There is a Blood Commentary on this article in this issue.
Key Points
There is no significant difference in PFS between UCB and haploidentical transplantation for leukemia or lymphoma.
Lower nonrelapse mortality and superior OS favor haploidentical marrow over UCB transplantation.
Abstract
Results of 2 parallel phase 2 trials of transplantation of unrelated umbilical cord blood (UCB) or bone marrow (BM) from HLA-haploidentical relatives provided equipoise for direct comparison of these donor sources. Between June 2012 and June 2018, 368 patients aged 18 to 70 years with chemotherapy-sensitive lymphoma or acute leukemia in remission were randomly assigned to undergo UCB (n = 186) or haploidentical (n = 182) transplant. Reduced-intensity conditioning comprised total-body irradiation with cyclophosphamide and fludarabine for both donor types. Graft-versus-host disease prophylaxis for UCB transplantation was cyclosporine and mycophenolate mofetil (MMF) and for haploidentical transplantation, posttransplant cyclophosphamide, tacrolimus, and MMF. The primary end point was 2-year progression-free survival (PFS). Treatment groups had similar age, sex, self-reported ethnic origin, performance status, disease, and disease status at randomization. Two-year PFS was 35% (95% confidence interval [CI], 28% to 42%) compared with 41% (95% CI, 34% to 48%) after UCB and haploidentical transplants, respectively (P = .41). Prespecified analysis of secondary end points recorded higher 2-year nonrelapse mortality after UCB, 18% (95% CI, 13% to 24%), compared with haploidentical transplantation, 11% (95% CI, 6% to 16%), P = .04. This led to lower 2-year overall survival (OS) after UCB compared with haploidentical transplantation, 46% (95% CI, 38-53) and 57% (95% CI 49% to 64%), respectively (P = .04). The trial did not demonstrate a statistically significant difference in the primary end point, 2-year PFS, between the donor sources. Although both donor sources extend access to reduced-intensity transplantation, analyses of secondary end points, including OS, favor haploidentical BM donors. This trial was registered at www.clinicaltrials.gov as #NCT01597778.
Visual Abstract
Introduction
Allogeneic blood or marrow transplantation is a potentially curative treatment of patients with poor risk or relapsed hematologic malignancies. An HLA-matched sibling or unrelated donor is generally preferred as HLA mismatching between donor and recipient is, historically, associated with higher incidences of graft-versus-host disease (GVHD) and nonrelapse mortality, and inferior survival.1,2 For patients who lack an HLA-matched donor, alternative donor sources include unrelated donor umbilical cord blood or an HLA-mismatched (haploidentical) relative. Although individual cord blood units have low numbers of stem cells leading to unacceptably low cell doses in many adults, successful transplantation in adults is feasible with coinfusion of 2 units.3,4 Despite HLA mismatch, transplantation of bone marrow from a haploidentical relative is feasible with novel methods of GVHD prophylaxis, especially those that incorporate high-dose, posttransplantation cyclophosphamide after infusion of the graft.5 These improvements motivated the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) to conduct parallel phase 2 trials of haploidentical bone marrow (BMT CTN 0603, NCT 00849147) or double cord blood transplants (BMT CTN 0604, NCT 00864227) for adults with poor-risk acute leukemia or lymphoma.6 Three-year progression-free survival (PFS) was 36% (95% confidence interval [CI], 23% to 49%) after cord blood and 35% (95% CI, 21% to 48%) after haploidentical transplantation.7 The patterns of treatment failure (recurrent disease or death) differed between the donor sources. Nonrelapse mortality was more common after cord blood transplantation and relapse was more common after haploidentical transplantation.6,7 In light of the overlapping utility of the 2 donor sources and the uncertainty over their relative merits, the BMT CTN conducted a randomized multicenter phase 3 trial in the United States comparing double cord blood and haploidentical related donor transplantation for the treatment of leukemia and lymphoma in adults.
Methods
Study design
Randomization for the Blood and Marrow Transplant Clinical Trials Network protocol #1101 (BMT CTN 1101) was performed at Emmes Corporation at a 1:1 ratio using random block sizes stratified by transplant center. The primary end point was PFS 2 years after randomization, as assessed by intention-to-treat (ITT) analysis. Prespecified secondary end points included incidences of neutrophil and platelet recovery, acute and chronic GVHD, nonrelapse mortality, relapse/progression, and overall survival (OS). Enrollment began on 19 June 2012 and ended 30 June 2018 without reaching the accrual goal of 410 patients. The Data Safety Monitoring Board (DSMB) closed the trial in consultation with the study’s sponsor, the National Heart, Lung, and Blood Institute (NHLBI), due to slow accrual. Three hundred sixty-eight patients (90% of planned accrual) enrolled from 33 centers. Supplemental Table 1 (available on the Blood Web site) lists the participating centers and the site principal investigators. The analysis included data collected as of 1 August 2019.
Patients
Eligible patients were aged 18 to 70 years and had high-risk acute leukemia, Hodgkin or non-Hodgkin lymphoma, performance score of 70 or higher, adequate organ function, and availability of 2 cord blood units and at least 1 haploidentical relative (parent, full or half sibling, offspring or second-degree relative). Each cord blood unit had to have a cell dose of at least 1.5 × 107 nucleated cells per kilogram patient body weight at cryopreservation and be HLA matched to the patient at 4 or more loci (HLA-A, -B at the antigen level and -DRB1 at the allele level). The haploidentical relative had to be matched to the patient for at least 1 allele each of the HLA-A, -B, -C, and -DRB1 genes and was required to donate bone marrow. We excluded a cord blood unit or a haploidentical donor if the patient had donor-specific antibodies against the high-expression HLA-A, -B, -C, or -DRB1 alleles with mean fluorescence intensity >1000. Details of the inclusion and exclusion criteria are provided in the treatment protocol document, section 2.2 (supplemental Material). All patients provided written informed consent. One hundred eighty-six patients were randomly assigned to receive cord blood transplants and 182 patients to receive haploidentical transplants. Of the 186 patients randomized to the cord blood arm, 172 received the treatment as assigned; 8 patients relapsed before transplant, 3 withdrew consent, 1 crossed over to receive haploidentical bone marrow, and 2 were transplanted off protocol at the investigator’s discretion (Figure 1). Of the 182 patients randomized to the haploidentical bone marrow arm, 153 received the treatment as assigned; 10 patients relapsed before transplant, 1 died without relapse, 4 withdrew consent, 11 crossed over to receive cord blood, and 3 were transplanted off protocol at the investigator’s discretion.
Figure 1.
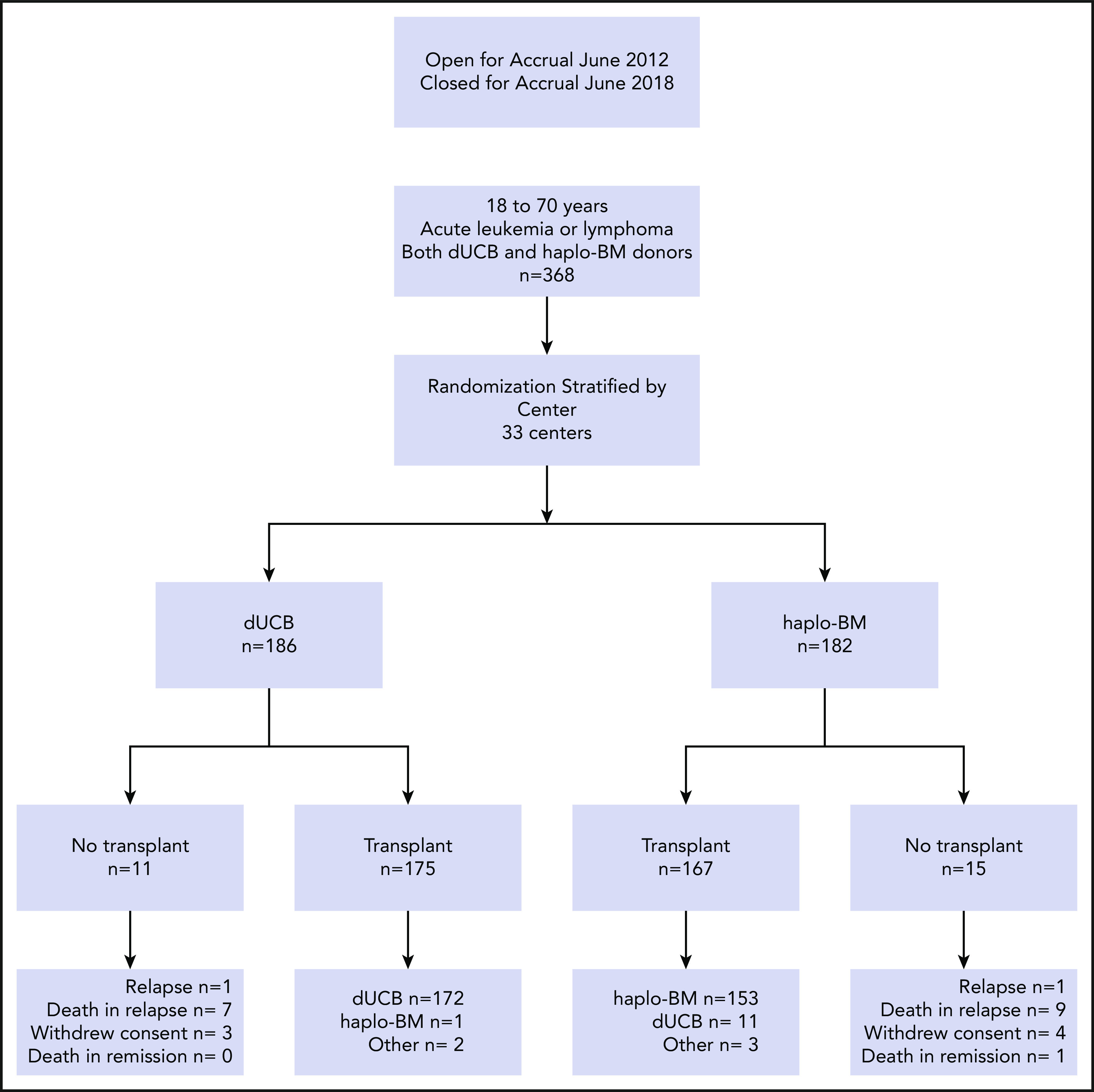
Randomization and treatment. dUCB, double umbilical cord blood; haplo-BM, HLA-haploidentical bone marrow.
Treatment
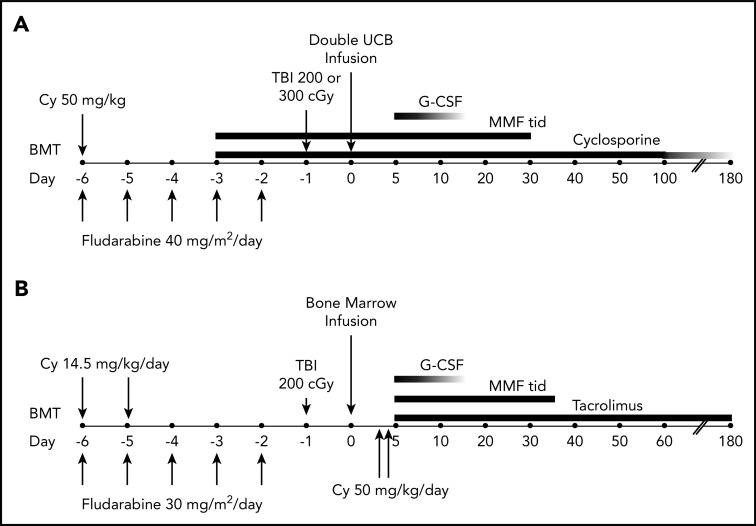
The treatment plans are shown in Figure 2. Patients randomized to the double cord arm received fludarabine, cyclophosphamide, and either 200 or 300 cGy total-body irradiation (TBI), with the higher TBI dose given to patients who had not received cytotoxic chemotherapy within 3 months of enrollment or an autologous hematopoietic stem cell transplant within 24 months of enrollment. GVHD prophylaxis commenced 3 days prior to transplantation and comprised 15 mg/kg mycophenolate mofetil 3 times daily (with a maximum daily dose of 3 g) through day 35 and cyclosporine adjusted to maintain a trough level of 200 to 400 ng/mL through day 100 and tapered by 10% weekly until discontinued between days 180 and 200. For patients receiving haploidentical bone marrow, the conditioning regimen included cyclophosphamide, fludarabine, and 200 cGy TBI. GVHD prophylaxis consisted of 50 mg/kg cyclophosphamide IV daily on days 3 and 4, 15 mg/kg mycophenolate mofetil 3 times daily from days 5 through 35, and tacrolimus adjusted to maintain a concentration of 5 to 15 ng/mL from day 5 through day 180.
Figure 2.
Treatment schema. For patients receiving umbilical cord blood (UCB) (A) or HLA-haploidentical bone marrow (B). BMT, blood or marrow transplantation; Cy, cyclophosphamide; G-CSF, granulocyte colony-stimulating factor; MMF, mycophenolate mofetil; TBI, total-body irradiation; tid, thrice daily.
Study oversight
A protocol review committee appointed by the NHLBI approved the research protocol, which was subsequently approved by the institutional review board at each participating center. A DSMB appointed by the NHLBI reviewed accrual, adverse events, and outcomes data at periodic intervals and the final analysis. An end point review committee, whose members were unaware of treatment assignments, adjudicated the primary and key secondary end points on 86 randomly selected patients to establish concordance between reviewed data and data reported by centers. As the overall concordance exceeded a prespecified threshold (>90%), adjudication of additional cases was terminated.
Statistical analysis
The primary analysis was a comparison of PFS at 2 years after randomization using ITT analysis. We selected a pointwise comparison due to concerns about potential nonproportional hazards. The sample-size target of 205 patients in each group aimed at providing 80% power for a 2-sided test to detect a 15% difference in 2-year PFS, assuming baseline PFS of 35% to 40% at 2 years, and accounting for up to 5% of patients not transplanted and 5% loss to follow-up by 2 years. PFS times were calculated from the day of randomization.
The χ2 statistic was used to compare categorical variables and the Mann-Whitney test to compare continuous variables between the treatment groups. PFS and OS were estimated using the Kaplan-Meier method.8 PFS was compared between treatment arms using a pointwise Z test of the difference in 2-year probabilities, whereas OS was compared using the log-rank test. The first occurrence of relapse or progression or death was an event for PFS and death from any cause an event for OS. Patients without an event were censored at last follow-up or at 2 years after randomization, whichever came first. We calculated the cumulative incidence of hematopoietic recovery, acute and chronic GVHD, nonrelapse mortality, and relapse/progression using the Aalen-Johansen estimator9 to accommodate competing risks. Neutrophil and platelet engraftment were compared between groups using pointwise estimates at day 56 and day 100, respectively, whereas acute and chronic GVHD, nonrelapse mortality, and relapse/progression were compared using the Gray test.
A Cox proportional hazards regression analysis identified predictors of PFS while assessing the treatment effect.10 Results are expressed as hazard ratios (HRs) with 95% CIs. All variables studied met the assumptions for proportionality. Multivariable modeling included disease, disease risk, age at randomization, and performance score. Other variables (sex and ethnic origin) did not meet the prespecified requirement for inclusion in the model (P < .1 between treatment arms). An effect of transplant center on the primary end point was explored using not only a piecewise exponential PFS model, adjusted for the same variables as in the Cox regression model mentioned earlier, but also adding random effects for both the intercept term as well as the treatment by center interaction effect. All P values are 2-sided; values of P < .05 were considered to indicate statistical significance. Analyses were performed with SAS software, version 9.4 (SAS Institute).
B.L. and P.D. were responsible for analyzing the data, and all authors had access to the primary clinical trial data.
Results
Patient characteristics
The baseline characteristics of patients randomly assigned to the treatment groups are shown in Table 1. The 2 treatment groups were balanced with respect to age, sex, performance score, recipient cytomegalovirus serostatus, and disease type at randomization. More patients assigned to receive cord blood identified as White, and fewer were in first remission of leukemia or partial remission of lymphoma. The median age at randomization was 58 years (range, 20-70 years) and 60 years (range, 20-70 years) for patients assigned to the cord blood and haploidentical treatment arms, respectively. Corresponding median follow-up times for surviving patients were 24 months (interquartile range [IQR], 23-25) and 24 months (IQR, 23-25).
Table 1.
Patient, disease, and transplant characteristics by donor source
| Umbilical cord blood, N = 186, N (%)* | Haploidentical relative, N = 182, N (%)* | |
|---|---|---|
| Sex | ||
| Female | 89 (48) | 76 (42) |
| Male | 97 (52) | 106 (58) |
| Age, median (range), y | 58 (20-70) | 60 (20-70) |
| Ethnic origin | ||
| White | 145 (78) | 126 (69) |
| African American | 27 (15) | 34 (19) |
| Other | 14 (7) | 22 (12) |
| Hispanic or Latino | ||
| Yes | 22 (12) | 21 (12) |
| No | 164 (88) | 159 (87) |
| Unknown or not answered | 0 | 2 (1) |
| Performance score | ||
| 90-100 | 126 (68) | 111 (61) |
| 80-70 | 60 (32) | 71 (39) |
| Hematopoietic comorbidity index | ||
| ≤2 | 104 (59) | 99 (59) |
| ≥3 | 71 (41) | 68 (41) |
| Cytomegalovirus serostatus | ||
| Positive | 98 (56) | 99 (59) |
| Negative | 76 (43) | 68 (41) |
| Not reported | 1 (<1) | |
| Disease | ||
| Acute lymphoblastic leukemia | 31 (17) | 32 (18) |
| Acute myeloid leukemia | 98 (53) | 98 (54) |
| Biphenotypic leukemia | 1 (<1) | 5 (3) |
| T-cell leukemia/lymphoma | 4 (2) | 3 (2) |
| Hodgkin lymphoma | 10 (6) | 8 (4) |
| Large cell lymphoma | 21 (11) | 19 (10) |
| Follicular non-Hodgkin lymphoma | 7 (4) | 5 (3) |
| Mantle cell lymphoma | 6 (3) | 5 (3) |
| Other lymphoma | 8 (4) | 7 (4) |
| Disease status at transplant | ||
| Acute leukemia | ||
| First complete remission | 99 (74) | 117 (85) |
| Second complete remission | 35 (26) | 20 (15) |
| Third complete remission | 1 (<1) | |
| Lymphoma | ||
| Complete remission | 20 (39) | 14 (32) |
| Partial remission | 25 (48) | 25 (57) |
| Follicular lymphoma | 7 (14) | 5 (11) |
| Cytogenetic risk (leukemia only) | ||
| Favorable | 17 (13) | 20 (15) |
| Intermediate | 61 (46) | 56 (41) |
| Poor | 43 (32) | 45 (33) |
| Not reported | 13 (10) | 17 (12) |
| Disease risk index | ||
| Low | 23 (12) | 14 (8) |
| Intermediate | 119 (64) | 120 (66) |
| High | 35 (19) | 37 (20) |
| Not evaluable | 9 (5) | 11 (6) |
| Infused nucleated cell dose, ×107/kg | ||
| Median (IQR) | 2.95 (1.85-4.32) | 26.82 (18.76-36.35) |
| Infused CD34+ dose, ×106/kg | ||
| Median (IQR) | 0.13 (0.07-0.23) | 2.87 (1.44-3.86) |
| Infused CD3+ dose, ×106/kg | ||
| Median (IQR) | 5.50 (1.90-8.20) | 29.66 (22.48-42.87) |
N (%) unless otherwise stated in the row headings.
Hematopoietic recovery and treatment toxicity
Cumulative incidences of neutrophil recovery by posttransplantation day 56 were 95% (95% CI, 90% to 97%) after cord blood transplantation with a median time to recovery of 15 days (range, 4-69 days) and 99% (95% CI, 94% to 100%) after haploidentical transplantation with a median time to recovery of 17 days (range, 1-87 days; Figure 3A). The day 56 cumulative incidence of neutrophil recovery was significantly higher after haploidentical than after cord blood transplantation (P = .05). Cumulative incidences of platelet recovery were 78% (95% CI, 71% to 84%) with a median time to recovery of 42 days (range, 7-147 days) after cord blood and 84% (95% CI, 78% to 89%) with a median time to recovery of 28 days (range, 12-173 days) after haploidentical transplantation (P = .15; Figure 3B).
Figure 3.
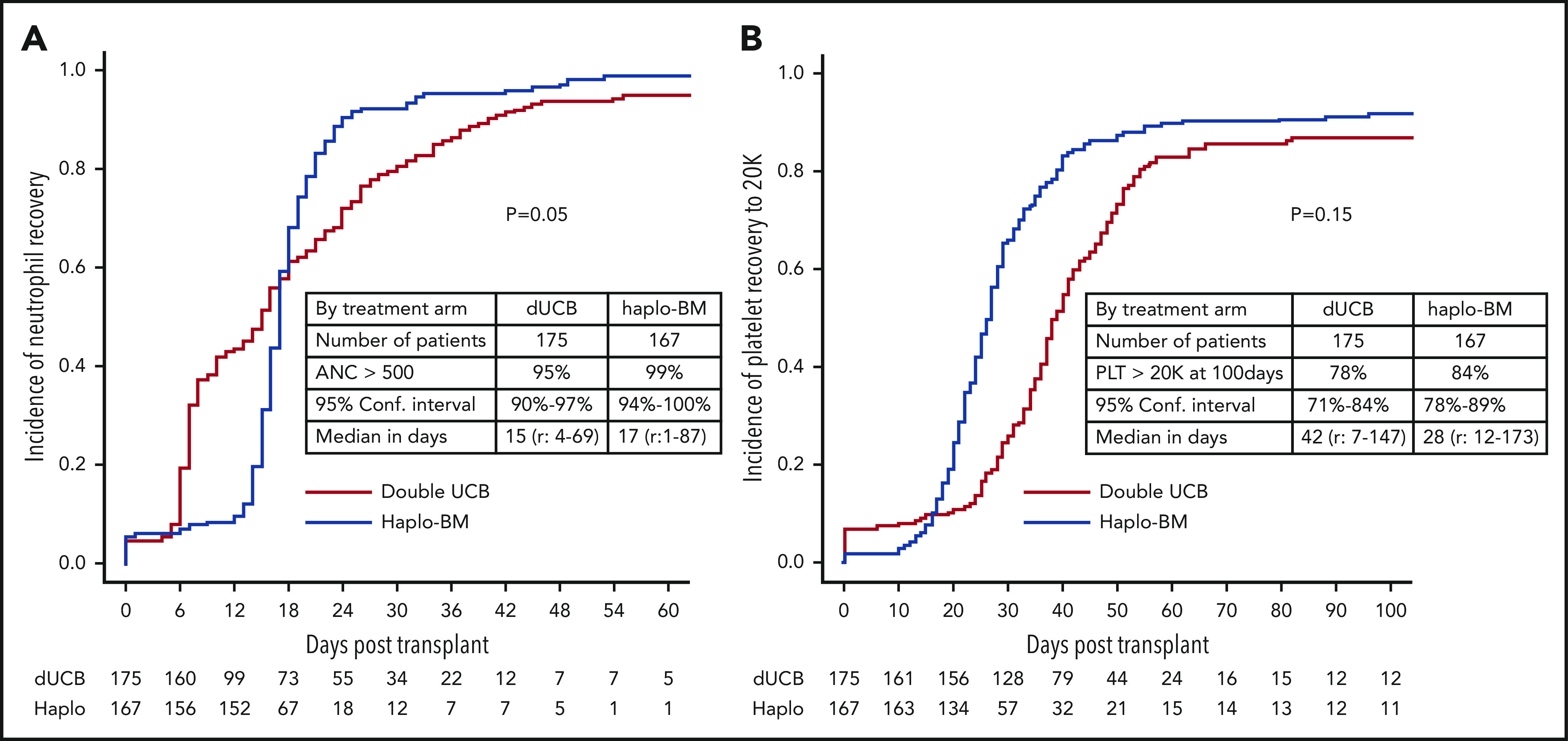
Cumulative incidences of recovery. (A) Neutrophil recovery. (B) Platelet recovery. ANC, absolute neutrophil count; Conf. Interval, confidence interval; Haplo, haploidentical; PLT, platelet.
Supplemental Table 2 shows the severe, grade 3-5, adverse events occurring in each treatment arm. Nine serious adverse events occurred in recipients of haploidentical bone marrow vs 4 such events among recipients of umbilical cord blood.
Acute and chronic GVHD
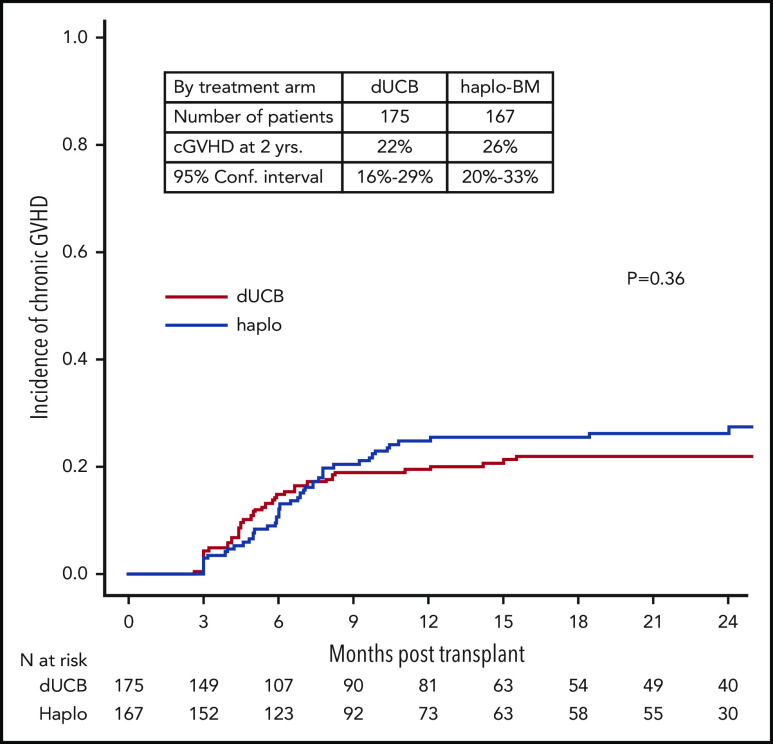
The day 180 incidences of grade II-IV acute GVHD were similar in the 2 groups, 35% (95% CI, 28% to 42%) and 28% (95% CI, 22% to 35%) after cord blood and haploidentical transplantation, respectively (P = .142; Figure 4A). The corresponding incidences of grade III-IV acute GVHD were 9% (95% CI, 5% to 13%) and 7% (95% CI, 4% to 12%), P = .604 (Figure 4B). The 2-year incidences of chronic GVHD were also similar in the 2 groups, 22% (95% CI, 16% to 29%) and 26% (95% CI, 20% to 33%) after cord blood and haploidentical transplantation, respectively (P = .361; Figure 5). The severity of chronic GVHD did not differ between treatment groups; the proportion of patients with moderate/severe chronic GVHD was 10% after both cord blood and haploidentical transplantation.
Figure 4.
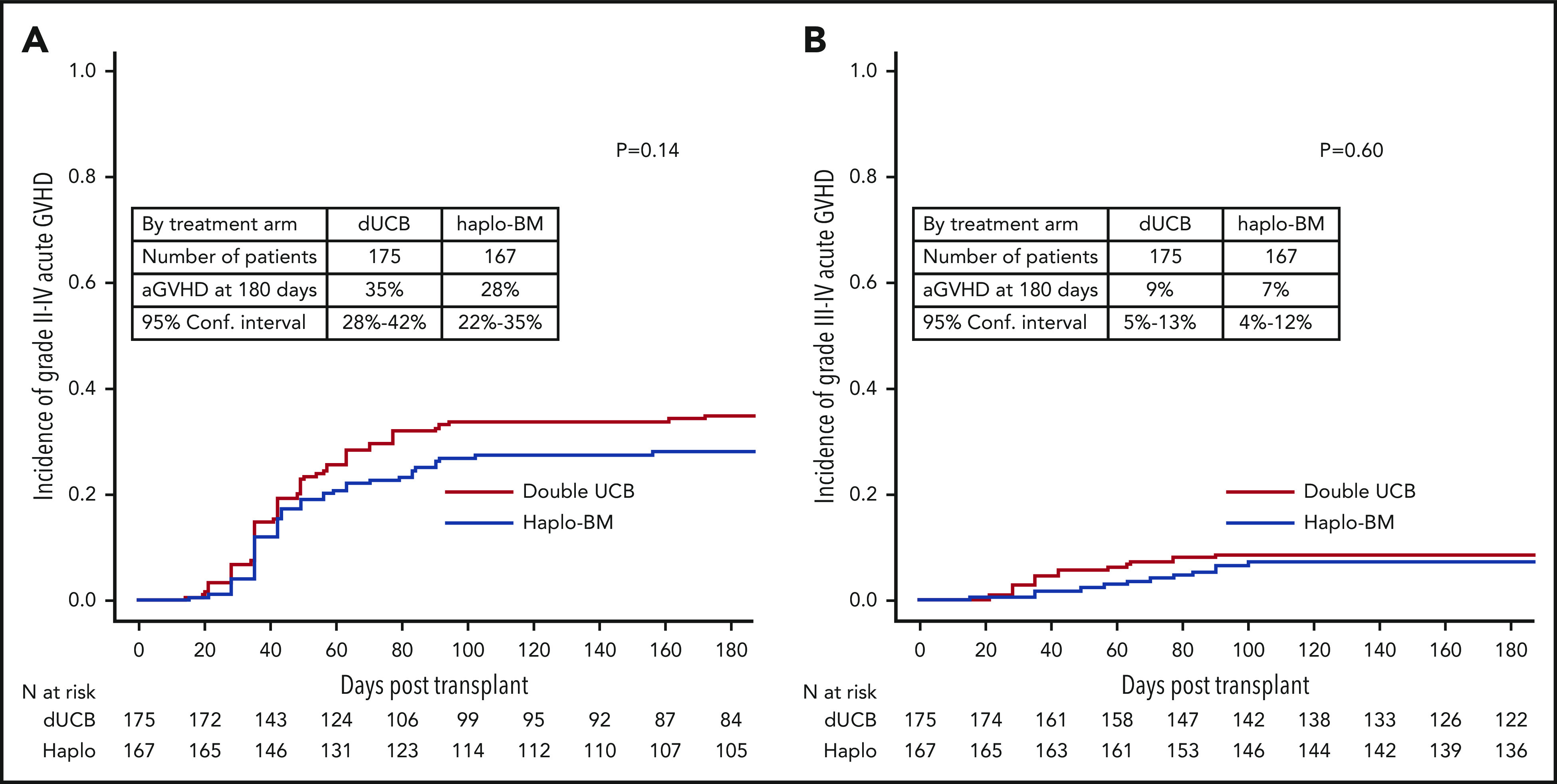
Cumulative incidences of acute GVHD. (A) Acute grades II-IV GVHD. (B) Acute grades III-IV GVHD. aGVHD, acute GVHD.
Figure 5.
Cumulative incidence of chronic GVHD. cGVHD, chronic GVHD.
PFS
The probabilities of 2-year PFS after randomization were 35% (95% CI, 28% to 42%) and 41% (95% CI, 34% to 48%) after cord blood and haploidentical transplantation, respectively (P = .409) (Figure 6A). In multivariable analysis, the HR of death or progression (inverse of PFS) was higher after cord blood transplantation but did not meet the level of significance set for the trial (HR, 1.30; 95% CI, 0.99-1.70; P = .060) after adjustment for age, performance score, and disease type. PFS was higher for patients in complete remission of lymphoma other than follicular lymphoma than for patients with acute leukemia in first complete remission (HR of death or progression, 0.58; 95% CI, 0.34-1.01; P = .053). PFS was lower among patients with lymphoma in partial remission than for acute leukemia patients in first remission (HR of death or progression, 1.51; 95% CI, 1.04-2.20; P = .023). No other variables tested were significantly associated with PFS and there was no difference in treatment effect by disease status (P = .290 for interaction). Additionally, there was not a statistically significant interaction between treatment effect and transplant center (P = .275).
Figure 6.
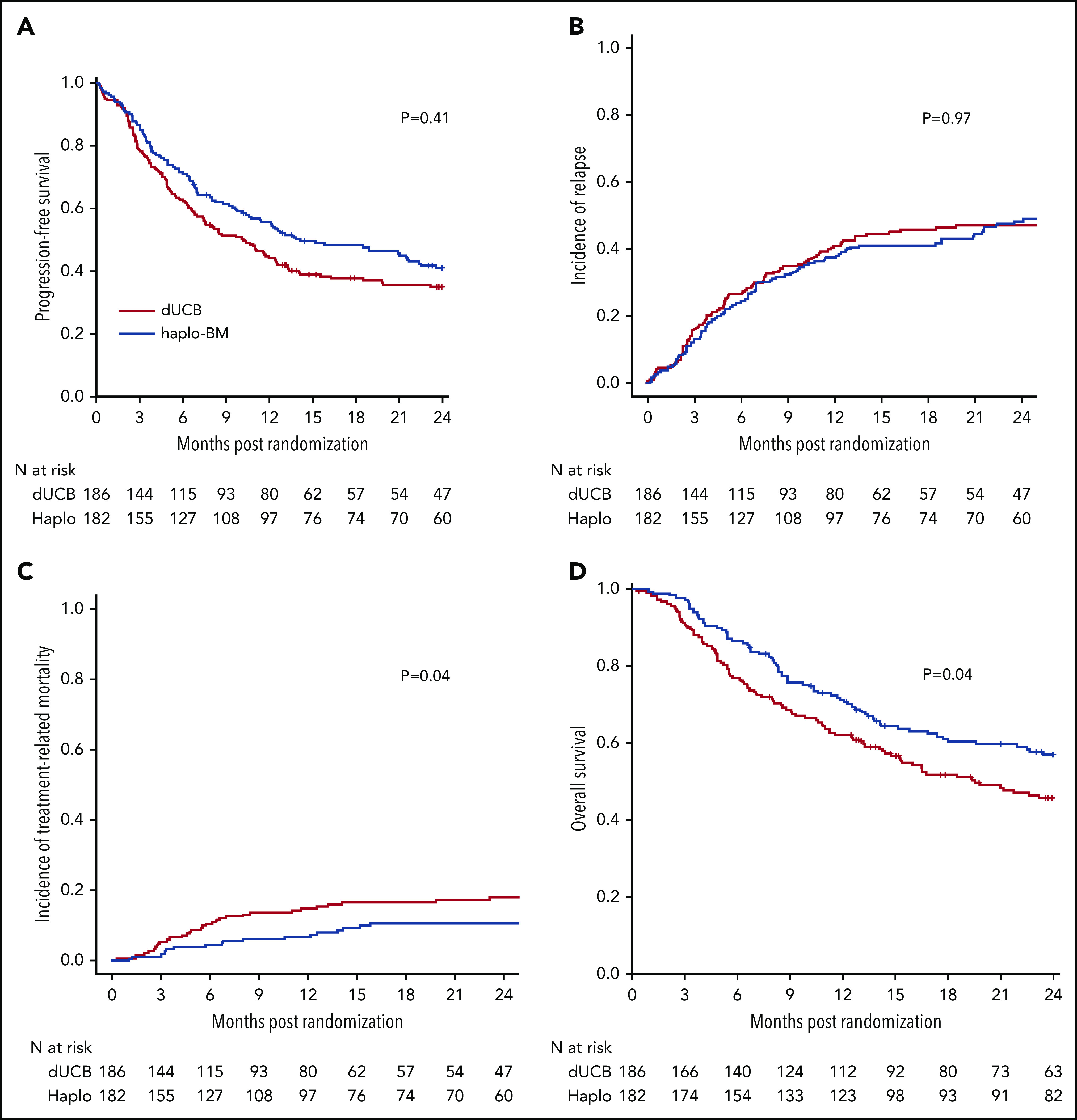
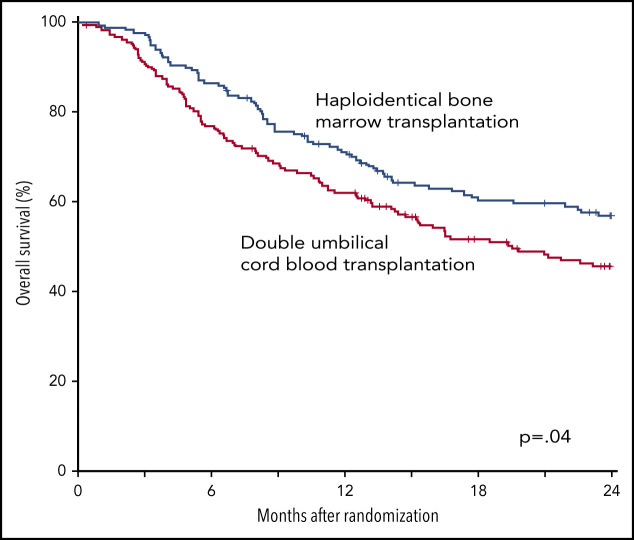
Outcomes of transplantation according to graft source. (A) PFS. (B) Relapse. (C) Nonrelapse mortality. (D) OS.
Relapse or progression, nonrelapse mortality, and OS
The 2-year incidence of relapse/progression did not differ significantly between treatment groups, 47% (95% CI, 40% to 54%) and 48% (95% CI, 41% to 56%) after cord blood and haploidentical transplantation, respectively (P = .968) (Figure 6B). The 2-year incidence of nonrelapse mortality was higher after cord blood (18%; 95% CI, 13% to 24%) compared with haploidentical (11%; 95% CI, 7% to 16%) transplantation (P = .039) (Figure 6C). Consequently, 2-year OS was lower after cord blood (46%; 95% CI, 38% to 53%) compared with haploidentical (57%; 95% CI, 49% to 64%) transplantation (P = .037) (Figure 6D). Table 2 shows the causes of death of patients on study. Recurrent disease was the most common cause of death in both treatment groups, and the proportion of deaths attributed to recurrent disease did not differ by treatment group. The proportion of death attributed to infection and hemorrhage (pulmonary or intracranial) was higher in the cord blood compared with the haploidentical treatment arm. Supplemental Table 3 lists the number and types of infection occurring in each treatment arm.
Table 2.
Causes of death
| Treatment arm, N (%) | ||
|---|---|---|
| UCB, n = 186 | Haplo relative, n = 182 | |
| Recurrent disease | 55 (29.6) | 46 (25.3) |
| Graft failure | 2 (1.1) | 2 (1.1) |
| Acute GVHD | 0 (0) | 2 (1.1) |
| Chronic GVHD | 2 (1.1) | 2 (1.1) |
| Infection | 17 (9.1) | 13 (7.1) |
| Organ failure | 9 (4.8) | 6 (3.3) |
| Hemorrhage | 4 (2.2) | 1 (0.5) |
| Interstitial pneumonia | 2 (1.1) | 2 (1.1) |
| Acute respiratory distress syndrome | 2 (1.1) | 0 (0) |
| Other | 2 (1.1) | 0 (0) |
| Missing | 1 (0.5) | 0 (0) |
Haplo, haploidentical; UCB, umbilical cord blood.
Discussion
This multicenter randomized trial of umbilical cord blood vs haploidentical relative transplantation with reduced-intensity conditioning did not show a statistically significant difference in 2-year PFS between the donor sources. This lack of a significant difference held in both an ITT analysis and an analysis of those patients actually transplanted (data not shown). We observed a 6% difference in PFS between treatment groups that was well short of the 15% difference used for sample-size determination. Although we cannot be certain why the trial failed to complete accrual, the time period of the trial was marked by a dramatic increase in the use of haploidentical donors, a decline in the use of umbilical cord blood grafts, and an increased use of peripheral blood over bone marrow as haploidentical donor graft source.11 These trends may have reflected a reluctance of patients or their physicians to accept donor selection based on randomization and/or the relative simplicity of haploidentical donor transplantation using the posttransplant cyclophosphamide platform for GVHD prophylaxis. Planned secondary analyses showed similar relapse incidences with the 2 donor sources but, importantly, lower nonrelapse mortality and higher OS after haploidentical bone marrow as compared with umbilical cord blood transplantation. Among patients who received transplantation, neutrophil recovery was less likely after umbilical cord blood transplantation but there were no significant differences in platelet recovery or in acute or chronic GVHD.
To our knowledge, this is the first randomized trial to compare these 2 donor sources in the setting of reduced-intensity conditioning. A small randomized trial of the 2 donor sources using a uniform myeloablative regimen (thiotepa, busulfan, and fludarabine with antithymocyte globulin) did not show significant differences in relapse, disease-free, or OS.12 Although that trial included only 45 patients, nonrelapse mortality was higher after umbilical cord blood transplantation, which is consistent with our observation.12
Transplantation of 2 umbilical cord blood units overcame the cell dose barrier to engraftment in this adult population but nonrelapse mortality was higher when compared with haploidentical relative transplantation. We hypothesize that several factors contribute to higher nonrelapse mortality after umbilical cord blood transplantation. In the current analysis, the double cord blood grafts contained fivefold to sixfold fewer CD3+ T cells than haploidentical marrow. Furthermore, umbilical cord blood T cells lack immunologic memory against opportunistic pathogens that are a source of morbidity and mortality after hematopoietic stem cell transplantation. Indeed, mortality from bacterial or viral infections accounted for 9 of the 13 additional nonrelapse deaths in recipients of cord blood transplantation. In a report that studied infections after umbilical cord blood, HLA-matched, and HLA-mismatched unrelated donor transplantations, bacterial and viral infections were higher after umbilical cord blood compared with HLA-mismatched adult donor transplants.13 Bacterial infections were also associated with higher mortality.13 Hemorrhage was also a significant contributor to mortality in recipients of umbilical cord blood. Lowering nonrelapse mortality after umbilical cord blood transplantation will require development of methods to improve hematopoietic and immune reconstitution, especially in the early posttransplantation period.14
Historically, HLA-haploidentical relative transplantation was limited by the high frequencies of alloreactive donor and host T cells, which contribute to high incidences of graft failure, GVHD, and nonrelapse mortality, resulting in poor survival.1 Rigorous ex vivo depletion of donor T cells reduces GVHD at the expense of increased graft failure, infection, and possibly relapse, with no improvement in survival.15 High-dose posttransplantation cyclophosphamide (100 mg/kg) achieves selective in vivo allodepletion that reduces graft failure and GVHD while sparing memory T cells that confer resistance to infection.16 The effect of posttransplantation cyclophosphamide on the graft-versus-leukemia effect is the subject of controversy but our study demonstrated similar risks of relapse after umbilical cord blood and haploidentical transplantation. This is in contrast to the earlier parallel phase 2 BMT CTN trials where nonrelapse mortality was higher after umbilical cord blood transplantation and relapse higher after haploidentical donor transplantation.6 The predominant disease type in both treatment arms was acute myeloid leukemia. Other reports including a randomized clinical trial favor myeloablative transplant conditioning regimens for acute myeloid leukemia.17 We hypothesize that the higher relapse/progression recorded in our trial is explained by the low-intensity regimens prescribed for both treatment groups.17,18
Banked umbilical cord blood and HLA-haploidentical relatives are rapidly and readily available sources of hematopoietic stem cells for patients lacking HLA-matched siblings or HLA-matched unrelated donors. Over one-quarter of the patients on this study were from ethnic minorities that are underrepresented in the volunteer unrelated donor registries worldwide.19 HLA-haploidentical donor availability can be compromised by donor-specific anti-HLA antibodies in the recipient20 or by medical or psychological conditions in the donor that preclude donation.21 Of note, umbilical cord blood was used for 11 of 182 patients randomized to receive haploidentical bone marrow (9 donors were ineligible and 2 donors declined to donate) whereas only 1 of 186 patients randomized to receive cord blood crossed over to receive haploidentical bone marrow instead (patient preferred a haploidentical donor).
Although our trial did not show a statistically significant difference in the primary end point, 2-year PFS, there were statistically significant differences in important secondary end points, nonrelapse mortality and OS. A retrospective report that focused on patients with Hodgkin (n = 283) or non-Hodgkin (n = 457) lymphoma also demonstrated lower nonrelapse mortality and higher OS after haploidentical vs cord blood transplantation.22 In that study, the difference in PFS, assessed at 4 years, was somewhat larger and statistically significant (46% and 36%, P = .002), respectively.21 The absence of a significant difference in PFS in our trial vs that study may be explained by lower patient numbers in the current trial or by differences in the patients treated, including the inclusion of patients with acute leukemia. However, we did not find a significant effect of disease type on treatment effect. It is possible that the difference in PFS between the 2 arms of our study will increase with longer follow-up; continued follow-up of the cohort is planned.
Our trial confirms that both donor sources expand access to transplantation, which means that few if any patients should be denied transplantation for lack of a donor. Despite the lack of a statistically significant difference in PFS, the primary end point, the observed lower nonrelapse mortality, and higher survival in the context of reduced-intensity conditioning suggest that transplantation of bone marrow from a haploidentical relative and GVHD prophylaxis including posttransplantation cyclophosphamide is the preferred approach.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by grants U10HL069294 and U24HL138660 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute and the National Cancer Institute of the National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Deidentified individual participant data that underlie the reported results will be made available upon publication on the Web site of the NHLBI’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J.F., P.V.O., M.E., B.L., and C.G.B. designed research; P.D. and B.L. performed statistical analysis; E.J.F. wrote the manuscript; and all authors performed research, collected and interpreted data, and critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Blood and Marrow Transplant Clinical Trials Network appears in the supplemental Appendix.
Correspondence: Ephraim J. Fuchs, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 289 Cancer Research Building, 1650 Orleans St, Baltimore, MD 21287; e-mail: fuchsep@jhmi.edu.
REFERENCES
- 1.Szydlo R, Goldman JM, Klein JP, et al. . Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767-1777. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein J, Haagenson M, et al. . High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576-4583. [DOI] [PubMed] [Google Scholar]
- 3.Barker JN, Weisdorf DJ, DeFor TE, et al. . Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343-1347. [DOI] [PubMed] [Google Scholar]
- 4.Brunstein CG, Gutman JA, Weisdorf DJ, et al. . Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13(2):132-132. [DOI] [PubMed] [Google Scholar]
- 6.Brunstein CG, Fuchs EJ, Carter SL, et al. ; Blood and Marrow Transplant Clinical Trials Network . Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eapen M, O’Donnell P, Brunstein CG, et al. . Mismatched related and unrelated donors for allogeneic hematopoietic cell transplantation for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2014;20(10):1485-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-480. [Google Scholar]
- 9.Aalen O, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat. 1978;5:141-150. [Google Scholar]
- 10.Cox DR. Regression models and life-tables. J R Stat Soc Series B Methodol. 1972;34(2):187-220. [Google Scholar]
- 11.D’Souza A, Fretham C, Lee SJ, et al. . Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26(8):e177-e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanz J, Montoro J, Solano C, et al. . Prospective randomized study comparing myeloablative unrelated umbilical cord blood transplantation versus HLA-haploidentical related stem cell transplantation for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2020;26(2):358-366. [DOI] [PubMed] [Google Scholar]
- 13.Ballen K, Woo Ahn K, Chen M, et al. . Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(9):1636-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bejanyan N, Brunstein CG, Cao Q, et al. . Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv. 2018;2(8):909-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ash RC, Horowitz MM, Gale RP, et al. . Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991;7(6):443-452. [PubMed] [Google Scholar]
- 16.Kanakry CG, Coffey DG, Towlerton AMH, et al. . Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight. 2016;1(5):e86252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott BL, Pasquini MC, Logan BR, et al. . Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eapen M, Brazauskas R, Hemmer M, et al. . Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic syndrome: conditioning regimen intensity. Blood Adv. 2018;2(16):2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gragert L, Eapen M, Williams E, et al. . HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladstone DE, Zachary AA, Fuchs EJ, et al. . Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19(4):647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosuri S, Wolff T, Devlin SM, et al. . Prospective evaluation of unrelated donor cord blood and haploidentical donor access reveals graft availability varies by patient ancestry: practical implications for donor selection. Biol Blood Marrow Transplant. 2017;23(6):965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fatobene G, Rocha V, St Martin A, et al. . Nonmyeloablative alternative donor transplantation for Hodgkin and non-Hodgkin lymphoma: from the LWP-EBMT, Eurocord, and CIBMTR. J Clin Oncol. 2020;38(14):1518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.