Abstract
Background
Annual human immunodeficiency virus (HIV) diagnoses in the United States (US) have plateaued since 2013. We assessed whether there is an association between uptake of pre-exposure prophylaxis (PrEP) and decreases in HIV diagnoses.
Methods
We used 2012–2016 data from the US National HIV Surveillance System to estimate viral suppression (VS) and annual percentage change in diagnosis rate (EAPC) in 33 jurisdictions, and data from a national pharmacy database to estimate PrEP uptake. We used Poisson regression with random effects for state and year to estimate the association between PrEP coverage and EAPC: within jurisdictional quintiles grouped by changes in PrEP coverage, regressing EAPC on time; and among all jurisdictions, regressing EAPC on both time and jurisdictional changes in PrEP coverage with and without accounting for changes in VS.
Results
From 2012 to 2016, across the 10 states with the greatest increases in PrEP coverage, the EAPC decreased 4.0% (95% confidence interval [CI], −5.2% to −2.9%). On average, across the states and District of Columbia, EAPC for a given year decreased by 1.1% (95% CI, −1.77% to −.49%) for an increase in PrEP coverage of 1 per 100 persons with indications. When controlling for VS, the state-specific EAPC for a given year decreased by 1.3% (95% CI, −2.12% to −.57%) for an increase in PrEP coverage of 1 per 100 persons with indications.
Conclusions
We found statistically significant associations between jurisdictional increases in PrEP coverage and decreases in EAPC independent of changes in VS, which supports bringing PrEP use to scale in the US to accelerate reductions in HIV infections.
Keywords: HIV prevention, PrEP, prophylaxis, HIV diagnoses
This study provides the first evidence of an association between increasing pre-exposure prophylaxis coverage and decreasing HIV diagnoses at the state and national level in the United States when controlling for changes in viral suppression rates.
Efforts to bring delivery of antiretroviral human immunodeficiency virus (HIV) prevention to scale are under way in the United States (US) to reduce the number of new HIV infections. Since 2008, the annual number of diagnoses in the United States has been decreasing slowly overall but has been stable or increasing among certain population groups [1, 2]. HIV incidence (annual new infections) has been stable from 2013 to 2016 after several years of decline [3]. Efforts to reduce new infections have focused on expanding (1) sustained antiretroviral therapy (ART) for persons with diagnosed HIV infection and (2) daily oral antiretroviral pre-exposure prophylaxis (PrEP) with coformulated tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) for persons without HIV who engage in sexual or drug injection behaviors that place them at significant risk of acquiring HIV infection. Since the US Food and Drug Administration’s approval of TDF/FTC for PrEP in 2012, the estimated number of US persons prescribed PrEP has increased from 8768 in 2012 to 77 120 in 2016 and 100 282 in 2017 [4]. During this period, ART delivery to all persons with HIV infection has increased as have efforts to retain them in care and to support high medication adherence in order to achieve viral suppression levels associated with essentially no sexual transmission to others [5, 6]. Randomized trials, cohort studies, and ecologic studies have demonstrated HIV diagnosis reductions associated with scale-up of either ART [7, 8] or PrEP [9–11] or with a combination [12] of the 2 strategies, but other studies have demonstrated only minimal change [13]. Additionally, mathematical models have indicated that increasing HIV testing and coverage of ART [14] and PrEP [15] alone or in combination [16–18] for indicated groups can result in substantial decreases in HIV incidence. We conducted an ecologic analysis at the US jurisdiction level to assess whether increasing PrEP prescription was associated with decreases in the rate of HIV diagnoses, taking into account (for a subset of states with available data) the changes in the observed state-specific levels of HIV viral suppression resulting from increased ART coverage.
METHODS
Data Sources
To assess HIV diagnosis trends, we analyzed HIV infection cases diagnosed during 2012–2016 among persons aged ≥13 years reported to the Centers for Disease Control and Prevention (CDC) through December 2017 from each US state and the District of Columbia (hereafter “jurisdictions”). Data about persons with reported diagnoses were identified by health department personnel conducting active surveillance and reviewing medical records. To account for change in HIV viral suppression rates, we used National HIV Surveillance System (NHSS) data reported to CDC from the 33 jurisdictions (32 states and the District of Columbia) with complete laboratory reporting of HIV type 1 viral load test results reported to NHSS for ≥3 years from 2013 through 2015. Viral suppression was defined as <200 copies/mL at the most recent test result reported during a given calendar year.
Data from the US Census Bureau’s American Community Survey 1-year population estimates for each calendar year in each jurisdiction were used as the denominator for computing HIV diagnosis rates. American Community Survey data were also the source for jurisdiction-specific social and demographic variables.
For our analysis, data for unique persons prescribed TDF/FTC were extracted from a larger Source Healthcare Analytics (SHA) data set containing approximately 82% of all TDF/FTC prescriptions in the US linked to anonymized medical or hospital claims data for all payment types (eg, public or private insurance, medication assistance programs, or cash). These data were then filtered by a validated algorithm that used medical procedure and diagnosis code data to select the TDF/FTC prescriptions that were provided for PrEP and to exclude uses of TDF/FTC for treatment of HIV infection, active hepatitis B infection, or HIV postexposure prophylaxis [4, 19]. PrEP coverage per 100 persons with an indication were calculated by using the number of persons prescribed PrEP in a calendar year as numerators for each state and the number of persons with a PrEP indication in each state during each calendar year as denominators, estimated using the method previously reported [20].
Statistical Analyses
Temporal trends in HIV diagnosis rates, 2012–2016, were modeled with year as an independent continuous variable. From this model, we reported state-specific estimated annual percentage change (EAPC) in HIV diagnosis rates. EAPC measures the relative change in HIV diagnoses rates per year [21].
To describe the relationship between changes in PrEP coverage and HIV diagnoses rates, we divided the 50 states into quintiles based on the changes in state-specific PrEP coverage calculated as the difference from 2012 to 2016. Within each quintile, we fit the temporal trends model and reported the quintile-specific EAPC and corresponding 95% confidence intervals.
We also estimated the association of state-level PrEP coverage with HIV diagnoses rates (2013–2016) while controlling for yearly trends in these rates, by including 1-year lagged PrEP coverage (2012–2015) as a continuous covariate in our temporal-trend models. The lagged covariate assessed the potential impact of PrEP coverage in a given year on HIV diagnoses during the following year.
We reported the relative change in state-specific HIV diagnoses rates for both a 1 per 100 and 5 per 100 increase in PrEP coverage over the previous year. Finally, for a subset of 33 jurisdictions with available data, we added to the model 1-year lagged state-specific viral suppression rates as a continuous covariate. This last model allowed assessment of the impact of PrEP coverage while controlling for viral suppression and temporal trends in HIV diagnosis rates.
State-level covariates for poverty, insurance coverage, education, income, and race were included, one at a time, to assess the robustness of PrEP coverage association while controlling for yearly trends in diagnosis rates and these demographic and social factors. In addition, because most PrEP use was in men (94% in 2017) [4] and men accounted for 81% of HIV diagnoses in 2017 [2], we refit our models using data for only males.
We used multilevel Poisson regression of rates with random effects for state and year. Random effects allowed for between-state variation in HIV diagnosis rates at the start of the study as well as between-state variation in how HIV diagnoses rates changed over the study period. In all models, an indicator, and its interaction with year, was included for the District of Columbia, because the District of Columbia was a notable outlier with respect to HIV diagnoses rate in the first year of the study period (2012) and in rate of decline over time.
All models were fit in SAS version 9.4 software (SAS Institute, Cary, North Carolina) using the GLIMMIX procedure.
Data Sharing
HIV diagnosis data are available at https://www.cdc.gov/nchhstp/atlas/index.htm and HIV viral suppression data at https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-22-2.pdf; TDF/FTC for PrEP prescription data from SHA are available at https://aidsvu.org/.
RESULTS
The HIV diagnosis rate averaged across states decreased from 13.1/100 000 persons during 2012 to 11.8/100 000 during 2016. Conversely, PrEP coverage among those estimated to have indications for its use, when averaged across 50 states and the District of Columbia, increased from 0.7 per 100 in 2012 to 5.8 per 100 in 2016 (Table 1).
Table 1.
Descriptive Statistics for Human Immunodeficiency Virus Diagnosis Rates and Pre-exposure Prophylaxis Coverage in 50 States and the District of Columbia (N = 51)
| Year | HIV Diagnosis Rate per 100 000 | PrEP Coverage per 100 | Viral Suppressiona,b | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| 2012 | 13.13 (14.78) | 103.20–1.47 | 0.72 (0.52) | 3.55–0.17 | 40.17 (6.43) | 52.20–28.10 |
| 2013 | 12.57 (12.83) | 87.74–1.92 | 1.03 (0.71) | 4.78–0.31 | 45.43 (8.20) | 62.20– 27.70 |
| 2014 | 12.36 (11.09) | 71.96–1.63 | 2.18 (1.38) | 8.90–0.50 | 47.54 (7.92) | 60.00– 29.00 |
| 2015 | 11.94 (10.05) | 62.45–2.18 | 4.64 (2.52) | 15.38–0.99 | 49.49 (7.12) | 62.00–36.40 |
| 2016 | 11.83 (9.26) | 56.83–0.92 | 5.80 (2.84) | 16.90–1.71 | NA | NA |
Abbreviations: HIV, human immunodeficiency virus; NA, not available; PrEP, pre-exposure prophylaxis; SD, standard deviation.
a2012: n = 27; 2013–2015: n = 33.
bCoverage is defined as persons prescribed PrEP during the prior year per estimated 100 persons with an indication for PrEP use.
EAPC in HIV diagnosis rates during 2012–2016 varied across states, from a 14.4% decrease in the District of Columbia to a 4.3% increase in Nevada. The change in PrEP coverage also varied across states, from an increase of 16.0 per 100 in New York to an increase of 1.5 per 100 in Wyoming (Table 2).
Table 2.
Jurisdiction-specific Estimated Annual Percentage Change in Human Immunodeficiency Virus Diagnosis Rates and Change in Pre-exposure Prophylaxis Coverage, United States, 2012–2016
| Statea | EAPC in HIV Diagnosis Rates (Lower, Upper 95% CI) | Change in PrEP Coverage Rates per 100 Persons With Indications | Quintile of Change in PrEP Coverage Rates | |
|---|---|---|---|---|
| DC | −14.43 | (−16.98, −11.79) | 10.49 | NA |
| Pennsylvania | −5.20 | (−6.79, −3.58) | 6.59 | 1 |
| New York | −5.05 | (−6.07, −4.02) | 15.60 | 1 |
| Maryland | −4.91 | (−6.51, −3.28) | 4.57 | 3 |
| Oregon | −3.77 | (−6.81, −.63) | 4.95 | 2 |
| Illinois | −3.75 | (−5.20, −2.27) | 7.61 | 1 |
| Washington | −3.46 | (−5.89, −.95) | 6.91 | 1 |
| Tennessee | −3.33 | (−5.32, −1.30) | 3.53 | 4 |
| Massachusetts | −3.31 | (−5.43, −1.15) | 11.05 | 1 |
| Delaware | −3.04 | (−6.69, .75) | 4.19 | 3 |
| Connecticut | −2.83 | (−5.68, .11) | 8.76 | 1 |
| Vermont | −2.36 | (−6.96, 2.47) | 3.05 | 5 |
| New Jersey | −2.32 | (−3.95, −.65) | 6.03 | 1 |
| Kentucky | −2.25 | (−4.95, .52) | 3.25 | 5 |
| Rhode Island | −2.24 | (−6.17, 1.86) | 7.12 | 1 |
| Minnesota | −2.14 | (−4.96, .76) | 5.25 | 2 |
| New Hampshire | −2.07 | (−6.36, 2.41) | 4.50 | 3 |
| Ohio | −2.05 | (−3.85, −.22) | 5.47 | 2 |
| West Virginia | −2.04 | (−5.98, 2.06) | 3.27 | 5 |
| Michigan | −1.77 | (−3.77, .28) | 4.41 | 3 |
| Montana | −1.62 | (−6.13, 3.11) | 5.45 | 5 |
| Nebraska | −1.59 | (−5.48, 2.47) | 5.02 | 2 |
| Georgia | −1.57 | (−2.76, −.37) | 5.03 | 2 |
| Virginia | −1.26 | (−3.11, .62) | 3.46 | 4 |
| Missouri | −1.16 | (−3.57, 1.30) | 4.26 | 2 |
| Kansas | −1.12 | (−4.61, 2.49) | 5.51 | 2 |
| South Carolina | −0.90 | (−2.97, 1.22) | 3.98 | 3 |
| Oklahoma | −0.83 | (−3.67, 2.09) | 3.27 | 4 |
| Texas | −0.81 | (−1.72, .11) | 3.60 | 4 |
| California | −0.80 | (−1.66, .07) | 5.62 | 2 |
| Mississippi | −0.63 | (−3.08, 1.90) | 4.26 | 3 |
| Wisconsin | −0.58 | (−3.71, 2.64) | 5.15 | 2 |
| Hawaii | −0.50 | (−4.34, 3.49) | 3.47 | 4 |
| Utah | −0.36 | (−4.03, 3.45) | 7.95 | 1 |
| Alabama | −0.15 | (−2.32, 2.06) | 4.72 | 3 |
| Alaska | −0.13 | (−4.62, 4.57) | 2.66 | 5 |
| Maine | −0.11 | (−4.42, 4.40) | 2.93 | 5 |
| Idaho | −0.04 | (−4.49, 4.61) | 3.13 | 5 |
| South Dakota | 0.02 | (−4.49, 4.74) | 4.46 | 3 |
| New Mexico | 0.16 | (−3.49, 3.94) | 3.03 | 5 |
| Florida | 0.27 | (−.64, 1.19) | 3.91 | 4 |
| Wyoming | 0.27 | (−4.47, 5.25) | 1.54 | 5 |
| Iowa | 0.29 | (−3.46, 4.18) | 9.28 | 1 |
| Louisiana | 0.57 | (−1.18, 2.34) | 4.04 | 3 |
| Arizona | 0.96 | (−1.19, 3.15) | 3.04 | 5 |
| North Carolina | 1.22 | (−.43, 2.89) | 3.84 | 4 |
| Indiana | 1.43 | (−1.03, 3.95) | 3.91 | 4 |
| Colorado | 1.57 | (−1.20, 4.42) | 3.68 | 4 |
| Arkansas | 2.40 | (−.72, 5.62) | 5.30 | 2 |
| North Dakota | 2.47 | (−2.59, 7.79) | 3.50 | 4 |
| Nevada | 4.32 | (1.53, 7.18) | 4.66 | 3 |
Quintiles of change in PrEP coverage: 1 = highest increase; 2 = second highest; 3 = third highest; 4 = fourth highest; 5 = lowest.
Abbreviations: CI, confidence interval; DC, District of Columbia; EAPC, estimated annual percentage change; HIV, human immunodeficiency virus; NA, not applicable; PrEP, pre-exposure prophylaxis.
aFor this study, data for the District of Columbia were analyzed as if equivalent to state data.
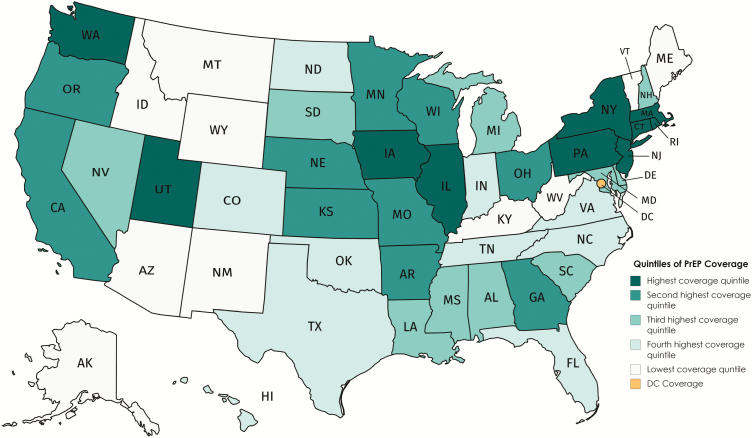
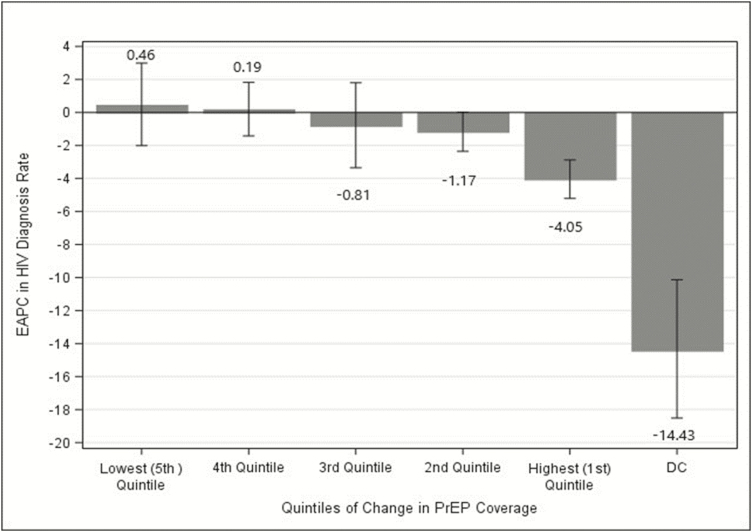
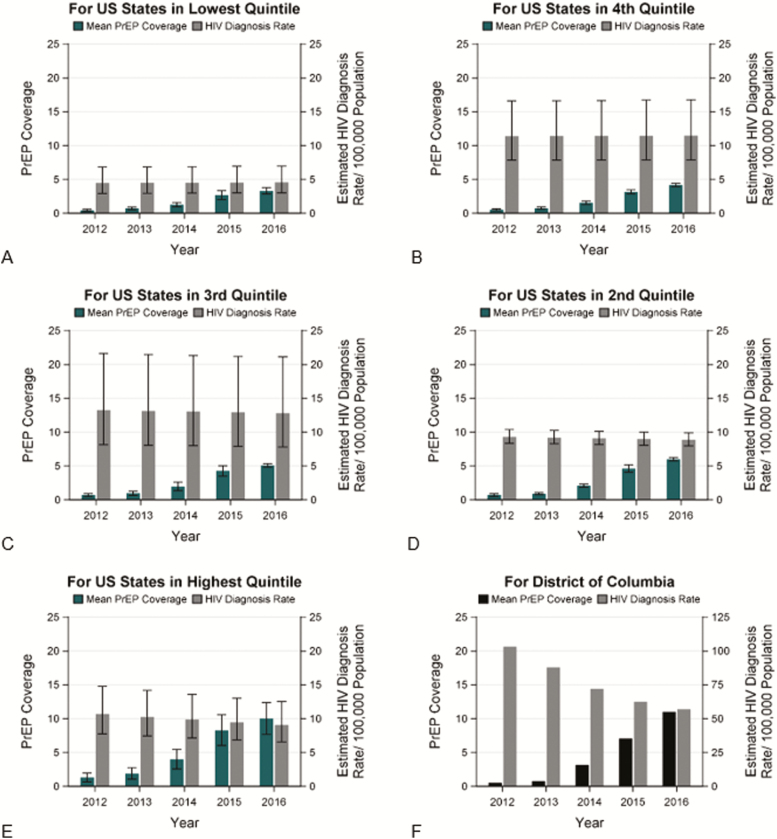
The 50 states were grouped into quintiles based on their change in PrEP coverage during 2012–2016 (Figure 1). For the 10 states with the greatest change in PrEP coverage, the average EAPC of HIV diagnosis rates decreased 4.0% and the average EAPC decreased by 1.2% in the 10 states with the second highest quintile of change in PrEP coverage (both statistically significant) (Figure 2). Though the changes were not statistically significant, in the third quintile the average EAPC decreased slightly and in the fourth and fifth quartiles, the average EAPC increased slightly. Overall, the pattern of quintile-specific estimates of average EAPC of HIV diagnosis rates suggest that, as change in PrEP coverage increases, EAPC of HIV diagnose rates decreases (Figure 3 and Supplementary Data 1.3.4).
Figure 1.
Quintiles of change in PrEP coverage, by state, United States, 2012–2016. State abbreviations can be found at https://www.50states.com/abbreviations.htm. Abbreviation: PrEP, pre-exposure prophylaxis.
Figure 2.
Average EAPC stratified by quintiles of change in PrEP coverage, United States, 2012–2016. Abbreviations: DC, District of Columbia; EAPC, estimated annual percentage change; HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis.
Figure 3.
Changes in tenofovir disoproxil fumarate/emtricitabine PrEP coverage and HIV diagnosis rates, in the District of Columbia and 5 state quintiles, United States (US), 2012–2016. Abbreviations: DC, District of Columbia; EAPC, estimated annual percentage change; HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis.
To model the impact on HIV diagnoses of increasing PrEP use, we incorporated a 1-year lag so that coverage during a given year was assessed for potential impact on diagnoses during the following year. When data were modeled from all 50 states and the District of Columbia, predicted EAPC in HIV diagnosis rate averaged across the 50 states decreased by 0.9% (Table 3). For the District of Columbia, the predicted EAPC in HIV diagnosis rates decreased by 13.7%. When yearly state-specific PrEP coverage was added to the model, on average, HIV diagnosis rates for a given year decreased by 1.1% for an increase in PrEP coverage of 1 per 100 and 5.6% decrease when PrEP coverage increased by 5 per 100 over the previous year. After controlling for PrEP coverage in the model, no statistically significant effect of time alone existed on HIV diagnosis rates.
Table 3.
Predicted Percentage Change in Human Immunodeficiency Virus Diagnosis Rates per Unit Increase in Pre-exposure Prophylaxis Coverage (Includes a 1-Year Lag), Without Accounting for Changes in Viral Suppression Rates
| Variable (Unit) | 50 US States and the District of Columbia (N = 51) | |
|---|---|---|
| Temporal Trend Model Percentage Change (95% CI) | PrEP Model Percentage Change (95% CI) | |
| Time (per 1 y) | ||
| 50 states | −0.95 (−1.90 to .02) | 0.56 (−.68, 1.81) |
| District of Columbia | −13.69 (−17.46 to −9.76) | −11.59 (−15.63, −7.36) |
| a(PrEP coverage)t−1 | NA | |
| (1 per 100) | −1.14 (−1.77 to −.49) | |
| (5 per 100) | −5.56 (−8.57 to −2.45) | |
| b(VS)t−1 | NA | NA |
| (1 per 100) | ||
| (5 per 100) |
t−1 was measured during the previous year.
Abbreviations: CI, confidence interval; NA, not applicable; PrEP, pre-exposure prophylaxis; VS, viral suppression.
aPrEP coverage rate during the previous year.
bVS rate among persons with diagnosed human immunodeficiency virus during the previous year.
When controlling for viral suppression in the 33 areas with ≥3 years of viral suppression data, the state-specific HIV diagnosis rate for a given year decreased by 1.3% for an increase in PrEP coverage of 1 per 100 and by 6.6% for an increase in PrEP coverage of 5 per 100 (Table 4). Similar to the model without viral suppression, no effect of time alone existed on HIV diagnosis rate. Additionally, we observed no impact of viral suppression on the HIV diagnosis rate when PrEP coverage was accounted for.
Table 4.
Predicted Percentage Change in Human Immunodeficiency Virus Diagnosis Rates per Unit Increase in Pre-exposure Prophylaxis Coverage (Includes a 1-Year Lag), When Accounting for Changes in Viral Suppression Rates
| Variable (Unit) | Jurisdictions With VS Data Available (n = 33) |
|---|---|
| PrEP and VS Model Percentage Change (95% CI) | |
| Time (per 1 year) | |
| 50 states | 1.11 (−1.06 to 3.33) |
| District of Columbia | −12.34 (−17.94 to −6.37) |
| a(PrEP coverage)t−1 | |
| (1 per 100) | −1.34 (−2.12 to −.57) |
| (5 per 100) | −6.55 (−10.15 to −2.80) |
| b(VS)t−1 | |
| (1 per 100) | −0.11 (−.53 to .32) |
| (5 per 100) | −0.54 (−2.63 to 1.59) |
t−1 was measured during the previous year.
Abbreviations: CI, confidence interval; PrEP, pre-exposure prophylaxis; VS, viral suppression.
aPrEP coverage rate during the previous year.
bVS rate among persons with diagnosed human immunodeficiency virus during the previous year.
Sensitivity analyses to assess difference in effect of PrEP in the male population and difference of PrEP effect when controlling for specific demographic or social factors revealed no difference from the results reported in Table 3 (see Supplementary Data). Estimates of PrEP impact in the male population were similar to those in Table 4 for the entire population. HIV diagnosis rates among men for a given year decreased by 0.94% for an increase in PrEP coverage of 1 per 100 and a 4.6% decrease when PrEP coverage increases by 5 per 100 over the previous year.
Discussion
Our results reveal associations between state-level increases in PrEP coverage and decreases in annual HIV diagnoses. States in the quintile with the greatest increase in PrEP coverage during 2012–2016 had the greatest average decrease in EAPC in HIV diagnosis rates. Conversely, states in the quintile with the lowest increase in PrEP coverage had no change in EAPC of HIV diagnosis rate.
In the 33 areas with HIV viral suppression data for ≥3 years, when controlling for changes in viral suppression, increases in PrEP coverage were independently associated with decreases in EAPC of HIV diagnosis rates. The lack of demonstrated impact of viral suppression on EAPC of HIV diagnosis after accounting for PrEP coverage may be due to the relatively small annual changes in viral suppression during the study period.
During the study period, a substantial increase occurred in the number of PrEP prescriptions recorded. The proportion of the population with indications for PrEP did not change markedly (data not shown). Variability was observed across states in the amount of change in PrEP coverage and the amount of change in EAPC of HIV diagnosis rates, both across individual states and within quintiles. The national and quintile EAPC reported here uses an unweighted average of state values and differs from values calculated with Poisson regression models using weighted averages [21]. Our estimates are a result of averaging effects in the few states with rapidly increasing PrEP coverage and the many states with more limited coverage. At this relatively large population level (ie, state), stronger associations concentrated among smaller populations are likely obscured. The higher decrease observed in the District of Columbia in EAPC of HIV diagnosis rates associated with PrEP coverage is an illustration of this possibility. Our next step is to analyze county-level data to determine if this obscuring effect can be reduced.
Our data and study have certain limitations. As an ecologic analysis, it may be subject to unmeasured bias; therefore, we cannot prove definitively that the use of PrEP led to the changes in the HIV diagnosis rates for any given state. However, the direction of our finding is consistent with the biologic mechanism of PrEP’s effectiveness for HIV prevention that has been proven in multiple clinical trials [22], and our findings are consistent with the low rate of HIV infections reported for observational cohorts of persons prescribed PrEP [23]. Estimated HIV incidence data were unavailable for all states during all years of interest; therefore, we used annual HIV diagnosis rates. HIV diagnosis data do not include persons with undiagnosed HIV infection, including acute infection, which is the source of approximately 40% of new infections [24]. HIV diagnosis data include persons infected in earlier years and first testing HIV positive in the year of diagnosis and reported to NHSS. Using incidence rates would be preferable, but these data were not available for all states and annual HIV diagnoses are the only available alternative. However, with current levels of laboratory reporting of HIV diagnoses, and an assumption of stable HIV testing rates, the HIV diagnosis rate tracks closely with modeled national HIV incidence rates. Another limitation is that pharmacy databases (eg, SHA) are a principal source of data used to estimate PrEP prescriptions. However, these data can be an underestimate because of missing prescription or diagnosis data or might overestimate actual usage because persons with a prescription might not take the medication as prescribed. Reliance on an indirect though validated algorithm to detect prescriptions for PrEP is due to the lack of a specific diagnosis code (eg, International Classification of Diseases, Tenth Revision, Clinical Modification code) and may contribute imprecision to our estimate of PrEP prescriptions. Moreover, no data regarding transmission risk group are available in pharmacy databases, and race/ethnicity data are limited, both of which are important for understanding disparities in HIV infection and use of PrEP as prevention. HIV viral load suppression data are unavailable for several states or for all study years for some states. Additionally, definitions of viral suppression use a single viral load measure per year (the last measure during a year). However, 40%–50% of persons with multiple viral load measures during a year are unsuppressed at some time during that year [25], and using a single annual level can overestimate viral suppression during that entire year.
Despite these limitations, our results are consistent with a recent report from Australia where very rapid PrEP expansion in a community that had already achieved high rates of viral suppression among persons with HIV infection resulted in a marked 31.5% reduction in diagnoses of recent HIV infection when comparing the 12 months before PrEP roll-out with the 12 months after PrEP roll-out [11].
In the United States, annual HIV diagnoses have occurred at the highest rates among black men and women and men who have sex with men of all race/ethnicities, yet PrEP coverage is highest among white men [26] and women [27]. Our results strongly support the urgency of expanding PrEP delivery to as many as possible of the 1.2 million persons at substantial risk for HIV acquisition, including all transmission risk groups, providing equitable access for all racial/ethnic groups, and focusing on the states and regions where the rates of HIV infections are highest. For that reason, the federal government is including PrEP provision as a focus activity in a new initiative, “Ending the HIV Epidemic: A Plan for America” [28], with a goal to reduce new HIV infections by 75% in the first 5 years and 90% within 10 years. Under this “whole of government” initiative, scale-up and broadening the population coverage of PrEP are being supported in multiple ways, including through the Federally Qualified Healthcare Center Program (https://findahealthcenter.hrsa.gov/), a new Department of Health and Human Services program to provide free PrEP medication to the uninsured with indications (https://www.getyourprep.com/) and the application of implementation science and program evaluation to disseminate strategies that prove to be effective.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D. K. S. and S. M. initiated the study; A. S. and R. M.-G. provided the data; B. C. conducted the statistical analyses in consultation with R. M.-G., and L. A. W., D. K. S., P. S. S., and B. C. wrote the first draft. All authors contributed to the study design and interpretation of the data, critically reviewed the manuscript, and approved its final version.
Acknowledgments. The authors thank Kay C. Smith for providing services as a scientific editor for this paper.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC).
Financial support. The CDC provided funds to all states and the District of Columbia for conducting human immunodeficiency virus (HIV) surveillance that generated data regarding annual HIV diagnoses and viral suppression used in this study (cooperative agreement number PS 13–1302). Truvada prescription data were purchased by Gilead Sciences from Source Healthcare Analytics and provided for this study under a multiyear memorandum of understanding between the CDC, Emory University, and Gilead Sciences. Emory University provided salaries for P. S. S. and L. A. W. The CDC provided salaries for authors D. K. S., B. C., A. S., N. S. H., K. W. H., and X. H., and they were not paid by any other party to write this article.
Potential conflicts of interest. P. S. S. has received grants and personal fees from the National Institutes of Health (NIH) and Gilead Sciences during the conduct of the study; has received grants and personal fees from the NIH, CDC, and Gilead Sciences; has received personal fees from the Ontario HIV Research Network; and is on the Board of Directors for the M·A·C AIDS Fund, outside the submitted work. S. M. and R. M.-G. are employees and shareholders of Gilead Sciences. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Atlas Plus 2019. Available at: https://www.cdc.gov/nchhstp/atlas/index.htm. Accessed 19 February 2019.
- 2. Centers for Disease Control and Prevention. HIV surveillance report, 2017 Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf. Accessed 2 January 2019.
- 3. Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report 2019 Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-24-1.pdf. Accessed 3 March 2019.
- 4. Sullivan PS, Giler RM, Mouhanna F, et al. Trends in the use of oral emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis against HIV infection, United States, 2012–2017. Ann Epidemiol 2018:28:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. CDC fact sheet: trends in U.S. HIV diagnoses, 2005-2014 Available at: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-data-trends-fact-sheet-508.pdf. Accessed 10 June 2018.
- 6. Centers for Disease Control and Prevention. Understanding the HIV care continuum 2018. Available at: https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum.pdf. Accessed 2 January 2019.
- 7. Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okano JT, Robbins D, Palk L, Gerstoft J, Obel N, Blower S. Testing the hypothesis that treatment can eliminate HIV: a nationwide, population-based study of the Danish HIV epidemic in men who have sex with men. Lancet Infect Dis 2016; 16:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cairns G. The UK’s largest sexual health clinic saw a 40% drop in new HIV infections this year. AIDSMap2016. Available at: http://www.aidsmap.com/The-UKs-largest-sexual-health-clinic-saw-a-40-drop-in-new-HIV-infections-this-year/page/3106754/. Accessed 10 June 2018.
- 10. Grulich A. Rapid reduction in HIV diagnoses after targeted PrEP implementation in NSW, Australia 2018. Available at: http://www.croiwebcasts.org/console/player/37190?mediaType=audio&&crd_fl=1&ssmsrq=1528669149203&ctms=5000&csmsrq=2324. Accessed 10 June 2018.
- 11. Grulich AE, Guy R, Amin J, et al. Expanded PrEP Implementation in Communities New South Wales (EPIC-NSW) Research Group Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study. Lancet HIV 2018; 5:e629–37. [DOI] [PubMed] [Google Scholar]
- 12. Brown AE, Mohammed H, Ogaz D, et al. Fall in new HIV diagnoses among men who have sex with men (MSM) at selected London sexual health clinics since early 2015: testing or treatment or pre-exposure prophylaxis (PrEP)? Euro Surveill 2017; 22:30553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwuji CC, Orne-Gliemann J, Larmarange J, et al. ANRS 12249 TasP Study Group Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV 2017; 5:e116–25. [DOI] [PubMed] [Google Scholar]
- 14. Phillips AN, Cambiano V, Miners A, et al. Potential impact on HIV incidence of higher HIV testing rates and earlier antiretroviral therapy initiation in MSM. AIDS 2015; 29:1855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenness SM, Sharma A, Goodreau SM, et al. Individual HIV risk versus population impact of risk compensation after HIV preexposure prophylaxis initiation among men who have sex with men. PLoS One 2017; 12:e0169484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott N, Stoové M, Kelly SL, Wilson DP, Hellard ME. Achieving 90-90-90 human immunodeficiency virus (HIV) targets will not be enough to achieve the HIV incidence reduction target in Australia. Clin Infect Dis 2018; 66:1019–23. [DOI] [PubMed] [Google Scholar]
- 17. Khurana NP, Yaylali EP, Farnham PGP, et al. Impact of improved HIV care and treatment on PrEP effectivenesss in the United States, 2016–2020. J Acquir Immune Defic Syndr 2018; 78:399–405. [DOI] [PubMed] [Google Scholar]
- 18. Kusejko K, Marzel A, Hampel B, et al. Swiss HIV Cohort Study Quantifying the drivers of HIV transmission and prevention in men who have sex with men: a population model-based analysis in Switzerland. HIV Med 2018; 19:688–97. [DOI] [PubMed] [Google Scholar]
- 19. Mera-Giler RMT, Magnuson D, Bush S, Piontkowsky D. Validation of a Truvada for PrEP algorithm through chart review from an electronic medical record. In: National HIV Prevention Conference, Atlanta, GA, 2015. [Google Scholar]
- 20. Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol 2018; 28:850–7.e9. [DOI] [PubMed] [Google Scholar]
- 21. Chapin-Bardales J, Rosenberg ES, Sullivan PS. Trends in racial/ethnic disparities of new AIDS diagnoses in the United States, 1984–2013. Ann Epidemiol 2017; 27:329–34.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016; 30:1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marcus JL, Hurley LB, Nguyen DP, Silverberg MJ, Volk JE. Redefining human immunodeficiency virus (HIV) preexposure prophylaxis failures. Clin Infect Dis 2017; 65:1768–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gopalappa C, Farnham PG, Chen Y-H, Sansom SL. Progression and transmission of HIV/AIDS (PATH 2.0): a new, agent-based model to estimate HIV transmissions in the United States. Med Decis Making 2017; 37:224–33. [DOI] [PubMed] [Google Scholar]
- 25. Crepaz N, Dong X, Wang X, Hernandez AL, Hall HI. Racial and ethnic disparities in sustained viral suppression and transmission risk potential among persons receiving HIV care—United States, 2014. Morb Mortal Wkly Rep 2018; 67:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marcus JL, Hurley LB, Hare CB, Silverberg MJ, Volk JE. Disparities in uptake of HIV preexposure prophylaxis in a large integrated health care system. Am J Public Health 2016; 106:e2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith DK, MVH, Grey JA. By race/ethnicity, blacks have highest number needing PrEP in the United States, 2015 2018. Available at: http://www.croiwebcasts.org/p/2018croi/86. Accessed 9 January 2019.
- 28. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.