Abstract
Cancer cells are abnormal cells that can reproduce and regenerate rapidly. They are characterized by unlimited proliferation, transformation and migration, and can destroy normal cells. To meet the needs for cell proliferation and migration, tumor cells acquire molecular materials and energy through unusual metabolic pathways as their metabolism is more vigorous than that of normal cells. Multiple carcinogenic signaling pathways eventually converge to regulate three major metabolic pathways in tumor cells, including glucose, lipid, and amino acid metabolism. The distinct metabolic signatures of cancer cells reflect that metabolic changes are indispensable for the genesis and development of tumor cells. In this review, we report the unique metabolic alterations in tumor cells which occur through various signaling axes, and present various modalities available for cancer diagnosis and clinical therapy. We further provide suggestions for the development of anti‐tumor therapeutic drugs.
Keywords: cancer metabolism, metabolic pathway, metabolomic profiling, therapeutic implication
Abbreviations
- 1‐MT
1‐methyl tryptophan
- 2DG
2‐deoxyglucose
- 3‐BP
3‐bromopyruvat
- AEA
endocannabinoids anandamide
- AML
acute myeloid leukemia
- AMPK
AMP‐activated protein kinase
- AR
adrenergic receptor
- ASNase1
asparaginase 1
- ASS1
argininosuccinate synthetase 1
- AXIN
axis inhibition protein
- BA
bile acid
- BCAR4
breast cancer anti‐estrogen resistance 4
- CAC
colitis‐associated cancer
- CAD
carbamoyl‐phosphate synthetase 2, aspartate transcarbamylase and dihydrooratase
- CAF
cancer‐associated fibroblast
- CCAR1
cell division cycle and apoptosis regulator protein 1
- CKB
creatine kinase, brain‐type
- CRC
colorectal cancer
- CRL
cullin‐RING E3 ligase
- CRP
C‐reactive protein
- CSC
cancer stem cell
- CTP
citrate transporter protein
- CUEDC2
CUE domain‐containing protein 2
- D2HG
D‐2‐hydroxyglutarate
- DCA
deoxycholic acid
- EDP
epoxydocosapentaenoic acid
- EGFR
epidermal growth factor receptor
- EMT
epithelial‐mesenchymal transition
- ER
endoplasmic reticulum
- ERα
estrogen receptor α
- EV
extracellular vesicle
- FXR
farnesoid X receptor
- G6PD
glucose‐6‐phosphate dehydrogenase
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- GBM
glioblastoma multiforme
- GBTC
gallbladder and biliary tract cancer
- Gln
glutamine
- GLUT3
glucose transporter 3
- GPT2
glutamic pyruvate transaminase 2
- GR
glucocorticoid receptor
- HCC
hepatocarcinoma
- HCV
hepatitis C virus
- HISLA
HIF‐1alpha‐stabilizing lncRNA
- HK2
hexokinase 2
- HMGCL
3‐hydroxy‐3‐methylglutaryl‐CoA lyase
- HMOX1
heme oxygenase 1
- HMW
high‐molecular‐weight
- HSP90
heat shock protein 90
- IBD
intrahepatic bile duct
- IDH
isocitrate dehydrogenase
- IDH1
isocitrate dehydrogenase 1
- IDH2
isocitrate dehydrogenase 2
- IDO
indoleamine 2,3‐dioxygenase
- IL‐6
interleukin‐6
- KDM8
lysine demethylase 8
- KYN
trigger kynurenine
- LAPTM4B
lysosomal protein transmembrane 4 beta
- LDH
lactate dehydrogenase
- LDHA
lactate dehydrogenase A
- LIC
leukemia‐initiating cell
- lncRNA
long non‐coding RNA
- LSC
leukemia stem cell
- lysoPC
lysophosphatidylcholine
- MCT1
monocarboxylate transporter‐1
- MM
multiple myeloma
- MPC
mitochondrial pyruvate carrier
- mTOR
mammalian target of rapamycin
- mTORC2
mTOR complex 2
- NMDA
N‐methyl‐D‐aspartic acid
- NNMT
methyltransferase nicotinamide N‐methyltransferase
- NSCLC
non‐small cell lung carcinoma
- NSE
neuron‐specific enolase
- OOA
oxaloacetic acid
- OXCT1
3‐oxoacid CoA‐transferase 1
- PCK1
phosphoenolpyruvate carboxykinase 1
- PCK2
phosphoenolpyruvate carboxykinase 2
- PDAC
pancreatic ductal adenocarcinoma
- PDGFRA
platelet‐derived growth factor receptor alpha
- PDH
pyruvate dehydrogenase
- PDK1
pyruvate dehydrogenase kinase 1
- PDK2
pyruvate dehydrogenase kinase isozyme 2
- PD‐L1
programmed death‐ligand 1
- PEA
palmitylethanolamide
- PEP
phosphoenolpyruvate
- PFKFB3
6‐phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase 3
- PFKFB4
6‐phosphofructo‐2‐kinase/fructose‐2,6‐bisphosphatase 4
- PHD2
prolyl hydroxylase 2
- PHGDH
phosphoglycerate dehydrogenase
- pHGG
pediatric high‐grade glioma
- PI3K
phosphoinositide 3‐kinase
- PKB
protein kinase B
- PKM2
pyruvate kinase M2
- Plk1
polo‐like kinase 1
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
- PPAR
peroxisome proliferator‐activated receptor
- PPP
pentose phosphate pathway
- PRMT5
protein arginine methyltransferase 5
- PUMA
p53 upregulated modulator of apoptosis
- R5P
ribose 5‐phosphate
- RIF1
replication timing regulatory factor 1
- ROS
reactive oxygen species
- SCC
squamous cell carcinoma
- SDH
serine dehydratase
- SIRT5
sirtuin5
- SNS
sympathetic nervous system
- SRC‐3
steroid receptor coactivator‐3
- SREBP
serol regulatory element‐binding protein
- SRSF5
serine and arginine‐rich splicing factor 5
- STAT3‐Socs3
signal transducers and activators of transcription 3‐suppressor of cytokine signaling 3
- TAM
tumor‐associated macrophage
- T‐betaMCA
tauro‐beta‐muricholic acid
- TCA
tricarboxylic acid
- TFEB
transcription factor EB
- TGF‐β
transforming growth factor‐beta
- TIL
tumor‐infiltrating T‐lymphocyte
- TKT
transketolase
- TME
tumor microenvironment
- Trp
tryptophan
- UC
urea cycle
- UGDH
UDP‐glucose 6‐dehydrogenase
- VEGF
vascular endothelial growth factor
- WTp53
wild‐type p53
- YAP
Yes‐associated protein
- ZIP14
ZRT‐ and IRT‐like protein 14
1. INTRODUCTION
The Warburg effect (aerobic glycolysis) indicates that tumor cells prefer high‐activity glycolysis to meet their survival demands [1]. However, until recently, this pathologic phenomenon has not been extensively researched and discussed. An epoch‐making finding revealed that 6‐phosphofructo‐2‐kinase/fructose‐2,6‐bisphosphatase 4 (PFKFB4), a leading metabolic enzyme, activates glycolysis steroid receptor coactivator‐3 (SRC‐3) transcriptional activity and drives glucose flux towards the pentose phosphate pathway (PPP) functionally, which explains why tumors do not show active mitochondrial metabolic activity (Table 1) [2]. High tumor‐associated glucose consumption frequently involves alterations at both molecular and cellular levels, which drive the malignant features of solid tumors. Thus, such metabolic pathways provide promising clinical targets.
TABLE 1.
The functions of specific metabolic enzymes in cancer progression
| Metabolic enzyme | Cancer type | Function | Reference |
|---|---|---|---|
| PFKFB4 | Breast cancer | Activate the transcriptional activity of SRC‐3 and drive the pentose phosphate pathway. | Dasgupta et al., 2018 [2] |
| TRIM11 | Sustain the ubiquitination of estrogen receptor αand metastasis behavior. | Tang et al., 2020 [76] | |
| PHGDH | Breast cancer | Decrease the integration with nucleotides of one‐carbon units. | Pacold et al., 2016 [91] |
| Breast cancer | Support tumorigenesis in serine‐limited conditions. | Sullivan et al., 2019 [89] | |
| Melanoma | |||
| PCK2 | Melanoma | PCK2 deletion regulates glucose metabolism and alleviates oxidative phosphorylation. | Luo et al., 2017 [22] |
| HMGCL | Melanoma | Respond to the MEK‐ERK pathway and induce acetoacetate production. | Kang et al., 2015 [63] |
| Leukemia | |||
| PCK1 | Melanoma | Activate T cells to overexpress glycolytic phosphoenolpyruvate. | Ho et al., 2015 [26] |
| Hepatocarcinoma | Prevent INSIG1/2 from integrated with intracellular lipid and trigger sterol regulatory element‐binding protein signaling. | Xu et al, 2020 [60] | |
| PFKFB3 | Breast cancer | Trigger mitotic‐associated translation and oxidative respiration. | Domenech et al., 2015 [5] |
| Cervical cancer | Prevent DNA from degradation and sustain glycolysis. | Li et al., 2018 [11] | |
| Plk1 | Cervical cancer | Support the pentose phosphate pathway and increase macromolecules' biosynthesis. | Ma et al.,2017 [4] |
| G6PD | Osteosarcoma | Trigger NADPH and ribose synthesis and attenuated reactive oxygen species. | Du et al., 2013 [52] |
| ASNase1 | Allow conversion between asparagine and aspartate. | Sullivan et al., 2018 [99] | |
| UGDH | Lung cancer | Induce epithelial‐mesenchymal transition and metastatic growth. | Wang et al., 2019 [6] |
| GAPDH | Respond to glucose signal. | Li et al., 2014 [12] | |
| PP1 | Dephosphorylate axis inhibition protein and stimulate Wnt/β‐catenin signaling. | Mei et al., 2018 [71] | |
| HMOX1 | Stabilize Bach1 and support metastasis. | Lignitto et al., 2019 [126] | |
| CKB | Colorectal cancer | Induce liver‐colonization. | Loo et al., 2015 [7] |
| GLUD1 | Facilitate glutamine to be incorporated into the tricarboxylic acid cycle. | Wang et al., 2018 [79] | |
| GPT2 | Colon cancer | Regulate glutamine catabolism. | Smith et al., 2016 [80] |
| HK2 | Lung cancer | Promote the Warburg effect. | Kim et al., 2019 [25] |
| Glioblastoma | Impair central nervous system with activated glycolysis and mitochondrial translocation | Wolf et al., 2011 [13] | |
| Cdc25A | Glioblastoma | Sustain glycolysis. | Liang et al., 2016 [9] |
| IDH1 mutant | Glioblastoma/glioma | Impair chromosomal topology and activate platelet‐derived growth factor receptor alpha. | Flavahan et al.,2016 [42] |
| Induce D2HG production to trigger neuronal activity. | Chen et al., 2017 [43] | ||
| Alleviate pyruvate dehydrogenase phosphorylation, upregulate HIF‐1 and pyruvate dehydrogenase kinase‐3, and induce glutamate generation. | Izquierdo‐Garcia et al., 2015 [45] | ||
| PKM2 | Ovarian cancer | Control glycolytic rate. | Chao et al., 2017 [14] |
| Methyltransferase nicotinamide N‐methyltransferase | Induce cancer‐associated fibroblast phenotype alterations and cause depletion of S‐adenosyl methionine. | Eckert et al., 2019 [95] | |
| PDK2 | Leukemia | Regulate glycolysis, home leukemia‐initiating cells, and trigger symmetric cell division. | Hao et al., 2019 [15] |
| KDM8 | Prostate cancer | Interact with pyruvate kinase M2 and modulate glycolytic gene activities under hypoxic conditions. | Wang et al., 2019 [17] |
| CAD | Prostate cancer | Induce both pyrimidine synthesis and transversion mutations at the nucleic acid and protein levels. | Lee et al.,2018 [116] |
| Lung cancer | |||
| Breast cancer | |||
| LDHA | Hepatocarcinoma | Modulate aerobic glycolysis. | Zhong et al., 2017 [16] |
| PDK1 | Regulate the tricarboxylic acid cycle in hypoxia‐inducible factor‐1 independent pathway. | Ma et al., 2014 [18] | |
| PRMT5 | Activate the sterol regulatory element‐binding protein methylation and lipogenesis. | Liu et al., 2016 [59] | |
| OXCT1 | Drive ketone metabolism to the tricarboxylic acid cycle and inactivate AMP‐activated protein kinase. | Huang et al., 2016 [62] | |
| TKT | Regulate R5P expression and nucleotide synthesis. | Li et al., 2019 [51] | |
| P5C synthase and P5C reductase 1 | Kidney cancer | Direct proline biosynthesis from glutamine. | Liu et al., 2012 [82] |
| Prostate cancer | |||
| Burkitt lymphoma | |||
| Cytochrome c oxidase | Pancreatic cancer | Mediate oxidative phosphorylation and ATP production. | Ishida et al., 2013 [114] |
| SDH | Induce pyruvate production. | Yu et al., 2019 [90] | |
| PP2A | Gastric cancer | Target MST1/2, trigger YAP signaling and suppress the Hippo tumor suppressor with the regulatory subunit, STRN3. | Tang et al., 2020 [73] |
| CRLs | Colitis‐associated cancer | Induce ST7 ubiquitination in an inflammatory tumor microenvironment. | Liu et al., 2019 [77] |
| IDO | Multiple myeloma | Block T cells and decrease antitumor bioactive substances production. | Yan et al., 2019 [101] |
| LDH | Squamous cell carcinoma | LDH defectiveness sustains glycolysis. | Flores et al., 2019 [33] |
Abbreviations: ASNase1, asparaginase 1; CAD, carbamoyl‐phosphate synthetase 2, aspartate transcarbamylase and dihydrooratase; CKB, creatine kinase, brain‐type; CRL, cullin‐RING E3 ligase; G6PD, glucose‐6‐phosphate dehydrogenase; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; GLUD1, glutamate dehydrogenase 1; GPT2, glutamic pyruvate transaminase 2; HK2, hexokinase 2; HMGCL, 3‐hydroxy‐3‐methylglutaryl‐CoA lyase; HMOX1, heme oxygenase 1; IDH1, isocitrate dehydrogenase 1; IDO, indoleamine 2,3‐dioxygenase; KDM8, lysine demethylase 8; LDH, isocitrate dehydrogenase.; LDHA, lactate dehydrogenase A; OXCT1, 3‐oxoacid CoA‐transferase 1; PCK1, phosphoenolpyruvate carboxykinase 1; PCK2, phosphoenolpyruvate carboxykinase 2; PDK1, pyruvate dehydrogenase kinase 1; PDK2, pyruvate dehydrogenase kinase isozyme 2; PFKFB3, 6‐phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase 3; PFKFB4, 6‐phosphofructo‐2‐kinase/fructose‐2,6‐bisphosphatase 4; PHGDH, phosphoglycerate dehydrogenase; PKM2, pyruvate kinase M2; Plk1, polo‐like kinase 1; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A; PRMT5, protein arginine methyltransferase 5; SDH, serine dehydratase; TKT, transketolase; TRIM11, tripartite motif containing 11; UGDH, UDP‐glucose 6‐dehydrogenase.
Cancer cells possess multiple prime metabolic signaling pathways, sometimes re‐routing glucose flux consumption to other metabolic pathways, which contributes to maintain an aerobic microenvironment in the tumor. For example, unlike proliferating leukemia cell populations that switch cellular respiration to aerobic glycolysis, oxidative phosphorylation, and proliferation pathways of leukemia stem cells (LSCs) are very reliant on amino acid metabolism [3]. We have previously reported that regulatory alterations of the metabolic network in cancer cells extend beyond the Warburg effect and individual metabolic flux. Thus, it is imperative to interrogate the metabolome of tumors to elucidate the properties associated with clinical intervention. In this review, we summarize the unique metabolic alterations in tumor cells which occur through various signaling axes. Based on these metabolic alterations, we present various modalities available for cancer diagnosis and clinical therapy and further provide suggestions for the development of anti‐tumor therapeutic drugs.
2. GLUCOSE METABOLISM
2.1. Phosphorylation
Phosphorylation is vital to coordinate cancer metabolism as well as the biosynthesis of cancer‐related metabolites. Ma et al. [4] showed that the active dimer of polo‐like kinase 1 (Plk1) and phosphorylated glucose‐6‐phosphate dehydrogenase (G6PD) caused sustained activation of the PPP and resulted in increased biosynthesis of macromolecules in HeLa cells (cervical cancer cells cultured in vitro) (Table 1). Additionally, the metabolic enzyme 6‐phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase 3 (PFKFB3) is highly regulated by phosphorylation. In breast cancer cells, it has been reported that prolongation of mitotic arrest causes AMP‐activated protein kinase (AMPK)‐dependent phosphorylation of PFKFB3, and thereby induce mitosis‐associated translation. This results in an increase in glycolysis which replaces oxidative respiration in breast cancer (Table 1) [5].
Phosphorylation often accelerates cancer invasion and metastasis. Wang et al. [6] found that the activation of epidermal growth factor receptor (EGFR) in metastatic lung cancer induced the phosphorylation of UDP‐glucose 6‐dehydrogenase (UGDH) at tyrosine 473 and stimulated the expression of SNAI1 mRNA; activated EGFR also promoted epithelial‐mesenchymal transition (EMT) (Table 1). Loo et al. [7] identified multiple miRNAs, including miR‐551a and miR‐483, which stimulated creatine kinase, brain‐type (CKB) and thereby induced the phosphorylation of creatine to produce phosphocreatine, leading to sustained ATP production and the colonization of colorectal cancer (CRC) cells in the liver (Table 1 and Figure 1A).
FIGURE 1.
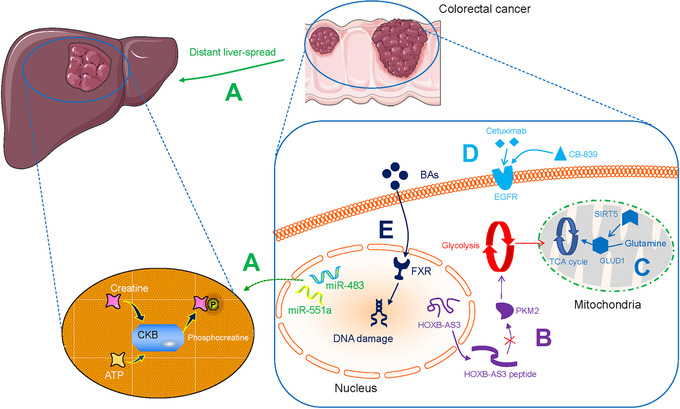
Metabolic alterations in colorectal cancer. A. miR‐551a and miR‐483 induce CKB‐directed creatine phosphorylation and ATP‐sustaining liver colonization. B. LncRNA HOXB‐AS3‐encoded peptide alleviates the formation of PKM2 and downregulates glycolysis. C. SIRT5 activates GLUD1 and facilitates Gln to be incorporated into the TCA cycle. D. EGFR‐targeted cetuximab in combination with CB‐839 can improve therapeutic efficacy. E. BAs impair intestinal FXR and induce subsequent DNA damage. Abbreviations: CKB, creatine kinase brain‐type; LncRNA, long non‐coding RNA; PKM2, pyruvate kinase M2; SIRT5, sirtuin5; GLUD1, glutamate dehydrogenase 1; Gln, glutamine; TCA, tricarboxylic acid; EGFR, epidermal growth factor receptor; BA, bile acid; FXR, farnesoid X receptor;
Phosphorylation is also associated with converting physiological signaling factors to cancer‐promoting factors. Kim et al. [8] showed that the nuclear oncogenic wild‐type p53 (WTp53) downregulated pyruvate‐directed oxidative phosphorylation by triggering a p53 upregulated modulator of apoptosis (PUMA)‐inactivated mitochondrial pyruvate carrier (MPC) in hepatocarcinoma (HCC; Figure 2A). Liang et al. [9] discovered that the phosphatase Cdc25A is activated by phosphorylation at Y59 by EGFR‐c‐Src signaling, and regulates the cell cycle. They further demonstrated that the activated Cdc25A sustains glycolysis and is related to the grades of human glioblastoma malignancy (Table 1). Phosphorylation‐dependent networks cause metabolic abnormalities and instigate malignancies, and thus can be explored to identify potential therapeutic targets.
FIGURE 2.
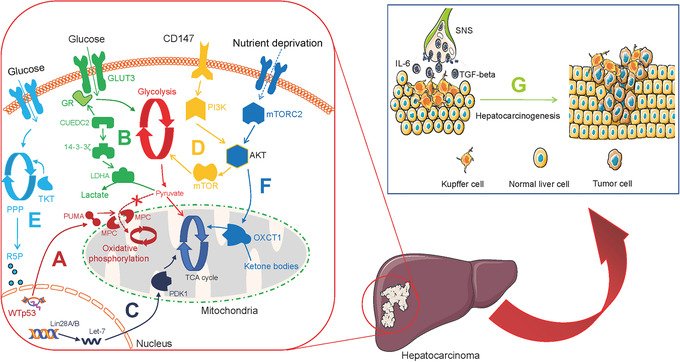
Metabolic alterations in hepatocarcinoma. A. WTp53 downregulates pyruvate‐directed oxidative phosphorylation by triggering PUMA‐inactivated MPC. B. CUEDC2 triggers the GR‐GLUT3 peptide axis and 14‐3‐3ζ‐LDHA pathway to produce lactate. C. Lin28A and Lin28B express aberrantly while let‐7 downregulates, which contributes to activate PDK1 to TCA cycle. D. CD147 sustains the Warburg effect through PI3K/Akt/mTOR axis. E. TKT activates R5P and subsequent nucleotide production. F. mTORC2‐AKT axis triggers OXCT1 to drive ketolysis to the TCA cycle and inactivate AMPK. G. SNS regulates liver inflammation and secretory IL‐6 and TGF‐β production to trigger hepatocarcinogenesis. Abbreviations: WTp53, wild‐type p53; PUMA, p53 upregulated modulator of apoptosis; MPC, mitochondrial pyruvate carrier; CUEDC2, CUE domain‐containing protein 2; GR, glucocorticoid receptor; GLUT3, glucose transporter 3; LDHA, lactate dehydrogenase A; PDK1, pyruvate dehydrogenase kinase 1; TCA, tricarboxylic acid; TKT, transketolase; R5P, ribose 5‐phosphate; PPP, pentose phosphate pathway; mTORC2, mTOR complex 2; OXCT1, 3‐oxoacid CoA‐transferase 1; AMPK, AMP‐activated protein kinase; SNS, sympathetic nervous system; IL‐6, interleukin‐6; TGF‐β, transforming growth factor‐beta
2.2. Acetylation
Acetylation of various targets promotes cancer development and is thought to occur as a response to glucose consumption. Chen et al. [10] discovered that serine and arginine‐rich splicing factor 5 (SRSF5) was hyperacetylated in lung carcinoma, blocked Smurf1‐coordinated ubiquitylation, sustained the splicing of cell division cycle and apoptosis regulator protein 1 (CCAR1), and responded to increased acetyl CoA biosynthesis and upregulated glucose consumption. Li et al. [11] demonstrated that cisplatin triggered the acetylation of accumulated PFKFB3 in the cytoplasm, contributing to the prevention of DNA degradation in glycolytic HeLa cells (Table 1). Li et al. [12] proposed that acetylation at lysine 254 increased glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) activity, and responded to intracellular glucose consumption in lung cancer (Table 1). Therefore, acetylation significantly supports cancer cell proliferation and aggressive invasion.
2.3. Enzyme catalysis
Aberrant overexpression of glycolysis‐associated enzymes in tumor tissue is common and is associated with the Warburg effect. In human glioblastoma multiforme (GBM), hexokinase 2 (HK2) promotes the migration of chemotherapy‐resistant GBM cells into the central nervous system, this effect is associated with protein kinase B (PKB)‐stimulated glycolysis and mitochondrial translocation (Table 1) [13]. In ovarian cancer, pyruvate kinase M2 (PKM2) has been shown to control glycolytic rate (Table 1) [14]. In leukemia, pyruvate dehydrogenase kinase isozyme 2 (PDK2) in the bone marrow regulates the levels of glycolysis and recruits leukemia‐initiating cells (LICs), which triggers symmetric cell division (Table 1) [15]. Multiple glycolysis enzymes jointly coordinate the Warburg effect. For example, Zhong et al. [16] proposed that the CUE domain‐containing protein 2 (CUEDC2) is regulated by the glucocorticoid receptor (GR)‐glucose transporter 3 (GLUT3) peptide axis as well as the 14‐3‐3ζ‐lactate dehydrogenase A (LDHA) pathway, and promotes the conversion of pyruvate to be converted to lactate in HCC (Table 1, Figure 2B and Figure 3).
FIGURE 3.
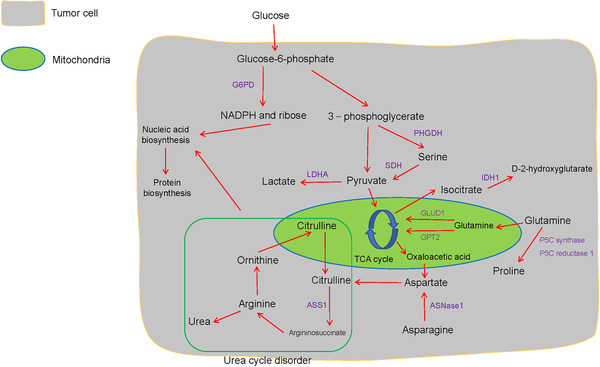
The correlation between glucose metabolism and amino acid metabolism in tumor cells. Aerobic glycolysis contributes to serine metabolism, and glutamine metabolism and Warburg effect transmit precursors to the TCA cycle. Arginine is cleaved to produce urea and ornithine. Ornithine supports citrulline biosynthesis in the mitochondria, and subsequently to arginine renewal, and this series of reactions is termed as the urea cycle. And the urea cycle disorder and pentose phosphate pathway alter nucleic acid level. Abbreviations: G6PD, glucose‐6‐phosphate dehydrogenase; PHGDH, phosphoglycerate dehydrogenase; SDH, serine dehydratase; LDHA, lactate dehydrogenase A; IDO, indoleamine 2,3‐dioxygenase; IDH1, isocitrate dehydrogenase 1; GLUD1, glutamate dehydrogenase 1; GPT2, glutamic pyruvate transaminase 2; ASNase1, asparaginase 1; ASS1, argininosuccinate synthetase 1
Furthermore, the concentration of oxygen in the tumor microenvironment (TME) affects glycolytic enzymes indirectly. Wang et al. [17] found that the histone lysine demethylase lysine demethylase 8 (KDM8) interacts with PKM2 and subsequently modulates hypoxia‐inducible factor‐1 alpha (HIF‐1α) to upregulate glycolytic gene activities in prostate cancer cells under hypoxic conditions (Table 1). Furthermore, Ma et al. [18] reported that aberrantly expressed Lin28A and Lin28B and decreased let‐7 results in upregulating pyruvate dehydrogenase kinase 1 (PDK1) in the HIF‐1 independent pathway and modulating tricarboxylic acid (TCA) cycle in the aerobic tumor environment of HCC (Table 1 and Figure 2C). Proliferating tumors enable the Warburg effect in both ambient oxygen levels and in the anaerobic TME via glycolytic‐associated genes and enzymes. Previously, this aspect has received little attention.
2.4. Glycolysis and long non‐coding RNAs (lncRNAs)
lncRNAs are RNA molecules that are not translated into proteins. However, they regulate the metabolic characteristics of cancers such as CRC. Tang et al. [19] discovered that in CRC cells, upregulated lncRNA GLCC1 in conditions of glucose depletion protected the ubiquitination of c‐MYC transcriptional factor by integration with heat shock protein 90 (HSP90). In addition, the peptides encoded by lncRNAs are involved in regulating metabolism in CRC tumor cells. The lncRNA, HOXB‐AS3‐encoded peptide (HOXB‐AS3 peptide) inhibited the expression of PKM2 and downregulated glycolysis, suggesting that HOXB‐AS3 peptide deficiency is a critical oncogenic event in CRC (Figure 1B) [20]. Zheng et al. [21] found that Yes‐associated protein (YAP)‐upregulated lncRNA breast cancer anti‐estrogen resistance 4 (BCAR4), coordinated Hedgehog signaling and enhanced the transcription of HK2 and PFKFB3 to generate secretory lactate (Figure 4A). These observations not only validate lncRNAs as regulators of tumors with potential clinical value but also reveal regulatory mechanisms for controlling tumors proliferation and energy metabolism.
FIGURE 4.
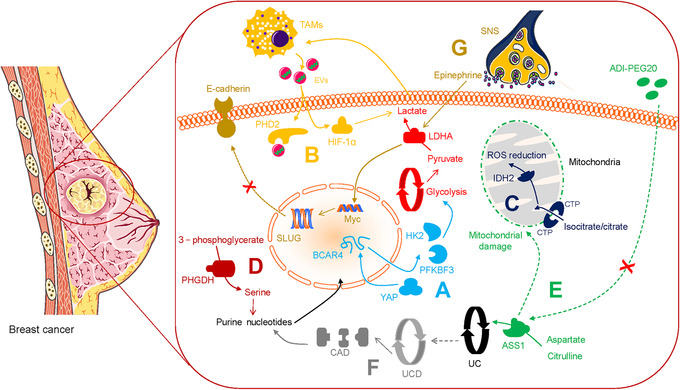
Metabolic alterations in breast cancer. A. YAP‐upregulated lncRNA BCAR4 enhances the transcription of HK2 and PFKFB3 to generate lactate. B. TAM‐derived EVs deliver HISLA, which antagonizes the binding of PHD2 and HIF‐1α. C. Isocitrate/citrate enters the mitochondria via CTP and enables ROS stress downregulation. D. PHGDH improves serine levels and sustains purine and nucleotides biosynthesis. E. ADI‐PEG20 induces ASS1 deprivation and attenuates arginine synthesis with mitochondrial damage. F. UC disorder activates CAD and contributes to subsequent nucleic acid synthesis. G. SNS releases epinephrine to trigger LDHA and stabilize Myc, subsequently triggers SLUG and inactivates E‐cadherin. Abbreviations: YAP, Yes‐associated protein; LncRNA, long non‐coding RNA; BCAR4, breast cancer anti‐estrogen resistance 4; HK2, hexokinase 2; PFKFB3, 6‐phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase 3; TAM, tumor‐associated macrophage; EV, extracellular vesicle; HISLA, HIF‐1alpha‐stabilizing lncRNA; PHD2, prolyl hydroxylase 2; HIF‐1α, hypoxia‐inducible factor‐1 alpha; CTP, citrate transporter protein; ROS, reactive oxygen species; IDH2, isocitrate dehydrogenase 2; PHGDH, phosphoglycerate dehydrogenase; ADI‐PEG20, pegylated arginine deiminase; ASS1, argininosuccinate synthetase 1; UC, urea cycle; CAD, carbamoyl‐phosphate synthetase 2, aspartate transcarbamylase and dihydrooratase; SNS, sympathetic nervous system; LDHA, lactate dehydrogenase A
2.5. Glycolysis and mitochondria
The unique metabolic profile of cancer cells is characterized by suppressed mitochondrial glucose oxidation. Luo et al. [22] demonstrated that reduced mitochondrial phosphoenolpyruvate carboxykinase 2 (PCK2) led to glucose carbon flow via oxaloacetic acid (OOA)‐malate‐pyruvate and acetyl‐CoA‐fatty acid axis and suppressed the oxidative phosphorylation in melanoma (Table 1). Altered mitochondrial metabolism is a crucial factor in pediatric high‐grade gliomas (pHGGs). Shen et al. [23] found that dichloroacetate can stimulate mitochondrial oxidation and reduce the Warburg effect, and consequently interrupt the metabolism of tumor cells (Table 2). Reversing mitochondrial oxidation suppression holds promise in metabolic oncology.
TABLE 2.
Anti‐tumor effectors in targeting cancer metabolism
| Anti‐tumor effector | Molecular mechanism | Reference |
|---|---|---|
| Dichloroacetate | Reduce the Warburg effect. | Shen et al., 2020[23] |
| 2DG | Combining 2DG with rapamycin inhibits glycolysis. | Zhao et al., 2016 [37] |
| Clomifene | Induce mutant enzyme downregulations and occupy the allosteric site of mutations. | Zheng et al., 2017 [47] |
| Enasidenib | Enhance molecular remissions and mitigate hematopoietic differentiation damage in acute myeloid leukemia. | Stein et al., 2019 [48] |
| AGI‐5198 | Decrease D2HG, inhibit metastasis, impair cell cycling, and activate apoptosis in chondrosarcoma. | Li et al., 2015 [49] |
| Polydatin | Act as glucose‐6‐phosphate dehydrogenase inhibition and block pentose phosphate pathway. | Mele et al., 2018 [54] |
| EDPs | Block angiogenesis as well as endothelial cell transmission and vascular endothelial growth factor receptor 2‐induced protease generation. | Zhang et al., 2013 [68] |
| Hakai | Inhibit E‐cadherin ubiquitination and suppress EMT | Martinez‐Iglesias et al., 2020 [78] |
| CB‐839 | Improve therapeutic efficacy in cetuximab‐resistant colorectal cancer. | Cohen et al., 2020 [86] |
| Combining arsenic trioxide or homoharringtonine with CB‐839 decreases glutathione synthesis and upregulate mitochondrial reactive oxygen species | Gregory et al., 2019 [87] | |
| 1‐MT | Improve the therapeutic efficacy of Doxorubicin in cancer treatment. | Lan et al., 2020 [102] |
| ADI‐PEG20 | Induce argininosuccinate synthetase deprivation. | Huang et al., 2013 [104] |
| Be sensitive to prostate cancer cells with mitigated argininosuccinate synthetase and suppress early protective autophagy. | Kim et al., 2009 [105] | |
| Induce autophagy‐dependent death of breast cancer cells with the loss of argininosuccinate synthetase 1. | Qiu et al., 2014 [106] | |
| Vitamin D/25‐hydroxyvitamin D | Decrease the risk of colorectal cancer and induce an immune response. | Song et al., 2016 [110, 111] |
| Promote T‐cell function and decrease inflammation of tumor microenvironment in breast cancer. | Karkeni et al., 2019 [112] | |
| Propranolol | Synergize with anti‐cancer vaccine in cancer immunotherapies | Daher et al., 2019 [123] |
Abbreviations: 2DG, 2‐deoxyglucose; EDP, epoxydocosapentaenoic acid; EMT, epithelial‐mesenchymal transition.
2.6. Glycolysis and T‐cell
It is acknowledged that aerobic glycolysis restricts T‐cell‐mediated tumoricidal effector functions. Li et al. [24] reported that CD147 sustained the Warburg effect in HCC through the phosphoinositide 3‐kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) axis, which is correlated with T‐cell immunosuppression (Figure 2D). Kim et al. [25] found that programmed death‐ligand 1 (PD‐L1) triggered HK2 to promote the Warburg effect in non‐small cell lung carcinoma (NSCLC), a low expression of CD8+ T‐cell function‐associated genes was noted (Figure 5A and Table 1). However, aerobic glycolysis promotes effector T‐cells from immunopathological impairments triggered by the TME. Ho et al. [26] validated that T‐cells overexpressed the glycolytic metabolite phosphoenolpyruvate (PEP) in melanoma via phosphoenolpyruvate carboxykinase 1 (PCK1) activity, which bolstered T‐cell functions (Table 1). Further exploration of metabolic checkpoints for T‐cells could contribute to the elucidation of new forms of tumor immunity.
FIGURE 5.
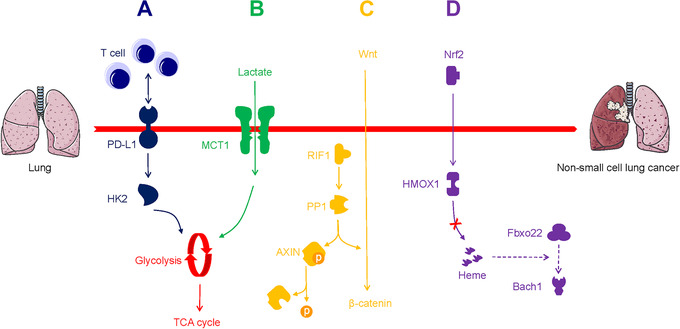
Metabolic alterations in non‐small cell lung cancer progression and metastasis. A. PD‐L1 triggers HK2 to promote the Warburg effect with immunosuppression. B. Lactate is driven to the TCA cycle via MCT1. C. PP1 to dephosphorylate AXIN and stimulates Wnt/β‐catenin axis. D. Nrf2 suppresses the heme‐induced and Fbxo22‐induced degradation of Bach1 through HMOX1. Abbreviations: PD‐L1, programmed death‐ligand 1; HK2, hexokinase 2; TCA, tricarboxylic acid; MCT1, monocarboxylate transporter‐1; PP1, RIF1 triggers protein phosphatase 1; AXIN, axis inhibition protein; HMOX1, heme oxygenase 1
2.7. Glycolysis and macrophage
The TME contains abundant infiltrating immune cells, including tumor‐associated macrophages (TAMs) [27]. Chen et al. [28] reported that to sustain the Warburg effect in breast cancer, extracellular vesicles (EVs) delivered HIF‐1alpha‐stabilizing lncRNA (HISLA), which antagonized the binding of prolyl hydroxylase 2 (PHD2) to HIF‐1α to stabilize HIF‐1α (Figure 4B). This consequently led TAM to sustain aerobic glycolysis and exhibit anti‐apoptotic ability, while secretory lactate remarkably upregulated HISLA in TAMs reciprocally. In addition, metastatic lung adenocarcinoma‐derived lactate stimulated mTORC1/transcription factor EB (TFEB)/ATP6V0d2/HIF‐2α and promoted the expression of vascular endothelial growth factor (VEGF) [29]. Within the TME, altered TAMs acquire invasive properties which can be correlated with aerobic glycolysis.
2.8. Glycolysis and cancer stem cell (CSC)
CSCs possess self‐renewal properties and the capability to differentiate into various tumor cell subpopulations [30]. Due to the Warburg effect, a high level of glucose depletion is observed in the TME. Tumor cells tend to increase stem cells phenotype to adapt to microenvironmental changes [31]. Significantly, the Warburg effect is implicated in the invasive activity of CSCs. Luo et al. [32] validated the critical role of breast CSCs, and showed that in these cells glycolysis was active while under dormancy, whereas antioxidant responses were exhibited in the proliferating state. These observations suggest that the metabolic vulnerabilities of CSCs may provide novel ways to target cancer cells.
2.9. Glycolysis and lactate metabolism
Recently many approaches have been attempted to target the Warburg effect, though valid therapeutic outcomes were not achieved. Flores et al. [33] reported that squamous cell carcinoma (SCC) cells with defective activity of lactate dehydrogenase (LDH) showed decreased glucose uptake and glycolytic metabolite production (Table 1). It is possible that tumorigenesis does not require active aerobic glycolysis, which overrides the dogma that tumors must rely on high glucose metabolically. Faubert et al. [34] provided evidence to prove that compared with glucose, lactate was the dominant carbon source for the TCA cycle in NSCLC, and was transported using the monocarboxylate transporter‐1 (MCT1; Figure 5B). Hensley et al. [35] observed and further identified the value of lactate as an available non‐glucose carbon source in well‐perfused tumor areas. These studies strongly indicate that tumors can use lactate as a fuel in vivo, and may not rely exclusively on aerobic glycolysis.
2.10. The promise of glucose metabolism‐glycolysis inhibition
Targeting aerobic glycolysis has become a promising chemotherapeutic strategy in the clinic. The glycolysis inhibitor 2‐deoxyglucose (2DG) is a potent anti‐cancer agent, and can function as an adjuvant drug in chemotherapy [36]. Zhao et al. [37] reported that rapamycin inhibited mTORC1 with LDHA downregulation, and the combination of rapamycin with 2DG potently influenced glycolysis in malignancies (Table 2). Nonetheless, glycolysis inhibitors including 2DG have not been used in clinical applications owing to nonspecific distribution to tumor tissue, and low bioavailability. Dong et al. [38] developed a nanoplatform that could target cancer cells and further modulate the TME based on photodynamic therapy and 2DG‐triggered endoplasmic reticulum (ER) stress. Zhang et al. [39] designed a liposomal nanocarrier that readily transmitted 3‐bromopyruvate (3‐BP), another glycolysis inhibitor, into cancer cells without distinct side effects. Delivering glycolysis inhibitors using nanoscale functional materials represents a crucial step forward in the precise treatment of malignancies.
2.11. Mutant isocitrate dehydrogenase (IDH)‐dependent pathway
Previously, an isocitrate dehydrogenase 1 (IDH1) mutant subset was reported in prostate cancer [40]. Cytosolic IDH mutations that convert isocitrate into D‐2‐hydroxyglutarate (D2HG) initiate events that allow primary clinical detection and prognosis prediction in gliomas (Figure 3) [41]. Flavahan et al. [42] identified IDH mutants that contribute to gliomagenesis via impaired chromosomal topology and trigger activities of the platelet‐derived growth factor receptor alpha (PDGFRA) oncogene (Table 1). Chen et al. [43] discovered that D2HG induced neuronal activity by simulating the binding of glutamate to the N‐methyl‐D‐aspartic acid (NMDA) receptor, and indicated that the IDH1 mutant caused a high risk of seizures in glioma patients (Table 1). Jiang et al. [44] reported that isocitrate/citrate could enter the mitochondria via the citrate transporter protein (CTP), this metabolite was involved in isocitrate dehydrogenase 2 (IDH2)‐induced oxidation, resulting in NADPH production, reactive oxygen species (ROS) stress downregulation, and stabilized the TME in lung, colon, and breast cancer (Figure 4C). Also, Izquierdo‐Garcia et al. [45] proposed that IDH1 mutations inhibited the pyruvate dehydrogenase (PDH) phosphorylation, upregulated HIF‐1 and pyruvate dehydrogenase kinase‐3 in glioblastoma cells, and reprogrammed pyruvate metabolism to aid glutamate generation (Table 1).
Significantly, Okoye‐Okafor et al. [46] revealed that inhibitors of mutant IDH1 contributed to D‐2HG reduction, myeloid differentiation, and granulocytic differentiation regulated by precursor cells in acute myeloid leukemia (AML). Zheng et al. [47] also showed that clomifene, an IDH1 mutant inhibitor, occupied the allosteric site of mutations in a dose‐dependent non‐competitive manner, and led to the downregulation of the mutant enzyme (Table 2). Another oral IDH2 mutant inhibitor, Enasidenib, is reported to enhance molecular remissions and mitigate hematopoietic differentiation damage in patients with AML (Table 2) [48]. IDH mutations are also present in chondrosarcoma. Li et al. [49] indicated that another dose‐dependent IDH1 mutant inhibitor, AGI‐5198, decreased the level of D‐2HG, downregulated colony formation and metastasis, derailed cell cycle regulation, and activated apoptosis (Table 2). In short, the therapeutic effects of novel mutant IDH inhibitors are very useful for cancer patients displaying mutations in IDH.
2.12. PPP
The PPP physiologically regulates redox homeostasis via G6PD that transforms glucose‐6‐phosphate and contributes to ribose‐5‐phosphate and NADPH production (Figure 3) [50]. Though PPP is related to the Warburg effect, its precise role in cancer metabolism remains unclear. Li et al. [51] reported that transketolase (TKT), which is a part of a branch of the PPP that is known to sustain HCC progression, modulates ribose 5‐phosphate (R5P) expression and nucleotide synthesis (Table 1 and Figure 2E). Du et al. [52] found that the p53 family member TAp73 activates the expression of the G6PD and triggers the biosynthesis of NADPH and ribose with attenuated ROS in osteosarcoma (Table 1 and Figure 3). Importantly, the PPP also plays a critical role in the regional spread and distant metastasis of cancers. McDonald et al. [53] revealed that the oxidative branch of the PPP propelled regional migration, while its suppression partly alleviated chromatin rewiring, oncogenic gene activity, and tumorigenesis in pancreatic ductal adenocarcinoma (PDAC). This indicates that an increase in PPP flux promotes tumor proliferation via a metabolic‐epigenetic signaling pathway. Clinical analysis indicates that PPP blockade can be recognized as a reliable anti‐tumor tool and the G6PD inhibitor polydatin functions as a novel anti‐PPP agent of cancers, such as tongue cancer [54] (Table 2).
3. LIPID METABOLISM
3.1. Oncogenic effect
Lipid metabolism is a critical event in the survival of malignancies [55, 56, 57]. Serol regulatory element‐binding protein (SREBP) has been historically recognized as a crucial element in lipid metabolism [58]. Liu et al. [59] proposed that SREBP activated high‐level de novo lipogenesis in HCC via protein arginine methyltransferase 5 (PRMT5)‐mediated methylation, which prevented ubiquitin‐proteasome‐mediated degradation of SREBP (Table 1). Xu et al. [60] reported that activated PCK1 prevented INSIG1/2 from complexing with intracellular lipid and trigged SREBP signaling related to lipogenesis in HCC, suggesting that SREBP has a central role in lipid metabolism (Table 1). However, in lung adenocarcinoma, hepatic metabolism is reprogrammed through an altered pro‐inflammatory response via signal transducers and activators of transcription 3‐suppressor of cytokine signaling 3 (STAT3‐Socs3) signaling, with the disruption of AKT, AMPK, and SREBP signaling, altered lipid metabolism is noted in these cells [61]. Active tumors present hyperactivation of lipid metabolism and reprogramming of physiological metabolic processes in distant organs.
Huang et al. [62] found that in HCC cells under nutrient stress, mitochondrial ketolytic promoter 3‐oxoacid CoA‐transferase 1 (OXCT1) was activated by serum starvation‐stimulated mTOR complex 2 (mTORC2)‐AKT axis, and drove ketone metabolism to the TCA cycle, while also inactivating AMPK. This observation suggests that HCC cells with poor nutrition status may rely on ketone bodies to support survival (Table 1 and Figure 2F). Human primary melanoma and hairy cell leukemia cells also show metabolic ketogenesis. Kang et al. [63] found that 3‐hydroxy‐3‐methylglutaryl‐CoA lyase (HMGCL) was activated by BRAF V600E‐expressing melanoma and leukemia cells, resulting in the activation of the MEK‐ERK pathway, the production of acetoacetate (Table 1). Ketolysis and ketogenesis are important for cancer cell activities, and targeting ketone body metabolism can be a novel strategy to develop drug candidates against malignancies.
3.2. Anti‐cancer effect
There is abundant evidence showing that fatty acids possess anti‐cancer properties. In previous studies, T‐cells pretreated with fatty acids were injected into patients to identify changes in functional T‐cells [64]. It has been revealed that fatty acids may be used by CD8+ T‐cells as energy sources. Thus, anti‐tumor effects were observed when cells were simultaneously subjected to hypoxic and hypoglycemic environments [65]. Zhang et al. [66] also found that CD8+ tumor‐infiltrating T‐lymphocytes (TILs) improved peroxisome proliferator‐activated receptor (PPAR)‐alpha signaling and degradation of fatty acids under hypoglycemia and hypoxia. Further enhancing fatty acid catabolism sustains the effector function of T‐cells, and improves antitumor efficacy.
Fatty acid metabolism is of great therapeutic interest in cancer patient populations. Omega‐3 polyunsaturated fatty acids in the diet can partially inhibit CRC development [67]. Zhang et al. [68] discovered that epoxydocosapentaenoic acids (EDPs) derived from omega‐3 fatty acids could block angiogenesis as well as endothelial cell migration and VEGF receptor 2‐induced protease generation (Table 2). Omega‐3 fatty acids may also be involved in certain anti‐cancer metabolism pathways and exhibit potential preventive and inhibitory effects in cancer.
4. PROTEIN MODIFICATION
4.1. Dephosphorylation
Serine/threonine‐protein phosphatase‐mediated dephosphorylation is a key process in physiological conditions, though mutations in phosphatases are found to sustain malignant progression [69, 70]. Mei et al. [71] reported that the replication timing regulatory factor 1 (RIF1) triggered protein phosphatase 1 (PP1) to dephosphorylate axis inhibition protein (AXIN), and stimulated the Wnt/β‐catenin axis to support the proliferative invasion of NSCLC cells (Table 1 and Figure 5C). Another phosphatase, protein phosphatase 2A (PP2A), modulates most of the protein dephosphorylation events in the cell by distinct regulatory subunits. Alterations in the subunits disturbed PP2A function and induce oncogenic signaling [72]. Tang et al. [73] found that a PP2A regulatory subunit, STRN3, recruited and dephosphorylated MST1/2, which helps trigger the YAP signaling and suppress the Hippo tumor suppressor of gastric cancer (Table 1). Recently, Liu et al. [74] identified that PP2A inhibitors synergize with chemotherapeutic agents to suppress aberrant signal transduction in cancer. Targeting aberrant protein phosphatase represents a promising therapeutic strategy in cancer treatment.
4.2. Ubiquitination
E3 ubiquitin ligase‐induced ubiquitination significantly modulates protein posttranslational modifications in tumor maintenance [75]. Tang et al. [76] found that tripartite motif‐containing 11 (TRIM11) sustained tumor‐promoting estrogen receptor α (ERα) ubiquitination to further stabilize this receptor in metastatic breast carcinoma (Table 1). Liu et al. [77] demonstrated that in an inflammatory microenvironment, upregulation of Cullin‐RING E3 Ligases (CRLs) and activation of c‐Myc induced ST7 ubiquitination in colitis‐associated cancer (CAC) (Table 1). Blocking the E3 ubiquitin‐ligase may be a reasonable anti‐tumor strategy in the clinic. Remarkably, Hakin‐1, which functions as a specific inhibitor of the E3 ubiquitin‐ligase Hakai, was found to inhibit the ubiquitination of E‐cadherin and suppress EMT (Table 2), implying a potential strategy to restrict CRC [78]. The ubiquitination of some pivotal molecules by E3 ubiquitin‐ligase modulates the activity of oncogenic signaling, and this feature may be potentially explored for designing molecularly targeted drugs for cancer treatment.
5. AMINO ACID METABOLISM
5.1. Glutamine metabolism
High levels of glutamine (Gln) in the blood provide a readily available source of carbon and nitrogen to support cancer cell activities. Wang et al. [79] reported that mitochondrial sirtuin5 (SIRT5) activated glutamate dehydrogenase 1 (GLUD1) and consequently facilitated the incorporation of Gln into the TCA cycle. This indicates the critical role of Gln metabolic rewiring in the malignant phenotypes of CRC (Table 1 and Figure 1C). Similarly, colon cancer‐derived mitochondrial glutamic pyruvate transaminase 2 (GPT2)‐regulated Gln catabolism is involved in the Warburg effect, which takes advantage of the fact that Gln is the dominant carbon source Gln for the TCA cycle (Figure 3 and Table 1) [80].
The oncogenic transcription factor c‐MYC is regulated by the Menin gene, and drives cancer cell proliferation and metabolism in vitro and in vivo [81]. In kidney cancer, prostate cancer, and Burkitt lymphoma, c‐MYC also triggers the upregulation of glutaminolysis to fuel tumor growth by increasing P5C synthase and P5C reductase 1‐mediated proline biosynthesis from Gln; suggesting a metabolic link between Gln and proline (Table 1 and Figure 3) [82].
Alteration of Gln metabolism contributes to nucleotide biosynthesis and attenuates DNA damage. This leads to an increase in conventional radiotherapy resistance [83]. Targeting Gln metabolic pathways, such as glutaminolysis, may provide a therapeutic strategy to kill tumor cells. The oral glutaminase inhibitor CB‐839 was reported to suppress Gln‐derived metabolite production during tumor development [84, 85]. Cohen et al. [86] found that cetuximab (which targets EGFR) in combination with CB‐839 improved therapeutic efficacy in cetuximab‐resistant CRC (Table 2 and Figure 1D). Gregory et al. [87] reported that combining CB‐839 with arsenic trioxide or homoharringtonine decreased Gln synthesis in hematologic malignancies, upregulated ROS in the mitochondria, and triggered tumor cell death (Table 2). Dual targeting of oncogenic signaling by combining CB‐839 with another anti‐tumor agent may be a potential strategy for reversing conventional resistance to therapy.
5.2. Serine metabolism
Compared to normal tissue, cancer cells exhibit altered metabolism with the overexpression of serine synthesis pathway enzymes, such as phosphoglycerate dehydrogenase (PHGDH), which converts 3‐phosphoglycerate derived from glucose‐6‐phosphate into serine [88] (Figure 3). Sullivan et al. [89] found that the overexpression of PHGDH improved serine levels and sustained purine and nucleotide biosynthesis, which supported melanoma and breast cancer growth under serine deprivation (Table 1 and Figure 4D). Activation of serine synthesis resulted in the conversion of glucose to pyruvate via serine dehydratase (SDH), and rewired glycolysis in a pyruvate kinase‐independent manner in pancreatic cancer cells [90] (Table 1 and Figure 3). Pacold et al. [91] reported that PHGDH suppression inhibited the production of nucleotides which derived from one‐carbon units from glycolytic serine, even in the presence of exogenous serine, demonstrating that serine was a vital source of one‐carbon for purine as well as deoxythymidine synthesis in breast cancer (Table 1). Thus, PHGDH might be a novel metabolic vulnerability, and the preclinical evaluation of PHGDH inhibitor could contribute to further therapeutic applications [92].
5.3. Methionine metabolism
For mammalian cells, methionine metabolism is essential for epigenetic reprogramming and cell growth [93]. The methionine cycle flux also impacts the epigenetic state of tumor cells. Metabolomic studies indicated that in NSCLC stem cells, methionine cycle activity is dysregulated, and methionine consumption exceeded methionine regeneration. [94]. Proteomic and genomic analyses showed that methyltransferase nicotinamide N‐methyltransferase (NNMT) expression contributed to the cancer‐associated fibroblast (CAF) phenotype and caused depletion of S‐adenosyl methionine, which is critical for ovarian cancer metastasis [95] (Table 1). Methionine‐related interventions are potentially used to treat diseases of metabolic origin. Gao et al. [96] revealed that methionine limitation induced clinical responses in pathological models of chemotherapy‐tolerated RAS‐driven CRC and soft‐tissue sarcoma. Targeting methionine uptake can partly influence cancer metabolism, which ultimately regulates multiple aspects of therapeutic outcomes in cancer.
5.4. Aspartate metabolism
Unlike other amino acids, aspartate is not readily available in the blood, so proliferating cells, such as tumor cells, produce aspartate by themselves. For example, TCA cycle‐derived OOA mediates aspartate biosynthesis, which plays a pivotal role in cellular processes [97] (Figure 3). Surprisingly, mitochondrial respiration generates sufficient electron acceptors for sustaining aspartate production [98]. However, aspartate has poor cell permeability, while the amino acid asparagine is available in tumors. Sullivan et al. [99] found that asparaginase 1 (ASNase1) allowed inter‐conversion between asparagine and aspartate in 143B cells (human osteosarcoma cells in vitro), and subsequently provided sufficient endogenic aspartate, leading to a significant acceleration of tumor growth (Table 1 and Figure 3). These studies revealed that facilitating metabolic aspartate depletion could help to treat cancer.
5.5. Tryptophan metabolism
Indoleamine 2,3‐dioxygenase (IDO) in tumor cells can metabolize tryptophan (Trp) and trigger kynurenine (KYN) biosynthesis, which suppresses T‐cell proliferative growth and promotes an immunosuppressive TME [100]. Notably, intratumoral metabolites instigate other tumor‐associated cell populations to stimulate Trp catabolism and impair immune function. In multiple myeloma (MM), tumor cells release IL‐32 and trigger TAMS to produce IDO. This further antagonizes CD4+ T‐cell proliferative growth, which decreases antitumor bioactive substance secretion [101] (Table 1). IDO modulates the immunosuppressive function via depletion of Trp and the accumulation of KYN. Thus, a combination of IDO inhibitors including 1‐methyl tryptophan (1‐MT) and chemotherapeutic agents containing doxorubicin could be considered as a potential treatment option [102] (Table 2).
5.6. Amino acid metabolism‐arginine deprivation
Arginine accelerates tumorigenesis via the enzyme argininosuccinate synthetase 1 (ASS1) which catalyzes the synthesis of argininosuccinate from citrulline and aspartate, and is the rate‐limiting enzyme in arginine biosynthesis [103] (Figure 3). This enzyme can be targeted for developing arginine deprivation therapy in multiple tumor types. Huang et al. [104] identified that ASS1‐deleted myxofibrosarcoma cells were sensitive to PEGylated arginine deiminase (ADI‐PEG20) therapy, and ASS1 deletion was associated with unfavorable clinical results in patients. The feasibility of ADI‐PEG20 targeted treatment therapy may be directly linked to the loss of ASS 1 (Table 2).
The availability of ADI‐PEG20 is directly implicated in ASS deficiency in other cancer types. Kim et al. [105] pointed out that prostate cancer cells with mitigated ASS were susceptible to autophagy suppression and ADI‐PEG20 therapy, while early autophagy was reported to exert an initial protective effect for cancer cells. The above evidence suggests that ASS deletion, autophagy, and ADI‐PEG20 efficiency could be interrelated (Table 2). ADI‐PEG20 also induced autophagy‐dependent death of breast cancer cells with the loss of ASS1, and attenuated de novo arginine synthesis derived from aspartate and citrulline, and arginine deprivation also caused mitochondrial damage and cytotoxic autophagy in cancer cells [106] (Table 2 and Figure 4E). Thus, robust arginine deprivation therapy merits exploration as a potential therapeutic strategy to treat cancer.
6. OTHER METABOLIC PATHWAYS
6.1. Bile acid metabolism
In most cases of sporadic CRC, intestinal bile acids (BAs) are risk factors linked to worse clinical outcomes. Fu et al. [107] showed that APC mutants could change the structure of BA, leading to the production of BAs such as tauro‐beta‐muricholic acid (T‐betaMCA) and deoxycholic acid (DCA). This can lead to an impairment of intestinal farnesoid X receptor (FXR) function, resulting in DNA damage and adenoma‐to‐adenocarcinoma transformation in CRC (Figure 1E). BA metabolism alters the composition of the intestinal microbiome in the TME of CRC. Yachida et al. [108] observed increased BAs including deoxycholate in polypoid adenomas and/or intramucosal carcinomas, indicating a link between the human gut microbiome, BA metabolism, and the early stage of the development of CRC.
6.2. Vitamin D metabolism
Epidemiological data have shown that vitamin D has anti‐cancer function as it was associated with a decrease in CRC risk. Maalmi et al. [109] reported that CRC patients with plasma 25‐hydroxyvitamin D (25‐(OH)‐D3) levels < 30 nmol/L had an extremely high mortality, and 25‐(OH)‐D3 deprivation led to a poor clinical prognosis. Song et al. [110, 111] analyzed a large number of samples and discovered that elevated blood 25‐(OH)‐D3 levels were associated with a decreased risk of CRC (Table 2). In addition to CRC, vitamin D boosts CD8+ T‐cell function and decreases inflammation of the TME in breast cancer tissue (Table 2) [112]. Due to its anti‐inflammatory and anti‐tumor properties, vitamin D may be a promising candidate for use as an adjuvant in standard cancer immunotherapies.
6.3. Trace element metabolism
Metabolic imbalances in trace elements are associated with multiple cancer‐induced pathological conditions. For example, zinc homeostasis damage causes muscle wasting in cancer patients. Wang et al. [113] discovered that the activated zinc transporter ZRT‐ and IRT‐like protein 14 (ZIP14) in metastatic cancer cells facilitated zinc uptake in muscle progenitor cells and inhibited MyoD and Mef2c, leading to mitigated muscle‐cell differentiation. Another trace element, copper, enables cancer cell proliferation, especially in primary pancreatic cancer. Ishida et al. [114] highlighted that the tumor promoter copper interacts with cytochrome c oxidase and mediates oxidative phosphorylation and ATP production (Table 1). Trace elements in the TME may be capable of partially altering tumor metabolic phenotypes.
6.4. Urea cycle metabolism
Arginine is the main source of urea. Arginine is cleaved to produce urea and ornithine. Ornithine supports citrulline biosynthesis in the mitochondria, and subsequently to arginine renewal, and this series of reactions is termed as the urea cycle (UC) [115] (Figure 3). UC dysregulation is a general metabolic feature of multiple cancer types. Lee et al. [116] reported the critical role of the UC disorder in nitrogen conversion toward the activation of carbamoyl‐phosphate synthetase 2, aspartate transcarbamylase and dihydrooratase (CAD). This contributes to subsequent pyrimidine synthesis and a detectable hallmark constituted by transversion mutations at the nucleotides, nucleic acid and protein levels in prostate cancer, breast cancer, and lung carcinoma models (Figure 4F, Figure 3, and Table 1). The UC is a metabolic vulnerability in EGFR‐driven NSCLC, and sustains cellular energetics upon inhibiting EGFR [117].
6.5. Stress‐mediated adrenaline metabolism
Stress mediates neurochemical and endocrinological alterations, and leads to increased levels of adrenaline in metastatic tumor initiation [118]. Chronic behavioral stress induces the sympathetic nervous system (SNS) and contributes to greater tumor burden and invasive growth in HCC and breast cancer. Huan et al. [119] identified that SNS supported HCC behaviors by targeting alpha1‐adrenergic receptors (ARs) in Kupffer cells to modulate chronic liver inflammation, secretory interleukin‐6 (IL‐6) production, and transforming growth factor‐beta (TGF‐β) production (Figure 2G). Cui et al. [120] demonstrated that stress‐activated SNS stimulated the production of epinephrine which triggered LDHA to generate lactate and stabilize Myc, subsequently triggered SLUG and further sustained breast cancer stem‐like properties (Figure 4G). Activated SLUG inactivates E‐cadherin and accelerates breast cancer cell metastasis (Figure 4G) [121, 122]. Significantly, Daher et al. [123] identified that β‐blockers enhanced CD8+ T‐cell responses and propranolol could synergize with anti‐cancer vaccines in cancer immunotherapies (Table 2). Thus, chronic stress‐induced adrenaline signaling regulates metabolic rewiring in cancer progression, and this complements existing therapeutic strategies with beta‐blockade.
6.6. HMOX1‐dependent pathway
In cancer development, the superinduction of oxidative stress response often causes depletion of tumor suppressors, with the concomitant accumulation of heme oxygenase 1 (HMOX1) and Bach1, a sensor and effector of heme [124]. HMOX1 contributes to create a microenvironment that promotes tumor initiation. In addition, HMOX1 and Nrf2 significantly correlate with specific organ colonization of malignancies [125]. Lignitto et al. [126] discovered that Nrf2 was presented in nearly 30% of lung cancer cases, and enabled metastasis by stimulating HMOX‐1 activity which subsequently downregulated heme and suppressed Fbxo22 (a ubiquitin ligase)‐induced degradation of Bach1 in NSCLC (Table 1 and Figure 5D). Potent HMOX‐1 inhibitors may be effective in alleviating malignancies.
6.7. Autophagy pathway
Autophagy predominates in cellular catabolic responses under nutrient or energy depletion [127], while tumor cell metabolism strikingly affects autophagic processes of the cell. Thomas et al. [128] found that genetic defects in mitochondrial complex I suppressed autophagy induced by mTOR inhibitors in cells with increased mitochondrial metabolism. However, the lack of the autophagy gene Atg5 in KRAS‐driven cancer led to impaired asparagine metabolism, reduced mitochondrial function, and caused excessive mitochondrial fragmentation. This evidence supports the critical role of autophagy in KRAS‐driven progression [129]. In addition, upon serum starvation or metabolic stress, the oncoprotein lysosomal protein transmembrane 4 beta (LAPTM4B) and the exocyst subcomplex Sec5 stimulated inactive EGFR to interact with the autophagy inhibitor Rubicon, resulting in a disassociation of the Beclin 1‐Rubicon complex. Subsequently, initial autophagic activity was triggered, which correlated with tumorigenesis [130]. Autophagy may be positioned to tune tumor metabolism under poor nutritional conditions within the TME.
7. METABOLOMIC PROFILING
Metabolic profiling helps in understanding the molecular mechanisms involved in individual tumors in cancers such as HCC. Tan et al. [131] provided serum profiling data and identified the diagnostic usefulness of taurocholic acid, lysophosphoethanolamine, and lysophosphatidylcholine, which indicate the aberrant metabolic properties in all HCCs. Metabolomics can help in the identification of underlying inflammatory metabolite markers for early HCC diagnosis. Aleksandrova et al. [132] conducted metabolic profiling in HCC, intrahepatic bile duct (IBD), gallbladder, and biliary tract cancers (GBTC), and identified the diagnostic role of C‐reactive protein (CRP), IL‐6, non‐high‐molecular‐weight (HMW) adiponectin, and C‐peptide, suggesting that inflammation and hyperinsulinemia are involved in hepatocarcinogenesis (Table 3).
TABLE 3.
Diagnostic metabolic biomarkers in cancer
| Metabolic biomarker | Cancer type | Reference |
|---|---|---|
| Taurocholic acid, lysophosphoethanolamine and lysophosphatidylcholine | Hepatocarcinoma | Tan et al., 2013 [131] |
| CRP, IL‐6, non HMW adiponectin, and C‐peptide | Aleksandrova et al., 2014 [132] | |
| AEA and PEA | Hepatocarcinoma infected with HCV | Zhou et al., 2012[133] |
| Ethanolamine, lactic acid, phenylalanine, and ribose | Hepatocarcinoma after surgical resection. | Ye et al., 2012 [134] |
| Chain lengths ceramides and sphingomyelins | Epithelial ovarian cancer | Kozar et al., 2018 [135] |
| N‐oleoyl taurine | Prostate cancer | Huang et al., 2019 [136] |
| Acetate | Colorectal cancer | Lin et al., 2019 [137] |
| 2‐hydroxyisobutyrate, 3‐indoxylsulfate, and alanine | Gastric cancer | Chan et al., 2016 [138] |
Abbreviations: AEA, endocannabinoids anandamide; CRP, C‐reactive protein; HCV, hepatitis C virus.; HMW, high‐molecular‐weight; IL‐6, interleukin‐6; NSE, neuron‐specific enolase; PEA, palmitylethanolamide.
Zhou et al. [133] performed serum metabolic profiling and reported that higher concentrations of arachidonic acid and lower levels of lysophosphatidylcholines (lysoPCs) with polyunsaturated fatty acid acyl chains, led to HCC development in conditions of chronic inflammation, and elevated serum endocannabinoids anandamide (AEA), and palmitoylethanolamide (PEA) could lead to HCC in hepatitis C virus (HCV)‐positive patients (Table 3).
In addition, metabolomics method sheds light on the metabolic regulation of HCC after surgical resection. Ye et al. [134] proposed that post‐surgical HCC patients displayed complex metabolic responses that promoted anabolic metabolism; they further identified 16 elevated metabolites in HCC recurrence as well as five feasible diagnostic markers including ethanolamine, lactic acid, phenylalanine, and ribose (Table 3).
Metabolomic profiling is diverse and flexible, and provides opportunities to also identify metabolic markers in other cancer types. Kozar et al. [135] utilized serum metabolomic profiling and identified ceramides and sphingomyelin compounds of specific chain lengths as diagnostic markers in epithelial ovarian cancer (Table 3). In addition, serum metabolomics indicated that N‐oleoyl taurine correlated with impaired metabolism and contributed to prostate cancer pre‐diagnosis [136] (Table 3). Additionally, metabolomics based on HNMR revealed that 2‐hydroxyisobutyrate, 3‐indoxylsulfate, and alanine from urine in gastric cancer and acetate from fecal metabolites in patients with CRC exhibited higher diagnostic superiority than other metabolites in corresponding specimens (Table 3) [137, 138]. Metabolic signatures reflect the tumor tissue microenvironment, and metabolomics can enable the identification of sensitive diagnostic biomarkers for malignancies in a clinical setting.
8. CONCLUSIONS AND OUTLOOK
In this review, we summarized a variety of sufficiently unrecognized metabolic signaling pathways in tumorigenesis, invasive metastasis, aggressive growth, and immunosuppressive reactions. Research on cancer metabolism has made great progress, and the genetic background and metabolic signatures of tumors, including the idiographic concepts mentioned above, are worth further exploration.
Cellular metabolism is a key link between the extracellular environment and intracellular processes, and cancer‐specific metabolic rewiring frequently converts normal physiological metabolic processes, such as protein modification and diverse enzymatic reactions, into necessary protective barriers for proliferating tumors [139, 140, 141]. Tumor cell populations compete with the normal tissue for glucose, and rate‐limiting stimulators subsequently coordinate the Warburg effect. In addition, increased anaerobic glycolysis provides sufficient building blocks of bioactive substances including purines and pyrimidines in combination with other equally crucial metabolites. In condition of glucose starvation, other metabolites are metabolized which not only provides energy supplies but also stimulates oncogenic alterations.
Therefore, identifying and further targeting the metabolic nodes of tumor cells is the promising therapeutic interventions in patients with malignancies. Compared with most conventional diagnostic methods, metabolomics effectively analyzes accessible biofluid which contain abundant metabolic information therein in cancers. Some malignancies are lack of early diagnostic platform, and metabolomics contributes to search for circulating metabolic biomarkers and achieve clinical therapies such as early surgical resection of the cancer [142, 143]. New discoveries into cancer metabolic reprogramming provide metabolic vulnerabilities which promote cancer treatments. Increasing small molecule metabolic inhibitors loaded by nanomaterials are employed as adjuvants with conventional anti‐tumor agents, which can improve biosafety of metabolic inhibitors. Furthermore, nanoplatform induces apoptosis of cancer cells by enhancing ROS production that suppresses tumor growth [144]. Nanoscale functional materials not only achieve anti‐tumor functions of carried drugs but also improve cancer treatments.
In brief, tumor cells exhibit high metabolic activity and energy consumption, which causes exhaustion in cancer patients. Thus, it may be necessary to design therapeutic strategies to reduce the metabolic rate of tumor cells to decrease cancerous phenotypes caused by oncogenic mutations.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was supported partly by the National Natural Science Foundation of China (81541153 and 81772404), The Guangdong Science and Technology Department (2016A050503046, 2015A050502048, 2016B030309002 and 2019B090905011), The Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (ZJW‐2019‐007), and The Public Service Platform of South China Sea for R&D Marine Biomedicine Resources (GDMUK201808).
AUTHORS' CONTRIBUTIONS
Z.T. wrote the manuscript. Z.X. and J.Z. discussed and edited the manuscript. X.Z. and J.Z. designed the concept, edited the manuscript. X.Z. and J.Z. supervised the project. All authors agree on the final version.
ACKNOWLEDGEMENTS
Not applicable.
Tang Z, Xu Z, Zhu X, Zhang J. New insights into molecules and pathways of cancer metabolism and therapeutic implications. Cancer Commun. 2021;41:16–36. 10.1002/cac2.12112
Contributor Information
Xiao Zhu, Email: zhangjf06@gzucm.edu.cn.
Jinfang Zhang, Email: xzhu@gdmu.edu.cn.
REFERENCES
- 1. Wang Y, Xia Y, Lu Z. Metabolic features of cancer cells. Cancer Commun (Lond). 2018;38(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dasgupta S, Rajapakshe K, Zhu B, Nikolai BC, Yi P, Putluri N, et al. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC‐3 to drive breast cancer. Nature. 2018;556(7700):249‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones CL, Stevens BM, D'Alessandro A, Reisz JA, Culp‐Hill R, Nemkov T, et al. Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell. 2018;34(5):724‐40 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma X, Wang L, Huang, Li Y , Yang D, Li T, et al. Polo‐like kinase 1 coordinates biosynthesis during cell cycle progression by directly activating pentose phosphate pathway. Nat Commun. 2017;8(1):1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Domenech E, Maestre C, Esteban‐Martinez L, Partida D, Pascual R, Fernandez‐Miranda G, et al. AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nat Cell Biol. 2015;17(10):1304‐16. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Liu R, Zhu W, Chu H, Yu H, Wei P, et al. UDP‐glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis. Nature. 2019;571(7763):127‐31. [DOI] [PubMed] [Google Scholar]
- 7. Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, et al. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160(3):393‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim J, Yu L, Chen W, Xu Y, Wu M, Todorova D, et al. Wild‐Type p53 promotes cancer metabolic switch by inducing PUMA‐dependent suppression of oxidative phosphorylation. Cancer Cell. 2019;35(2):191‐203 e8. [DOI] [PubMed] [Google Scholar]
- 9. Liang J, Cao R, Zhang Y, Xia Y, Zheng Y, Li X, et al. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat Commun. 2016;7:12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y, Huang Q, Liu W, Zhu Q, Cui CP, Xu L, et al. Mutually exclusive acetylation and ubiquitylation of the splicing factor SRSF5 control tumor growth. Nat Commun. 2018;9(1):2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li FL, Liu JP, Bao RX, Yan G, Feng X, Xu YP, et al. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin‐induced apoptosis. Nat Commun. 2018;9(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li T, Liu M, Feng X, Wang Z, Das I, Xu Y, et al. Glyceraldehyde‐3‐phosphate dehydrogenase is activated by lysine 254 acetylation in response to glucose signal. J Biol Chem. 2014;289(6):3775‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chao TK, Huang TS, Liao YP, Huang RL, Su PH, Shen HY, et al. Pyruvate kinase M2 is a poor prognostic marker of and a therapeutic target in ovarian cancer. PLoS One. 2017;12(7):e0182166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hao X, Gu H, Chen C, Huang D, Zhao Y, Xie L, et al. Metabolic imaging reveals a unique preference of symmetric cell division and homing of leukemia‐initiating cells in an endosteal niche. Cell Metab. 2019;29(4):950‐65 e6. [DOI] [PubMed] [Google Scholar]
- 16. Zhong X, Tian S, Zhang X, Diao X, Dong F, Yang J, et al. CUE domain‐containing protein 2 promotes the Warburg effect and tumorigenesis. EMBO Rep. 2017;18(5):809‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang HJ, Pochampalli M, Wang LY, Zou JX, Li PS, Hsu SC, et al. KDM8/JMJD5 as a dual coactivator of AR and PKM2 integrates AR/EZH2 network and tumor metabolism in CRPC. Oncogene. 2019;38(1):17‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma X, Li C, Sun L, Huang D, Li T, He X, et al. Lin28/let‐7 axis regulates aerobic glycolysis and cancer progression via PDK1. Nat Commun. 2014;5:5212. [DOI] [PubMed] [Google Scholar]
- 19. Tang J, Yan T, Bao Y, Shen C, Yu C, Zhu X, et al. LncRNA GLCC1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c‐Myc. Nat Commun. 2019;10(1):3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang JZ, Chen M, Chen, Gao XC , Zhu S, Huang H, et al. A Peptide Encoded by a Putative lncRNA HOXB‐AS3 Suppresses Colon Cancer Growth. Mol Cell. 2017;68(1):171‐84 e6. [DOI] [PubMed] [Google Scholar]
- 21. Zheng X, Han H, Liu GP, Ma YX, Pan RL, Sang LJ, et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36(22):3325‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo S, Li Y, Ma R, Liu J, Xu P, Zhang H, et al. Downregulation of PCK2 remodels tricarboxylic acid cycle in tumor‐repopulating cells of melanoma. Oncogene. 2017;36(25):3609‐17. [DOI] [PubMed] [Google Scholar]
- 23. Shen H, Yu M, Tsoli M, Chang C, Joshi S, Liu J, et al. Targeting reduced mitochondrial DNA quantity as a therapeutic approach in pediatric high‐grade gliomas. Neuro Oncol. 2020;22(1):139‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Zhang Y, Ma W, Fu Q, Liu J, Yin G, et al. Enhanced glucose metabolism mediated by CD147 contributes to immunosuppression in hepatocellular carcinoma. Cancer Immunol Immunother. 2020;69(4):535‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim S, Jang JY, Koh J, Kwon D, Kim YA, Paeng JC, et al. Programmed cell death ligand‐1‐mediated enhancement of hexokinase 2 expression is inversely related to T‐cell effector gene expression in non‐small‐cell lung cancer. J Exp Clin Cancer Res. 2019;38(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti‐tumor t cell responses. Cell. 2015;162(6):1217‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(Pt 23):5591‐6. [DOI] [PubMed] [Google Scholar]
- 28. Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle‐packaged HIF‐1alpha‐stabilizing lncRNA from tumour‐associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21(4):498‐510. [DOI] [PubMed] [Google Scholar]
- 29. Liu N, Luo J, Kuang D, Xu S, Duan Y, Xia Y, et al. Lactate inhibits ATP6V0d2 expression in tumor‐associated macrophages to promote HIF‐2alpha‐mediated tumor progression. J Clin Invest. 2019;129(2):631‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo Y, Li B, Yan X, Shen X, Ma J, Liu S, et al. Bisphenol A and polychlorinated biphenyls enhance the cancer stem cell properties of human ovarian cancer cells by activating the WNT signaling pathway. Chemosphere. 2020;246:125775. [DOI] [PubMed] [Google Scholar]
- 31. Yoshikawa N, Saito Y, Manabe H, Nakaoka T, Uchida R, Furukawa R, et al. Glucose depletion enhances the stem cell phenotype and gemcitabine resistance of cholangiocarcinoma organoids through AKT phosphorylation and reactive oxygen species. Cancers (Basel). 2019;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo M, Shang L, Brooks MD, Jiagge E, Zhu Y, Buschhaus JM, et al. Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 2018;28(1):69‐86 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Flores A, Sandoval‐Gonzalez S, Takahashi R, Krall A, Sathe L, Wei L, et al. Increased lactate dehydrogenase activity is dispensable in squamous carcinoma cells of origin. Nat Commun. 2019;10(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, et al. Lactate metabolism in human lung tumors. Cell. 2017;171(2):358‐71 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hensley CT, Faubert B, Yuan Q, Lev‐Cohain N, Jin E, Kim J, et al. Metabolic heterogeneity in human lung tumors. Cell. 2016;164(4):681‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang B, Chen Y, Shi J. Tumor‐specific chemotherapy by nanomedicine‐enabled differential stress sensitization. Angew Chem Int Ed Engl. 2020;59(24):9693‐701. [DOI] [PubMed] [Google Scholar]
- 37. Zhao X, Jiang P, Deng X, Li Z, Tian F, Guo F, et al. Inhibition of mTORC1 signaling sensitizes hepatocellular carcinoma cells to glycolytic stress. Am J Cancer Res. 2016;6(10):2289‐98. [PMC free article] [PubMed] [Google Scholar]
- 38. Dong M, Xiao XZ, Su ZG, Yu ZH, Qian CG, Liu JH, et al. Light‐induced ROS Generation and 2‐DG‐activated endoplasmic reticulum stress by antitumor nanosystems: An effective combination therapy by regulating the tumor microenvironment. Small. 2019;15(17):e1900212. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Wei J, Xu J, Leong WS, Liu G, Ji T, et al. Suppression of tumor energy supply by liposomal nanoparticle‐mediated inhibition of aerobic glycolysis. ACS Appl Mater Interfaces. 2018;10(3):2347‐53. [DOI] [PubMed] [Google Scholar]
- 40. Cancer Genome Atlas Research N . The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bangalore Yogananda CG, Shah BR, Vejdani‐Jahromi M, Nalawade SS, Murugesan GK, Yu FF, et al. A novel fully automated MRI‐based deep‐learning method for classification of IDH mutation status in brain gliomas. Neuro Oncol. 2020;22(3):402‐11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer‐Rachamimov AO, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529(7584):110‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen H, Judkins J, Thomas C, Wu M, Khoury L, Benjamin CG, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang L, Shestov AA, Swain P, Yang C, Parker SJ, Wang QA, et al. Reductive carboxylation supports redox homeostasis during anchorage‐independent growth. Nature. 2016;532(7598):255‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Izquierdo‐Garcia JL, Viswanath P, Eriksson P, Cai L, Radoul M, Chaumeil MM, et al. IDH1 mutation induces reprogramming of pyruvate metabolism. Cancer Res. 2015;75(15):2999‐3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Okoye‐Okafor UC, Bartholdy B, Cartier J, Gao EN, Pietrak B, Rendina AR, et al. New IDH1 mutant inhibitors for treatment of acute myeloid leukemia. Nat Chem Biol. 2015;11(11):878‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng M, Sun W, Gao S, Luan S, Li D, Chen R, et al. Structure based discovery of clomifene as a potent inhibitor of cancer‐associated mutant IDH1. Oncotarget. 2017;8(27):44255‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular remission and response patterns in patients with mutant‐IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li L, Paz AC, Wilky BA, Johnson B, Galoian K, Rosenberg A, et al. Treatment with a small molecule mutant IDH1 inhibitor suppresses tumorigenic activity and decreases production of the oncometabolite 2‐hydroxyglutarate in human chondrosarcoma cells. PLoS One. 2015;10(9):e0133813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gao X, Zhao L, Liu S, Li Y, Xia S, Chen D, et al. gamma‐6‐Phosphogluconolactone, a byproduct of the oxidative pentose phosphate pathway, contributes to AMPK activation through inhibition of PP2A. Mol Cell. 2019;76(6):857‐71 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li M, Lu Y, Li Y, Tong L, Gu XC, Meng J, et al. Transketolase deficiency protects the liver from DNA damage by increasing levels of ribose 5‐phosphate and nucleotides. Cancer Res. 2019;79(14):3689‐701. [DOI] [PubMed] [Google Scholar]
- 52. Du W, Jiang P, Mancuso A, Stonestrom A, Brewer MD, Minn AJ, et al. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat Cell Biol. 2013;15(8):991‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McDonald OG, Li X, Saunders T, Tryggvadottir R, Mentch SJ, Warmoes MO, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet. 2017;49(3):367‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mele L, Paino F, Papaccio F, Regad T, Boocock D, Stiuso P, et al. A new inhibitor of glucose‐6‐phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018;9(5):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carroll PA, Diolaiti D, McFerrin L, Gu H, Djukovic D, Du J, et al. Deregulated Myc requires MondoA/Mlx for metabolic reprogramming and tumorigenesis. Cancer Cell. 2015;27(2):271‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond). 2018;38(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kuo CY, Ann DK. When fats commit crimes: fatty acid metabolism, cancer stemness and therapeutic resistance. Cancer Commun (Lond). 2018;38(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Loregger A, Raaben M, Nieuwenhuis J, Tan JME, Jae LT, van den Hengel LG, et al. Haploid genetic screens identify SPRING/C12ORF49 as a determinant of SREBP signaling and cholesterol metabolism. Nat Commun. 2020;11(1):1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu L, Zhao X, Zhao L, Li J, Yang H, Zhu Z, et al. Arginine methylation of SREBP1a via PRMT5 promotes de novo lipogenesis and tumor growth. Cancer Res. 2016;76(5):1260‐72. [DOI] [PubMed] [Google Scholar]
- 60. Xu D, Wang Z, Xia Y, Shao F, Xia W, Wei Y, et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580(7804):530‐5. [DOI] [PubMed] [Google Scholar]
- 61. Masri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, et al. Lung adenocarcinoma distally rewires hepatic circadian homeostasis. Cell. 2016;165(4):896‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang, Li T , Wang L, Zhang L, Yan R, Li K, et al. Hepatocellular carcinoma redirects to ketolysis for progression under nutrition deprivation stress. Cell Res. 2016;26(10):1112‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kang HB, Fan J, Lin R, Elf S, Ji Q, Zhao L, et al. Metabolic rewiring by oncogenic BRAF V600E links ketogenesis pathway to BRAF‐MEK1 signaling. Mol Cell. 2015;59(3):345‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bradley CA. Immunotherapy: CD8(+) T cells ‐ burn fat, get fit. Nat Rev Cancer. 2017;17(11):635. [DOI] [PubMed] [Google Scholar]
- 65. Bailis W, Shyer JA, Chiorazzi M, Flavell RA. No oxygen? no glucose? no problem: Fatty acid catabolism enhances effector CD8(+) TILs. Cancer Cell. 2017;32(3):280‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang G, Hudaihed A, et al. Enhancing CD8(+) T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell. 2017;32(3):377‐91 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang W, Yang J, Nimiya Y, Lee KSS, Sanidad K, Qi W, et al. omega‐3 Polyunsaturated fatty acids and their cytochrome P450‐derived metabolites suppress colorectal tumor development in mice. J Nutr Biochem. 2017;48:29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110(16):6530‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Paul D, Bargale AB, Rapole S, Shetty PK, Santra MK. Protein phosphatase 1 regulatory subunit SDS22 inhibits breast cancer cell tumorigenesis by functioning as a negative regulator of the AKT signaling pathway. Neoplasia. 2019;21(1):30‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ward MP, Spiers JP. Protein phosphatase 2A regulation of markers of extracellular matrix remodelling in hepatocellular carcinoma cells: functional consequences for tumour invasion. Br J Pharmacol. 2017;174(10):1116‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mei Y, Liu YB, Cao S, Tian ZW, Zhou HH. RIF1 promotes tumor growth and cancer stem cell‐like traits in NSCLC by protein phosphatase 1‐mediated activation of Wnt/beta‐catenin signaling. Cell Death Dis. 2018;9(10):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hein AL, Seshacharyulu P, Rachagani S, Sheinin YM, Ouellette MM, Ponnusamy MP, et al. PR55alpha subunit of protein phosphatase 2A supports the tumorigenic and metastatic potential of pancreatic cancer cells by sustaining hyperactive oncogenic signaling. Cancer Res. 2016;76(8):2243‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tang Y, Fang G, Guo F, Zhang H, Chen X, An L, et al. Selective inhibition of STRN3‐containing PP2A phosphatase restores hippo tumor‐suppressor activity in gastric cancer. Cancer Cell. 2020;38(1):115‐28 e9. [DOI] [PubMed] [Google Scholar]
- 74. Liu L, Wang H, Cui J, Zhang Q, Zhang W, Xu W, et al. Inhibition of protein phosphatase 2A sensitizes mucoepidermoid carcinoma to chemotherapy via the PI3K‐AKT pathway in response to insulin stimulus. Cell Physiol Biochem. 2018;50(1):317‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang D, Sun B, Zhang X, Cheng D, Yu X, Yan L, et al. Huwe1 sustains normal ovarian epithelial cell transformation and tumor growth through the histone H1.3‐H19 cascade. Cancer Res. 2017;77(18):4773‐84. [DOI] [PubMed] [Google Scholar]
- 76. Tang J, Luo Y, Tian Z, Liao X, Cui Q, Yang Q, et al. TRIM11 promotes breast cancer cell proliferation by stabilizing estrogen receptor alpha. Neoplasia. 2020;22(9):343‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu H, Lu W, He H, Wu J, Zhang C, Gong H, et al. Inflammation‐dependent overexpression of c‐Myc enhances CRL4(DCAF4) E3 ligase activity and promotes ubiquitination of ST7 in colitis‐associated cancer. J Pathol. 2019;248(4):464‐75. [DOI] [PubMed] [Google Scholar]
- 78. Martinez‐Iglesias O, Casas‐Pais A, Castosa R, Diaz‐Diaz A, Roca‐Lema D, Concha A, et al. Hakin‐1, a new specific small‐molecule inhibitor for the E3 ubiquitin‐ligase hakai, inhibits carcinoma growth and progression. Cancers (Basel). 2020;12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang YQ, Wang HL, Xu J, Tan J, Fu LN, Wang JL, et al. Sirtuin5 contributes to colorectal carcinogenesis by enhancing glutaminolysis in a deglutarylation‐dependent manner. Nat Commun. 2018;9(1):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Smith B, Schafer XL, Ambeskovic A, Spencer CM, Land H, Munger J. Addiction to coupling of the warburg effect with glutamine catabolism in cancer cells. Cell Rep. 2016;17(3):821‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wu G, Yuan M, Shen S, Ma X, Fang J, Zhu L, et al. Menin enhances c‐Myc‐mediated transcription to promote cancer progression. Nat Commun. 2017;8:15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c‐MYC. Proc Natl Acad Sci U S A. 2012;109(23):8983‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fu S, Li Z, Xiao L, Hu W, Zhang L, Xie B, et al. Glutamine synthetase promotes radiation resistance via facilitating nucleotide metabolism and subsequent DNA damage repair. Cell Rep. 2019;28(5):1136‐43 e4. [DOI] [PubMed] [Google Scholar]
- 84. Ruan JJ, Yu Y, Hou W, Chen Z, Fang J, Zhang J, et al. Kidney‐type glutaminase inhibitor hexylselen selectively kills cancer cells via a three‐pronged mechanism. ACS Pharmacol Transl Sci. 2019;2(1):18‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lee P, Malik D, Perkons N, Huangyang P, Khare S, Rhoades S, et al. Targeting glutamine metabolism slows soft tissue sarcoma growth. Nat Commun. 2020;11(1):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cohen AS, Geng L, Zhao P, Fu A, Schulte ML, Graves‐Deal R, et al. Combined blockade of EGFR and glutamine metabolism in preclinical models of colorectal cancer. Transl Oncol. 2020;13(10):100828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gregory MA, Nemkov T, Park HJ, Zaberezhnyy V, Gehrke S, Adane B, et al. Targeting glutamine metabolism and redox state for leukemia therapy. Clin Cancer Res. 2019;25(13):4079‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yoshino H, Enokida H, Osako Y, Nohata N, Yonemori M, Sugita S, et al. Characterization of PHGDH expression in bladder cancer: potential targeting therapy with gemcitabine/cisplatin and the contribution of promoter DNA hypomethylation. Mol Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sullivan MR, Mattaini KR, Dennstedt EA, Nguyen AA, Sivanand S, Reilly MF, et al. Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab. 2019;29(6):1410‐21 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yu L, Teoh ST, Ensink E, Ogrodzinski MP, Yang C, Vazquez AI, et al. Cysteine catabolism and the serine biosynthesis pathway support pyruvate production during pyruvate kinase knockdown in pancreatic cancer cells. Cancer Metab. 2019;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pacold ME, Brimacombe KR, Chan SH, Rohde JM, Lewis CA, Swier LJ, et al. A PHGDH inhibitor reveals coordination of serine synthesis and one‐carbon unit fate. Nat Chem Biol. 2016;12(6):452‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mullarky E, Lucki NC, Beheshti Zavareh R, Anglin JL, Gomes AP, Nicolay BN, et al. Identification of a small molecule inhibitor of 3‐phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc Natl Acad Sci U S A. 2016;113(7):1778‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tang S, Fang Y, Huang G, Xu X, Padilla‐Banks E, Fan W, et al. Methionine metabolism is essential for SIRT1‐regulated mouse embryonic stem cell maintenance and embryonic development. EMBO J. 2017;36(21):3175‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang Z, Yip LY, Lee JHJ, Wu Z, Chew HY, Chong PKW, et al. Methionine is a metabolic dependency of tumor‐initiating cells. Nat Med. 2019;25(5):825‐37. [DOI] [PubMed] [Google Scholar]
- 95. Eckert MA, Coscia F, Chryplewicz A, Chang JW, Hernandez KM, Pan S, et al. Proteomics reveals NNMT as a master metabolic regulator of cancer‐associated fibroblasts. Nature. 2019;569(7758):723‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572(7769):397‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Melendez‐Rodriguez F, Urrutia AA, Lorendeau D, Rinaldi G, Roche O, Bogurcu‐Seidel N, et al. HIF1alpha Suppresses Tumor Cell Proliferation through Inhibition of Aspartate Biosynthesis. Cell Rep. 2019;26(9):2257‐65 e4. [DOI] [PubMed] [Google Scholar]
- 98. Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162(3):552‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sullivan LB, Luengo A, Danai LV, Bush LN, Diehl FF, Hosios AM, et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat Cell Biol. 2018;20(7):782‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kudo T, Prentzell MT, Mohapatra SR, Sahm F, Zhao Z, Grummt I, et al. Constitutive expression of the immunosuppressive tryptophan dioxygenase TDO2 in glioblastoma is driven by the transcription factor C/EBPbeta. Front Immunol. 2020;11:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yan H, Dong M, Liu X, Shen Q, He D, Huang X, et al. Multiple myeloma cell‐derived IL‐32gamma increases the immunosuppressive function of macrophages by promoting indoleamine 2,3‐dioxygenase (IDO) expression. Cancer Lett. 2019;446:38‐48. [DOI] [PubMed] [Google Scholar]
- 102. Lan Y, Liang Q, Sun Y, Cao A, Liu L, Yu S, et al. Codelivered chemotherapeutic doxorubicin via a dual‐functional immunostimulatory polymeric prodrug for breast cancer immunochemotherapy. ACS Appl Mater Interfaces. 2020;12(28):31904‐21. [DOI] [PubMed] [Google Scholar]
- 103. Kim SS, Xu S, Cui J, Poddar S, Le TM, Hayrapetyan H, et al. Histone deacetylase inhibition is synthetically lethal with arginine deprivation in pancreatic cancers with low argininosuccinate synthetase 1 expression. Theranostics. 2020;10(2):829‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Huang HY, Wu WR, Wang YH, Wang JW, Fang FM, Tsai JW, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19(11):2861‐72. [DOI] [PubMed] [Google Scholar]
- 105. Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, et al. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase‐independent apoptosis. Cancer Res. 2009;69(2):700‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Qiu F, Chen YR, Liu X, Chu CY, Shen LJ, Xu J, et al. Arginine starvation impairs mitochondrial respiratory function in ASS1‐deficient breast cancer cells. Sci Signal. 2014;7(319):ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fu T, Coulter S, Yoshihara E, Oh TG, Fang S, Cayabyab F, et al. FXR regulates intestinal cancer stem cell proliferation. Cell. 2019;176(5):1098‐112 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct stage‐specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25(6):968‐76. [DOI] [PubMed] [Google Scholar]
- 109. Maalmi H, Walter V, Jansen L, Chang‐Claude J, Owen RW, Ulrich A, et al. Relationship of very low serum 25‐hydroxyvitamin D3 levels with long‐term survival in a large cohort of colorectal cancer patients from Germany. Eur J Epidemiol. 2017;32(11):961‐71. [DOI] [PubMed] [Google Scholar]
- 110. Song M, Nishihara R, Wang M, Chan AT, Qian ZR, Inamura K, et al. Plasma 25‐hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2016;65(2):296‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Song M, Konijeti GG, Yuan C, Ananthakrishnan AN, Ogino S, Fuchs CS, et al. Plasma 25‐Hydroxyvitamin D, Vitamin D Binding Protein, and Risk of Colorectal Cancer in the Nurses' Health Study. Cancer Prev Res (Phila). 2016;9(8):664‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Karkeni E, Morin SO, Bou Tayeh B, Goubard A, Josselin E, Castellano R, et al. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front Immunol. 2019;10:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang G, Biswas AK, Ma W, Kandpal M, Coker C, Grandgenett PM, et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat Med. 2018;24(6):770‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ishida S, Andreux P, Poitry‐Yamate C, Auwerx J, Hanahan D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci U S A. 2013;110(48):19507‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Franko A, Shao Y, Heni M, Hennenlotter J, Hoene M, Hu C, et al. Human prostate cancer is characterized by an increase in urea cycle metabolites. Cancers (Basel). 2020;12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee JS, Adler L, Karathia H, Carmel N, Rabinovich S, Auslander N, et al. Urea cycle dysregulation generates clinically relevant genomic and biochemical signatures. Cell. 2018;174(6):1559‐70 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pham‐Danis C, Gehrke S, Danis E, Rozhok AI, Daniels MW, Gao D, et al. Urea cycle sustains cellular energetics upon EGFR inhibition in EGFR‐mutant NSCLC. Mol Cancer Res. 2019;17(6):1351‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939‐44. [DOI] [PubMed] [Google Scholar]
- 119. Huan HB, Wen XD, Chen XJ, Wu L, Wu LL, Zhang L, et al. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1‐adrenergic receptors of Kupffer cells. Brain Behav Immun. 2017;59:118‐34. [DOI] [PubMed] [Google Scholar]
- 120. Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y, et al. Stress‐induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem‐like cells. J Clin Invest. 2019;129(3):1030‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hajra KM, Chen DY, Fearon ER. The SLUG zinc‐finger protein represses E‐cadherin in breast cancer. Cancer Res. 2002;62(6):1613‐8. [PubMed] [Google Scholar]
- 122. Tray N, Taff J, Adams S. Therapeutic landscape of metaplastic breast cancer. Cancer Treat Rev. 2019;79:101888. [DOI] [PubMed] [Google Scholar]
- 123. Daher C, Vimeux L, Stoeva R, Peranzoni E, Bismuth G, Wieduwild E, et al. Blockade of beta‐Adrenergic Receptors Improves CD8(+) T‐cell Priming and Cancer Vaccine Efficacy. Cancer Immunol Res. 2019;7(11):1849‐63. [DOI] [PubMed] [Google Scholar]
- 124. Zhu X, Fan WG, Li DP, Lin MC, Kung H. Heme oxygenase‐1 system and gastrointestinal tumors. World J Gastroenterol. 2010;16(21):2633‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chang LC, Fan CW, Tseng WK, Chein HP, Hsieh TY, Chen JR, et al. The ratio of Hmox1/Nrf2 mRNA Level in the tumor tissue is a predictor of distant metastasis in colorectal cancer. Dis Markers. 2016;2016:8143465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of bach1. Cell. 2019;178(2):316‐29 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pavlinov I, Salkovski M, Aldrich LN. Beclin 1‐ATG14L protein‐protein interaction inhibitor selectively inhibits autophagy through disruption of VPS34 complex I. J Am Chem Soc. 2020;142(18):8174‐82. [DOI] [PubMed] [Google Scholar]
- 128. Thomas HE, Zhang Y, Stefely JA, Veiga SR, Thomas G, Kozma SC, et al. Mitochondrial complex I activity is required for maximal autophagy. Cell Rep. 2018;24(9):2404‐17 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lin HH, Chung Y, Cheng CT, Ouyang C, Fu Y, Kuo CY, et al. Autophagic reliance promotes metabolic reprogramming in oncogenic KRAS‐driven tumorigenesis. Autophagy. 2018;14(9):1481‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tan X, Thapa N, Sun Y, Anderson RA. A kinase‐independent role for EGF receptor in autophagy initiation. Cell. 2015;160(1‐2):145‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tan Y, Yin P, Tang L, Xing W, Huang Q, Cao D, et al. Metabolomics study of stepwise hepatocarcinogenesis from the model rats to patients: potential biomarkers effective for small hepatocellular carcinoma diagnosis. Mol Cell Proteomics. 2012;11(2):M111010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Aleksandrova K, Boeing H, Nothlings U, Jenab M, Fedirko V, Kaaks R, et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60(3):858‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhou L, Ding L, Yin P, Lu X, Wang X, Niu J, et al. Serum metabolic profiling study of hepatocellular carcinoma infected with hepatitis B or hepatitis C virus by using liquid chromatography‐mass spectrometry. J Proteome Res. 2012;11(11):5433‐42. [DOI] [PubMed] [Google Scholar]
- 134. Ye G, Zhu B, Yao Z, Yin P, Lu X, Kong H, et al. Analysis of urinary metabolic signatures of early hepatocellular carcinoma recurrence after surgical removal using gas chromatography‐mass spectrometry. J Proteome Res. 2012;11(8):4361‐72. [DOI] [PubMed] [Google Scholar]
- 135. Kozar N, Kruusmaa K, Bitenc M, Argamasilla R, Adsuar A, Goswami N, et al. Metabolomic profiling suggests long chain ceramides and sphingomyelins as a possible diagnostic biomarker of epithelial ovarian cancer. Clin Chim Acta. 2018;481:108‐14. [DOI] [PubMed] [Google Scholar]
- 136. Huang J, Weinstein SJ, Moore SC, Derkach A, Hua X, Mondul AM, et al. Pre‐diagnostic Serum Metabolomic Profiling of Prostate Cancer Survival. J Gerontol A Biol Sci Med Sci. 2019;74(6):853‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lin Y, Ma C, Bezabeh T, Wang Z, Liang J, Huang Y, et al. (1) H NMR‐based metabolomics reveal overlapping discriminatory metabolites and metabolic pathway disturbances between colorectal tumor tissues and fecal samples. Int J Cancer. 2019;145(6):1679‐89. [DOI] [PubMed] [Google Scholar]
- 138. Chan AW, Mercier P, Schiller D, Bailey R, Robbins S, Eurich DT, et al. (1)H‐NMR urinary metabolomic profiling for diagnosis of gastric cancer. Br J Cancer. 2016;114(1):59‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 2020;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yao D, Huang L, Ke J, Zhang M, Xiao Q, Zhu X. Bone metabolism regulation: Implications for the treatment of bone diseases. Biomedicine & Pharmacotherapy. 2020;129. [DOI] [PubMed] [Google Scholar]
- 141. Guo B, Li D, Du L, Zhu X. piRNAs: biogenesis and their potential roles in cancer. Cancer Metastasis Rev. 2020;39(2):567‐75. [DOI] [PubMed] [Google Scholar]
- 142. Luo X, Liu J, Wang H, Lu H. Metabolomics identified new biomarkers for the precise diagnosis of pancreatic cancer and associated tissue metastasis. Pharmacol Res. 2020;156:104805. [DOI] [PubMed] [Google Scholar]
- 143. Martin‐Blazquez A, Jimenez‐Luna C, Diaz C, Martinez‐Galan J, Prados J, Vicente F, et al. Discovery of pancreatic adenocarcinoma biomarkers by untargeted metabolomics. Cancers (Basel). 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kou L, Sun R, Jiang X, Lin X, Huang H, Bao S, et al. Tumor microenvironment‐responsive, multistaged liposome induces apoptosis and ferroptosis by amplifying oxidative stress for enhanced cancer therapy. ACS Appl Mater Interfaces. 2020;12(27):30031‐43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


