Abstract
Objective
To identify early life adversity (ELA) risk factors for earlier pubertal timing, itself a risk factor for poor cardiometabolic health, and to determine whether such ELA-related risk may be mediated by pre-pubertal body mass index (BMI).
Methods
Subjects included 426 female participants in a prospective birth cohort study, the NICHD Study of Early Child Care and Youth Development. Survival analysis models were fit to examine ELA exposures, representing childhood socioeconomic status (SES), maternal sensitivity, mother–child attachment, and negative life events, along with child health indicators and covariates, in relation to pubertal timing outcomes, including age at menarche and ages at Tanner stage II for breast and pubic hair development.
Results
Higher childhood SES emerged as an independent predictor of older age at menarche, showing each one standard deviation increase in childhood SES corresponded to a 1.3% increase in age at menarche (factor change = 1.013; 1.003–1.022; p < .01), but did not predict breast or pubic hair development (ps > .05). In mediation analyses, indirect (mediated) effects of mother–child attachment on the pubertal timing outcomes, via pre-pubertal BMI, were all statistically significant (ps < .05).
Conclusions
Higher childhood SES predicted directly, and secure (vs. insecure) mother–child attachment predicted indirectly (via pre-pubertal BMI), later pubertal timing, suggesting these factors may protect girls from earlier pubertal development. By extension, clinical implications are that intervention strategies designed to lessen ELA- and pre-pubertal obesity-related risk may be effective in remediating life course pathways linking ELA, accelerated pubertal development, and cardiometabolic risk.
Keywords: cardiometabolic disease, chronic illness, endocrinology, family functioning, health behavior, health promotion and prevention, longitudinal research, obesity, parenting, prevention/control, psychosocial functioning, pubertal timing, stress
Introduction
The timing of pubertal development in girls has a profound impact on their longer-term cardiometabolic health and disease risk. Earlier pubertal timing predicts the emergence of cardiovascular risk factors in adolescence and a worsening of these risk factors over time, as well as risk for obesity and type 2 diabetes in adulthood (Frontini et al., 2003; He et al., 2010; Widen et al., 2012). In one study, for example, girls who were younger at menarche experienced greater increases in insulin, glucose, and blood pressure over a 13-year study period (ages 8–21) (Remsberg et al., 2005). Earlier pubertal timing also predicts incident cardiovascular disease and early mortality in adulthood. Studies, for example, show younger age at menarche predicted higher incident cardiovascular disease and all-cause mortality among 15,807 women followed over 10.6 years as well as increased risk for cardiac-specific, stroke-specific, and all-cause mortality among 61,319 women followed over 37 years (Jacobsen et al., 2007, 2009; Lakshman et al., 2009).
The robust association between pubertal development and cardiometabolic risk highlights the importance of identifying pre-pubertal risk factors for earlier pubertal timing that, if mitigated, could plausibly improve trajectories of cardiometabolic health over time. A growing literature points broadly to the role of early life adversity (ELA) exposures in shaping adulthood cardiometabolic health (Suglia et al., 2018, 2020). One of the origins of this literature stems from early findings in The Adverse Childhood Experiences Study in which multiple categories of adverse childhood exposures were related to adulthood diseases, including cardiovascular disease (Felitti et al., 1998). ELA exposures represent a range of experiences that threaten a child’s physical or emotional security and are hypothesized to disrupt typical development, thereby promoting underlying disease processes in neuroendocrine, immune, and metabolic systems (Berens et al., 2017). ELA exposures are common in the population with 60% of adults reporting that they experienced at least one type of adversity [Centers for Disease Control and Prevention (CDC), 2010], highlighting the potential for ELA’s far-reaching impact on health.
With respect to ELA exposures and pubertal development specifically, ELA exposures, reflecting variability in parenting quality, parent–child relationships, and family stressors, including socioeconomic disadvantage, have all been related to the timing of puberty (Belsky et al., 2007, 2010; Bleil et al., 2013; Deardorff et al., 2014; Ellis & Essex, 2007; Hiatt et al., 2017). For example, greater maternal parenting support and higher socioeconomic status (SES), assessed in preschool, were both related prospectively to later pubertal development indexed by a composite of mother ratings of breast and pubic hair development in fifth grade (Ellis & Essex, 2007). In addition, evidence suggests higher pre-pubertal body mass index (BMI), a well-established predictor of earlier pubertal timing (Biro et al., 2013; Juul et al., 2017), is itself influenced by ELA exposures (Suglia et al., 2012). This suggests BMI may play a mediating role in transmitting ELA-related risk for earlier pubertal timing, although this potential pathway, leading ultimately to worse cardiometabolic health, remains poorly understood.
The current study included 426 female participants in the landmark NICHD Study of Early Child Care and Youth Development (SECCYD). Prospective models examined ELA exposures, representing early life SES, maternal sensitivity, mother–child attachment, and negative life events, along with annual health assessments and relevant covariates, in relation to state-of-the-art assessments of pubertal timing outcomes. The study objectives were (a) to identify specific ELA risk factors for earlier pubertal timing and (b) to determine whether ELA-related risk for earlier pubertal timing may be mediated by pre-pubertal BMI.
Methods
Participants
Subjects in the current study were participants in the NICHD SECCYD, a prospective study of children and their families recruited at birth to examine trajectories of health and development across early childhood (birth to 54 months), middle childhood (kindergarten through to fifth grade), and adolescence (sixth grade through 15 years of age). Families were recruited from 10 study sites in the United States: Charlottesville, VA; Irvine, CA; Lawrence, KS; Little Rock, AR; Madison, WI; Morganton, NC; Philadelphia, PA; Pittsburgh, PA; Seattle, WA; and Wellesley, MA. In the first 11 months of 1991, all mother–infant dyads of babies born within pre-selected 24-hr intervals at participating hospitals were screened. Families were excluded if the (a) mother was <18 years old; (b) mother was non-English speaking; (c) family was re-locating within 1 year; (d) infant or mother had a serious medical problem; (e) mother had a substance use disorder; (f) infant was placed for adoption; (g) family lived >1 hr away from the study site; (h) family was already participating in another study; and/or (i) family refused to participate in the initial study interview. Additional sampling requirements (e.g., 10% recruitment of single-parent households) ensured that the socio-demographic composition of the final sample [N = 1,364 families; n = 659 girls (48.3%) and n = 705 boys (51.7%)] was similar to the population for families living in the same geographic regions, according to the 1990 U.S. Census. Retained for analysis in the current study were 426 girls (64.6% of the 659 girls originally recruited) who participated in at least one assessment of pubertal development between ages 9 and 15.5 years and who agreed to be followed for future assessments. In attrition analyses, logistic models regressing the retention indicator onto race/ethnicity and SES indicators (maternal and paternal/partner education, family income-to-needs ratio) showed only maternal education had a significant, independent effect [odds ratio (OR) = 1.10; confidence interval (CI): 1.03–1.17; p < .01], reflecting higher odds of retention in the current sample among girls with more educated mothers. Informed consent was obtained from parents and assent was obtained from children when they were old enough to do so. The study was approved by the Institutional Review Boards of each university-based study site, including the Human Subjects Division of the University of Washington.
Measures
Pubertal Timing
Medical providers (physicians or nurse practitioners) were trained and re-certified annually to conduct a physical exam each year between child ages 9.5 and 15.5 years (Susman et al., 2010) in which stage of sexual maturity was determined using Tanner criteria (Dorn et al., 2006; Marshall & Tanner, 1969), augmented by breast bud palpation. In this staging system, photographs are used as the point of comparison by which stages of sexual maturity for breast and pubic hair development are rated separately (I–V), ranging between stage I (pre-puberty) and stage V (full sexual maturity) with girls who are between stages assigned to the earlier stage (Herman Giddens et al., 1997). Annual evaluations continued until menarche and full sexual maturity for breast and pubic hair development were reached. In addition, age at menarche was determined by querying the girls and their mothers. Mothers’ reports were used if girls’ reports were missing.
Derived from these exams, three indicators of pubertal timing, all measured in years, were examined as the primary outcomes of interest in the current study: (a) age at menarche; (b) age at Tanner stage II for breast development, marking the initiation of gonadarche; and (c) age at Tanner stage II for pubic hair development, marking the initiation of adrenarche. The Tanner stage outcomes were both left and interval censored, as described below.
ELA Exposures
Child SES in early childhood was indexed by a composite of mother and father/partner’s education and family income-to-needs ratio. Educational attainment of the mother and father/partner at child’s age 1 month was ascertained by self-report: 1 = less than high school; 2 = high school or general education diploma; 3 = some college or vocational degree; 4 = college degree; 5 = some graduate school or master’s degree; and 6 = graduate degree greater than a master’s degree. Income-to-needs ratio (i.e., family income expressed as a proportion of the federal poverty line for a family of a particular size) was derived by self-report of family income at child’s ages 1, 6, 15, 24, and 36 months. Separate means, taken across the indicated time points for parental education and income-to-needs ratio, were then standardized, averaged, and re-standardized to produce a single composite of SES in early childhood. Mother–child attachment in early childhood was assessed using the Strange Situation Procedure (SSP) at age 15 months, the Attachment Q-Sort (AQS) at age 24 months, and the Modified Strange Situation Procedure (MSSP) at age 36 months (Ainsworth et al., 1978; Cassidy & Marvin, 1992; Waters & Deane, 1985). The SSP and MSSP both entailed videotaped separation–reunion tasks designed to evoke attachment behaviors in the child that were then coded secure (vs. insecure). The AQS included 90 items that were sorted by similarity to the child based on a 2-hr home observation; sorts correlated with the criterion sort at .30 or above were then coded secure (vs. insecure). Interrater agreement was 83% (к = .69) and 76% (к = .58) for the SSP and the MSSP, respectively, and the interclass correlation was .96 for the AQS. The validity of early attachment measures has been well-established as shown in meta-analyses focused on their antecedents (De Wolff & van IJzendoorn, 1997) and predictive and discriminant validity (Groh et al., 2017). Next, utilizing these dichotomized variables, the proportion of times the child was coded secure (vs. insecure) across all assessments (ages 15, 24, and 36 months) was computed to produce a composite of mother–child attachment status in early childhood (Groh et al., 2014). Maternal sensitivity in early childhood was assessed according to 15-min semi-structured interactions between mothers and their children at child ages 6, 15, 24, and 36 months which were videotaped and coded by trained raters; intraclass correlations, reflecting intercoder reliability, were .87, .83, .84, and .84 for each assessment, respectively (Appelbaum et al., 1999). Segments involved developmentally appropriate play-based tasks in which the mother provided assistance to her child, enabling the observation of maternal attentiveness and appropriate responding to child cues. The mean of observational ratings across all assessments (ages 6, 15, 24, and 36 months) was computed to produce a composite of maternal sensitivity in early childhood. Predictive validity of this early maternal sensitivity measure is well-established (Fraley et al., 2013). Negative life events in early childhood were assessed using the Life Experiences Survey (LES) (Sarason et al., 1978) completed by mothers at child age 54 months. The LES includes a list of 57 life events, ranging in nature from routine (e.g., start of school) to traumatic (e.g., death of a parent). Mothers reported whether each event occurred in the past year, rating the impact of endorsed events on a 7-point scale (−3 = very negative… 0 = neutral… 3 = very positive). All items with a negative impact (rated −3 to −1) were then summed to produce a composite score reflecting the number of negative life events during this year period in early childhood. The negative life events scale has demonstrated adequate test–retest reliability (r = 0.56–0.88) and validity as supported by its correlation with related constructs (Sarason et al., 1978).
Pre-Pubertal Health Indicators
BMI: pre-pubertal BMI (weight in kg/height in m2) was derived using measurements of height and weight at child age 24 months and grade 1. BMI z-scores (zBMI) were then calculated using the 2000 CDC BMI-for-age clinical growth charts for girls. Mother-rated child health: pre-pubertal health was assessed using mother reports of child health problems [i.e., ear infections requiring medication, respiratory problems (runny nose, cough, or cold), and intestinal problems (vomiting, diarrhea, not eating)], summed to produce a count of total health problems. Assessments occurred at child ages 1, 3, 6, 9, 12, 15, 24, 36, 42, 50, 54, 60, 66 months and in kindergarten. Means were taken in periods: infancy (0–36 months), preschool (42–54 months), and early school (60 months–kindergarten) to produce composites of pre-pubertal child health problems.
The ELA exposures and health indicators were measured at time points between periods of infancy, early childhood, and early school (i.e., kindergarten) to ensure the pre-pubertal measures did not overlap with the pubertal period. In addition, zBMI, measured in grade 1, was selected for testing as a potential mediator of ELA effects on pubertal timing. Its slightly later time point (grade 1) was chosen to ensure temporal separation between the ELA exposures, zBMI (grade1) as a potential mediator, and the pubertal timing outcomes.
Covariates
Covariates included maternal menarcheal age, child race/ethnicity, and study recruitment site. Maternal menarcheal age (in years) was assessed by self-report of mothers queried at three different assessments from which the mean was computed. Child race/ethnicity was coded according to mother reports in five categories: Asian, African American, Latina, ‘other’ race, and white. Study recruitment site was coded in 10 categories, reflecting the locations from which the participants were recruited.
Analytical Plan
Survival analysis models examined predictors of age at key pubertal timing events. Specifically, separate accelerated failure time models with Weibull distributions were fit to each of three pubertal timing outcomes: age at menarche; age at Tanner stage II for breast development; and age at Tanner stage II for pubic hair development. Accelerated failure time models accommodated left and interval censoring of outcomes describing ages at transition to Tanner stage II for breast and pubic hair development. The outcome describing age at menarche was modeled as uncensored. All specified predictors were modeled simultaneously, including the covariates and each of the ELA exposures and child health indicators. The final models reflect the variables remaining after forced retention of covariates and backward elimination of ELA and child health effects with p > .05. Factor change (FC) coefficients are reported, which represent multiplicative effects on pubertal timing outcomes per one-unit increase in the corresponding predictor. Next, to examine the role of zBMI (grade 1) as a potential mediator of effects of the ELA exposures on the pubertal timing outcomes, multivariate linear models were fit to estimate the effects of each ELA exposure on zBMI (grade 1), conditional on covariates and the child health indicators; linear regression parameter estimates are reported. Combining results of the zBMI (grade 1) outcome model with each of the pubertal timing outcome models, we then estimated indirect effects of the ELA exposures on pubertal timing outcomes, via zBMI (grade 1); FC coefficients are reported for the indirect effects as well as partial posterior p-values and hierarchical Bayes 95% CIs (Falk & Biesanz, 2016). Across all participants and variables included in analyses, 10.9% of all data values were missing. Missing values were multiply imputed to create 30 completed data sets; parameter and standard errors were estimated by combining results across imputed data sets (Rubin, 1987).
Results
Descriptive Analyses
In Table I, sample characteristics, including covariates, ELA exposures, and child health indicators are reported. The racial/ethnic composition included 1.2% Asian, 11.0% African American, 4.9% Latina, 3.8% ‘other’ race, and 79.1% white children. For both mothers and fathers/partners, 39% received a college degree or higher with 12.0% of families reporting incomes below the federal poverty line. Consistent with previous studies of attachment security (Moullin et al., 2014), 63.4%, 59.3%, and 57.5% of children were classified as ‘secure’ (vs. insecure) at the 15-, 24-, and 36-month assessments, respectively. The maternal sensitivity composite score averaged 9.5 (range: 4.8–12.0) and children experienced 3.3 (range: 0–27) negative life events on average over a 1-year period between ages 3 and 4 years. With respect to the child health indicators, zBMI mean values at child age 24 months and grade 1 were 0.11 (SE = 0.044) and 0.41 (SE = 0.044), respectively. In addition, also at child age 24 months and grade 1, 86.8% and 79.0% of girls fell between 0 and 84.9 BMI percentile, 8.6% and 12.2% between 85 and 94.9 BMI percentile, and 4.6% and 8.8% at 95+ BMI percentile, respectively. Mother-reported number of child health problems was just greater than 1 on average across assessments in all developmental periods. With respect to the pubertal timing outcomes, age at menarche averaged 12.4 years (range: 9.0–15.6). Derived from accelerated failure time models, the predicted median age at Tanner stage II for breast development was 9.9 (CI: 9.611–10.232) years and the predicted median age at Tanner stage II for pubic hair development was 10.5 (CI: 10.284–10.820) years. In this sample of predominately white girls, these mean and median ages align closely with similar samples (Biro et al., 2013; Chumlea et al., 2003).
Table I.
Sample Characteristics (n = 426)
| Covariates | Mean (SE) | Range | n (%) |
|---|---|---|---|
| Maternal menarcheal age | 12.76 (0.073) | 9.0–18.1 | – |
| Child race/ethnicity | |||
| Asian (%) | – | – | 5 (1.2) |
| African American (%) | – | – | 47 (11.0) |
| Latina (%) | – | – | 21 (4.9) |
| Other (%) | – | – | 16 (3.8) |
| White (%) | – | – | 337 (79.1) |
| ELA exposures | |||
| Mother, college degree+ (%) | – | – | 166 (39.0) |
| Father/partner, college degree+ (%) | – | – | 167 (39.2) |
| Families below poverty (%) | – | – | 51 (12.0) |
| Attachment 15 months (% secure) | – | – | 270 (63.4) |
| Attachment 24 months (% secure) | – | – | 253 (59.3) |
| Attachment 36 months (% secure) | – | – | 245 (57.5) |
| Maternal sensitivity | 9.50 (0.062) | 4.8–12.0 | – |
| Negative life events | 3.30 (0.170) | 0.0–27.0 | – |
| Child pre-pubertal health | |||
| zBMI, 24 months | 0.11 (0.044) | −2.6 to 2.6 | – |
| zBMI, grade 1 | 0.41 (0.044) | −1.8 to 2.6 | – |
| Health problems count (infancy) | 1.25 (0.027) | 0.0–3.0 | – |
| Health problems count (preschool) | 1.31 (0.032) | 0.0–3.0 | – |
| Health problems count (early school) | 1.25 (0.040) | 0.0–3.0 | – |
Note. SE = standard error; zBMI = BMI z-score.
Unadjusted Models
In Table II, results of unadjusted accelerated failure time models are reported for effects of ELA exposures and child health indicators on pubertal timing outcomes. Results showed higher childhood SES predicted older age at menarche (FC = 1.018; CI: 1.010–1.027; p < .0001) and older age at Tanner stage II breast development (FC = 1.020; CI: 1.006–1.034; p < .01). Each one standard deviation increase in childhood SES corresponded to a 1.8% increase in age at menarche and a 2.0% increase in age at Tanner stage II breast development. Greater maternal sensitivity also predicted older age at menarche (FC = 1.013; CI: 1.006–1.019; p < .001) and older age at Tanner stage II breast development (FC = 1.012; CI: 1.000–1.023; p < .05). Each one-unit increase in maternal sensitivity corresponded to a 1.3% increase in age at menarche and a 1.2% increase in age at Tanner stage II breast development. Mother–child attachment and negative life events, however, did not predict pubertal timing outcomes. With respect to child health, zBMI assessed at 24 months predicted age at Tanner stage II breast development only (FC = 0.978; CI: 0.963–0.994; p < .01), while zBMI, assessed more proximally in grade 1, predicted all three pubertal timing outcomes. Each one standard deviation increase in BMI in grade 1 corresponded to a 2.1% decrease in age at menarche (FC = 0.979; CI: 0.970–0.989; p < .0001), a 4.5% decrease in age at Tanner stage II for breast development (FC = 0.955; CI: 0.941–0.970; p < .0001), and a 2.0% decrease in age at Tanner stage II for pubic hair development (FC = 0.980; 0.967–0.992; p < .01). Mother reports of child health problems did not predict the pubertal timing outcomes.
Table II.
Unadjusted FC Coefficients Examining ELA Exposures and Child Pre-Pubertal Health Indicators in Relation to Pubertal Timing Outcomes
| Age at menarche |
Age at Tanner stage II breast development |
Age at Tanner stage II pubic hair development |
||||
|---|---|---|---|---|---|---|
| FC | 95% CI | FC | 95% CI | FC | 95% CI | |
| ELA composites | ||||||
| Childhood SES | 1.018**** | 1.010–1.027 | 1.020** | 1.006–1.034 | 1.008 | 0.996–1.020 |
| Mother–child attachment | 1.018 | 0.990–1.047 | 1.016 | 0.971–1.063 | 1.016 | 0.979–1.053 |
| Maternal sensitivity | 1.013*** | 1.006–1.019 | 1.012* | 1.000–1.023 | 1.006 | 0.971–1.063 |
| Negative life events | 0.999 | 0.997–1.002 | 0.997 | 0.993–1.002 | 1.002 | 0.996–1.015 |
| Child pre-pubertal health | ||||||
| zBMI, 24 months | 0.992 | 0.981–1.002 | 0.978** | 0.963–0.994 | 0.988 | 0.975–1.001 |
| zBMI, grade 1 | 0.979**** | 0.970–0.989 | 0.955**** | 0.941–0.970 | 0.980** | 0.967–0.992 |
| Health problems (infancy) | 1.006 | 0.999–1.023 | 0.992 | 0.962–1.023 | 1.005 | 0.982–1.029 |
| Health problems (preschool) | 1.013 | 0.999–1.028 | 1.001 | 0.977–1.025 | 1.006 | 0.987–1.026 |
| Health problems (early school) | 1.008 | 0.996–1.021 | 1.001 | 0.981–1.021 | 1.000 | 0.983–1.017 |
Note. CI = confidence interval; ELA = early life adversity; FC = factor change; SES = socioeconomic status; zBMI = BMI z-score.
p < .05;
p < .01;
p < .001;
p < .0001.
Final Adjusted Models
In Table III, results of final, adjusted accelerated failure time models are reported for effects of ELA exposures, child health indicators, and covariates on each pubertal timing outcome: (a) age at menarche (model 1); (b) age at Tanner stage II for breast development (model 2); and (c) age at Tanner stage II for pubic hair development (model 3). Outcome values were left censored for 46.3% and 30.3% of the sample for Tanner stage II breast and pubic hair development, respectively; all other values on those outcomes were interval censored. With respect to the ELA exposures, in the final, adjusted models, only childhood SES predicted age at menarche. That is, higher childhood SES was related significantly to older age at menarche (FC = 1.013; CI: 1.003–1.022; p < .01). Each one standard deviation increase in childhood SES corresponded to a 1.3% increase in age at menarche. The other ELA exposures did not directly predict the pubertal timing outcomes. With respect to the child health indicators, in the final, adjusted models, higher zBMI assessed in grade 1 predicted all three pubertal timing outcomes. Each one standard deviation increase in BMI in grade 1 corresponded to a 1.9% decrease in age at menarche (FC = 0.981; CI: 0.972–0.990; p < .0001), a 3.9% decrease in age at Tanner stage II for breast development (FC = 0.961; CI: 0.948–0.976; p < .0001), and a 1.7% decrease in age at Tanner stage II for pubic hair development (FC = 0.983; CI: 0.971–0.995; p < .01). Finally, with respect to the covariates, maternal menarcheal age and child race/ethnicity also predicted all three pubertal timing outcomes. Each 1-year increase in maternal age at menarche corresponded to a 1.9% increase in age at menarche (FC = 1.019; CI: 1.014–1.024; p < .0001), a 1.3% increase in age at Tanner stage II breast development (FC = 1.013; CI: 1.004–1.021; p < .01), and a 1.2% increase in age at Tanner stage II pubic hair development (FC = 1.012; CI: 1.005–1.019; p < .001). Comparison of racial/ethnic groups showed that African American (vs. white) girls had median transition ages that were 3.3% younger at menarche (FC = 0.967; CI: 0.939–0.996; p < .05), 8.8% younger at Tanner stage II breast development (FC = 0.912; CI: 0.874–0.952; p < .0001), and 9.3% younger at Tanner stage II pubic hair development (FC = 0.907; CI: 0.874–0.940; p < .0001).
Table III.
Adjusted FC Coefficients Examining Effects of Pre-Pubertal Adversity and Health Factors on Pubertal Timing Outcomes Following Backward Elimination of Effects With p > .05 and With Adjustment, Through Forced Retention, of Covariates
| Adversity effects on pubertal timing outcomes |
||||||
|---|---|---|---|---|---|---|
| Model 1: Age at menarche |
Model 2: Age at Tanner stage II (breast) |
Model 3: Age at Tanner stage II (pubic hair) |
||||
| FC | 95% CI | FC | 95% CI | FC | 95% CI | |
| Predictors | ||||||
| Child adversity exposures | ||||||
| Childhood SES | 1.013** | 1.003–1.022 | – | – | – | – |
| Mother–child attachment | – | – | – | – | – | – |
| Maternal sensitivity | – | – | – | – | – | – |
| Negative life events | – | – | – | – | – | – |
| Child health factorsa | ||||||
| zBMI (24 months) | – | – | – | – | – | – |
| zBMI (grade 1) | 0.981**** | 0.972–0.990 | 0.961**** | 0.948–0.976 | 0.983** | 0.971–0.995 |
| Covariatesb | ||||||
| Maternal menarcheal age | 1.019**** | 1.014–1.024 | 1.013** | 1.004–1.021 | 1.012*** | 1.005–1.019 |
| Child race/ethnicity: | ||||||
| Asian (vs. white) | 0.954 | 0.891–1.022 | 0.941 | 0.836–1.059 | 0.988 | 0.897–1.089 |
| African American (vs. white) | 0.967* | 0.939–0.996 | 0.912**** | 0.874–0.952 | 0.907**** | 0.874–0.940 |
| Latina (vs. white) | 0.990 | 0.952–1.029 | 0.985 | 0.930–1.043 | 1.000 | 0.951–1.052 |
| Other (vs. white) | 0.973 | 0.933–1.016 | 0.992 | 0.920–1.070 | 1.019 | 0.963–1.078 |
Note. CI = confidence interval; FC = factor change; SES = socioeconomic status; zBMI = BMI z-score. Cells with a dash ‘-’ indicate effects that were dropped from the corresponding model via backward elimination.
For brevity, variables pertaining to mother-reported child health problems are not listed; all effects were p > .05.
All covariates (maternal menarcheal age, child race/ethnicity, and recruitment site) were forced to remain in the models; effects of recruitment site are not tabled.
p < .05;
p < .01;
p < .001;
p < .0001.
Mediation Analyses
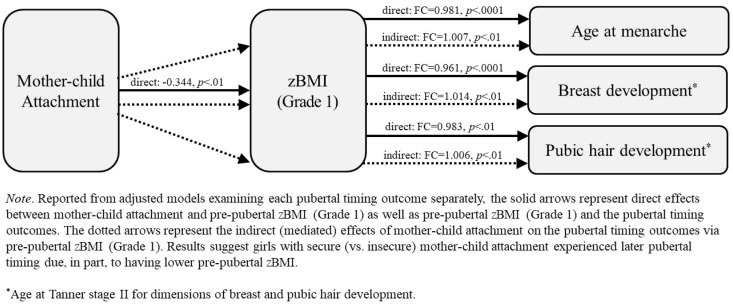
To examine the role of zBMI (grade 1) as a potential mediator of ELA exposure effects on the pubertal timing outcomes, multivariate linear models were first fit to estimate the effects of each ELA exposure on zBMI, conditional on the child health indicators and covariates. Results showed that having a secure (vs. insecure) mother–child attachment pattern predicted lower zBMI (estimate = −0.344 standard deviations; CI: −0.572 to −0.116; p < .01). Combining results of the zBMI outcome model with each of the pubertal timing outcome models, indirect (mediated) effects were then calculated, revealing mother–child attachment effects on each pubertal timing outcome via zBMI were statistically significant: age at menarche (indirect effect: FC = 1.007; CI: 1.002–1.013; p < .01), age at Tanner stage II breast development (indirect effect: FC = 1.014; CI: 1.004–1.025; p < .01), and age at Tanner stage II pubic hair development (indirect effect: FC = 1.006; CI: 1.001–1.013; p < .01). These findings suggest that girls with secure (vs. insecure) mother–child attachment tended to be older at menarche and to have later breast and pubic hair development due, in part, to having lower pre-pubertal BMI. Specifically, having a secure (vs. insecure) attachment pattern at all three assessments (ages 15, 24, and 36 months) corresponded to a 0.7% increase in age at menarche, a 1.4% increase in age at Tanner stage II breast development, and a 0.6% increase in age at Tanner stage II pubic hair development. These pathways are represented in Figure 1 with solid and dotted arrows for the direct and indirect effects, respectively. Importantly, no other ELA exposures were related to zBMI (grade 1). Therefore, zBMI did not mediate effects of any of the other ELA exposures, including childhood SES which was shown previously to be an independent predictor of age at menarche.
Figure 1.
Mediated effects of mother–child attachment via pre-pubertal body mass index on pubertal timing outcomes.
Discussion
The current study tested a series of life course models with a dual purpose: (a) to identify specific ELA risk factors for earlier pubertal timing, itself a risk factor for poor cardiometabolic health and (b) to characterize the role of pre-pubertal BMI in potentially mediating effects of ELA-related risk on pubertal timing. In unadjusted analyses, higher childhood SES and greater maternal sensitivity were related to older ages at menarche and the initiation of breast development. However, in adjusted, multivariate analyses, only higher childhood SES predicted older age at menarche. In addition, older maternal menarcheal age was related to older ages for all three pubertal timing outcomes, and African American (vs. white) race/ethnicity and greater pre-pubertal BMI were related to younger ages at all three pubertal timing outcomes—consistent with prior studies (Biro et al., 2013; Chumlea et al., 2003; Juul et al., 2017; Morris et al., 2011). Finally, in analyses of indirect effects, significant indirect (mediated) effects of mother–child attachment on the pubertal timing outcomes via pre-pubertal BMI were observed, suggesting girls with secure (vs. insecure) mother–child attachment patterns were older at all three pubertal timing outcomes due, in part, to having lower pre-pubertal BMI values (grade 1). In summary, direct and indirect effects of ELA exposures were limited to childhood SES and mother–child attachment, respectively, and were small in magnitude. The current study findings provide support for a modest role of ELA exposures when considering the early life origins of pathways linking ELA, accelerated pubertal development, and cardiometabolic risk.
SES and Pubertal Timing
A key finding was that childhood SES, indexed by a composite of mother and father/partner’s education and family income-to-needs ratio, emerged as an independent predictor of pubertal timing in analyses adjusted for well-established predictors of pubertal timing, including pre-pubertal BMI, maternal menarcheal age, and race/ethnicity. Higher childhood SES predicted older age at menarche but was unrelated to ages at the initiation of breast or pubic hair development, assessed by Tanner staging. Specifically, each one standard deviation increase in childhood SES corresponded to a 1.3% increase in age at menarche. Relative to the mean menarcheal age (12.4 years), a 1.3% increase would add about 2 months to the expected age at menarche—an effect that is small but on par with effects of other predictors such as maternal menarcheal age when examined in a multivariate framework.
The role of SES in predicting pubertal timing is consistent with findings in prior studies. In the Wisconsin Study of Family and Work, for example, higher SES in preschool was an independent predictor of later initiation of secondary sexual characteristics (Ellis & Essex, 2007). In two recent studies, higher SES similarly predicted later initiation of breast development assessed by Tanner staging (Hiatt et al., 2017) and later pubertal timing indexed by parent reports (Sun et al., 2017). In several other longitudinal studies, indicators of higher SES were shown to extend to the prediction of older age at menarche (Campbell & Udry, 1995; Deardorff et al., 2014; James-Todd et al., 2010; Windham et al., 2004). Non-significant findings, however, have also been reported (Ellis & Garber, 2000; Moffitt et al., 1992). In studies reporting significant associations with pubertal timing outcomes, the measurement of childhood SES typically included indicators of family income and parental education, either singly or in composites. In contrast, childhood SES was indexed by the Hollingshead index (reflecting educational attainment but also dimensions of marriage and occupation) and by parental occupation, respectively, in two studies reporting non-significant findings (Ellis & Garber, 2000; Moffitt et al., 1992), possibly explaining differences in results.
Future work is needed both to clarify nuances in the dimensions of childhood SES that may be most relevant to pubertal development and to identify mechanisms of childhood SES effects on pubertal timing. Effects of childhood SES in the current study were not mediated by pre-pubertal BMI, although it is plausible that other SES-related lifestyle or nutrition factors that were not measured may play a role. It is also plausible that SES may operate through other pathways. Lower SES, for example, has been related to greater exposure to endocrine-disrupting chemicals (Tyrrell et al., 2013) known to have deleterious impacts on reproductive health, including links to earlier pubertal timing (Buttke et al., 2012).
Mother–Child Attachment, BMI, and Pubertal Timing
Another key finding was that a significant, albeit indirect, role of mother–child attachment emerged, suggesting girls with a secure (vs. insecure) attachment pattern may experience reduced risk for earlier pubertal timing due, in part, to having lower pre-pubertal BMI. Specifically, having a secure (vs. insecure) attachment pattern at all three assessments (ages 15, 24, and 36 months) corresponded to a 0.7% increase in age at menarche and a 1.4% and 0.6% increase in age at Tanner stage II for breast and pubic hair development, respectively. Although these mediated effects are small, findings are intriguing in highlighting the role of BMI as a potential mechanism in explaining how certain ELA exposures may influence pubertal timing and for directing intervention efforts to consider child health behaviors associated with secure (vs. insecure) attachment patterns.
In a recent review, Bergmeier et al. (2020) proposed that the quality of early parent–child relationships, well-studied in relation to areas of child socio-emotional development, should also be examined in relation to trajectories of childhood weight gain and obesity risk. This assertion is supported by longitudinal studies reporting mother–child attachment as well as a composite of mother–child attachment and maternal sensitivity predicted obesity risk in childhood (age 4.5) (Anderson & Whitaker, 2011) and adolescence (age 15) (Anderson et al., 2012), as well as an increased likelihood of having a physical illness in adulthood 30 years later, albeit as assessed by self-reports of health conditions (Puig et al., 2013). An insecure mother–child attachment relationship among preadolescents also predicted changes in maladaptive eating behaviors and increases in BMI over a 1-year period (Goossens et al., 2012). Attachment theory posits that the ability of the primary caregiver to appropriately respond to the needs of the child, especially in times of distress, facilitates the development of self-regulatory processes in the child (Ainsworth et al., 1978). This has clear implications for understanding a host of behavioral outcomes including eating behaviors (Bergmeier et al., 2020). In fact, lower self-regulation has been related to maladaptive eating behaviors (Stoeckel et al., 2017), including decreased sensitivity to satiety (vs. external) food cues, emotional eating, and binge eating (Braden et al., 2014; Dingemans et al., 2017; Frankel et al., 2012).
Implications for Clinical Intervention
The clinical implications of the current study point to the possibility that interventions designed to mitigate the influence of ELA- and pre-pubertal obesity-related risk may be effective in reducing risk for earlier pubertal development and, thereby, cardiometabolic risk. In a recent scientific statement from the American Heart Association, recommendations included testing interventions which act on upstream ELA exposures to prevent cardiometabolic disease (Suglia et al., 2018). A review of early life interventions targeting the caregiving environments of ELA-exposed children, including children living in poverty, reported positive intervention-related impacts on a range of biological and health outcomes (Boparai et al., 2018). The authors concluded that interventions occurring earlier (vs. later) and those focused on enhancing parenting quality were most effective (Boparai et al., 2018), with specific intervention benefits observed in relation to metabolic syndrome, prediabetes, inflammation, and health behaviors (Brody et al., 2017, 2019; Chen et al., 2018; Miller et al., 2014). In addition, a recent intervention focused on ‘responsive parenting’ in areas of feeding, sleep, interactive play, and self-regulation showed intervention benefits related to the reduction of rapid weight gain and overweight status, improved infant feeding, and reduced screen time (Adams et al., 2018; Hohman et al., 2017; Savage et al., 2016). Together, these findings align with and extend the results of the current study, pointing to (a) parenting interventions among ELA-exposed children to improve cardiometabolic health outcomes and to (b) the augmentation of specific attachment-based parenting behaviors to prevent or reduce obesity risk in children. Extending these findings to pubertal timing outcomes, it is plausible that interventions to improve parenting may be health protective either by mitigating direct impacts of ELA exposures, such as socioeconomic disadvantage, on earlier pubertal development, or by enhancing parenting behaviors (e.g., responsive parenting) which, in turn, improve healthy weight maintenance and, thereby reduce risk for earlier pubertal development and cardiometabolic disease.
Directions for Future Research
Beyond the role of obesity, future studies should examine more broadly the biological connections among ELA exposures, pubertal development, and cardiometabolic risk. Although not fully elucidated, pathobiological processes, including hyperandrogenism and insulin resistance may play a role. For example, higher pre-pubertal androgen levels have been shown to predict earlier pubertal timing, independent of birthweight, fat mass, and maternal overweight (Remer et al., 2010) and, in adult women, higher androgens predict increased cardiometabolic risk (e.g., adverse lipid profiles, inflammation, insulin resistance, metabolic syndrome, and type 2 diabetes) (Oh et al., 2002; Sutton-Tyrrell et al., 2005; Torrens et al., 2009). In addition, insulin resistance increases during puberty, even among non-obese girls, suggesting an apparent vulnerability during this time (Ball et al., 2006). Insulin resistance predicts post-pubertal weight gain and increased cardiometabolic risk (Lustig, 2006; Saklayen, 2018) and is correlated with hormones, including lower sex hormone binding globulin and higher androgens which themselves predict poorer cardiovascular risk factor profiles and subclinical atherosclerosis, independent of BMI (El Khoudary et al., 2012; Sutton-Tyrrell et al., 2005). In parallel, ELA exposures themselves have been linked to deleterious hormone profiles, insulin resistance, and inflammation (Bleil et al., 2015; Miller & Chen, 2010; Rich-Edwards et al., 2010). In sum, more mechanistic work is needed to understand how pubertal timing, itself partially influenced by ELA exposures, appears to both reflect and additionally promote hormonal, metabolic, and immunological changes that have profound long-term implications for compromised health maintenance and increased risk for cardiometabolic disease.
Strengths and Weaknesses
Notable strengths of the current study are its longitudinal design and rigorous measurement of the variables of interest. ELA composites were derived from repeated assessments of key pre-pubertal adversity exposures, including videotaped mother–child interactions in well-validated study tasks coded to assess mother–child attachment patterns and maternal sensitivity as well as real-time mother reports of socioeconomic conditions and early life stressors. Pubertal timing assessments were similarly comprehensive. Tanner staging was derived from annual physical exams (with breast bud palpation) performed by trained medical providers to assess dimensions of breast development and pubic hair growth as well as real-time self-reports to assess age at menarche.
Notable weaknesses of the current study design are the somewhat infrequent (annual) and late (beginning at age 9.5) Tanner staging assessments of pubertal development, leading to interval- and left-censored observations. However, censored observations were accommodated by fitting accelerated time models. Another weakness is the small number of non-white girls, limiting the generalizability of the results. Attrition analyses, comparing the girls retained in the current sample to the girls excluded because they did not participate in the Tanner staging assessments, in fact, showed disproportionate loss among girls with mothers who were less educated. A related weakness is the exclusive focus on girls, albeit consistent with the broader literature in which there are few similar studies of boys. Future studies in boys are warranted, however, as some links between ELA and pubertal timing (Brown et al., 2004) and pubertal timing and cardiometabolic risk (Hardy et al., 2006; Widen et al., 2012) have also been observed in boys. In addition, the current study lacks adequate assessment of pre-pubertal health variables. For example, pre-pubertal blood samples were not obtained which would have enabled the examination of relevant underlying biological processes (e.g., insulin resistance). Finally, the potential connection of the current findings to cardiometabolic health in the post-pubertal period is only speculative. A follow-up study of the NICHD SECCYD, however, is currently underway in which the participants, who are now adults, are being assessed on relevant dimensions of cardiometabolic risk, enabling the examination of links between ELA exposures, pubertal development, and cardiometabolic health over the life course.
Conclusions
Results of the current study showed higher childhood SES was related directly, and secure (vs. insecure) mother–child attachment was related indirectly (via pre-pubertal BMI), to later pubertal timing, suggesting these factors may protect girls from earlier pubertal development. Results point to potential intervention opportunities to reduce ELA- and pre-pubertal obesity-related risk, either by mitigating direct impacts of ELA exposures or by augmenting specific attachment-based parenting behaviors to enhance healthy weight maintenance, thereby, reducing risk for earlier puberty and subsequent cardiometabolic disease.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10HD025447, R01HD091132) and the National Heart, Lung, and Blood Institute (R01HL130103) at the National Institutes of Health.
Conflicts of interest: None declared.
References
- Adams E. L., Marini M. E., Stokes J., Birch L. L., Paul I. M., Savage J. S. (2018). INSIGHT responsive parenting intervention reduces infant's screen time and television exposure. International Journal of Behavioral Nutrition and Physical Activity, 15(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth M., Blehar M., Waters E., Wall S. (1978). Patterns of attachment: A psychological study of the strange situation. Lawrence Erlbaum Associates. [Google Scholar]
- Anderson S. E., Gooze R. A., Lemeshow S., Whitaker R. C. (2012). Quality of early maternal-child relationship and risk of adolescent obesity. Pediatrics, 129(1), 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. E., Whitaker R. C. (2011). Attachment security and obesity in US preschool-aged children. Archives of Pediatrics & Adolescent Medicine, 165(3), 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum M., Batten D. A., Belsky J., Booth C., Bradley R., Brownell C. A., Burchinal, M., Caldwell, B., Campbell, S. B., Clarke-Stewart, A., Cox, M., Friedman, S. L., Hirsh-Pasek, K., Huston, A., Jaeger, E., Knoke, B., Marshall, N., McCartney, K., O'Brien, M., &… Weinraub, M. , (1999). Child care and mother-child interaction in the first 3 years of life. Developmental Psychology, 35(6), 1399–1413. [PubMed] [Google Scholar]
- Ball G. D. C., Huang T. T. K., Gower B. A., Cruz M. L., Shaibi G. Q., Weigensberg M. J., Goran M. I. (2006). Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. The Journal of Pediatrics, 148(1), 16–22. [DOI] [PubMed] [Google Scholar]
- Belsky J., Houts R. M., Fearon R. M. P. (2010). Infant attachment security and the timing of puberty: Testing an evolutionary hypothesis. Psychological Science, 21(9), 1195–1201. [DOI] [PubMed] [Google Scholar]
- Belsky J., Steinberg L. D., Houts R. M., Friedman S. L., DeHart G., Cauffman E., Roisman G. I., Halpern-Felsher B. L., Susman E.; The NICHD Early Child Care Research Network (2007). Family rearing antecedents of pubertal timing. Child Development, 78(4), 1302–1321. [DOI] [PubMed] [Google Scholar]
- Berens A. E., Jensen S. K. G., Nelson C. A. (2017). Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Medicine, 15(1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeier H., Paxton S. J., Milgrom J., Anderson S. E., Baur L., Hill B., Lim S., Green R., Skouteris H. (2020). Early mother-child dyadic pathways to childhood obesity risk: A conceptual model. Appetite, 144, 104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro F. M., Greenspan L. C., Galvez M. P., Pinney S. M., Teitelbaum S., Windham G. C., Deardorff J., Herrick R. L., Succop P. A., Hiatt R. A., Kushi L. H., Wolff M. S. (2013). Onset of breast development in a longitudinal cohort. Pediatrics, 132(6), 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil M. E., Adler N. E., Appelhans B. M., Gregorich S. E., Sternfeld B., Cedars M. I. (2013). Childhood adversity and pubertal timing: Understanding the origins of adulthood cardiovascular risk. Biological Psychology, 93(1), 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil M. E., Appelhans B. M., Latham M. D., Irving M. A., Gregorich S. E., Adler N. E., Cedars M. I. (2015). Neighborhood socioeconomic status during childhood versus puberty in relation to endogenous sex hormone levels in adult women. Nursing Research, 64(3), 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boparai S. K. P., Au V., Koita K., Oh D. L., Briner S., Harris N. B., Bucci M. (2018). Ameliorating the biological impacts of childhood adversity: A review of intervention programs. Child Abuse & Neglect, 81, 82–105. [DOI] [PubMed] [Google Scholar]
- Braden A., Rhee K., Peterson C. B., Rydell S. A., Zucker N., Boutelle K. (2014). Associations between child emotional eating and general parenting style, feeding practices, and parent psychopathology. Appetite, 80, 35–40. [DOI] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Chen E., Miller G. E. (2017). Family-centered prevention ameliorates the association between adverse childhood experiences and prediabetes status in young black adults. Preventive Medicine, 100, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Yu T. Y., Miller G. E., Ehrlich K. B., Chen E. (2019). Preventive parenting intervention during childhood and young black adults' unhealthful behaviors: A randomized controlled trial. Journal of Child Psychology and Psychiatry, 60(1), 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Cohen P., Chen H., Smailes E., Johnson J. G. (2004). Sexual trajectories of abused and neglected youths. Journal of Developmental and Behavioral Pediatrics, 25(2), 77–82. [DOI] [PubMed] [Google Scholar]
- Buttke D. E., Sircar K., Martin C. (2012). Exposures to endocrine-disrupting chemicals and age of menarche in adolescent girls in NHANES (2003-2008). Environmental Health Perspectives, 120(11), 1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. C., Udry J. R. (1995). Stress and age at menarche of mothers and daughters. Journal of Biosocial Science, 27(2), 127–134. [DOI] [PubMed] [Google Scholar]
- Cassidy J., Marvin R. (1992). Attachment organization in preschool children: Coding guidelines (4th edn). Unpublished manuscript. University of Virginia.
- Centers for Disease Control and Prevention (CDC) (2010). Adverse childhood experiences reported by adults: Five states. MMWR Morbidity & Mortality Weekly Report, 59(49), 1609–1613. [PubMed] [Google Scholar]
- Chen E., Miller G. E., Yu T., Brody G. H. (2018). Unsupportive parenting moderates the effects of family psychosocial intervention on metabolic syndrome in African American youth. International Journal of Obesity, 42(4), 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumlea W. C., Schubert C. M., Roche A. F., Kulin H. E., Lee P. A., Himes J. H., Sun S. S. (2003). Age at menarche and racial comparisons in US girls. Pediatrics, 111(1), 110–113. [DOI] [PubMed] [Google Scholar]
- De Wolff M., van IJzendoorn M. H. (1997). Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Development, 68(4), 571–591. [PubMed] [Google Scholar]
- Deardorff J., Abrams B., Ekwaru J. P., Rehkopf D. H. (2014). Socioeconomic status and age at menarche: An examination of multiple indicators in an ethnically diverse cohort. Annals of Epidemiology, 24(10), 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans A., Danner U., Parks M. (2017). Emotion regulation in binge eating disorder: A review. Nutrients, 9(11), 1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn L. D., Dahl R. E., Woodward H. R., Biro F. (2006). Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science, 10(1), 30–56. [Google Scholar]
- El Khoudary S. R., Wildman R. P., Matthews K., Thurston R. C., Bromberger J. T., Sutton-Tyrrell K. (2012). Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis, 225(1), 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B. J., Essex M. J. (2007). Family environments, adrenarche, and sexual maturation: A longitudinal test of a life history model. Child Development, 78(6), 1799–1817. [DOI] [PubMed] [Google Scholar]
- Ellis B. J., Garber J. (2000). Psychosocial antecedents of variation in girls' pubertal timing: Maternal depression, stepfather presence, and marital and family stress. Child Development, 71(2), 485–501. [DOI] [PubMed] [Google Scholar]
- Falk C. F., Biesanz J. C. (2016). Two cross-platform programs for inferences and interval estimation about indirect effects in mediational models. SAGE Open, 6(1), 215824401562544. [Google Scholar]
- Felitti V. J., Anda R. F., Nordenberg D., Williamson D. F., Spitz A. M., Edwards V., Koss M. P., Marks J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults—The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Fraley R. C., Roisman G. I., Haltigan J. D. (2013). The legacy of early experiences in development: Formalizing alternative models of how early experiences are carried forward over time. Developmental Psychology, 49(1), 109–126. [DOI] [PubMed] [Google Scholar]
- Frankel L., Hughes S., O'Connor T., Power T., Fisher J., Hazen N. (2012). Parental influences on children's self-regulation of energy intake: Insights from developmental literature on emotion regulation. Journal of Obesity, 2012, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini M. G., Srinivasan S. R., Berenson G. S. (2003). Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: The Bogalusa Heart Study. International Journal of Obesity, 27(11), 1398–1404. [DOI] [PubMed] [Google Scholar]
- Goossens L., Braet C., Van Durme K., Decaluwe V., Bosmans G. (2012). The parent-child relationship as predictor of eating pathology and weight gain in preadolescents. Journal of Clinical Child & Adolescent Psychology, 41(4), 445–457. [DOI] [PubMed] [Google Scholar]
- Groh A. M., Narayan A. J., Bakermans-Kranenburg M. J., Roisman G. I., Vaughn B. E., Fearon R. M. P., Van IJzendoorn M. H. (2017). Attachment and temperament in the early life course: A meta-analytic review. Child Development, 88(3), 770–795. [DOI] [PubMed] [Google Scholar]
- Groh A. M., Roisman G. I., Booth-LaForce C., Fraley R. C., Owen M. T., Cox M. J., Burchinal M. R. (2014). Stability of attachment security from infancy to late adolescence. Monographs of the Society for Research in Child Development, 79(3), 51–66. [DOI] [PubMed] [Google Scholar]
- Hardy R., Kuh D., Whincup P. H., Wadsworth M. E. J. (2006). Age at puberty and adult blood pressure and body size in a British birth cohort study. Journal of Hypertension, 24(1), 59–66. [DOI] [PubMed] [Google Scholar]
- He C. Y., Zhang C. L., Hunter D. J., Hankinson S. E., Louis G. M. B., Hediger M. L., Hu F. B. (2010). Age at menarche and risk of type 2 diabetes: Results from 2 large prospective cohort studies. American Journal of Epidemiology, 171(3), 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman Giddens M. E., Slora E. J., Wasserman R. C., Bourdony C. J., Bhapkar M. V., Koch G. G., Hasemeier C. M. (1997). Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings Network. Pediatrics, 99(4), 505–512. [DOI] [PubMed] [Google Scholar]
- Hiatt R. A., Stewart S. L., Hoeft K. S., Kushi L. H., Windham G. C., Biro F. M., Pinney, S. M., Wolff, M. S., Teitelbaum, S. L., & … Braithwaite D. (2017). Childhood socioeconomic position and pubertal onset in a cohort of multiethnic girls: Implications for breast cancer. Cancer Epidemiology Biomarkers & Prevention, 26(12), 1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman E. E., Paul I. M., Birch L. L., Savage J. S. (2017). INSIGHT responsive parenting intervention is associated with healthier patterns of dietary exposures in infants. Obesity, 25(1), 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen B. K., Heuch I., Kvale G. (2007). Association of low age at menarche with increased all-cause mortality: A 37-year follow-up of 61,319 Norwegian women. American Journal of Epidemiology, 166(12), 1431–1437. [DOI] [PubMed] [Google Scholar]
- Jacobsen B. K., Oda K., Knutsen S. F., Fraser G. E. (2009). Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: The Adventist Health Study, 1976-88. International Journal of Epidemiology, 38(1), 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd T., Tehranifar P., Rich-Edwards J., Titievsky L., Terry M. B. (2010). The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Annals of Epidemiology, 20(11), 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul F., Chang V. W., Brar P., Parekh N. (2017). Birth weight, early life weight gain and age at menarche: A systematic review of longitudinal studies. Obesity Reviews, 18(11), 1272–1288. [DOI] [PubMed] [Google Scholar]
- Lakshman R., Forouhi N. G., Sharp S. J., Luben R., Bingham S. A., Khaw K.-T., Wareham N. J., Ong K. K. (2009). Early age at menarche associated with cardiovascular disease and mortality. The Journal of Clinical Endocrinology & Metabolism, 94(12), 4953–4960. [DOI] [PubMed] [Google Scholar]
- Lustig R. H. (2006). Childhood obesity: Behavioral aberration or biochemical drive? Reinterpreting the first law of thermodynamics. Nature Clinical Practice. Endocrinology & Metabolism, 2(8), 447–458. [DOI] [PubMed] [Google Scholar]
- Marshall W. A., Tanner J. M. (1969). Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood, 44(235), 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Brody G. H., Yu T., Chen E. (2014). A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proceedings of the National Academy of Sciences, 111(31), 11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Chen E. (2010). Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science, 21(6), 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T. E., Caspi A., Belsky J., Silva P. A. (1992). Childhood experience and the onset of menarche—A test of a sociobiological model. Child Development, 63(1), 47–58. [DOI] [PubMed] [Google Scholar]
- Morris D. H., Jones M. E., Schoemaker M. J., Ashworth A., Swerdlow A. J. (2011). Familial concordance for age at menarche: Analyses from the Breakthrough Generations Study. Paediatric and Perinatal Epidemiology, 25(3), 306–311. [DOI] [PubMed] [Google Scholar]
- Moullin S., Waldfogel J., Washbrook E. (2014). Baby bonds: Parenting, attachment and a secure base for children. The Sutton Trust. [Google Scholar]
- Oh J. Y., Barrett-Connor E., Wedick N. M., Wingard D. L. (2002). Endogenous sex hormones and the development of type 2 diabetes in older men and women: The Rancho Bernardo study. Diabetes Care, 25(1), 55–60. [DOI] [PubMed] [Google Scholar]
- Puig J., Englund M. M., Simpson J. A., Collins W. A. (2013). Predicting adult physical illness from infant attachment: A prospective longitudinal study. Health Psychology, 32(4), 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer T., Shi L. J., Buyken A. E., Maser-Gluth C., Hartmann M. F., Wudy S. A. (2010). Prepubertal adrenarchal androgens and animal protein intake independently and differentially influence pubertal timing. The Journal of Clinical Endocrinology & Metabolism, 95(6), 3002–3009. [DOI] [PubMed] [Google Scholar]
- Remsberg K. E., Demerath E. W., Schubert C. M., Chumlea W. C., Sun S. M. S., Siervogel R. M. (2005). Early menarche and the development of cardiovascular disease risk factors in adolescent girls: The Fels Longitudinal Study. The Journal of Clinical Endocrinology & Metabolism, 90(5), 2718–2724. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards J. W., Spiegelman D., Hibert E. N. L., Jun H. J., Todd T. J., Kawachi I., Wright R. J. (2010). Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. American Journal of Preventive Medicine, 39(6), 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. (1987). Multiple imputation for nonresponse in surveys. John Wiley & Sons. [Google Scholar]
- Saklayen M. G. (2018). The global epidemic of the metabolic syndrome. Current Hypertension Reports, 20(2), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarason I. G., Johnson J. H., Siegel J. M. (1978). Assessing the impact of life changes—Development of Life Experiences Survey. Journal of Consulting and Clinical Psychology, 46(5), 932–946. [DOI] [PubMed] [Google Scholar]
- Savage J. S., Birch L. L., Marini M., Anzman-Frasca S., Paul I. M. (2016). Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: A randomized clinical trial. JAMA Pediatrics, 170(8), 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel L. E., Birch L. L., Heatherton T., Mann T., Hunter C., Czajkowski S., Onken L., Berger P. K., Savage C. R. (2017). Psychological and neural contributions to appetite self-regulation. Obesity, 25, S17–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia S. F., Campo R. A., Brown A. G. M., Stoney C., Boyce C. A., Appleton A. A., Bleil M. E., Boynton-Jarrett R., Dube S. R., Dunn E. C., Ellis B. J., Fagundes C. P., Heard-Garris N. J., Jaffee S. R., Johnson S. B., Mujahid M. S., Slopen N., Su S., Watamura S. E. (2020). Social determinants of cardiovascular health: Early life adversity as a contributor to disparities in cardiovascular diseases. The Journal of Pediatrics, 219, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia S. F., Duarte C. S., Chambers E. C., Boynton-Jarrett R. (2012). Cumulative social risk and obesity in early childhood. Pediatrics, 129(5), e1173–e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia S. F., Koenen K. C., Boynton-Jarrett R., Chan P. S., Clark C. J., Danese A., Faith M. S., Goldstein B. I., Hayman L. L., Isasi C. R., Pratt C. A., Slopen N., Sumner J. A., Turer A., Turer C. B., Zachariah J. P. (2018). Childhood and adolescent adversity and cardiometabolic outcomes: A Scientific Statement from the American Heart Association. Circulation, 137(5), e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Mensah F. K., Azzopardi P., Patton G. C., Wake M. (2017). Childhood social disadvantage and pubertal timing: A national birth cohort from Australia. Pediatrics, 139(6), e20164099. [DOI] [PubMed] [Google Scholar]
- Susman E. J., Houts R. M., Steinberg L., Belsky J., Cauffman E., DeHart G., Friedman S. L., Roisman G. I., Halpern-Felsher B. L; for the Eunice Kennedy Shriver NICHD Early Child Care Research Network (2010). Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 1/2 and 15 1/2 years. Archives of Pediatrics & Adolescent Medicine, 164(2), 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton-Tyrrell K., Wildman R. P., Matthews K. A., Chae C., Lasley B. L., Brockwell S., Pasternak R. C., Lloyd-Jones D., Sowers M. F., Torréns J. I. (2005). Sex hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation, 111(10), 1242–1249. [DOI] [PubMed] [Google Scholar]
- Torrens J. I., Sutton-Tyrrell K., Zhao X., Matthews K., Brockwell S., Sowers M., Santoro N. (2009). Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: Study of Women's Health Across the Nation. Menopause, 16(2), 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell J., Melzer D., Henley W., Galloway T. S., Osborne N. J. (2013). Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001-2010. Environment International, 59, 328–335. [DOI] [PubMed] [Google Scholar]
- Waters E., Deane K. E. (1985). Defining and assessing individual differences in attachment relationships—Q methodology and the organization of behavior in infancy and early childhood. Monographs of the Society for Research in Child Development, 50(1/2), 41–65. [Google Scholar]
- Widen E., Silventoinen K., Sovio U., Ripatti S., Cousminer D. L., Hartikainen A.-L., Laitinen J., Pouta A., Kaprio J., Jarvelin M.-R., Peltonen L., Palotie A. (2012). Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care, 35(4), 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham G. C., Bottomley C., Birner C., Fenster L. (2004). Age at menarche in relation to maternal use of tobacco, alcohol, coffee, and tea during pregnancy. American Journal of Epidemiology, 159(9), 862–871. [DOI] [PubMed] [Google Scholar]