Abstract
Background:
Laparoscopic cholecystectomy (LC) is associated with moderate-to-severe pain in immediate postoperative period. Some patients even suffer from prolonged pain long after surgery.
Aims:
The aim of present study is to determine the efficacy of ultrasound-guided bilateral erector spinae plane block (ESPB) in patients undergoing LC, time to ambulation after surgery, and incidence of prolonged pain up to 6 months later.
Settings and Design:
This was a double-blinded prospective randomized controlled trial.
Materials and Methods:
Eighty-five adults posted for elective LC were randomized to receive bilateral ESPB at T7 level with either 20 mL of 0.375% ropivacaine or 20 mL normal saline. Postoperative static and dynamic pain score as per the visual analog scale (VAS), intraoperative requirement of fentanyl, postoperative use of diclofenac, time to ambulation after surgery, and presence of any pain after surgery were noted.
Statistical Analysis:
Independent t-test and Mann–Whitney U-test were used for quantitative data, while Chi-square test was used for comparing qualitative data.
Results:
Static and dynamic VAS scores were significantly lower in ESPB group (P < 0.05). Intraoperative fentanyl requirement (165 ± 30.72 – ESPB, 180.95 ± 29.12 – controls, P = 0.020) and number of patients requiring diclofenac (28/42 – ESPB, 37/42 – controls, P = 0.019) were lower, while number of patients ambulating by 4 hours (20/42 – ESPB, 9/42 – control, P = 0.012) were higher in ESPB group. Patients suffering from pain at 1 week (22/42 – ESPB and 34/42 – control, P = 0.005) and 1 month (9/42 – ESPB and 13/42 – control, P = 0.207) were lower in ESPB group.
Conclusion:
ESPB provides effective analgesia and early ambulation after LC. The benefit extends to 1 week thereafter.
Keywords: Ambulation, chronic pain, erector spinae plane block, laparoscopic cholecystectomy
INTRODUCTION
Laparoscopic cholecystectomy (LC) is a gold standard minimally invasive technique in the management of cholelithiasis. Pain continues to be the most common medical causes of delayed discharge (17%–40%) or readmissions.[1] Few patients may also develop prolonged or chronic pain following the surgery.[2]
Various modalities of analgesia have been advocated for postoperative pain. Patient-controlled analgesia with opioids, port-site infiltration, intraperitoneal instillation with local anesthetics (LA), transversus abdominis plane (TAP) block,[3] and oblique subcostal transversus abdominis plane (OSTAP) block, paravertebral block have been tried.[4] Except paravertebral block, these techniques affect only somatic pain and can be inadequate in some cases.[2] Thus, the quest for a better analgesic modality is ongoing.
Erector spinae plane block (ESPB) was described in 2016[5] as a novel truncal interfascial regional analgesia technique which affects both the ventral and dorsal rami, thus leading to blockage of both visceral and somatic pain. A few randomized controlled trials (RCTs)[6,7,8] have shown ultrasound guided (USG) ESPB to be effective in reducing postoperative pain in patients undergoing LC. None of the RCTs however have been conducted in the Indian subcontinent. Hence, we decided to determine the efficacy of ESPB in Indian patients undergoing LC. In addition to the pain scores in immediate postoperative period, this study also intends to see the effect of block on requirement of rescue analgesics, time to ambulation after surgery, and incidence of prolonged pain up to 6 months later.
MATERIALS AND METHODS
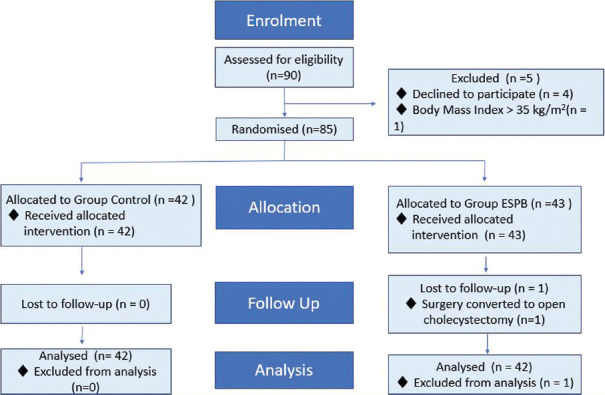
We present a double-blinded, prospective RCT conducted from May 2019 to February 2020 in a tertiary care center after Institutional Ethics Committee approval (IEC: 2018-215-IP-107; Dated: March 8, 2019) and registering the trial in Clinical Trial Registry of India (ctri.nic.in; Registration No.: CTRI/2019/05/019184; Registered on: 16/05/2019). Written informed consent was obtained from all participants. CONSORT checklist was used for enrollment and allocation of patients [Figure 1].
Figure 1.
Consort flow chart
Patients aged 18–60 years and belonging to the American Society of Anesthesiologists (ASA) physical status Classes I and II listed for elective LC were included in the study. Exclusion criteria included patient refusal, morbid obesity (Body mass index [BMI] >35 Kg.sqm −1), any lesion obscuring sonoanatomy, allergies to LA, bleeding diathesis, use of anticoagulants, and psychiatric disorders.
Patients included were randomly assigned into two groups to avoid patient selection bias. Randomization was done by computer-generated random numbers and sealed envelope technique. ESPB Group received ESPB with 20 mL of 0.375% ropivacaine on either side and control group received 20 mL normal saline (NS) in the same plane. On the day of surgery, an anesthesiologist not participating in the study opened the envelope and prepared either two 20 mL syringes of 0.375% ropivacaine or two 20 mL syringes of NS accordingly. The drugs, prepared in identical syringes maintaining full sterile precautions, were labeled “study drug” and placed on the sterilized trolley to be used for performance of the block. The patient, anesthesiologist performing ESPB, and outcome assessor were unaware of the group allocation and drug given to achieve double blinding.
The night before LC, patients enrolled were explained the method of assessment of postoperative pain using the visual analog scale (VAS) from 0 to 10 where 0 is no pain at all and 10 is the worst possible pain. A standard balanced general anesthesia (GA) was administered with propofol (1–2 mg.kg−1 titrated to effect), fentanyl (2 μg.kg−1), and vecuronium 0.1 mg.kg−1. It was maintained with sevoflurane in oxygen and air mixture.
Performance of block
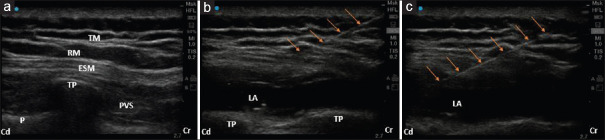
After the administration of GA, patients were turned to the right lateral position. Under aseptic precautions, a 6–13 MHz linear ultrasound transducer (Edge II, Fujifilm Sonosite, Inc., Bothell, WA 98021, USA) was placed longitudinally at the level of T7 spinous process and then moved 3 cm para-sagittally. The US landmarks, T7 transverse process and overlying erector spinae, rhomboid, and trapezius muscles were identified. A 45 mm 18G intravenous (i.v.) cannula stylet (BD Venflon™, Franklin Lakes, NJ USA 07417) was inserted in-plane, in cranial-to-caudal direction until the tip contacted the T7 transverse process. After careful aspiration to exclude vascular puncture, hydrodissection with 2 mL of saline solution was done to confirm the correct needle tip position. Under real-time ultrasonography, 20 mL prefilled “study drug” was injected lifting the erector spinae muscle off the bony shadow of the transverse process [Figure 2]. The same procedure was repeated on the contralateral side.
Figure 2.
Ultrasonographic images of erector spinae plane block. (a) Sonoanatomy before performing the block. (b) During the block. (c) After completion of the block with needle in situ. TP = Transverse process, ESM = Erector spinae muscle, RM = Rhomboid muscle, TM = Trapezius muscle, PVS = Paravertebral space, LA = Local anesthetic, P = Pleura, Cr = Cranial, Cd = Caudal, Arrows = Showing needle trajectory
The patients were then turned supine and a conventional four-port LC was performed. Intrabdominal pressures were kept ≤12 mmHg. One gram i.v. paracetamol was given preemptively before incision. 1 μg.kg −1 i.v. fentanyl was administered 5 min before the first port insertion, and then, 0.5 μg.kg −1 was repeated as and when required. After completion of surgery, 8 mg dexamethasone and 4 mg ondansetron were given to prevent postoperative nausea and vomiting (PONV). Neuromuscular blockade was reversed, trachea extubated when appropriate criteria were met and the patient was transferred to the recovery room. Paracetamol (1 g) i.v. was continued 8 hourly for next 24 hours. Aqueous diclofenac 75 mg i.v. was used as rescue analgesic given by paramedics on demand of patients.
Outcome assessment
The primary outcome measured was postoperative pain, as assessed by VAS scores, from 0 to 10, at rest (static pain) and on coughing (dynamic pain). The time points on which this outcome was assessed were 0 minutes, that is on arrival in recovery room and then at 1, 6, 12, 24 and 48 hours.
The secondary outcomes measured were intraoperative fentanyl consumption, presence of PONV, time to ambulation (able to walk with or without support), and total need for rescue analgesic in the first 24 hours after surgery. The presence of any pain (at the site of incision, generalized or localized abdominal or shoulder pain) was inquired and the average intensity of cumulative pain was asked on verbal rating scale (VRS) of 0–10 through telephonic conversation at 1 week. The presence or absence of any component of pain[2] (incision site, abdominal, or shoulder pain) at 1, 3, and 6 months postoperatively was also asked telephonically.
Sample size estimation
The sample size was calculated based on data from a previous study.[6] We considered a reduction in pain score by 2 or more in treatment group as compared to control, to be statistically significant, with a pooled standard deviation of 1.76 (effect size = 1.136). At 99.9% power of study and minimum two-sided 95% confidence interval, estimated sample size for each of the two groups came out to be 41. Finally, in this study, we have analyzed 42 patients in each of the two groups. The sample size was estimated using software Power analysis and sample size version-16 (PASS-16, NCSS, LLC, USA).
Statistical analysis
Normality of the continuous variables was assessed. Data were considered normally distributed when Z-score of the skewness was within ± 3.29. Descriptive statistics of continuous variables were presented as mean ± standard deviation or median (interquartile range), whereas categorical data in frequency (%). Independent sample t-test was used to compare the means, whereas Mann–Whitney U-test was used to compare the nonnormal continuous variables between the two groups. Friedman test was used for repeated measure analysis of VAS pain score from baseline to 48 h. Multiple comparisons with Bonferroni correction were done (when Friedman test had P < 0.05). Box plots were drawn to present the median, minimum, maximum, first, and third quartiles of the VAS score data. Chi-square test was used to test the association between categorical variables. P < 0.05 was considered as statistically significant. Statistical Package for the Social Sciences (SPSS) version-23 (SPSS-23, IBM, Chicago, IL, USA) and MedCalc (MedCalc Software Ltd, Ostend, Belgium) softwares were used for the data analysis.
RESULTS
Ninety patients were assessed for enrollment. Five were excluded as four refused to participate and one was obese. The remaining 85 patients were randomized into two groups (43 in ESPB group; 42 in control group) and received interventions accordingly. Surgical plan was changed to open cholecystectomy in one patient. Subsequently, 84 patients (42 in each group) completed the study and were analyzed [Figure 1].
Both the groups were comparable with respect to patient demographics (age, BMI, gender, and ASA status) and duration of surgery [Table 1]. All blocks were performed smoothly with no complications as local bleeding, hematoma, subcutaneous emphysema, pneumothorax, or signs of LA toxicity.
Table 1.
Patient demographics and duration of surgery
| Parameter | ESPB group (n=42) | Control group (n=42) | P |
|---|---|---|---|
| Age (years)* | 41.88±6.17 | 43.26±11.35 | 0.491 |
| BMI (kg/m2)* | 26.22±3.98 | 25.55±3.55 | 0.428 |
| Gender (male/female)† | 10/32 | 16/26 | 0.157 |
| ASA status (I/II)† | 29/13 | 24/18 | 0.258 |
| Duration of surgery (min)* | 89.17±27.20 | 99.38±26.56 | 0.085 |
P<0.05 is significant. Data presented as mean±SD or number of patients. *Independent Student’s t-test or †Chi-square test/Fischer’s exact test were used as appropriate. BMI=Body mass index, ASA=American Society of Anesthesiologists, ESPB=Erector spinae plane block, SD=Standard deviation
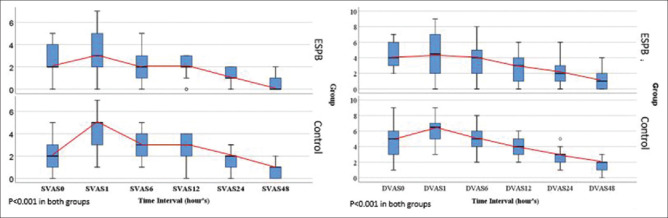
Static and dynamic VAS scores at 0 hour were similar in both the groups. Thereafter, ESPB group had significantly less static pain at 1, 6, 12, and 24 hours. Dynamic pain was significantly lower at 1, 6, 12, 24, and 48 hours. Friedman test was used to assess change in pain score in repeated observations. VAS showed significant (each P < 0.001) decreasing trend over the time in ESPB and control group for both static and dynamic pain. Multiple comparisons (using Bonferroni correction) showed that pain score was significantly reduced in static pain (ESPB: Between 0 hour and 48 hours and control group: 0 hour and 1 hour, 48 hour) and in dynamic pain (ESPB: Between 0 hour and 12 hours, 24 hours, and 48 hours and control group: Between 0 hour and 1 hour, 24 hours, and 48 hours). For the rest of other paired groups, differences were statistically insignificant [Tables 2, 3 and Figure 3].
Table 2.
Median static visual analog scale scores at different time points
| Time points | Median (IQR) | P* | |
|---|---|---|---|
| ESPB group (n=42) | Control group (n=42) | ||
| At 0 h | 2 (1.75-4.00) | 2 (1.00-3.00) | 0.622 |
| At 1 h | 3 (2.00-5.00) | 5 (3.00-5.00) | 0.019 |
| At 6 h | 2 (1.00-3.00) | 3 (2.00-4.00) | 0.025 |
| At 12 h | 2 (1.75-3.00) | 3 (1.75-4.00) | 0.031 |
| At 24 h | 1 (1.00-2.00) | 2 (1.00-2.00) | 0.025 |
| At 48 h | 0 (0.00-1.00) | 1 (0.00-1.00) | 0.059 |
| Friedman test (P) | <0.001 | <0.001 | |
| Multiple comparisons† (P<0.05) | Between 0 h and 48 h | Between 0 h and 1 h and 48 h | |
P<0.05 is significant. Data presented as median (IQR). *Mann– Whitney U-test used, †Friedman test with Bonferroni correction used. ESPB=Erector spinae plane block, IQR=Interquartile range
Table 3.
Median dynamic visual analog scale score at different time points
| Time points | Median (IQR) | P* | |
|---|---|---|---|
| ESPB group (n=42) | Control group (n=42) | ||
| At 0 h | 4 (3.00-6.00) | 5 (3.00-6.00) | 0.188 |
| At 1 h | 4.5 (2.00-7.00) | 6.5 (5.00-7.00) | 0.014 |
| At 6 h | 4 (2.00-5.25) | 5 (4.00-6.00) | 0.024 |
| At 12 h | 3 (1.00-4.25) | 4 (3.00-5.00) | 0.015 |
| At 24 h | 2 (0.75-3.00) | 3 (2.00-3.00) | 0.023 |
| At 48 h | 1 (0.00-2.00) | 2 (1.00-2.00) | 0.025 |
| Friedman test (P) | <0.001 | <0.001 | |
| Multiple comparisons† (P<0.05) | Between 0 h and 12 h, 24 h, and 48 h | Between 0 h and 1 h, 24 h, and 48 h | |
P<0.05 is significant. Data presented as median (IQR). *Mann– Whitney U-test used, †Friedman test with Bonferroni correction used. ESPB=Erector spinae plane block, IQR=Interquartile range
Figure 3.
Trend of the static and dynamic pain score at various time points. Median visual analog scale scores at rest (SVAS) and on coughing (DVAS) for erector spinae plane block and control group at different time points. Repeated measures analysis done by Friedman test. In ESPB group all values of SVAS and DVAS (except at 1 hour) were less than or equal to clinically significant value of 4. SVAS = Static VAS score, DVAS = Dynamic VAS score
Intraoperative fentanyl requirement was significantly lower in patients receiving ESPB. The mean difference between ESPB and control group was − 15.476 (95% confidence interval: −28.46–−2.48). Postoperatively, the number of patients requiring diclofenac was significantly more in controls than in ESPB group, 37 and 28, respectively (P = 0.019). Total diclofenac consumption, though more in controls, was not significant. There was no difference observed in PONV [Table 4].
Table 4.
Intraoperative and postoperative analgesic requirements and postoperative nausea and vomiting
| Parameter | ESPB group (n=42) | Control group (n=42) | P |
|---|---|---|---|
| Intraoperative fentanyl consumption (µg)* | 165.47±30.72 | 180.95±29.12 | 0.02 |
| Diclofenac required† (yes/no) | 28/14 | 37/5 | 0.019 |
| Postoperative diclofenac consumption (mg)‡ | 75 (0.00-150.00) | 75 (75.00-150.00) | 0.074 |
| PONV (yes/no)† | 3/39 | 2/40 | 0.645 |
P<0.05 is significant. *Data presented as mean±SD, compared by independent Student’s t-test, †Data presented as frequency and compared by Chi-square test/Fisher exact test, ‡Data presented as median (IQR) compared by Mann–Whitney U-test. SD=Standard deviation, IQR=Interquartile range, PONV=Postoperative nausea and vomiting, ESPB=Erector spinae plane block
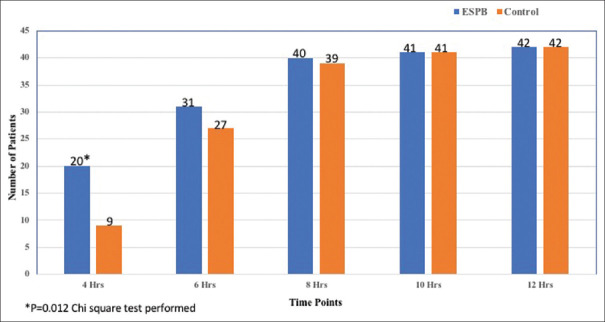
Twenty patients in ESPB group were able to ambulate within 4 hours in contrast to nine in the control group (P = 0.012). By 8 hours, 95.2% (40/42) in ESPB group and 92.8% (39/42) in controls were mobilizing. By 10 hours, 41 patients (97.6%) and by 12 hours all patients ambulated without support in both the groups. There was no difference among the groups in frequency of patients ambulating after 4 hours [Figure 4].
Figure 4.
Ambulation at various time points
The incidence of cumulative pain at 1 week was 56/84 (66.66%). Of these, 22 patients had received ESPB and 34 were in control group (P = 0.005). Most patients had mild pain of VRS score <4, but ten patients had pain with VRS ≥4. Among them, three had received the block and seven had placebo (P = 0.510).
At 1 month, nine in ESPB and 13 patients in control had some form of pain (total incidence: 26.19%). The frequency (%) was 3 (3.57%) and 1 (1.19%) at 3 and 6 months, respectively. At 3 months, one patient was from ESPB and two were from control group. One patient of ESPB group had pain at 6 months. The data were not statistically significant on intergroup comparison.
DISCUSSION
The present study shows that patients receiving bilateral single-shot USG-ESPB just before LC had significantly lower static and dynamic VAS from 1st hour after surgery to 24 hours thereafter in comparison to patients in control group. The beneficial effect of ESPB extended to the 1st week postsurgery. The secondary outcomes such as intraoperative requirement of fentanyl and number of patients requiring rescue analgesics postoperatively were also lower in ESPB group. The patients receiving the block ambulated earlier than patients not receiving it.
Inadequate pain relief, apart from being unethical, results in increased morbidity and directly correlates with increased time to discharge.[9] Barazanchi et al.[10] recommended the use of paracetamol or nonsteroidal anti-inflammatory drugs preoperatively and LA infiltration at port sites to curb pain after LC. The other modalities such as TAP[11] and OSTAP block[4] tried so far mostly cover the somatic component and pain from parietal peritoneum, but the visceral pain of dissection of the gallbladder from the liver bed is left uncovered. The paravertebral block affects both somatic and visceral pain[12] but has its own set of disadvantages. The deep-seated injection of drugs makes the block more difficult to perform with increased risk of vascular puncture, hematoma, hypotension, and pneumoperitoneum.[13,14] The ESPB arrived as an effective respite in these circumstances.
ESPB is a relatively superficial block where LA is deposited superficial to transverse process and below the erector spinae muscle. The drug then spreads in all directions. It seeps medially to paravertebral space and is known to block both somatic and visceral sensations. The LA targets the ventral and dorsal rami and rami communicantes of the spinal nerves.[15] This block has been effectively used as a part of multimodal analgesia in patients undergoing LC.[6,7,8,16]
Tulgar et al.[6] were the first to show that bilateral ESPB with 20 mL of 0.375% bupivacaine at the level of T9 transverse process leads to a significant decrease in pain from 20th min to 3 hours postoperatively. Similarly, Altiparmak et al.[7] claimed that bilateral ESPB at T7 level with 20 mL of 0.25% bupivacaine decreased pain significantly at 15 minutes, 30 minutes, 12 hours and 24 hours postoperatively. The volume of drug and level of injection in our study was similar to them. We chose a concentration of 0.375% like Tulgar et al.,[6] but the drug used was ropivacaine instead of bupivacaine because of its enhanced safety profile.[17,18] Our study arrived at the result, that patients receiving ESPB had lower pain scores up to 24 hours postoperatively. This result was similar to that achieved by Altiparmak.7 The dynamic VAS scores were significantly lower till 48 hours after surgery. The difference mentioned was statistically and clinically significant, as the average dynamic VAS scores were >4 in controls from 1 to 12 hours postsurgery. Later, the clinical VAS scores settled in both the groups (VAS decreased to <4) but remained statistically more in controls.
The intraoperative fentanyl requirement was also significantly lower in patients receiving the block. Similar to previous studies,[6,7] the number of patients requiring rescue analgesics in postoperative period was far less in ESPB group than in controls. The amount of rescue analgesic required was also lower, though not statistically significant. The incidence of PONV, much like previous studies, was similar in both the groups.
LC is increasingly been done as day care surgery, and one of the key components of early discharge is ability of a patient to walk without support.[1] Significantly more number of patients were able to ambulate by 4 hours in the ESPB group as compared to controls in our study. The mean time to discharge after day-care LC in a study by Singh et al.[1] was 9 ± 0.72 hours, implying that patients were walking without support by then. Similarly, 94% of our patients had been mobilized by 8 hours and almost all were walking by 10 hours after surgery. Indeed, the application of ESPB helped us to achieve early mobility in a greater number of patients.
The incidence of pain at 1 week was 66.66% in our study. Arora et al.[19] followed 207 Indian patients after LC for 6 months. Their incidence of postcholecystectomy syndrome at 1 week after surgery was 58%, though they did not mention the number of patients having pain alone. Significantly less number of patients (22 as compared to 34 in controls) of ESPB group had pain at 1 week. Despite extensive literature search, we were unable to find any study mentioning the effect of abdominal or fascial plane blocks on pain at 1 week after LC. Ten of 84 patients (11.90%) had overall pain ≥4. Blichfeldt-Eckhardt et al.[2] showed that 9% of patients had moderate-to-severe cumulative pain at 1 week. Variation in surgical and analgesia protocols at different Institutes may be the reason behind the discrepancies in the incidence of pain at 1 week in our study.
The incidence of pain at 1, 3, and 6 months was 26.19%, 3.57%, and 1.19%, respectively. Arora et al.[19] have reported an incidence of 39.1%, 23.2%, and 13.0% of postcholecystectomy syndrome at 1, 3, and 6 months after LC in Indian patients. Again, this was incidence of postcholecystectomy syndrome as a whole which includes nausea, vomiting, bloating, jaundice, diarrhea, or abdominal pain. The incidence of abdominal or overall pain was not mentioned separately. Blichfeldt-Eckhardt et al.[2] had reported the presence of prolonged abdominal pain in 23% and 17% of patients on 3- and 6-month follow-up. Bansal et al.[20] showed that 4% of patients had pain in the right upper quadrant or epigastric area on follow-up to 11 months. Application of ESPB in 50% of our patients may have resulted in lower incidence of pain at 3 and 6 months in our study. However, the study is not adequately powered to definitely comment on this aspect.
The ESPB is not free of side effects. A rare case of pneumothorax on attempting block at thoracic level has been reported.21 Performing the block in lower thoracic or lumbar area may lead to weakness in lower limbs due to the involvement of the lumbar plexus. The large amount of drug used in the bilateral block increases the risk of LA toxicity.[21] Complications such as perioral numbness, dizziness, and transient loss of consciousness have been reported.[15] No such complications were seen in our study.
The study design of double-blinded RCT with a placebo group where NS was instilled in controls at the same site as cases, was the main strength of our study. Till date, only one such RCT[7] is published where a sham group acted as control and the sample size in it was only 21 in each arm, while we had double the size in each group.
The main limitation of our study was that the sample size was calculated on the basis of acute pain scores and this may be a reason of similarity in PONV numbers or absence of shoulder pain in both the groups. The block was performed under GA, so a mapping of dermatomes involved and onset of action of block could not be tested. Therefore, any block failure[21] or patchy blocks[22] might have been missed. The documentation on prolonged pain was limited to the presence or absence of any type of pain. Detailed questions on the nature of pain and follow-up on abdominal examination and findings were not done. This is the reason we have refrained from using the term chronic pain even though the pain was noted till 6 months postsurgery. The current definition of chronic postsurgical pain includes continuation of postoperative pain or development of new-onset pain after a brief asymptomatic period till 3 months after surgery. All other possible causes of pain like infection and malignancy must be ruled out,[23] which was not done in our study.
CONCLUSION
Nevertheless, this study reverberated that single-shot bilateral ESPB with 20 mL of LA effectively reduced the postoperative pain and analgesic requirement in patients undergoing LC. In addition, ESPB also aided early mobilization of patients and improved pain up to 1 week after surgery. Further RCTs are required to focus on beneficial effects of ESPB on postsurgical ambulation and chronic pain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Singh AD, Sood D, Jain A, Kumar A, Sood SH. Day care laproscopic cholecystectomy – A prospective study. IOSR J Dent Med Sci. 2017;16:47–61. [Google Scholar]
- 2.Blichfeldt-Eckhardt MR, Ording H, Andersen C, Licht PB, Toft P. Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain. 2014;155:2400–7. doi: 10.1016/j.pain.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Mitra S, Khandelwal P, Roberts K, Kumar S, Vadivelu N. Pain relief in laparoscopic cholecystectomy – A review of the current options. Pain Pract. 2012;12:485–96. doi: 10.1111/j.1533-2500.2011.00513.x. [DOI] [PubMed] [Google Scholar]
- 4.Suseela I, Anandan K, Aravind A, Kaniyil S. Comparison of ultrasound-guided bilateral subcostal transversus abdominis plane block and port-site infiltration with bupivacaine in laparoscopic cholecystectomy. Indian J Anaesth. 2018;62:497–501. doi: 10.4103/ija.IJA_55_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 6.Tulgar S, Kapakli MS, Senturk O, Selvi O, Serifsoy TE, Ozer Z. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: A prospective, randomized, controlled clinical trial. J Clin Anesth. 2018;49:101–6. doi: 10.1016/j.jclinane.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Altiparmak B, Toker MK, Uysal AI, Kuscu Y, Demirbilek SG. Efficacy of ultrasound-guided erector spinae plane block for analgesia after laparoscopic cholecystectomy: A randomized controlled trial. Rev Bras Anestesiol. 2019;69:561–8. doi: 10.1016/j.bjane.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altiparmak B, Toker MK, Uysal AI, Kuscu Y, Demirbilek SG. Ultrasound-guided erector spinae plane block versus oblique subcostal transversus abdominis plane block for postoperative analgesia of adult patients undergoing laparoscopic cholecystectomy: Randomized, controlled trial. J Clin Anesth. 2019;57:31–3. doi: 10.1016/j.jclinane.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar S, Rastogi A, Haldar R, Kumar A, Singh S. An audit to study pain after laparoscopic cholecystectomy with the use of nonopioid analgesics. Indian J Pain. 2017;31:170–4. [Google Scholar]
- 10.Barazanchi AW, MacFater WS, Rahiri JL, Tutone S, Hill AG, Joshi GP. Evidence-based management of pain after laparoscopic cholecystectomy: A PROSPECT review update. Br J Anaesth. 2018;121:787–803. doi: 10.1016/j.bja.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Peng K, Ji FH, Liu HY, Wu SR. Ultrasound-guided transversus abdominis plane block for analgesia in laparoscopic cholecystectomy: A systematic review and meta-analysis. Med Princ Pract. 2016;25:237–46. doi: 10.1159/000444688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydin G, Aydin O. The efficacy of ultrasound-guided paravertebral block in laparoscopic cholecystectomy. Medicina (Kaunas) 2018;54:75. doi: 10.3390/medicina54050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung JH, Gates S, Naidu BV, Wilson MJ, Smith FG. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2:CD009121. doi: 10.1002/14651858.CD009121.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Ghamry MR, Amer AF. Role of erector spinae plane block versus paravertebral block in pain control after modified radical mastectomy. A prospective randomised trial. Indian J Anaesth. 2019;63:1008–14. doi: 10.4103/ija.IJA_310_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tulgar S, Ahiskalioglu A, de Cassai A, Gurka Y. Efficacy of bilateral erector spinae plane block in the management of pain: Current insights. J Pain Res. 2019;12:2597–613. doi: 10.2147/JPR.S182128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannig KE, Jessen C, Soni UK, Børglum J, Bendtsen TF. Erector spinae plane block for elective laparoscopic cholecystectomy in the ambulatory surgical setting? Case Rep Anesthesiol. 2018:e5492527. doi: 10.1155/2018/5492527. doi:10.1155/2018/5492527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohmura S, Kawada M, Ohta T, Yamamoto K, Kobayashi T. Systemic toxicity and resuscitation in bupivacaine-, levobupivacaine-, or ropivacaine-infused rats. Anesth Analg. 2001;93:743–8. doi: 10.1097/00000539-200109000-00039. [DOI] [PubMed] [Google Scholar]
- 18.Marganella C, Bruno V, Matrisciano F, Reale C, Nicoletti F, Melchiorri D. Comparative effects of levobupivacaine and racemic bupivacaine on excitotoxic neuronal death in culture and N-methyl-D-aspartate-induced seizures in mice. Eur J Pharmacol. 2005;518:111–5. doi: 10.1016/j.ejphar.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Arora D, Kaushik R, Kaur R, Sachdev A. Post-cholecystectomy syndrome: A new look at an old problem. J Minim Access Surg. 2018;14:202–7. doi: 10.4103/jmas.JMAS_92_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal S, Jain S, Daga RN, Vyas KC. Prevalence of postcholecystectomy symptoms: Long term outcome after open versus laparoscopic cholecystectomy. World J Surg Res. 2014;3:6–12. doi: 10.1007/s002680010243. [DOI] [PubMed] [Google Scholar]
- 21.Tulgar S, Selvi O, Senturk O, Serifsoy TE, Thomas DT. Ultrasound-guided erector spinae plane block: Indications, complications, and effects on acute and chronic pain based on a single-center experience. Cureus. 2019;11:e3815. doi: 10.7759/cureus.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taketa Y, Irisawa Y, Fujitani T. Ultrasound-guided erector spinae plane block elicits sensory loss around the lateral, but not the parasternal, portion of the thorax. J Clin Anesth. 2018;47:84–5. doi: 10.1016/j.jclinane.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Lavand'homme P. ‘Why me?’ The problem of chronic pain after surgery? Br J Pain. 2017;11:162–5. doi: 10.1177/2049463717722119. [DOI] [PMC free article] [PubMed] [Google Scholar]