Abstract
A set of 4-(R2-imino)-3-mercapto-5-(R1)-4H-1,2,4-triazoles derivatives were synthesized, characterized and evaluated for their ability to inhibit nitric oxide (NO) production in PAM212 mouse keratinocytes, which led to the discovery and the subsequent evaluation of their growth inhibitory cytotoxic potency toward that same mouse cell line together with a number of human cells lines (PC3, HT-29 and HeLa). Some limited SAR could be established for both NO production inhibition potency and growth inhibition cytotoxicity. Noticeably, the compounds designed to be nitrofurantoin mimics were the most potent anti-neoplastic agents.
Graphical Abstract
Introduction
In our studies on interventional pharmaceuticals to treat sulfur mustard-induced injury, inhibitors of inducible nitric oxide synthase (iNOS) have shown promise.(1) A number of five-membered ring nitrogen heterocyclics have been found to function as anti-inflammatory agents (Figure 1) and a NOS-inhibitory mechanism has been invoked.(1–3) Imidazoles such as itraconazole, ketoconazole, and miconazole are known inhibitors of iNOS in intact cells.(4) In addition, itraconazole has recently been reported to be an effective eNOS inhibitor although its exact mechanism of action was not elucidated.(5) Aryl-substituted imidazoles in particular SB203580 have been established as inhibitors of iNOS at high concentrations(6) while certain indazoles and triazoles (of both the 1,2,3- and the 1,2,4- isomeric types) have also displayed impressive iNOS inhibition.(7–9)
Figure 1:
Some examples of imidazole, triazole and Nitrofurantoin derivatives with demonstrated biological activity
The ability of cells to synthesize NO is part of their natural immune defense mechanism. However, excessive NO production can lead to tissue damage and, therefore, drugs that suppress NO production are helpful in those situations. For host cells, exposure to many bacterial pathogens can lead to over-production of NO. This may also explain the fact that some successful antibacterial agents possess simultaneous iNOS inhibition as part of their antibacterial activity.(10,11) The nitrofuran antibacterials, e.g., nitrofurantoin (NF) and nitrofurazone, have been classically employed against urinary tract infections and there are reports of their concurrent anti-inflammatory activity.(12–16)
In our earlier studies of 1,2,4-triazole imines we observed significant anti-bacterial activity against urogenital pathogens such as G. vaginalis and E. coli and also made note of a structural similarity of our system to nitrofurantoin.(17,18) The question then arises that if these imines possess activity against urinary tract infectious organisms do they also manifest an ability to inhibit NO production. The focus of this work was therefore centered on evaluating a small library of such compounds for NO inhibition. It is important to note that although the compounds presented are designed as partial mimics of Nitrofurantoin and Nitrofurazone, known for their adverse side effects and toxicity; we do not anticipate such problems because we envision a different route of administration, namely topical, which should lead to reduced toxicity.
Discussion and results
Synthesis
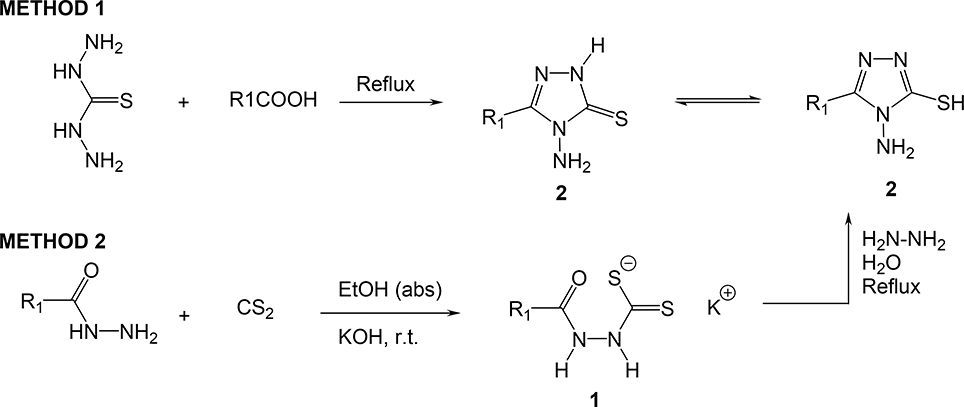
The starting 4-amino-5-R1-2,4-dihydro-3H-1,2,4-triazole-3-thiones (general structure 2) were prepared according to the procedure by Reid (Scheme 1).(19) The synthetic route employed to obtain the target 1,2,4-triazole imines, compounds 3(a-z), is outlined in Scheme 2. Structures of R1 and R2 for 3(a-z) are given in Table 1. Compounds 3(a-z) were prepared by reacting 4amino-2,4-dihydro-5-R1-3H-1,2,4-triazole-3-thiones with various aldehydes at reflux in absolute ethanol (EtOH) or tetrahydrofuran (THF) to give the expected corresponding imine in moderate to excellent yields (45 – 97%). Usually the compounds were obtained by precipitation upon cooling, and purified by recrystallization in some cases. However, when a nitro-aldehyde derivative was utilized, the purification was not so straightforward. Most of the time, an excess of aldehyde had to be used to drive the reaction to completion. At the end of the reaction, upon cooling, both excess of aldehyde and expected compound precipitated. In those cases, the crude solid had to be purified by flash silica gel column chromatography followed by recrystallization and in these conditions, pure material was obtained in lower yields (22 – 60%).
Scheme 1:
Synthesis of 4-amino-5-R1-2,4-dihydro-3H-1,2,4-triazole-3-thione
Scheme 2:
Synthesis of compounds 3(a-z)
Table 1:
iNOS & growth inhibition data obtained with PAM 212 keratinocytes for compounds 3(a-z).
| Compound | R1 | R2 | Nitrite inhibition IC50 (μM) | Growth inhibition IC50 (μM) |
|---|---|---|---|---|
| 3a |  |
22 | > 100(*) | |
| 3b |  |
 |
> 100(*) | > 100(*) |
| 3c |  |
 |
14 | 59 |
| 3d |  |
 |
NA | 4.6 |
| 3e | 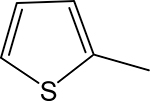 |
 |
26 | 93 |
| 3f |  |
 |
NA | 8.1 |
| 3g |  |
 |
NA | 4.2 |
| 3h | Me- | 15 | > 100(*) | |
| 3i |  |
15 | > 100(*) | |
| 3j | Me- |  |
NA | 6.2 |
| 3k |  |
26 | 97 | |
| 3l |  |
 |
NA | 2.9 |
| 3m | Me- |  |
NA | 12 |
| 3n | 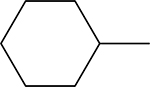 |
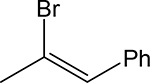 |
NA | 5.1 |
| 3o | 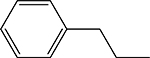 |
 |
NA | 5.9 |
| 3p |  |
 |
NA | 5.2 |
| 3q | 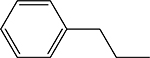 |
23 | > 100(*) | |
| 3r |  |
34 | 102 | |
| 3s |  |
28 | 61 | |
| 3t |  |
NA | 13 | |
| 3u |  |
 |
NA | 15 |
| 3v |  |
NA | 2.9 | |
| 3w |  |
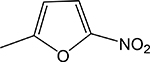 |
NA | 3.2 |
| 3x |  |
 |
NA | 15 |
| 3y |  |
 |
NA | 3.1 |
| 3z |  |
 |
NA | 1.5 |
NA: Not Available (compound was too cytotoxic to cell line);
Maximum [ ] tested and for which 50% nitrite inhibition was not reached and 50% growth inhibition was not attained.
Functional assays
NO production inhibition in PAM212 cells
The ability of the compounds synthesized to inhibit nitric oxide production was assessed in vitro. Nitric oxide production by IFN-γ stimulated PAM212 mouse keratinocytes was quantified by the accumulation of nitrite in the cell culture medium using the Greiss reaction, with sodium nitrite as the standard as previously described.(20) In brief, 24 hours after treatment, the culture medium was collected, 100 μL was incubated with 100 μL of Griess reagent (0.1% napthalethylenediamine dihydrochloride, 1% sulfanilamide, 2.5% H3PO4) and the absorbance was measured at 540 nm. The concentration of nitrite was calculated with sodium nitrite as a standard.(21)
Cellular Growth Inhibition
The primary in vitro screen for the anti-proliferative activity was based on the ability of the beneficial therapeutic compound to inhibit the growth of tumor cells grown in culture. This type of growth assay can be used with mammalian cancer cells as well as with pathogenic and non-pathogenic microbes including, but not limited to, yeasts and bacteria. In our case, to assay the candidate triazoles for anti-proliferative activity, PAM212 keratinocytes grown in vitro in monolayer culture were used as previously described.(20, 22–25). Briefly, cells were plated at low density (104 cells /well) in 6 well tissue culture dishes in 2 mL of growth medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% calf serum, and allowed to adhere overnight. The medium was then changed to growth medium supplemented with increasing concentrations of the triazoles (compounds 3(a-z)). After 5 days, the medium was drained from the culture dishes and the cells washed with phosphate buffered saline. The cells from each well were removed by trypsin treatment and the number of cells in each culture dish was determined using a Coulter Counter (Coulter Electronics Inc., Hialeah, FL). The effects of compound 3d was evaluated in HT-29, PC3 and HeLa human cell lines using the same technique as described above.
Structure-activity Relationships
Nitrite inhibition
While low μM IC50s were observed for NO production inhibition for some of the compounds, it was generally not in the nitro-containing analogs (Table 1). Compounds (3d, 3f-g, 3j, 3l-p, 3t-z) proved to be too toxic to the PAM212 cells used to generate the iNOS inhibition data and, as a result, their inhibition could not be measured. However, in compounds (3a, 3c, 3e, 3h-i, 3k, 3q-3s) wherein cytotoxicity was less manifest, clear evidence of NO production inhibition was observed. All compounds except 3c inhibited NO production with IC50’s ranging from from 14 to 34 μM while possessing an R2 moiety (see Scheme 1) derived from a simple cinnamaldehyde or the α-substituted-cinnamaldehyde. The most active iNOS inhibitor was 3c, derived from o-nitrocinnamaldehyde and an electronic structural contrast to 3b (from o-methoxycinnamaldehyde). The latter manifested neither growth inhibition – over the maximum concentration range through which it could be tested – nor NOS inhibition. In view of the cytotoxicity of some of these compounds toward PAM212 cells, we decided to investigate further the potential utility of these triazole imines as anti-neoplastic agents.
Growth inhibition
Results obtained are summarized in Table 1. From the data collected and displayed in Table 1, we could establish the following structure-activity relationships. Keeping the R1 group unchanged, modification of the structure of R2 had a significant impact on cytotoxicity. Compounds bearing a substituent on the cinnamyl chain display increased cytotoxicity, as can be seen for examples with R1 = 2-thienyl. In this series, the addition of a halogen (Br or Cl) resulted in an increase of cytotoxicity greater than when a methyl or methoxy group were present instead; as exemplified by compounds 3d (Br, IC50 = 4.6 μM) < 3f (Cl, IC50 = 8.1 μM) < 3e (Me, IC50 = 93 μM) < 3a (H, IC50 > 100 μM). Similar trends could be observed when comparing 3o (Br, IC50 = 5.9 μM) < 3q (H, IC50 > 100 μM); 3j (Br, IC50 = 6.2 μM) < 3m (Me, IC50 = 12 μM) < 3h (H, IC50 > 100 μM); 3p (Br, IC50 = 5.2 μM) < 3s (H, IC50 = 61 μM); 3l (Br, IC50 = 2.9 μM) < 3k (H, IC50 = 97 μM) and 3g (Br, IC50 = 4.2 μM) < 3i (H, IC50 > 100 μM). While keeping R2 constant as in compounds (3d, 3g, 3j, 3l, 3n, 3o, 3p), where R2 is an α-bromocinnamyl group, it is interesting to note that R1 seemed to have little impact on the potency of the compounds, with IC50s very narrowly falling between 2 and 7 μM. Independent from R1, the presence of a nitro group on R2 also increased the cytotoxicity, as exemplified by 3c (NO2, IC50 = 59 μM) < 3a (H, IC50 > 100 μM). Of special interest, are compounds (3t-3z), designed as nitrofurantoin mimics, which displayed the most enhanced activity with IC50s as low as 1.5 to 3.5 μM (3v-w and 3y-z). Lastly for the same R2 group (cinnamyl), there is no significant change for the IC50 values when one modifies R1 as exemplified by compounds 3a, 3h, 3i, 3k, 3q and 3r.
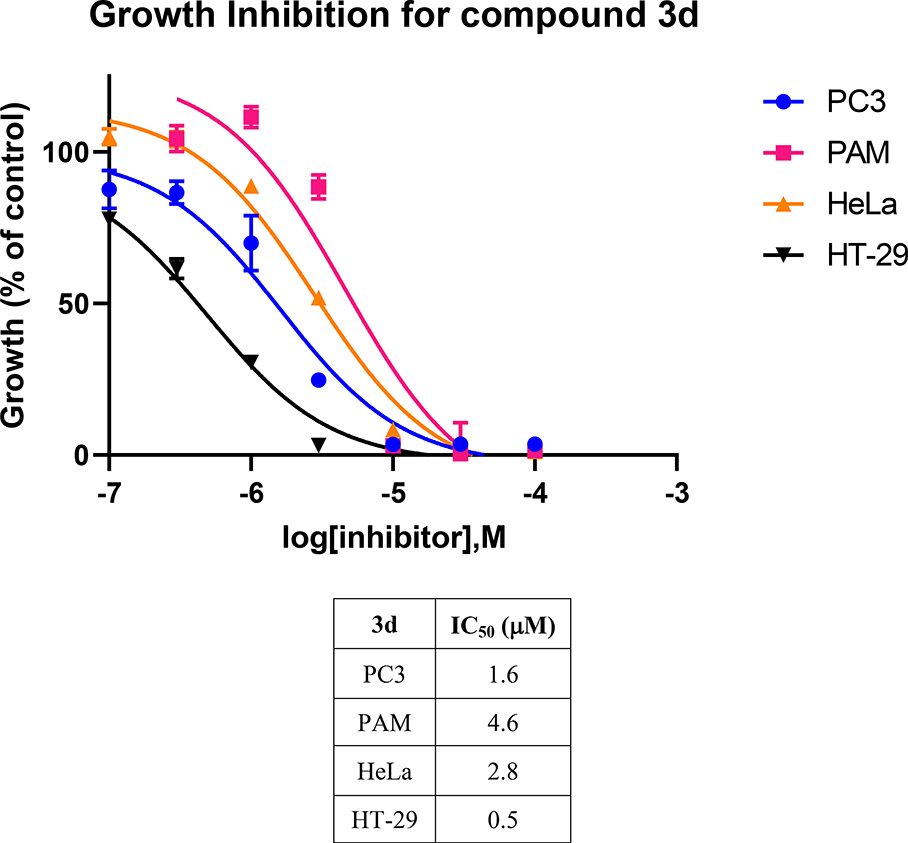
Following the discovery of the cytotoxicity of the triazole imines against the murine keratinocyte cell line PAM212, we selected compound (3d) for additional in vitro testing against human cancer cell lines HT-29 (colorectal cancer), PC3 (prostate cancer) and HeLa (cervical cancer). The results, displayed in Figure 2, demonstrated the marked potency of 3d to inhibit in vitro the growth of all three human cell lines with IC50’s ranging from 0.5 μM for the HT-29 (colon), 1.6 μM for PC3 (prostate), and 2.8 μM for HeLa (cervix). For comparison, the IC50 for 3d in mouse keratinocytes was 4.6 μM.
Figure 2:
Cellular growth inhibition curves for compound 3d in various cancer cell lines such as (HT-29: human colon), (PC3: human prostate), (HeLa: human cervical) and (PAM212: mouse keratinocytes)
Experimental
Synthesis
Unless otherwise indicated, all materials were purchased from commercial suppliers and used without further purification.
IR spectra were recorded using a Mattson Polaris FT-IR spectrophotometer. Solids compounds were analyzed using the KBr disk methods, or solubilized in nujol. Liquids were analyzed as thin film between two NaCl blocks.
1H spectra were recorded at 360 MHz on a Brucker AMX 360 spectrometer. Chemical shifts were measured relative to CDCl3 (δ 7.24 ppm), CD3OD (δ 3.30 ppm), acetone (d6) (δ 2.04 ppm) and DMSO (d6) (δ 2.49 ppm). The following abbreviations are used to describe the signal multiplicities: s (singlet), d (doublet), t (triplet), q (quadruplet), m (multiplet). Chemical shifts are expressed in ppm and listed as follows: shift in ppm (multiplicity, integration, and coupling).
Thin-layer chromatography (TLC) was performed on plates (0.25 mm) precoated with fluorescent silica gel GF (Analtech). Reaction components were then visualized under UV light and/or following staining with iodine and/or with a saturated solution of KMnO4 in methanolic NaOH (1N). Silica gel (230–400 mesh, EM Sciences) was used for flash chromatography separations. Uncorrected melting points (mp) were determined with a Thomas Hoover capillary melting point apparatus. Elemental analyses were provided by Intertek.
Synthesis of 4-(R2-imino)-3-mercapto-5-(R1)-4H-1,2,4-triazoles, 3(a-y)
General Procedure
The corresponding 4-amino-3-mercapto-5-(R1)-4H-1,2,4-triazole (general structure 2) and aldehyde were solubilized in absolute ethanol or anhydrous tetrahydrofuran. The α-bromo and α-chlorocinnamaldehydes used were the (E)-isomers whereas the (Z)-isomer was used for α-methylcinnamaldehyde. The mixture was stirred at reflux until completion was shown by TLC (CH2Cl2/MeOH, 96/4, v/v), usually 6 hours, and a solid precipitated out of solution. The flask was cooled down to room temperature, and then put in the freezer (−20°C) overnight. The precipitate was filtered and rinsed with cold ethanol or tetrahydrofuran to give the expected 4(R2-imino)-3-mercapto-5-(R1)-4H-1,2,4-triazole. Variations of this general procedure are described for each individual compound.
4-(((1Z,2E)-3-phenylallylidene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3thione 3a

Compound 3a was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.1 g, 0.50 mmol), trans-cinnamaldehyde (0.146 g, 1.16 mmol) and 4 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 101 mg (0.323 mmol, 64 %) of 3a as a yellow solid. 1H NMR (DMSO-d6): 7.22 (t, 1H, J = 4.9 Hz); 7.30 (dd, 1H, J = 9.5 Hz and J = 16.0 Hz); 7.43–7.52 (m, 4H); 7.767.84 (m, 4H); 9.58 (d, 1H, J = 9.5 Hz). IR (nujol): 1457 cm-1. m.p.= 206–207°C. Analysis Calculated for C15H12N4S2: C, 57.67; H, 3.87; N, 17.93. Found: C, 57.76; H, 3.65; N, 17.56.
4-(((1Z,2E)-3-(2-methoxyphenyl)allylidene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3b

Compound 3b was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.1 g, 0.50 mmol), trans-2-methoxycinnamaldehyde (0.123 g, 0.76 mmol) and 4 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 129 mg (0.378 mmol, 75 %) of 3b as a yellow solid. 1H NMR (DMSO-d6): 3.89 (s, 3H); 7.02 (t, 1H, J = 7.51 Hz); 7.11 (d, 1H, J = 8.4 Hz); 7.22 (t, 1H, J = 4.50 Hz); 7.28 (dd, 1H, J = 9.6 Hz and J = 16.0 Hz); 7.43 (t, 1H, J = 7.2 Hz); 7.63 (d, 1H, J = 16.0 Hz); 7.79–7.83 (m, 3H); 9.50 (d, 1H, J = 9.5 Hz). IR (nujol): 1459 cm-1. m.p.= 227–228°C. Analysis Calculated for C16H14N4OS2.0.5 H2O: C, 54.71; H, 4.30; N, 15.95. Found: C, 54.70; H, 3.94; N, 16.01.
4-(((1Z,2E)-3-(2-nitrophenyl)allylidene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3c

Compound 3c was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.1 g, 0.50 mmol), trans-2-nitrocinnamaldehyde (0.268 g, 1.51 mmol) and 4 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 150 mg (0.42 mmol, 83 %) of 3c as a yellow solid. 1H NMR (DMSO-d6): 7.21 (t, 1H, J = 3.9 Hz); 7.31 (dd, 1H, J = 9.0 Hz and J = 16.0 Hz); 7.68 (t, 1H, J = 7.9 Hz); 7.74–7.85 (m, 5H); 8.09 (dd, 2H, J = 7.9 Hz and J = 18.0 Hz); 9.76 (d, 1H, J = 9.0 Hz). IR (nujol): 1457 cm-1. m.p.= 214–215°C. Analysis Calculated for C15H11N5O2S2: C, 50.41; H, 3.10; N, 19.60. Found: C, 50.50; H, 2.77; N, 19.29.
4-(((1Z,2Z)-2-bromo-3-phenylallylidene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3d

Compound 3d was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.1 g, 0.50 mmol), α-bromocinnamaldehyde (0.32 g, 1.51 mmol) and 4 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 174 mg (0.44 mmol, 88 %) of 3d as a yellow solid. 1H NMR (DMSO-d6): 7.25 (t, 1H, J = 4.1 Hz); 7.51–7.54 (m, 3H); 7.86 (d, 1H, J = 4.9 Hz); 7.96–8.02 (m, 3H); 8.11 (s, 1H); 10.11 (s, 1H). IR (nujol): 1457 cm-1. m.p.= 197–198°C. Analysis Calculated for C15H11BrN4S2: C, 46.06; H, 2.83; N, 14.32. Found: C, 46.14; H, 2.83; N, 14.26.
4-(((1Z,2E)-2-methyl-3-phenylallylidene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3e

Compound 3e was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.1 g, 0.50 mmol), α-methylcinnamaldehyde (0.221 g, 1.51 mmol) and 4 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 74 mg (0.23 mmol, 45 %) of 3e as a yellow solid. 1H NMR (DMSO-d6): 2.28 (s, 3H); 7.22–7.30 (m, 2H); 7.38–7.50 (m, 3H); 7.59 (d, 2H, J = 7.6 Hz); 7.827.86 (m, 2H); 9.62 (s, 1H). IR (nujol): 1457 cm-1. m.p.= 200–201°C. Analysis Calculated for C16H14N4S2: C, 58.87; H, 4.32; N, 17.16. Found: C, 58.51; H, 4.47; N, 17.14.
4-(((1Z,2Z)-2-chloro-3-phenylallylidene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3f

Compound 3f was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.1 g, 0.50 mmol), α-chlorocinnamaldehyde (0.252 g, 1.51 mmol) and 4 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 114 mg (0.33 mmol, 65 %) of 3f as a yellow solid. 1H NMR (DMSO-d6): 7.24 (t, 1H, J = 3.8 Hz); 7.53 (t, 3H, J = 8.6 Hz); 7.81–8.00 (m, 5H); 10.18 (s, 1H). IR (nujol): 1457 cm-1. m.p.= 223–224°C. Analysis Calculated for C15H11ClN4S2: C, 51.94; H, 3.20; N, 16.15. Found: C, 51.66; H, 2.86; N, 15.85.
4-(((1Z,2Z)-2-bromo-3-phenylallylidene)amino)-5-(furan-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3g
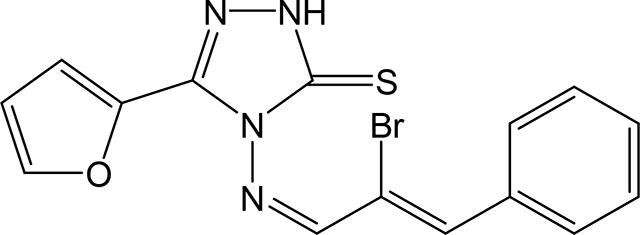
Compound 3g was prepared according to the above procedure using 4-amino-5-(furan-2-yl)-2,4dihydro-3H-1,2,4-triazole-3-thione (0.141 g, 0.77 mmol), α-bromocinnamaldehyde (0.216 g, 1.02 mmol) and 6 mL of absolute ethanol. After filtration, the product was purified by recrystallization in CHCl3/Hexanes to give 244 mg (0.65 mmol, 84%) of 3g as a yellow solid. 1H NMR (Acetone-d6): 6.71 (dd, 1H, J = 3.4 Hz and J = 1.8 Hz); 7.47–7.56 (m, 3H); 7.61 (dd, 1H, J = 3.7 Hz and J = 0.7 Hz); 7.83 (dd, 1H, J = 1.7 Hz and J = 0.7 Hz); 8.00 (s, 1H); 8.04–8.07 (m, 2H); 10.59 (s, 1H). m.p.= 184–185°C. Analysis Calculated for C15H11BrN4OS: C, 48.01; H, 2.96; N, 14.93. Found: C, 48.07; H, 3.02: N, 14.80.
5-methyl-4-(((1Z,2E)-3-phenylallylidene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione 3h
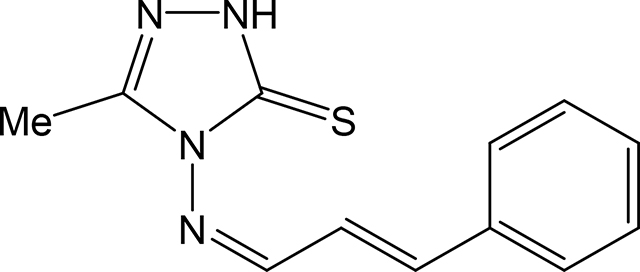
Compound 3h was prepared according to the above procedure using 4-amino-5-methyl-2,4dihydro-3H-1,2,4-triazole-3-thione (0.13 g, 0.99 mmol), trans-cinnamaldehyde (0.396 g, 2.99 mmol) and 6 mL of absolute ethanol. After filtration, the product was purified by column chromatography (CHCl3) on silica gel to give 144 mg (0.59 mmol, 59 %) of 3h as a yellow solid. 1H NMR (Acetone-d6): 2.36 (s, 3H); 7.09 (dd, 1H, J = 16.0 Hz and J = 9.5 Hz); 7.39 (d, 1H, J = 15.8 Hz); 7.42–7.48 (m, 3H); 7.72 (dd, 1H, J = 6.3 Hz and J = 1.6 Hz); 10.14 (d, 1H, J = 9.4 Hz). m.p.= 193–194°C. Analysis Calculated for C12H12N4S.0.2 H2O: C, 58.13; H, 5.04; N, 22.60. Found: C, 58.08; H, 5.05; N, 22.83.
4-(((1Z,2E)-3-phenylallylidene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3thione 3i

Compound 3i was prepared according to the general procedure using 4-amino-5-(furan-2-yl)-2,4dihydro-3H-1,2,4-triazole-3-thione (0.192 g, 1.05 mmol), trans-cinnamaldehyde (0.545 g, 4.12 mmol) and 8 mL of absolute ethanol. The reaction mixture was dry evaporated, the residue solubilized in CHCl3 and the orange solid obtained by precipitation with hexanes was recrystallized in acetone/hexanes to give 262 mg (0.64 mmol, 84 %) of 3i as an orange solid. 1H NMR (CD3OD): 6.63 (dd, 1H, J = 3.4 Hz and J = 1.8 Hz); 7.15–7.23 (m, 2H); 7.37–7.45 (m, 4H); 7.64–7.73 (m, 3H), 9.70 (d, 1H, J = 9.4 Hz). m.p.= 181–182°C. Analysis Calculated for C15H12N4OS: C, 60.79; H, 4.08; N, 18.91. Found: C, 60.91; H, 4.33; N, 18.50.
4-(((1Z,2Z)-2-bromo-3-phenylallylidene)amino)-5-methyl-2,4-dihydro-3H-1,2,4-triazole-3thione 3j

Compound 3j was prepared according to the general procedure using 4-amino-5-methyl-2,4dihydro-3H-1,2,4-triazole-3-thione (0.13 g, 0.99 mmol), α-bromocinnamaldehyde (0.634 g, 3.00 mmol) and 5 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 174 mg (0.54 mmol, 54 %) of 3j as an orange solid. 1H NMR (Acetone-d6): 2.42 (s, 3H); 7–46-7.52 (m, 3H); 7.86 (s, 1H); 7.99–8.02 (m, 2H); 10 49 (s, 1H). m.p.= 194195°C. Analysis Calculated for C12H11BrN4S.0.3H2O: C, 43.86; H, 3.56; N, 17.05. Found: C, 43.83; H, 3.38; N, 17.05.
5-(4-fluorophenyl)-4-(((1Z,2E)-3-phenylallylidene)amino)-2,4-dihydro-3H-1,2,4-triazole-3thione 3k
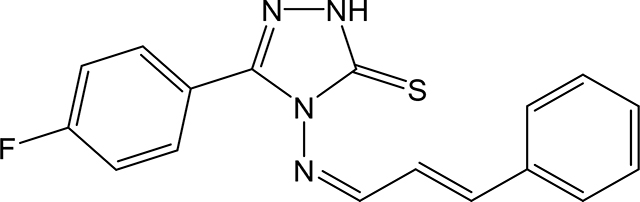
Compound 3k was prepared according to the general procedure using 4-amino-5-(4fluorophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (0.105 g, 0.49 mmol), trans-cinnamaldehyde (0.198 g, 1.49 mmol) and 5 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 100 mg (0.31 mmol, 620 %) of 3k as a yellow solid. 1H NMR (Acetone-d6): 7.16 (dd, 1H, J = 16.1 Hz and J = 9.5 Hz); 7.30–7.38 (m, 2H); 7.43–7.48 (m, 4H); 7.22–7.45 (m, 2H); 8.02–8.07 (m, 2H); 9.81 (d, 1H, J = 9.5 Hz). m.p.= 216–217°C. Analysis Calculated for C17H13FN4S.0.3H2O: C, 61.92; H, 4.16; N, 16.99. Found: C, 61.83; H, 4.05; N, 16.64.
4-(((1Z,2Z)-2-bromo-3-phenylallylidene)amino)-5-(4-fluorophenyl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3l

Compound 3l was prepared according to the above procedure using -amino-5-(4-fluorophenyl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.106 g, 0.5 mmol), α-bromocinnamaldehyde (0.32 g, 1.51 mmol) and 5 mL of absolute ethanol. After filtration, the crude product obtained was purified by recrystallization in CHCl3/Hexanes to give 104 mg (0.25 mmol, 51%) of 3l as a yellow solid. 1H NMR (Acetone-d6): 7.32 ( t, 2H, J = 8.9 Hz); 7.48–7.54 (m, 3H); 7.98 (s, 1H); 8.00–8.06 (m, 2H); 8.18 (dd, 2H, J = 9.0 Hz and J = 5.2 Hz); 10.38 (s, 1H). m.p.= 212–213bromocinnamaldehyde C. Analysis Calculated for C17H12FN4S.0.55H2O: C, 49.42; H, 3.20; N, 13.56. Found: C, 49.44; H, 3.09; N, 13.23.
4-(((1Z,2Z)-2-chloro-3-phenylallylidene)amino)-5-methyl-2,4-dihydro-3H-1,2,4-triazole-3thione 3m

Compound 3m was prepared according to the above procedure using 4-amino-5-methyl-2,4dihydro-3H-1,2,4-triazole-3-thione (0.15 g, 1.15 mmol), α-chlorocinnamaldehyde (0.384 g, 2.30 mmol) and 5 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 283 mg (1.01 mmol, 88 %) of 3m as an off-white solid. 1H NMR (CD3OD): 2.41 (d, 3H, J = 3.5 Hz); 7.41–7.51 (m, 4H); 7.91–7.97 (m, 3H); 10.46 (s, 1H). m.p.= 221–222°C. Analysis Calculated for C17H19ClN4S: C, 51.70; H, 3.98; N, 20.10. Found: C, 51.88; H, 3.93; N, 19.97.
4-(((1Z,2Z)-2-bromo-3-phenylallylidene)amino)-5-cyclohexyl-2,4-dihydro-3H-1,2,4-triazole3-thione 3n

Compound 3n was prepared according to the above procedure using 4-amino-5-cyclohexyl-2,4dihydro-3H-1,2,4-triazole-3-thione (0.146 g, 0.74 mmol), α-bromocinnamaldehyde (0.39 g, 1.84 mmol) and 5 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 279 mg (0.71 mmol, 97 %) of 3n as a pale yellow solid. 1H NMR (Acetone-d6): 1.28–1.50 (m, 3H); 1.51–1.65 (m, 2H); 1.70–1.80 (m, 1H); 1.81–1.90 (m, 2H); 2.01–2.10 (m, 2H); 2.98 (tt, 1H, J = 3.4 Hz and J = 11.5 Hz); 7.42–7.49 (m, 1H); 7.77 (s, 1H); 7.93–8.00 (m, 1H); 10.46 (s, 1H). m.p.= 199–200°C. Analysis Calculated for C17H19BrN4S: C, 52.18; H, 4.89; N, 14.32. Found: C, 52.36; H, 4.82; N, 14.14.
4-(((1Z,2Z)-2-bromo-3-phenylallylidene)amino)-5-phenethyl-2,4-dihydro-3H-1,2,4-triazole3-thione 3o

Compound 3o was prepared according to the above procedure using 4-amino-5-phenethyl-2,4dihydro-3H-1,2,4-triazole-3-thione (0.15 g, 0.68 mmol), α-bromocinnamaldehyde (0.287 g, 1.36 mmol) and 5 mL of absolute ethanol. A pale yellow solid was isolated after filtration and was purified by a column chromatography on silica (CHCl3) followed by recrystallization in CHCl3/Hexanes to give 189 mg (0.47 mmol, 67 %) of 3o as a pale yellow solid. 1H NMR (CD3OD): 3.03–3.13 (m, 4H); 7.15–7.25 (m, 5H); 7.25–7.30 (m, 4H); 7.43–7.52 (m, 3H); 7.74 (s, 1H); 7.93–8.00 (m, 1H); 10.30 (s, 1H). m.p.= 175–176°C. Analysis Calculated for C19H17BrN4S: C, 55.21; H, 4.15; N, 13.55. Found: C, 54.96; H, 3.99; N, 13.15.
4-(((1Z,2Z)-2-bromo-3-phenylallylidene)amino)-5-phenyl-2,4-dihydro-3H-1,2,4-triazole-3thione 3p

Compound 3p was prepared according to the above procedure using 4-amino-5-phenyl-2,4dihydro-3H-1,2,4-triazole-3-thione (0.151 g, 0.78 mmol), α-bromocinnamaldehyde (0.333 g, 1.56 mmol) and 5 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 269 mg (0.70 mmol, 89 %) of 3p as a pale yellow solid. 1H NMR (Acetone-d6): 1.28–1.50 (m, 3H); 1.51–1.65 (m, 2H); 1.70–1.80 (m, 1H); 1.81–1.90 (m, 2H); 2.01–2.10 (m, 2H); 2.98 (tt, 1H, J = 3.4 Hz and J = 11.5 Hz); 7.42–7.49 (m, 1H); 7.77 (s, 1H); 7.93–8.00 (m, 1H); 10.46 (s, 1H). m.p.= 195–196°C. Analysis Calculated for C17H13BrN4S: C, 53.00; H, 3.40; N, 14.54. Found: C, 52.94; H, 3.46; N, 14.28.
5-phenethyl-4-(((1Z,2E)-3-phenylallylidene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione 3q
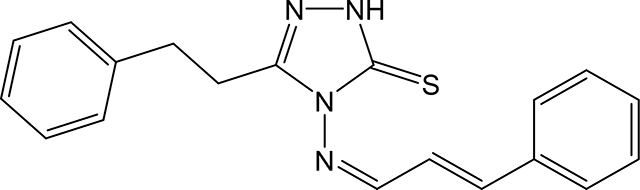
Compound 3q was prepared according to the general procedure using 4-amino-5-phenethyl-2,4dihydro-3H-1,2,4-triazole-3-thione (0.15 g, 0.68 mmol), trans-cinnamaldehyde (0.18 g, 1.34 mmol) and 5 mL of absolute ethanol. After filtration, the product was recrystallized with acetone/hexanes to give 0.128 g (0.383 mmol, 56 %) of 3q as a pale yellow solid. 1H NMR (Acetone-d6): 3.03–3.06 (m, 4H); 7.10 (dd, 1H, J = 16.1 Hz and J = 9.4 Hz); 7.16–7.22 (m, 1H); 7.24–7.29 (m, 4H); 7.37 (d, 1H, J = 16.1 Hz); 7.40–7.47 (m, 3H); 7.70–7.74 (m, 2H); 10.05 (d, 1H, J = 9.4 Hz). m.p.= 150–151°C. Analysis Calculated for C19H18N4S.0.25 H2O: C, 67.33; H, 5.50; N, 16.53. Found: C, 67.43; H, 5.50; N, 16.37.
4-(((1Z,2E)-3-phenylallylidene)amino)-5-(pyridin-3-yl)-2,4-dihydro-3H-1,2,4-triazole-3thione 3r

Compound 3r was prepared according to the general procedure using 4-amino-5-(pyridin-3-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.122 g, 0.63 mmol), trans-cinnamaldehyde (0.168 g, 1.27 mmol) and 5 ml of absolute ethanol. The reaction mixture was dry evaporated, and the yellow residue was purified by column chromatography (CHCl3/MeOH, 95/5, v/v) on silica gel to give 0.117 g (0.38 mmol, 60 %) of 3r as a yellow solid. 1H NMR (CD3OD): 7.14 (dd, 1H, J = 15.9 Hz and J = 9.5 Hz); 7.38–7.44 (m, 4H); 7.57–7.59 (m, 1H); 7.60–7.68 (m, 2H); 8.39 (dt, 1H, J =1.1 Hz and J = 8.1 Hz); 8.66 (dd, 1H, J = 1.6 Hz and J = 4.9 Hz); 9.08 (d, 1H, J = 1.6 Hz); 9.77 (d, 1H, J = 9.5 Hz). m.p.= 198–199°C. Analysis Calculated for C16H13N5S: C, 62.52; H, 4.26; N, 22.78. Found: C, 62.28; H, 4.16; N, 22.60.
5-phenyl-4-(((1Z,2E)-3-phenylallylidene)amino)-2,4-dihydro-3H-1,2,4-triazole-3-thione 3s

Compound 3s was prepared according to the general procedure using 4-amino-5-phenyl-2,4dihydro-3H-1,2,4-triazole-3-thione (0.179 g, 0.93 mmol), trans-cinnamaldehyde (0.188 g, 1.43 mmol) and 5 mL of absolute ethanol. The product was isolated via suction filtration and washed with cold ethanol to give 0.191 mg (0.62 mmol, 67 %) of 3s as a yellow solid. 1H NMR (Acetone-d6): 7.17 (dd, 1H, J = 16.0 Hz and J = 9.5 Hz); 7.42–7.55 (m, 7H); 7.73–7.76 (m, 2H); 7.94–7.98 (m, 2H); 9.76 (d, 1H, J = 9.4 Hz). m.p.= 191–193°C. Analysis Calculated for C17H14N4S: C, 66.64; H, 4.61; N, 18.29. Found: C, 66.56; H, 4.68; N, 17.93.
4-(((1Z,2E)-3-(5-nitrofuran-2-yl)allylidene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3t
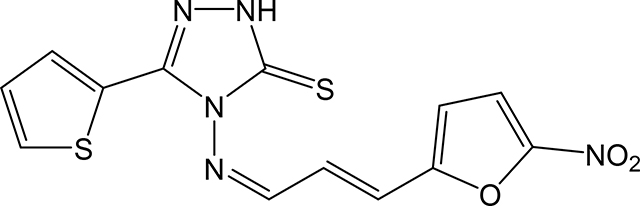
Compound 3t was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.14 g, 0.71 mmol), 5-nitro-2-furanacrolein (0.26 g, 1.54 mmol) and 6 mL of absolute ethanol. After filtration, the crude product was purified by recrystallization in acetone to give 92 mg (0.26 mmol, 37%) of 3t as a yellow solid. 1H NMR (Acetone-d6): 7.10–7.35 (m, 4H); 7.44 (d, 1H, J = 15.9 Hz); 7.62 (d, 1H, J = 3.9 Hz); 7.77 (d, 1H, J = 5.0 Hz); 7.96 (d, 1H, J = 2.8 Hz); 10.29 (d, 1H, J = 9.4 Hz). IR (KBr): 1369 cm-1. m.p.= 205–207°C. Analysis Calculated for C13H9N5O3S2.0.5 H2O: C, 43.81; H, 2.82; N, 19.65. Found: C, 43.29; H, 2.56; N, 19.36.
(Z)-4-(((5-nitrothiophen-2-yl)methylene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3u

Compound 3u was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.138 g, 0.696 mmol), 5-nitro-2thiophenecarboxaldehyde (0.218 g, 1.39 mmol) and 6 mL of anhydrous THF. The reaction mixture was dry evaporated and the crude product was recrystallized in acetone to give 0.142 g (0.42 mmol, 60%) of 3u as an orange solid. 1H NMR (Acetone-d6): 7.13–7.28 (m, 1H); 7.73 (dd, 2H, J = 14.9 Hz and J = 4.2 Hz); 7.90 (d, 1H, J = 3.6 Hz); 8.07 (d, 1H, J = 4.3 Hz); 10.95 (s, 1H). IR (KBr): 1368 cm-1. m.p.= 241–243°C. Analysis Calculated for C11H7N5O2S3: C, 39.16; H, 2.09; N, 20.75. Found: C, 39.38; H, 1.82; N, 20.43.
5-(furan-2-yl)-4-(((1Z,2E)-3-(5-nitrofuran-2-yl)allylidene)amino)-2,4-dihydro-3H-1,2,4triazole-3-thione 3v

Compound 3v was prepared according to the above procedure using 4-amino-5-(furan-2-yl)-2,4dihydro-3H-1,2,4-triazole-3-thione (0.234 g, 1.28 mmol), 5-nitro-2-furanacrolein (0.322 g, 1.92 mmol) and 8 mL of anhydrous THF. The reaction mixture was dry evaporated and the crude product was recrystallized in acetone to give 0.184 g (0.56 mmol, 44%) of 3v as an orange solid. 1H NMR (Acetone-d6): 6.69 (m, 1H); 7.19 (d, 1H, J = 3.8 Hz); 7.26–7.46 (m, 3H); 7.62 (d, 1H, J = 8.6 Hz); 7.82 (d, 1H, J =1.5 Hz); 10.23 (d, 1H, J = 10.2 Hz). IR (KBr): 1369 cm-1. m.p.= 217–218°C. Analysis Calculated for C13H9N5O4S. 0.2 acetone: C, 47.67; H, 3.00; N, 20.42. Found: C, 48.08; H, 3.16; N, 20.50. HRMS: ESI, (M+H) formula: C13H10N5O4S, Calculated 332.0448, Found 332.0457.
(Z)-4-(((5-nitrofuran-2-yl)methylene)amino)-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4triazole-3-thione 3w
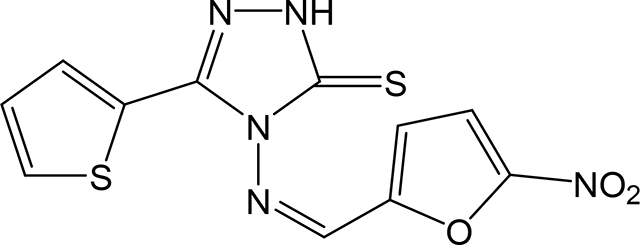
Compound 3w was prepared according to the above procedure using 4-amino-5-(thiophen-2-yl)2,4-dihydro-3H-1,2,4-triazole-3-thione (0.196 g, 1.00 mmol), 5-nitro-2-furaldehyde (0.278 g, 1.97 mmol) and 6 mL of anhydrous THF. The reaction mixture was dry evaporated and the crude product was purified by a column chromatography on silica (CHCl3) and then recrystallized in CHCl3/Hexanes to give 72 mg (0.224 mmol, 23%) of 3w as a light orange solid. 1H NMR (Acetone-d6): 7.24 (m, 1H); 7.62 (d, 1H, J = 3.8 Hz); 7.71 (d, 1H, J = 3.9 Hz); 7.79 (d, 1H, J = 5.0 Hz); 8.00 (d, 1H, J = 4.0 Hz); 10.83 (s, 1H). IR (KBr): 1363 cm-1. m.p. = 190–191°C. Analysis Calculated for C11H7N5O3S2: C, 41.12; H, 2.19; N, 21.79. Found: C, 41.00; H, 2.34; N, 21.48.
(Z)-5-(furan-2-yl)-4-(((5-nitrothiophen-2-yl)methylene)amino)-2,4-dihydro-3H-1,2,4triazole-3-thione 3x
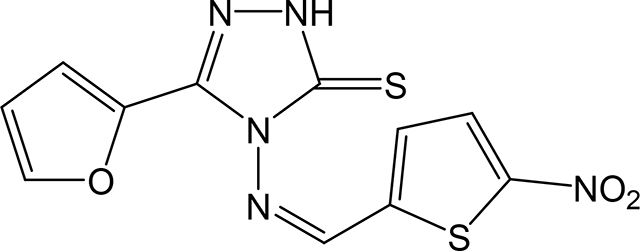
Compound 3x was prepared according to the above procedure using 4-amino-5-(furan-2-yl)-2,4dihydro-3H-1,2,4-triazole-3-thione (0.2 g, 1.09 mmol), 5-nitro-2-thiophenecarboxaldehyde (0.256 g, 1.63 mmol) and 8 mL of anhydrous THF. The reaction mixture was dry evaporated and the crude product was recrystallized in acetone to give 80 mg (0.25 mmol, 22%) of 3x as an orange solid. 1H NMR (Acetone-d6): 6.70–6.80 (m, 1H); 7.28 (d, 1H, J = 3.7 Hz); 7.80–7.95 (m, 2H); 8.14 (d, 1H, J = 4.2 Hz); 10.84 (s, 1H). IR (KBr): 1365 cm-1. m.p.= 244–245°C. Analysis Calculated for C11H7N5O3S2: C, 41.12; H, 2.19; N, 21.79. Found: C, 40.79; H, 2.06; N, 21.58.
(Z)-5-(4-hydroxyphenyl)-4-(((5-nitrothiophen-2-yl)methylene)amino)-2,4-dihydro-3H-1,2,4triazole-3-thione 3y
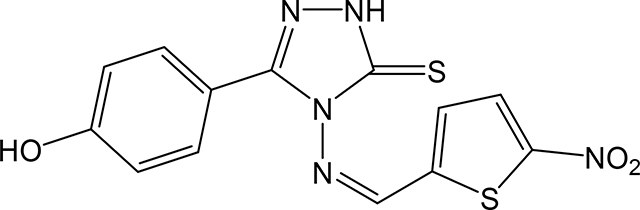
Compound 3y was prepared according to the above procedure using 4-amino-5-(4hydroxyphenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (0.1 g, 0.48 mmol), 5-nitro-2thiophenecarboxaldehyde (0.204 g, 1.29 mmol) and 4 mL of absolute ethanol. After filtration, the crude product was purified by column chromatography on silica (CH2Cl2) to give 89 mg (0.26 mmol, 53%) of 3y as an orange solid. 1H NMR (Acetone-d6): 6.99–7.03 (m, 2H); 7.80–7.85 (m, 2H); 7.86 (d, 1H, J = 4.3 Hz); 8.12 (d, 1H, J = 2.2 Hz). m.p.= 275–276°C. Analysis Calculated for C13H9N5O3S2: C, 44.95; H, 2.61; N, 20.16. Found: C, 45.57; H, 2.76; N, 19.46.
(Z)-5-(furan-2-yl)-4-(((5-nitrofuran-2-yl)methylene)amino)-2,4-dihydro-3H-1,2,4-triazole-3thione 3z
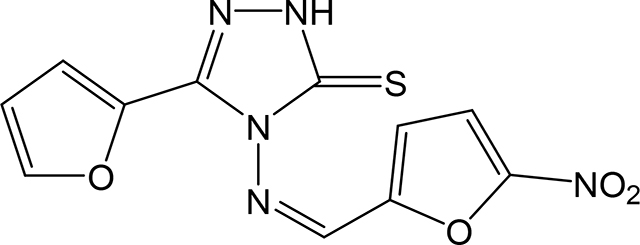
Compound 3z was prepared according to the above procedure using 4-amino-5-(furan-2-yl)-2,4dihydro-3H-1,2,4-triazole-3-thione (0.2 g, 1.09 mmol), 5-nitro-2-furaldehyde (0.385 g, 2.72 mmol) and 8 mL of anhydrous THF. The reaction mixture was dry evaporated and the crude product was recrystallized in acetone to give 82 mg (0.27 mmol, 25%) of 3z as an orange solid. 1H NMR (Acetone-d6): 6.73 (s, 1H); 7.43 (d, 1H, J = 3.6 Hz); 7.62 (d, 1H, J = 3.9 Hz); 7.71 (d, 1H, J = 3.9 Hz); 7.84 (s, 1H); 10.77 (s, 1H). m.p.= 177–179°C. Analysis Calculated for C11H7N5O4S: C, 43.28; H, 2.31; N, 22.94. Found: C, 43.36; H, 2.44; N, 22.30.
Conclusion
While investigating a class 4-(R2-imino)-5-(R1)-2,4-dihydro-3H-1,2,4-triazole-3-thiones, potentially useful as inhibitors of NO production in PAM212 cells, we also established their potential usefulness as anti-neoplastic agents. The initial findings were obtained with a murine keratinocyte cell line (PAM212) but were also extended to human cancer cell lines (HT-29, PC3 and HeLa), demonstrating the potency of such compounds to efficiently inhibit the growth of such malignant cell lines. Amongst some of the most active compounds were the nitrofuran and the isosteric nitrothiophenes, designed to structurally mimic nitrofurantoin. It should be noted that the mechanism of iNOS inhibition by our compounds is not known. Further studies are needed to determine if the mechanism involves direct interaction with the iNOS protein (e.g., competitive inhibition, inhibition of active iNOS dimerization) or inhibition of iNOS expression.(26)
A set of synthesized and fully characterized 1,2,4-triazole imines were evaluated as iNOS inhibitors
A sub-set, within that group, also showed promising inhibitory growth properties against a range of human cancer cell lines (PC3, HeLa, HT-29).
Within that subset, the compounds designed to be nitrofurantoin mimics showed the best anti-neoplastic activity.
Acknowledgments
This work was funded in part by the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH Grant number U54AR055073). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government.
Footnotes
The authors listed on the submitted article entitled, have no conflict of interest, financial or otherwise, to declare.
All authors are academics and no one is in the employment of a corporation. These candidate compounds are not under consideration for commercial development.
Christophe Guillon, on behalf of the other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).Lacey CJ, Wohlman I, Guillon C, Saxena J, Heindel ND et al. , Multi-inhibitor prodrug constructs for simultaneous delivery of anti-inflammatory agents to mustard-induced skin injury. Ann. N Y Acad. Sci. 1378 (1) (2016) 174–179. 10.1111/nyas.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Egolf RA, Heindel ND, Structure-activity relationships in NOS inhibitors,” in, Laskin JD, Laskin DJ (Eds.), Cellular & Molecular Biology of Nitric Oxide, Marcel Dekker, NY, 1999, pp 125–136. [Google Scholar]
- 3).Joubert J, Malan SF, Novel nitric oxide synthase inhibitors: a patent review, Expert. Opin. Ther. Pat. 21 (4) 2011. 537–560. 10.1517/13543776.2011.556619. [DOI] [PubMed] [Google Scholar]
- 4).Vermuyten K, Laurijssens L, Vanden Bossche H, Azole antifungals as weak inhibitors of inducible nitric oxide synthase in mouse and human cells, Mycoses 40 1997, 40 119–125. 10.1111/j.1439-0507.1997.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 5).Del Carratore R, Carpi A, Beffy P, Lubrano V, Giorgetti L, Maserti BE, Carluccio MA, Simili M, Iervasi G, Balzan S Itraconazole inhibits HMEC-1 angiogenesis. Biomed. Pharmacother. 66 (4) 2012. 312–317. 10.1016/j.biopha.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 6).Gutiérrez-Venegas G, Maldonado-Frías S, Ontiveros-Granados A, Kawasaki-Cárdenas P, Role of P p38 in nitric oxide synthase and cyclooxygenase expression, and nitric oxide and PGE2 synthesis in human gingival fibroblasts stimulated with lipopolysaccharides, Life Sci. 77 (1) 2005. 60–73, 10.1016/j.lfs.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 7).Jeong YH, Kim Y, Song H, Chung YS, Park SB, Kim HS, Anti-Inflammatory effects of α-Galactosylceramide analogs in activated microglia: Involvement of the p38 MAPK signaling pathway PLoS One 9 (2) 2014. e87030 10.1371/journal.pone.0087030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Buchmüller-Rouiller Y, Schneider P, Betz-Corradin S, Smith J, Mauël J, 3-Amino-1,2,4triazole inhibits macrophase NO synthase, Biochem. Biophys. Res. Commun. 183 1992. 150–155. 10.1016/0006-291X(92)91621-V. [DOI] [PubMed] [Google Scholar]
- 9).Stefani HA, Gueogjan K, Manarin F, Farsky SH, Zukerman-Schpector J, Caracelli I, Rodrigues SR, Muscará MN, Teixeira SA, Santin JR, Machado ID, Bolonheis SM, Curi R, Vinolo MA, Synthesis, biological evaluation and molecular docking studies of 3(triazolyl)-coumarin derivatives: Effect on inducible nitric oxide synthase, Eur. J. Med. Chem. 58 2012. 117–127. 10.1016/j.ejmech.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 10).Holden JK, Li H, Jing Q, Kang S, Richo J, Silverman RB, Poulos TL, Structural and biological studies on bacterial nitric oxide synthase inhibitors, PNAS 110 (45) 2013. 18127– 18131. 10.1073/pnas.1314080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Xu JF, Qu JM, Li HP, N-Acetylcysteine moderates acute lung injury induced by Pseudomonas aeruginosa in rats, Clin. Exp. Pharmacol. Physiol. 38 (5) 2011. 345–351. 10.1111/j.1440-1681.2011.05515.x. [DOI] [PubMed] [Google Scholar]
- 12).Wang A, Nizran P, Malone MA, Riley T, Urinary tract infections, Prim. Care 40 (3) 2013. 687–706. 10.1016/j.pop.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 13).Chen YH, Ko WC, Hseuj PR, Emerging resistance problems and future perspectives in pharmacotherapy for complicated urinary tract infections, Expert. Opin. Pharmacother. 14 (5) 2013. 587–596. 10.1517/14656566.2013.778827. [DOI] [PubMed] [Google Scholar]
- 14).Abu-al-Basal MA, Histological evaluation of the healing properties of Dead Sea black mud on full-thickness excision cutaneous wounds in BALB/c mice, Pak. J. Bio. Sci. 15 (7) 2012. 306–315. 10.3923/pjbs.2012.306.315. [DOI] [PubMed] [Google Scholar]
- 15).Sandifer SH, Clinical trial of topical nitrofurazone, with or without hydrocortisone, in 252 children with skin infections, J. S. C. Med. Assoc 66 (10) 1970. 363–365. [PubMed] [Google Scholar]
- 16).Galimberti E, Forlani A, D’Atri G, Protection against experimental neuritis caused by Nitrofurantoin, Boll. Chim. Farm. 119 (3) 1980. 164–71. [PubMed] [Google Scholar]
- 17).Laskin JD, Heck DE, Heindel ND, Vetrano AM, Guillon CD, DeMatteo P, Fluorescent fused-ring triazoles that inhibit cell proliferation, 2006. U.S. Patent 7,105,511. [Google Scholar]
- 18).Laskin JD, Heck DE, Guillon GB, Finetti T, Hunter A, Guillon CD, Rapp RD, Vetrano AM, Heindel ND, Furyl and thienyl triazole derivatives and therapeutic Uses thereof, 2016. U. S. Patent 9,290,484. [Google Scholar]
- 19).Reid JR, Heindel ND, Improved syntheses of 5-substituted-4-amino-3-mercapto-(4H)1,2,4-triazoles, J. Heterocycl. Chem. 13 (4) 1976. 925–926. 10.1002/jhet.5570130450. [DOI] [Google Scholar]
- 20).Heck DE, Laskin DL, Gardner CR, Laskin JD, Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. Potential role for nitric oxide in the regulation of wound healing, J. Biol. Chem. 267 (30) 1992. 21277–21280. https://www.jbc.org/content/267/30/21277.long. [PubMed] [Google Scholar]
- 21).Lahti A, Kankaanranta H, Moilanen E P38 mitogen-activated protein kinase inhibitor SB203580 has a bi-directional effect on iNOS expression and NO production. Europ. J. Pharmacol 454 (2–3) 2002. 115–123. 10.1016/S0014-2999(02)02490-1. [DOI] [PubMed] [Google Scholar]
- 22).Zheng R, Heck DE, Mishin V, Black AT, Shakarjian MP, Kong AN, Laskin DL, Laskin JD, Modulation of keratinocyte expression of antioxidants by 4-hydroxynonenal, a lipid peroxidation end product, Toxicol. Appl. Pharmacol. 275 (2) 2014. 113–21. 10.1016/j.taap.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Wang Y, Gray JP, Mishin V, Heck DE, Laskin DL, Laskin JD, Role of cytochrome P450 reductase in nitrofurantoin-induced redox cycling and cytotoxicity, Free Radic. Biol. Med. 44 (6) 2008. 1169–1179. 10.1016/j.freeradbiomed.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Gray J, Mishin V, Wang Y, Heck DE and Laskin JD, Structurally diverse phytochemicals block xenobiotic-induced oxidative stress in prostate tumor cells by inhibiting flavin-containing NADPH oxidases, Cancer Res. 67 (9 Supplement) 2007. 4453. [Google Scholar]
- 25).Yurkow EJ, Laskin JD, Characterization of a photoalkylated psoralen receptor in HeLa cells, J. Biol. Chem. 262 (18) 1987. 8439–42. https://www.jbc.org/content/262/18/8439.long. [PubMed] [Google Scholar]
- 26).Cinelli MA, Do HT, Miley GP, Silverman RB Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 40 (1) 2020. 158–189. 10.1002/med.21599 [DOI] [PMC free article] [PubMed] [Google Scholar]