Supplemental Digital Content is Available in the Text.
The INJECT trial of ocriplasmin for vitreomacular traction in clinical practice showed safety and efficacy results that are consistent with previous studies, including the randomized controlled MIVI-TRUST and OASIS trials.
Key words: macular hole, ocriplasmin, symptomatic vitreomacular adhesion, vitrectomy, vitreomacular traction
Abstract
Purpose:
Randomized clinical trials have demonstrated the safety and efficacy of ocriplasmin in patients with vitreomacular traction (VMT), including those with macular hole (MH). The INJECT study prospectively evaluated ocriplasmin in the setting of clinical practice.
Methods:
INJECT was a Phase 4, multicenter, prospective observational study. Patients were followed up for 12 months. Assessments included nonsurgical VMT resolution, nonsurgical MH closure, best-corrected visual acuity, occurrence of vitrectomy, and adverse events.
Results:
The efficacy population (N = 395) received an ocriplasmin injection and had optical coherence tomography–confirmed VMT at baseline. At Day 28, the rate of nonsurgical VMT resolution was 40.7% in the overall group, and the rate of nonsurgical MH closure was 36.0% in the VMT with MH group. At Month 12, the rate of ≥2-line best-corrected visual acuity gain (irrespective of vitrectomy) was 36.8% in the overall group and 59.6% in the VMT with MH group. The percentage of patients who underwent vitrectomy in the study eye was 29.1% in the overall group and 55.6% in the VMT with MH group. Photopsia (9.8%) and vitreous floaters (6.8%) were the most frequent adverse events.
Conclusion:
The INJECT study showed that ocriplasmin is effective in a clinical setting in patients with VMT, with or without MH. No new safety signals were identified from this large and surgeon-selected patient group, although the significant limitations of the study design without an image reading center and scheduled study visit timings should be noted.
Posterior vitreous detachment is a normal age-related process. Abnormal detachment of the vitreous during this process can result in vitreomacular traction (VMT, also referred to as symptomatic vitreomacular adhesion) and/or macular hole (MH).1 Established management options include watchful waiting, vitrectomy, and ocriplasmin. Expansile gas to achieve “pneumatic vitreolysis” has also recently been proposed as an alternative; however, prospective trial data to guide clinical practice are limited to date.2 Ocriplasmin is a recombinant truncated form of human plasmin that is approved by the European Medicines Agency for the treatment of adults with VMT, including when associated with an MH of diameter ≤400 µm.
The safety and efficacy of ocriplasmin for VMT, with and without MH, were demonstrated in Phase 3 randomized clinical trials (MIVI-TRUST and OASIS).3,4 Further analysis identified baseline predictors associated with increased odds of treatment success, with retrospective case series and Phase 4 studies (ORBIT and OVIID-1) supporting the use of these predictors in optimizing outcomes.5–16 Small case series and reports have described adverse events (AEs), including transient vision loss and transient changes in the ellipsoid zone on optical coherence tomography (OCT).17–28 However, the long-term effects of ocriplasmin in a clinical practice setting in a large, diverse population outside the United States have not been widely documented.
To evaluate the efficacy and safety of ocriplasmin in the clinical practice setting in Canada and Europe, we designed a multicenter, prospective, observational trial, the INvestigation of JETREA in Patients with Confirmed Vitreomacular Traction (INJECT) study. Results are reported herein.
Materials and Methods
Study Design
INJECT was a Phase 4, multicenter, prospective, observational study of patients treated with ocriplasmin for VMT. Patients enrolled at European (n = 20 Germany, n = 30 United Kingdom, n = 5 Portugal, n = 4 Spain, n = 1 Norway, and n = 1 The Netherlands) and Canadian sites (n = 7) with VMT, including when associated with an MH of diameter ≤400 µm, according to the European Medicines Agency and Health Canada indications.
Patients were aged ≥18 years and treated with ocriplasmin 0.125 mg at the physician's discretion according to the local product label. Patients were excluded if they received ocriplasmin for medical conditions outside the product labeling or if they had any contraindications to ocriplasmin.
After initial diagnosis at screening, the presence of VMT was confirmed by OCT on the day of ocriplasmin injection. The eye that received ocriplasmin was termed the study eye. In patients in whom both eyes were eligible for injection, the eye that received ocriplasmin first was the study eye. Data were collected for both eyes, but this report includes results for study eyes alone.
The investigator performed all OCT assessments according to local practice and without a central reading center. Optical coherence tomography equipment used is listed in Supplemental Digital Content 1, http://links.lww.com/IAE/B239. Three follow-up visits were scheduled at the discretion of each investigator per local practice, although investigators were encouraged to complete all visits within 12 months (±2 months). To facilitate analysis, each visit was assigned to one of the following time points: Baseline was the date of injection; Day 14 included any visit after baseline to ≤Day 20; Day 28 included any visit >Day 20 to ≤Day 60; Month 6 included any visit >Day 60 to ≤Day 240; and Month 12 included any visit >Day 240. If more than one record fell into the same time frame, the one closest to Day 14, Day 28, Month 6, or Month 12 was used.
Institutional review boards and/or independent ethics committees approved the study protocol. All patients provided written informed consent. The study was conducted in compliance with the Declaration of Helsinki.
Outcomes and Assessments
Assessments included monocular distance best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, OCT, fundus photography, intraocular pressure, AEs and ocular symptoms, and vision-related quality of life measured with the 25-item National Eye Institute Visual Function Questionnaire (VFQ-25). Efficacy outcomes included nonsurgical VMT resolution (without vitrectomy), nonsurgical MH closure (without vitrectomy), BCVA (irrespective of vitrectomy), and ≥2-line gain in BCVA (irrespective of vitrectomy). Other outcomes included the occurrence of vitrectomy and VFQ-25 composite score. Safety outcomes included the type, frequency, seriousness, severity, and treatment relationship (as assessed by the investigator) of AEs and the frequency of ocular symptoms. Investigators were required to report all AEs regardless of causality or severity within 24 hours.
Statistical Analysis
Demographics, baseline characteristics, and safety analysis included all patients who received an ocriplasmin injection in the study eye. The efficacy analysis was limited to patients who received an ocriplasmin injection in the study eye and had OCT-confirmed VMT diagnosed at baseline by the investigator. Descriptive statistics for continuous variables were reported as the number of available observations and medians with interquartile ranges where appropriate. Categorical data were reported as available observations, including the frequency and percentage for each category, where appropriate. The last observation carried forward method was used to impute missing data. In addition to the analysis by defined time points, an analysis of the last observed value was also performed to systematically capture the status under which patients left the study.
Subgroup analyses were performed based on the presence of baseline MH and, among those with baseline MH, based on small (≤250 µm) or medium size (>250 to ≤400 µm) at baseline. Patients with VMT and a large MH (>400 µm) were included in the overall VMT with MH group (n = 8). Patients with no baseline MH information (n = 42) were included in the overall category but not the subgroups. Investigators used OCT to measure the MH size according to local practice. Additional subgroup analyses were performed for patients who received or did not receive rescue therapy, that is, vitrectomy, to investigate the influence of vitrectomy on the final BCVA result at Month 12.
A multivariable logistic regression analysis was performed on the efficacy population to identify baseline factors associated with outcomes after ocriplasmin injection. Candidate baseline variables included the following: VMT with MH (yes or no), sex (women or men), age (<65 or ≥65 years), subretinal fluid (SRF, yes or no on OCT), MH size at baseline (≤250 µm [small] or >250 to ≤400 µm [medium] on OCT as assessed by the investigator according to local practice), epiretinal membrane (ERM) (yes or no), and cataract detected on slit-lamp examination (yes or no). Outcomes tested were nonsurgical VMT resolution at Day 28, MH closure at Day 28, and ≥2-line BCVA gain at the last observation. A stepwise procedure was used in which all candidate variables needed to satisfy a criterion of α < 0.05 in a univariable model to enter the multivariable model and α < 0.05 to remain in the model. Summary results were reported as odds ratios, 95% confidence intervals, and P values based on the Wald chi-square test. SAS software, Version 9.4 (SAS Institute, Cary, NC), was used for analysis.
Results
Patient Populations and Demographics
INJECT enrolled 452 patients across 68 sites (median of five patients per site) (see Supplemental Digital Content 2, http://links.lww.com/IAE/B240). Of these, 428 (safety population) received an injection of ocriplasmin, which included 33 patients with missing information for VMT and 26 patients with missing information for both VMT and MH. Baseline characteristics are reported in Table 1. Overall, 27.3% (117/428) of patients had a baseline MH, and 5.2% (20/382) had an ERM as per investigator assessment.
Table 1.
Demographics and Baseline Ocular Characteristics Based on the Investigator Assessment (Safety Population)
| Characteristics | Overall* | VMT Without MH | VMT With MH† | ||
| (N = 428) | (N = 269) | VMT With MH of Any Size (N = 117) | VMT With Small MH (≤250 µm) (N = 49) | VMT With Medium MH (>250 to ≤400 µm) (N = 54) | |
| Study population (percentage of total), n (%) | 428 (100) | 269 (62.9) | 117 (27.3) | 49 (11.4) | 54 (12.6) |
| Age (years) | |||||
| Median (Q1, Q3) | 74.0 (67.0, 79.0) | 75.0 (70.0, 81.0) | 68.0 (64.0, 74.0) | 67.0 (63.0, 72.0) | 69.0 (64.0, 75.0) |
| Gender, n (%) | |||||
| Male | 143 (33.4) | 103 (38.3) | 27 (23.1) | 10 (20.4) | 15 (27.8) |
| Female | 285 (66.6) | 166 (61.7) | 90 (76.9) | 39 (79.6) | 39 (72.2) |
| Race, n (%) | |||||
| n | 427 | 268 | 117 | 49 | 54 |
| White | 411 (96.3) | 257 (95.9) | 113 (96.6) | 48 (98.0) | 51 (94.4) |
| Black | 11 (2.6) | 6 (2.2) | 4 (3.4) | 1 (2.0) | 3 (5.6) |
| Asian | 5 (1.2) | 5 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Comorbid ocular condition, n (%) | |||||
| n | 428 | 269 | 117 | 49 | 54 |
| AMD | 24 (5.6) | 20 (7.4) | 2 (1.7) | 1 (2.0) | 0 (0.0) |
| Cataract | 31 (7.2) | 17 (6.3) | 11 (9.4) | 4 (8.2) | 7 (13.0) |
| DR | 15 (3.5) | 13 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Glaucoma | 12 (2.8) | 11 (4.1) | 1 (0.9) | 0 (0.0) | 1 (1.9) |
| RVO | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ERM, n (%) | |||||
| n | 382 | 262 | 111 | 46 | 54 |
| Present | 20 (5.2) | 18 (6.9) | 2 (1.8) | 0 (0.0) | 2 (3.7) |
| Absent | 362 (94.8) | 244 (93.1) | 109 (98.2) | 46 (100.0) | 52 (96.3) |
| VMT data available at baseline, n (%) | |||||
| n | 396 | 267 | 114 | 47 | 54 |
| Present | 301 (76.0) | 208 (77.9) | 80 (70.2) | 35 (74.5) | 37 (68.5) |
| VMT ≤1,500 µm at baseline, n (%) | |||||
| n | 291 | 204 | 78 | 35 | 36 |
| Yes | 284 (97.6) | 198 (97.1) | 78 (100.0) | 35 (100.0) | 36 (100.0) |
| VMT width at baseline (µm) | |||||
| n | 284 | 204 | 78 | 35 | 36 |
| Median (Q1, Q3) | 355.5 (227.0, 510.0) | 411.0 (273.0, 537.0) | 251.0 (152.0, 361.0) | 246.0 (119.0, 351.0) | 251.5 (170.0, 382.5) |
| Monocular distance BCVA (ETDRS) | |||||
| n | 409 | 263 | 110 | 47 | 52 |
| Median (Q1, Q3) | 61.0 (53.0, 70.0) | 65.0 (55.0, 70.0) | 55.0 (46.0, 60.0) | 59.0 (50.0, 65.0) | 50.0 (38.5, 56.5) |
Overall includes 26 patients missing information for both VMT and MH, 9 patients with VMT but missing information for MH, and 7 patients with both VMT and MH recorded as “No” who were not included in the VMT without MH, or VMT with any size MH, small MH, or medium MH subgroups.
The VMT with MH of any size subgroup includes eight patients with a large MH and six patients missing information for MH size who were not included in the VMT without MH, VMT with small MH, or VMT with medium MH subgroups.
AMD, age-related macular degeneration; DR, diabetic retinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study; Q1, interquartile range, first quartile; Q3, interquartile range, third quartile; RVO, retinal vein occlusion.
Nonsurgical Vitreomacular Traction Resolution and Macular Hole Closure
The rate of VMT resolution among evaluable patients in the overall group was 40.7% (120/295) at Day 28 (Figure 1A). In the VMT with MH group, the rate of MH closure was 36.0% (32/89) at Day 28 (Figure 1B). The rate of MH closure was highest in the VMT with small MH group (Day 28: 48.6% [17/35]). Patients who required vitrectomy were considered nonresponders.
Fig. 1.
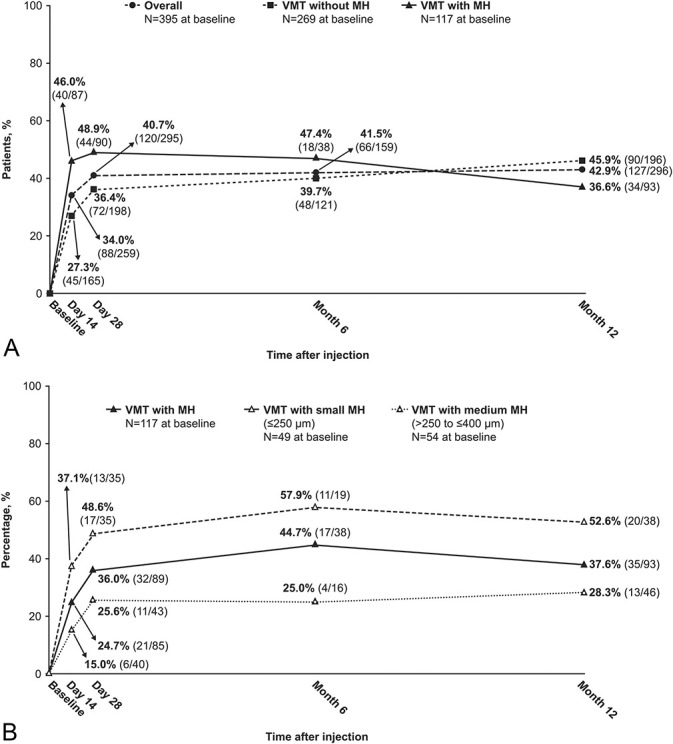
Resolution of VMT and closure of MH over the course of the study (efficacy population): A. Rates of nonsurgical VMT resolution at each visit for the overall, VMT without MH, and VMT with MH groups. B. Rates of nonsurgical MH closure at each visit for the VMT with MH, VMT with small MH, and VMT with medium MH groups. Patients who required vitrectomy were considered nonresponders.
Macular hole closure with VMT resolution occurred in 22.5% (20/89) of the VMT with MH group at Day 28. This happened in 31.4% (11/35) and 44.7% (17/38) of the VMT with small MH group and in 14.0% (6/43) and 23.9% (11/46) of the VMT with medium MH group at Day 28 and Month 12, respectively. In 13.5% (12/89) of cases, the MH closed without VMT resolution at Day 28.
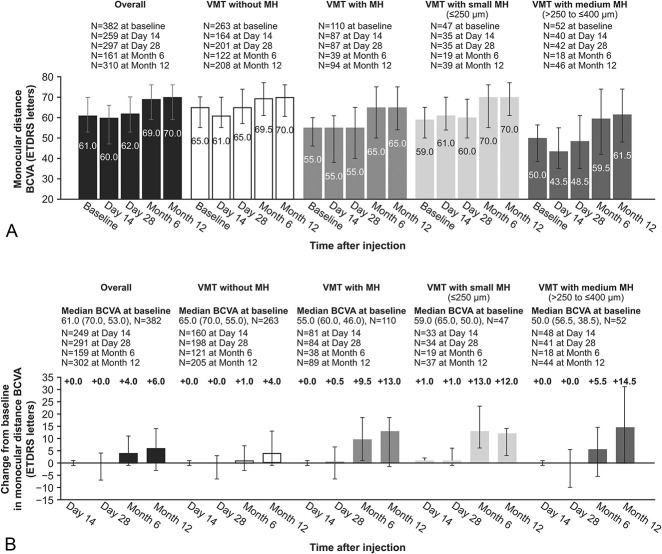
Best-Corrected Visual Acuity and Vision-Related Quality of Life
The BCVA increased above baseline by Day 14 in the VMT with small MH group and by Month 6 in all other groups (Figure 2, A and B). Overall, the median BCVA change from baseline at Month 12 was 6.0 letters; the change from baseline in BCVA is shown for all groups in Figure 2B. VFQ-25 composite scores increased from baseline to Month 12 in all groups (see Supplemental Digital Content 3, http://links.lww.com/IAE/B241).
Fig. 2.
Best-corrected visual acuity from baseline to Month 12 (efficacy population): A. Monocular distance BCVA was assessed over the study period, irrespective of vitrectomy. Best-corrected visual acuity was measured in Early Treatment Diabetic Retinopathy Study letters. The graph shows median values, as well as top and bottom error bars show interquartile ranges Q1 and Q3, respectively. B. Change from baseline in monocular distance BCVA at each visit after ocriplasmin injection, irrespective of vitrectomy. The graph shows median values, as well as top and bottom error bars show interquartile ranges Q1 and Q3, respectively.
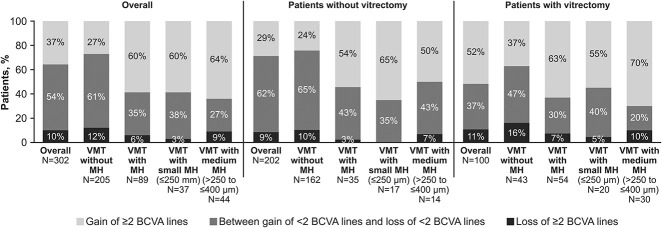
In the subgroup of patients who did not require rescue treatment, that is, vitrectomy, the median BCVA change from baseline to Month 12 was equal to 4.85 overall, with median changes equal to 3.0, 10.21, 10.12, and 14.00 in the VMT, VMT with MH, VMT with medium MH, and VMT with small MH subgroups, respectively. In the subgroup of patients who did require vitrectomy, the median BCVA change from baseline to Month 12 was equal to 10.13 overall, with median changes equal to 4.05, 13.50, 14.95, and 11.95 in the VMT, VMT with MH, VMT with medium MH, and VMT with small MH subgroups, respectively. The proportion of patients who gained or lost at least two lines in the BCVA from baseline to Month 12 is shown in Figure 3. Patients with and without vitrectomy showed a similar trend in the BCVA gain and loss.
Fig. 3.
Monocular distance BCVA, categorized by a ≥2-line gain, ≥2-line decrease, or between a <2-line gain and <2-line decrease at Month 12, irrespective of vitrectomy.
Occurrence of Vitrectomy
The percentages of patients with ≥1 vitrectomy on the study and the median (range) time to the first vitrectomy are shown in Figure 4. The most frequent indications for the first vitrectomy were MH and VMT (see Supplemental Digital Content 4, http://links.lww.com/IAE/B242).
Fig. 4.
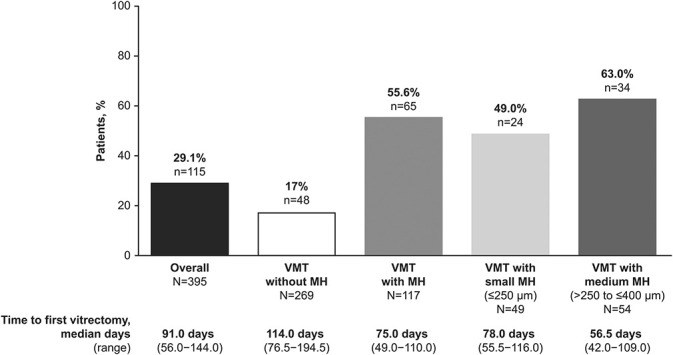
Rates of vitrectomy in the study eye at Month 12 (efficacy population).
Baseline Predictors of Anatomic and Visual Outcomes
Sex, age, and baseline MH size were significantly associated with ocriplasmin treatment outcomes. Female patients were more likely to have a ≥2-line BCVA gain at the last observation compared with male patients (reference: male sex, odds ratio: 1.8, 95% confidence interval 1.0–3.0, P = 0.03). Patients younger than 65 years were more likely to have nonsurgical VMT resolution at Day 28 compared with patients 65 years of age or older (reference: <65 years, odds ratio: 0.4, 95% confidence interval 0.2–0.6, P < 0.01). Patients with VMT and a small MH were more likely to have nonsurgical MH closure at Day 28 compared with patients with VMT and a medium MH (reference: VMT with medium MH, odds ratio: 0.3, 95% confidence interval 0.1–0.7, P < 0.01).
Adverse Events and Ocular Symptoms
Ocular AEs in the study eye were reported in 46.3% (198/428) of the overall group (Table 2). The most frequent AEs suspected to be related to the study drug as assessed by the investigator were photopsia (9.6%, 41/428) and vitreous floaters (6.5%, 28/428). For patients with MH reported as an AE in the study eye (n = 25), 22 already had baseline MH by OCT assessment and three were newly formed. Of the 22 MHs that were already present at baseline, seven were recorded as “worsening MH” during the study. The remaining 15 patients had baseline MH, and the investigator reported MH as an AE without specifying “worsening MH.” Therefore, at least 10 patients had a new or worsening MH, and the other 15 may have had an enlargement of their MH after ocriplasmin treatment, making the incidence of new or worsening MH potentially in 25/423 (5.9%) of the total population and 22/117 (18.9%) of the MH at baseline population. Time to onset and resolution of new or worsening MH was not reported. The rate of a ≥2-line BCVA decrease (overall, and according to vitrectomy status) is shown in Figure 3. No data were recorded regarding the number of patients with a ≥2-line BCVA decrease who also had cataract surgery.
Table 2.
Ocular AEs and Ocular Symptoms of Interest Reported in the Study Eye (Safety Population)
| Preferred Term | Overall (N = 428) |
| Ocular AEs, no. of patients (%) | 198 (46.3) |
| Suspected to be related to the study drug* | 160 (37.4) |
| Photopsia | 42 (9.8) |
| Vitreous floaters | 29 (6.8) |
| MH | 25 (5.8) |
| Newly occurring or worsening MH | 10 (2.3)† |
| Metamorphopsia | 25 (5.8) |
| Visual impairment | 16 (3.7) |
| Eye pain | 15 (3.5) |
| Visual acuity reduced | 15 (3.5) |
| Retinal detachment | 13 (3.0) |
| Had vitrectomy for retinal detachment | 8 (1.9) |
| Serious ocular AEs, no. of patients (%) | 39 (9.1) |
| Suspected to be related to the study drug* | 26 (6.1) |
| MH | 10 (2.3) |
| Photopsia | 2 (0.5) |
| Metamorphopsia | 2 (0.5) |
| Retinal detachment | 2 (0.5) |
| Retinal tear | 2 (0.5) |
| Blindness‡ | 2 (0.5) |
| Blindness unilateral§ | 1 (0.2) |
| Visual acuity reduced§ | 1 (0.2) |
| Foreign-body sensation in eyes§ | 1 (0.2) |
| Sudden visual loss¶ | 1 (0.2) |
| Vision blurred | 1 (0.2) |
| Vitreous adhesion | 1 (0.2) |
| Vitreous hemorrhage | 1 (0.2) |
| Endophthalmitis** | 1 (0.2) |
| Infection | 1 (0.2) |
| Ocular symptoms | |
| Flashes of light, no. of patients (%) | |
| Baseline | 16/295 (5.4) |
| Day 28 | 42/226 (18.6) |
| Month 6 | 7/135 (5.2) |
| Month 12 | 8/228 (3.5) |
| Color vision abnormalities††, no. of patients (%) | |
| Baseline | 19/286 (6.6) |
| Day 28 | 26/219 (11.9) |
| Month 6 | 14/131 (10.7) |
| Month 12 | 11/220 (5.0) |
| Micropsia, no. of patients (%) | |
| Baseline | 23/284 (8.1) |
| Day 28 | 27/220 (12.3) |
| Month 6 | 7/131 (5.3) |
| Month 12 | 9/227 (4.0) |
| Metamorphopsia, no. of patients (%) | |
| Baseline | 166/317 (52.4) |
| Day 28 | 101/239 (42.3) |
| Month 6 | 46/142 (32.4) |
| Month 12 | 63/248 (25.4) |
Estimated.
As assessed by the investigator.
There were two cases in which the investigator recorded the term “blindness.” One patient reported and had resolution on Day 1. The other patient reported on Day 2 and had resolution at a visit outside of the follow-up period (Day 582).
Visual acuity reduced, blindness unilateral, and foreign-body sensation in the eye were reported in the same patient. All were reported on Day 2 and resolved on Day 358.
Sudden visual loss was reported in a patient separately from those who reported blindness, unilateral blindness, or visual acuity reduced. The event was reported on Day 1 and resolved on Day 3.
Endophthalmitis was reported on Day 4 and resolved on Day 59 after treatment with an unspecified medication.
Included chromatopsia, xanthopsia, color blindness, and acquired color blindness.
The percentage of patients with a serious AE in the study eye was 9.1% (39/428), with 6.1% (26/428) suspected to be related to the study drug as assessed by the investigator (Table 2). There were no reports of zonular dehiscence or lens subluxation. Five patients (1.2%) withdrew from the study because of ocular AEs, two of which were serious AEs, both of which were retinal detachments and both treated successfully by vitrectomy. Seven patients (1.6%) withdrew from the study because of nonocular AEs.
Investigators reported 13 patients with retinal detachment in the study eye, of which 10 were considered by investigators to be treatment related. The median time to onset was 8 days, and the median time to resolution was 62 days. Of the eight patients who had vitrectomy, six reattached within the follow-up period, one reattached at a visit after the follow-up period (Day 701), and one was unresolved at the last observation. Five cases of retinal detachment resolved without intervention; the exact extent and etiology of these cases were uncertain.
The percentage of patients with submacular SRF increased during the month after ocriplasmin injection and then decreased below baseline levels at Month 12. In the overall group at baseline, 12.6% (47/374) of patients had SRF. After ocriplasmin injection, 24.9% (69/277) had SRF at Day 28, 14.6% (23/157) at Month 6, and 8.1% (23/284) at Month 12. The extent of SRF was not quantified.
The incidence of some ocular symptoms (e.g., flashes of light; micropsia; and color vision abnormalities, including chromatopsia, xanthopsia, color blindness, and acquired color blindness) increased during the month after ocriplasmin injection and then decreased to baseline levels or lower at Month 6 (Table 2). By contrast, the incidence of metamorphopsia decreased at each subsequent time point after ocriplasmin.
Discussion
Ocriplasmin is a pharmacologic alternative to vitrectomy for the treatment of VMT, including when associated with an MH of ≤400 µm, especially for patients who have early VMT and for whom a surgical intervention seems not yet to be appropriate. INJECT was a Phase 4 observational study of patients with VMT who received ocriplasmin injection. Results were consistent with the MIVI-TRUST, OASIS, ORBIT, and OVIID-1 trials (see Supplemental Digital Content 5, http://links.lww.com/IAE/B243).3,4,7,8
The absence of an ERM is another baseline predictor of ocriplasmin success.5,6 Few patients had a baseline ERM in INJECT (5.2% on the investigator assessment). This is different from trials that used a central reading center, suggesting that the actual rate of ERM was likely higher in INJECT (see Supplemental Digital Content 5, http://links.lww.com/IAE/B243).3,4,7 In OASIS, a double-masked, randomized, sham-controlled, multicenter study, 22.7% of patients were discovered to have a baseline ERM by a central reading center analysis, although an ERM was an exclusion criterion and the rate was 0.0% by investigator assessment.3 In light of this, the lack of a predictive effect of ERM on functional outcomes in INJECT is not surprising, given the small sample size and the potential for false negatives in the absence of a central reading center.
The rate of nonsurgical MH closure in INJECT was consistent with other trials (see Supplemental Digital Content 5, http://links.lww.com/IAE/B243).3,4,7,8 Patients with a small MH had almost twice the rate of nonsurgical MH closure compared with patients with a medium MH, similar to findings from other trials.3,5,7,8
The median BCVA improved from baseline to Month 12 in the subgroup of patients who received ocriplasmin without vitrectomy and those who received vitrectomy as a rescue therapy. Both treatment procedures show comparable effects on a BCVA gain in patients with VMT alone and those with VMT plus MH at baseline, respectively. Not surprisingly, in both treatment subgroups, this effect was greater for patients with MHs because resolution of VMT and closure of MH are normally associated with substantial visual improvement compared with what could be seen in patients with VMT alone. The greater overall effect in the subgroup having vitrectomy could be explained by the fact that more than 50% of patients underwent vitrectomy as a rescue therapy due to persistent MHs after ocriplasmin injection.
Visual outcomes in INJECT were consistent with other trials. The rate of a ≥2-line BCVA gain was parallel to MIVI-TRUST and ORBIT (see Supplemental Digital Content 5, http://links.lww.com/IAE/B243).5,7 In INJECT, the percentage of patients who gained ≥2 lines in BCVA was higher in groups with MH compared with the group without MH. This is similar to the ocriplasmin group from OASIS, which showed larger 24-month gains in patients with MH (+12.2 Early Treatment Diabetic Retinopathy Study letters ocriplasmin group) versus those without MH (+7.7 letters ocriplasmin group).3
Safety findings from INJECT were consistent with previous studies,3,4,7,8 and serious AEs were infrequent and mostly transient. Overall, AE rates in this study were lower than those seen in randomized controlled trials but were in line with rates observed in the ORBIT observational study.3,4,7 It is likely that this is a characteristic of the study design and the absence of frequent, scheduled follow-ups. Indeed, significant visual AEs have been reported by several authors, including the occurrence of ellipsoid line abnormalities suggestive of outer retinal dysfunction. The fact that these were not observed in this study likely reflects the lack of centralized reading center and lack of specific case report forms asking investigators to specify the presence or absence of outer retinal changes. There remains no doubt that the uptake of ocriplasmin has been low partly based on these concerns.29 The percentage of patients who withdrew from the study because of ocular AEs was low (1.2%) and similar to MIVI-TRUST (0.9% owing to AEs in the ocriplasmin group) and OVIID-1 (0.2% owing to treatment-emergent AEs).8,30
The incidence of retinal detachment requiring surgery in INJECT was 1.8% (8/428), consistent with other ocriplasmin trials (0.4% in MIVI-TRUST to 3.6% in OVIID-1).3,4,7,8 Six of the 8 cases requiring surgery were successfully treated by Month 12. Five patients were reported to have retinal detachment that did not require surgery. These events may have been adverse side effects of ocriplasmin. The occurrence of SRF has been noted after ocriplasmin injection, most typically localized in the macular area associated with the area of VMT, but more widespread retinal changes have been described, and it is conceivable that the areas of retinal detachment described were more extensive.18,31 Unfortunately, we do not have more information on their exact nature and extent, but it was reported that they all spontaneously resolved. The percentage of patients experiencing flashes of light, micropsia, or color vision abnormalities increased in the month after ocriplasmin injection, but then decreased to a baseline level or lower during the study. A similar trend was observed for submacular SRF, which also resolved to a baseline level by Month 12. This is consistent with a recent subanalysis of the ORBIT study that showed that both SRF and ellipsoid changes after ocriplasmin injection were resolved by the end of the study and were not associated with the final visual acuity.32 By contrast, the percentage of patients with metamorphopsia gradually decreased after ocriplasmin injection, consistent with the release of VMT. Electroretinography abnormalities have been reported with ocriplasmin.33 Although electroretinograms were not obtained as part of INJECT, it is worth noting that the recent OASIS electroretinography substudy reported an association between electroretinography reductions, VMT resolution, and greater visual improvement by the study end.34
Limitations of the study include those typical of a noninterventional observational design. Follow-up schedules were at the physician's discretion, as long as procedures were performed according to the product label, and all visits were completed within the 12-month (±2 months) study period. The study did not use a central reading center for image analysis and depended on investigators to perform and report on OCT assessments. The only patients excluded from the study were those with contraindications or those treated outside of the product label, which means that INJECT enrolled a complex range of patients as is the case in observational trials reflecting real-world situations, including some with age-related macular degeneration and diabetic retinopathy. This is in contrast with MIVI-TRUST, OASIS, and OVIID-1, which excluded patients with these ocular comorbidities.3,4,8
INJECT showed that ocriplasmin is effective in a clinical setting in patients with VMT, with or without MH. Outcomes are consistent with a previous observational study (ORBIT), a single-arm open-label study (OVIID-1), and two randomized controlled trials (MIVI-TRUST and OASIS).3,4,7,8 No new safety signals were identified from this large and surgeon-selected patient group, although the significant limitations of the study design without an image reading center and scheduled study visit timings should be noted.
Acknowledgments
The authors wish to acknowledge the principal investigators and study site staff who participated in the INJECT clinical study as well as all patients enrolled in the study. Medical writing assistance was provided by Zachary Harrelson and Duprane Pedaci Young from Fishawack Communications Inc. and was funded by Oxurion NV (formerly ThromboGenics).
Footnotes
The sponsor of this study was Alcon Research, Ltd. (6,201 South Freeway, Fort Worth, TX 76134–2099). Oxurion NV (formerly ThromboGenics NV) participated in the study design, data analysis and interpretation, writing the manuscript, and the decision to submit the paper for publication.
Interim results from INJECT were presented previously at the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO), Denver, CO, May 3 to 7, 2015.
D. H. W. Steel is a consultant for Alcon and Orbit Biomedical and has received research funding from Alcon, Astellas, Bayer, and Novartis. N. Patton is an advisory board member for Alcon. H. Hoerauf is a consultant for Alcon/Novartis, Alimera, Allergan, Bayer, and Oxurion; has received grants and/or funding from Alcon/Novartis, Allergan, Bayer, Bioeq/Formycon, Boehringer Ingelheim, Carl Zeiss Meditec, Heidelberg Engineering, Lutronic, Ophthotech, Regeneron, and Roche/Genentech; has received speaker fees and related travel expenses from Alcon/Novartis, Alimera, Allergan, Bayer, Heidelberg Engineering, Oxurion, and Thea Pharma; and has equity investments in 3M, Amgen, BASF, Bayer, GlaxoSmithKline, Johnson & Johnson, Medtronic, Merck, Novartis, Roche, and Siemens. N. Patel has been a consultant for Alcon, Bayer, Novartis, Roche, Allergan, Bausch & Lomb, and Zeiss. J. Wachtlin has received speaker fees from Alcon, Allergan, Novartis, and Bayer; and is an advisory board member for Bayer and Novartis. T. Raber and P. Kozma-Wiebe are full-time employees of Oxurion NV. The remaining authors have no conflicting interests to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
References
- 1.Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol 2010;149:371–382 e371. [DOI] [PubMed] [Google Scholar]
- 2.Neffendorf JE, Simpson ARH, Steel DHW, et al. Intravitreal gas for symptomatic vitreomacular adhesion: a synthesis of the literature. Acta Ophthalmol 2018;96:685–691. [DOI] [PubMed] [Google Scholar]
- 3.Dugel PU, Tolentino M, Feiner L, et al. Results of the 2-year ocriplasmin for treatment for symptomatic vitreomacular adhesion including macular hole (OASIS) randomized trial. Ophthalmology 2016;123:2232–2247. [DOI] [PubMed] [Google Scholar]
- 4.Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med 2012;367:606–615. [DOI] [PubMed] [Google Scholar]
- 5.Haller JA, Stalmans P, Benz MS, et al. Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: subgroup analyses from two randomized trials. Ophthalmology 2015;122:117–122. [DOI] [PubMed] [Google Scholar]
- 6.Jackson TL, Regillo CD, Girach A, et al. Baseline predictors of vitreomacular adhesion/traction resolution following an intravitreal injection of ocriplasmin. Ophthalmic Surg Lasers Imaging Retina 2016;47:716–723. [DOI] [PubMed] [Google Scholar]
- 7.Khanani AM, Duker JS, Heier JS, et al. Ocriplasmin treatment leads to symptomatic vitreomacular adhesion/vitreomacular traction resolution in the real-world setting: the phase IV ORBIT study. Ophthalmol Retina 2019;3:32–41. [DOI] [PubMed] [Google Scholar]
- 8.Tadayoni R, Holz FG, Zech C, et al. Assessment of anatomical and functional outcomes with ocriplasmin treatment in patients with vitreomacular traction with or without macular holes: results of OVIID-1 trial. Retina 2019;39:3241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BT, Schwartz SG, Smiddy WE, et al. Initial outcomes following intravitreal ocriplasmin for treatment of symptomatic vitreomacular adhesion. Ophthalmic Surg Lasers Imaging Retina 2013;44:334–343. [DOI] [PubMed] [Google Scholar]
- 10.Singh RP, Li A, Bedi R, et al. Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. Br J Ophthalmol 2014;98:356–360. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Juhn A, Houston SK, et al. Efficacy of intravitreal ocriplasmin on vitreomacular traction and full-thickness macular holes. Am J Ophthalmol 2015;159:861–867 e862. [DOI] [PubMed] [Google Scholar]
- 12.Figueira J, Martins D, Pessoa B, et al. The Portuguese experience with ocriplasmin in clinical practice. Ophthalmic Res 2016;56:186–192. [DOI] [PubMed] [Google Scholar]
- 13.Schumann RG, Langer J, Compera D, et al. Assessment of intravitreal ocriplasmin treatment for vitreomacular traction in clinical practice. Graefes Arch Clin Exp Ophthalmol 2017;255:2081–2089. [DOI] [PubMed] [Google Scholar]
- 14.Feng HL, Roth DB, Hasan A, et al. Intravitreal ocriplasmin in clinical practice: predictors of success, visual outcomes, and complications. Retina 2018;38:128–136. [DOI] [PubMed] [Google Scholar]
- 15.Muqit MMK, Hamilton R, Ho J, et al. Intravitreal ocriplasmin for the treatment of vitreomacular traction and macular hole- A study of efficacy and safety based on NICE guidance. PLoS One 2018;13:e0197072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul C, Heun C, Müller HH, et al. Calculating the individual probability of successful ocriplasmin treatment in eyes with VMT syndrome: a multivariable prediction model from the EXPORT study. Br J Ophthalmol 2018;102:1092–1097. [DOI] [PubMed] [Google Scholar]
- 17.Casswell E, Fernandez-Sanz G, Mitry D, et al. Macular hole progression following ocriplasmin intravitreal injection. Case Rep Ophthalmol Med 2014;2014:403461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahim AT, Khan NW, Johnson MW. Acute panretinal structural and functional abnormalities after intravitreous ocriplasmin injection. JAMA Ophthalmol 2014;132:484–486. [DOI] [PubMed] [Google Scholar]
- 19.Haynes RJ, Yorston D, Laidlaw DA, et al. Real world outcomes of ocriplasmin use by members of the British and Eire Association of Vitreoretinal Surgeons. Eye (Lond) 2017;31:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller J, Haynes RJ. Zonular dehiscence at the time of combined vitrectomy and cataract surgery after intravitreal ocriplasmin injection. JAMA Ophthalmol 2015;133:1091–1092. [DOI] [PubMed] [Google Scholar]
- 21.Khoshnevis M, Nguyen-Cuu J, Sebag J. Floaters and reduced contrast sensitivity after successful pharmacologic vitreolysis with ocriplasmin. Am J Ophthalmol Case Rep 2016;4:54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madi HA, Haynes RJ, Depla D, et al. Rhegmatogenous retinal detachment following intravitreal ocriplasmin. Graefes Arch Clin Exp Ophthalmol 2016;254:2333–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quezada Ruiz C, Pieramici DJ, Nasir M, et al. Severe acute vision loss, dyschromatopsia, and changes in the ellipsoid zone on SD-OCT associated with intravitreal ocriplasmin injection. Retin Cases Brief Rep 2015;9:145–148. [DOI] [PubMed] [Google Scholar]
- 24.Silva RA, Moshfeghi DM, Leng T. Retinal breaks due to intravitreal ocriplasmin. Clin Ophthalmol 2014;8:1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steel DH, Parkes C, Papastavrou VT, et al. Predicting macular hole closure with ocriplasmin based on spectral domain optical coherence tomography. Eye (Lond) 2016;30:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steel DH, Wong D. Ocriplasmin—variable efficacy? Graefes Arch Clin Exp Ophthalmol 2016;254:1245–1246. [DOI] [PubMed] [Google Scholar]
- 27.Tibbetts MD, Reichel E, Witkin AJ. Vision loss after intravitreal ocriplasmin: correlation of spectral-domain optical coherence tomography and electroretinography. JAMA Ophthalmol 2014;132:487–490. [DOI] [PubMed] [Google Scholar]
- 28.Zhang TY, Vachon-Joannette E, Proulx S, et al. Delayed transient corneal edema after intravitreal injection of ocriplasmin. Can J Ophthalmol 2018;53:e77–e79. [DOI] [PubMed] [Google Scholar]
- 29.Grinton M, Steel DH. Cochrane Corner: ocriplasmin—why isn't it being used more? Eye 2019;33:1195–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser PK, Kampik A, Kuppermann BD, et al. Safety profile of ocriplasmin for the pharmacologic treatment of symptomatic vitreomacular adhesion/traction. Retina 2015;35:1111–1127. [DOI] [PubMed] [Google Scholar]
- 31.Hager A, Seibel I, Riechardt A, et al. Does ocriplasmin affect the RPE-photoreceptor adhesion in macular holes? Br J Ophthalmol 2015;99:635–638. [DOI] [PubMed] [Google Scholar]
- 32.Lavine JA, Srivastava SK, Dukles N, et al. Longitudinal ellipsoid zone and subretinal fluid mapping following ocriplasmin injection in the prospective observational ORBIT trial. Br J Ophthalmol 2020;104:410–415. [DOI] [PubMed] [Google Scholar]
- 33.Hahn P, Chung MM, Flynn HW, Jr, et al. Safety profile OF ocriplasmin for symptomatic vitreomacular adhesion: a comprehensive analysis of premarketing and postmarketing experiences. Retina 2015;35:1128–1134. [DOI] [PubMed] [Google Scholar]
- 34.Birch DG, Benz MS, Miller DM, et al. Evaluation of full-field electroretinogram reductions after ocriplasmin treatment: results of the OASIS trial ERG substudy. Retina 2018;38:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]