use of fluid bolus and rescue vasopressors/inotropes (both bolus and infusion), during induction were noted. Significant hypotension was defined as MAP <60 mmHg or the use of rescue vasopressors.
This analysis was done using receiver operating characteristic (ROC) curve analysis and Chi-square test. Numerical variables were presented using mean and standard deviation. ROC curve was used to find the ideal cutoff for finding the significant hypotension for the variables IVC-CI and IVCmax. Chi-square test was used to find the statistical significance of the association of IVC-CI and IVCmax with MAP. Diagnostic measures such as sensitivity, specificity, positive and negative predictive values, and accuracy were calculated. Statistical analyses were done using SPSS version 20.0 for Windows (IBM Corporation Armonk, NY, USA).
RESULTS
We included 50 adult patients in the study. Among these 50 patients, 15 patients developed significant hypotension. Out of these 15 patients, 7 patients developed a MAP <60 mmHg after 3 min of induction with propofol. The remaining 8 patients received vasopressor/inotrope as a rescue measure. Hence, we got two groups of patients: 35 who did not develop significant hypotension and 15 who developed significant hypotension. In our study, age, gender, and body mass index (BMI) were statistically comparable between the two groups. The dose of propofol used for induction was also comparable between the two groups. It was 111 ± 13 mg in the hypotension group and 107 ± 17 mg in the other group.
An IVC collapsibility of more than 50% is generally accepted as a sign of hypovolemia. Our study compared the hemodynamic response to induction of general anesthesia using propofol. ROC analysis [Figure 1] was done to analyze the relation between significant hypotension and IVC-CI, and we found an area under curve to be 0.959 and a cutoff of 0.43 (or 43%). The association of significant hypotension with IVC-CI of 0.43 was calculated and found to be statistically significant (P < 0.001) [Table 1]. The sensitivity of IVC-CI of 0.43 in predicting significant hypotension developing was 86.67% and the specificity was 94.29%. It had very high negative and positive predictive values (94.29% and 86.67%, respectively). The accuracy was 92% [Table 2 and Figure 2]. The association of significant hypotension and the universally accepted IVC collapsibility (50%) or IVC-CI of 0.50 in predicting significant hypotension was also statistically significant (P < 0.001). The sensitivity of IVC-CI of 0.50 in predicting significant hypotension, though, was very low at 53.33%. The specificity was 100% [Table 3 and Figure 3].
Figure 1.
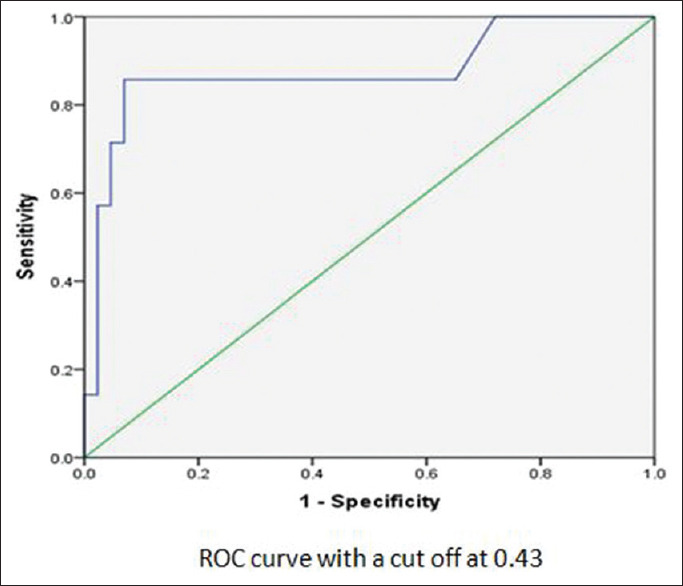
Receiver operating characteristic curve of inferior vena cava-collapsibility index
Table 1.
Association of mean arterial pressure with inferior vena cava-collapsibility index of 0.43
| IVC CI | Significant hypotension | P | |
|---|---|---|---|
| Yes (%) | No (%) | ||
| >0.43 (15) | 13 (86.7) | 2 (13.3) | 0.001 |
| ≤0.43 (35) | 2 (5.7) | 33 (94.3) | |
IVC - CI=Inferior vena cava-collapsibility index
Table 2.
Statistics of using collapsibility index of 0.43
| Statistics | Value (%) | 95% CI |
|---|---|---|
| Sensitivity | 86.67 | 59.54-98.34 |
| Specificity | 94.29 | 80.84-99.30 |
| Positive predictive value | 86.67 | 62.52-96.20 |
| Negative predictive value | 94.29 | 81.91-98.36 |
| Accuracy | 92.00 | 80.77-97.78 |
CI=Confidence interval
Figure 2.
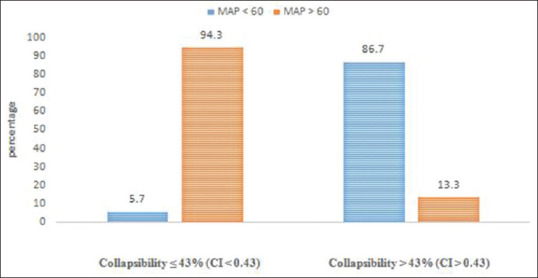
Association between inferior vena cava collapsibility of 43% and development of significant hypotension
Table 3.
Statistics of using collapsibility index of 0.50
| Statistics | Value (%) | 95% CI |
|---|---|---|
| Sensitivity | 53.33 | 26.59-78.73 |
| Specificity | 100.00 | 90.00-100.00 |
| Positive predictive value | 100.00 | 51.80-100.00 |
| Negative predictive value | 83.33 | 74.43-89.97 |
| Accuracy | 86.00 | 73.26-94.18 |
CI=Confidence interval
Figure 3.
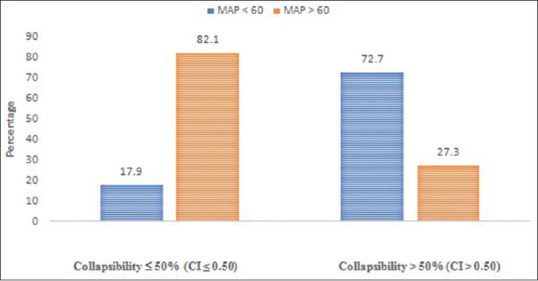
Association between inferior vena cava collapsibility of 50% and development of significant hypotension
DISCUSSION
Hemodynamic fluctuations are a common occurrence during anesthesia. Both uncontrolled hypertension and hypotension can be dangerous. Both can lead to not just increased morbidity but mortality as well.[1] Hypotension can lead to underperfusion in the different vascular beds of the body including brain, heart, liver, and kidney.[1]
There are various factors that can lead to hypotension during anesthesia. Some of the anesthetic drugs can cause myocardial depression or vascular dilatation and hence can lead on to hypotension. This effect of anesthetic drugs can be potentiated by various other factors. One very important factor is the volume status of the patient. The patients coming for surgery can be hypovolemic for various reasons. Assessing the volume status accurately is not simple. Various methods have been suggested for the same.
The use of IVC size and collapsibility to assess the volume status has been suggested as a useful bedside tool.[8] Variation of IVC diameter with respiration has an important value in assessing volume status.[9] A greater collapsibility of IVC during spontaneous respiration suggests low volume status.[10,11]
The present diagnostic accuracy study was undertaken to determine whether the IVC collapsibility index can predict hypotension in patients following induction with propofol. We did this study in patients who were posted for elective surgery under general anesthesia.
Bijker et al.[12] have found 140 definitions in the literature of different reported indices of hypotension. We chose a mean blood pressure <60 mmHg as our definition of significant hypotension. We also included patients who received a bolus dose or infusion of vasopressor/inotropic agent as a rescue measure in the group who developed significant hypotension.
We included 50 adult patients in the study. Among these 50 patients, 15 patients developed significant hypotension. Out of these 15 patients, 7 patients developed a MAP <60 mmHg after 3 min of induction with propofol. The remaining 8 patients received vasopressor/inotrope as a rescue measure. Hence, we got two groups of patients: 35 who did not develop significant hypotension and 15 who developed significant hypotension.
In our study, age, gender, and BMI were statistically comparable between the two groups. The dose of propofol used for induction was comparable between the two groups. It was 111 ± 13 mg in the hypotension group and 107 ± 17 mg in the other group.
An IVC collapsibility of more than 50% is generally accepted as a sign of hypovolemia. Our study compared the hemodynamic response of the induction of general anesthesia using propofol. ROC curve analysis was done to analyze the relation between significant hypotension and IVC-CI, and we found an area under curve to be 0.959 and a cutoff of 0.43 or 43%. This is in agreement with previous studies.[5,11,13,14,15] Our study found an optimal cutoff value of 43% for predicting significant hypotension after induction with propofol.
The association of significant hypotension with IVC-CI of 43% was calculated and found to be statistically significant (P < 0.001). The sensitivity of IVC-CI of 43% in predicting significant hypotension developing was 86.67% and the specificity was 94.29%. It had very high negative and positive predictive values (94.29% and 86.67%, respectively). The accuracy was 92%. The sensitivity and specificity were similar to that of Zhang and Critchley,[5] who got a cutoff of 43% (78.6% and 91.7%, respectively).
We also analyzed the results keeping IVC-CI as 50%, which is the universally accepted cutoff to predict fluid responsiveness. This was also found to be statistically significant (P < 0.001). The sensitivity, though, was very low at 53.33% which is in stark contrast to the sensitivity obtained while using a cutoff of 43% (86.67%). The specificity when using a cutoff of 50% is, however, 100%, which means all patients whose IVC collapses by more than 50% developed significant hypotension after induction with propofol. The accuracy of this was lower at 86%, compared to the accuracy while using 43% cutoff, which was 92%.
The results from our study suggest that using the universal IVC-CI cutoff of more than 50% will lead to missing almost 50% of cases who can develop significant hypotension after induction with propofol as the sensitivity is very low (53.33%). This finding was also seen in the studies done by Szabó et al.[13] (sensitivity of 45.5%) and Au et al.[6] (sensitivity 66.67%).
We have few limitations of our study. Due to the smaller sample size (50 patients), the allowable error turns out to be 20% for our results. We did not do a subgroup analysis of our patients. There were some patients who had systemic hypertension and were on vasoactive agents. These drugs could affect the degree of hypotension after induction. Hence, ideally, we should have either excluded such patients or done a subgroup analysis.
A larger sample size can be adopted to obtain more accurate results. Ultrasound assessment can become a simpler, noninvasive method to predict fluid responsiveness in patients undergoing propofol-induced general anesthesia.
CONCLUSION
We conclude that patients with an IVC collapsibility of more than 43%, as assessed by ultrasonography, are more likely to develop significant hypotension after propofol induction. Before induction of general anesthesia with propofol, ultrasound assessment of IVC collapsibility can be a potential tool to predict postinduction hypotension.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8. doi: 10.1213/01.ANE.0000175214.38450.91. [DOI] [PubMed] [Google Scholar]
- 2.Mehandale SG, Rajasekhar P. Perfusion index as a predictor of hypotension following propofol induction-A prospective observational study. Indian J Anaesth. 2017;61:990–5. doi: 10.4103/ija.IJA_352_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijker JB, Persoon S, Peelen LM, Moons KG, Kalkman CJ, Kappelle LJ, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: A nested case-control study. Anesthesiology. 2012;116:658–64. doi: 10.1097/ALN.0b013e3182472320. [DOI] [PubMed] [Google Scholar]
- 4.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Critchley LA. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after Induction. Anesthesiology. 2016;124:580–9. doi: 10.1097/ALN.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 6.Au AK, Steinberg D, Thom C, Shirazi M, Papanagnou D, Ku BS, et al. Ultrasound measurement of inferior vena cava collapse predicts propofol-induced hypotension. Am J Emerg Med. 2016;34:1125–8. doi: 10.1016/j.ajem.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 7.Finnerty NM, Panchal AR, Boulger C, Vira A, Bischof JJ, Amick C, et al. Inferior vena cava measurement with ultrasound: What is the best view and best mode.? West J Emerg Med. 2017;18:496–501. doi: 10.5811/westjem.2016.12.32489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan JM, Ronan A, Goonewardena S, Blair JE, Hammes M, Shah D, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749–53. doi: 10.2215/CJN.00310106. [DOI] [PubMed] [Google Scholar]
- 9.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Bortolotti P, Colling D, Colas V, Voisin B, Dewavrin F, Poissy J, et al. Respiratory changes of the inferior vena cava diameter predict fluid responsiveness in spontaneously breathing patients with cardiac arrhythmias. Ann Intensive Care. 2018;8:79. doi: 10.1186/s13613-018-0427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaydu A, Gokcek E. Preoperative and postoperative assessment of ultrasonographic measurement of inferior vena cava: A prospective, observational study. J Clin Med. 2018;7:145. doi: 10.3390/jcm7060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: Literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107:213–20. doi: 10.1097/01.anes.0000270724.40897.8e. [DOI] [PubMed] [Google Scholar]
- 13.Szabó M, Bozó A, Darvas K, Horváth A, Iványi ZD. Role of inferior vena cava collapsibility index in the prediction of hypotension associated with general anesthesia: an observational study. BMC Anesthesiol. 2019;19:139. doi: 10.1186/s12871-019-0809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salama ER, Elkashlan M. Preoperative ultrasonographic evaluation of inferior vena cava collapsibility index and caval aorta index as new predictors for hypotension after induction of spinal anaesthesia: A prospective observational study. Eur J Anaesthesiol. 2019;36:297–302. doi: 10.1097/EJA.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 15.Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16:R188. doi: 10.1186/cc11672. [DOI] [PMC free article] [PubMed] [Google Scholar]


