Abstract
Background
BK polyomavirus (BKPyV) is associated with symptomatic hemorrhagic cystitis after hematopoietic cell transplantation (HCT). Little is known about the host immune response, effectiveness of antiviral treatment, or impact of asymptomatic replication on long-term kidney function.
Methods
In children and young adults undergoing allogeneic HCT, we quantified BKPyV viruria and viremia (pre-HCT and at Months 1–4, 8, 12, and 24 post-HCT) and tested associations of peak viremia ≥10 000 or viruria ≥109 copies/mL with estimated kidney function (glomerular filtration rate, eGFR) and overall survival at 2 years posttransplant. We examined the factors associated with viral clearance by Month 4, including BKPyV-specific T cells by enzyme-linked immune absorbent spot at Month 3 and cidofovir use.
Results
We prospectively enrolled 193 participants (median age 10 years) and found that 18% had viremia ≥10 000 copies/mL and 45% had viruria ≥109 copies/mL in the first 3 months post-HCT. Among the 147 participants without cystitis (asymptomatic), 58 (40%) had any viremia. In the entire cohort and asymptomatic subset, having viremia ≥10 000 copies/mL was associated with a lower creatinine/cystatin C eGFR at 2 years post-HCT. Viremia ≥10 000 copies/mL was associated with a higher risk of death (adjusted hazard ratio, 2.2; 95% confidence interval, 1.1–4.2). Clearing viremia was associated with detectable BKPyV-specific T cells and having viremia <10 000 copies/mL, but not cidofovir exposure.
Conclusions
Screening for BKPyV viremia after HCT identifies asymptomatic patients at risk for kidney disease and reduced survival. These data suggest potential changes to clinical practice, including prospective monitoring for BKPyV viremia to test virus-specific T cells to prevent or treat BKPyV replication.
Keywords: BK polyomavirus, stem cell transplantation, pediatrics, kidney
BK polyomavirus (BKPyV) viremia, whether symptomatic or not, is associated with morbidity and mortality after hematopoietic cell transplantation. Detectable BKPyV-specific T cells, but not cidofovir, are associated with clearance of viremia. This suggests potential benefits in screening for asymptomatic viremia.
BK polyomavirus (BKPyV) is associated with nephropathy after kidney transplantation and hemorrhagic cystitis after hematopoietic cell transplantation (HCT) [1, 2]. Hemorrhagic cystitis occurs in up to 25% of patients, leading to pain, urinary obstruction, prolonged hospitalizations, and possibly increased mortality [3–5]. In immunosuppressed patients, BKPyV replication is detected by nucleic acid testing of blood (viremia) or urine (viruria). Asymptomatic viruria is common after HCT and solid organ transplant, while viremia, especially high-level replication ≥10 000 copies/mL, is more specific for kidney and bladder disease [1, 4, 6].
Single-center studies have reported viremia or viruria incidences in the first 100 days after HCT [3, 7, 8] but not the longer-term risk of asymptomatic BKPyV replication on kidney function or the host immune response. We defined the natural history of BKPyV replication and outcomes for 2 years after HCT among patients at 2 large children’s hospitals and examined antiviral immunity. We hypothesized that BKPyV was associated with decreased kidney function and that the measurement of BKPyV T cells would predict viral clearance. Consensus guidelines do not recommend screening for BKPyV after HCT [5, 9] perhaps missing a risk factor for reduced kidney function or cystitis. Moreover, antivirals do not have proven efficacy, but third-party BKPyV T cells can be infused to promote viral clearance and more data are needed to define appropriate therapeutic use.
METHODS
Patient Population
We prospectively enrolled children and young adults ≥2 years of age who were undergoing an allogeneic HCT at the Children’s Hospital of Philadelphia (CHOP) or Cincinnati Children’s Hospital Medical Center (CCHMC) from April 2013–May 2018, with follow-up until October 2018. The Institutional Review Boards at CHOP and CCHMC approved the study and participants provided informed consent and assent.
Laboratory Methods
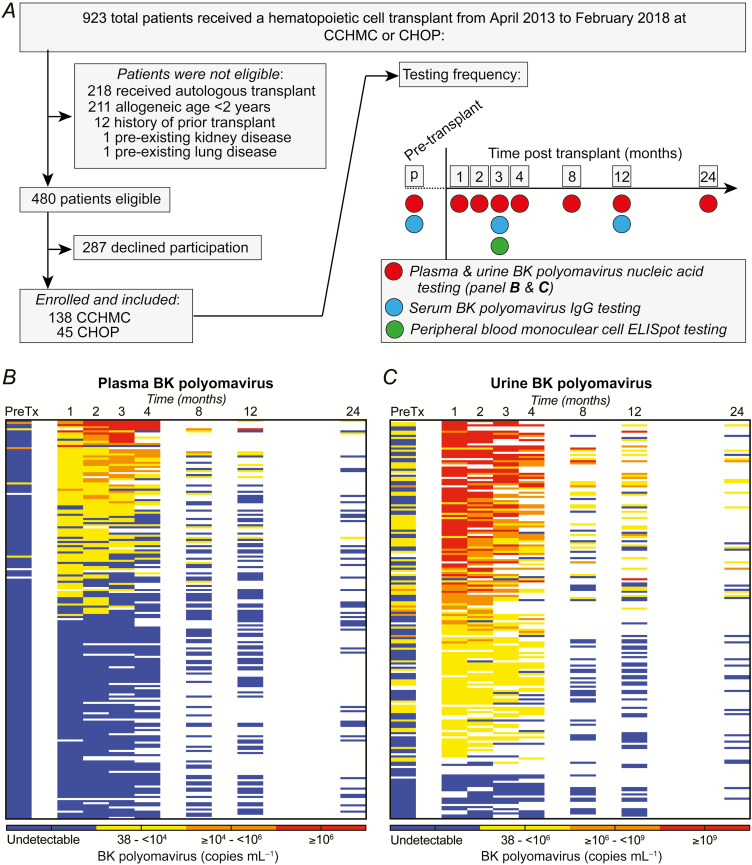
Plasma, serum, peripheral blood mononuclear cells (PBMCs), and urine were frozen prior to HCT and at Months 1–4, 8, 12, and 24 (the research methods are available in the Supplementary Material). Nucleic acid testing for BKPyV was performed on all plasma and urine samples (Viracor-Eurofins, Lee’s Summit, MO). Blood BKPyV immunoglobulin G (IgG) was measured at baseline and Months 3 and 12 (Viracor-Eurofins). Enzyme-linked immunosorbent spot (ELISPOT) testing to detect BKPyV-specific T cells was performed on Month 3 PBMC samples (Figure 1).
Figure 1.
Study cohort, sampling, and testing frequency and BK polyomavirus (BKPyV) viremia and viruria after hematopoietic cell transplant. A, We tested 193 children and young adults for BK polyomavirus in the blood (viremia) and urine (viruria) in the first 2 years after hematopoietic cell transplant (exclusion criteria in Supplementary Methods). Samples were collected and analyzed prior to transplant and at Months 1, 2, 3, 4, 8, 12, and 24 after transplant. B, Viremia was rare prior to transplant, occurred most frequently in the first 4 months, and, among survivors, was less frequent >8 months after transplant. C, Viruria was common prior to transplant, occurred frequently in the first 4 months, and persisted among a high proportion of survivors >8 months after transplant. Note that the scales are different for viremia and viruria. Abbreviations: CCHMC, Cincinnati Children’s Hospital Medical Center; CHOP, Children’s Hospital of Philadelphia; ELISPOT, enzyme-linked immune absorbent spot; IgG, immunoglobin G; PreTX, pretransplant.
Clinical Data
Clinical data were abstracted from the medical record, including serum creatinine and absolute lymphocyte counts within ±1 day of when 1049 of the 1070 (98.0%) research blood samples were collected (Supplementary Table 1). We recorded participants receiving cidofovir for any indication. Epstein-Barr virus, cytomegalovirus, adenovirus, and human herpes virus 6 viremia were captured from center-specific monitoring; positivity was defined using ≥2 consecutive nucleic acid results at any time posttransplant. Blood cystatin C was measured clinically or on stored samples at baseline and at Months 8, 12, and 24 [10].
BK Polyomavirus Definitions, Outcome Variables, and Covariates
BKPyV replication was defined as a positive nucleic acid test, with or without clinical symptoms, and was categorized as peak viremia ≥10 000 copies/mL [1, 4, 6] and/or viruria ≥109 copies/mL [11, 12] among participants with ≥2 samples during the first 3 months post-HCT. The outcomes included cystitis, estimated glomerular filtration rate (eGFR), dialysis, and all-cause death. Cystitis was defined as grade ≥2 (presence of BKPyV, symptoms, and visible hematuria [2, 5]) and was assessed retrospectively by reviewing clinical documentation. GFR was estimated at baseline and at Months 8, 12, and 24. For participants <18 years of age [13] and ≥18 years of age [14], eGFRs were separately calculated using serum creatinine alone and cystatin C and creatinine together. We analyzed eGFR in the whole cohort and in the subset with asymptomatic viral replication (no cystitis). Covariates included demographic and transplant characteristics, graft-versus-host disease (GVHD), thrombotic microangiopathy (TMA), cidofovir for any indication, and other viremias. TMA was only assessed at CCHMC due to an established screening guideline [15]. As a risk factor for BKPyV replication, other viremias, GVHD, and TMA were not analyzed as time-dependent variables and could have occurred before or after detectable BKPyV. As a risk factor for cystitis, acute GVHD was only considered if it occurred prior to cystitis.
Immune Response and Viral Clearance
We examined whether the baseline IgG predicted posttransplant replication. We tested the Month 3 PBMC sample for BKPyV-specific T cells, defined as those with ELISPOT counts >5 or >10 above the negative control. We hypothesized that among participants with persistent BKPyV replication (≥2 positive samples) in the first 3 months posttransplant, a positive ELISPOT would predict viral clearance (undetectable viral DNA) at Month 4. We also assessed other factors potentially associated with clearance, including the absolute lymphocyte count, IgG, and cidofovir use.
Statistical Analyses
Continuous variables were presented as medians and interquartile ranges (IQR) and were examined with the Wilcoxon rank sum or 2-sample t test, as appropriate. Categorical variables were examined with the Chi-square or Fisher’s exact test, as appropriate. Logistic regression examined the factors associated with viral clearance. We excluded those participants developing cystitis prior to their Month 1 sample, to examine whether BKPyV replication predicted later cystitis. Linear regression examined the factors associated with eGFR. Multivariable Cox proportional hazards regression assessed the associations among BKPyV, cystitis, and mortality. Model selection for cystitis was performed with backward elimination using likelihood ratio testing and for mortality included all significant plausible variables. Given the high mortality rate, we also examined cystitis in a competing risk regression with death. We examined the overall survival rate instead of the nonrelapse mortality rate, given the high proportion of nonmalignant transplant indications. The study was originally designed to have 85% power to detect a clinically meaningful difference in a 1-year eGFR of 20 ml/min/1.73m2 between participants with and without BKPyV viremia ≥10 000 copies/mL and assumed a sample size of 100 participants surviving to 1 year and a 10% prevalence of viremia. Analyses were performed using STATA Version 15.1 and R 3.5.1, and a 2-sided P value <.05 was considered statistically significant.
RESULTS
Cohort
We enrolled 193 participants (Figure 1; Table 1), who had a median age of 10 years (IQR, 6–15 years; range, 2–32 years). The most common underlying diagnoses were malignancy (37%), bone marrow failure (27%), or immunodeficiency (21%). At CCHMC (n = 148), a greater proportion of participants had bone marrow failure or immunodeficiency, compared to CHOP (n = 45), where most participants had malignancy. The cohort characteristics by center, specific underlying diagnoses, and conditioning regimens are shown in Supplementary Tables 2–4.
Table 1.
Cohort Characteristics and Variables Associated with Peak BK Polyomavirus Viremia ≥10 000 Copies/mL in the First 3 Months After Hematopoietic Cell Transplant
| Variable | Entire Cohort | <10 000 Copies/mL | ≥10 000 Copies/mL | P a |
|---|---|---|---|---|
| N = 193 | N = 178b | |||
| n = 146 | n = 32 | |||
| Age at transplant, years | 10 (6–15) | 10 (6–14) | 12 (9–17) | .02 |
| Recipient male gender | 112 (58.0%) | 82 (56.2%) | 21 (65.6%) | .33 |
| Diagnosis group | .63 | |||
| Malignancy | 72 (37.3%) | 52 (35.6%) | 12 (37.5%) | |
| Bone marrow failure | 52 (26.9%) | 38 (26.0%) | 11 (34.4%) | |
| Immunodeficiency | 40 (20.7%) | 30 (20.5%) | 6 (18.8%) | |
| Other | 29 (15.0%) | 26 (17.8%) | 3 (9.4%) | |
| Unrelated donor, versus related | 130 (67.4%) | 94 (64.4%) | 28 (87.5%) | .01 |
| Donor age, years | 24 (16–31) | 24 (15–29) | 28 (23–36) | .01 |
| Donor/recipient gender mismatch | 86 (44.6%) | 68 (46.6%) | 15 (46.9%) | .98 |
| 10/10 HLA match, versus another match | 143 (74.1%) | 115 (78.8%) | 20 (62.5%) | .05 |
| Donor HLA C7 allele | 107 (55.4%) | 80 (54.8%) | 15 (46.9%) | .42 |
| Cell product | ‑ | .45 | ||
| Bone marrow | 123 (63.7%) | 96 (65.8%) | 18 (56.3%) | |
| Peripheral blood stem cells | 64 (33.2%) | 45 (30.8%) | 14 (43.8%) | |
| Cord bloodc | 6 (3.1%) | 5 (3.4%) | 0 (0%) | |
| Conditioning chemotherapy | ||||
| Received alemtuzumab | 51 (26.4%) | 40 (27.4%) | 6 (18.8%) | .31 |
| Received antithymocyte globulin | 80 (41.5%) | 54 (37.0%) | 20 (62.5%) | .01 |
| Received cyclophosphamide | 109 (56.5%) | 76 (52.1%) | 23 (71.9%) | .04 |
| Received busulfan | 84 (43.5%) | 65 (44.5%) | 14 (43.8%) | .94 |
| Received total body irradiation | 45 (23.3%) | 30 (20.5%) | 9 (28.1%) | .35 |
| Myeloablative conditioning | 129 (66.8%) | 95 (65.1%) | 23 (71.9%) | .46 |
| Graft-versus-host disease prophylaxis | ||||
| Received calcineurin inhibitor | 157 (81.4%) | 118 (80.8%) | 26 (81.3%) | .96 |
| Received ex vivo T-cell depletion | 55 (28.5%) | 40 (27.4%) | 12 (37.5%) | .26 |
| Epstein-Barr virus viremiad | 85 (44.0%) | 65 (44.5%) | 15 (46.9%) | .81 |
| Cytomegalovirus viremiad | 60 (31.1%) | 49 (33.6%) | 9 (28.1%) | .55 |
| Adenovirus viremiad | 33 (17.1%) | 21 (14.4%) | 5 (15.6%) | .79 |
| Human herpes virus 6 viremiad | 12 (6.2%) | 8 (5.5%) | 1 (3.1%) | 1.00 |
| Detectable pretransplant viruria | 69 (39.7%) | 46 (34.3%) | 19 (63.3%) | .003 |
| Month 1 absolute lymphocyte count | 410 (190–720) | 460 (220–790) | 383 (195–595) | .35 |
| Month 2 absolute lymphocyte count | 530 (280–900) | 610 (300–1090) | 375 (150–620) | .01 |
| Month 3 absolute lymphocyte count | 726 (400–1140) | 740 (430–1270) | 695 (260–980) | .15 |
Data are shown as either a median (interquartile range) or n (%).
Abbreviation: HLA, human leukocyte antigen.
a P value by Wilcoxon rank sum, 2-sample t test, Chi-square, or Fisher’s exact test, as appropriate, comparing viremia with <10 000 copies/mL to those with ≥10 000 copies/mL.
bIncludes 178 participants with ≥2 blood tests (n = 10 with <2 blood tests) in the first 3 months and negative pretransplant viremia (n = 5 with detectable pretransplant viremia).
cOf 6 cord blood transplant recipients, 5 also received bone marrow–derived cells.
dOccurring at any point posttransplant.
BK Polyomavirus Replication
BKPyV results are shown in Figure 1 and Tables 2 and 3. We tested 1070 blood samples (median, 6 samples/participant; IQR, 5–7 samples). Only 5 of 188 (2.7%) participants had viremia pre-HCT. Of 190 participants, 94 (49.5%) had ≥1 sample with detectable viremia after transplant. Among participants with ≥2 samples in the first 3 months after transplant and with negative pre-HCT viremia, the viral load was ≥10 000 copies/mL in 32 of 178 (18.0%). In univariate analyses, the risk factors significantly associated with viremia ≥10 000 copies/mL included an older recipient age, unrelated donor, older donor age, antithymocyte globulin or cyclophosphamide conditioning (but not alemtuzumab), detectable pre-HCT viruria, and a lower Month 2 absolute lymphocyte count (Table 1). Viremia ≥10 000 copies/mL was associated with acute GVHD grade ≥2 (odds ratio [OR], 2.5; 95% confidence interval [CI], 1.2–5.4; P = .02) at any time relative to transplant (not time-dependent). Among the CCHMC participants, viremia ≥10 000 copies/mL was associated with TMA (OR, 7.0; 95% CI, 2.4–20.0; P < .001) at any time relative to transplant (not time-dependent). At Months 8, 12, and 24, 12 of 85 (14.1%), 9 of 91 (9.9%), and 3 of 44 (6.8%) participants had detectable viremia, respectively.
Table 2.
BK Polyomavirus Viremia Sample Results by Time Point
| Pre-HCT | Month 1 | Month 2 | Month 3 | Month 4 | Month 8 | Month 12 | Month 24 | |
|---|---|---|---|---|---|---|---|---|
| Copies/mL | n = 188 | n = 185 | n = 178 | n = 165 | n = 134 | n = 85 | n = 91 | n = 44 |
| 0 | 183 (97.3%) | 112 (60.5%) | 105 (59.0%) | 110 (66.7%) | 101 (75.4%) | 73 (85.9%) | 82 (90.1%) | 41 (93.2%) |
| 1–9999 | 3 (1.6%) | 67 (36.2%) | 52 (29.2%) | 30 (18.2%) | 20 (14.9%) | 10 (11.8%) | 8 (8.8%) | 3 (6.8%) |
| 10 000–99 999 | 2 (1.1%) | 3 (1.6%) | 16 (9.0%) | 15 (9.1%) | 8 (6.0%) | 2 (2.4%) | 0 (0%) | 0 (0%) |
| ≥100 000 | 0 (0%) | 3 (1.6%) | 5 (2.8%) | 10 (6.1%) | 5 (3.7%) | 0 (0%) | 1 (1.1%) | 0 (0%) |
There were 191 participants with at least 1 blood sample tested for viremia: n represents the number of participants tested at each time point.
Abbreviation: HCT, hematopoietic cell transplant.
Table 3.
BK Polyomavirus Viruria Sample Results by Time Point
| Copies/mL | Pre-HCT | Month 1 | Month 2 | Month 3 | Month 4 | Month 8 | Month 12 | Month 24 |
| n = 174 | n = 162 | n = 146 | n = 128 | n = 99 | n = 63 | n = 81 | n = 37 | |
| 0 | 105 (60.3%) | 17 (10.5%) | 23 (15.8%) | 20 (15.6%) | 26 (26.3%) | 43 (68.3%) | 52 (64.2%) | 24 (64.9%) |
| 1–999 999 999 | 63 (36.2%) | 65 (40.1%) | 51 (34.9%) | 53 (41.4%) | 37 (37.4%) | 15 (23.8%) | 21 (25.9%) | 11 (29.7%) |
| ≥106–109 | 6 (3.5%) | 21 (13.0%) | 32 (21.9%) | 27 (21.1%) | 24 (24.2%) | 3 (4.8%) | 6 (7.4%) | 2 (5.4%) |
| ≥109 | 0 (0%) | 59 (36.4%) | 40 (27.4%) | 28 (21.9%) | 12 (12.1%) | 2 (3.2%) | 2 (2.5%) | 0 (0%) |
There were 189 participants with at least 1 urine sample tested for viruria: n represents the number of participants tested at each time point.
Abbreviation: HCT, hematopoietic cell transplant.
We tested 890 urine samples (median, 5 samples/participant; IQR, 3–6 samples). Pre-HCT, one-third of participants (69 of 174, 39.7%) had detectable viruria, with a median of 3500 copies/mL (IQR, 1000–4 242 400 copies/mL). Of 184 participants, 160 (87.0%) had ≥1 sample with viruria after transplant. The peak urine viral load was ≥109 copies/mL in 70 of 157 (44.6%) participants with ≥2 urine samples in the first 3 months posttransplant. Approximately half of participants with viruria also experienced viremia (90 of 160, 56.3%). Viremic participants essentially all also had viruria, with only 1 participant with viremia (a single value of 112 copies/mL) having no viruria.
Clinical Outcomes
Hemorrhagic Cystitis
BKPyV-associated cystitis was identified in 43 of 193 (22.3%) participants at a median of 34 days (IQR, 25–54 days) post-HCT. Any detectable viremia or viruria ≥109 copies/mL significantly predicted subsequent cystitis (Table 4). Of note, exposure to busulfan was also associated with an increased risk of cystitis (39.3% with no exposure versus 58.1% with exposure; P = .03), but cyclophosphamide was not (54.0% versus 65.1%; P = .2). In a multivariable model adjusting for significant covariates (Table 4), detectable viremia was independently associated with cystitis (adjusted hazard ratio [HR], 7.8; 95% CI, 3.1–19.3; P < .01). The model results were similar when including viruria ≥109 copies/mL instead of viremia (adjusted HR, 5.7; 95% CI, 2.4–13.4; P < .01). In a competing risk regression with death, adjusted for the same covariates as shown in the final Cox models in Table 4, detectable viremia and viruria ≥109 copies/mL remained independently associated with cystitis (adjusted HRs 7.9 [95% CI 3.1–20.4; P < .01] and 5.7 [95% CI, 2.4–13.2; P < .01], respectively.)
Table 4.
Variables Associated With Grade ≥ 2 Hemorrhagic Cystitis After Hematopoietic Cell Transplant
| No Grade ≥ 2 Cystitis n = 150 |
Grade ≥ 2 Cystitis n = 43 |
Univariate P | Multivariable Cox Models, Data Shown as Hazard Ratios for Cystitis (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Detectable BKPyV Viremia Pre-cystitis | BKPyV Viruria >109 Copies/mL Pre-cystitis | ||||||
| Full Model | Final Model | Full Model | Final Model | ||||
| Age at transplant, years | 10 (6–14) | 11 (8–17) | .04 | 1.0 (.9–1.1) | … | 1.0 (.9–1.1) | … |
| Male gender | 85 (56.7%) | 27 (62.8%) | .47 | … | … | … | … |
| Diagnosis group | .83 | … | … | … | … | ||
| Malignancy | 54 (36.0%) | 18 (41.9%) | … | … | … | … | |
| Bone marrow failure | 40 (26.7%) | 12 (27.9%) | … | … | … | … | |
| Immunodeficiency | 33 (22.0%) | 7 (16.3%) | … | … | … | … | |
| Other | 23 (15.3%) | 6 (14.0%) | … | … | … | … | |
| Unrelated donor, versus related | 98 (65.3%) | 32 (74.4%) | .26 | … | … | … | … |
| 10/10 HLA match, versus another match | 117 (78.0%) | 26 (60.5%) | .02 | .6 (.3–1.3) | … | .7 (.3–1.7) | … |
| Peripheral blood stem cell product, versus bone marrow/cord | 43 (28.7%) | 21 (48.8%) | .01 | .5 (.05–5.1) | … | .4 (.1–3.0) | … |
| Conditioning chemotherapy | |||||||
| Received alemtuzumab | 48 (32.0%) | 3 (7.0%) | <.001 | .1 (0–.9)a | .1 (.01–.7)a | b | b |
| Received antithymocyte globulin | 56 (37.3%) | 24 (55.8%) | .03 | 1.0 (.4–2.6) | … | 1.7 (.7–4.2) | … |
| Received cyclophosphamide | 81 (54.0%) | 28 (65.1%) | .20 | … | … | … | … |
| Received busulfan | 59 (39.3%) | 25 (58.1%) | .03 | 2.7 (1.0–7.6) | 3.0 (1.3–6.7)c | 1.9 (.7–4.9) | … |
| Total body irradiation | 35 (23.3%) | 10 (23.3%) | .99 | … | … | … | … |
| Myeloablative conditioning | 92 (61.3%) | 37 (86.0%) | .002 | .7 (.2–3.0) | … | 5.5 (1.1–27.4)a | 11.1 (2.6–48.0)c |
| Graft-versus-host disease prophylaxis | |||||||
| Received calcineurin inhibitor | 123 (82.0%) | 34 (79.1%) | .66 | … | … | … | … |
| Received ex vivo T-cell depletion | 36 (24.0%) | 19 (44.2%) | .01 | 2.2 (.2–23.8) | … | 2.6 (.4–18.4) | … |
| Detectable pre-HCT BKPyV viruria | 51/135 (37.8%) | 18/39 (46.2%) | .35 | … | … | … | … |
| Detectable pre-HCT BKPyV viremia | 3/146 (2.1%) | 2/42(4.8%) | .31 | … | … | … | … |
| Epstein-Barr virus viremiad | 71 (47.3%) | 14 (32.6%) | .09 | … | … | … | … |
| Cytomegalovirus viremiad | 41 (27.3%) | 19 (44.2%) | .04 | 3.4 (1.5–7.9)c | 3.4 (1.6–7.4)c | 3.2 (1.4–7.3)c | 2.9 (1.3–6.5)c |
| Adenovirus viremiad | 28 (18.7%) | 5 (11.6%) | .36 | … | … | … | … |
| HHV6 viremiad | 9 (6.0%) | 3 (7.0%) | .73 | … | … | … | … |
| Acute graft-versus-host disease pre-cystitise | 50 (33.3%) | 15 (34.9%) | .85 | … | … | … | … |
| BKPyV viruria >109 copies/mL pre-cystitise | 48/141 (34.0%) | 17/25 (68.0%) | .001 | … | … | 6.4 (2.5–15.9)c | 5.7 (2.4–13.4)c |
| Detectable BKPyV viremia pre-cystitise | 58/147 (39.5%) | 21/28 (75.0%) | <.001 | 7.4 (2.9–19.3)c | 7.8 (3.1–19.3)c | … | … |
| BKPyV viremia >10 000 copies/mL pre-cystitise | 19/147 (12.9%) | 2/28 (7.1%) | .53 | … | … | … | … |
Total N = 193. Univariate data are shown as a median (interquartile range) or n (%) with a univariate P value by Wilcoxon rank sum, 2-sample t test, Chi-square, or Fisher’s exact test, as appropriate.
Abbreviations: BKPyV, BK polyomavirus; CI, confidence interval; HCT, hematopoietic cell transplant; HHV6, human herpes virus 7; HLA, human leukocyte antigen.
a P < .05.
bNot receiving alemtuzumab conditioning predicted failure perfectly and therefore was not included in the Cox models.
c P < .01.
dOccurring at any point posttransplant.
eWe excluded participants without an available posttransplant research sample before the diagnosis of cystitis so that the variable had to precede the diagnosis of cystitis or occur at any time after transplant among those without cystitis.
Kidney Outcomes
Participants with viremia ≥10 000 copies/mL in the first 3 months after transplant had a significantly lower eGFR at 12 and 24 months (Table 5) and a higher risk of receiving dialysis (OR, 6.2; 95% CI, 1.8–21.6; P = .004). Age, total body irradiation, cyclophosphamide, calcineurin inhibitor prophylaxis, GVHD, cystitis, and cidofovir were not associated with the Month 24 eGFR (Supplementary Table 5). Adjusting for only the baseline eGFR, participants with viremia ≥10 000 copies/mL had a Month 24 creatinine/cystatin C eGFR that was 20.2 ml/min/1.73m2 lower (95% CI, −38.9 to −1.6 ml/min/1.73m2; P = .03), compared to those with viremia <10 000 copies/mL. Viruria ≥109 copies/mL was not associated with Month 8, 12, or 24 eGFR or dialysis (Table 5).
Table 5.
Kidney Outcomes Associated With BK Polyomavirus Viremia and Viruria After Hematopoietic Cell Transplant
| Peak Viremia in First 3 Months After Transplant | Peak Viruria in First 3 Months After Transplant | |||||||
|---|---|---|---|---|---|---|---|---|
| n | <10 000 Copies/mL | ≥10 000 Copies/mL | P a | n | <109 Copies/mL | ≥109 Copies/mL | P a | |
| n = 149b | n = 34b | n = 87c | n = 70c | |||||
| Baseline | ||||||||
| Creatinine | 183 | 131 (112–153) | 132 (108–152) | .41 | 157 | 130 (108–155) | 131 (113–145) | .77 |
| Creatinine/cystatin | 183 | 125 (110–141) | 118 (108–143) | .48 | 156 | 120 (109–141) | 123 (112–140) | .42 |
| Month 8 | ||||||||
| Creatinine | 82 | 121 (104–138) | 98 (84–116) | .10 | 74 | 125 (98–147) | 116 (100–132) | .35 |
| Creatinine/cystatin | 82 | 106 (92–120) | 92 (82–111) | .47 | 74 | 106 (90–121) | 104 (92–119) | .53 |
| Month 12 | ||||||||
| Creatinine | 89 | 126 (104–148) | 110 (78–125) | .02 | 77 | 119 (97–150) | 124 (104–134) | .70 |
| Creatinine/cystatin | 88 | 110 (97–131) | 100 (88–107) | .04 | 76 | 104 (94–129) | 104 (91–126) | .74 |
| Month 24 | ||||||||
| Creatinine | 43 | 125 (110–141) | 98 (75–111) | .005 | 35 | 125 (108–137) | 112 (105–131) | .34 |
| Creatinine/cystatin | 43 | 113 (100–127) | 97 (73–104) | .003 | 35 | 115 (103–127) | 106 (95–118) | .39 |
| Received dialysis | … | 5 (3.4%) | 6 (17.7%) | .006 | … | 3 (3.5%) | 6 (8.6%) | .19 |
| Alive at last follow-up | … | 122 (81.9%) | 19 (55.9%) | .001 | … | 70 (80.5%) | 50 (71.4%) | .19 |
Data are shown as a median (interquartile range) or n (%). Data are for the unadjusted, estimated glomerular filtration rate (mL/min/1.73m2)
a P value by Wilcoxon rank sum, 2-sample t test, Chi-square, or Fisher’s exact test, as appropriate.
bIncludes the 183 participants with at least 2 samples in the first 3 months after transplant.
cIncludes the 157 participants with at least 2 urine samples in the first 3 months after transplant.
Among those not developing cystitis, 58 of 147 (39.5%) had viremia, including 19 of 58 (32.8%) with viremia ≥10 000 copies/mL. When limiting the data to these participants with asymptomatic viremia, both the creatinine (median, 83 versus 125 ml/min/1.73m2, respectively; P = .01) and creatinine/cystatin C (median, 78 versus 117 ml/min/1.73m2, respectively; P = .004) eGFRs were significantly lower at 24 months among those with viremia ≥10 000 copies/mL (n = 4), compared to those without (n = 32). Adjusting only for the baseline eGFR, participants with asymptomatic viremia ≥10 000 copies/mL in the first 3 months after transplant had a creatinine/cystatin C eGFR that was 29.4 ml/min/1.73m2 lower (95% CI, −55.3 to −3.6 ml/min/1.73m2, respectively; P = .03) than those with viremia <10 000 copies/mL (Supplementary Table 5).
Mortality
After a median follow-up of 2.2 years (IQR, .8–3.8 years), 49 of 193 (25.4%) participants died. Participants with viremia ≥10 000 copies/mL in the first 3 months after transplant had a 3-fold increased risk of all-cause mortality (HR, 2.8; 95% CI, 1.5–5.2; P = .002). The HR for death associated with grade ≥2 cystitis was 2.0 (95% CI, 1.1–3.6; P = .02). Adjusting for cystitis and other factors associated with mortality, including age, gender, underlying diagnosis, and acute GVHD grade ≥2, viremia ≥10 000 copies/mL was independently associated with mortality (HR, 2.2; 95% CI, 1.1–4.2; P = .02).
Immune Response and Clearance of Viremia
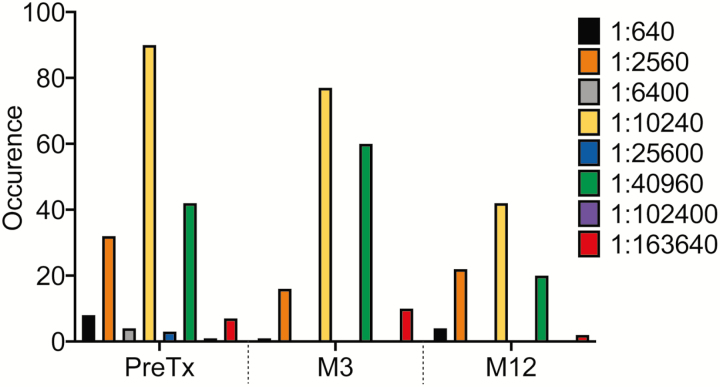
The distribution of BKPyV IgG is shown in Figure 2. The baseline IgG was not associated with post-HCT viruria or viremia, as participants with both the highest and lowest levels developed viremia after transplant (data not shown). The Month 3 IgG was not associated with viral clearance (Table 6 and below).
Figure 2.
Distribution of BK polyomavirus immunoglobulin G titers after transplant. There were 8 participants that inadvertently had 2 separate samples run pretransplant with results that differed by one 4-fold dilution, and their titers were averaged. Abbreviations: M, month; PreTX, pretransplant.
Table 6.
Factors Associated With Clearance of BK Polyomavirus Viremia at 4 Months After Hematopoietic Cell Transplant
| Did Not Clear Viremia at Month 4 | Cleared Viremia at Month 4 | P a | |
|---|---|---|---|
| n = 31b | n = 21b | ||
| Peak viremia ≥10 000 copies/mL in the first 3 months after transplant | 21 (67.7%) | 3 (14.3%) | <.001 |
| Month 3 BK polyomavirus IgG | n = 31 | n = 20 | .49 |
| 1:2560 | 2 (6.5%) | 2 (10.0%) | |
| 1:10 240 | 17 (54.8%) | 7 (35.0%) | |
| 1:40 960 | 10 (32.3%) | 8 (40.0%) | |
| 1:163 840 | 2 (6.5%) | 3 (15.0%) | |
| Month 3 absolute lymphocyte count, cells/µL | 646 (260–880) | 780 (500–970) | .15 |
| Received cidofovir in first 3 months after transplant | 17 (54.8%) | 3 (14.3%) | .004 |
| Month 3 ELISPOT spots above negative control | n = 25 | n = 18 | |
| Number of spots | 1.3 (0–8.4) | 20.1 (2.9–48.5) | .007 |
| >5 spots | 9 (36.0%) | 12 (66.7%) | .05 |
| >10 spots | 6 (24.0%) | 11 (61.1%) | .01 |
Data are shown as a median (interquartile range) or n (%).
Abbreviation: ELISPOT, enzyme-linked immune absorbent spot.
a P value by Wilcoxon rank sum, 2-sample t test, Chi-square, or Fisher’s exact test, as appropriate.
bThe included participants had detectable viremia on ≥2 samples in the first 3 months after transplant and had a Month 4 blood sample to assess for viral clearance. Clearance was defined as having undetectable viremia at Month 4.
Month 3 PBMC samples were tested for BKPyV-specific T cells in 104 of 193 (53.9%) participants. We examined factors associated with viral clearance by Month 4 among the subset of 52 participants with persistent viremia in the first 3 months after transplant and with an available Month 4 sample to assess clearance (Tables 6 and 7). Clearance of viremia at Month 4 occurred in 21 of these 52 (40.4%) and was less likely among participants with prior viremia ≥10 000 copies/mL. The Month 3 absolute lymphocyte count did not predict clearance. Receiving cidofovir for any indication (cystitis or adenoviremia) was less likely among participants with a 10/10 matched donor and more common among those developing acute GVHD, BKPyV viremia >10 000, and viruria ≥109 copies/mL (Supplementary Table 6). Participants with BKPyV replication who had received cidofovir were significantly less likely to clear viremia, but those with an ELISPOT >10 spots above the control were more likely to clear viremia (OR, 5.0; 95% CI, 1.3–18.6; P = .02; Table 6). Finally, 6 of 7 participants with persistent viremia at both Months 8 and 12 did not have detectable BKPyV-specific T cells at Month 3 (Supplementary Table 7).
Table 7.
BK Polyomavirus Viremia Clearance by Month 4 and Enzyme-linked Immune Absorbent Spot Results
| Participant | Month 1 Viremia Copies/mL | Month 2 Viremia Copies/mL | Month 3 Viremia Copies/mL | Month 3 ELISPOT Spots Above Control | Month 4 Viremia Copies/mL |
|---|---|---|---|---|---|
| Cleared viremia | |||||
| 1 | 2000 | 7600 | 3400 | 0 | 0 |
| 2 | 417 | 168 | 0 | 2.9 | 0 |
| 3 | 1200 | 51 | 0 | 20.4 | 0 |
| 4 | 3000 | 500 | 0 | 149 | 0 |
| 5 | 0 | 6800 | 196 | 7 | 0 |
| 6 | 190 | 5300 | 600 | 65.7 | 0 |
| 7 | 0 | 1100 | 900 | 44.3 | 0 |
| 8 | 38 | 102 | 0 | 3.3 | 0 |
| 9 | 700 | 172 | 0 | 1.7 | 0 |
| 10 | 5900 | 11 900 | 8700 | 0 | 0 |
| 11 | No sample | 201 | 185 | 1.9 | 0 |
| 12 | 1 300 000 | 58 700 | 98 | 127.3 | 0 |
| 13 | 0 | 2900 | 148 | 82.5 | 0 |
| 14 | 2400 | 2100 | 0 | 48.5 | 0 |
| 15 | 4200 | 1500 | 0 | 38.2 | 0 |
| 16 | 16 100 | 316 | 0 | 26.3 | 0 |
| 17 | 67 | 1600 | 1100 | 19.8 | 0 |
| 18 | 1300 | 70 | 0 | 14.3 | 0 |
| Did not clear viremia | |||||
| 1 | 175 | 1200 | 0 | 0 | 89 |
| 2 | 1400 | 26 500 | 10 400 | 8.4 | 109 |
| 3 | 1300 | 7200 | 900 | 153.6 | 193 |
| 4 | 900 | 500 | 429 | 14.5 | 800 |
| 5 | 0 | 1400 | 339 | 2.1 | 1000 |
| 6 | 1100 | 23 300 | 42 500 | 0 | 2700 |
| 7 | 1700 | 600 | 700 | 1.6 | 2800 |
| 8 | 900 | 2100 | 66 400 | 0 | 2900 |
| 9 | 38 | No sample | 35 000 | 0 | 3100 |
| 10 | 800 | 700 | 1700 | 17.4 | 3300 |
| 11 | 1700 | 57 500 | 166 000 | .8 | 3600 |
| 12 | 6400 | 60 000 | 800 | 40.3 | 4000 |
| 13 | 3000 | 900 | 7300 | 0 | 5500 |
| 14 | 222 | 1300 | 5700 | 0 | 6700 |
| 15 | 1500 | 12 900 | 93 000 | 1.3 | 13 500 |
| 16 | 209 | 3900 | 16 400 | 0 | 23 500 |
| 17 | 4700 | 8500 | 27 800 | −3.8 | 34 500 |
| 18 | 900 | 21 600 | 48 800 | 4.1 | 57 400 |
| 19 | 600 | 3600 | 25 700 | 1.2 | 68 900 |
| 20 | 800 | 1100 | 1900 | −1.7 | 97 000 |
| 21 | 184 000 | 496 000 | 2 000 000 | 5.9 | 460 000 |
| 22 | No sample | 574 000 | 682 000 | −1.3 | 463 000 |
| 23 | 79 900 | 2 700 000 | 8 500 000 | 8.2 | 1 400 000 |
| 24 | 87 | 73 000 | 168 000 | 22.3 | 2 400 000 |
| 25 | 26 100 | 287 000 | 14 000 000 | 64.9 | 13 000 000 |
These 43 selected participants had persistent viremia in the first 3 months after transplant, had a Month 4 sample to assess for clearance, and had a Month 3 ELISPOT result. Participants with peak viremia <10 000 copies/mL and ELISPOT counts >10 above the negative control (bold and highlighted) were more likely to clear viremia by Month 4.
Abbreviation: ELISPOT, enzyme-linked immune absorbent spot.
DISCUSSION
We report the natural history of BKPyV replication in almost 200 children and young adults undergoing HCT at 2 large centers. A number of novel findings have the potential to change practice. First, we identified frequent asymptomatic viremia and found that high levels of viremia, whether symptomatic or not, were associated with significant reductions in later kidney function. Second, we found that children and young adults with BKPyV replication who received cidofovir were not more likely to clear viremia. Last, we identified BKPyV-specific T cells as a marker of clearing viremia but no benefit from antibody responses. The management of BKPyV at most centers currently includes testing for viral reactivation only in symptomatic cases, with the provision of additional intravenous immunoglobulin and cidofovir. While our data suggest that none of these approaches may be beneficial, randomized trials would be needed to validate the effectiveness of treatments. The screening of asymptomatic patients for BKPyV will only be beneficial if a therapy is available. Our data show that the recovery of endogenous BKPyV-specific T cells is associated with viral clearance, similar to prior studies after kidney transplantation [16–19]. To our knowledge, our study is the largest systematic evaluation of cellular responses to BKPyV after HCT [20–22]. The efficacy and safety of third-party BKPyV-specific T cells has recently been reported after HCT [23–25].
Our findings expand on single-center studies examining BKPyV in the first 100 days after HCT, which are generally without a comprehensive assessment of kidney outcomes or immune responses [11, 12, 26, 27]. Hill et al [7, 8] measured the impact of viremia from 5 viruses, including BKPyV, on mortality among 400 HCT recipients. The incidence of BKPyV viremia was 54%, most episodes were persistent, and, similar to our findings, viremia was associated with mortality. BKPyV viremia occurred a median of 10 days before cystitis. Others have reported that viremia or viruria predict cystitis, which we also confirmed [3, 6].
About 25% of our cohort developed cystitis [5, 7, 11]. BKPyV viremia was associated with a higher risk of receiving dialysis and an eGFR that was, on average, 20 ml/min/1.73m2 lower by 2 years after transplant. O’Donnell et al [27] monitored 57 adults after HCT and observed that viremia was independently associated with higher peak creatinine, similar to retrospective studies in children [4]. In contrast to antithymocyte globulin, alemtuzumab was not associated with the risk of BKPyV replication. It is possible that these data are confounded by an unmeasured variable or that qualitative, BKPyV-specific T-cell recovery after alemtuzumab is importantly different than recovery after antithymocyte globulin.
Although we and others have shown that BKPyV viremia can predict cystitis, the positive predictive value of viremia remains low, implying that other factors, perhaps related to the host response or viral diversity, are important to determining which patients will develop disease [11]. We observed that BKPyV viremia was associated with dialysis, TMA, acute GVHD, and death. More research is needed to determine whether these associations are causal, time-dependent, associated with poor immune reconstitution, or are confounded by higher degrees of immunosuppression. In vitro studies indicate that BKPyV infection can induce host endothelial cell production of interferon [28], which may precipitate both TMA and acute GVHD, supporting a possible direct, causative effect of BKPyV viremia on these other, significant posttransplant complications [7].
The strengths of our approach include the multi-center design, collection of samples and clinical data for 2 years after transplant, and centralized lab testing. We assessed both the humoral and cellular host immune response to BKPyV. Finally, we examined the association between BKPyV replication and eGFR for the first time in the HCT population using both serum creatinine and cystatin C, which has advantages in patients with decreased muscle mass [10]. Participants with viremia >10 000 copies/mL had an 18–26% decrease in baseline eGFR by 2 years post-HCT (Table 5). In older adults, a 30% reduction in eGFR over 2 years has been strongly associated with end-stage kidney disease and death [29]. While similar data are not available in children, it is plausible that the eGFR decline we observed would also be associated with poor outcomes in a younger population. Our study was limited by a lack of biopsy data to confirm nephropathy. Nevertheless, BKPyV viremia ≥10 000 copies/mL has been classified as presumptive nephropathy after kidney transplantation, even without a biopsy [30]. Patient characteristics, most notably the high proportion of nonmalignant indications, may not reflect the risk for BKPyV infection at other centers. Not all centers have the resources to perform centralized, quantitative testing for viremia, and semi-quantitative detection of high-level viremia may have produced the same observations. Our monthly sampling frequency may have missed earlier windows of detection associated with clinical outcomes, including TMA and GVHD. The potential benefits and risks of treatments, such as intravenous immune globulin and cidofovir, would need to be tested in randomized trials. Finally, clinical information was abstracted from the medical record, possibly influencing the capture of outcomes, such as cystitis and GVHD.
In conclusion, BKPyV viremia was associated with significant kidney and bladder disease and mortality after HCT. Moreover, asymptomatic viremia was common and was associated with decreased kidney function. Assessments of novel interventions, such as the infusion of virus-specific T cells, are needed to determine whether preventing or treating BKPyV infection can improve morbidity and mortality, as our study does not suggest the utility of cidofovir. Patients with persistent, high-level symptomatic or asymptomatic viremia may benefit from infusion of BKPyV-specific T cells, and this hypothesis can be tested in future clinical trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgement. The authors thank Dr Ulf Beier for his assistance with illustrating the figures.
Financial support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (grant numbers K23 DK101600 to B. L. L. and K23 DK093556 to M. R. D.) and the National Center for Advancing Translational Sciences (grant number UL1 TR001878). Viracor-Eurofins tested the samples for BK polyomavirus viremia, viruria, and antibody at no charge.
Potential conflicts of interest. B. L. L and S. J. are coinventors of a patent application under review: Compositions and Methods for Treatment of HSCT-Associated Thrombotic Microangiopathy (United States Patent Number PCT/US2014/055922, 2014). B. L. L. has received consulting fees from Jazz Pharmaceuticals and Bioporto. S. K. and M. A. are employees of Viracor-Eurofins. M. R. D. has received research funding from Mallinckrodt, unrelated to this study. S. M. D. has received research support from Alexion Pharmaceuticals, personal fees from Novartis and Anthem, and research grants from Prolacta, outside the submitted work. S. J. has received research support from Alexion Pharmaceuticals; has received grants from the National Institutes of Health; has received personal fees from Omeros, Arcus Medica, and Magnolia Innovations, outside the submitted work; and has the following patents pending: 61/878,119, 62/094,802, 62/172,987, and 62/593,401. T. O. has received personal fees from Bluebird Bio, Miltenyi, and Novartis, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 2002; 347:488–96. [DOI] [PubMed] [Google Scholar]
- 2. Bedi A, Miller CB, Hanson JL, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol 1995; 13:1103–9. [DOI] [PubMed] [Google Scholar]
- 3. Cesaro S, Tridello G, Pillon M, et al. A prospective study on the predictive value of plasma BK virus-DNA load for hemorrhagic cystitis in pediatric patients after stem cell transplantation. J Pediatric Infect Dis Soc 2015; 4:134–42. [DOI] [PubMed] [Google Scholar]
- 4. Haines HL, Laskin BL, Goebel J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17:1512–9. [DOI] [PubMed] [Google Scholar]
- 5. Cesaro S, Dalianis T, Hanssen Rinaldo C, et al. ; European Conference on Infections in Leukemia (ECIL)-6 Group. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother 2018; 73:12–21. [DOI] [PubMed] [Google Scholar]
- 6. Erard V, Kim HW, Corey L, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood 2005; 106:1130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill JA, Mayer BT, Xie H, et al. The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood 2017; 129:2316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill JA, Mayer BT, Xie H, et al. Kinetics of double-stranded DNA viremia after allogeneic hematopoietic cell transplantation. Clin Infect Dis 2018; 66:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laskin BL, Nehus E, Goebel J, Furth S, Davies SM, Jodele S. Estimated versus measured glomerular filtration rate in children before hematopoietic cell transplantation. Biol Blood Marrow Transplant 2014; 20:2056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayden RT, Gu Z, Liu W, et al. Risk factors for hemorrhagic cystitis in pediatric allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis 2015; 17:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong AS, Chan KH, Cheng VC, Yuen KY, Kwong YL, Leung AY. Relationship of pretransplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clin Infect Dis 2007; 44:830–7. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 2012; 82:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood 2014; 124:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leboeuf C, Wilk S, Achermann R, et al. BK polyomavirus-specific 9mer CD8 T cell responses correlate with clearance of BK viremia in kidney transplant recipients: first report from the swiss transplant cohort study. Am J Transplant 2017; 17:2591–600. [DOI] [PubMed] [Google Scholar]
- 17. Ginevri F, Azzi A, Hirsch HH, et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant 2007; 7:2727–35. [DOI] [PubMed] [Google Scholar]
- 18. Schachtner T, Müller K, Stein M, et al. BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. Am J Transplant 2011; 11:2443–52. [DOI] [PubMed] [Google Scholar]
- 19. Schaenman JM, Korin Y, Sidwell T, et al. Increased frequency of BK virus-specific polyfunctional CD8+ T cells predict successful control of BK viremia after kidney transplantation. Transplantation 2017; 101:1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Apiwattanakul N, Hongeng S, Anurathapan U, et al. Viral-specific T-cell response in hemorrhagic cystitis after haploidentical donor stem cell transplantation. Transpl Infect Dis 2017; 19:e12775. doi: 10.1111/tid.12775. [DOI] [PubMed] [Google Scholar]
- 21. Schneidawind D, Schmitt A, Wiesneth M, et al. Polyomavirus BK-specific CD8+ T cell responses in patients after allogeneic stem cell transplant. Leuk Lymphoma 2010; 51:1055–62. [DOI] [PubMed] [Google Scholar]
- 22. Saliba RM, Rezvani K, Leen A, et al. General and virus-specific immune cell reconstitution after double cord blood transplantation. Biol Blood Marrow Transplant 2015; 21:1284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2017; 35:3547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pello OM, Innes AJ, Bradshaw A, et al. BKV-specific T cells in the treatment of severe refractory haemorrhagic cystitis after HLA-haploidentical haematopoietic cell transplantation. Eur J Haematol 2017; 98:632–4. [DOI] [PubMed] [Google Scholar]
- 25. Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014; 6:242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee YJ, Zheng J, Kolitsopoulos Y, et al. Relationship of BK polyoma virus (BKV) in the urine with hemorrhagic cystitis and renal function in recipients of T Cell-depleted peripheral blood and cord blood stem cell transplantations. Biol Blood Marrow Transplant 2014; 20:1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Donnell PH, Swanson K, Josephson MA, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Transplant 2009; 15: 1038–48 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. An P, Sáenz Robles MT, Duray AM, Cantalupo PG, Pipas JM. Human polyomavirus BKV infection of endothelial cells results in interferon pathway induction and persistence. PLOS Pathog 2019; 15:e1007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311:2518–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):179–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.