Abstract
Background and Aim:
To assess the quality and effectiveness of postoperative pain relief after fast-tracking tracheal extubation in cardiac surgery intensive care unit, effected by a single-shot modified parasternal intercostal nerve block compared with routine in-hospital analgesic protocol, when administered before sternotomy.
Design:
A prospective, randomized, double-blinded interventional study.
Setting:
Single-center tertiary teaching hospital.
Participants:
Ninety adult patients undergoing elective coronary artery bypass grafting surgery under cardiopulmonary bypass.
Materials and Methods:
Patients were randomized into two groups. Patients in the parasternal intercostal block group (PIB) (n = 45) received ultrasound-guided modified parasternal intercostal nerve block with 0.5% levobupivacaine after anesthesia induction at 2nd–6th intercostal space along postinduction using standardized anesthesia drugs with routine postoperative hospital analgesic protocol with intravenous morphine. Patients in the group following routine hospital analgesia protocol (HAP) (n = 45) served as controls, with standardized anesthesia drugs and routine hospital postoperative analgesic protocol with intravenous morphine. The primary study outcome aimed to evaluate pain at rest and when doing deep breathing exercises with spirometry, coughing expectorations using a 11-point numerical rating scale.
Results:
The postoperative pain score at rest and during breathing exercises was compared between the two groups at different time durations (15 min after extubation and every 4th hourly for 24 h). Patients in the PIB group had significantly lower pain scores and better quality of analgesia during the entire study period at rest and during breathing exercise (P < 0.0001). Furthermore, the side effect profile and need of rescue analgesics were better in the PIB group than the HAP group at different time intervals.
Conclusion:
PIB is safe for presternotomy administration and provided significant quality of pain relief postoperatively, as seen after tracheal extubation for a period of 24 h, on rest as well as with deep breathing, coughing, and chest physiotherapy exercises when compared to intravenous morphine alone after sternotomy. This study further emphasizes the role of preemptive analgesia in mitigating postoperative sternotomy pain and it's role as a plausible safe analgesic adjunct facilitating fast tracking with sternotomies on systemic heparinization.
Keywords: Modified parasternal block, postoperative pain mitigation, poststernotomy pain, preemptive analgesia, regional analgesia for systemic heparinization, regional blocks for fast tracking, ultrasound guided
INTRODUCTION
Fear of uncontrolled pain is among the primary concerns of many patients who are about to undergo cardiac surgery. Pain affects patients physiological and psychological recovery.[1] An aggressive implementation of well-planned pain management strategy is thereby crucial for decreasing poststernotomy pain and the resultant morbidity and mortality in cardiac surgeries.[2] In recent years, a multimodal approach utilizing combined regional and systemic analgesics with different mechanisms of action has been found to be promising and beneficial in treating acute and chronic pain after cardiac surgery.[3] Preemptive analgesia regional assumes a critical role in blunting the surgical damage induced stimulus modification in both peripheral and central nervous systems.[4,5] Hence, we designed a randomized controlled trial with a primary objective to assess the quality of pain relief with preemptive analgesia technique from a single-shot modified parasternal intercostal block (PIB) administered before sternotomy and its role in fast tracking after cardiac surgery. Secondary objectives of the study were to assess the need of rescue analgesics, duration of mechanical ventilation, and the side effects in the postoperative period.
MATERIALS AND METHODS
After obtaining approval from the institutional ethics committee (IEC No: SCT/IEC/1267/2018) and registering with Clinical Trial Registry of India (CTRI REF/2019/07/027397), this prospective randomized controlled trial was conducted. This trial complies with the CONSORT 2011 statement guidelines. After obtaining written informed consent, patients undergoing elective coronary artery bypass surgery (CABG) with normal left ventricular function candidates for fast tracking were recruited for the study by the principal investigator during preanesthetic evaluation. Exclusion criteria were (1) hemodynamically unstable patients, (2) patients on preoperative inotropic support or intra-arterial balloon pump, (3) emergency surgery, (4) redo surgery, (5) left ventricular ejection fraction <35%, (6) liver or kidney disease, (7) allergy to local anesthetics, and (8) candidates requiring reexplorative surgeries after shifting to postoperative care.
In the operation room, during the induction of general anesthesia as per the institute protocol, study participants were randomly assigned to one of the two groups using a computer-generated random number table: (1) PIB group (n = 45): patients receiving ultrasound-guided modified PIB with 0.5% levobupivacaine and intravenous infusion of morphine at 60 μg.kg−1.h−1 in the intraoperative period and (2) hospital analgesia protocol (HAP) group (n = 45): patients receiving in-hospital analgesic protocol of intravenous morphine at 60 μg.kg−1.h−1 during the intraoperative period and both the groups received morphine infusions at 20 μg.kg−1.h−1 in the postoperative period. Placebo-controlled group with block using normal saline as intervention was not approved by the Technical Advisory Committee and Institutional Ethics Committee.
Modified parasternal intercostal block
The PIB was performed under ultrasound guidance using a linear probe of 6–12 MHz (Esaote MyLab One, Ginova, Italy). The intercostal muscles, pleura, and internal thoracic vessels were identified at 3–4 cm from the midline along the lower border of the rib. Under real-time ultrasound guidance [Figure 1a and b and Video 1], 1.5 ml of 0.5% levobupivacaine was injected below the internal intercostal muscles to block the terminal anterior branch of the intercostal nerve and allow the drug to spread into the perivascular nerve plexus. Spread of the local anesthetic is identified by the separation of the fascial plane and depression of pleura. T2 to T6 intercostal nerves were blocked bilaterally on either side of the sternum. Furthermore, 3 ml of 0.5% levobupivacaine was infiltrated above the manubrium sternum to block the superficial sensory nerves from T1 and supraclavicular nerve.
Figure 1.
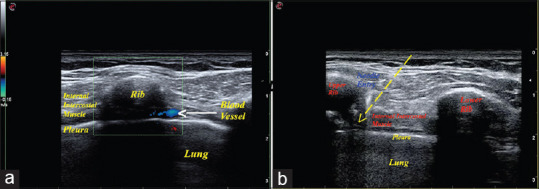
(a) Ultrasound image of the parasternal intercostal space using color Doppler shows to avoid the blood vessel at the lower border of the rib and (b) ultrasound image showing the needle entry into the lower border of the rib for parasternal intercostal nerve block administration
After the completion of surgical procedure, patients were transferred to the intensive care unit. Patients were weaned off from ventilator and the trachea was extubated when the extubation criteria were met. Physiotherapy and incentive spirometry were given to all patients and pain scores were noted when performing deep breathing, coughing–expectoration, and incentive spirometry exercises. When the documented numerical rating scale (NRS) pain score was more than 3 or when the patient demanded extra analgesia intravenous tramadol 100 mg was given. Side effects such as nausea, vomiting, pruritus, excessive sedation (Ramsay sedation scale >3), and respiratory depression were monitored and treated accordingly. The following parameters were noted: (a) intraoperative period – total fentanyl consumption and (b) postoperative period – (1) NRS pain score at rest (15 min posttracheal extubation and every 4th hourly for the next 24 h) and during breathing exercises, coughing, and expectoration (4th h after tracheal extubation and every 4th hourly till 24 h), (2) duration of mechanical ventilation, (3) rescue analgesia, and (4) incidence of side effects. The study participants, data entry anesthesiologist–intensivist, data collectors, and the outcome assessor were blinded to the study groups.
Statistical analysis
The sample size was calculated based on a primary outcome variable of pain scores using NRS from previous studies.[6] A sample size of 45 patients in each group was required to detect a 20% difference in the mean NRS pain scores between the two groups to achieve 80% power and type I alpha error of 0.05. Numerical data were expressed as mean ± standard deviation or median with interquartile range and categorical data as frequencies and percentage. Comparison of categorical variables was tested using the Chi-square test. Continuous data between the two groups were compared using unpaired Student's t-test or the Mann–Whitney U-test. The results were considered statistically significant if P < 0.05. Statistical Package for the Social Sciences (SPSS, Version 21.0; SPSS Inc., IBM, Chicago, IL, USA) was used to analyze the data.
RESULTS
Ninety patients, 45 in each group, were included in the study during the period of 8 months. Demographic profile and duration of surgery were comparable between the two groups [Table 1]. Both the groups had equal distribution of male and female patients. Analyzing the consumption of analgesics, the intraoperative fentanyl consumption was significantly less in the PIB group than the HAP group, whereas there was no significant difference in the duration of mechanical ventilation between the two groups [Table 1].
Table 1.
The demographic profile, total intraoperative fentanyl consumption, and duration of mechanical ventilation of patients in both groups
| PIB group (n=45) | HAP group (n=45) | P | |
|---|---|---|---|
| Age (years) | 59.9±9 | 59.6±7.5 | 0.8491 |
| Weight (kg) | 67.7±10 | 67.3±10.9 | 0.8654 |
| Height (cm) | 166.7±7.4 | 165.6±7.5 | 0.4722 |
| Total fentanyl consumption (mg) | 675.6±188.8 | 976.7±169.1 | <0.0001 |
| Duration of mechanical ventilation (hours) | 5.38±0.43 | 5.54±0.40 | 0.069 |
HAP=Hospital analgesia protocol, PIB=Parasternal intercostal block
The NRS pain scores at rest and during breathing exercises, coughing, and expectorations were significantly less in the PIB group than the HAP group at different time points in the first postoperative day [Figures 2 and 3]. The pain scores were not severe at different time intervals in both the groups. The need of rescue analgesic was significantly lower in the PIB group than the HAP group except at the end of 24th h where rescue analgesic needs showed no statistically significant difference and was comparable between the two groups [Table 2]. The incidence of nausea, vomiting, pruritus, and excessive sedation was significantly higher in the HAP group than the PIB group [Table 3]. None of the patients in both the groups had respiratory depression.
Figure 2.
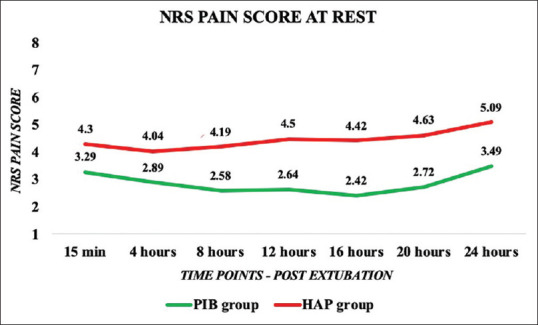
Comparison of numerical rating scale pain score at rest between parasternal intercostal block group and hospital analgesic protocol group at different time points
Figure 3.
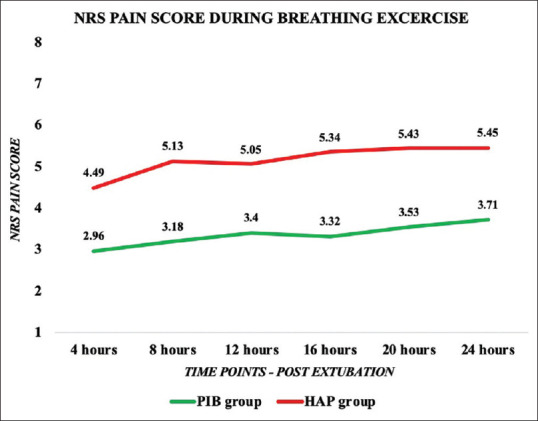
Comparison of numerical rating scale pain score during breathing exercise between parasternal intercostal block group and hospital analgesic protocol group at different time points
Table 2.
Comparison of need of rescue analgesics between parasternal intercostal block group and hospital analgesic protocol group at different time points
| Time (postextubation) | PIB group (n=45), n (%) | HAP group (n=45), n (%) | P |
|---|---|---|---|
| 15 min | 0 (0) | 4 (8.8) | 0.0420 |
| 4 ho | 1 (2.2) | 8 (17.8) | 0.0142 |
| 8 ho | 2 (4.4) | 10 (22.2) | 0.0134 |
| 12 h | 2 (4.4) | 8 (17.8) | 0.0442 |
| 16 h | 1 (2.2) | 9 (20) | 0.0075 |
| 20 h | 2 (4.4) | 8 (17.8) | 0.0442 |
| 24 h | 4 (8.8) | 8 (17.8) | 0.2112 |
PIB=Parasternal intercostal block, HAP=Hospital analgesia protocol
Table 3.
Comparison of side effect profile parasternal intercostal block group and hospital analgesic protocol group at different time points
| Side effects | PIB group, n (%) | HAP group, n (%) | P |
|---|---|---|---|
| Nausea | 2 (4.44) | 10 (22.2) | 0.0137 |
| Vomiting | 1 (2.22) | 8 (17.7) | 0.0148 |
| Pruritis | 3 (6.66) | 13 (28.8) | 0.0062 |
| Excessive sedation (Ramsay sedation scale >3) | 2 (4.44) | 11 (24.4) | 0.0074 |
PIB=Parasternal intercostal block, HAP=Hospital analgesia protocol
DISCUSSION
Pain following sternotomy is described as very severe and the analgesia management of such postoperative situation is predominantly challenging.[1] Opioids as a sole postoperative analgesia has its own drawbacks such as nausea, vomiting, respiratory depression, and poor control of breakthrough pain.[7] Regional anesthesia techniques involving intrathecal and epidural local anesthetics with adjuvants carry huge risk of hematoma formation in anticoagulated patients, dural CSF leaks, hypotension, and high failure rates.[8] To overcome these disadvantages of neuraxial techniques, recently, various interfascial blocks such as pectoral nerve block, serratus anterior plane block, parasternal block, transversus thoracic muscle plane block, intercostal nerve block, and erector spinae block have been described.[9,10,11]
Parasternal intercostal nerve blocks with local anesthetic have traditionally been practiced in cardiac surgeries as a single-shot local anesthetic infiltration in each intercostal space under vision by the surgeon before sternal closure.[6] This technique has been studied widely to provide superior analgesia than the conventional opioid analgesic regimen and can be administered even in anticoagulated patients.[12] The limitations were about the concerns of confounding effect produced by the general anesthesia drugs, intraoperative opioids, and activation of local incisional inflammatory responses, which could have masked the benefits and results under various clinical studies. We chose to investigate the concept of preemptive analgesia by administering the parasternal intercostal nerve block before the surgical incision and evaluated the quality of intraoperative and postoperative analgesia in patients undergoing CABG surgery.
In our study, patients who received intercostal nerve block had significantly lower intraoperative fentanyl requirement and postoperative mean pain scores (NRS < 4) at rest and during breathing exercise in the entire study period. This demonstrates that patients in the PIB group had better quality of analgesia than the HAP group, supporting the beneficial effect of this preemptive analgesic strategy. The analgesic efficacy has extended beyond the intended duration of pharmacological profile of local anesthetic. The results of our study are consistent with the similar studies by McDonald et al. and Barr et al.[6,13]
Ultrasound-guided parasternal intercostal nerve block was proposed by Thomas et al. as a method of analgesia in a case of sternal fracture.[14] We modified and implemented this technique in our study by considering the extent of sternotomy incision and left internal mammary artery graft for CABG surgeries. Our needle entry point was 3–4 cm away from sternum, and the needle progression was done under real-time ultrasound guidance utilizing the color Doppler. No adverse event of vascular injury or hematoma formation, or any damage to internal mammary artery, was documented intraoperatively during the course of our study in both the groups.
In our study, patients in the HAP group required rescue analgesia within 4 h of tracheal extubation. There was significant difference in the number of patients requiring rescue analgesia between the two groups at different time intervals except at the end of 24th h posttracheal extubation period. Patients in the PIB group were more comfortable and additional analgesics were less taken when offered, showing improved quality of postoperative analgesia. This may due to breaking the pain cycle early by the preemptive analgesia technique with the parasternal intercostal nerve block before the surgical incision.[15]
Main disadvantages of sole opioid-based analgesic are the incidence of side effects such as nausea, vomiting, pruritis, and respiratory depression.[16,17] The incidence of side effects was high in the HAP who received higher dose of opioids when compared to the PIB group. Patients in the PIB group had adequate and arousable level of sedation with only two cases of oversedation. The difference in the incidence of side effects may be due to the agonistic properties of morphine against μ opioid receptors.[18,19]
There is a limitation with this study, as it was not a placebo-controlled study. Giving intercostal block with placebo (normal saline) in the control group was not considered by Technical advisory committee and not permitted by Institutional Ethics, because all the pain assessment was being followed up in postoperative Intensive Care Unit after fast tracking and no study documentation was being done in any point of time intraoperatively. We have not followed up the patients postdischarge from hospital and also no data was collected regarding the complete analgesic efficacy of both groups on long-term analgesic outcome and prevalence of chronic pain conditions arising in this settings. This is because we thought that the preemptive PIB may not be effective for more than 24 h.
Further research studies on preemptive PIB with large sample size and longer follow-up period should be done, so that analgesia technique with high efficacy and less side effect profile can be safely provided for poststernotomy pain in patients undergoing cardiac surgeries. Future trails should take much more detailed consideration of the pulmonary morbidity and outcome with respect to better postoperative analgesia on sternotomy with systemic heparinization.
CONCLUSION
PIB is safe for presternotomy administration and provided significant quality of pain relief postoperatively, as seen after tracheal extubation for a period of 24 h, on rest as well as with deep breathing, coughing, and chest physiotherapy exercises when compared to intravenous morphine alone after sternotomy. This emphasizes the role of preemptive analgesia and its importance with fast tracking in cardiac surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.aeronline.org
REFERENCES
- 1.Kianfar A, Shadvar K, Mahoori A, Azarfarin R. Pain after cardiac surgery. Crit Care. 2007;11:429. [Google Scholar]
- 2.Ferguson J, Gilroy D, Puntillo K. Dimensions of pain and analgesic administration associated with coronary artery bypass grafting in an Australian intensive care unit. J Adv Nurs. 1997;26:1065–72. doi: 10.1111/j.1365-2648.1997.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 3.Ziyaeifard M, Azarfarin R, Golzari SE. A review of current analgesic techniques in cardiac surgery? Is epidural worth it. J Cardiovasc Thorac Res. 2014;6:133–40. doi: 10.15171/jcvtr.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaney MA. How important is postoperative pain after cardiac surgery? J Cardiothorac Vasc Anesth. 2005;19:705–7. doi: 10.1053/j.jvca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Mittnacht AJC, Shariat A, Weiner MM, Malhotra A, Miller MA, Mahajan A, et al. Regional techniques for cardiac and cardiac-related procedures. J Cardiothorac Vasc Anesth. 2019;33:532–46. doi: 10.1053/j.jvca.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Barr AM, Tutungi E, Almeida AA. Parasternal intercostal block with ropivacaine for pain management after cardiac surgery: A double-blind, randomized, controlled trial. J Cardiothorac Vasc Anesth. 2007;21:547–53. doi: 10.1053/j.jvca.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Kwanten LE, O'Brien B, Anwar S. Opioid-based anesthesia and analgesia for adult cardiac surgery: History and narrative review of the literature. J Cardiothorac Vasc Anesth. 2019;33:808–16. doi: 10.1053/j.jvca.2018.05.053. [DOI] [PubMed] [Google Scholar]
- 8.Chaney MA. Intrathecal and epidural anesthesia and analgesia for cardiac surgery. Anesth Analg. 2006;102:45–64. doi: 10.1213/01.ane.0000183650.16038.f6. [DOI] [PubMed] [Google Scholar]
- 9.Ueshima H, Hara E, Marui T, Otake H. The ultrasound-guided transversus thoracic muscle plane block is effective for the median sternotomy. J Clin Anesth. 2016;29:83. doi: 10.1016/j.jclinane.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 11.Perttunen K, Nilsson E, Heinonen J, Hirvisalo EL, Salo JA, Kalso E. Extradural, paravertebral and intercostal nerve blocks for post-thoracotomy pain. Br J Anaesth. 1995;75:541–7. doi: 10.1093/bja/75.5.541. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhary V, Chauhan S, Choudhury M, Kiran U, Vasdev S, Talwar S. Parasternal intercostal block with ropivacaine for postoperative analgesia in pediatric patients undergoing cardiac surgery: A double-blind, randomized, controlled study. J Cardiothorac Vasc Anesth. 2012;26:439–42. doi: 10.1053/j.jvca.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 13.McDonald SB, Jacobsohn E, Kopacz DJ, Desphande S, Helman JD, Salinas F, et al. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: The effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg. 2005;100:25–32. doi: 10.1213/01.ANE.0000139652.84897.BD. [DOI] [PubMed] [Google Scholar]
- 14.Thomas KP, Sainudeen S, Jose S, Nadhari MY, Macaire PB. Ultrasound-guided parasternal block allows optimal pain relief and ventilation improvement after a sternal fracture. Pain Ther. 2016;5:115–22. doi: 10.1007/s40122-016-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: A meta-analysis. Anesth Analg. 2005;100:757–73. doi: 10.1213/01.ANE.0000144428.98767.0E. table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–84. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Paqueron X, Lumbroso A, Mergoni P, Aubrun F, Langeron O, Coriat P, et al. Is morphine-induced sedation synonymous with analgesia during intravenous morphine titration? Br J Anaesth. 2002;89:697–701. [PubMed] [Google Scholar]
- 18.Young-McCaughan S, Miaskowski C. Definition of and mechanism for opioid-induced sedation. Pain Manag Nurs. 2001;2:84–97. doi: 10.1053/jpmn.2001.25012. [DOI] [PubMed] [Google Scholar]
- 19.Aubrun F, Mazoit JX, Riou B. Postoperative intravenous morphine titration. Br J Anaesth. 2012;108:193–201. doi: 10.1093/bja/aer458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


