Abstract
Background: Transthyretin amyloid cardiomyopathy is a progressive disease with a poor prognosis. There had been no specific treatment for transthyretin amyloid cardiomyopathy until tafamidis received expanded approval in March 2019 in Japan. However, the clinical efficacy of tafamidis remains unknown.
Methods and Results: We initiated tafamidis treatment in 9 patients (median age 78 years; 89% male) from May 2019 to April 2020. Within 6 months after initiation, 1 patient discontinued prematurely and 2 patients were hospitalized due to worsening heart failure, with 1 of these patients discontinuing therapy. There were no significant changes in plasma B-type natriuretic peptide and serum troponin I concentrations over the 6-month treatment period, but interventricular septum thickness increased in 3 of 6 patients.
Conclusions: Further evaluation of tafamidis therapy in a larger patient cohort with transthyretin amyloid cardiomyopathy is warranted to determine the optimal therapeutic strategy.
Key Words: Atrial fibrillation, Cardiac hypertrophy, Congestive heart failure
Survival has improved for patients with heart failure with reduced ejection fraction (EF) owing to guideline-directed medical therapy, including the use of angiotensin-converting enzyme inhibitors, β-blockers, and mineralocorticoid receptor antagonists, whereas the prognosis of patients with heart failure with preserved EF (HFpEF) remains unsatisfactory thus far. Among cases of HFpEF, there are many occult wild-type transthyretin amyloid cardiomyopathies, which, in some cases, are not correctly diagnosed1 and, in other cases, undergo aortic valve replacement.2
Tafamidis (Pfizer, New York, NY, USA) binds to the thyroxine-binding site of transthyretin and stabilizes transthyretin tetramers.3 In the Safety and Efficacy of Tafamidis in Patients With Transthyretin Cardiomyopathy (ATTR-ACT) trial, tafamidis reduced all-cause mortality and cardiovascular hospitalization compared with placebo.4 Of note, patients with transthyretin amyloid cardiomyopathy and New York Heart Association (NYHA) Class III had a higher rate of cardiovascular-related hospitalizations than patients with NYHA Class I–II.4 In that study, a reduction of interventricular septal wall thickness was not observed and the efficacy of tafamidis in treating conductance disturbances was not investigated, with patients with pacemakers excluded from the trial.4 The cost-effectiveness of tafamidis was reported to be 0% of 10,000 probabilistic simulations in the US.5 However, the clinical implication of tafamidis therapy in real-world practice remains unknown.
In the present study we investigated the clinical features of transthyretin amyloid cardiomyopathy treated by tafamidis and discuss appropriate patient selection.
Methods
Patient Selection
Nine consecutive patients with advanced heart failure who were diagnosed as having transthyretin amyloid cardiomyopathy by the presence of amyloid deposits with transthyretin in the myocardium (wild-type, n=8; Val30Met mutation of the transthyretin [TTR] gene, n=1) were prospectively enrolled in this study. All patients received 80 mg tafamidis daily (at a cost of JPY 5,240,640 per month) and were followed-up between May 2019 and April 2020.
Written informed consent was obtained from all participants before study enrolment. This study was approved by the Clinical Research Review Board, University of Toyama (IRB no. R2019166).
Management of Heart Failure
All patients received guideline-directed medical therapy in addition to tafamidis treatment. If a patient without a pacemaker complained of bradycardia, β-blockers were reduced or discontinued with a priority on maintaining heart rate.
Baseline Characteristics
Data on baseline demographic characteristics, including NYHA classifications, the number of previous heart failure hospitalizations, and the duration between heart failure diagnosis and tafamidis administration, were collected.
Measurements of Biomarkers
Plasma B-type natriuretic peptide (BNP), serum N-terminal pro BNP (NT-proBNP), and serum troponin I concentrations were measured just before starting tafamidis and after 6 months treatment. Plasma BNP and serum troponin I concentrations were measured using a commercially available assay (Abbott Japan, Matsudo, Japan). Serum NT-proBNP concentrations were determined using the Elecsys NT-proBNP immunoassay (Roche Diagnostics, Rotkreuz, Switzerland).
Measurements of Electrocardiographic and Echocardiographic Data
Electrocardiographic data, including rhythm and QRS duration, before and after 6 months treatment with tafamidis were examined. Echocardiographic data, including interventricular septum thickness, left ventricular EF (LVEF), left ventricular mass index (LVMI), stroke volume (SV) index, and the ratio of early transmitral flow velocity to early mitral annular velocity septal ratios, were also investigated. Echocardiography measurements were performed by physiological technicians and verified by echocardiologists in a standard manner as recommended by the American Society of Echocardiography.6,7 The interventricular septum thickness was measured using M-mode echocardiography in the parasternal long-axis view. LVEF and SV were measured by the modified Simpson method in apical 4- and 2-chamber views. LVMI was calculated by the linear method using the Deverereux and Reichek “cube” formula.
Statistical Analysis
Statistical analyses were performed using JMP pro ver.14.0 (SAS Institute, Cary, NC, USA). Two-sided P<0.05 was considered significant. Continuous data are described as the median and interquartile range (IQR) and were compared between 2 groups using the Mann-Whitney U-test. Categorical data were compared between 2 groups using the Chi-squared test or Fischer’s exact test, as appropriate. In 6 patients who continued tafamidis treatment for more than 6 months, clinical variables were compared between baseline and 6 months using a Wilcoxon signed-rank test. In addition, rates of cardiovascular-related hospitalizations per year were calculated.
Results
Baseline Characteristics
The baseline characteristics of the 9 patients in this study are given in Table. The median age of patients was 78 years, and 8 (89%) were male. At baseline, 1 patient was NYHA Class IV, 3 patients were NYHA Class III, and 5 patients were NYHA Class II. The median number of previous heart failure hospitalizations was 1. The median duration between heart failure diagnosis and tafamidis administration was 32 months. Four patients (44%) had permanent pacemakers, including an implantable cardioverter-defibrillator and cardiac resynchronization therapy with cardioverter-defibrillator placement in 1 patient each. Median concentrations of BNP and NT-proBNP were 289 and 3,035 pg/mL, respectively.
Table.
Baseline Characteristics
| Total (n=9) | HF hospitalization within 1 year | P-value | ||
|---|---|---|---|---|
| Yes (n=3) | No (n=6) | |||
| Age (years) | 78 [74, 82] | 78 [77, 81] | 78 [68, 85.3] | 1.000 |
| Male sex | 8 (89) | 2 (67) | 6 (100) | 0.330 |
| Wild-type TAC | 8 (89) | 2 (67) | 6 (100) | 0.330 |
| Body surface area (m2) | 1.6 [1.5, 1.74] | 1.5 [1.3, 1.5] | 1.7 [1.6. 1.8] | 0.020 |
| Body mass index (kg/m2) | 21.0 [20.0, 23.7] | 19.0 [18.2, 21.4] | 22.4 [20.5, 24.4] | 0.121 |
| NYHA functional class | 0.021 | |||
| Class I | 0 (0) | 0 (0) | 0 (0) | |
| Class II | 5 (56) | 0 (0) | 5 (83) | |
| Class III | 3 (33) | 2 (66) | 1 (17) | |
| Class IV | 1 (11) | 1 (33) | 0 (0) | |
| No. previous HF hospitalizations | 1 [1, 3] | 3 [1, 4] | 1 [1, 1.75] | 0.217 |
| Duration of HF (months) | 32 [12, 50] | 60 [44, 60] | 16 [5.8, 37] | 0.038 |
| Atrial fibrillation (%) | 4 (33) | 1 (33) | 3 (50) | 1.000 |
| Cardiac pacemaker (%) | 4 (44) | 2 (67) | 2 (33) | 0.524 |
| Troponin I (ng/mL) | 95 [78, 251] | 251 [89, 660] | 86 [66, 508] | 0.302 |
| Plasma BNP (pg/mL) | 289 [213, 618] | 735 [349, 1,405] | 215 [165, 462] | 0.053 |
| Serum NT-proBNP (pg/mL) | 3,053 [1,286, 3,368] | 7,779 [6,155, 9,403] | 3,085 [1,501, 4,729] | 0.064 |
| Serum creatinine (mg/dL) | 1.25 [0.94, 1.43] | 1.73 [0.94, 1.77] | 1.19 [0.9, 1.37] | 0.197 |
| LVEF (%) | 55 [48, 57] | 55 [15, 57] | 54 [32, 62] | 0.606 |
| IVST (mm) | 15 [14, 17] | 17 [15, 18] | 13.5 [12, 15.3] | 0.051 |
| LV SV (mL) | 43 [36, 50] | 36.0 [16.1, 58.0] | 44.0 [39.8, 56.8] | 0.439 |
| LV SVI (mL/m2) | 28.9 [27.3, 29.3] | 27.3 [10.7, 29.4] | 29.0 [25.7, 32.7] | 0.439 |
Unless indicated otherwise, data are given as the median [interquartile range] or n (%). *P<0.05 by Mann-Whitney U-test or Fisher’s exact test as appropriate. BNP, B-type natriuretic peptide; HF, heart failure; IVST, interventricular septum thickness; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SV, stroke volume; SVI, stroke volume index; TAC, transthyretin amyloid cardiomyopathy.
Clinical Course
The median duration of tafamidis treatment was 260 days. One patient with NYHA Class IV also had monoclonal gammopathy of undetermined significance and had undergone cardiac resynchronization therapy with cardioverter-defibrillator placement for reduced EF and complete atrioventricular block 5 years previously. This patient had received scheduled infusions of inotropes twice a week and was hospitalized for worsening heart failure 34 days after starting tafamidis. Tafamidis was discontinued after the patient was admitted to hospital. Another 2 of 3 patients with NYHA Class III were hospitalized for worsening heart failure at 281 and 196 days, although they had not been hospitalized for 1 year prior to starting tafamidis. Of these 2 patients, 1 discontinued tafamidis after hospital admission at the physician’s discretion. Five patients with NYHA Class II were not hospitalized. Thus, patients rehospitalized for heart failure were in more advanced NYHA functional classes and had a longer duration of heart failure before starting tafamidis therapy (Table).
There were no other cardiovascular-related events except for heart failure. The rate of cardiovascular-related hospitalization was 0.66 per year (2.14 patients with NYHA Class III and IV; 0 patients with NYHA Class II). No patients died. A few patients complained of digestive symptoms during tafamidis therapy, but no patients required additional treatment. No patients complained of headache or urinary tract infections.
Changes in Biomarkers and QRS Duration
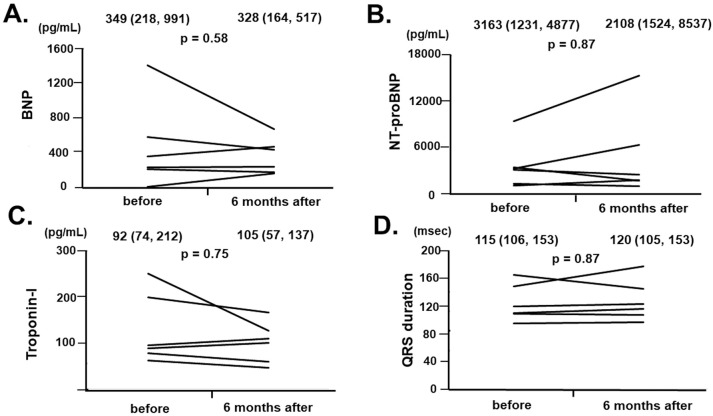
There were no significant changes in plasma BNP (Figure 1A), serum NT-proBNP (Figure 1B), and troponin I (Figure 1C) concentrations or in the QRS duration (Figure 1D) from baseline to 6 months in the 6 patients who received tafamidis therapy for 6 months (P>0.05 for all).
Figure 1.
Trends in (A) B-type natriuretic peptide (BNP), (B) N-terminal pro B-type natriuretic peptide (NT-proBNP), and (C) troponin I concentrations, as well as (D) QRS duration before and 6 months after the initiation of tafamidis in 6 patients who continued on tafamidis for more than 6 months. Median (interquartile rage) values for each of the parameters before and at 6 months are given at the top of each graph.
Changes in Echocardiographic Parameters
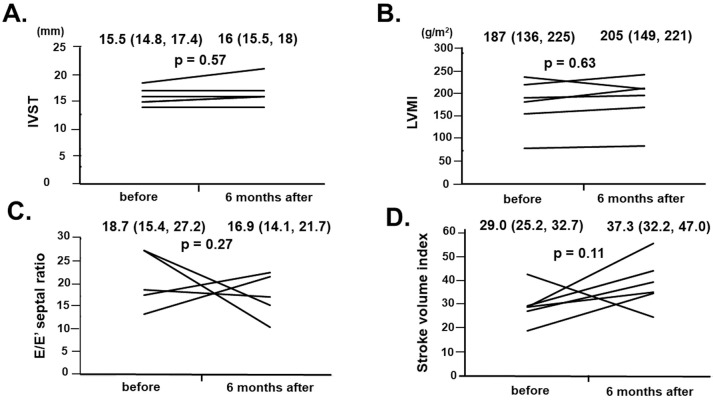
There were no significant changes from baseline to 6 months in interventricular septum thickness, LVMI, E/e′, or the SV index in the 6 patients who received tafamidis therapy for 6 months (Figure 2; P>0.05 for all). Of note, interventricular septum thickness remained unchanged in 3 of 6 patients and increase in the remaining 3 patients. One patient showed rapid progression of cardiac hypertrophy, but was not hospitalized after tafamidis initiation. This patient had atrial fibrillation, familial spastic paraplegia, spinal neurilemoma, and benign prostatic hypertrophy.
Figure 2.
Trends in (A) interventricular septum thickness (IVST), (B) left ventricular mass index (LVMI), (C) the ratio of early transmitral flow velocity to early mitral annular velocity (E/e′), and (D) left ventricular stroke volume index before and 6 months after the initiation of tafamidis in 6 patients who continued on tafamidis for more than 6 months. Median (interquartile rage) values for each of the parameters before and at 6 months are given at the top of each graph.
Discussion
In this study we investigated the clinical course of patients diagnosed with transthyretin amyloid cardiomyopathy who received tafamidis treatment. Most of the patients were elderly men and had wild-type disease.
The main finding of this study is that there were no significant changes in the clinically important surrogates of the severity of amyloid cardiomyopathy, namely BNP, NT-proBNP, troponin I, QRS duration, interventricular septum thickness, LVMI, and E/e′, over the 6-month period of tafamidis treatment.
Clinical Presentation
Wild-type transthyretin amyloid cardiomyopathy is known to be an underdiagnosed cause of HFpEF, presenting with high concentrations of NT-proBNP, cardiac troponin, left ventricle maximum wall thickness, and disturbances in the conduction system,8,9 as seen in the patients in the present study. Median survival from the time of biopsy-proven diagnosis is 46.7 months, and 78% of deaths are due to cardiac causes (heart failure or ventricular arrhythmias). Others have reported that 5-year survival following the diagnosis of transthyretin amyloid cardiomyopathy is 35.7%. Multivariate predictors of 5-year mortality were higher plasma BNP and serum uric acid concentrations, lower LVEF, and left ventricular wall thickness at baseline.10
Tafamidis Treatment
Tafamidis binds to transthyletin, preventing tetramer dissociation and amyloidogenesis. In the ATTR-ACT trial, long-term tafamidis therapy reduced all-cause mortality and cardiovascular hospitalization compared with placebo.4 Of note, in that trial the survival curves for the tafamidis and placebo arms were comparable for the first 18 months.4
In the present study, BNP and NT-proBNP concentrations, interventricular septum thickness, and LVMI remained unchanged during the 6-month period of tafamidis therapy, although it should be highlighted that statistical non-significance does not indicate “similarity”, particularly in such a small cohort. Nevertheless, individual data were unchanged or worsened numerically. Of note, interventricular septum thickness worsened numerically in 3 of 6 patients.
In the ATTR-ACT trial, the median baseline NT-proBNP concentration in the tafamidis and control arms was 2,996 and 3,161 pg/mL, respectively,4 which is comparable to the findings in the present study. Furthermore, the rates of cardiovascular-related hospitalizations per year were 0.55 and 0.77 in the tafamidis and placebo arms, respectively, in the ATTR-ACT trial.4 In the present study, the rate of cardiovascular-related hospitalizations per year was 0.66, which is higher than that of the tafamidis arm in the ATTR-ACT trial, probably due to the severity of disease in the present cohort, including many patients with NYHA Class III or IV. In the ATTR-ACT trial,4 NT-proBNP concentrations at 12 and 30 months increased in both the tafamidis and placebo arms, but the increase was smaller in the tafamidis than the placebo arm. Furthermore, at 30 months, interventricular septal wall thickness had decreased −0.11 mm in the tafamidis arm and increased +0.30 mm in the placebo arm in the ATTR-ACT trial.4 The clinical implications of such a very small improvement in the tafamidis arm require further investigation.
Given that these parameters are important surrogates of the deposition of amyloid, the present data may indicate no significant effect of tafamidis on suppressing the continuous deposition of amyloid. In contrast, it should be noted that tafamidis theoretically just suppresses the further deposition of amyloid, and we cannot expect any regression of amyloid that has already been deposited.
Appropriate Patient Selection
In the ATTR-ACT trial,4 patients with transthyretin amyloid cardiomyopathy and NYHA Class III had a higher rate of cardiovascular-related hospitalizations than those with NYHA Class I–II.
In the present study, patients with NYHA Class III or IV had early hospitalization (within 1 year) with worsening heart failure. Tafamidis therapy in patients with NYHA Class I–II may have clinical benefit in suppressing mortality and morbidity. The cost-effectiveness of tafamidis therapy in patients who are relatively less sick warrants further comparison studies. Longer-term tafamidis therapy >6 months may also have clinical benefits compared to conventional therapy without tafamidis, as observed in the ATTR-ACT trial. However, individual patients receiving tafamidis may not be sufficiently satisfied with the outcomes (e.g., unchanged surrogate markers and clinical outcomes as observed in the present study) given the considerable cost (JPY 5,240,640 per month). Of note, the attending institute should pay all the medical costs for tafamidis therapy during hospitalization, given the diagnosis procedure combination system. One patient in the present study showed rapid progression of cardiac hypertrophy despite tafamidis therapy. The underlying mechanism remains uncertain, but a way to predict responses to tafamidis should be investigated in future studies.
Study Limitations
This study had a relatively small sample size and was performed at a single institute with an observation period of only 6 months. There were no data regarding exercise capacity and quality of life. In addition, we do not have any global myocardial strain data. It should be emphasized that this study is a proof-of-concept study and that larger-scale multicenter studies should be conducted in the future. Statistical non-significance does not indicate “similarity” between the groups. We cannot exclude the effects of medications that were administered concomitantly. Nevertheless, given the accumulating concern regarding tafamidis therapy and its relatively high cost, we decided to present the findings of this study and discuss the need for appropriate patient selection against such a small sample size. We are proud to have reported real-world clinical data for such a severe and rare disease for the first time in Japan.
Conclusions
Tafamidis therapy for patients with transthyretin amyloid cardiomyopathy may be optimal for those with NYHA Class I or II, but not appropriate for NYHA Class III patients, given that tafamidis does not induce the regression of already deposited amyloid. Nevertheless, further studies are warranted to investigate the cost-effectiveness of longer-term tafamidis therapy for a less sick cohort, given the extremely high medical costs.
Disclosure
K.K. is a member of Circulation Reports ’ Editorial Team.
Acknowledgments / Sources of Funding
None.
References
- 1. Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, et al.. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014; 2: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Treibel TA, Fontana M, Gilbertson JA, Castelletti S, White SK, Scully PR, et al.. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: Prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging 2016; 9: e005066. [DOI] [PubMed] [Google Scholar]
- 3. Emdin M, Aimo A, Rapezzi C, Fontana M, Perfetto F, Seferovic PM, et al.. Treatment of cardiac transthyretin amyloidosis: An update. Eur Heart J 2019; 40: 3699–3706. [DOI] [PubMed] [Google Scholar]
- 4. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al.. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 5. Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, et al.. Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation 2020; 141: 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Emande L, et al.. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al.. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, De Haro-Del Moral FJ, Cobo-Marcos M, Robles C, et al.. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015; 36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- 9. Marume K, Takashio S, Nishi M, Hirakawa K, Yamamoto M, Hanatani S, et al.. Combination of commonly examined parameters is a useful predictor of positive 99 mTc-labeled pyrophosphate scintigraphy findings in elderly patients with suspected transthyretin cardiac amyloidosis. Circ J 2019; 83: 1698–1708. [DOI] [PubMed] [Google Scholar]
- 10. Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, et al.. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: A prospective, observational cohort study. Circulation 2016; 133: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]