Abstract
Introduction
Inflating endotracheal tube cuffs using water instead of air before hyperbaric oxygen treatment (HBOT) is common. The objective of this study was to assess cuff pressure (Pcuff), when the cuff was inflated using water, in normobaric conditions and during HBOT.
Methods
This was a prospective, observational study taking place in hyperbaric centre and intensive care unit of the University Hospital of Lille. Every patient who required tracheal intubation and HBOT at 253.3 kPa (2.5 atmospheres absolute [atm abs]) was included. Pcuff was measured using a pressure transductor connected to the cuff inflating port. Measurements were performed at 'normobaria' (1 atm abs) and during HBOT at 2.5 atm abs.
Results
Thirty patients were included between February and April 2016. Recordings were analysable in 27 patients. Mean Pcuff at normobaria was 60.8 (SD 42) cmH2O. Nineteen (70%) of patients had an excessive Pcuff (higher than 30 cmH2O). Coefficient of variation was 69%. Mean Pcuff at 2.5 atm abs was 51.6 (40.7) cmH2O, significantly lower than at normobaria (P < 0.0001). Coefficient of variation was 79%. In only five (18%) patients was Pcuff < 20 cmH2O at 2.5 atm abs.
Conclusions
In normobaric conditions, when the cuff was inflated using water and not specifically controlled Pcuff was not predictable. The cuff was typically over-inflated exceeding safe pressure. During HBOT Pcuff decreased slightly.
Keywords: Intensive care medicine, Mechanical ventilation, Cuff pressure, Patient monitoring, Ventilators
Introduction
Endotracheal tube cuff pressure (Pcuff) monitoring is recommended for ventilated patients.[ 1] Target levels for Pcuff should be between 20 and 30 cmH2O, to avoid both under-pressure, causing micro-inhalations and ventilator associated pneumonia (VAP), and overpressure, which is a risk factor for tracheal mucosa ischaemia and tracheal stenosis.[ 2 - 3] In prehospital clinical practice, Pcuff is not routinely measured. In a series of 107 patients with air inflation of the cuff, a systematic analysis of Pcuff showed over-inflation in 79% of cases. Pcuff was 56 (SD 34) cmH2O when intubation was performed outside the hospital and 69 (37) cmH2O when intubation was performed within the hospital.[ 4] In another study in the operating theatre, when not controlled, Pcuff was measured at 58 (31) cmH2O.[ 5] Without manometric control, cuff air inflating pressure is unpredictable and varies from one patient to another, however, over-inflation is usually observed.
During hyperbaric oxygen treatment (HBOT), patients are subjected to ambient pressure variations. If cuffs remain inflated with air, which is compressible, cuff volume will decrease during compression – following the Boyle -Mariotte Law – leading to leakage during positive pressure ventilation. During decompression, an increase in cuff volume can cause cuff rupture. Water is non-compressible, and replacing cuff air with water before HBOT sessions is the accepted and usual technique.[ 6] Ventilatory leakage is inversely correlated with Pcuff.[ 7] In current practice, the quantity of water injected into the cuff is whatever volume is required to prevent ventilatory leakage.
This technique is empirical. To our knowledge, Pcuff of water-inflated endotracheal tubes has never been evaluated, and Pcuff is not monitored in HBOT conditions. Initial pressure levels in normobaric and hyperbaric conditions are unknown. Moreover, the completeness of cuff air removal under these conditions is unknown. Air bubbles remaining in the inflation system could cause unexpected pressure variations. The aim of this observational study was to determine Pcuff in normobaric and hyperbaric conditions, when endotracheal tube cuffs are inflated with water within a standard care protocol. As water-filled cuffs have long been used in our unit and in other hyperbaric treatment centres with no adverse effects, we were expecting to find equivalent pressures in cuffs, whether water or air-inflated.
Methods
This prospective observational pilot study was performed in the intensive care unit (ICU) and the hyperbaric centre of the Lille University Hospital in collaboration with the Clinical Investigation Centre – Technological Innovation of Lille (INSERM CIC-IT 807). Our study was considered by the Ethics Commission of the French Language Resuscitation Society (SRLF) as a low-risk, usual-care study for which waiver for consent was granted (CE SRLF 13-31). No changes in patient management were caused by our study since it was descriptive by design. Patients (or their families) were nevertheless informed orally and in writing. Although their consent was not required, they were free to refuse to be included at any time. The data were collected and processed anonymously.
The primary objective of this study was to evaluate the median Pcuff at 101.3 kPa ('normobaria', 1.0 atmosphere absolute [atm abs]) in water-inflated cuffs as compared to that in air-inflated cuffs. Secondary objectives were the assessment of median Pcuff at 253.3 kPa (2.5 atm abs) in both conditions, and any Pcuff variations during HBOT sessions.
During a three-month period, all intubated and ventilated adult patients receiving HBOT at 2.5 atm abs during working hours were included, whether intubated within the ICU or before admission.
The HBOT protocol used involved a 15-min pressure rise from normobaria to 2.5 atm abs, maintaining this pressure over 90 min, followed by a 15-min decompression period back to normobaria. As water is non-compressible, replacing air with water in endotracheal tube cuffs before HBOT sessions is the accepted and usual technique in our unit. The usual practice before compression is to aspirate the air and replace it with sterile water whilst maintaining the endotracheal tube in situ. The amount of water injected into the cuff is whatever volume is required to prevent ventilatory leakage.
Pcuff and airway pressure (Paw) were monitored continuously during HBOT sessions beginning at normobaria, 15 min prior to compression (initial Pcuff), 15 min after the session was over (final Pcuff) and during treatment (120 min). Pcuff was measured in the way we measure invasive arterial pressure. The cuff was connected to an arterial pressure transducer (Edwards Lifesciences) connected to the Physiotrace® (Physiotrace 1.0, Estaris Monitoring, Lille, France) data acquisition board (Figure 1).[ 8] Pressure transducers and tubing were purged with water, then connected to the cuff using the inflation valve. Airway pressure (Paw) was monitored continuously via a pressure transducer (Edwards Lifesciences) connecting the breathing circuit filter to the Physiotrace® acquisition station (Figure 1). Physiotrace® includes a blood pressure measurement module which enables calibration (performed before each measurement), acquisition and processing of the blood pressure transducer data. To meet HBOT safety requirements and reduce fire risks as much as possible, the acquisition station was placed outside the hyperbaric chamber and connected to the pressure transducers via conventional electrical wiring through sealed bushings. Signals were post-analysed by an expert in signal processing. Low quality signals or with artifacts were excluded from the study.
Figure 1.
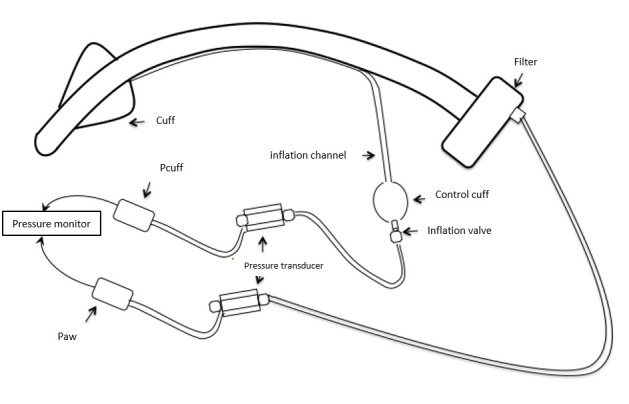
Schematic of the cuff pressure (Pcuff) and airway pressure (Paw) monitoring system
To answer a question raised by our initial results, we performed further experimental tests (cylindrical cuffed endotracheal tubes) at 1.0 and 2.5 atm abs under two conditions: 1) usual practice: aspirating the cuff air out then replacing it with water; 2) aspirating absolutely all the air present in the cuff, its inflation channel, as well as in the control cuff by performing multiple fluid purges. The tests were performed in ‘static’ conditions, without ventilation, and in ‘dynamic’ conditions, with ventilation, on a test lung, at 1 and 2.5 atm abs.
The data collected were demographic (sex, age, weight) and clinical (HBOT indication, endotracheal tube used, mechanical ventilation (MV) specifics). Patient follow-up was continued until ICU discharge and clinical events related to the management of Pcuff such as clinical tracheal ischeamia, days without MV, length of stay in the ICU, and outcome were collected. Statistical analysis was performed using SPSS software (version 20.0, SPSS, Chicago, IL). Results for qualitative variables are presented as numbers (percentage) and for quantitative variables are expressed in median with interquartile range. Pressures before, during and after HBOT were compared using the Wilcoxon signed-rank method.
Results
Between 01 February and 28 April 2016, 59 ventilated patients received HBOT. Twenty-nine patients could not be included because HBOT was urgent or because the session pressure was above 2.5 atm abs. Thus, 30 patients were included in the study; owing to artifacts, only the Pcuff and Paw of 27 patients could be analysed.
The median age of patients was 48 (IQR 35–67) years, 81% were men. The median simplified acute physiology score (SAPS-2) was 59 (IQR 39–64). The median time between ICU admission and HBOT was 1 (IQR 1–4) day. In eight of the 27 patients, HBOT was prescribed for cervical necrotizing soft tissue infections, in 11 for necrotizing soft tissue infections in other locations, in 6 for post-anoxic encephalopathy following self-attempted hanging, and in two for other indications (Table 1).
Table 1. General demographic and clinical data. Data are n (%) or median [IQR]. ACV – assist-control ventilation mode; CO – carbon monoxide; HBOT – hyperbaric oxygen treatment; IP – inspiratory pressure; MV – mechanical ventilation; NSTI – necrotizing soft tissue infection; PEEP – positive end-expiratory pressure; PSV = pressure support ventilation mode; RR – respiratory rate; SAPS-2 – simplified acute physiology score; VAP – ventilator-associated pneumonia .
| Patients | 27 |
| Age (years) | 48 [35−67] |
| SAPS-2 | 59 [39−64] |
| Weight (kg) | 79 [67.5−88] |
| Male | 22 (81) |
| HBOT indication | |
| Cervical NSTI | 8 (30) |
| NSTI, other locations | 11 (41) |
| Anoxic encephalopathy | 6 (22) |
| Air embolism | 1 (4) |
| CO intoxication | 1 (4) |
| Delay between ICU admission and inclusion (days) | 1 [1−4] |
| Intubation | |
| Orotracheal | 24 (89) |
| Nasotracheal | 3 (11) |
| Endotracheal tube size (mm) | 7.5 [7−7.5] |
| Ventilator | |
| Siarétron® | 24 (89) |
| Maquet® | 3 (11) |
| ACV | 25 (93) |
| Tidal volume (mL) | 440 [420−480] |
| RR (breaths·min-1) | 20 [16−25] |
| PSV | 2 (7) |
| IP (cmH2O) | 14 and 16 |
| PEEP (cmH2O) | 6 [6−8] |
| VAP | 2 (22) |
| MV duration (days) | 11 [5−16.5] |
| Time spent without MV (days) | 2 [0−7.5] |
| ICU stay duration (days) | 14 [8.5−23.5] |
| Mortality | 8 (30) |
INTUBATION AND VENTILATION
Intubation was orotracheal in 24 (89%) patients, whilst three were intubated with a nasotracheal tube because of airway compression due to cervical necrotizing soft tissue infection. These three patients also required a reinforced endotracheal tube (Mallinckrodt™ Lo-Contour reinforced). The median internal diameter of the endotracheal tube was 7.5 (IQR 7–7.5) mm. All patients were intubated before admission to our ICU, which is why nine different endotracheal tubes were identified. The Rüschelit® super safety clear™ tube was the most widely used (12/27, 44% of patients). All endotracheal tubes were made of polyvinyl chloride (PVC). Cuffs were cylindrical in 22 (81%) of cases, oval in four (Mallinckrodt™ Lo-Contour reinforced) and conical in one (Mallinckrodt™ Taperguard™ Evac). Mallinckrodt™ Lo-Contour reinforced tubes were the only tubes with high-pressure cuffs. A tube with subglottic suction (Mallinckrodt™ Taperguard™ Evac and Portex®SACETT™) was used for two patients (Table 2).
Table 2. Endotracheal tube characteristics and manufacturers .
| Endotracheal tubes | Patients | Cuff shape | Subglottic suction |
| Rüschelit® super safety clear™ Teleflex, Wayne, USA | 12 | cylindrical | – |
| Rüsch® safety clear plus™ Teleflex, Wayne, USA | 2 | cylindrical | – |
| Sheridan/HVT® Teleflex, Wayne, USA | 1 | cylindrical | – |
| Mallinckrodt™ Lo-Contour reinforced Covidien, Dublin, Ireland | 4 | oval | – |
| Mallinckrodt™ Hi-Contour Covidien, Dublin, Ireland | 2 | cylindrical | – |
| Mallinckrodt™ oral/nasal tracheal tube cuffed Covidien, Dublin, Ireland | 1 | cylindrical | – |
| Mallinckrodt™ Taperguard™ Evac Covidien, Dublin, Ireland | 1 | conical | + |
| Pre-Portex® clear PVC oral/nasal soft seal® cuff Smith Medical, Minneapolis, USA | 3 | cylindrical | – |
| Portex® SACETT™ Smith Medical, Minneapolis, USA | 1 | cylindrical | + |
A Maquet Servo-i HBO® (Getinge, Solna, Sweden) ventilator was used for ventilating three patients; two in pressure support ventilation (PSV) mode because of ventilator weaning and one under assist-control ventilation (ACV) mode. Twenty-four patients were ventilated with a Siaretron 1000 IPER® ventilator (Siare Engineering International Group, Crespellano-Valsamoggia, Italy) in ACV mode. When the ventilatory mode was ACV, the median tidal volume (TV) was 440 (IQR 420–480) mL and the median respiratory rate (RR) was 20·min-1 (IQR 16–25). Median positive end-expiratory pressure (PEEP) was 6 (IQR 6–8) cmH2O.
Median duration of MV was 11 (IQR 5–16.5) days and time spent without MV was 2 (IQR 0–7.5) days. The median stay in ICU was 14 (IQR 8.5–23.5) days; eight of the 27 patients died.
CUFF PRESSURE DATA
Before HBOT, the initial Pcuff was 52.9 (IQR 27.6–84.8) cmH2O with a lowest value of 6.1 cmH2O, and a highest value of 203 cmH2O. Back at atmospheric pressure, the final Pcuff was 57.1 (IQR 24.6–84.5) cmH2O. Initial and final Pcuff were not statistically different. The median normobaric Pcuff was 53.9 (IQR 24.6–84.7) cmH2O with a lowest value of 7.8 cmH2O and a highest value of 199 cmH2O. At 1 atm abs, Pcuff exceeded the usual limit of 30 cmH2O in 19 (70%) of patients, between 20 and 30 cmH2O in five of patients (5/27) and < 20 cmH2O in three patients. The average recording time at 2.5 atm abs was 90 min. At 2.5 atm abs the median Pcuff was 38.9 (IQR 22.6−61.5) cmH2O, significantly lower than at 1 atm abs before and after the HBOT session (P < 0.001). At 2.5 atm abs, 18 (67%) of patients had a Pcuff > 30 cmH2O, four between 20 and 30 cmH2O and five < 20 cmH2O. The median Paw was 11 (IQR 9.1−17) cmH2O at 1 atm abs and 12.2 (IQR 8.7−19.2])cmH2O at 2.5 atm abs (P = 0.024) (Table 3).
Table 3. Endotracheal cuff (Pcuff) and airway (Paw) pressures at 1 and 2.5 atm abs for all patients (n = 27); Min = minimum; Max = maximum .
| Parameter | 1 atm abs | 2.5 atm abs | P | ||||
| Median [IQR] | Min | Max | Median [IQR] | Min | Max | ||
| Pcuff (cmH2O) | 53.9 [24.6−84.7] | 7.8 | 199 | 38.9 [22.6−61.5] | 6.2 | 191 | < 0.001 |
| Paw (cmH2O) | 11 [9.1−17] | 6 | 21 | 12.2 [8.7−19.2] | 6 | 23 | 0.024 |
PRESSURE DATA FOR RÜSCHELIT® SUPER SAFETY CLEAR™ TUBES
Initial Pcuff was 47.7 (IQR 27.1−67.3) cmH2O. Final Pcuff was 47.6 (IQR 23.4–68.9) cmH2O. Initial and final Pcuff were not statistically different. The median normobaric Pcuff was 47.2 (IQR 24.4–68.1) cmH2O with a lowest value of 16.2 cmH2O and a highest value of 102 cmH2O. At 2.5 atm abs, the median Pcuff was 34.8 (IQR 18.7–49.2) cmH2O, significantly lower than at 1 atm abs before and after the session (P = 0.002). The median Paw was 12.5 (IQR 9.4–17.3) cmH2O at 1 atm abs and 14 (IQR 9.1–19.4]) cmH2O at 2.5 atm abs (P = 0.022).
Discussion
In complete contrast with the expected results, the Pcuff was high in water-inflated cuffs. The median Pcuff at 1 atm abs when cuffs were water-filled was 53.9 (IQR 24.6–84.7]) cmH2O (n = 27) and 47.2 (IQR 24.4–68.1) cmH2O in the 12-patient group with Rüschelit® super safety clear™ tubes. To our knowledge, this was the first time that this parameter was assessed in these conditions. The gauges usually used to control and adjust the Pcuff operate only when the cuffs are inflated with air. The experimental system (Figure 1) devised to measure Pcuff with water is actually simple and could be made available on a routine basis.
The results are consistent with other data.[ 4] Without manometric control, cuff inflation with air or water is unpredictable from one patient to another and, therefore, tends to over-inflation.[ 4] This over-inflation might be explained by the lack of clinical detection. Since air leaks during mechanical ventilation are inversely correlated with Pcuff, cuff underinflation can be clinically detected as an audible leak, loss of volume from the ventilation circuit, or detected by the ventilator monitor.[ 7] This method for assessing pressure is unreliable and does not ensure that a level of Pcuff above 20 cmH2O will be maintained as recommended to avoid microinhalations.[ 1] In contrast, the tracheal ischaemia potentially induced by cuff over-inflation is not clinically detectable. Nurses palpating the external (‘control’) cuff on the inflation tube to estimate Pcuff is not a reliable method.[ 9]
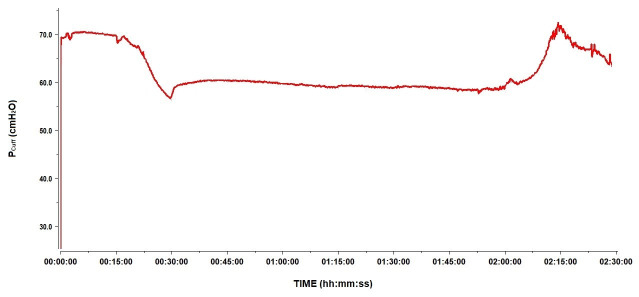
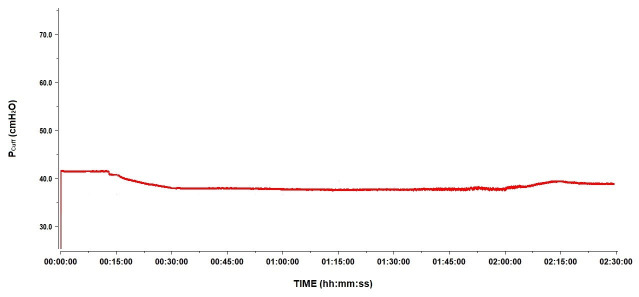
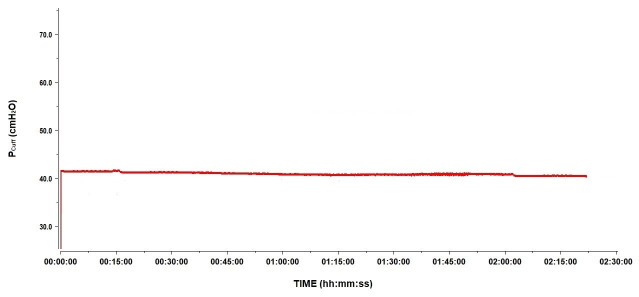
A secondary objective of the study was to describe the changes in Pcuff during HBOT sessions. At 2.5 atm abs, on average, Pcuff decreased by 15 cmH2O to reach a median Pcuff of 38.9 (IQR 22.6–61.5) cmH2O. This was also observed in the 12 patients intubated with the Rüschelit® endotracheal tube. Back at 1 atm abs, Pcuff returned to its original level (Figure 2). Water being non-compressible, we hypothesised an incomplete aspiration of air before replacing it with water in the cuffs. Since any remaining air bubbles are compressible, according to Boyle-Mariotte’s Law, their presence results in the decrease in recorded Pcuff. To explore this hypothesis, we performed tests as explained in the Methods section. The results are shown in Figure 3 and Figure 4. Pcuff remains stable at an ambient pressure of 1 atm abs, but there is a small but clear decrease in Pcuff when ambient pressure increases to 2.5 atm abs, with a recovery of the original Pcuff after returning to 1 atm abs, whether conditions were static or dynamic (Figure 3). Conversely, after repetitive air removal manoeuvers, Pcuff was totally stable, whether at 1 or 2.5 atm abs, under static or dynamic conditions (Figure 4).
Figure 2.
An example of Pcuff evolution as recorded in one patient during a session of HBOT at 2.5ATA
Figure 3.
Pcuff variation at 1 and 2.5ATA following typical cuff air removal procedure
Figure 4.
Pcuff variation at 1 and 2.5ATA following repeated cuff air removal procedures
Besides the volume of air or water injected, Pcuff can be influenced by other factors such as tracheal size and the ratio of tracheal diameter and cuff diameter, cuff type (high or low pressure), thickness, compliance, geometry, curvature of the tracheal tube and position in the trachea.[ 10 - 12] Since none of these factors vary during HBOT sessions, they cannot be blamed for hyperbaric Pcuff variations. Patient temperature also influences Pcuff.[ 13] In theory, patient cooling could lower cuff pressure, but since ambient temperature increases during a hyperbaric exposure this is unlikely. We conclude that imperfect purging of air from the cuff, is the most plausible cause of Pcuff reduction during the period at 2.5 atm abs.
High Pcuff appears to be a major risk factor for tracheal ischaemia. The main complication of tracheal ischaemia is the occurrence of tracheal stenosis. The occurrence of tracheomalacia, false obstructive tracheal membranes, tracheo-oesophageal or tracheo-innominate fistulas is unusual. Tracheal mucosa perfusion was reduced at a Pcuff > 30 cmH2O and completely suppressed at > 50 cmH2O.[ 3] An animal study found that superficial lesions appeared after 15 min of intubation at 27 cmH2O tracheal pressure.[ 14] The median Pcuff at 1 atm abs for our patients was 53.9 (IQR 24.6–84.7) cmH2O and median intubation time was 11 days (IQR 5–16.5). In our treatment protocol, most patients requiring HBOT were given two sessions a day. Their cuff remained inflated with water throughout the treatment, for several days. Yet no clinical event related to possible tracheal ischaemia was reported. Literature analysis shows that clinically detected consequences involving tracheal ischaemia are rare events, especially when tracheal ischaemia is not systematically sought. Its incidence has not been evaluated recently. But in tomography of 47 patients after tracheal intubation, a tracheal size reduction greater than 10% was found in 9 cases. None of those tracheal stenoses were symptomatic.[ 15] In another study, when tracheal stenosis was routinely sought three months after intubation, the incidence was 11%.[ 16]
One mitigating factor is that the measured Pcuff may be different to the tracheal mucosa pressure applied by the cuff. While for a high pressure endotracheal tube, a 30 cmH2O Pcuff generates an equivalent pressure on the tracheal mucosa, the Pcuff for a low pressure tube, generates a lower pressure on the tracheal mucosa because of the elastic forces of the cuff material.[ 17] Half of our patients were intubated with tubes with high-volume low-pressure cuffs (Rüschelit® super safety clear™ and Mallinckrodt™ Taperguard™ Evac). Cuff inflation with water instead of air may still induce a tracheal mucosa pressure different from that expected with air for the same Pcuff. However, this has never been studied. Despite the very high Pcuff we measured, no clinical events due to tracheal ischaemia occurred. Since this study was observational, it lacks medium-term and long-term bronchoscopy follow-up to screen for tracheal stenosis.
Our study was observational and has not shown any clinical impact, neither regarding cuff overpressure during HBOT nor for Pcuff decrease at 2.5 atm abs. Nevertheless, in the light of previous clinical studies and current recommendations, a change in our practice is under consideration, with an evaluation of the effect this change would have. We feel our results should undergo validation by other centres following the same practice.
An alternative would be replacing water with air at the end of each HBOT session. However, if two HBOT sessions are performed per day, this means replacing water or air in the cuff four times a day. These manipulations are known to be a risk factor for ventilator acquired pneumonia and are thus avoided in our practice. Water is replaced by air if hyperbaric treatment is stopped and the patient remains intubated. Alternatively, an endotracheal tube with an expansile foam cuff could be used (Bivona®Fome Cuff Wire Reinforced, Smith Medical, Minneapolis, USA). The foam cuff is connected to ambient air, thus inflating itself. Under standard conditions with air inflation, this type of endotracheal tube would create fewer tracheal ischaemic lesions than would a high-volume low-pressure cuff tube.[ 18 , 19] This system, however, has not been evaluated under hyperbaric conditions. Moreover if used, many ICU patients would require re-intubation since this may not be the usual device with which the patients were intubated. As an alternative, a smart Cuff Manager which monitors and regulates the internal pressure of high-volume, low-pressure cuffs is being tested. This seems promising for sessions at 2.5 atm abs but inefficient at 4 atm abs.
Several devices for the continuous control of tracheal cuff pressure have been successfully tested in ICU patients. They allow a more reliable control of Pcuff around a target value than intermittent control.[ 20 - 22] Among these devices, only pneumatic pressure regulators, unlike electrical pressure regulators, have shown effectiveness in reducing ventilator acquired pneumonia risk.[ 23] Such a regulator could be an interesting alternative in the control of initial Pcuff and could limit the depression of the Pcuff during HBOT. If the cuff is inflated with air, the device must be extremely reactive in order to avoid a major cuff overpressure and rupture during decompression. However, this system requires evaluation during HBOT. This device has never been tested when the cuff is inflated with water.
Finally a pressure transducer could be used to control Pcuff as in the present study. This method has previously been described outside HBOT.[ 24] Pcuff control could be continuous during HBOT sessions, with a detection of under- and over-pressure episodes, to which the inside attendant could provide the necessary adjustments. An initial pressure measurement at 1 atm abs may suffice if a complete purge of the air by the method described in the protocol is performed. This method has the advantage of being simple, inexpensive and does not require buying or testing any additional hardware.
There are some limitations to our study. The sample size is small. Also, patients were intubated with many different endotracheal tubes. For ethical reasons, we wished to provide a general overview of some points in our daily practice. The next stage should be to perform a larger study in terms of number of patients all fitted with the same endotracheal tubes. Our results, even though they are not necessarily generalizable, need to be confirmed by further study with a view to avoiding high Pcuff and potential tracheal ischaemias.
Conclusions
The median Pcuff at 1 atm abs, when the cuff is inflated with water and is not controlled by a dedicated device, is not predictable and usually far above the recommended standards. During HBOT sessions, the Pcuff drops, probably due to incomplete air purging of the inflation system. The clinical consequences of these observations have not been evaluated. Measuring water-inflated Pcuff is easy. It now remains to be proved whether a complete purge of air from the inflation system could reliably avoid the Pcuff drop observed at 2.5 atm abs.
Footnotes
Conflicts of interest and funding: nil
Contributor Information
Younès Benzidi, Intensive Care Unit and Hyperbaric Center, Lille University Hospital, Lille, France.
Thibault Duburcq, Intensive Care Unit and Hyperbaric Center, Lille University Hospital, Lille, France.
Daniel Mathieu, Intensive Care Unit and Hyperbaric Center, Lille University Hospital, Lille, France.
Erika Parmentier-Decrucq, Intensive Care Unit and Hyperbaric Center, Lille University Hospital, Lille, France.
References
- American Thoracic Society, Infectious Diseases Society of America . Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia . Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- Rello J, Soñora R, Jubert P, Artigas A, Rué M, Vallés J. Pneumonia in intubated patients: Role of respiratory airway care . Am J Respir Crit Care Med. 1996;154:111–5. doi: 10.1164/ajrccm.154.1.8680665. [DOI] [PubMed] [Google Scholar]
- Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: Endoscopic study of effects of four large volume cuffs . Br Med J (Clin Res Ed). 1984;288(6422):965–8. doi: 10.1136/bmj.288.6422.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski M, Tréoux V, Garrigue B, Lapostolle F, Borron SW, Adnet F. Intracuff pressures of endotracheal tubes in the management of airway emergencies: The need for pressure monitoring . Ann Emerg Med. 2006;47:545–7. doi: 10.1016/j.annemergmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang X, Gong W, Li S, Wang F, Fu S, et al. Correlations between controlled endotracheal tube cuff pressure and postprocedural complications: A multicenter study . Anesth Analg. 2010;111:1133–7. doi: 10.1213/ANE.0b013e3181f2ecc7. [DOI] [PubMed] [Google Scholar]
- Weaver LK. Hyperbaric oxygen treatment for the critically ill patient . Diving Hyperb Med. 2015;45:1. [PubMed] [Google Scholar]
- Pitts R, Fisher D, Sulemanji D, Kratohvil J, Jiang Y, Kacmarek R, et al. Variables affecting leakage past endotracheal tube cuffs: A bench study . Intensive Care Med. 2010;36:2066–73. doi: 10.1007/s00134-010-2048-5. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J, Logier R, Dassonneville A, Delmar G, Vasseur C. PhysioTrace: An efficient toolkit for biomedical signal processing . Conf Proc IEEE Eng Med Biol Soc. 2005;7:6739–41. doi: 10.1109/IEMBS.2005.1616051. [DOI] [PubMed] [Google Scholar]
- Hoffman RJ, Parwani V, Hahn I-H. Experienced emergency medicine physicians cannot safely inflate or estimate endotracheal tube cuff pressure using standard techniques . Am J Emerg Med. 2006;24:139–43. doi: 10.1016/j.ajem.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Hoffman RJ, Dahlen JR, Lipovic D, Stürmann KM. Linear correlation of endotracheal tube cuff pressure and volume . West J Emerg Med. 2009;10(3):137–9. [PMC free article] [PubMed] [Google Scholar]
- Lichtenthal P, Borg U. Endotracheal cuff pressure: Role of tracheal size and cuff volume . Crit Care. 2011;15(Suppl1):P147. doi: 10.1186/cc9567. [DOI] [Google Scholar]
- Bernhard WN, Yost L, Joynes D, Cothalis S, Turndorf H. Intracuff pressures in endotracheal and tracheostomy tubes. Related cuff physical characteristics . Chest. 1985;87:720–5. doi: 10.1378/chest.87.6.720. [DOI] [PubMed] [Google Scholar]
- Souza Neto EP, Piriou V, Durand PG, George M, Evans R, Obadia JF, et al. Influence of temperature on tracheal tube cuff pressure during cardiac surgery . Acta Anaesthesiol Scand. 1999;43:333–7. doi: 10.1034/j.1399-6576.1999.430315.x. [DOI] [PubMed] [Google Scholar]
- Nordin U. The trachea and cuff-induced tracheal injury. An experimental study on causative factors and prevention . Acta Oto Laryngol Suppl. 1977;345:1–71. [PubMed] [Google Scholar]
- Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients . Am J Med. 1981;70:65–76. doi: 10.1016/0002-9343(81)90413-7. [DOI] [PubMed] [Google Scholar]
- Kastanos N, Estopá Miró R, Marín Perez A, Xaubet Mir A, Agustí-Vidal A. Laryngotracheal injury due to endotracheal intubation: Incidence, evolution, and predisposing factors. A prospective long-term study . Crit Care Med. 1983;11:362–7. doi: 10.1097/00003246-198305000-00009. [DOI] [PubMed] [Google Scholar]
- Doyle A, Santhirapala R, Crowe M, Blunt M, Young P. The pressure exerted on the tracheal wall by two endotracheal tube cuffs: A prospective observational bench-top, clinical and radiological study . BMC Anesthesiol. 2010;10:21. doi: 10.1186/1471-2253-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman DS, Klein EF, Drury WD, Donnelly WH, Applefeld JJ, Chapman RL, et al. A comparison of foam and air-filled endotracheal-tube cuffs . Anesth Analg. 1974;53:521–6. [PubMed] [Google Scholar]
- Gordin A, Chadha NK, Campisi P, Luginbuehl I, Taylor G, Forte V. Effect of a novel anatomically shaped endotracheal tube on intubation-related injury . Arch Otolaryngol Head Neck Surg. 2010;136:54–9. doi: 10.1001/archoto.2010.195. [DOI] [PubMed] [Google Scholar]
- Weiss M, Doell C, Koepfer N, Madjdpour C, Woitzek K, Bernet V. Rapid pressure compensation by automated cuff pressure controllers worsens sealing in tracheal tubes . Br J Anaesth. 2009;102:273–8. doi: 10.1093/bja/aen355. [DOI] [PubMed] [Google Scholar]
- Valencia M, Ferrer M, Farre R, Navajas D, Badia JR, Nicolas JM, et al. Automatic control of tracheal tube cuff pressure in ventilated patients in semirecumbent position: A randomized trial . Crit Care Med. 2007;35:1543–9. doi: 10.1097/01.CCM.0000266686.95843.7D. [DOI] [PubMed] [Google Scholar]
- Duguet A, D’Amico L, Biondi G, Prodanovic H, Gonzalez-Bermejo J, Similowski T. Control of tracheal cuff pressure: A pilot study using a pneumatic device . Intensive Care Med. 2007;33:128–32. doi: 10.1007/s00134-006-0417-x. [DOI] [PubMed] [Google Scholar]
- Nseir S, Zerimech F, Fournier C, Lubret R, Ramon P, Durocher A, et al. Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients . Am J Respir Crit Care Med. 2011;184:1041–7. doi: 10.1164/rccm.201104-0630OC. [DOI] [PubMed] [Google Scholar]
- Ganigara A, Ramavakoda CY. Continuous real time endotracheal tube cuff pressure waveform . J Clin Monit Comput. 2014;28:433–4. doi: 10.1007/s10877-014-9584-4.. [DOI] [PubMed] [Google Scholar]