Abstract
Research suggests that thyroid cancer incidence rates are increasing, and environmental exposures have been postulated to be playing a role. To explore this possibility, we conducted a pilot study to investigate the thyroid disrupting bioactivity of chemical mixtures isolated from personal silicone wristband samplers within a thyroid cancer cohort. Specifically, we evaluated TRβ antagonism of chemical mixtures extracted from wristbands (n = 72) worn by adults in central North Carolina participating in a case-control study on papillary thyroid cancer. Sections of wristbands were solvent-extracted and analyzed via mass spectrometry to quantify a suite of semivolatile chemicals. A second extract from each wristband was used in a bioassay to quantify TRβ antagonism in human embryonic kidney cells (HEK293/17) at concentrations ranging from 0.1 to 10% of the original extract (by volume). Approximately 70% of the sample extracts tested at a 1% extract concentration exhibited significant TRβ antagonism, with a mean of 30% and a range of 0–100%. Inhibited cell viability was noted in >20% of samples that were tested at 5 and 10% concentrations. Antagonism was positively associated with wristband concentrations of several phthalates, organophosphate esters, and brominated flame retardants. These results suggest that personal passive samplers may be useful in evaluating the bioactivities of mixtures that people contact on a daily basis. We also report tentative associations between thyroid receptor antagonism, chemical concentrations, and papillary thyroid cancer case status. Future research utilizing larger sample sizes, prospective data collection, and measurement of serum thyroid hormone levels (which were not possible in this study) should be utilized to more comprehensively evaluate these associations.
Graphical Abstract
INTRODUCTION
The gold standard for measuring human exposure to organic contaminants is via measurement of biomarkers in human blood and urine. However, these assessments are often both difficult and expensive to collect, with high costs and logistical limitations (e.g. scheduling difficulties and small volumes of biospecimens). As such, there is a critical need to develop and validate external, noninvasive sampling devices that can effectively recapitulate human exposures. Silicone wristbands have been increasing in popularity and have shown promise in measuring personal exposure to complex mixtures of volatile and semivolatile contaminants (SVOCs), including polycyclic aromatic hydrocarbons,1–3 brominated flame retardants and organophosphate esters (BFRs and OPEs, respectively),2,4,5 pesticides,6–8 and other chemicals,1,7,9 with good performance in recovery and stability tests across a wide range of chemical classes.9 These personal passive samplers can be used to estimate exposure to contaminants via both dermal and inhalation routes10 and can be utilized to create personalized exposure profiles encompassing multiple microenvironments.11 Moreover, measurements from silicone wristband extracts have been demonstrated to correlate well with biomarkers of exposure in human biospecimens (serum and urine),4,5,12 suggesting a robust association between external wristband chemical concentrations and internal chemical concentrations. These samplers also have superior performance in predicting the internal dose for some compounds relative to external exposure methods, such as hand wipes, household dust,5,12 or air monitoring samplers.3 Wristbands have been deployed in several human studies and have been used to evaluate chemical exposures for diverse populations across multiple continents,8,13 suggesting utility in interrogating exposure to chemical mixtures. They also have been successfully utilized with children6,14 and even pets,15,16 demonstrating their versatility.
Many of the contaminants detected by wristbands are considered to be endocrine disrupting chemicals (EDCSs) that can disrupt normal hormone action and contribute to adverse outcomes in humans, wildlife, and laboratory animals.17–19 The proper and unimpeded function of hormones is essential to normal development, maturation, and prevention of chronic diseases. While EDCs have been found to disrupt a number of receptor systems, disruption of nuclear receptors is better described than most receptors and has been linked to adverse outcomes at environmentally relevant levels.19–25 Notably, chemicals that disrupt thyroid receptor (TR) signaling have been widely reported across the literature. Receptor agonists are infrequently reported in environmental matrices; these typically include endogenous hormones and pharmaceuticals, which have low bioactivities.26,27 Receptor antagonists, in contrast, come from diverse chemical classes and have been widely reported in diverse environmental matrices,28,29 including natural and drinking water sources,30,31 varied source wastewaters,32–35 and indoor household dust.36,37 Chemicals that inhibit thyroid signaling have been linked to disrupted thyroid function; impaired neurodevelopment; behavioral modifications;28,38–40 metabolic health, adipogenesis, lipogenesis, and thermogenesis;41–44 and the development of thyroid cancer.45,46
Thyroid cancer rates have increased approximately 3.6% per year in the US,47 with current incidence rates of greater than 14 per 100,000;48 this is mirrored internationally, with rates rising similarly in most other countries.49 Thyroid cancer is classified into four histological types, including papillary (~80% of cases), follicular (~15% of cases), anaplastic, and medullary.50 While some researchers and clinicians have posited that these increases are due to improved surveillance and diagnostic changes,51 several factors suggest otherwise: (1) there has been an increase in papillary thyroid cancer (PTC) cases even with large tumor sizes that would presumably not be impacted by diagnostic changes, and (2) there has been a parallel increase in thyroid cancer mortality that would be unexpected if there is improved surveillance and diagnosis.52 This worsening public health trend has promoted increased attention to assess potential factors. We have long appreciated a role for environmental contamination in thyroid cancer influence, reporting sharp spikes in incidence following radioactive iodine exposure in Chernobyl53 and Fukushima54 accidents (though importantly, increases in thyroid cancer incidence and mortality were evident before these disasters47) and higher rates of incidence for populations living in volcanic regions.55 These and findings of elevated thyroid cancer incidence near National Priority Contaminated Sites56 have spurred investigations into the role of EDCs, particularly, TR disrupting chemicals. A recent review summarized evidence for elevated thyroid cancer incidence from occupational exposures in various industries (e.g. construction, papermaking and wood processing, agricultural activities, etc.) as well as evidence for diverse SVOCs (particularly, phthalates, bisphenols, and certain heavy metals) in contributing to thyroid cancer incidence.46 The causal mechanism of action for these effects has been posited to be TR disruption.46 In support of this, controlled exposure studies in vivo with well-characterized TR antagonists [including potassium perchlorate, propylthiouracil (PTU), and carbimazole] have demonstrated a causal role in the development and/or progression of PTC.57–62 However, research on causative contaminants in the development of thyroid cancer has primarily focused on single contaminants rather than environmentally relevant mixtures.
Thyroid disrupting chemicals also have been implicated in thyroid dysfunction and disease. Similar to cancer incidence, rates of thyroid disease also have been increasing, with >12% of US adults estimated to experience some form of thyroid disease in their lifetimes. In particular, hypothyroidism (characterized by high thyroid stimulating hormone [TSH] and low thyroxine [T4] concentrations) affects approximately 5% of the US population aged ≥12 years,63 and rates have been increasing globally over the last several decades.64,65 We previously demonstrated that >40% of household dust extracts promoted TRβ antagonism in vitro.37 The potency of TRβ antagonism was positively correlated with the serum-free thyroxine (T4) concentrations of residents,37 suggesting that residents in households with more potent TRβ antagonist activities in dust had higher serum T4 levels. Other recent work evaluating companion felines reported that greater tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) concentrations on silicone tags (worn on the collar) were associated with increased incidence of hyperthyroidism in domestic cats (higher free triiodothyronine [T3] and thyroxine [T4] concentrations).15 In sum, these studies provide evidence for a contributory role of TR antagonists in thyroid hormone dysregulation and dysregulated health in exposed humans and animals.
Herein, we conducted a pilot study to evaluate the potential to measure TRβ antagonism of mixtures isolated from silicone wristbands that were worn for one week by adults from central North Carolina who were participating in a research study focused on PTC. One gram sections of wristband were solvent extracted and analyzed via mass spectrometry to quantify a range of SVOCs (BFRs, OPEs, phthalates, and pesticides). Separate wristband extracts were reconstituted in tissue culture media and tested for their ability to antagonize TRβ using a reporter gene assay in human kidney cells. Bioactivity was assessed across a range of extract concentrations and was supported by dual cell viability measurements to ensure toxicity-independent effects. Dose-related responses were observed, and bioactivities (efficacies and potencies) were estimated and utilized to assess associations with SVOCs and PTC case status (i.e. thyroid cancer patient or control). Notably, this is the first study to assess the bioactivity of mixtures of chemicals isolated from silicone wristbands on nuclear receptor activation and may suggest that there are additional benefits from these passive sampling devices.
MATERIALS AND METHODS
Chemicals.
Chemicals purchased for use in bioassays are as follows: triiodothyronine (T3; VWR cat #80057–656, ≥98%) and 1–850 (Millipore cat #609315, ≥98%). Stock solutions were prepared in 100% cell-culture grade dimethyl sulfoxide (DMSO) (Sigma cat #D2650) and stored at − 20 °C between use. Wristband extraction and mass spectrometry solvents of high-performance liquid chromatography grade (>99.5%) are as follows, unless otherwise specified: ethyl acetate (Fisher Sci #E196SK-4), hexane (J.T. Baker #9262–03, reagent grade, >95%), methanol (Fisher Sci #A456–4), acetone (VWR #BDH20067.400), and dichloromethane (Fisher Sci #D151–4).
Study Population.
Silicone wristbands (n = 72) were purchased from a commercial source (black in color, https://24hourwristbands.com) and were collected and processed as described previously.4,5,12 Briefly, wristbands were collected from participants in a case-control study assessing environmental exposures and thyroid cancer.66 Patients were newly diagnosed with PTC between 2017 and 2019 and referred to endocrinology or endocrine surgery at the Duke Cancer Institute or Duke University Hospital; physicians approached and ascertained willingness to participate. Willing participants were consented and enrolled by the study team (n = 36), and age- and sex-matched control participants (n = 36) were recruited from other Duke patients undergoing routine wellness care or for unrelated medical conditions. Controls had no known history of malignant or benign thyroid disease. Serum samples were not collected from any study participants, and no serum thyroid hormone data were available for use in this study. Inclusion of participants was restricted to individuals between 20 and 80 years of age; individuals living in Durham, Orange, Granville, Alamance, Person, Wake, or Chatham County, North Carolina (USA); individuals who had lived in the same household for at least two years; and individuals who were not pregnant. Study participants completed questionnaires with study staff and provided information on age, race/ethnicity, educational status, height, and weight for body mass index (BMI) calculations and other general health information. Participants were given a silicone wristband and directed to wear throughout all activities for one week; at the completion of the study period, wristbands were wrapped in foil, sealed in a zip top bag, and mailed to our laboratory, where they were stored at − 20 °C until analysis. All study protocols were reviewed and approved by the Duke University Health System Institutional Review Board.
Wristbands were precleaned via two 12 h Soxhlet extractions with 1:1 ethyl acetate/hexane and 1:1 ethyl acetate/methanol and then allowed to dry in a fume hood. Wristbands were then wrapped in aluminum foil and placed in amber glass jars to distribute to study participants. Field blanks were prepared as above, not worn, and were stored at room temperature until extraction.
Wristband Sample Collection and Processing.
Silicone wristbands (n = 72 unique samples) were collected and processed based on previously described methods.4,5 Participants were asked to wear wristbands for one week, after which they were wrapped in foil and stored at −20 °C until analysis. Half-gram sections were cut from each wristband and field blank, weighed, and placed into glass centrifuge tubes. Samples were extracted via a 20 min sonication in 3.0 mL of 1:1 hexane/acetone and then evaporated to complete dryness under nitrogen gas. Samples were then reconstituted in 1 mL of cell culture assay media and sonicated for a further 10 min and then transferred to vials and stored at −20 °C. These extracts were used in bioassays and tested for their ability to antagonize TRβ, using a reporter gene assay in human kidney cells.
Targeted Chemical Analysis.
Separate 1 g sections of each wristband were analyzed via mass spectrometry to quantify BFRs, OPEs, pesticides, and phthalates. Samples were extracted and processed based on a modified version of previously described methods.4,5 Wristbands were spiked with a suite of isotopically labeled standards that were used for quantification of all analytes (Table S1). Samples were then extracted via sonication in 10 mL of a 50:50 (v/v) mixture of hexane/dichloromethane for 15 min. The extraction was repeated three times, and the extracts were combined, concentrated to dryness, and reconstituted in 1 mL of hexane. Extracts were then purified using 8 g of deactivated, 100–200 mesh Acros Organics Florisil (Thermo Fisher Scientific, Waltham, MA, USA). Both an F1 (40 mL hexane) and an F2 fraction (40 mL ethyl acetate) were eluted, collected, and combined before being concentrated to 1 mL in a TurboVap benchtop concentrator (TurboVap II, Caliper Life Sciences). Sample elutes were then concentrated to near dryness and reconstituted in 1 mL of hexane. A different set of isotopically labeled standards were then spiked into each sample prior to mass spectrometry analysis to measure the recovery of the first set of isotopically labeled standards. Samples were analyzed for OPEs, pesticides, and phthalates using a Q Exactive GC Hybrid Quadrupole-Orbitrap GC–MS/MS system (Thermo Fisher Scientific, Waltham, MA, USA) operated in full-scan electron ionization mode. Samples were analyzed for BFRs using single quadrupole GC-MS (Agilent 6890N and 5975, respectively) (Agilent Technologies, Inc., Santa Clara, CA, USA) operated in electron capture negative chemical ionization mode. Method detection limits were calculated as three times the standard deviation of the field and lab blank responses. MDL values for all target analytes and percent recoveries for all internal standards are presented in the Supporting Information (Tables S1 and S2).
Reporter Gene Activity Bioassays.
Human embryonic kidney (HEK293/17) cells were obtained through the Duke Cell Culture Facility (ATCC cat #CRL-11268, lot #3579061) and were maintained as described previously.36 Cells were maintained in growth media (DMEM-HG, Gibco #11995, with 10% fetal bovine serum and 1% penicillin/streptomycin), ensuring that cells did not reach confluency. Cells were switched to white media two days prior (DMEM-HG without phenol red, Gibco cat #31053; 10% charcoal-stripped fetal bovine serum, Gibco #A33821; and 1% penicillin and streptomycin) to transfection. Near confluent cells were transfected in a flask as described previously.35,36,67 Briefly, cells were transfected in flasks using Lipofectamine LTX & Plus (Invitrogen cat #15338–100) according to manufacturer instructions and plasmids: receptor (6 μg; hTRβ1-pSG5), reporter gene (9 μg; pGL4-TK-2X-TADR4; receptor response element linked to the firefly luciferase gene), and a constitutively active normalization vector (3 μg; CMV-β-Gal; all plasmids were generous gifts of the Donald McDonnell Lab, Duke University). After 5 h of transfection, 15 mL of white media was added to the flasks for overnight recovery. The next day, the transfected cells were seeded at approximately 60,000 cells per well into 96-well tissue culture plates (Midsci cat #TP92696) and allowed to settle for 4 h. Cells were then induced with a graded dose series of positive/negative controls and/or wristband extracts using a 0.1% DMSO vehicle. Four blank bands were spiked with 1/10 (low/high) μmol T3 or 1/10 μmol 1–850 to serve as recovery controls for the bioassays. Cells were induced for approximately 18 h, then were lysed with buffer (10% glycerol, 0.25 M tris base, 2 mM CDTA, 0.5% Triton X-100, and 2 mM DTT), and the lysate was used for luciferase and β-galactosidase (β-gal) assays. Receptor bioactivities were calculated as a fold induction relative to the 0.1% DMSO solvent control and were then used to determine relative responses to control chemicals. For TRβ antagonism, activity is presented as a percent enhancement or inhibition of triiodothyronine (T3) response at its EC50 concentration (1 nM). Significant bioactivities were only determined in the absence of significant toxicity (≥15% change in response of constitutively an active β-gal promoter relative to the solvent control) as described previously.35,67 An additional cell viability assay was performed to validate inhibited cell health using the CellTiter-Glo assay (cell viability via ATP content; Promega cat #G7572), as described previously.68 Briefly, cells were rinsed with DPBS, and all but 30 μL was removed from wells, with the remaining 30 μL mixed with 30 μL of CellTiter-Glo reagent. Plates were incubated for 10 min and then read for luminescence; viability was assessed as a percent change from solvent controls. Inhibited cell health was measured via deviations of ≥15% in cell viability (ATP) assays. Four technical replicates (within each assay) and three biological replicates (separate assays/cell passages) were utilized for every test chemical and concentration. Positive and negative controls were utilized to assess efficacy, potency, and sensitivity of assays and compared to historical and literature values to ensure consistency (Figure S1). EC10/20 values were estimated using curves generated from raw luminescence data using a 4-parameter variable-slope Hill model in GraphPad Prism 8.0.
QA/QC.
Field blanks were extracted and purified as described above using cleaned wristbands that had not been worn by study participants. Laboratory or solvent blanks were prepared by performing the extraction process in the absence of a wristband to control for solvents and procedures utilized throughout extraction. To ensure that there was no bias in chemical extractions using hexane/dichloromethane (DCM) versus hexane/acetone (i.e. how wristbands were extracted for chemical analysis vs bioassays), we performed a small experiment in the laboratory. Six clean wristbands were spiked with a small volume (100 μL) of iso-octane containing 24 SVOCs (~30 ng each) ranging in vapor pressure from approximately 3.0 × 10−7 to 3.0 × 10−3 mmHg. Two clean wristbands were used as processing blanks and were placed next to the spiked wristbands. The wristbands were allowed to dry in a hood overnight to evaporate off any residual solvent. The following day, three spiked wristbands, and one blank, were extracted in 50:50 hexane/DCM, and three spiked wristbands, and one blank, were extracted in 50:50 hexane/acetone, as described above for all samples. Extracts were blown to dryness with purified nitrogen and reconstituted in 1 mL of hexane for gas chromatography-mass spectrometry analysis as described above. The measured concentrations for each SVOC are presented in the Supporting Information (Figure S2). Our results demonstrate no bias between the two methods.
Statistical Analysis.
Data for nuclear receptor bioactivities are presented as mean ± SEM from four technical replicates of two or three independent biological replicates. All in vitro experiments were performed and analyzed prior to receiving analytical and participant health outcome data, ensuring appropriate blinding procedures until data analysis. Relationships between bioactivities and the concentrations of each of the individual chemicals in wristband extracts were assessed for chemicals detected on greater than 60% of wristbands using Spearman’s correlations due to non-normal distributions. For these analyses, chemical concentrations below the method detection limit (MDL) were imputed as the MDL divided by two. Logistic regressions were also performed to better assess potential relationships between bioactivities and chemical concentrations on the wristbands using log-transformed chemical concentrations as a continuous measure. Though our sample size was quite small, we additionally conducted exploratory analyses investigating bioactivities and chemical concentrations in association with PTC using logistic regressions. Based on our a priori expectation of the potential for socioeconomic status and race to confound these associations,69–71 we adjusted for educational attainment (less than college degree or at least college degree), race (non-Hispanic white, non-Hispanic black, or other), and BMI. Correlation and regression analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc., Cary, NC), and all results were assessed at α = 0.05 for significance.
RESULTS
Seventy-two silicone wristbands, worn for one week by participants, were solvent-extracted and tested at concentrations of 0.1, 1, 5, and 10% (of the original extract concentration or ~ 1 g of wristband extracted into 1 mL of media) in cell assays. Two separate measurements of cell viability were used to ensure activity was occurring independently of inhibited cell health. Analytical measurements of select SVOCs were made from separate extracts from separate sections of the same wristbands, and relationships between TRβ antagonism, chemical concentrations, and health outcomes were assessed.
Concentrations of SVOCs in Wristband Extracts.
Concentrations of 49 chemicals were measured in wristband extracts (Table 1), including OPEs, novel (EH-TBB and BEH-TEBP) and legacy (polybrominated diphenyl ethers, PBDEs) BFRs, phthalates, and pesticides. Thirty-six of these chemicals were detected in >60% of wristband extracts, and concentrations ranged over 2 orders of magnitude. The concentrations of PBDEs generally ranged from 4–75 ng/g, and both novel BFRs had geometric mean levels of approximately 70 ng/g and detection frequencies ≥80%. The concentrations of OPEs were generally much greater, ranging from approximately 4–470 ng/g, although more than 20% of the OPEs were detected in <60% of wristband extracts. Concentrations of pesticides ranged from 4–87 ng/g, and four of nine were detected at <60% frequency. Phthalates were reported more consistent and at greater concentrations, ranging from approximately 50–63,000 ng/g.
Table 1.
Wristband Concentrations of Brominated Flame Retardants and Organophosphate Estersa
| targeted contaminant | acronym | detection frequency (%) | range (ng/g) | geometric mean (ng/g) |
|---|---|---|---|---|
| Brominated Flame Retardants BFRs | ||||
| 2,4,4′-tribromodiphenyl ether, 2′,3,4-tribromodiphenyl ether | BDE-28,33 | 94.4 | <MDL–76.1 | 4.1 |
| 2,2′,4,4′-tetrabromodiphenyl ether | BDE-47 | 94.4 | <MDL–2356.8 | 75.0 |
| 2,2′,4,4′,6-pentabromodiphenyl ether | BDE-100 | 79.2 | <MDL–562.5 | 12.6 |
| 2,2′,4,4′,5,5′-hexabromodiphenyl ether | BDE-153 | 100.0 | 0.9–203.6 | 4.3 |
| 2,2′,4,4′,5,6′-hexabromodiphenyl ether | BDE-154 | 93.1 | <MDL–118.0 | 3.2 |
| decabromodiphenyl ether | BDE-209 | 100.0 | 12.9–276.3 | 31.4 |
| 2-ethylhexyl tetrabromobenzoate | EH-TBB | 100.0 | 4.5–2033.2 | 72.8 |
| bis(2-ethylhexyl) tetrabromophthalate | BEH-TEBP | 95.8 | 8.2–814.0 | 70.0 |
| Organophosphate Esters OPEs | ||||
| tri(n-butyl) phosphate | TnBP | 100.0 | 12.7–2397.9 | 52.6 |
| tris(2-carboxyethyl) phosphine | TCEP | 68.9 | <MDL–258.8 | 22.4 |
| tris(1-chloro-isopropyl) phosphate | TCIPP | 98.6 | <MDL–7907.4 | 319.1 |
| tris(1,3-dichloro-2-propyl) phosphate | TDCIPP | 100.0 | 26.4–16272.7 | 359.6 |
| tris(2-butoxyethyl) phosphate | TBOEP | 82.4 | <MDL–11376.6 | 470.8 |
| triphenyl phosphate | TPHP | 100.0 | 22.2–2168.6 | 267.1 |
| ethylhexyl diphenyl phosphate | EHDPP | 100.0 | 10.0–739.9 | 49.0 |
| 2-isopropylphenyl diphenyl phosphate | 2-IPPDPP | 100.0 | 7.9–1610.9 | 114.5 |
| 3-isopropylphenyl diphenyl phosphate | 3-IPPDPP | 95.9 | <MDL–240.7 | 10.5 |
| 2-tert-butylphenyl diphenyl phosphate | 2tBPDPP | 20.3 | <MDL–0.6 | <MDL |
| bis(2-isopropylphenyl) phenyl phosphate | B2IPPPP | 98.6 | <MDL–567.5 | 44.2 |
| 4-isopropylphenyl diphenyl phosphate | 4-IPPDPP | 98.6 | <MDL–666.3 | 36.4 |
| 2,4-diisopropylphenyl diphenyl phosphate | 2,4-DIPPDPP | 86.5 | <MDL–544.9 | 36.6 |
| 4-tert-butylphenyl diphenyl phosphate | 4tBPDPP | 98.6 | <MDL–708.9 | 59.2 |
| bis(3-isopropylphenyl) phenyl phosphate | B3IPPPP | 28.4 | <MDL–10.6 | <MDL |
| bis(2-tert-butylphenyl) phenyl phosphate | B2tBPPP | 13.5 | <MDL–0.9 | <MDL |
| bis(4-isopropylphenyl) phenyl phosphate | B4IPPPP | 95.9 | <MDL–83.2 | 4.4 |
| tris(3-isopropylphenyl) phosphate | T3IPPP | 14.9 | <MDL–10.2 | <MDL |
| bis(2,4-diisopropylphenyl) phenyl phosphate | B24DIPPPP | 29.7 | <MDL–49.2 | <MDL |
| bis(4-tert-butylphenyl) phenyl phosphate | B4tBPPP | 91.9 | <MDL–307.2 | 23.4 |
| tris(4-isopropylphenyl) phosphate | T4IPPP | 10.8 | <MDL–3.2 | <MDL |
| tris(4-tert-butylphenyl) phosphate | T4tBPP | 79.7 | <MDL–57.8 | 3.2 |
| Pesticides | ||||
| lindane | 23.0 | <MDL–4.6 | <MDL | |
| chlorpyrifos | 71.6 | <MDL–103.2 | 3.6 | |
| trans-chlordane | 82.4 | <MDL–523.1 | 11.9 | |
| cis-chlordane | 48.6 | <MDL–301.6 | <MDL | |
| chlorfenapyr | 8.1 | <MDL–12.5% | <MDL | |
| cis-permethrin | 100.0 | 1.8–7018.6 | 60.3 | |
| trans-permethrin | 100.0 | 3.1–9000.4 | 86.6 | |
| cypermethrin | 35.1 | <MDL–1930.6 | <MDL | |
| azoxystrobin | 55.4 | <MDL–64.8 | 4.3 | |
| Phthalates | ||||
| dimethyl phthalate | DMP | 54.1 | <MDL–167.7 | 51.5 |
| diethyl phthalate | DEP | 100.0 | 176.9–16813.8 | 1635.3 |
| diisobutyl phthalate | DiBP | 13.5 | <MDL–11168.1 | <MDL |
| dibutyl phthalate | DBP | 100.0 | 313.7–5425.9 | 1314.0 |
| butyl benzyl phthalate | BBP | 100.0 | 101.1–11894.4 | 631.3 |
| di(2-ethylhexyl) adipate | DEHA | 100.0 | 160.1–21834.4 | 1674.6 |
| bis(2-ethylhexyl) phthalate | DEHP | 100.0 | 3103.4–56566.2 | 13,456.9 |
| bis(2-ethylhexyl) terephthalate | DEHT | 100.0 | 2896.0–59366.6 | 12,425.3 |
| diisononyl phthalate | DiNP | 100.0 | 5852.1–1360295.9 | 62,942.5 |
| tris(2-ethylhexyl) trimellitate | TOTM | 100.0 | 47.7–5450.4 | 480.3 |
Descriptive statistics and detection frequencies for wristband extract concentrations of various semivolatile organic contaminants following blank correction as described in Materials and Methods.
Correlations across chemical classes were examined to assess the degree of co-occurrence in wristband extracts (Figure 1). PBDEs demonstrated high concordance across the chemical class, while weak or nonsignificant relationships were observed with newer BFRs (EH-TBB and BEH-TEBP). The OPEs also demonstrated particularly strong concordance, although these associations were weaker for TnBP, TCEP, and TCIPP. TCEP and TCIPP were more highly correlated with each other than the other OPEs, and TnBP was only correlated with TDCIPP. Many of the BFRs were also significantly and positively associated with most OPEs, most notably for BDE-209 and EH-TBB (Figure 1). Pesticides were not appreciably correlated with each other, with some exceptions. Chlorpyrifos was positively correlated with trans-chlordane, and permethrins were highly correlated with each other. The phthalates were highly intercorrelated with the exception of DEP, which was correlated with none of the other chemicals measured herein (one negative correlation with BEH-TEBP). Phthalates were also positively correlated with most OPEs and several BFRs.
Figure 1.
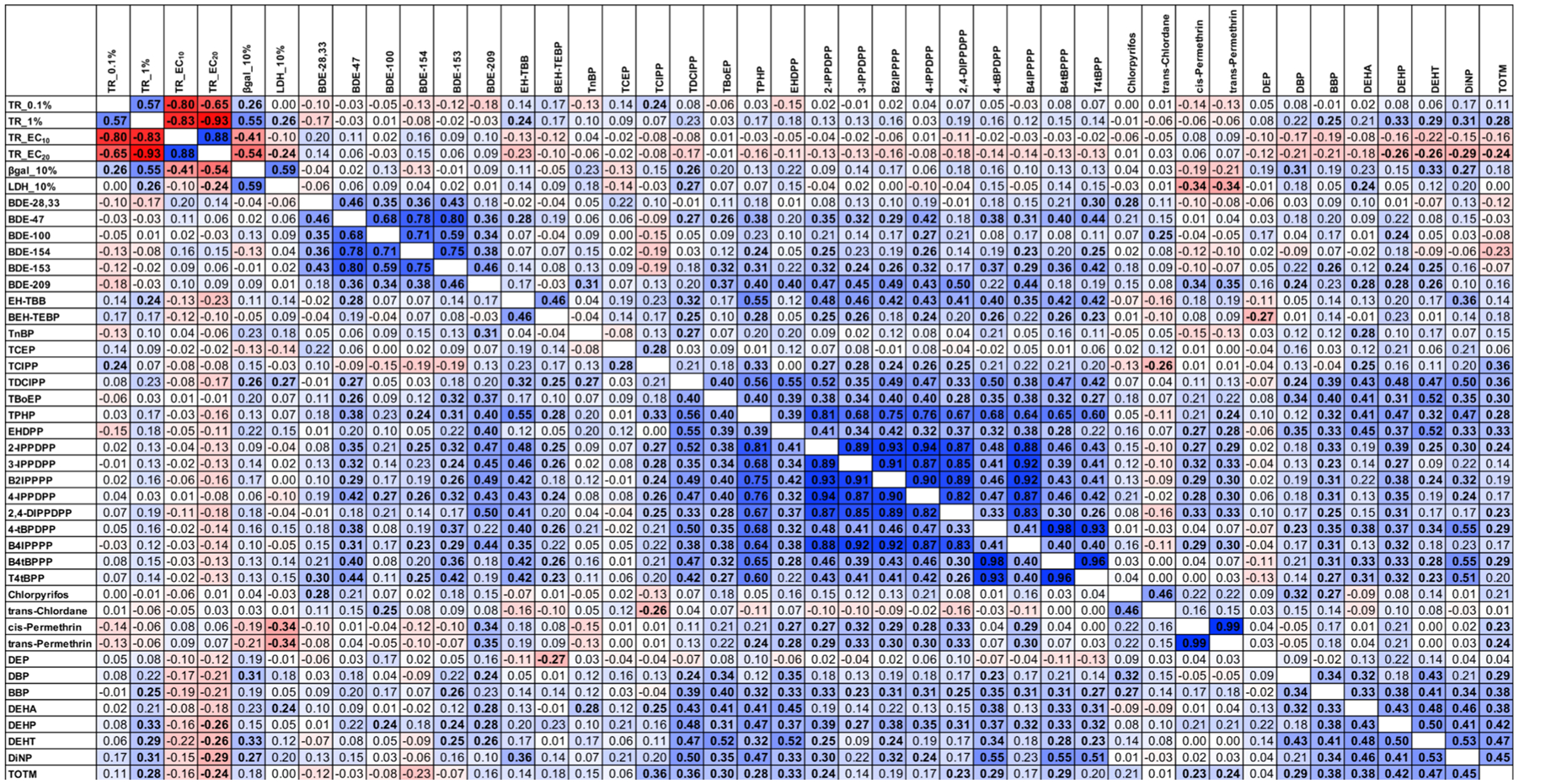
Correlations among bioactivities and targeted chemical concentrations in wristband extracts. Spearman’s correlations between concentrations of targeted SVOC concentrations (only performed for chemicals with ≥60% detection) in silicone wristband extracts. Correlations performed using SAS 9.4; bolded samples represent significant (p < 0.05) correlations, and the color depicts the strength and direction of the correlation. Darker colors represent a stronger correlation, blue coloration depicts positive correlations, and red depicts negative correlations. TR_0.1% and TR_1% represent the efficacy/magnitude of TR antagonism at 0.1 and 1% wristband extract concentration, respectively. TR_EC10 and TR_EC20 represent the potencies of TR antagonism (concentration of wristband extract at which 10 or 20% TR antagonism was observed). β-gal and LDH represent the magnitude of cell viability inhibition for each of these assays at 10% wristband concentration.
Cell Viability.
Significant inhibition of cell viability was noted for >20% of samples in both viability assays at 5 and 10% wristband extract concentrations (Table 2, Figure 2). Therefore, these concentrations were largely not utilized for bioactivity determinations. Only one sample exhibited significant inhibition in 1% extract concentration and none in 0.1% (Figure 2). No significant inhibition was observed in either wristband field blanks or laboratory (solvent) blanks (Figure S3).
Table 2.
Thyroid Antagonism and Cell Viability across Wristband Extract Concentrationsa
| TR antagonism | Cell viability | ||||
|---|---|---|---|---|---|
| wristband extraction concentration (%) | efficacy range (% activity) | mean (%) | median (%) | % toxic (β-Gal) | % toxic (LDH) |
| 0.1 | 0.0–63.2 | 10.2 | 4.9 | 0.0 | 0.0 |
| 1 | 0.0–100.0 | 29.7 | 22.7 | 1.4 | 1.4 |
| 5 | 0.0–100.0 | 66.5 | 69.2 | 20.8 | 23.6 |
| 10 | 0.0–100.0 | 83.0 | 99.8 | 23.6 | 38.9 |
Descriptive statistics for TR antagonism and cell viability across each wristband extract concentration. TR antagonism provided as a range of percent antagonism across extracts as well as mean and median antagonism. Inhibited cell viability provided as the percentage of toxic samples via indirect cell viability measures as per significant reduction in a constitutively active promoter via β-gal assay and per LDH release assay. Statistics provided for n = 72 samples.
Figure 2.
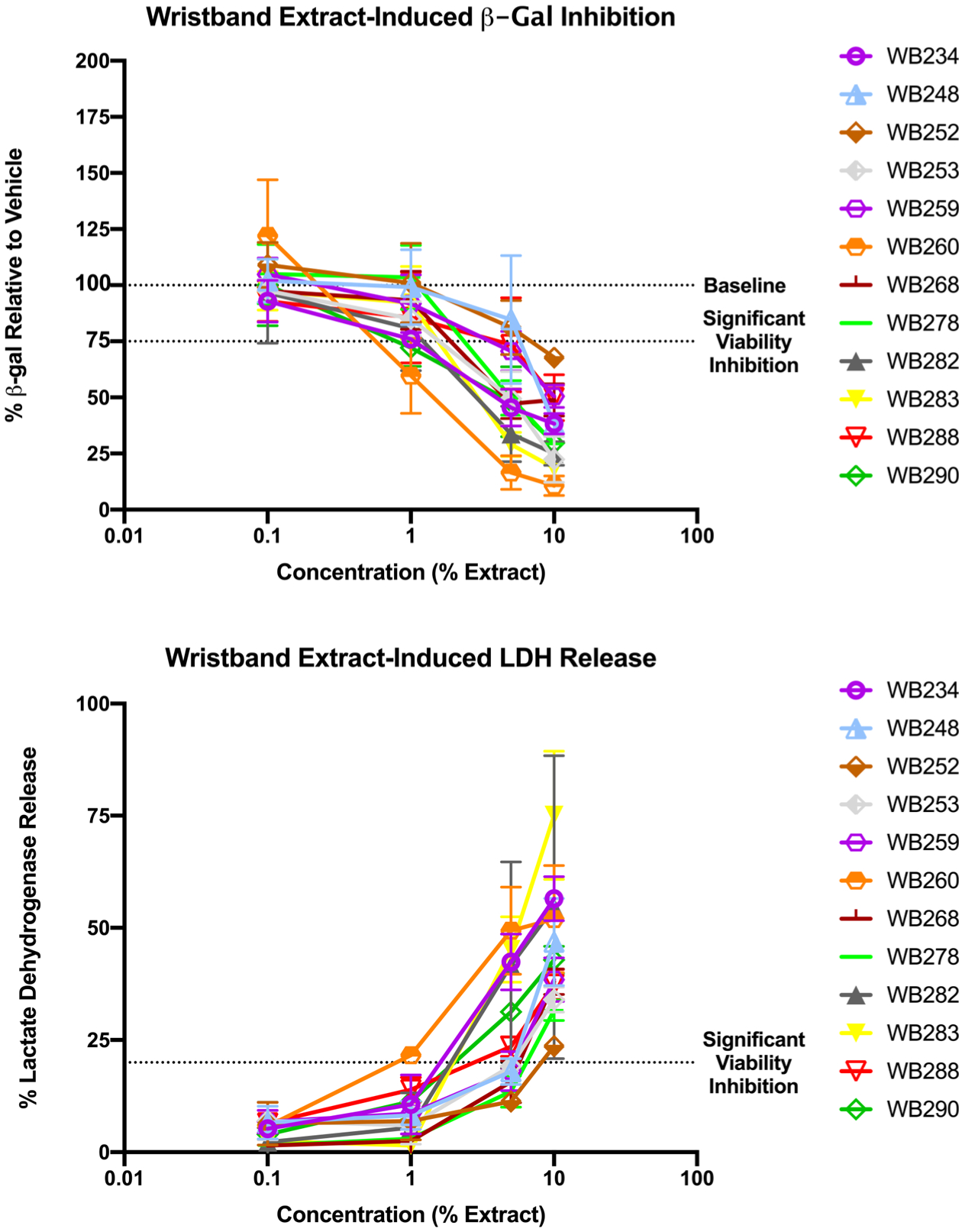
Representative wristband extract-induced inhibited cell viability. HEK293T cells were transfected with plasmids and induced with wristband extracts and/or control chemicals as described in the Materials and Methods. Representative dose response curves are provided based on concentration of extraction in contact with the cells (0.1% = 1000-fold dilution of the extract into the exposure media). Cells were induced for approximately 18 h, after which cell viability was assessed by two different measures: via significant reduction in β-gal activity as per constitutively active promoter plasmid relative to vehicle/solvent control (A) and via significant release of LDH relative to a cytotoxic control (B). Data presented as mean ± SEM from two or three independent experiments and four technical replicates of each concentration within each.
TRβ Antagonism of Silicone Wristband Extracts.
TR antagonism at the 0.1% concentration ranged from 0–63.2% (percent inhibition of added EC50 T3), with 42% of the samples exhibiting significant antagonism, and a mean of 10% (Table 2, Figure 3). Receptor antagonism at 1% concentration ranged from 0–100%, with 82% of the samples exhibiting significant antagonism, and a mean of 30% (Table 2). No significant receptor antagonism was observed in either wristband field blanks or laboratory (solvent) blanks (Figure S3).
Figure 3.
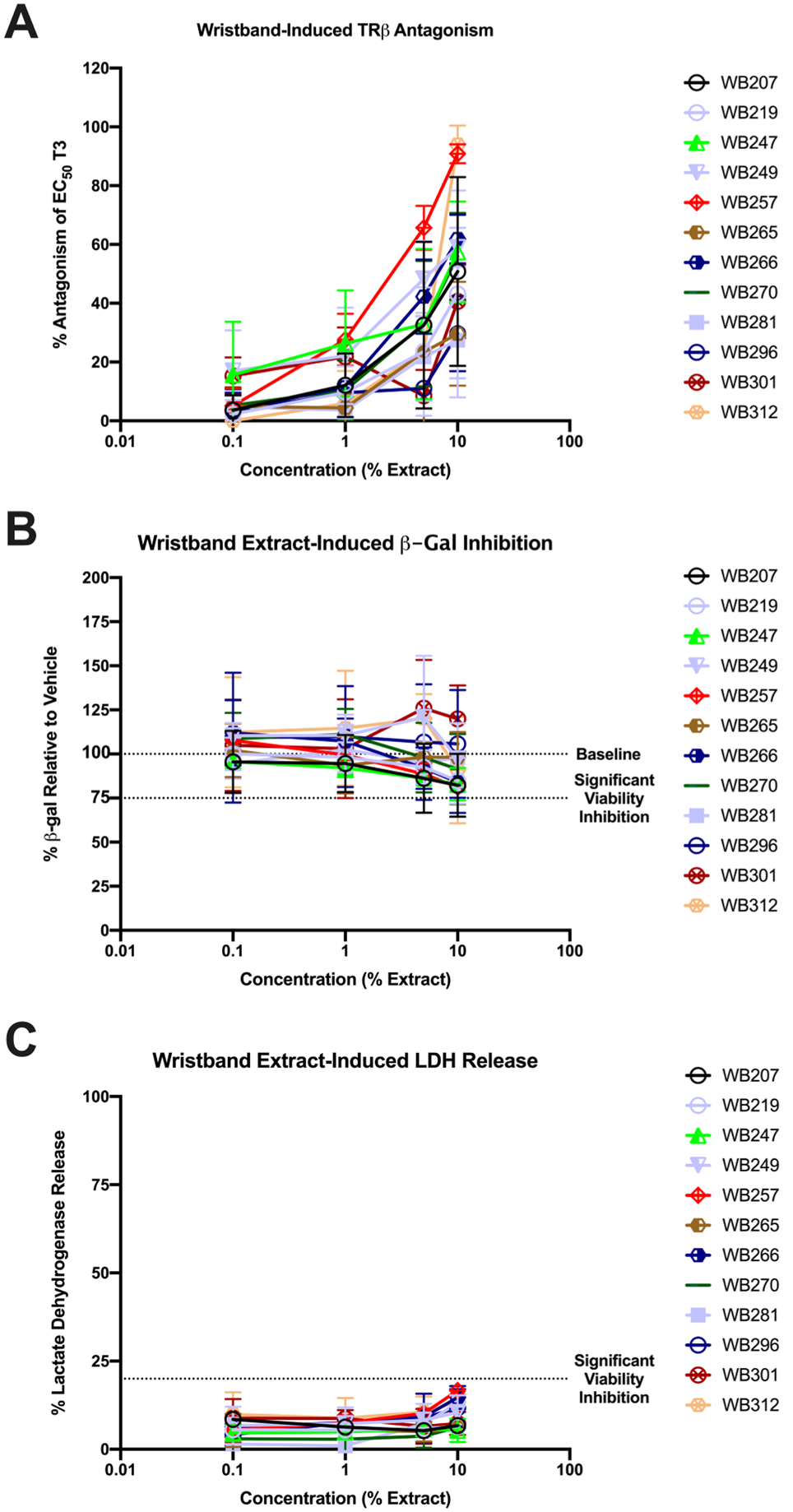
Representative wristband extract-induced TRβ antagonism. HEK293T cells were transfected with plasmids and induced with wristband extracts and/or control chemicals as described in the Materials and Methods. Representative dose response curves are provided for antagonistic extracts that did not exhibit inhibited cell viability based on concentration of extraction in contact with the cells (0.1% = 1000-fold dilution of the extract into the exposure media). Cells were induced for approximately 18 h, after which TR β antagonism was assessed via reduction in luciferase luminescence relative to EC50 (1 nM) concentration of T3 (A). Cell viability was assessed with the same cells in the same plates by two different measures: via significant reduction in β-gal activity as per constitutively active promoter plasmid relative to vehicle/solvent control (B) and via significant release of LDH relative to a cytotoxic control (C). Data presented as mean ± SEM from two or three independent experiments and four technical replicates of each concentration within each.
Correlations across bioactivity measures (at various dilutions) were calculated to assess relationships among samples (Figure 1). Measures of TR efficacy (the magnitude of TR inhibition at 0.1 and 1%) were strongly and positively correlated (Rs = ~0.6) and strongly negatively correlated with measures of TR potency (EC10 and EC20; Rs = 0.6–0.9), as would be expected. Both cell viability measures [β-gal and lactate dehydrogenase (LDH) at 10%] were positively correlated (Rs = ~0.6). TR efficacy at 1% was positively correlated with both measures of inhibited cell viability (Rs = ~0.25–0.6), while TR efficacy at 0.1% was correlated with neither. A similar relationship was observed with potencies, as the EC20 values were negatively correlated with both cell viability measures (Rs = ~ −0.25 to (−0.5)), although the EC10 values were only correlated with the β-gal cell viability results (Figure 1).
Associations of TRβ Antagonism with Chemical Concentrations in Wristband Extracts.
Overall, chemical concentrations were not highly correlated with bioactivities. TCIPP concentrations were positively correlated with TR efficacy at 0.1% but not at 1%; in contrast, EH-TBB, BBP, DEHP, DEHT, DiNP, and TOTM concentrations were positively correlated with TR efficacy at 1% but not at 0.1% (Figure 1). Concentrations of DEHP, DEHT, DiNP, and TOTM were negatively correlated with TR potency at 20% activity (EC20), but these were not significantly correlated with EC10 values. TDCIPP concentrations were significantly and positively correlated with both cell viability measures. DBP, DEHT, and DiNP were positively correlated with inhibited cell viability in the β-gal assay but were not significantly associated in the LDH release assay. In contrast, DEHA was positively and cis/trans-permethrin was negatively correlated with inhibited cell viability in the LDH assay but not in the β-gal assay.
Logistic regressions also were performed to further assess relationships between bioactivities (classifying samples as either active or inactive) and chemical concentrations on the wristbands (Figure 4) using log-normalized chemical concentrations as a continuous measure. Concentrations of BEH-TEBP, TCIPP, and TDCIPP were significantly associated with TR antagonism in these models, with odd ratios of 2.9 (95% confidence intervals, 1.11–7.41, p < 0.05), 3.0 (1.08–8.52, p < 0.05), and 3.0 (1.04–8.51, p < 0.05), respectively. This can be roughly translated to indicate that each log unit increase in concentration results in a sample being ~3 times as likely to be active for TR antagonism (TR efficacy at 0.1%). Associations with TR efficacy at 1% were not evident. Concentrations of DEP and DiNP were positively associated and BEH-TEBP was negatively associated with inhibited cell viability via the β-gal assay (Figure S4). Concentrations of DiNP and DEHA were positively associated and cis/trans-permethrin were negatively associated with inhibited cell viability in the LDH assay (Figure S5).
Figure 4.
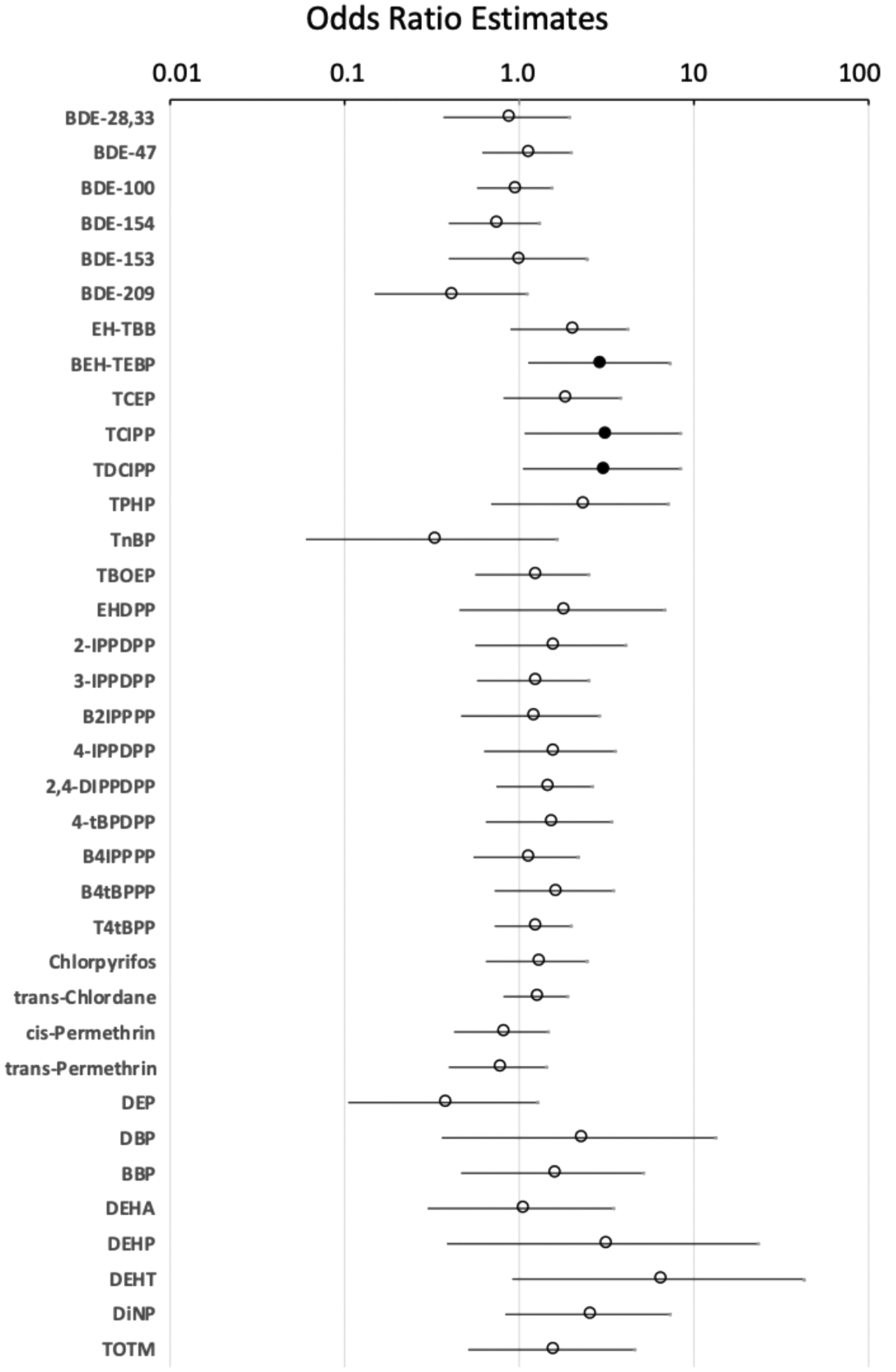
TR antagonism regressions by chemical concentrations. Results of logistic regression models performed in SAS 9.4 using chemical concentrations in wristband extracts (log-transformed) and TR β antagonism as measured by reporter gene assay at 0.1% extract concentration. Log-transformed chemicals were included as a continuous measure, and antagonism as a logistic measure (inactive or active for significant antagonism). Filled circles denote significant difference between inactive to active samples for specific chemicals, and open circles denote insignificance. Odds ratios indicate the likelihood that the extracts will be active for TR antagonism with each log increase in chemical concentrations. Interpretation: TCIPP with an odds ratio of 3.0 suggests that for each log unit increase in TDCIPP, the likelihood that a sample will be active is approximately 3 times as high.
Associations of TRβ Antagonism, Chemical Concentrations, and Health Outcomes.
Regressions were also performed to assess relationships between the TRβ bioactivity measures, chemical concentrations, and PTC. Across the four TRβ measures (maximal efficacy at 0.1 and 1% extract concentrations and potency/concentration at 10 or 20% inhibition of positive control), consistent trends were observed with elevated odds for PTC with increased bioactivity (Figure 5) after controlling for potential confounding by educational attainment, BMI, and race/ethnicity. Specifically, participants with wristbands active for TRβ antagonism were 1.49 and 2.13 (p = 0.12) as likely to have PTC using TR efficacies of 1 and 0.1%, respectively; however, ORs were not statistically significant. For regression analyses, potency was separated into tertiles. The low tertile represents the least potent samples (highest values and the weakest TR potencies). Odds ratios were higher for the high tertile and PTC (highest tertile for EC20 values consisting of the most potent samples; OR = 1.91); however, they were not statistically significant. While not significant, both TR activity measures demonstrated trends for increased odds of PTC with increased wristband TRβ antagonism.
Figure 5.
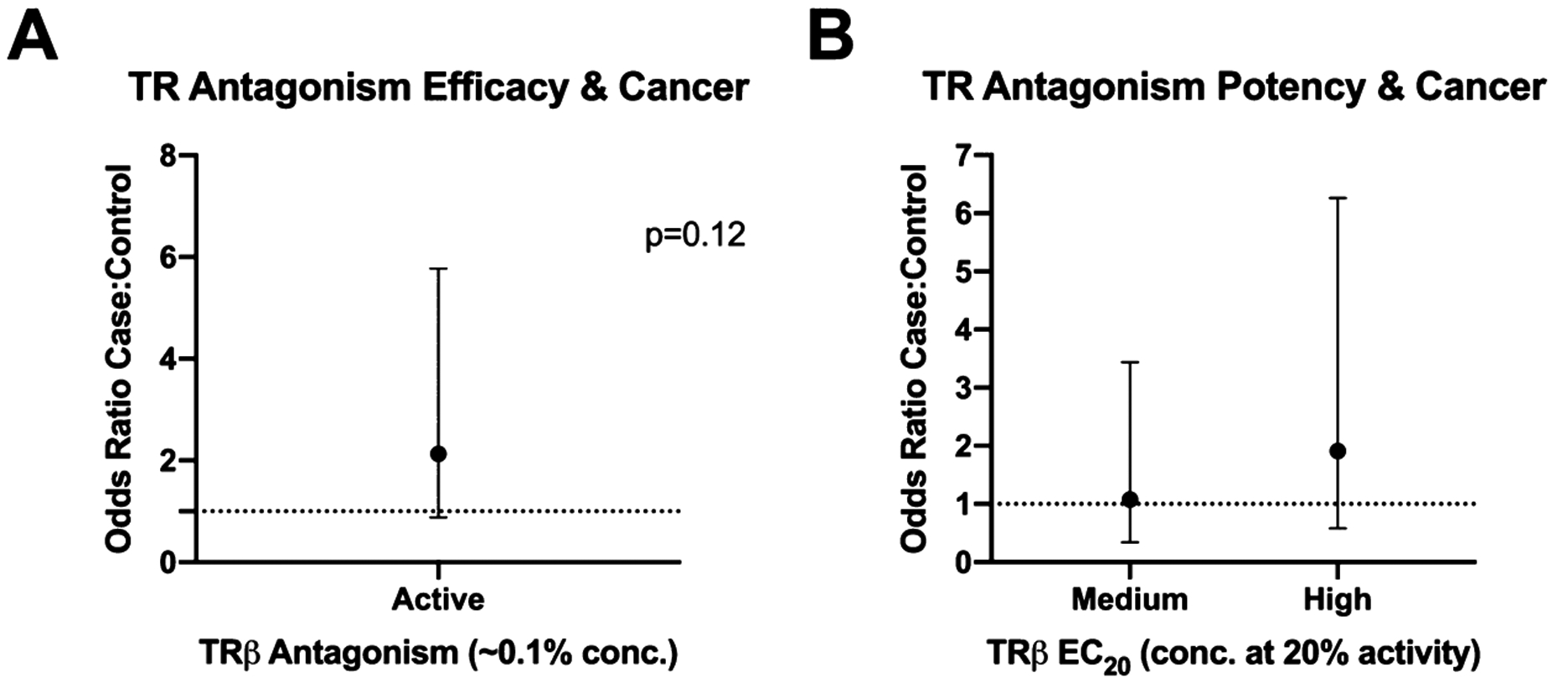
TR antagonism and PTC case status. Logistic regression models performed in SAS 9.4 to assess relationships between TR antagonism metrics (maximal inhibition at 0.1% wristband extract concentration, potency of inhibition via concentration at which 20% of added agonist control was inhibited; EC20) and participant PTC status. These models controlled for potential confounding by race, BMI, and educational status, which we anticipated could be related to both exposure and health outcomes. Interpretation: odds ratio of 2.1 (A) suggest that active samples are approximately twice as likely to come from cases.
Regression analyses were also performed between individual chemical contaminants on wristbands and the odds of PTC. Concentrations of TCEP, TDCIPP, 4-tBPDPP, B4tBPPP, T4tBPP, DiNP, and TOTM were significantly associated with increased odds of PTC case status: odds ratios of 2.3 (1.02–5.05, p < 0.05), 3.5 (1.20–10.47, p < 0.05), 4.6 (1.67–12.71, p < 0.01), 5.6 (2.03–15.34, p < 0.001), 3.6 (1.74–7.37, p < 0.001), 9.5 (2.37–38.29, p < 0.01), and 3.5 (1.09–11.15, p < 0.05), respectively. For each log unit increase in DiNP in wristband extracts, for example, samples were ~9.5 times as likely to be from a PTC patient (Figure 6). In contrast, BDE-100 concentrations had an odds ratio that was significantly less than one (OR = 0.5, 0.30–0.94, p < 0.05), suggesting an inverse association.
Figure 6.
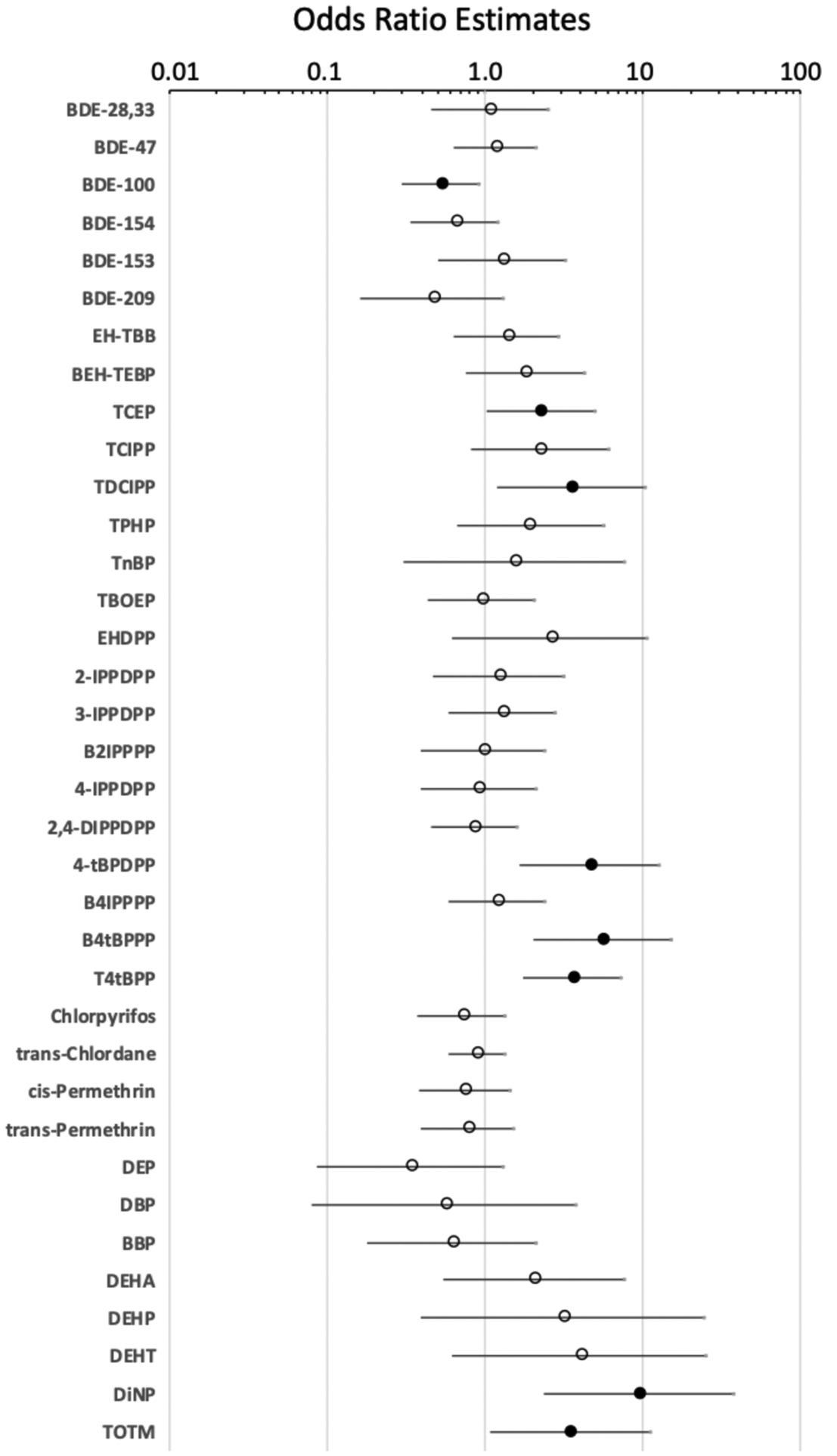
Targeted chemical concentrations and PTC case status. Results of logistic regression models performed in SAS 9.4 using chemical concentrations in wristband extracts (log-transformed) and PTC case status of participants. Log-transformed chemicals are included as a continuous variable. Filled circles denote significant p < 0.05, and open circles denote p > 0.05. Odds ratios represent the likelihood that a participant will be a PTC case with each log increase in chemical concentrations. Interpretation: DiNP with odds ratio of 9.5 suggests an 8.5-times greater likelihood of having PTC with each log increase in DiNP concentration.
DISCUSSION
These results demonstrate for the first time the viability of measuring nuclear receptor bioactivity from silicone wristband extracts worn by study participants. We report that up to 80% of wristband extracts was able to significantly antagonize TRβ at levels up to 100% inhibition of an EC50 T3 agonist, independent of inhibited cell viability. Notably, we measured these bioactivities at extract concentrations of 0.1–1% (of the extract from 1.0 g of wristband) in contact with the cells, as 5–10% concentrations exhibited significant inhibited cell viability. These low concentrations allow for greater use in a range of different bioassays, limiting the need for extracting large sections of the wristbands.
TR antagonism was positively correlated with concentrations of TCIPP, EH-TBB, BBP, DEHP, DEHT, DiNP, and TOTM on wristbands. We also observed significant associations via logistic regressions with BEH-TEBP, TCIPP, and TDCIPP (p < 0.05) promoting increased odds of TR antagonism. A number of phthalate esters have been rigorously demonstrated to act as TR antagonists in diverse models and/or be associated with thyroid dysfunction in humans. DEHT was demonstrated to disrupt thyroxine and TSH levels in a rodent model,72 and direct TR antagonism has been demonstrated in vitro for DEHP,30,73 DiNP,30,74 and BBP.30 While we have not found literature evaluating impacts of TOTM on modulation of thyroid hormone signaling, there is evidence for effects on estrogen receptor α and β activation in a reporter gene assay75 and it demonstrated higher binding affinity to sex hormone binding globulin than dihydrotestosterone did in molecular docking experiments.76 Our laboratory previously reported that these OPEs/BFRs (TCIPP, TDCIPP, EH-TBB, and BEH-TEBP) were incapable of significantly antagonizing TRβ using a stably transfected human construct in human bone cells,37 though there are conflicting results in the literature. Other research supported an absence of activity for both TR α and β in a Chinese hamster ovary cell reporter gene model,77 with some research in a thyroid hormone-dependent cell proliferation assay reporting agonistic effects.78 Conversely, others have reported antagonistic effects for EH-TBB and BEH-TEBP using a stable reporter assay (rat pituitary cells constitutively expressing both TRα/β),79 and the Tox21 database reports TR antagonist activity for TDCIPP. Outside of direct receptor testing, treatment with TDCIPP has been demonstrated to significantly inhibit thyroid hormone concentrations in developmentally exposed80,81 or adult-exposed82 zebrafish and in developmentally exposed chickens83 and disrupt thyroid hormone synthesis and signaling in rodents.84 TCIPP previously has been reported to modulate thyroid-dependent gene expression in chicken embryonic hepatocytes85 and alter thyroid hormone signaling in developmentally exposed chickens.83 BEH-TEBP has been reported to be associated with thyroid hormone (T3/T4) concentrations in humans,86,87 and its metabolite, mono(2-ethyhexyl) tetrabromophthalate (TBMEHP), reduced thyroid hormone concentrations in the rat model88 and deiodinase activity in a rat liver microsome model described previously REF. Developmental exposures to both BEH-TEBP and EH-TBB have been demonstrated to inhibit thyroid hormone (T3/T4) concentrations and thyroid-dependent gene expression in zebrafish.89 As such, while some mechanistic assays do not demonstrate direct receptor antagonism at the level of the receptor, there is evidence for the majority of these phthalates, OPEs, and BFRs directly or indirectly interfering with thyroid hormone signaling as described above.
We also report that concentrations of TCEP, TDCIPP, 4-tBPDPP, B4tBPPP, T4tBPP, DiNP, and TOTM were significantly associated with PTC, with a ~8.5 times greater likelihood of being a case relative to a control with each log increase in DiNP concentrations on wristbands. Multiple studies have demonstrated thyroid cancer cell proliferation in vitro and in vivo90 and also significant associations between DEHP and thyroid cancer incidence and/or malignancy in human cohorts.91–93 DEHT was shown to increase thyroid C-cell hyperplasia in female rats chronically exposed to all doses.94 While there is no evidence of direct carcinogenicity for DiNP, it has been demonstrated to promote autoimmune thyroid disease through increased oxidative stress and activation of the Akt/mTOR pathway.95 Notably, previous work from our laboratory reported significant associations between concentrations of TCEP in household dust and PTC, particularly for larger, more aggressive tumors,66 although a separate study measuring urinary TCEP at the time of diagnosis did not report an association.96 We also report a protective effect for BDE-100, which has been observed previously for serum concentrations in two separate studies,97,98 although only in the middle tertile or quartile of exposure and not in the highest exposure groups. We did not observe a significant protective effect in our previous study66 but did report exacerbated risk with increasing BDE-209 exposure, which we did not observe here.
Many of these and/or similar chemicals have been described as TR antagonists. While not statistically significant, the extent of TR antagonism measured herein was also positively associated with PTC. All four TR metrics (efficacy at 0.1 and 1% wristband extract concentration and potency at 10 and 20% antagonism) demonstrated a consistent positive relationship between the PTC status and TR efficacy and potency, suggesting a potential role for TR antagonism in the PTC associations. Thyroid hormones have long had well-appreciated roles in angiogenesis, proliferation, and thyroid cancer,99 with thyroid hormone receptor mutations in particular linked to thyroid hormone resistance and cancer.99 Researchers have previously demonstrated in mice that inducing a dominant negative mutation in the TRβ gene disrupts the thyroid pituitary axis, increases TSH and thyroid hormone concentrations, and subsequently leads to hyperplasia of the thyroid follicular epithelium.100 More detailed analysis of the progression to metastasis of this follicular carcinoma suggested activation of TSH signaling pathways and repression of peroxisome proliferator-activated receptor gamma (PPARγ) signaling,101,102 suggesting that TRβ might act as a tumor suppressor gene. To evaluate a causal role for elevated TSH, a major stimulator for thyrocyte proliferation, wildtype mice were treated with PTU to inhibit thyroid hormones; these mice exhibited enlarged thyroids but no metastatic thyroid cancer,103 suggesting that TSH-induced growth is a prerequisite but not sufficient for metastasis. Notably, a range of thyroid disruption can potentially contribute to thyroid dysfunction, disease, and subsequent development of cancer, including iodide uptake, TSH signaling, deiodination/sulfation/glucuronidation enzyme activity modulation, disruption of transporters, and more.50 There is also an apparent contributory role for PPARγ, as mice with PPARγ insufficiency demonstrated increased cell proliferation and carcinogenesis and treatment with a PPARγ agonist-delayed thyroid cancer progression,41,104 which may help explain the exacerbated cancer risk in obese individuals/animals.105
While no research previously has assessed bioactivities from wristband extracts, a number of studies have measured TRβ antagonism in household dust extracts. Research by our laboratory using a stably transfected human construct in human bone cells reported significant antagonism for 42% of dust extracts,37 while another study from our group reported that 76% of samples exhibited significant TRβ antagonism when tested using the transient transfection reporter assay used in the current experiments, which has a greater dynamic range.36 Other researchers have assessed TR bioactivities in dust extracts, reporting antagonism from both indoor and outdoor environments106 but at considerably higher concentrations than our previous research. We report a similar frequency of antagonism (~80%) to what we detected previously in household dust using the same transient transfection reporter assay, although this was achieved with much lower concentrations of wristband extracts relative to those required for household dust. As such, these extracts could be utilized to interrogate a diversity of nuclear receptors and interactions (agonism and antagonism) that would not be possible using household dust, for which the sample size also can be limiting.
We have previously reported concentrations for a number of these OPEs and BFRs on wristbands in other human cohorts. Levels of BFRs were previously measured on wristbands from a similar geographic region (central NC) in August 2016, with geometric mean concentrations of BFRs ranging from 2 to 56 ng/g wristband.4 Geometric means herein ranged from 3 to 73 ng/g, with ≤2-fold variances observed relative to previous ones. Levels of the OPEs were previously measured in a separate study of children (August 2015 to April 2016) from a similar geographic region.12 This study did not provide geometric means, but comparing median concentrations to those here revealed equivalent levels of TCEP, five-times higher TCIPP levels, two-times higher TDCIPP levels, and three-times lower levels of TPHP on current adult wristbands relative to previous child wristbands.12 Recent collaborative work from our group examined 22 OPEs on wristbands, with concentrations ranging from 20 to 520 ng/g.107 Most OPEs exhibited equivalent concentrations to present ones (within two or three-fold), although we reported 5–15-times higher detection frequencies here relative to previous ones.107 While previous studies have reported the presence and detection of phthalates and pesticides on wristbands, they have not provided quantitative measures per mass of wristband for the purpose of comparison to the values reported here.
We also reported significant inhibition of cell viability by the wristband extracts with increasing concentrations, with 20–40% exhibiting significant inhibition at wristband concentrations of 5 and 10%. Moreover, we reported associations between our indirect measures of toxicity and concentrations of several contaminants in the wristband extracts: TDCIPP was positively correlated with both cell viability measures; DBP, DEHT, and DiNP were positively correlated in the β-gal assay only; and DEHA was positively and cis/trans-permethrin were negatively correlated in the LDH assay only. We previously assessed the toxicity and inhibited cell viability for each of the chemicals examined herein at concentrations up to 10 μM and did not report any significant cell viability impacts, suggesting potent impacts from the mixtures of contaminants isolated from wristbands. Consistent findings of toxicity for TDCIPP between assays suggest a potential causal role for this contaminant. While the use of TDCIPP has increased in the residential indoor environment following the PBDE phase-out in 2005, it has been in use since the 1960s. It was phased out of use in children’s pajamas in 1977 after it was described as mutagenic108 but has become one of the most widely used flame retardants in polyurethane foam over the last 10 years.109
While this research presents some novel information and insights, it is not without several limitations. For cancer associations, it is important to note that PTC is an indolent cancer, with estimated latency believed to be years to decades. Therefore, while we observed significant associations between specific chemicals and the odds of concurrent PTC in this cross-sectional study, these trends should be interpreted with caution. Because our sample size was relatively small and our study population was drawn from central North Carolina, our results may not be generalizable to the broader U.S. population. Nonetheless, we do not anticipate that the sample size or the heterogeneity of our study samples will impact the validity of the comparisons made herein. Moreover, the limited data available for the participants in this study limited our ability to account for a range of other potential confounding variables. Additional research in a larger population with prospective data collection is needed to confirm these findings. While we assessed 49 SVOCs herein, with 36 detected in >60% of wristband extracts, it is likely that there are hundreds to thousands of chemicals that we are exposed to daily. For example, household dust is estimated to contain thousands of chemicals.110,111 As such, it is likely that there are other active constituents that are yet to be identified and measured, and future research should evaluate other potential contributory contaminants via both target and nontargeted analytical methods. A larger cohort would also support the use of a mixture model approach, which might be more informative than examining associations with individual chemicals present in the mixture. It is also possible that some associations observed here are due to co-occurring contaminants or mixture effects, which should be evaluated in future studies. We also appreciate the potential limited application of a direct TR binding screen, particularly given the small ligand binding domain of TRβ relative to other nuclear receptors.112 Previous work has described limited direct TR binding in the Tox21 library,113 though this analysis had less success in evaluating TR binding related to antagonism. These high throughput screens are often limited in species and tissue diversity, potentially limiting their generalizability. However, it should be noted that diverse mechanisms of TR disruption can contribute to thyroid cancer development and progression, as noted above50 and that despite these limitations, we report significant antagonistic effects in our in vitro model.
In closing, this study is the first to demonstrate the potential to measure nuclear receptor bioactivities in mixtures isolated from silicone wristbands. Wristbands provide a completely noninvasive and comprehensive marker for exposure to environmental contaminants present in the home, work, and outdoor environments. Trends observed in TR antagonism are similar to what we observed in our previous study using house dust. However, wristbands may be a better sampling tool, as they help account for exposures across multiple environments (encompassing home, work, and outside life), allow for a greater diversity of bioassay and analytical measurement testing, and are amenable to citizen science projects (stable at room temperature and/or mail back to the study laboratory).9 These passive samplers have been demonstrated to capture both dermal and inhalation exposures10 and reflect significant correlations with internal biomarkers of exposure for a range of contaminant classes. While we have described the ability to utilize wristband extracts to measure TR antagonism, this extraction method and analysis protocol could be broadly applicable to various receptor-based tests and should be explored further in future research. We also report significant associations between concentrations of specific semivolatile chemicals on wristbands and the odds of concurrent PTC and trends between wristband TR antagonism and PTC. These results provide support for the role of TR antagonists in the development and/or progression of PTC. Given the promising literature associating bioactivities derived from human tissues or biospecimens with various human health conditions,114–117 using these external noninvasive samplers may present new opportunities to assess potential relationships between contaminant mixtures and human health..
Supplementary Material
Funding
This research project was primarily supported by a pilot grant from the Duke Cancer Institute. Additional support was provided by a grant [R01 ES016099] and an award [K99 ES030405] from the National Institute of Environmental Health Sciences.
Footnotes
The authors declare the following competing financial interest(s): JAS is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by GlaxoSmithKline, Novo Nordisk, Astra Zeneca and Eli Lilly. She receives institutional research funding from Exelixis and Eli Lilly.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.0c05972.
Positive control agonist and antagonist dose responses; comparison of extraction solvent recoveries for SVOCs; inhibited cell viability regressions by chemical concentrations; inhibited cell viability regressions by chemical concentrations; surrogate standards used for analytical assessments; and method detection limits for target analytes (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.est.0c05972
Contributor Information
Christopher D. Kassotis, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States
Nicholas J. Herkert, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States
Stephanie C. Hammel, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States
Kate Hoffman, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States.
Qianyi Xia, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States.
Seth W. Kullman, Toxicology Program, North Carolina State University, Raleigh, North Carolina 27695, United States
Julie Ann Sosa, Department of Surgery, University of California at San Francisco, San Francisco, California 94143, United States.
Heather M. Stapleton, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States.
REFERENCES
- (1).O’Connell SG; Kincl LD; Anderson KA Silicone wristbands as personal passive samplers. Environ. Sci. Technol 2014, 48, 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Romanak KA; Wang S; Stubbings WA; Hendryx M; Venier M; Salamova A Analysis of brominated and chlorinated flame retardants, organophosphate esters, and polycyclic aromatic hydrocarbons in silicone wristbands used as personal passive samplers. J. Chromatogr. A 2019, 1588, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dixon HM; Scott RP; Holmes D; Calero L; Kincl LD; Waters KM; Camann DE; Calafat AM; Herbstman JB; Anderson KA Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem 2018, 410, 3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hammel SC; Phillips AL; Hoffman K; Stapleton HM Evaluating the Use of Silicone Wristbands To Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol 2018, 52, 11875–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hammel SC; Hoffman K; Webster TF; Anderson KA; Stapleton HM Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol 2016, 50, 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Harley KG; Parra KL; Camacho J; Bradman A; Nolan JES; Lessard C; Anderson KA; Poutasse CM; Scott RP; Lazaro G; Cardoso E; Gallardo D; Gunier RB Determinants of pesticide concentrations in silicone wristbands worn by Latina adolescent girls in a California farmworker community: The COSECHA youth participatory action study. Sci. Total Environ 2019, 652, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bergmann AJ; North PE; Vasquez L; Bello H; del Carmen Gastañaga Ruiz M; Anderson KA Multi-class chemical exposure in rural Peru using silicone wristbands. J. Expo. Sci. Environ. Epidemiol 2017, 27, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Donald CE; Scott RP; Blaustein KL; Halbleib ML; Sarr M; Jepson PC; Anderson KA Silicone wristbands detect individuals’ pesticide exposures in West Africa. R. Soc. Open Sci 2016, 3, 160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Anderson KA; Points GL III; Donald CE; Dixon HM; Scott RP; Wilson G; Tidwell LG; Hoffman PD; Herbstman JB; O’Connell SG Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Expo. Sci. Environ. Epidemiol 2017, 27, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wang S; Romanak KA; Stubbings WA; Arrandale VH; Hendryx M; Diamond ML; Salamova A; Venier M Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs). Environ. Int 2019, 132, 105104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Aerts R; Joly L; Szternfeld P; Tsilikas K; De Cremer K; Castelain P; Aerts J-M; Van Orshoven J; Somers B; Hendrickx M; Andjelkovic M; Van Nieuwenhuyse A Silicone Wristband Passive Samplers Yield Highly Individualized Pesticide Residue Exposure Profiles. Environ. Sci. Technol 2018, 52, 298–307. [DOI] [PubMed] [Google Scholar]
- (12).Hammel SC; Hoffman K; Phillips AL; Levasseur JL; Lorenzo AM; Webster TF; Stapleton HM Comparing the use of silicone wristbands, hand wipes, and dust to evaluate children’s exposure to flame retardants and plasticizers. Environ. Sci. Technol 2020, 54, 4484, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Dixon HM; Armstrong G; Barton M; Bergmann AJ; Bondy M; Halbleib ML; Hamilton W; Haynes E; Herbstman J; Hoffman P; Jepson P; Kile ML; Kincl L; Laurienti PJ; North P; Paulik LB; Petrosino J; Points GL III; Poutasse CM; Rohlman D; Scott RP; Smith B; Tidwell LG; Walker C; Waters KM; Anderson KA Discovery of common chemical exposures across three continents using silicone wristbands. R. Soc. Open Sci 2019, 6, 181836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kile ML; Scott RP; O’Connell SG; Lipscomb S; MacDonald M; McClelland M; Anderson KA Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res 2016, 147, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Poutasse CM; Herbstman JB; Peterson ME; Gordon J; Soboroff PH; Holmes D; Gonzalez D; Tidwell LG; Anderson KA Silicone Pet Tags Associate Tris(1,3-dichloro-2-isopropyl) Phosphate Exposures with Feline Hyperthyroidism. Environ. Sci. Technol 2019, 53, 9203–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wise CF; Hammel SC; Herkert N; Ma J; Motsinger-Reif A; Stapleton HM; Breen M Comparative exposure assessment using silicone passive samplers indicates domestic dogs are sentinels to support human health research. Environ. Sci. Technol 2020, 54, 7409–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Guillette LJ Jr.; Gross TS; Masson GR; Matter JM; Percival HF; Woodward AR Developmental Abnormalities of the Gonad and Abnormal Sex Hormone Concentrations in Juvenile Alligators from Contaminated and Control Lakes in Florida. Environ. Health Perspect 1994, 102, 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Ottinger MA; Lavoie E; Thompson N; Barton A; Whitehouse K; Barton M; Abdelnabi M; Quinn M; Panzica G; Viglietti-Panzica C Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res. Rev 2008, 57, 376–385. [DOI] [PubMed] [Google Scholar]
- (19).Vandenberg LN; Colborn T; Hayes TB; Heindel JJ; Jacobs DR Jr.; Lee D-H; Shioda T; Soto AM; Vom Saal FS; Welshons WV; Zoeller RT; Myers JP Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev 2012, 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Vandenberg LN Low-dose effects of hormones and endocrine disruptors. Vitam. Horm 2014, 94, 129–165. [DOI] [PubMed] [Google Scholar]
- (21).Welshons WV; Thayer KA; Judy BM; Taylor JA; Curran EM; vom Saal FS Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect 2003, 111, 994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).vom Saal FS; Akingbemi BT; Belcher SM; Birnbaum LS; Crain DA; Eriksen M; Farabollini F; Guillette LJ Jr.; Hauser R; Heindel JJ; Ho S-M; Hunt PA; Iguchi T; Jobling S; Kanno J; Keri RA; Knudsen KE; Laufer H; LeBlanc GA; Marcus M; McLachlan JA; Myers JP; Nadal A; Newbold RR; Olea N; Prins GS; Richter CA; Rubin BS; Sonnenschein C; Soto AM; Talsness CE; Vandenbergh JG; Vandenberg LN; Walser-Kuntz DR; Watson CS; Welshons WV; Wetherill Y; Zoeller RT Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol 2007, 24, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Diamanti-Kandarakis E; Bourguignon J-P; Giudice LC; Hauser R; Prins GS; Soto AM; Zoeller RT; Gore AC Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev 2009, 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gore AC; Chappell VA; Fenton SE; Flaws JA; Nadal A; Prins GS; Toppari J; Zoeller RT EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev 2015, 36, E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Bhandari RK; Deem SL; Holliday DK; Jandegian CM; Kassotis CD; Nagel SC; Tillitt DE; Vom Saal FS; Rosenfeld CS Effects of the Environmental Estrogenic Contaminants Bisphenol A and 17a-ethinylestradiol on Sexual Development and Adult Behaviors in Aquatic Wildlife Species. Gen. Comp. Endocrinol 2015, 214, 195–219. [DOI] [PubMed] [Google Scholar]
- (26).Shi W; Zhang F-X; Hu G-J; Hao Y-Q; Zhang X-W; Liu H-L; Wei S; Wang X-R; Giesy JP; Yu H-X Thyroid hormone disrupting activities associated with phthalate esters in water sources from Yangtze River Delta. Environ. Int 2012, 42, 117–123. [DOI] [PubMed] [Google Scholar]
- (27).Jugan ML; Oziol L; Bimbot M; Huteau V; Tamisier-Karolak S; Blondeau JP; Lévi Y In vitro assessment of thyroid and estrogenic endocrine disruptors in wastewater treatment plants, rivers and drinking water supplies in the greater Paris area (France). Sci. Total Environ 2009, 407, 3579–3587. [DOI] [PubMed] [Google Scholar]
- (28).Boas M; Feldt-Rasmussen U; Main KM Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol 2012, 355, 240–248. [DOI] [PubMed] [Google Scholar]
- (29).Crofton KM Thyroid disrupting chemicals: mechanisms and mixtures. Int. J. Androl 2008, 31, 209–223. [DOI] [PubMed] [Google Scholar]
- (30).Shi W; Hu X; Zhang F; Hu G; Hao Y; Zhang X; Liu H; Wei S; Wang X; Giesy JP; Yu H Occurrence of thyroid hormone activities in drinking water from eastern China: contributions of phthalate esters. Environ. Sci. Technol 2012, 46, 1811–1818. [DOI] [PubMed] [Google Scholar]
- (31).Chen C-H; Chou P-H; Kawanishi M; Yagi T Occurrence of xenobiotic ligands for retinoid X receptors and thyroid hormone receptors in the aquatic environment of Taiwan. Mar. Pollut. Bull 2014, 85, 613–618. [DOI] [PubMed] [Google Scholar]
- (32).Shi W; Wang X; Hu W; Sun H; Shen O; Liu H; Wang X; Giesy JP; Cheng S; Yu H Endocrine-disrupting equivalents in industrial effluents discharged into Yangtze River. Ecotoxicology 2009, 18, 685–692. [DOI] [PubMed] [Google Scholar]
- (33).Kassotis CD; Vu DC; Vo PH; Lin C-H; Cornelius-Green JN; Patton S; Nagel SC Endocrine Disrupting Activities and Organic Contaminants Associated with Oil and Gas Operations in Wyoming Groundwater. Arch. Environ. Contam. Toxicol 2018, 75, 247–258. [DOI] [PubMed] [Google Scholar]
- (34).Kassotis CD; Iwanowicz LR; Akob DM; Cozzarelli IM; Mumford AC; Orem WH; Nagel SC Endocrine disrupting activities of surface water associated with a West Virginia oil and gas industry wastewater disposal site. Sci. Total Environ 2016, 557–558, 901–910. [DOI] [PubMed] [Google Scholar]
- (35).Kassotis CD; Klemp KC; Vu DC; Lin C-H; Meng C-X; Besch-Williford CL; Pinatti L; Zoeller RT; Drobnis EZ; Balise VD; Isiguzo CJ; Williams MA; Tillitt DE; Nagel SC Endocrine-Disrupting Activity of Hydraulic Fracturing Chemicals and Adverse Health Outcomes After Prenatal Exposure in Male Mice. Endocrinology 2015, 156, 4458–4473. [DOI] [PubMed] [Google Scholar]
- (36).Kassotis CD; Hoffman K; Phillips AL; Zhang S; Webster TF; Stapleton HM, Characterization of Adipogenic, PPARy, and TRb Activities in House Dust Extracts and Their Associations with Organic Contaminants. 2020, DOI: 10.1021/acs.est.7b01788.s001, Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kollitz EM; Kassotis CD; Hoffman K; Ferguson PL; Sosa JA; Stapleton HM Chemical mixtures isolated from house dust disrupt thyroid receptor β (TRβ) signaling. Environ. Sci. Technol 2018, 52, 11857–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zoeller RT Environmental chemicals impacting the thyroid: targets and consequences. Thyroid 2007, 17, 811–817. [DOI] [PubMed] [Google Scholar]
- (39).Blanck HM; Marcus M; Tolbert PE; Rubin C; Henderson AK; Hertzberg VS; Zhang RH; Cameron L Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology 2000, 11, 641–647. [DOI] [PubMed] [Google Scholar]
- (40).Colborn T Clues from wildlife to create an assay for thyroid system disruption. Environ. Health Perspect 2002, 110, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Lu C; Cheng S-Y Thyroid hormone receptors regulate adipogenesis and carcinogenesis via crosstalk signaling with peroxisome proliferator-activated receptors. J. Mol. Endocrinol 2010, 44, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kassotis CD; Masse L; Kim S; Schlezinger JJ; Webster TF; Stapleton HM Characterization of adipogenic chemicals in three different cell culture systems: implications for reproducibility based on cell source and handling. Sci. Rep 2017, 7, 42104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Bryzgalova G; Effendic S; Khan A; Rehnmark S; Barbounis P; Boulet J; Dong G; Singh R; Shapses S; Malm J; Webb P; Baxter JD; Grover GJ Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. J. Steroid Biochem. Mol. Biol 2008, 111, 262–267. [DOI] [PubMed] [Google Scholar]
- (44).Obregon M-J Thyroid Hormone and Adipocyte Differentiation. Thyroid 2008, 18, 185–195. [DOI] [PubMed] [Google Scholar]
- (45).Hoffman K; Sosa JA; Stapleton HM Do flame retardant chemicals increase the risk for thyroid dysregulation and cancer? Curr. Opin. Oncol 2017, 29, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Marotta V; Malandrino P; Russo M; Panariello I; Ionna F; Chiofalo MG; Pezzullo L Fathoming the link between anthropogenic chemical contamination and thyroid cancer. Crit. Rev. Oncol. Hematol 2020, 150, 02950, In press [DOI] [PubMed] [Google Scholar]
- (47).Lim H; Devesa SS; Sosa JA; Check D; Kitahara CM Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).American Cancer Society. Cancer Statistics Center. Available at: https://cancerstatisticscenter.cancer.org/?_ga=2.243837317.1823865298.1588107384-1945183083.1588107384#!/ [accessed April 28, 2020].
- (49).James BC; Mitchell JM; Jeon HD; Vasilottos N; Grogan RH; Aschebrook-Kilfoy B An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control 2018, 29, 465–473. [DOI] [PubMed] [Google Scholar]
- (50).Tang Z; Zhang J; Zhou Q; Xu S; Cai Z; Jiang G Thyroid Cancer ″Epidemic″: A Socio-Environmental Health Problem Needs Collaborative Efforts. Environ. Sci. Technol 2020, 54, 3725–3727. [DOI] [PubMed] [Google Scholar]
- (51).Fujitani T; Soleman SR; Harada KH; Kobayashi H Comment on, ″Thyroid Cancer ″Epidemic″: A Socio-Environmental Health Problem Needs Collaborative Efforts″. Environ. Sci. Technol 2020, 54, 9713. [DOI] [PubMed] [Google Scholar]
- (52).Tang Z; Zhang J; Zhou Q; Xu S; Cai Z; Jiang G Response to Comments on ″Thyroid Cancer ‘Epidemic’: A Socio-Environmental Health Problem Needs Collaborative Efforts″. Environ. Sci. Technol 2020, 54, 9711. [DOI] [PubMed] [Google Scholar]
- (53).Kazakov VS; Demidchik EP; Astakhova LN Thyroid cancer after Chernobyl. Nature 1992, 359, 21. [DOI] [PubMed] [Google Scholar]
- (54).Yamashita S; Suzuki S; Suzuki S; Shimura H; Saenko V Lessons from Fukushima: Latest Findings of Thyroid Cancer After the Fukushima Nuclear Power Plant Accident. Thyroid 2018, 28, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Vigneri R; Malandrino P; Gianì F; Russo M; Vigneri P Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell. Endocrinol 2017, 457, 73–80. [DOI] [PubMed] [Google Scholar]
- (56).Benedetti M; Zona A; Beccaloni E; Carere M; Comba P Incidence of Breast, Prostate, Testicular, and Thyroid Cancer in Italian Contaminated Sites with Presence of Substances with Endocrine Disrupting Properties. Int. J. Environ. Res. Publ. Health 2017, 14, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Hiasa Y; Kitahori Y; Kato Y; Ohshima M; Konishi N; Shimoyama T; Sakaguchi Y; Hashimoto H; Minami S; Murata Y Potassium perchlorate, potassium iodide, and propylthiouracil: promoting effect on the development of thyroid tumors in rats treated with N-bis(2-hydroxypropyl)-nitrosamine. Jpn. J. Cancer Res 1987, 78, 1335–1340. [PubMed] [Google Scholar]
- (58).Zhang L; Fang C; Liu L; Liu X; Fan S; Li J; Zhao Y; Ni S; Liu S; Wu Y A case-control study of urinary levels of iodine, perchlorate and thiocyanate and risk of papillary thyroid cancer. Environ. Int 2018, 120, 388–393. [DOI] [PubMed] [Google Scholar]
- (59).Kitahori Y; Hiasa Y; Konishi N; Enoki N; Shimoyama T; Miyashiro A Effect of propylthiouracil on the thyroid tumorigenesis induced by N-bis(2-hydroxypropyl)nitrosamine in rats. Carcinogenesis 1984, 5, 657–660. [DOI] [PubMed] [Google Scholar]
- (60).Hood A; Liu J; Klaassen CD Effects of phenobarbital, pregnenolone-16alpha-carbonitrile, and propylthiouracil on thyroid follicular cell proliferation. Toxicol. Sci 1999, 50, 45–53. [DOI] [PubMed] [Google Scholar]
- (61).Redmond O; Tuffery AR Thyroid proliferation, body weight, thyrotropin and thyroid hormones in chronic antithyroid (carbimazole) treatment in rats. J. Anat 1981, 133, 37–47. [PMC free article] [PubMed] [Google Scholar]
- (62).Leux C; Guénel P Risk factors of thyroid tumors: role of environmental and occupational exposures to chemical pollutants. Rev. Epidemiol. Sante Publique 2010, 58, 359–367. [DOI] [PubMed] [Google Scholar]
- (63).Garber J; Cobin R; Gharib H; Hennessey J; Klein I; Mechanick J; Pessah-Pollack R; Singer P; Woeber K Clinical Practice Guidelines for Hypothyroidism in Adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract 2012, 18, 988–1028. [DOI] [PubMed] [Google Scholar]
- (64).McGrath N; Hawkes CP; McDonnell CM; Cody D; O’Connell SM; Mayne PD; Murphy NP Incidence of Congenital Hypothyroidism Over 37 Years in Ireland. Pediatrics 2018, 142, e20181199. [DOI] [PubMed] [Google Scholar]
- (65).Barry Y; Bonaldi C; Goulet V; Coutant R; Léger J; Paty A-C; Delmas D; Cheillan D; Roussey M Increased incidence of congenital hypothyroidism in France from 1982 to 2012: a nationwide multicenter analysis. Ann. Epidemiol 2016, 26, 100–105. [DOI] [PubMed] [Google Scholar]
- (66).Hoffman K; Lorenzo A; Butt CM; Hammel SC; Henderson BB; Roman SA; Scheri RP; Stapleton HM; Sosa JA Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: A case-control study. Environ. Int 2017, 107, 235–242. [DOI] [PubMed] [Google Scholar]
- (67).Kassotis CD; Tillitt DE; Davis JW; Hormann AM; Nagel SC Estrogen and Androgen Receptor Activities of Hydraulic Fracturing Chemicals and Surface and Ground Water in a Drilling-Dense Region. Endocrinology 2014, 155, 897–907. [DOI] [PubMed] [Google Scholar]
- (68).Kassotis CD; Hoffman K; Stapleton HM Characterization of Adipogenic Activity of House Dust Extracts and Semi-Volatile Indoor Contaminants in 3T3-L1 Cells. Environ. Sci. Technol 2017, 51, 8735–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Xu L; Port M; Landi S; Gemignani F; Cipollini M; Elisei R; Goudeva L; Müller JA; Nerlich K; Pellegrini G; Reiners C; Romei C; Schwab R; Abend M; Sturgis EM Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid 2014, 24, 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Altekruse S; Das A; Cho H; Petkov V; Yu M Do US thyroid cancer incidence rates increase with socioeconomic status among people with health insurance? An observational study using SEER population-based data. BMJ Open 2015, 5, No. e009843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Weeks KS; Kahl AR; Lynch CF; Charlton ME Racial/ethnic differences in thyroid cancer incidence in the United States, 2007–2014. Cancer 2018, 124, 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Wirnitzer U; Rickenbacher U; Katerkamp A; Schachtrupp A Systemic toxicity of di-2-ethylhexyl terephthalate (DEHT) in rodents following four weeks of intravenous exposure. Toxicol. Lett 2011, 205, 8–14. [DOI] [PubMed] [Google Scholar]
- (73).Shen O; Du G; Sun H; Wu W; Jiang Y; Song L; Wang X Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol. Lett 2009, 191, 9–14. [DOI] [PubMed] [Google Scholar]
- (74).Shi W; Wei S; Hu X.-x. Hu G.-j.; Chen C.-l.; Wang X.-r.; Giesy JP; Yu H.-x. Identification of thyroid receptor ant/agonists in water sources using mass balance analysis and monte carlo simulation. PloS One 2013, 8, No. e73883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).ter Veld MGR; Schouten B; Louisse J; van Es DS; van der Saag PT; Rietjens IMCM; Murk AJ Estrogenic potency of food-packaging-associated plasticizers and antioxidants as detected in ERalpha and ERbeta reporter gene cell lines. J. Agric. Food Chem 2006, 54, 4407–4416. [DOI] [PubMed] [Google Scholar]
- (76).Sheikh IA; Yasir M; Abu-Elmagd M; Dar TA; Abuzenadah AM; Damanhouri GA; Al-Qahtani M; Beg MA Human sex hormone-binding globulin as a potential target of alternate plasticizers: an in silico study. BMC Struct. Biol 2016, 16, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Kojima H; Takeuchi S; Itoh T; Iida M; Kobayashi S; Yoshida T In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 2013, 314, 76–83. [DOI] [PubMed] [Google Scholar]
- (78).Ren X; Cao L; Yang Y; Wan B; Wang S; Guo L In vitro assessment of thyroid hormone receptor activity of four organophosphate esters. J. Environ. Sci 2016, 45, 185–190. [DOI] [PubMed] [Google Scholar]
- (79).Klopcič I; Skledar DG; Masic f; Dolenc MS Comparison of in vitro hormone activities of novel flame retardants TBB, TBPH and their metabolites TBBA and TBMEPH using reporter gene assays. Chemosphere 2016, 160, 244–251. [DOI] [PubMed] [Google Scholar]
- (80).Wang Q; Liang K; Liu J; Yang L; Guo Y; Liu C; Zhou B Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat. Toxicol 2013, 126, 207–213. [DOI] [PubMed] [Google Scholar]
- (81).Song J TDCPP exposure affects the concentrations of thyroid hormones in zebrafish. 2017, bioRxiv: 10.1101/146092. [DOI] [Google Scholar]
- (82).Liu X; Cai Y; Wang Y; Xu S; Ji K; Choi K Effects of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish. Ecotoxicol. Environ. Saf 2019, 170, 25–32. [DOI] [PubMed] [Google Scholar]
- (83).Farhat A; Crump D; Chiu S; Williams KL; Letcher RJ; Gauthier LT; Kennedy SW In Ovo effects of two organophosphate flame retardants-TCPP and TDCPP-on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol. Sci 2013, 134, 92–102. [DOI] [PubMed] [Google Scholar]
- (84).Zhao F; Wang J; Fang Y; Ding J; Yang H; Li L; Xi Z; Qiao H Effects of tris(1,3-dichloro-2-propyl)phosphate on pathomorphology and gene/protein expression related to thyroid disruption in rats. Toxicol. Res 2016, 5, 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Porter E; Crump D; Egloff C; Chiu S; Kennedy SW Use of an avian hepatocyte assay and the avian Toxchip Polymerse chain reaction array for testing prioritization of 16 organic flame retardants. Environ. Toxicol. Chem 2014, 33, 573–582. [DOI] [PubMed] [Google Scholar]
- (86).Guo L-C; Xiao J; Zhang Y; Yu S; Lin H; Su G; Liu T; Li X; Lv S; Rutherford S; Ma W Association between serum polybrominated diphenyl ethers, new flame retardants and thyroid hormone levels for school students near a petrochemical complex, South China. Chemosphere 2018, 202, 476–482. [DOI] [PubMed] [Google Scholar]
- (87).Johnson PI; Stapleton HM; Mukherjee B; Hauser R; Meeker JD Associations between brominated flame retardants in house dust and hormone levels in men. Sci. Total Environ 2013, 445–446, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Springer C; Dere E; Hall SJ; McDonnell EV; Roberts SC; Butt CM; Stapleton HM; Watkins DJ; McClean MD; Webster TF; Schlezinger JJ; Boekelheide K Rodent thyroid, liver, and fetal testis toxicity of the monoester metabolite of bis-(2-ethylhexyl) tetrabromophthalate (tbph), a novel brominated flame retardant present in indoor dust. Environ. Health Perspect 2012, 120, 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Joeun J; Choi K Effects of alternative flame retardants, TBB and TBPH on thyroid hormone system in zebrafish (Danio rerio) larvae and GH3 and FRTL-5 cell lines. Master’s Thesis, Seoul National University, 2015. [Google Scholar]
- (90).Kim S; Park G-Y; Yoo YJ; Jeong JS; Nam KT; Jee S-H; Lim K-M; Lee Y-S Di-2-ethylhexylphthalate promotes thyroid cell proliferation and DNA damage through activating thyrotropin-receptor-mediated pathways in vitro and in vivo. Food Chem. Toxicol 2019, 124, 265–272. [DOI] [PubMed] [Google Scholar]
- (91).Benjamin S; Masai E; Kamimura N; Takahashi K; Anderson RC; Faisal PA Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard Mater 2017, 340, 360–383. [DOI] [PubMed] [Google Scholar]
- (92).Liu C; Deng Y-L; Zheng T-Z; Yang P; Jiang X-Q; Liu E-N; Miao X-P; Wang L-Q; Jiang M; Zeng Q Urinary biomarkers of phthalates exposure and risks of thyroid cancer and benign nodule. J. Hazard Mater 2020, 383, 121189. [DOI] [PubMed] [Google Scholar]
- (93).Miao H; Liu X; Li J; Zhang L; Zhao Y; Liu S; Ni S; Wu Y Associations of urinary phthalate metabolites with risk of papillary thyroid cancer. Chemosphere 2020, 241, 125093. [DOI] [PubMed] [Google Scholar]
- (94).Deyo JA Carcinogenicity and chronic toxicity of di-2-ethylhexyl terephthalate (DEHT) following a 2-year dietary exposure in Fischer 344 rats. Food Chem. Toxicol 2008, 46, 990–1005. [DOI] [PubMed] [Google Scholar]
- (95).Duan J; Deng T; Kang J; Chen M DINP aggravates autoimmune thyroid disease through activation of the Akt/mTOR pathway and suppression of autophagy in Wistar rats. Environ. Pollut 2019, 245, 316–324. [DOI] [PubMed] [Google Scholar]
- (96).Deziel NC; Yi H; Stapleton HM; Huang H; Zhao N; Zhang Y A case-control study of exposure to organophosphate flame retardants and risk of thyroid cancer in women. BMC Cancer 2018, 18, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Aschebrook-Kilfoy B; DellaValle CT; Purdue M; Kim C; Zhang Y; Sjodin A; Ward MH Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am. J. Epidemiol 2015, 181, 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Deziel NC; Alfonso-Garrido J; Warren JL; Huang H; Sjodin A; Zhang Y Exposure to Polybrominated Diphenyl Ethers and a Polybrominated Biphenyl and Risk of Thyroid Cancer in Women: Single and Multi-Pollutant Approaches. Cancer Epidemiol. Biomark. Prev 2019, 28, 1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Cheng S-Y; Leonard JL; Davis PJ Molecular aspects of thyroid hormone actions. Endocr. Rev 2010, 31, 139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Suzuki H; Willingham MC; Cheng S -y. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 2002, 12, 963–969. [DOI] [PubMed] [Google Scholar]
- (101).Kato Y; Ying H; Willingham MC; Cheng S-Y A tumor suppressor role for thyroid hormone beta receptor in a mouse model of thyroid carcinogenesis. Endocrinology 2004, 145, 4430–4438. [DOI] [PubMed] [Google Scholar]
- (102).Ying H; Suzuki H; Zhao L; Willingham MC; Meltzer P; Cheng SY Mutant thyroid hormone receptor beta represses the expression and transcriptional activity of peroxisome proliferator-activated receptor gamma during thyroid carcinogenesis. Cancer Res 2003, 63, 5274–5280. [PubMed] [Google Scholar]
- (103).Lu C; Zhao L; Ying H; Willingham MC; Cheng S.-y. Growth activation alone is not sufficient to cause metastatic thyroid cancer in a mouse model of follicular thyroid carcinoma. Endocrinology 2010, 151, 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Kato Y; Ying H; Zhao L; Furuya F; Araki O; Willingham MC; Cheng S -y. PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene 2006, 25, 2736–2747. [DOI] [PubMed] [Google Scholar]
- (105).Kim WG; Cheng S.-y. Mechanisms Linking Obesity and Thyroid Cancer Development and Progression in Mouse Models. Horm. Cancer 2018, 9, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Chou P-H; Lee C-H; Ko F-C; Lin Y-J; Kawanishi M; Yagi T; Li I-C Detection of Hormone-Like and Genotoxic Activities in Indoor Dust from Taiwan Using a Battery of in Vitro Bioassays. Aerosol Air Qual. Res 2015, 15, 1412–1421. [Google Scholar]
- (107).Reddam A; Tait G; Herkert N; Hammel SC; Stapleton HM; Volz DC Longer commutes are associated with increased human exposure to tris(1,3-dichloro-2-propyl) phosphate. Environ. Int 2020, 136, 105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Gold M; Blum A; Ames B Another flame retardant, tris-(1,3-dichloro-2-propyl)-phosphate, and its expected metabolites are mutagens. Science 1978, 200, 785–787. [DOI] [PubMed] [Google Scholar]
- (109).Stapleton HM; Klosterhaus S; Eagle S; Fuh J; Meeker JD; Blum A; Webster TF Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol 2009, 43, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Ferguson PL; Vogler B; Stapleton HM Non-targeted analysis to assess human exposure to semi-volatile organic contaminants in the indoor environment. Proceedings of the 63rd ASMS Conference on Mass Spectrometry and Allied Topics: St. Louis, MO, 2015, 31 May–4 June, 31 May–4 June, 2015. [Google Scholar]
- (111).Hilton DC; Jones RS; Sjödin A A method for rapid, nontargeted screening for environmental contaminants in household dust. J. Chromatogr. A 2010, 1217, 6851–6856. [DOI] [PubMed] [Google Scholar]
- (112).Gallastegui N; Mackinnon JAG; Fletterick RJ; Estébanez-Perpiñá E Advances in our structural understanding of orphan nuclear receptors. Trends Biochem. Sci 2015, 40, 25–35. [DOI] [PubMed] [Google Scholar]
- (113).Paul-Friedman K; Martin M; Crofton KM; Hsu C-W; Sakamuru S; Zhao J; Xia M; Huang R; Stavreva DA; Soni V; Varticovski L; Raziuddin R; Hager GL; Houck KA Limited Chemical Structural Diversity Found to Modulate Thyroid Hormone Receptor in the Tox21 Chemical Library. Environ. Health Perspect 2019, 127, 097009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Arrebola JP; Pumarega J; Gasull M; Fernandez MF; Martin-Olmedo P; Molina-Molina JM; Fernández-Rodríguez M; Porta M; Olea N Adipose tissue concentrations of persistent organic pollutants and prevalence of type 2 diabetes in adults from Southern Spain. Environ. Res 2013, 122, 31–37. [DOI] [PubMed] [Google Scholar]
- (115).ibarluzea j. m.; Fernandez MF; Fernández L; Serrano MF; Rivas AM; Aurrekoetxea JJ; Expósito; Lorenzo M; Torné P; Villalobos M; Pedraza V; Sasco AJ; Olea N Breast cancer risk and the combined effect of environmental estrogens. Cancer Causes Control 2004, 15, 591–600. [DOI] [PubMed] [Google Scholar]
- (116).Fernandez MF; Pumarega J; Porta M; Molina-Molina JM; Arrebola JP; Olea N Changes in the total effective xenoestrogen burden (TEXB) of breast cancer patients during an 18-month post-surgical follow-up. Reprod. Toxicol 2017, 69, 212–220. [DOI] [PubMed] [Google Scholar]
- (117).Pastor-Barriuso R; Fernandez MF; Castaño-Vinyals G; Whelan D; Pérez-Gómez B; Llorca J; Villanueva CM; Guevara M; Molina-Molina J-M; Artacho-Cordón F; Barriuso-Lapresa L; Tusquets I; Dierssen-Sotos T; Aragonés N; Olea N; Kogevinas M; Pollán M Total Effective Xenoestrogen Burden in Serum Samples and Risk for Breast Cancer in a Population-Based Multicase-Control Study in Spain. Environ. Health Perspect 2016, 124, 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


