Abstract
Study Objectives
To examine associations of social isolation and loneliness with sleep in older adults and whether associations differ for survey and actigraph sleep measures.
Methods
This study used data from the National Social Life, Health, and Aging Project (NSHAP), a nationally representative study of community-dwelling older adults born 1920–1947. A random one-third of participants in 2010–2011 were invited to participate in a sleep study (N = 759) that included survey questions, 72 hours of wrist actigraphy, and a sleep log. Perceived loneliness was measured using three questions from the UCLA Loneliness Scale. An index of social isolation was constructed from nine items that queried social network characteristics and social interactions. We used ordinary least squares and ordinal logistic regression to examine whether sleep measures were associated with loneliness and social isolation adjusted for potential sociodemographic confounders.
Results
Social isolation and loneliness had a low correlation (Spearman’s correlation = 0.20). Both loneliness and social isolation were associated with actigraphy measures of more disrupted sleep: wake after sleep onset and percent sleep. Neither was associated with actigraph total sleep time. Increased loneliness was strongly associated with more insomnia symptoms and with shorter sleep duration assessed by a single question, but social isolation was not. More isolated individuals spent a longer time in bed.
Conclusions
We found that both loneliness and social isolation were associated with worse actigraph sleep quality, but their associations with self-reported sleep differed. Only loneliness was associated with worse and shorter self-reported sleep.
Keywords: social isolation, disconnectedness, loneliness, sleep, actigraphy, aging
Statement of Significance.
This study uses a large, nationally representative sample of U.S. older adults to examine whether perceived loneliness and social isolation relate to sleep quality using both survey questions about sleep and actigraphy. “Social isolation” and “loneliness” are sometimes used interchangeably, and both have been previously linked to worse sleep quality in older adults. Loneliness refers to unmet social needs, while social isolation refers to observable social connections. We find that social isolation and loneliness are associated similarly with worse actigraph-estimated sleep, but only loneliness is associated with insomnia symptoms. Lonelier individuals also reported shorter sleep from a single survey question, but more socially isolated individuals averaged longer time in bed. Causal direction cannot be inferred from cross-sectional data, and future research needs to examine longitudinal effects.
Introduction
Older adults are at a higher risk of experiencing negative health consequences of social isolation and loneliness [1–3], including increased risk for cardiovascular heart disease, stroke, and mortality [4–7]. Although both social isolation and loneliness have been found to be related to poor health outcomes, they are distinct concepts and have a relatively low correlation [6, 8, 9]. Social isolation is described as a lack of contact with other people, which is measured through reported and observable social connections, such as social network characteristics, social interactions, and participation in groups [10]. Loneliness is the distressing feeling that one’s desired social needs are not being met [11]. Individuals with frequent social interactions may nonetheless feel lonely and, conversely, individuals with few social connections may not feel lonely. Despite their low correlation, the research literature sometimes conflates them using the terms interchangeably, although they may have different associations with health and well-being.
One of the mechanisms proposed for how loneliness and social isolation impact overall health status is through sleep quality [12, 13]. Sleep quality is multidimensional, and different measures are used to represent this complicated behavior. Both sleep duration and sleep quality or sleep problems may be measured by self-report, using survey questions or sleep logs, or by objective approaches using polysomnography or actigraphy. With the advantage of being able to assess sleep over several nights without affecting behavior, actigraphy has recently been added to a number of large population-based studies, such as Whitehall II and the UK Biobank [14, 15].
Actigraph measures of sleep and survey responses have only low to moderate correlations and likely capture different dimensions of sleep. Actigraph duration has a moderate correlation with self-reported sleep duration, and there is some evidence that self-reported duration may be influenced by factors other than sleep, including health or sociodemographics [16–19]. Insomnia symptom reports are significantly, but not strongly, associated with the sleep characteristics measured by actigraphy that they seem to reference, such as the insomnia symptom of reporting problems with waking up during the night and the actigraph estimate of minutes of wake after sleep onset (WASO) [20]. Therefore, associations between sleep and health may differ depending on how sleep is measured [21].
A recent systematic review assessed how measures of social connections and loneliness have been found to be related to self-reported sleep quality in older adults [22]. The review found that both loneliness and social isolation were associated with worse sleep quality but that loneliness had a stronger effect on sleep quality and insomnia symptoms than did social isolation [22]. The majority of the studies analyzed in this review measured sleep quality through the multidimensional Pittsburg Sleep Quality Index (PSQI) [22]. One previous study focused on examining the association between loneliness and sleep in a unique population in which everyone had similar and frequent social interactions: the Hutterites who live in communal farm villages [23]. This study found that higher reports of loneliness were associated with worse actigraph-measured sleep quality among this socially connected population but not with subjective sleep quality measured by the PSQI [23]. This study did not examine associations between social isolation and actigraph sleep metrics because no one is socially isolated in the community. There is little prior evidence of how both social isolation and loneliness are associated with sleep measured using actigraphy in the same study population.
There are reasons we might expect the associations of sleep with loneliness and social isolation to differ depending on how sleep is measured. Loneliness reflects social desires not being met and insomnia reflects sleep desires not being met; there may be common individual or contextual factors that tend to increase both kinds of dissatisfaction. If so, loneliness would have a stronger association with self-reported insomnia symptoms than with actigraph sleep metrics. We previously found, in the same study population, that married older adults have better sleep quality measured by actigraphy than unmarried but not better self-reported sleep quality [24]. There may be parallel associations between the density of social connections and sleep quality, with stronger associations for actigraphy metrics than self-reported sleep problems.
We use a national study of older adults in the United States, the National Social Life, Health and Aging Project (NSHAP), to examine how loneliness and social isolation are related to sleep. We construct a comprehensive measure of social isolation that includes social network size, complexity, and frequency of interactions. Loneliness is measured with a shortened version of the widely used UCLA loneliness scale. We examine whether each is associated with sleep, measured with actigraphy metrics of duration and disruption, self-reported duration and insomnia symptoms, and with durations from a sleep log.
Methods
Study sample
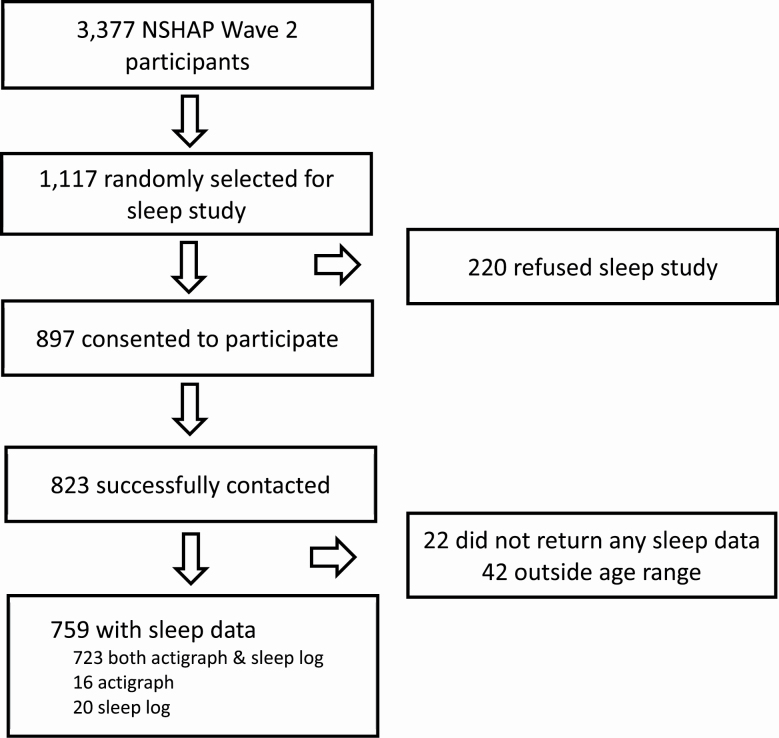
The NSHAP, a nationally representative longitudinal study of community-dwelling older adults (born 1920–1947) began in 2005–2006. Each wave of data collection used both in-person interviews and leave-behind survey booklets. In wave 2 (2010–2011), consenting partners of the original wave 1 participants were added to the cohort, regardless of their birth year. A full description of the wave 2 protocol and design can be found in Jaszczak et al. [25]. An ancillary sleep study was introduced in this wave. A randomly selected one-third of participants (n = 1117) were invited to participate, and 897 initially agreed. Of these, 759 were age eligible (born 1920–1947) and returned usable actigraph or sleep log data (see Figure 1) [26]. All respondents in NSHAP and the sleep study gave written informed consent and the study was approved by the institutional review boards at the University of Chicago and at NORC, who collected the data.
Figure 1.
Flowchart of NSHAP data used for analysis.
Actigraph-measured sleep metrics
Sleep study participants received a wrist actigraph (Actiwatch Spectrum model from Philips Respironics) by mail, along with a booklet that included a sleep log and additional sleep questions and a prepaid return mailer. They were instructed to wear the actigraph for 72 hours (three nights of sleep). This duration was selected because there was a concern on the part of the NSHAP data collection team that a longer sleep protocol might decrease participation in subsequent waves. The actigraphs include accelerometers, an ambient light meter, and event markers; the epochs for recording activity counts were set at 15 seconds [27, 28]. Data from the returned actigraphs were downloaded and analyzed using Philips Respironics software (version 5.59); a detailed description of the sleep data collection and data analysis protocol has been previously published [27, 29]. Sleep intervals initially set by the software using the activity counts were reviewed and revised by the investigators, who also considered the light data and event markers, which participants were asked to press each night when they started to try to sleep and each morning when they awoke. The actigraph sleep metrics used in this study are total sleep time (TST; the sum of all epochs scored as sleep), WASO (the sum of all epochs scored as wake during the sleep interval), and percent sleep (TST divided by the duration of the first to the last epochs scored as sleep). Sleep metrics were averaged over the number of nights with usable data, which was three nights for the great majority (93.5%). WASO and percent sleep were standardized. Agreeing to participate in the sleep study and wearing the actigraph the requested number of days were not associated with cognitive function [30].
Self-reported sleep measures
Insomnia symptoms
An insomnia symptom score was calculated by combining questions from the sleep questionnaire and core NSHAP survey [20]. The sleep questionnaire asked about the frequency (“rarely or never,” “sometimes,” or “most of the time”) of the participant (1) having trouble falling asleep, (2) waking up during the night and not being able to fall back asleep, and (3) waking up too early. These variables were combined with a reverse-coded NSHAP core question about the frequency of (4) feeling rested upon waking in the morning. Each of the four frequency scores (0, 1, or 2) were summed to create an insomnia symptom score, which could take values from 0 to 8, where higher scores indicate more frequent insomnia symptoms. None of these questions had a timeframe. The resulting insomnia symptom score had a Cronbach’s α of 0.66.
Duration
NSHAP includes three different self-reported sleep duration metrics [18]. The first was taken from a single question in the sleep questionnaire that asked, “How many hours do you usually sleep at night?” We refer to this as “single-question sleep duration.” The second was calculated from the sleep logs. Participants were asked to write down the time that they fell asleep and woke up on each of the three nights of the sleep study. Sleep duration was calculated from these times and averaged over the nights. We refer to this as “sleep log-calculated duration.” The last duration metric was calculated from four NSHAP core survey questions, which asked for the respondents’ usual bedtimes and waking times on weekdays and weekends. These were used to calculate an average nightly duration for weeknights and weekends and these were weighted to determine an average duration over the week, which we refer to as “calculated time in bed” [18, 31].
Actigraphy TST and the three survey-based measures are not expected to be perfectly correlated, in part, because they aim to measure somewhat different quantities. Both the single-question and actigraphy TST intend to measure time actually sleeping. Actigraphy TST is expected to be more accurate since typical sleep duration is difficult for respondents to estimate accurately. The other two measures are based on when people go to bed and get up in the morning, in general or for three specific nights; time awake during the night is explicitly not subtracted from either of them.
Loneliness
Loneliness in NSHAP is measured using three questions from the UCLA Loneliness Scale [32]: “How often do you feel that you lack companionship,” “How often do you feel left out,” and “How often do you feel isolated from others.” The scale reliability was 0.79 [1]. Responses were scored as 0 for “never” or “hardly ever,” 1 for “some of the time,” and 2 for “often.” These were summed creating a score that ranges from 0 to 6, where higher values represent more frequent feelings of loneliness.
Social isolation
The social isolation scale is a measurement of the richness of the participant’s social network and their social participation. This is an expanded version of the social disconnectedness score developed by Cornwell and Waite for the NSHAP study [10]. Frequency of religious services attendance was not originally included in the scale because its inclusion lowered the internal consistency [10]; however, we include it here because it has frequently been found to be salient for older adults’ social integration and support [33, 34]. Social isolation is composed of nine variables. They include four measures of social network (network size, network range, proportion of network living in household, and frequency of interaction with network), four measures of social participation (frequency of attending group meetings, frequency of socializing with friends/relatives, frequency of volunteering, and frequency of attending religious services), and number of friends. The social network variables are constructed from a name generator that asked respondents to name up to five people with whom they can discuss “important matters” (referred to as alters) [35]. Then respondents provided information about each alter, including their relationship type (i.e. family and friend), whether they live in the same household, their frequency of interaction, and the frequency of contact with the other alters [35]. Social participation is derived from four questions about how often the respondent attended group meetings, socialized with friends/relatives, volunteered, and attended religious services within the past year [36]. Lastly, respondents were asked about how many friends they had (“none,” “1,” “2–3,” “4–9,” “10–20,” and “more than 20”). Each variable is standardized and then all standardized values are averaged. The standardized inverse of the average score is used in this study to represent social isolation so that the direction of potential associations is the same as for the loneliness score. The expanded social isolation score had a Cronbach’s α of 0.69.
Additional covariates
Demographic variables have been previously shown to be associated with both self-reported and actigraph-measured sleep in NSHAP and other studies [24, 29, 37], and they are also associated with social isolation and loneliness [38, 39]. Because of these associations, we include age, sex, education, and race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, and other) as potential confounders in our models.
We considered including health indicators as confounders, such as cognitive function, frailty, pain, and obesity. An important consideration in deciding whether to include a variable as a confounder or not depends on the direction of the hypothesized causal associations [40]. The literature relating these health factors to sleep has primarily examined them as consequences of variation in sleep rather than causes of variation in sleep [26, 41–43]. There is also evidence that social isolation and loneliness influence health conditions, particularly frailty and cognitive decline [2, 44–46]. Controlling for health factors that are likely downstream from both the exposures and outcomes we are modeling could introduce collider bias [47]. Therefore, we do not include them as confounders.
Statistical analysis
Each sleep measure was modeled as an outcome in separate models. Two models were run for each of seven sleep outcome variables (WASO, TST, percent sleep, insomnia symptom score, single-question duration, sleep log-calculated duration, and calculated time in bed). One set of models examined social isolation as the exposure of interest, and another set examined loneliness as the exposure of interest. All outcomes were continuous variables, with the exception of the insomnia symptom score, which was an ordinal variable. For the continuous outcomes, we used ordinary least squares regression. Ordinal logistic regression was used for the insomnia symptom score. We present unadjusted models and models controlled for demographics of age, sex, education, and race/ethnicity.
The interpretation of the beta coefficient depends on both the predictor and sleep variable of interest. The coefficients in the loneliness models represent the change in sleep measure for every point increase on the UCLA loneliness score, while the coefficients of the social isolation models represent the change for every SD increase in social isolation. Both WASO and percent sleep were standardized. The four sleep duration measures, and their corresponding beta coefficients, are reported in hours. The beta coefficients for the ordinal logistic regression models were exponentiated to yield odds ratios, which are interpreted as the odds of having a point change in the insomnia symptom score for a unit change in either the loneliness score or the social isolation index.
The sample includes 164 coresiding couples, whose sleep characteristics may be correlated due to shared factors. We use design-based variance estimates due to the complex, multistage nature of the NSHAP sample. Specifically, we use the ultimate cluster method for variance estimation, which uses only the variation between primary sampling units (PSUs) and, therefore, assumes only that the PSUs are independent; no assumption is made about the form or magnitude of the correlation within PSUs. This is the recommended method for variance estimation for NSHAP [48]. Since, by definition, couples live in the same household (and are, therefore, in the same PSU), our design-based variance estimates automatically take into account any potential within-couple correlation. We carried out a sensitivity analysis in which we only included the primary spouse (sampled in wave 1 data collection before spouses were added in wave 2). The point estimates are very similar in this analysis to the full sample. Since the sample size was reduced (n = 595), confidence intervals (CIs) are somewhat wider. The sensitivity analysis is included in the Supplementary Tables; the models presented here are based on all of the available information. Stata Version 15.1 and its suite of commands for complex surveys were used throughout.
Results
Descriptive statistics
Descriptive statistics of the demographic and sleep measures can be found in Table 1. Population ages ranged from 62 to 90 years. The sample was 53.1% female. The mean of WASO was 40.4 minutes (SD: 24.1 minutes) and the mean for percent sleep was 91.5% (SD: 4.8%). Sleep duration differed by measurement type: actigraphy TST averaged 7.2 hours; single-question sleep duration averaged 7.4 hours; both sleep log-calculated duration and calculated time in bed averaged 8.2 hours. Table 2 presents a correlation matrix for the seven sleep measures. The Spearman correlation is also shown for the insomnia symptom score since it is not normally distributed. Correlation between actigraph WASO and percent sleep is high (−0.93). The correlations between the four duration measures are in the low-moderate range from 0.29 to 0.55. The insomnia symptom score has little correlation with actigraph measures.
Table 1.
Baseline characteristics of NSHAP age-eligible sleep study participants (N = 759)
| Mean (SD) or % | |
|---|---|
| Demographics | |
| Age | 72.6 (7.4) |
| Female | 53.2% |
| Education | |
| <High school | 17.9% |
| High school | 23.9% |
| Some college | 35.3% |
| College degree or higher | 22.9% |
| Ethnicity (n = 756) | |
| White | 73.4% |
| Black | 11.6% |
| Hispanic | 11.5% |
| Other | 3.5% |
| Marriage | |
| Married/living with partner | 69.8% |
| Divorced/separated | 7.9% |
| Widowed | 20.2% |
| Never married | 2.1% |
| Sleep outcomes | |
| Actigraph sleep measures [n = 739] | |
| Average WASO (min) | 40.4 (24.1) |
| Actigraph sleep duration (TST; h) | 7.2 (1.3) |
| Average percent sleep | 91.5% (4.8) |
| Self-reported sleep measures | |
| Insomnia symptom score (range: 0 – 8) | 2.8 (2.1) |
| Single-question sleep duration (h) [n = 627] | 7.4 (1.4) |
| Sleep log-calculated duration (h) [n = 716] | 8.2 (1.4) |
| Calculated time in bed (h) [n = 756] | 8.2 (1.5) |
Table 2.
Correlation matrix between seven sleep characteristics; all values represent Pearson correlation coefficients except where noted
| WASO | Percent sleep | Actigraph TST | Single-question duration | Sleep log- calculated duration | Calculated sleep interval | Insomnia symptom score | |
|---|---|---|---|---|---|---|---|
| WASO | 1.00 | ||||||
| Percent sleep | −0.93 | 1.00 | |||||
| Actigraph TST | 0.03 | 0.25 | 1.00 | ||||
| Single-question duration | −0.07 | 0.13 | 0.29 | 1.00 | |||
| Sleep log-calculated duration | 0.24 | −0.08 | 0.50 | 0.49 | 1.00 | ||
| Calculated sleep interval | 0.13 | −0.01 | 0.37 | 0.37 | 0.55 | 1.00 | |
| Insomnia symptom score* | 0.09 (Spearman= 0.07) |
−0.07 (Spearman = −0.07) |
0.01 (Spearman = −0.01) |
−0.34 (Spearman= −0.31) |
−0.02 (Spearman = −0.04) |
−0.01 (Spearman= 0.00) |
1.00 |
*Insomnia symptom scores had a positively skewed distribution and, therefore, Spearman correlations were also calculated.
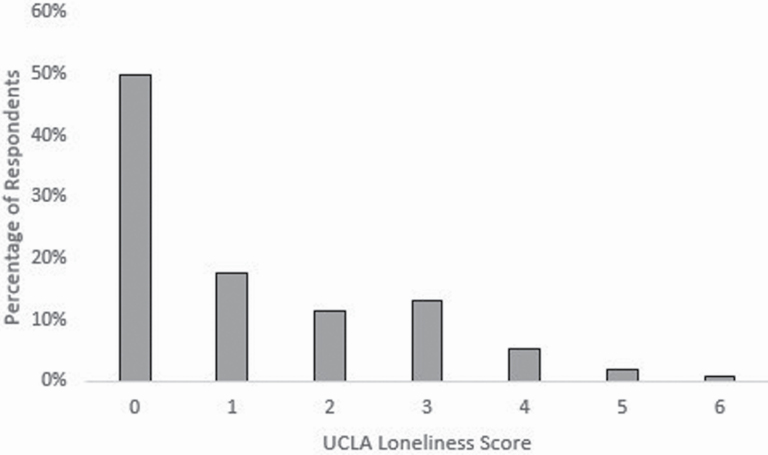
Figure 2 shows the distribution of loneliness scores. About half of respondents (49.8%) had a UCLA loneliness score of 0. The highest score was 6. The individual components of the standardized social isolation scale all showed variation. Forty-five percent of respondents named a maximum number of five people when asked to name the people with whom they could talk about important matters. About half of the participants socialized with friends or relatives at least once a week. Volunteering and group participation varied greatly, with about half of respondents participating in groups or volunteering very infrequently (less than once or twice a year) and about a fourth involved in these activities at least once a week. Social isolation and loneliness had a highly significant but low correlation (Spearman’s correlation = 0.20, p < 0.001).
Figure 2.
Distribution of UCLA loneliness scores in NSHAP age-eligible sleep study.
Regression results
The two actigraph measures of sleep disruption (WASO and percent sleep) were significantly associated with greater social isolation in adjusted models (Table 3). An SD increase in social isolation was associated with about a 0.13 SD increase in WASO (p < 0.01, 95% CI: 0.05, 0.21) in the adjusted model. For percent sleep, the association also indicated more disrupted sleep and was statistically significant (beta = −0.11; p < 0.01, 95% CI: −0.18, −0.03). Actigraphy TST and single-question sleep duration were not significantly associated with social isolation. However, the sleep log-calculated duration and the calculated time in bed were similarly and significantly associated with social isolation in adjusted models (Table 3). An SD increase in social isolation was associated with a 0.17 hour (about 10 minutes) increase in sleep log-calculated duration (p < 0.01, 95% CI: 0.04, 0.29) and a 0.14 hour (about 8 minutes) increase in calculated time in bed (p = 0.03, 95% CI: 0.01, 0.26).
Table 3.
Regression coefficients, 95% CIs, and p-values for the social isolation index from seven regression models in which each sleep outcome is separately regressed on social isolation and the indicated covariates
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| ß | 95% CI | P-value | ß | 95% CI | P-value | |
| Actigraph sleep measures | ||||||
| WASO | 0.17 | [0.08, 0.25] | <0.001 | 0.13 | [0.05, 0.21] | <0.01 |
| Percent sleep | −0.14 | [−0.22, −0.06] | <0.01 | −0.11 | [−0.18, −0.03] | <0.01 |
| Actigraph TST | 0.04 | [−0.08, 0.15] | 0.52 | 0.03 | [−0.08, 0.14] | 0.54 |
| Self-reported sleep measures | ||||||
| Single-question duration | −0.03 | [−0.16, 0.09] | 0.59 | −0.05 | [−0.18, 0.08] | 0.43 |
| Sleep log-calculated duration | 0.18 | [0.06, 0.30] | <0.01 | 0.17 | [0.04, 0.29] | <0.01 |
| Calculated sleep interval | 0.15 | [0.01, 0.28] | 0.03 | 0.14 | [0.01, 0.26] | 0.03 |
| Insomnia symptom score | 1.00† | [0.87, 1.17] | 0.95 | 1.00b | [0.87, 1.16] | 0.96 |
*Adjusted for demographics: age, sex, race/ethnicity, and education.
†The insomnia symptom score is modeled using ordinal logistic regression and values are odds ratios.
An additional set of social isolation models were then constructed to examine whether the longer calculated duration was associated specifically with bed times being earlier or wake times being later. Social isolation was significantly associated with later wake times (Table 4). An SD increase in social isolation was associated with a 0.16 hour (about 10 minutes) later wake time on weekdays (p = 0.02, 95% CI: 0.03, 0.29) and a 0.13 hour later wake time on weekends (about 8 minutes; p = 0.04, 95% CI: 0.00, 0.26).
Table 4.
Regression coefficients, 95% CI, and p-values for the social isolation index from four regression models in which each wake and bed time are separately regressed on social isolation, controlled for demographics (age, sex, race/ethnicity, and education)
| Social iolation β coefficient |
95% CI | P-value | |
|---|---|---|---|
| Wake times | |||
| Weekday | 0.16 | [0.03, 0.29] | 0.02 |
| Weekend | 0.13 | [0.00, 0.26] | 0.04 |
| Bed times | |||
| Weekday | 0.01 | [−0.09, 0.12] | 0.80 |
| Weekend | -0.01 | [−0.12, 0.10] | 0.85 |
Analysis of the age-eligible NSHAP sleep study participants. ordinary least squares regression was used for all models. All measures are in hours.
The two actigraph measures of sleep disruption (WASO and percent sleep) were significantly associated with greater loneliness in adjusted models (Table 5). An additional point on the loneliness scale was associated with about a 0.08 SD increase in WASO (p = 0.02, 95% CI: 0.01, 0.15) in the adjusted model. For percent sleep, the association similarly represented sleep disruption and was statistically significant (β = −0.07; p = 0.03, 95% CI: −0.13, −0.01). Actigraphy TST was not associated with loneliness. The insomnia symptom score was strongly associated with loneliness. A 1 point increase in loneliness score was associated with a 31% increase in the odds of a point higher insomnia symptom score (p < 0.001, 95% CI: 1.17, 1.41). Higher loneliness scores were associated with shorter self-reported sleep duration (about 7 minutes per point; β = −0.11; p = 0.03, 95% CI: −0.20, −0.01), but there was no evidence that sleep log duration or calculated duration measures were (Table 5). We also performed a robustness test by dichotomizing the loneliness scale based on prior research that uses this strategy to address the nonnormal distribution of the shortened UCLA scores [49–51]. The associations remained consistent to the more interpretable full scale and we include these findings in the Supplementary Tables.
Table 5.
Regression coefficients, 95% CIs, and p-values for loneliness score from seven regression models in which each sleep outcome is separately regressed on the loneliness score and the indicated covariates
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| ß | 95% CI | P-value | ß | 95% CI | P-value | |
| Actigraph sleep measures | ||||||
| WASO | 0.08 | [0.01, 0.15] | 0.02 | 0.08 | [0.01, 0.15] | 0.02 |
| Percent sleep | −0.07 | [−0.13, 0.00] | 0.04 | −0.07 | [−0.13, −0.01] | 0.03 |
| Actigraph TST | −0.02 | [−0.10, 0.06] | 0.56 | −0.05 | [−0.12, 0.03] | 0.25 |
| Self-reported sleep measures | ||||||
| Single-question duration | −0.11 | [−0.20, −0.02] | 0.02 | −0.11 | [−0.20, −0.01] | 0.03 |
| Sleep log-calculated duration | −0.05 | [−0.15, 0.04] | 0.27 | −0.05 | [−0.14, 0.04] | 0.26 |
| Calculated sleep interval | 0.02 | [−0.08, 0.12] | 0.72 | 0.02 | [−0.07, 0.12] | 0.67 |
| Insomnia symptom score | 1.32† | [1.18, 1.48] | <0.001 | 1.31b | [1.17, 1.47] | <0.001 |
*Adjusted for demographics: age, sex, race/ethnicity, and education.
†The Insomnia Symptom Score is modeled using ordinal logistic regression and values are odds ratios.
Discussion
In a large, nationally representative sample of older adults, we found that loneliness and social isolation scores, which have a significant but low correlation with each other, had similar associations with actigraph measures of sleep but different associations with self-reported sleep measures. Greater loneliness and social isolation were both associated with more disrupted sleep, measured by actigraphy WASO and sleep percent. Neither was associated with actigraph TST. Lonelier individuals reported more insomnia symptoms, but more socially isolated individuals did not. Lonelier individuals also reported shorter sleep, based on a single duration question, but social isolation was not associated with single-question duration. However, more socially isolated individuals had longer sleep durations when duration was calculated from bedtimes and wake times, either from direct questions about them or from a sleep log, and more lonely individuals did not. Greater time in bed for socially isolated individuals was specifically due to later waking times in the mornings, not earlier bedtimes.
Direct comparisons of our findings to previously published studies are limited by differences in how loneliness, social isolation, and sleep have been measured in each study population. Our results are similar to those from two previous studies that found an association between loneliness and objective sleep measures but differ with respect to their findings about self-reported sleep quality [12, 23]. Both of these studies used smaller, younger, and more homogenous populations than the present one. Kurina et al. examined 95 Hutterites (mean age 39.8 years) [23], and Cacioppo et al. studied 64 undergraduate students [12]. Additionally, our findings contrast with a recent cohort study of older adults in Taiwan [52]. Yu et al. found that social isolation, but not loneliness, was associated with worse self-reported sleep quality after controlling for demographic, health, cognitive, and depressive factors [52]. This inconsistent finding may be related to differences in how loneliness, social isolation, and sleep quality were measured, to differences in control variables, or to differences in the study populations. Yu et al. used the PSQI to determine self-reported sleep quality and used less comprehensive measures of both loneliness and social isolation [52]. Additionally, Yu et al. incorporated marital status into the social isolation measure [52].
Our finding that social isolation is associated with increased time in bed has not been previously reported. We found an association between social isolation and time in bed whether measured through the times recorded in a 3 day sleep log kept concurrently with the actigraph measurement or from answers to questions about routine bedtimes and wake times. People who are more socially disconnected report spending more time in bed in the mornings. This finding adds to results from two previous NSHAP studies. Chen et al. found that married adults report a longer calculated time in bed than those who are not married [24]. It is not clear why being married and greater social isolation both increase time in bed. Were one to speculate that, on average, people are seeking more connections to other people, then, for married people, being in bed is potentially a time for companionship, while more socially isolated adults could be spending more time in bed in the morning in order to moderate the effects of their greater sleep disruption or they could be spending more time in bed because it is a retreat from waking time, which includes few social interactions and activities. Chen et al. examined sleep correlates of a more limited measure of social interactions that only included group activity participation [31]. They did not find a significant association with the calculated sleep interval, although the direction of the effect was similar.
Many of our findings are consistent with previous studies. Our finding of a strong association between loneliness and more insomnia symptoms is consistent with the conclusions of a systematic review, which examined the impact of social isolation and loneliness on behavioral health in older adults [22]. This was also found in a recent study of community-dwelling older adults in southern California [53]. Past studies have failed to find an association between loneliness and sleep duration, whether measured by self-report or objective approaches [12, 23, 54], which is consistent with some of our results. Nonetheless, our findings confirm previous research that duration responses vary based on the way in which sleep questions are posed and that these measures capture different aspects of the sleep experience [18, 20, 26, 54]. Additionally, our results are consistent with Chen et al., which found that individuals with more group activity participation had better actigraph-measured sleep quality [31]. However, that study did not find that there was a longitudinal association between changes in group activity participation and actigraph-measured sleep after 5 years [31]. The 5 year interval between measures of group participation may be too long to observe an effect.
A key limitation of this study is that we cannot infer causal direction from these cross-sectional data, and both causal directions are plausible. While there is now sleep data from surviving and participating members of the third wave (2015–2016), the 5 year interval between waves is too long to capture what are likely to be shorter-term effects of loneliness and social isolation, as evidenced by the day-to-day effects of loneliness on sleep salubrity (daytime dysfunction) in one prior study [54]. An improved study design would need repeated measures at closer intervals. Generally, more than 3 days of actigraphy are recommended to assess sleep patterns, but we have previously found little day-of-the-week variation in this largely retired, older population [27]. Although our three-item loneliness measure is more robust than previous studies, it only represents one facet of loneliness rather than the three that the full 20-item UCLA Loneliness Scale describes [55]. The social isolation index is able to capture more variability across a wide range of social characteristics, while our loneliness measurement may be limited to this one factor of loneliness. Another important limitation is that the timeframe varies for our sleep measures. The actigraph sleep measures and the sleep log all reference the same three nights. However, the survey sleep measures have no explicit timeframe.
The strengths of this study include the large and nationally representative sample of older adults and the varied measures of social isolation, loneliness, and sleep. This study incorporates both actigraph and self-reported sleep measures that capture four distinct methods for measuring sleep duration. Actigraph TST is highly correlated with polysomnography, the “gold standard” for sleep measurement [56, 57]. Additionally, the social isolation score includes a number of measures that capture greater depth and range of social networks and interactions than has previously been examined in this context [24, 52, 53]. This study used the Three-Item Loneliness Scale, which has a high correlation with the full UCLA loneliness scale (r = 0.82) and offers a comprehensive assessment of feelings of loneliness without simply asking people whether they are lonely as some studies have done [32].
This study affirms the value of diverse sleep measures to assess this complicated multidimensional behavior and illustrates that loneliness and social isolation are associated with different dimensions of sleep experience. Only loneliness is associated with self-reported measures of more insomnia symptoms and shorter sleep duration. Both loneliness and social isolation are associated with worse actigraph-estimated sleep quality. Whether assessed by a sleep log or direct questions about bedtimes and wake times, more socially isolated individuals average longer time in bed in the morning, while not having longer sleep duration as measured either by self-report or actigraphy. Our study points to potential differences in how social isolation and loneliness may affect—and reflect—health and well-being at older ages.
Supplementary Material
Acknowledgment
The authors thank Phil Schumm for his assistance with data analysis.
Funding
This work was supported by grants for Wave 2 and Wave 3 of the National Social Life, Health, and Aging Project, which is supported by the National Institute on Aging (NIA) and National Institutes of Health (NIH; R01AG033903; R01AG043538; R01AG048511; R37AG030481), as well as by R01AG042164 from NIA and the OppNet at NIH. The funders had no role in designing the study, analyzing the data, or preparing the manuscript.
Disclosure Statements
Financial disclosure: None.
Nonfinancial disclosure: None.
References
- 1. Hawkley LC, et al. . Transitions in loneliness among older adults: a 5-year follow-up in the national social life, health, and aging project. Res Aging. 2018;40(4):365–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shankar A, et al. . Social isolation and loneliness: prospective associations with functional status in older adults. Health Psychol. 2017;36(2):179–187. [DOI] [PubMed] [Google Scholar]
- 3. Cacioppo JT, et al. . Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46(3 Suppl):S39–S52. [PubMed] [Google Scholar]
- 4. Valtorta NK, et al. . Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102(13):1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hawkley LC, et al. . Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging. 2006;21(1):152–164. [DOI] [PubMed] [Google Scholar]
- 6. Holt-Lunstad J, et al. . Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227–237. [DOI] [PubMed] [Google Scholar]
- 7. Leigh-Hunt N, et al. . An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health. 2017;152:157–171. [DOI] [PubMed] [Google Scholar]
- 8. Coyle CE, et al. . Social isolation, loneliness and health among older adults. J Aging Health. 2012;24(8):1346–1363. [DOI] [PubMed] [Google Scholar]
- 9. Perissinotto CM, et al. . Living alone, socially isolated or lonely—what are we measuring? J Gen Intern Med. 2014;29(11):1429–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cornwell EY, et al. . Measuring social isolation among older adults using multiple indicators from the NSHAP study. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i38–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hawkley LC, et al. . Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cacioppo JT, et al. . Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychol Sci. 2002;13(4):384–387. [DOI] [PubMed] [Google Scholar]
- 13. Cacioppo JT, et al. . Evolutionary mechanisms for loneliness. Cogn Emot. 2014;28(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Hees VT, et al. . A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One. 2015;10(11):e0142533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doherty A, et al. . Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS One. 2017;12(2):e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Girschik J, et al. . Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22(5):462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lauderdale DS, et al. . Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lauderdale DS, et al. . Sleep duration and health among older adults: associations vary by how sleep is measured. J Epidemiol Community Health. 2016;70(4):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lauderdale DS. Commentary on agreement between simple questions about sleep duration and sleep diaries in a large online survey. Sleep Health. 2015;1(2):138–139. [DOI] [PubMed] [Google Scholar]
- 20. Chen JH, et al. . Insomnia symptoms and actigraph-estimated sleep characteristics in a nationally representative sample of older adults. J Gerontol A Biol Sci Med Sci. 2015;70(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Den Berg JF, et al. . Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17(3):295–302. [DOI] [PubMed] [Google Scholar]
- 22. Choi H, et al. . Impact of social isolation on behavioral health in elderly: systematic review. World J Psychiatry. 2015;5(4):432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurina LM, et al. . Loneliness is associated with sleep fragmentation in a communal society. Sleep. 2011;34(11):1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen JH, et al. . Marriage, relationship quality, and sleep among U.S. older adults. J Health Soc Behav. 2015;56(3):356–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaszczak A, et al. . Continuity and innovation in the data collection protocols of the second wave of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl 2):S4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McSorley VE, et al. . Associations of sleep characteristics with cognitive function and decline among older adults. Am J Epidemiol. 2019;188(6):1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lauderdale DS, et al. . Assessment of sleep in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl 2):S125–S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huisingh-Scheetz M, et al. . Wrist accelerometry in the health, functional, and social assessment of older adults. J Am Geriatr Soc. 2016;64(4):889–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurina LM, et al. . Actigraphic sleep characteristics among older Americans. Sleep Health. 2015;1(4):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen JH, et al. . Cognitive function, consent for participation, and compliance with wearable device protocols in older adults. J Gerontol A Biol Sci Med Sci. 2019;74(2):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen JH, et al. . Social participation and older adults’ sleep. Soc Sci Med. 2016;149:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hughes ME, et al. . A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging. 2004;26(6):655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rote S, et al. . Religious attendance and loneliness in later life. Gerontologist. 2013;53(1):39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Curran E, et al. . Symptom profiles of late-life anxiety and depression: the influence of migration, religion and loneliness. Depress Anxiety. 2019;36(9):824–833. [DOI] [PubMed] [Google Scholar]
- 35. Cornwell B, et al. . Social networks in the NSHAP study: rationale, measurement, and preliminary findings. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i47–i55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cornwell EY, et al. . Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50(1):31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lauderdale DS, et al. . Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164(1):5–16. [DOI] [PubMed] [Google Scholar]
- 38. Cohen-Mansfield J, et al. . Correlates and predictors of loneliness in older-adults: a review of quantitative results informed by qualitative insights. Int Psychogeriatr. 2016;28(4):557–576. [DOI] [PubMed] [Google Scholar]
- 39. Menec VH, et al. . Examining individual and geographic factors associated with social isolation and loneliness using Canadian Longitudinal Study on Aging (CLSA) data. PLoS One. 2019;14(2):e0211143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300–306. [PubMed] [Google Scholar]
- 41. Ensrud KE, et al. . Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13(10):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Finan PH, et al. . The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van den Berg JF, et al. . Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond). 2008;32(7):1083–1090. [DOI] [PubMed] [Google Scholar]
- 44. Ožić S, et al. . Interventions aimed at loneliness and fall prevention reduce frailty in elderly urban population. Medicine (Baltim). 2020;99(8):e19145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuiper JS, et al. . Social relationships and cognitive decline: a systematic review and meta-analysis of longitudinal cohort studies. Int J Epidemiol. 2016;45(4):1169–1206. [DOI] [PubMed] [Google Scholar]
- 46. Lara E, et al. . Are loneliness and social isolation associated with cognitive decline? Int J Geriatr Psychiatry. 2019;34(11):1613–1622. [DOI] [PubMed] [Google Scholar]
- 47. Lederer DJ, et al. . Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. [DOI] [PubMed] [Google Scholar]
- 48. O’Muircheartaigh C, et al. . Sample design, sample augmentation, and estimation for Wave 2 of the NSHAP. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl 2):S15–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Steptoe A, et al. . Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110(15):5797–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Niedzwiedz CL, et al. . The relationship between wealth and loneliness among older people across Europe: is social participation protective? Prev Med. 2016;91:24–31. [DOI] [PubMed] [Google Scholar]
- 51. Mullen RA, et al. . Loneliness in primary care patients: a prevalence study. Ann Fam Med. 2019;17(2):108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu B, et al. . Prospective associations of social isolation and loneliness with poor sleep quality in older adults. Qual Life Res. 2018;27(3):683–691. [DOI] [PubMed] [Google Scholar]
- 53. Cho JH, et al. . Associations of objective versus subjective social isolation with sleep disturbance, depression, and fatigue in community-dwelling older adults. Aging Ment Health. 2018;23(9):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hawkley LC, et al. . Loneliness impairs daytime functioning but not sleep duration. Health Psychol. 2010;29(2):124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hawkley LC, et al. . How can I connect with thee? Let me count the ways. Psychol Sci. 2005;16(10):798–804. [DOI] [PubMed] [Google Scholar]
- 56. Marino M, et al. . Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jean-Louis G, et al. . Determination of sleep and wakefulness with the actigraph data analysis software (ADAS). Sleep. 1996;19(9):739–743. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.