Abstract
Background
The weight-band dosing in tuberculosis treatment regimen has been implemented in clinical practice for decades. Patients will receive different number of fixed dose combination tablets according to their weight-band. However, some analysis has shown that weight was not the best covariate to explain variability of rifampicin exposure. Furthermore, the rationale for using weight-band dosing instead of flat-dosing becomes questionable. Therefore, this study aimed to compare the average and the variability of rifampicin exposure after weight-band dosing and flat-dosing.
Methods
Rifampicin exposure were simulated using previously published population pharmacokinetics model at dose 10–40 mg/kg for weight-band dosing and dose 600–2400 mg for flat-dosing. The median area under the curve (AUC0–24 h) after day 7 and 14 were compared as well as the variability of each dose group between weight-band and flat-dosing.
Results
The difference of median AUC0–24 h of all dose groups between flat-dosing and weight-band dosing were considered low (< 20%) except for the lowest dose. At the dose of 10 mg/kg (600 mg for flat-dosing), flat-dosing resulted in higher median AUC0–24h compared to the weight-band dosing. A marginal decrease in between-patient variability was predicted for weight-band dosing compared to flat-dosing.
Conclusions
Weight-band dosing yields a small and non-clinically relevant decrease in variability of AUC0–24h.
Keywords: flat-dosing, weight-band dosing, simulation, pharmacokinetics, rifampicin
Rifampicin can be given as flat-dosing instead of current weight-band dosing. Clinical trial simulations revealed that weight-band dosing results in a small and not clinically relevant decrease in variability in AUC0–24h between patients compared to flat-dosing.
Rifampicin is one of the first-line drugs in tuberculosis (TB) treatment regimen. However, the current rifampicin dose of 10 mg/kg/day was not optimized using dose-finding studies but influenced by economic reasons, fears of toxicity, and pharmacokinetic considerations [1]. Studies have suggested that this particular rifampicin dose might be too low. In the past decade, higher doses of rifampicin up to 35 mg/kg/day resulting in up to 10-fold higher exposures in plasma have been investigated in patient with pulmonary TB and TB meningitis [2–11].
In current standard treatment for drug-susceptible TB, rifampicin is given as one formulation using fixed-dose combination (FDC) tablets together with other drugs including isoniazid, pyrazinamide, and ethambutol. The number of FDC tablets to be taken is dependent on the weight of the patient and is determined by so-called weight bands. There is, however, no clear reason why rifampicin is given as a weight-band dosing. It was assumed that by correcting the dose based on body weight, the patients would receive lower degree of toxicities but still result in desirable therapeutics effect. In general, body weight can reduce the variability of drug concentrations in plasma across individuals if body weight explains a large proportion of between-patient variability in pharmacokinetics (PK). However, some studies have suggested that fat-free mass (FFM) is a better covariate to explain the variability of rifampicin exposure than total body weight [12–14].
Despite the fact that the use of weight-band dosing is relatively simple, it becomes problematic when a patient shifts to another weight-band due to the change of patients’ body weight during the treatment. Some countries have also implemented different weight-band compared to current World Health Organization (WHO) guideline [15]. In contrast, flat-dosing, where all patients receive the same dose, is an easy way of administering the drug to patients. It is also indicated when the therapeutic window of a drug is broad and covariates do not explain between-patient variability. Therefore, we aimed to investigate average exposures achieved and between-patient variability of rifampicin after flat-dosing and weight-band dosing using clinical trial simulations at a standard dose and higher doses.
MATERIALS AND METHODS
Patients and Study Design in the Original Trial
The pharmacokinetic data of rifampicin for weight-band dosing was collected from HIGHRIF 1 trial (clinical trials registration NCT01392911). In summary, 83 adult patients with drug-susceptible tuberculosis in Cape Town (South Africa) were enrolled in the study. Patients received treatment with rifampicin as a single drug (monotherapy) for the first 7 days and followed with 7 days of rifampicin in combination with isoniazid, pyrazinamide, and ethambutol. The control group received 10 mg/kg (n = 8) rifampicin and the experimental group received 20, 25, 30, 35, and 40 mg/kg (n = 15/group) rifampicin according to weight-band dosing. The plasma concentrations were measured at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 hours on day 7 and 14 to obtain a full pharmacokinetic curve. More details about study design can be found in the original publication [2].
Population Pharmacokinetic Model
A previously developed population pharmacokinetic model of rifampicin using 10–40 mg/kg dose range was used in this study [16]. The predictive properties of the model have been evaluated and found to be superior to other published population pharmacokinetic models [17]. All model parameters are summarized in Table 1. The model consists of a 1-compartment disposition model, a transit compartment absorption model, dose-dependent bioavailability, enzyme auto-induction, and a concentration-dependent saturation of clearance. The relationship between dose and bioavailability was described using an Emax model where the bioavailability increases by the dose until it reaches maximum value. An enzyme turnover model was used to account for the time-course of the auto-induction. Michaelis-Menten kinetics was used to characterize the relationship between rifampicin concentration and clearance. To account for the variability within the population, inter-individual variability (IIV), inter-occasion variability (IOV), and additive residual error model on log scale was incorporated into the model. The occasion was defined as different regimen at week 1 and 2. FFM was used for allometric scaling of typical apparent clearance (CL/F) and typical apparent V/F, as shown in equations (1)–(2).
Table 1.
Parameters of the Population Pharmacokinetic Model of Rifampicin
| Parameter | Description | Estimate |
|---|---|---|
| V max (mg∙h−1∙70 kg−1) | Maximal elimination rate | 525 |
| k m (mg∙L−1) | Rifampicin concentration at which half Vmax is reached | 35.3 |
| V d (L∙70 kg−1) | Volume of distribution | 87.2 |
| k a (h−1) | Absorption rate constant | 1.77 |
| MTT (h) | Mean transit time | 0.513 |
| NN | Number of transits | 23.8 |
| E max | Maximal increase in enzyme production rate | 1.16 |
| EC50 (mg∙L−1) | Rifampicin concentration at which half the Emax is reached | 0.0699 |
| k ENZ (h−1) | First-order rate constant for enzyme pool degradation | 0.00603 |
| F max | Maximal increase in relative bioavailability at doses above 450 mg | 0.504 |
| ED50 (mg) | Difference in dose above 450 mg at which half the Fmax is reached | 67.0 |
| IIV Vmax (%) | Inter individual variability in Vmax | 30.0 |
| IIV km (%) | Inter individual variability in km | 35.8 |
| IIV Vd (%) | Inter individual variability in Vd | 7.86 |
| IIV ka (%) | Inter individual variability in ka | 33.8 |
| IIV MTT (%) | Inter individual variability in MTT | 38.2 |
| IIV NN (%) | Inter individual variability in NN | 77.9 |
| IOV km (%) | Inter occasion variability in km | 18.9 |
| IOV ka (%) | Inter occasion variability in ka | 31.4 |
| IOV MTT (%) | Inter occasion variability in MTT | 56.4 |
| IOV F (%) | Inter occasion variability in F | 15.7 |
| Correlation Vmax-km (%) | Correlation between Vmax-km | 38.9 |
| (%) | Additive error on log scale | 23.6 |
| (1) |
| (2) |
The noncompartmental analysis (NCA) derived area under the curve (AUC0–24h) based on observed concentrations in the original trial was compared to NCA derived AUC0–24h based on the simulated weight-band dosing in order to check whether the pharmacokinetic model used in this analysis was properly implemented.
Simulation of Exposures After Flat- and Weight-band Dosing
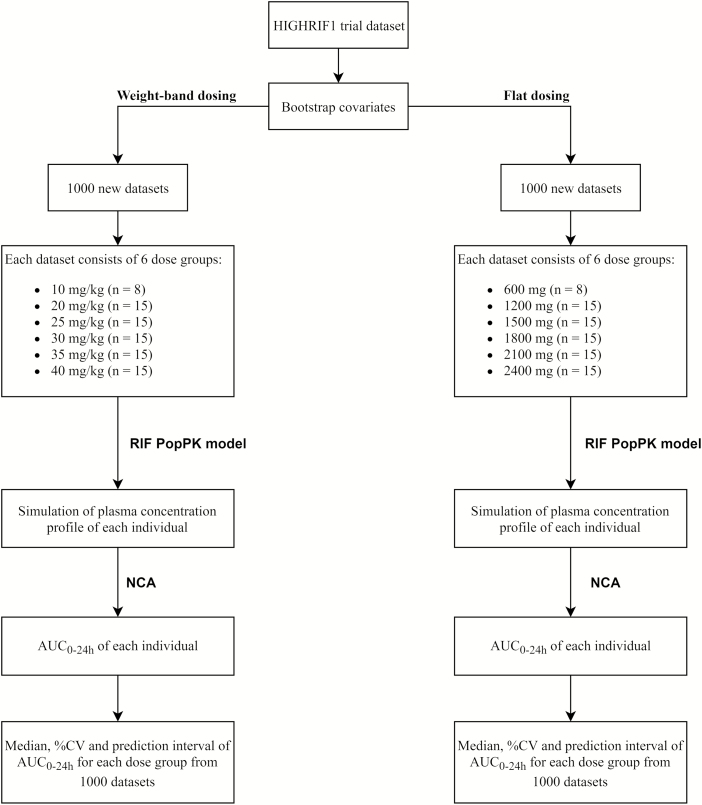
The flow chart of the simulation of rifampicin exposure in weight-band dosing and flat-dosing is shown in Figure 1. Patient covariates distribution from the original study were randomly sampled to reach 83 000 patients in order to create 1000 new trials mimicking the HIGHRIF1 trial. During covariates resampling, we maintained the correlation of the covariates including body weight and FFM. For each trial in weight-band dosing simulations, patients in each dose group received 10 (n = 8 subjects, reference group), 20, 25, 30, 35, or 40 (n = 15 subjects/group) mg/kg daily oral rifampicin for 14 days. The dose of rifampicin strictly followed the weight-band dosing as in the HIGHRIF1 study (Table S1A–H) where at dose 10 mg/kg followed weight-band dosing from WHO guideline. The number of tablets to be taken was based on the weight bands of the patients. However, for each dose group in the flat-dosing simulations, subjects received 600 (n = 8 subjects, reference group), 1200, 1500, 1800, 2100, or 2400 (n = 15 subjects/group) mg/day. The doses for flat-dosing were derived using the assumption that all patients in each dose group received the same dose as the patient with 60 kg body weight in weight-band dosing. Both flat- and weight-band dosing were considering the available dose strength as can be seen in Table S1. Because patients also received isoniazid, pyrazinamide, and ethambutol after day 7 in the HIGHRIF1 study, we assumed that there was no pharmacokinetic interaction with those companion drugs.
Figure 1.
Flow chart of the simulation of rifampicin exposure in weight-band dosing and flat-dosing. Abbreviations: AUC, area under the curve; CV, coefficient of variation; NCA, noncompartmental analysis; RIF PopPK, rifampicin population pharmacokinetics.
The plasma concentration profile for each individual was simulated at day 7 and day 14 with the same sampling schedule as in the original study. Individual rifampicin exposure (AUC0–24h) at day 7 and 14 were then calculated using noncompartmental analysis (NCA). The median and coefficient of variation (CV%) of AUC0–24h for each dose group were summarized based on the 1000 simulated trials. In addition, to reflect the variability in the simulations, the 90% prediction interval (PI) was calculated based on the 1000 simulated trials.
Software and Analysis
The simulations were performed using the software NONMEM (version 7.4). The limit of quantification (LOQ) was assumed to be 0.13 mg/L as in the original study. The M3 method was implemented in the simulation to handle samples below the LOQ [18]. Data management and visualization were performed in R (version 3.5.2). The AUC0–24h at day 7 and 14 were calculated using the “ncappc” package in R [19]. The data below LOQ was excluded while calculating the AUC0–24h using NCA. The comparison between flat-dosing and weight-band dosing simulation was performed based on the median, CV%, and PI of using simulated data.
RESULTS
Patients Characteristics in the Original Study
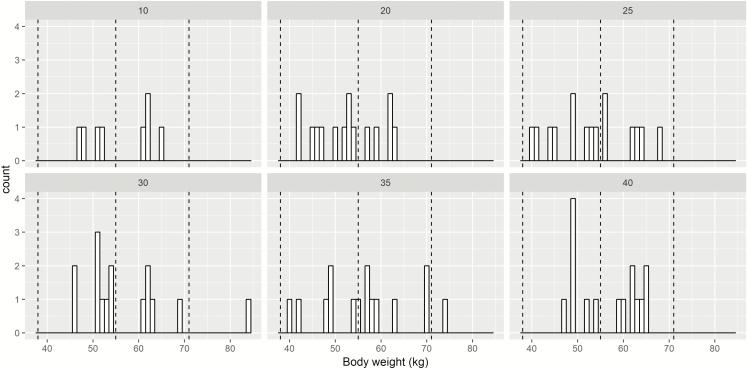
The patients’ demographics in the HIGHRIF1 trial are given in Table S2. The distribution of patients’ body weight involved in the HIGHRIF1 trial is visualized in Figure 2. Most of the patients involved in the original trial lied within 38–54 kg and 55–70 kg weight-band. In practice, only small proportion of adult TB patients fall within 30–37 kg or above 70 kg. Therefore, we assumed that this data is appropriate to represent distribution of body weight in general TB patient populations for further analysis.
Figure 2.
Distribution of body weight of patients involved in HIGHRIF1 trial stratified by mg/kg dose. Dashed line represented different weight-band according to World Health Organization guideline.
Population Pharmacokinetics Model
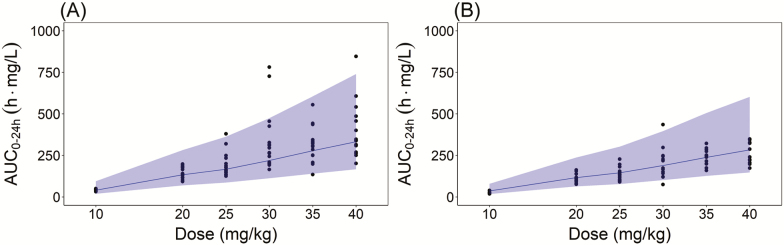
The previously developed population pharmacokinetic model well-predicted the observed AUC0–24h after doses range 10–40 mg/kg/day of weight-band dosing, as shown in Figure 3. The results indicated that the implementation of pharmacokinetic model used in the simulation of this study was appropriate.
Figure 3.
Comparison of noncompartmental analysis (NCA) area under the curve (AUC0–24h) after weight-band dosing simulations using the population pharmacokinetic model to the NCA AUC0–24h based on observed data from the HIGHRIF1 trial at (A) day 7 and (B) day 14. The dots represent the NCA AUC0–24h based on observed data from HIGHRIF1 trial. The black solid line represents the median of the NCA AUC0–24h based on simulated data. The gray shaded area represents the 5th and 95th percentile of NCA AUC0–24h based on simulated data.
Simulation of Exposures After Flat- and Weight-band Dosing
Table 2 shows the summary statistics for the simulated NCA AUC0–24h of each dose group for weight-band dosing compared to flat-dosing at day 7 and 14.
Table 2.
Comparison of Median and Between-Patient Variability (% CV) in AUC0–24h After Flat-Dosing and Weight-Band Dosing
| Median AUC0–24h (% CV) Day 7 | Median AUC0–24h (% CV) Day 14 | ||||||
|---|---|---|---|---|---|---|---|
| mg/kg Weight-Band Dose (mg Flat Dose) | Number of Simulated Subjects | Weight-Band Dosing | Flat-Dosing | Difference in Median (%) | Weight-Band Dosing | Flat-Dosing | Difference in Median (%) |
| 10 (600) | 8000 | 40.60 (53) | 56.85 (46) | 40.02 | 36.50 (50) | 50.77 (43) | 39.10 |
| 20 (1200) | 15 000 | 133.45 (48) | 144.36 (48) | 8.18 | 116.56 (45) | 126.72 (46) | 8.72 |
| 25 (1500) | 15 000 | 167.01 (50) | 195.12 (50) | 16.83 | 145.62 (46) | 168.84 (47) | 15.95 |
| 30 (1800) | 15 000 | 219.35 (50) | 253.59 (51) | 15.61 | 189.40 (46) | 218.71 (47) | 15.48 |
| 35 (2100) | 15 000 | 278.53 (53) | 283.39 (56) | 1.74 | 238.30 (49) | 242.94 (49) | 1.95 |
| 40 (2400) | 15 000 | 332.32 (58) | 385.68 (64) | 16.06 | 283.63 (49) | 329.15 (55) | 16.05 |
Abbreviations: AUC, area under the curve; CV, coefficient of variation.
The median of simulated NCA AUC0–24h at day 14 was lower than the median of predicted AUC0–24h at day 7 in both simulation settings, which can be ascribed to the phenomenon of auto-induction by rifampicin. The overall difference in median AUC0–24h of all dose groups after flat-dosing relative to weight-band dosing at day 7 and 14 were considered low (< 20%). However, for the lowest dose of 10 mg/kg (600 mg for flat-dosing), flat-dosing resulted in about 40% higher median exposure compared to the weight-band dosing. There are also some differences in the median value between observed AUC0–24h (Table 3) and simulated AUC0–24h (Table 2) of weight-band dosing. The differences in median between predicted and observed is due to that the observed is a sample of the true population for that dose. With high between-patient variability, the median of a sample will have some uncertainty. The predicted median based on the model, integrates information from all doses and as such, the precision in this estimate is higher than the sample for a single observed dose. Our work showed that the observed AUC0–24h can be predicted well (as shown in Figure 3) and as such, the predicted values are reliable despite some differences compared to the observed.
Table 3.
Median and Between-Patient Variability (% CV) in AUC0–24h From Observed HIGHRIF1 Trial Data
| Median AUC0–24h (% CV) | |||
|---|---|---|---|
| Dose (mg/kg) | Number of Subjects | Day 7 | Day 14 |
| 10 | 8 | 40.52 (18) | 24.80 (25) |
| 20 | 15 | 140.43 (23) | 110.28 (23) |
| 25 | 15 | 176.72 (37) | 125.38 (31) |
| 30 | 15 | 294.87 (54) | 170.11 (46) |
| 35 | 15 | 308.23 (34) | 240.49 (20) |
| 40 | 15 | 341.00 (42) | 232.04 (23) |
Abbreviations: AUC, area under the curve; CV, coefficient of variation.
The simulated variability in AUC0–24h ranged between 43–64% throughout all dose group (Table 2) and was simulated from the variances estimated from the observed concentrations in the original population PK model (Table 1). As such, the simulated exposures had a relatively consistent variability between the dose groups whereas the variability in observed exposure between the dose groups varied due to the low patient sample size per dose group. In the observed data, the variability of AUC0–24h at different doses were still relatively high even after the patients were given the dose based on body weight as presented in Table 3. In the simulated data, weight-band dosing at dose 10 mg/kg surprisingly gave higher variability than 600 mg flat dose. However, there was almost no difference in between-subject variability (% CV) after flat-dosing at doses between 20 and 35 mg/kg/day at day 7 and day 14 compared with weight-band dosing, although the variability of dose group 40 mg/kg was higher for flat-dosing than weight-band dosing.
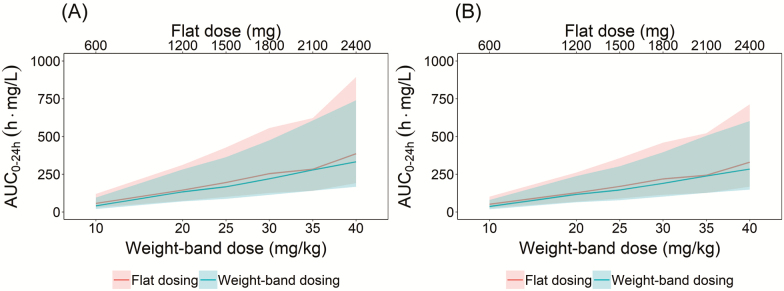
Figure 4 visualized the comparison of median and 90% prediction interval (5th to 95th percentile) of the simulated NCA AUC0–24h after different doses of rifampicin after flat-dosing and weight-band dosing at day 7 and day 14. The numerical comparison can be found in Table S3. The median and prediction interval after weight-band and flat-dosing almost overlapped at all dose levels. A marginally lower between-patient variability was predicted for weight-band dosing compared to flat-dosing.
Figure 4.
Comparison of median and the 90% prediction interval for area under the curve (AUC0–24h) after flat- and weight-band dosing simulation at (A) day 7 and (B) day 14. Solid line represents the median of the simulated data, shaded area represents the 5th and 95th percentile of simulated data (light gray: flat-dosing, dark gray: weight-band dosing).
DISCUSSION
In this study, the difference in rifampicin exposure (AUC0–24h) after weight-band dosing was compared to flat-dosing at standard and higher doses using clinical trial simulations. The overall difference in median AUC0–24h of doses above 10 mg/kg were considered low after flat-dosing compared to weight-band dosing at day 7 and 14 (< 20%). However, 10 mg/kg/day (600 mg for flat-dosing) resulted in an approximately 40% higher median AUC0–24h after flat-dosing compared to the weight-band dosing (Table 2). Higher median AUC0–24h for 600 mg on flat dosing than 10 mg/kg of weight-band dosing was considered good because it has been shown that higher exposure from higher dose gave better efficacy [11, 13].
We also showed that the between-patient variability of rifampicin exposure after flat-dosing and weight-band dosing is expected to be similar. The similarity in exposure between flat-dosing and weight-band dosing is translatable to similarity in efficacy. Several studies suggested that current rifampicin dose is not adequate and in the future higher doses of rifampicin will most likely be used. The exposure-response relationship for high dose rifampicin have also been identified in several studies [11, 13]. Future studies should study the safety and efficacy of a high flat-dosing regimen.
Because of the high inter-individual variability of rifampicin in relation to the low variability in exposure explained by body weight, the use of weight-band dosing will not reduce the variability in rifampicin exposure. There may be some unknown causes of rifampicin between-patient variability, which has not yet been explored and identified. More work should be undertaken in order to identify sources for the high between-patient variability. However, there is still a possibility to reduce between patient variability in exposure by optimising each individual dose in relation to a target exposure, that is, therapeutic drug monitoring (TDM) in order to maximize the benefit-risk for each patient.
The concern is then raising about the practical implication of rifampicin flat-dosing. In our study, we demonstrated that both regimens translate to the same exposure. Our analysis provides insight in the importance of flat-dosing and the future will most likely involve much higher doses of rifampicin compared to the current guideline and FDC, which is targeting the 10 mg/kg. This result could also be useful in developing new formulations, especially when information regarding the influence of body weight on the PK of the other drugs in the FDCs becomes available.
The limitation of this study is that the simulation data set was based on the HIGHRIF1 trial, in which the number of patients in the 10 mg/kg or 600 mg dose group was also less than other dose groups (8 subjects compared to 15 subjects). In the simulation, we simulated in total 8000 patients for the lowest dose group and 15 000 patients for the higher doses group. These numbers have no difference since both are big sample size. We also want to maintain the consistency with the original study so that we can compare it with the observed value. In this study, we used NCA analysis because we want to compare the simulated AUC0–24h with the observed AUC0–24h. Due to the rich sampling for each individual from the original study, it makes the NCA AUC0–24h not significantly different compared with AUC0–24h derived from the model. The other limitation of this study is that we used more weight-bands for dose higher than 10 mg/kg (6 weight-bands) compared to WHO guideline, which only has 4 weight-bands. However, this should not have an impact on the conclusions of this work. This study also only explored the rifampicin exposure in adult population and may not be applicable to children. In the original PK study, the trial only included limited number of patients at the extremes body weight as shown in Figure 2. For that particular patient groups, it is unclear if a flat dose dosing would result in similar exposure as after body weight adjusted dosing. Therefore, our result should not be extrapolated to patients with extreme body weight.
In conclusion, this work shows that weight-band dosing does not possess an advantage over flat-dosing for rifampicin as it does not reduce between-patient variability for the high-dose rifampicin which will be the future dosing of rifampicin. The impact of the result of this study in clinical practice is the possibility of rifampicin to be given as flat-dosing, that is, all patients will get the same dose in the beginning of therapy which will simplify the treatment.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the patients and site staff participating in the original study.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. van Ingen J, Aarnoutse RE, Donald PR, et al. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis 2011; 52:e195–9. [DOI] [PubMed] [Google Scholar]
- 2. Boeree MJ, Diacon AH, Dawson R, et al. ; PanACEA Consortium A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med 2015; 191:1058–65. [DOI] [PubMed] [Google Scholar]
- 3. Boeree MJ, Heinrich N, Aarnoutse R, et al. ; PanACEA consortium High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Velásquez GE, Brooks MB, Coit JM, et al. Efficacy and safety of high-dose rifampin in pulmonary tuberculosis. a randomized controlled trial. Am J Respir Crit Care Med 2018; 198:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seijger C, Hoefsloot W, De Guchteneire IB, et al. High-dose rifampicin in tuberculosis: experiences from a Dutch tuberculosis centre. PLoS One 2019; 14:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milstein M, Lecca L, Peloquin C, et al. Evaluation of high-dose rifampin in patients with new, smear-positive tuberculosis (HIRIF): study protocol for a randomized controlled trial. BMC Infect Dis 2016; 16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dian S, Yunivita V, Ganiem AR, et al. Double-blind, randomized, placebo-controlled phase II dose finding study to evaluate high-dose rifampin for tuberculous meningitis. Antimicrob Agents Chemother 2018; 62:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruslami R, Nijland HM, Alisjahbana B, Parwati I, van Crevel R, Aarnoutse RE. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother 2007; 51:2546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cresswell FV, Ssebambulidde K, Grint D, et al. High dose oral and intravenous rifampicin for improved survival from adult tuberculous meningitis: a phase II open-label randomised controlled trial (the RifT study). Wellcome Open Res 2018; 3:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yunivita V, Dian S, Ganiem AR, et al. Pharmacokinetics and safety/tolerability of higher oral and intravenous doses of rifampicin in adult tuberculous meningitis patients. Int J Antimicrob Agents 2016; 48:415–21. [DOI] [PubMed] [Google Scholar]
- 11. Svensson RJ, Svensson EM, Aarnoutse RE, et al. Greater early bactericidal activity at higher rifampicin doses revealed by modeling and clinical trial simulations. J Infect Dis 2018; 218:991–9. [DOI] [PubMed] [Google Scholar]
- 12. Denti P, Jeremiah K, Chigutsa E, et al. Pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PLoS One 2015; 10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Svensson EM, Svensson RJ, Te Brake LHM, et al. The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis 2018; 67:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeremiah K, Denti P, Chigutsa E, et al. Nutritional supplementation increases rifampin exposure among tuberculosis patients coinfected with HIV. Antimicrob Agents Chemother 2014; 58:3468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 16. Svensson RJ, Aarnoutse RE, Diacon AH, et al. A population pharmacokinetic model incorporating saturable pharmacokinetics and autoinduction for high rifampicin doses. Clin Pharmacol Ther 2018; 103:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Beek SW, Ter Heine R, Keizer RJ, Magis-Escurra C, Aarnoutse RE, Svensson EM. Personalized tuberculosis treatment through model-informed dosing of rifampicin. Clin Pharmacokinet 2019; 58:815–26. [DOI] [PubMed] [Google Scholar]
- 18. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28:481–504. [DOI] [PubMed] [Google Scholar]
- 19. Acharya C, Hooker AC, Türkyılmaz GY, Jönsson S, Karlsson MO. A diagnostic tool for population models using non-compartmental analysis: the ncappc package for R. Comput Methods Programs Biomed 2016; 127:83–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.