Abstract
Children’s poor sleep is a risk factor for lower cognitive functioning and internalizing and externalizing problems. It is unclear whether genetic and environmental influences vary based on sleep assessment and no studies to date have examined genetic and environmental contributions to links between multiple objective and subjective sleep indicators. Further, nearly all heritability studies rely on subjective parent- or self-report measures of sleep duration and problems. Given these gaps in the literature, we (1) modeled genetic and environmental influences on multiple objective and subjective sleep indicators and (2) estimated genetic and environmental covariances between objective and subjective sleep indicators in middle childhood. Participants were 608 twin children (MZ = 178, same-sex DZ = 234, opposite-sex DZ = 190) assessed at 8 years of age (SD = 0.63 years). Objective nighttime sleep duration, efficiency, sleep onset latency (SOL), midpoint time, and midpoint variability were collected from actigraph watches worn for 7 nights (Mnights = 6.83, SD = 0.62). Children’s nighttime sleep duration and daytime sleepiness were assessed via parent report. Findings suggested high additive genetic influence on objective sleep quantity and quality, whereas objective SOL, sleep midpoint time, midpoint variability, parent-reported sleep duration, and daytime sleepiness were largely influenced by the shared environment. Common genetic factors explained associations between objective sleep quantity and quality, but genetics did not account for links with parent-reported sleep duration, midpoint time, or midpoint variability. Thus, objective and parent-reported assessments of children’s sleep have unique genetic etiologies and should not be used interchangeably in the sleep literature.
Keywords: sleep, actigraphy, parent report, genetics, twins, children, middle childhood
Statement of Significance.
Our novel findings demonstrate that objectively measured sleep quantity and quality have high genetic influence during middle childhood, while objectively measured sleep timing and variability, as well as parent-reported sleep quantity and quality, are more environmentally influenced, suggesting that these indicators may be constrained by family routines and schedules. Shared genetics explain relations between objective sleep quantity and quality, but not between parent-reported sleep quantity, objective sleep timing, or sleep variability indicating that objective and parent-reported child sleep have unique genetic etiologies. Moving forward, it is essential to test how objective and parent-report sleep indicators differentially predict key developmental outcomes, as well as whether sociocontextual factors moderate genetic influences on objective and subjective sleep during middle childhood.
Introduction
Almost one-third of children and adolescents sleep eight or fewer hours per night, falling below recommended levels of sleep for these age groups (recommended levels: 9–12 h/24-h for children ages 6–12 and 8–10 h/24-h for adolescents ages 13–18) [1–3]. This suggests that children may regularly experience behavioral sleep problems (i.e. non- or sub-clinical sleep problems) like short duration, poor quality, irregular timing, and difficulty with sleep initiation or maintenance [4, 5]. These sleep indicators have been associated with numerous negative developmental outcomes including internalizing and externalizing problems and poorer cognitive functioning [5], signaling the need to understand which sleep indicators may be candidate targets for interventions seeking to improve children’s sleep and psychosocial functioning. However, sleep indicators like duration, quality, and timing are often examined as unique or independent predictors of psychopathology, which does not address shared etiology. Sleep indicators have underlying genetic or environmental covariances and modeling these shared factors can clarify etiology and point to potential targets for interventions aiming to improve sleep and well-being in children. As such, the current study aimed to test not only genetic and environmental influences on multiple individual aspects of objective and subjective sleep, but also estimate the extent to which objectively and subjectively measured sleep indicators may have shared etiology.
Heritability of sleep
Multiple studies show a considerable genetic contribution to unique subjective sleep indicators like sleep duration, quality, daytime sleepiness, sleep onset latency (SOL), sleep problems, and timing, while fewer studies have examined the heritability of objectively measured sleep in children and adolescents (e.g. via actigraphy). Prior twin studies also show different heritability estimates for various aspects of sleep in childhood depending on whether parents or children report sleep problems [6–9], indicating the importance of measurement when assessing sleep and related outcomes. However, there is some agreement across objective and subjective sleep studies in childhood regarding the heritability of various sleep indicators. For example, both objective (h2 = 0.13–0.65) [10, 11] and parent-reported sleep duration (h2 = 0.00–0.71) [12–14] show a wide range of additive genetic influence in prior studies. Similarly, daytime functioning and sleep quality are moderate to highly heritable in studies using objective (h2 = 0.52–0.70) [10, 11] and parent-reported (h2 = 0.32–0.47) [12, 14] sleep for children and adolescents, with objective sleep quality showing slightly higher heritability. Subjective and objective (self-reported) SOL has demonstrated similarly high heritability (h2 = 0.75–0.83) in samples of children during late childhood [10, 11], but lower heritability in late adolescence (parent-reported SOL; h2 = 0.21) [13], highlighting changes in heritability for various aspects of sleep like SOL across development and differences according to measurement. Regarding sleep timing, self-reported diary bedtimes and waketimes have shown relatively low heritability during late childhood (h2 = 0.26 and 0.14, respectively) [11], and objective waketime and sleep midpoint time demonstrate even lower additive genetic influence (h2 = 0.00 and 0.12, respectively) [11]. Yet, actigraphy-reported bedtime in the same study showed moderate heritability (h2 = 0.38) [10]. It should also be noted that both studies using objectively measured sleep had 50 participants or fewer [10, 11], so they likely did not have the power to obtain stable estimates [15–17]. Given some differences in the heritability of objective and subjective sleep across studies, the first aim of the current study was to estimate additive genetic, shared environmental, and nonshared environmental contributions to multiple objectively-and subjectively-assessed facets of sleep during middle childhood in a large twin sample.
Associations between objective and subjective sleep
The second aim of the current study was to estimate unique as well as shared additive genetic, shared environmental, and nonshared environmental covariances between various objectively-and subjectively-measured aspects of sleep during middle childhood. Testing links between objective and subjective sleep indicators, including examinations of shared or unique etiology, is critical due to research suggesting that different reporters (i.e. subjective and objective) may demonstrate differential prediction of developmental outcomes [18]. However, only one study to date has examined genetic and environmental covariance between different aspects of sleep, which points to shared underlying etiology. The study assessed subjective reports of sleep in a sample of over 1,000 late adolescent twins and siblings (Mage = 20 years) [13] and found that nonshared environmental factors primarily accounted for sleep duration links with quality (0.50), daytime dysfunction (0.63), and SOL (0.82), and for associations between SOL and sleep quality (0.63) [13]. On the other hand, shared additive genetic factors accounted for the majority of associations between sleep quality and daytime dysfunction (0.55) [13]. While this study elucidated genetic and environmental covariances between multiple indicators of sleep, no studies to our knowledge have examined genetic and environmental contributions to links beyond subjective sleep quality [13] or tested these associations during childhood.
Current study
Given multiple gaps in the literature, the current study had two main aims: (1) model additive genetic, shared environmental, and nonshared environmental influences on objective and subjective sleep indicators (objective sleep duration, sleep efficiency, SOL, sleep midpoint time, midpoint variability, parent-reported sleep duration, and daytime sleepiness) and (2) estimate genetic and environmental covariances between various objective and subjective sleep indicators during middle childhood. Based on prior empirical studies with twin children [6, 11–13], we expected the greatest proportion of the variance in objective sleep duration, efficiency, and SOL to be accounted for by additive genetic factors, with some contribution of nonshared environmental factors. Given prior studies showing strong environmental influence on sleep timing indicators and daytime functioning [10–13], we hypothesized that the greatest proportion of the variance in objective sleep midpoint time and midpoint variability to be accounted for by shared and nonshared environmental factors, with small influence from additive genetics. Finally, we expected that that greatest proportion of the variance in subjective (parent-reported) sleep duration and daytimes sleepiness in middle childhood would be explained by shared environmental factors with some small contributions from additive genetic factors [6, 11–13].
Regarding bivariate genetic and environmental links between sleep indicators, we expected that most of the covariance between objective sleep indicators to be accounted for by additive genetic factors and nonshared environmental influences. In contrast, we hypothesized that associations between subjective sleep indicators, as well as links between objective and subjective sleep indicators, would be accounted for by shared and nonshared environmental factors [10, 11, 13].
Methods
Participants
Participants were children from a large ongoing, longitudinal twin study [19]. Families were first assessed when twins were approximately 12 months old (N = 291 pairs; 50.5% male; 25.1% Hispanic/Latino) and were assessed again when children were 8 years old. Notably, new families with children born in the same years/cohort as the full sample were also recruited into the study at the eight-year assessment. Thus, the eight-year assessment included 608 twins (304 pairs; Mage = 8.52 years, SD = 0.63 years; data collected from 2016 to 2018). Of these families, there were 89 (29.6%) monozygotic or MZ twin pairs, 117 (38.9%) same-sex dizygotic or DZ twin pairs, 95 (31.6%) opposite-sex DZ twin pairs, and 3 pairs (1.0%) of unknown zygosity. At the eight-year assessment, participants were 49.2% male and ethnically diverse (approximately 56.6% non-Hispanic European American, 24.8% Hispanic/Latino, 3.6% Asian American, 4.0% African American, 2.6% Native American, 1.0% Native Hawaiian families, and 8.0% multiethnic or unknown ethnicity). The majority of primary caregivers reported either completing a college degree (36.8%; 33.3% for spouse/partner), a graduate or professional degree (22.5%; 20.2% for spouse/partner), or some college without graduating (27.5%; 26.7% for spouse/partner). Families in the eight-year assessment showed a broad range of socioeconomic status (SES; range = under $20,000 to over $150,000), with 9.1% reporting living in poverty, 23.0% living near the poverty line, 16.3% identifying as lower middle class, and 51.6% characterized as middle to upper class.
When comparing sample demographic information to state and national demographics and sleep statistics, current sample statistics are similar to state-level statistics particularly on ethnicity and income and poverty [20]. However, the current sample demonstrates higher levels of education for primary and secondary caregivers compared to state averages consisting of adults of all ages. Finally, parent-reported sleep duration and timing (e.g. bedtime and wake time) from at least one nationally representative sample of children assessed during middle childhood [21] are comparable to estimates of parent-reported sleep duration and objective sleep timing reported in the current study. Overall, sample statistics from the current study are similar to state and national data for demographic variables and some parent-reported sleep indicators, suggesting that the results from our study may be representative of the larger population in multiple respects.
Procedure
Parents of twins were recruited through birth records in Arizona when twins were approximately 12 months old. Primary caregivers (94.6% mothers) completed interviews via telephone regarding her pregnancy and twins’ development, zygosity, temperament, and health. Primary caregivers were contacted again when twins were approximately 8 years old and offered the opportunity to participate in an intensive assessment of child sleep and health, consisting of two home visits separated by a week-long study protocol. At each home visit, experimenters collected questionnaires and biological measures (e.g. height and weight), conducted cognitive tasks with the twins, and administered a parent–child interaction and an interview assessing the home environment. At each family’s first home visit, study staff also trained the primary caregiver (94.1% mothers) and twins for the week-long study protocol, in which the twins wore wrist-based accelerometers (actigraph watches) for seven nights and eight days, and primary caregivers completed web-based daily diaries about child sleep, activity, and food via smartphone or computer (90.9%), paper (7.2%), or both (1.5%; 0.4% missing diary data). Primary caregivers also reported each twin’s bedtimes and wake times on a daily assessment table as an additional report of child sleep used for cross-validation when cleaning actigraphy sleep data.
Families part of the full sample (beginning at 12 months of age) who lived outside the state of Arizona were also invited to participate in the eight-year assessment (N = 40 families). However, materials and assessments that typically occurred in home visits, including sleep assessment and biological measurements, were not collected from these families. Questionnaires and subjective reports of sleep were collected from these families. Analyses were conducted including and excluding families who completed the out-of-state protocol, and results were similar or the same across analyses. As such, out of state families were included in the analytic sample (N = 608).
Measures
Objective sleep
Objective sleep indicators were assessed using the Micro Motionlogger actigraph wristwatch (Ambulatory Monitoring, Inc., Ardsley, NY). The Micro Motionlogger contains an accelerometer, which captures small movement throughout the waking day and during sleep periods. Each child wore an actigraph watch on their nondominant wrist for seven nights (M = 6.83 nights, SD = 0.62). Researchers scored objective sleep data using the Action-W2 program (version 2.7.1), which uses a validated algorithm to measure sleep [22]. Researchers used the Sadeh algorithm to assess sleep [23, 24], with movement measured in 1-min epochs using a zero-crossing mode. Additionally, actigraph-measured ambient light and moderate to vigorous physical activity levels and parent-reported bedtimes and wake times from daily assessment tables and daily sleep diaries were used to cross-validate scoring of sleep data (i.e. sleep onset and offset times). Significant drops in actigraph-measured ambient light and physical activity, in correspondence with parent-reported bedtimes via assessment tables and diaries, were used to indicate when children were in bed and attempting to go to sleep at night, and first fell asleep (sleep onset). Significant increases in actigraph-measured ambient light and physical activity, as well as parent-reported wake times via assessment tables and diaries, were used to indicate when children were first waking from sleep (sleep offset). When these additional actigraph measures did not agree with parent-reported bedtimes and wake times, we relied on objective actigraph indicators to set sleep onset and offset markers.
The Sadeh algorithm calculates a variety of sleep parameters using 1-min epochs and based on significant movement after at least 20 min of inactivity. We used five indicators of average objective sleep: nighttime sleep duration, efficiency, SOL, midpoint time, and midpoint variability. In the current study, nighttime sleep duration represented the total number of hours and minutes asleep each night from sleep onset to sleep offset but excluding any minutes of waking or sleep latency before initial sleep onset. Sleep efficiency represented the percentage of time asleep each night (nighttime sleep duration) based on the total amount of time in bed. Notably, nighttime sleep duration represents true sleep time and excludes any periods of waking, whereas the total time spent in bed represents the period from sleep onset to offset including true sleep time, any minutes of waking during the sleep period, and minutes of sleep latency before initial sleep onset. SOL was the average number of minutes taken to fall asleep each night once in bed, or the period between bedtime and initial sleep onset. Sleep midpoint time was the time of night midway between sleep onset and offset on average. Sleep midpoint variability was the within-person standard deviation estimate of sleep midpoint time of night (time halfway between sleep onset and offset) across the study week.
As noted, study staff cross-checked objective actigraphy sleep periods with parent-reported bedtimes and wake times from daily assessment tables and daily sleep diaries as an additional sleep-period compliance measure to identify significant outliers and equipment malfunction. Compliance was high, with 9.8% (N = 48 children) of actigraphy data missing due to loss of actigraph watch or water damage (N = 4), watch mechanical malfunction (N = 15), children not wearing the watch but participating in other parts of the study week (N = 7), and the number of in-state families who participated only in the questionnaire and/or home visit portion of the study (N = 22). Of the families who had actigraphy data, 87.3% (N = 428 children) wore the watch for seven or more nights, 9.6% (N = 47) had six nights of data, 0.8% (N = 4) had five nights of data, 1.4% (N = 7) had four nights of data, and 0.8% (N = 4) had three nights of data. Research suggests that actigraphy is reliable when measuring five or more nights of sleep, and if an individual has fewer than five nights of actigraphy data, this may provide a poor estimation of regular sleep [25]. Thus, we conducted exploratory analyses excluding participants with fewer than five nights of sleep (N = 11 children) to determine whether results were similar compared to analyses including all children with available objective sleep data. Results excluding children with fewer than five nights of sleep did not differ from results including all children with available objective sleep data; as such, all cases were included in analyses.
Subjective sleep
Subjective sleep was measured using items from the primary caregiver-report of the Child Sleep Habits Questionnaire (CSHQ) [26]. Parent-reported sleep duration was assessed with one question (i.e. Typically, Twin A/B sleeps ___ hours and ___ minutes at night) and represented the total number of hours and minutes each twin slept at night on average. Additionally, the daytime sleepiness scale (7 items) assessed difficulty waking up in the morning and frequency of falling asleep during daytime activities (e.g. Twin A/B has a hard time getting out of bed in the morning). Daytime sleepiness items were summed to form a single score, where higher scores reflected greater daytime sleepiness. Items and scales in the CSHQ [26] are not necessarily highly correlated in community samples of children, given that individual items scales capture unique (possibly unrelated) child sleep problems; as such, alpha coefficients are not appropriate to report [26].
Zygosity
Zygosity was assessed at 12 months (α = 0.95) via primary-caregiver reports using the Zygosity Questionnaire for Young Twins [27], a 32-item measure that differentiates between MZ and DZ twins using parent report of birth and observable differences in physical appearance between the twins. This measure is between 93% and 98% accurate in characterizing twin zygosity compared to genotyping, making questionnaires a reliable alternative [28]. Families who did not report or participate at the 12-month assessment completed the questionnaire at 8 years of age.
Statistical analysis
Preliminary analyses were conducted to provide sample statistics, including descriptive statistics, zero-order correlations, and twin intra-class correlations. Variance and covariance between objective and subjective sleep indicators can be elucidated using genetically informed designs like the twin method [29]. Given that MZ twins share 100% of their segregating DNA, any differences observed between MZ twins can be attributed to environmental factors alone, while any differences between DZ twins (who share roughly 50% of their genetic composition) may be attributed to both genetic and environmental factors [29]. Using this logic, the ACE model is used to estimate additive genetic (A factor; MZ similarity set to 1.0, DZ set to 0.5), shared environmental (C factor; MZ and DZ similarity both set to 1.0 for twins reared together), and nonshared environmental factors (E factor; MZ and DZ similarity not correlated) that contribute to phenotype variance [15, 29].
Extending to the multivariate case, the Cholesky Decomposition estimates genetic and environmental influences on covariance in addition to variances [15]. For example, a bivariate Cholesky decomposition of associations between sleep duration (phenotype 1) and sleep quality (phenotype 2) would estimate unique additive genetic (A22), shared environmental (C22), and nonshared environmental (E22) influences on sleep quality, while accounting for shared additive genetic (A21), common environmental (C21), and nonshared environmental (E21) influences on sleep duration. As such, we can use the bivariate Cholesky decomposition to calculate or derive the percent of shared variance explained by additive genetic, shared environment, and/or nonshared environmental influences (e.g. A21, C21, and E21 paths). To do this, we: (1) used the unstandardized path estimate for genetic or environmental variance shared between two phenotypes, (2) then squared the unstandardized path estimate for the shared variance, and (3) finally, divided the squared shared variance by the total unstandardized variance in the second phenotype. For example, to calculate the percent of shared genetic variance between objective sleep duration and efficiency, we would use the unstandardized additive genetic path estimate shared between sleep duration and efficiency, square that number, and then divide it by the total unstandardized variance for sleep efficiency. This number can be transformed into a percentage by multiplying this estimate or number by 100. This same process was conducted for all shared variance paths in bivariate twin models to provide more comprehensible estimates or better understanding of these shared paths (see Figures 1 and 2). Finally, the bivariate twin model can also estimate the extent to which genetic (rG), shared environmental (rC), and nonshared environmental factors (rE) for sleep duration are correlated with genetic, shared environmental, and nonshared environmental factors for sleep quality.
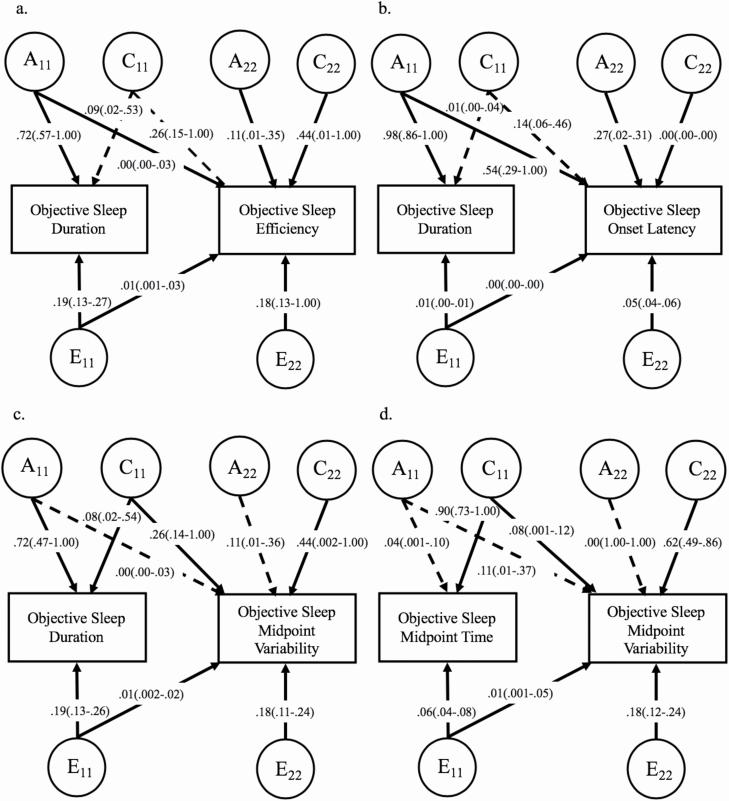
Figure 1.
Full bivariate Cholesky decompositions for associations between objective sleep indicators. Full bivariate Cholesky decomposition models are shown. Objective assessments were obtained using actigraphy. Sex and age were regressed out of variables prior to model fitting. Estimates are provided with variance-based confidence intervals provided in parentheses and are based on standardized path estimates. Standardized path estimates for the second phenotype in each model are adjusted after accounting for the covariance between phenotypes. Solid lines indicate significant (retained) paths in both the full and best-fitting models, whereas dashed lines signify paths that were dropped from the final, best-fitting models without significant loss of fit to the data (see Table 4 for best-fitting model standardized path estimates and corresponding confidence intervals, and Supplementary Tables S1–S4 for fit statistics and genetic and environmental correlations). A11, additive genetic components for first phenotype; C11, shared environment component for first phenotype, E11, nonshared environment component for first phenotype. A22, C22, E22, residual additive genetic, shared environmental, and nonshared environmental components for second phenotype not shared with first phenotype, respectively. (A) rG = 0.79, rC = 0.00, rE = 0.00. (B) rG = 0.94, rC = 1.00, rE = 0.26. (C) rG = 0.00, rC = 0.61, rE = 0.08. (D) rG = 1.00, rC = 0.35, rE = 0.28.
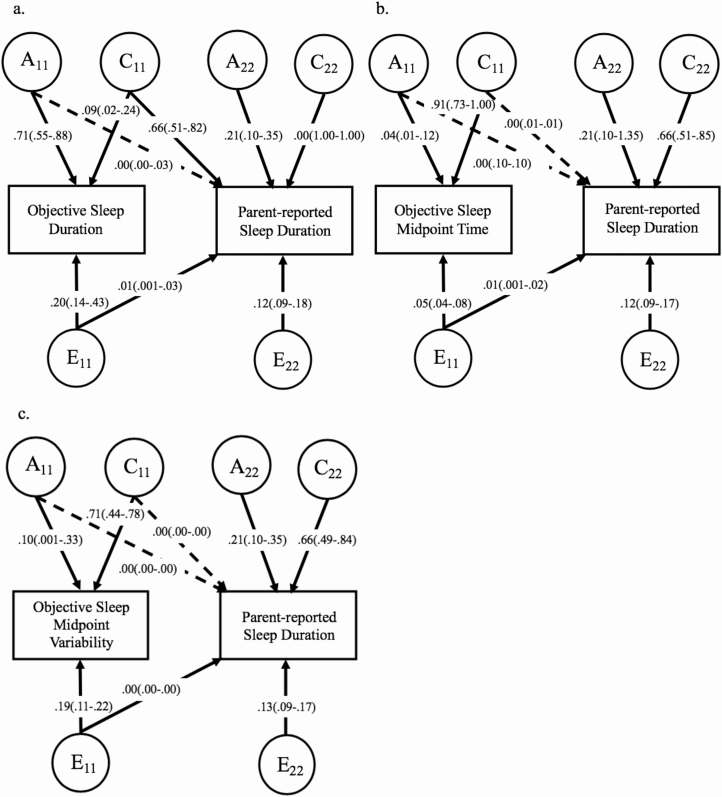
Figure 2.
Full bivariate Cholesky decompositions for associations between objective and subjective sleep indicators. Full bivariate Cholesky decomposition models are shown. Objective assessments were obtained using actigraphy. Sex and age were regressed out of variables prior to model fitting. Estimates are provided with variance-based confidence intervals provided in parentheses and are based on standardized path estimates. Standardized path estimates for the second phenotype in each model are adjusted after accounting for the covariance between phenotypes Solid lines indicate significant (retained) paths in both the full and best-fitting models, whereas dashed lines signify paths that were dropped from the final, best-fitting models without significant loss of fit to the data (see Table 4 for best-fitting model standardized path estimates and corresponding confidence intervals, and Supplementary Tables S5–S7 for fit statistics and genetic and environmental correlations). A11, additive genetic components for first phenotype; C11, shared environment component for first phenotype; E11, nonshared environment component for first phenotype. A22, C22, E22, residual additive genetic, shared environmental, and nonshared environmental components for second phenotype not shared with first phenotype, respectively. (A) rG = 0.02, rC = 0.89, rE = 0.09. (B) rG = 0.00, rC = 0.00, rE = 0.07. (C) rG = 0.00, rC = 0.00, rE = 0.14.
Twin ACE models were conducted with all variables to estimate genetic and environmental influences on variances and covariances (when two indicators were sufficiently correlated with one another). Bivariate models were not estimated for weak (less than 0.20) or uncorrelated sleep indicators [15]. Models were fit in OpenMx [30], an R-based program that estimates genetic and environmental variances and covariances using multigroup (MZ and DZ twins are modeled separately) structural equation models with maximum likelihood estimation and allowance for missing data. The effects of age and sex were regressed out of all sleep parameters before model fitting, as is standard practice for twin modeling [31]. Significance of all A and C parameters in each quantitative behavior genetic model was tested by systematically dropping the A parameter, C parameter, and then both A and C parameters from the model and comparing the fit of full and reduced models. Because the E parameter contains measurement error, it was not dropped from any models. Full and reduced models were compared using log-likelihood tests (indicated by ∆χ2) which compared model fit of nested models. Non-significant p-values for the χ2 difference test indicated that a reduced model did not fit the data significantly worse and the simpler model was adopted. Akaike’s Information Criterion (AIC) [32], which penalizes models with a larger number of parameters, was also used to assess model fit. Lower AIC values indicated a better model fit.
Model-fitting procedures
For univariate twin models, the full ACE model was fit, followed by systematically dropping the A parameter, C parameter, and then both A and C parameters from the model and comparing the fit of full and reduced models [33]. As noted, the E parameter was not dropped from any univariate twin models. For bivariate twin model fitting, we dropped paths in a similarly systematic way. We began by fitting the full ACE-ACE-ACE model and then we systematically dropped the following paths or combination of paths to determine parameter significance and arrive at the best-fitting models (See Supplementary Tables S1–S7 for results and more details on the order of path dropping): (1) ACE-ACE-ACE, (2) ACE-CE-ACE, (3) ACE-AE-ACE, (4) ACE-AC-ACE, (5) ACE-E-ACE, (6) ACE-C-ACE, (7) ACE-A-ACE, (8) CE-CE-CE, (9) AE-AE-AE, (10) ACE-CE-CE, (11) CE-CE-ACE, (12) ACE-AE-AE, and (13) AE-AE-ACE. As with univariate twin models, full and reduced models were compared and best-fitting models were determined by evaluating log-likelihood tests (indicated by ∆χ2), non-significant p-values for the χ2 difference test, and lower or change in AIC values.
Results
Preliminary analyses
On average, objective nighttime sleep duration measurement (excluding night waking minutes) showed that children slept about 8 h and 5 min each night (SD = 44 min; Table 1). Parents reported that children slept approximately 9 h and 39 min each night (SD = 52 min), with this measure including possible nighttime waking minutes. Children also showed adequate sleep quality, spending about 90% of their time in bed each night sleeping. On average, children took just over 20 min to fall asleep once in bed and showed an average midpoint time of sleep of 2:17 am (SD = 46 min). Objective nighttime sleep duration was positively correlated with objective sleep efficiency and negatively correlated with SOL, sleep midpoint time, and midpoint variability. Objective sleep efficiency was negatively correlated with SOL. Objective sleep midpoint time was also positively associated with SOL, sleep midpoint variability, and daytime sleepiness. Parent-reported sleep duration was negatively correlated with sleep midpoint time, midpoint variability, and subjective daytime sleepiness and positively correlated with objective sleep duration.
Table 1.
Zero-order correlations, descriptive statistics and twin intra-class correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | MZ | DZss | DZos | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Objective nighttime | – | 0.84 | 0.47 | 0.43 | |||||||||||
| Sleep duration (h) | |||||||||||||||
| 2. Objective sleep | 0.68*** | – | 0.84 | 0.50 | 0.43 | ||||||||||
| Efficiency (%) | |||||||||||||||
| 3. Objective sleep | −0.23*** | −0.15*** | - | 0.76 | 0.68 | 0.57 | |||||||||
| Latency (min)‡ | |||||||||||||||
| 4. Objective sleep | −0.17** | −0.05 | 0.12** | – | 0.95 | 0.97 | 0.86 | ||||||||
| Midpoint time§ | |||||||||||||||
| 5. Objective sleep | −0.20*** | −0.01 | 0.08 | 0.40*** | – | 0.83 | 0.83 | 0.69 | |||||||
| Midpoint time | |||||||||||||||
| Variability | |||||||||||||||
| 6. Parent-reported sleep | 0.36*** | −0.02 | −0.01 | −0.22*** | −0.24*** | – | 0.87 | 0.81 | 0.78 | ||||||
| Duration (h) | |||||||||||||||
| 7. Parent-reported | −0.05 | 0.05 | 0.09† | 0.17*** | 0.09† | −0.16*** | – | 0.93 | 0.88 | 0.62 | |||||
| Daytime sleepiness|| | |||||||||||||||
| 8. Sex | 0.15** | 0.12** | 0.01 | 0.03 | 0.02 | 0.04 | 0.09* | – | |||||||
| 9. Age (years) | −0.22*** | −0.02 | −0.04 | 0.19*** | 0.25*** | −0.26*** | −0.20*** | 0.04 | – | ||||||
| 10. SES | 0.18*** | 0.15*** | 0.06 | −0.08 | −0.08 | 0.09* | 0.16*** | 0.07 | −0.20*** | – | |||||
| 11. Ethnicity | 0.11* | −0.03 | 0.04 | −0.15*** | −0.12** | 0.29*** | −0.11* | −0.05 | −0.10* | 0.08* | – | ||||
| 12. Vacation | −0.05 | −0.06 | 0.05 | 0.33*** | 0.13** | 0.02 | −0.04 | −0.02 | −0.08 | 0.08 | −0.01 | – | |||
| Mean¶ | 8.08 | 89.89 | 21.10 | 2:17 | 0.58 | 9.65 | 2.50 | 49.2 | 8.52 | 0.00 | 56.6 | 25.7 | |||
| Standard deviation | 0.74 | 5.91 | 15.93 | 46 | 0.30 | 0.86 | 1.72 | – | 0.63 | 0.66 | – | – | |||
| Minimum | 4.46 | 55.90 | 2.17 | 12:21 | 0.08 | 6.33 | 1.00 | – | 6.97 | −1.20 | – | – | |||
| Maximum | 10.26 | 99.45 | 79.01 | 4:46 | 1.91 | 13.00 | 10.93 | – | 9.97 | 3.08 | – | – | |||
| Skewness | −0.72 | −1.37 | 1.82 | 0.64 | 1.24 | −0.24 | 3.99 | – | −0.21 | 1.12 | – | – | |||
| Kurtosis | 1.83 | 3.75 | 3.59 | 0.47 | 2.48 | 0.61 | 17.13 | – | −0.09 | 2.01 | – | – |
N = 608. MZ, Monozygotic twin pairs; DZss, same-sex dizygotic twin pairs; DZos, opposite-sex dizygotic twin pairs. Sex (1 = female), Race/ethnicity (1 = non-Hispanic European American), and Vacation (whether study was completed during a school vacation or during summer or not; 1 = study completed during a vacation) are reported to demonstrate sample demographics. Objective nighttime sleep duration, sleep efficiency, sleep latency, sleep midpoint time, and sleep midpoint variability were collected from each twin using wrist-based accelerometers during a week-long study protocol. Parent-reported nighttime sleep duration and daytime sleepiness were assessed using the CSHQ [26]. Objective sleep latency and parent-reported daytime sleepiness were windorized and log transformed for zero-order correlations and analyses given significant skew and kurtosis. SES was a standardized (z-scored) composite of primary caregiver highest education level, secondary caregiver highest education level, and family income to needs ratio at the eight-year assessment.
‡Log transformed values used for zero-order correlations and raw windorized values used for descriptive statistics.
§Sleep midpoint time presented on a 24-h time scale in descriptive statistics, with the mean, minimum, and maximum time representing AM, the standard deviation shown in minutes.
||Log transformed values used for zero-order correlations and raw windorized values used for descriptive statistics.
¶Mean for continuous variables and percentages of sample (for variables coded 1) for dichotomous variables.
†p < 0.10.
*p < 0.05.
**p < 0.01.
***p < 0.001.
Females showed longer objective sleep duration and greater sleep efficiency compared to male children. Non-Hispanic European American participants showed longer actigraph and parent-reported sleep duration, earlier sleep midpoint time, lower midpoint variability, and lower levels of parent-reported daytime sleepiness compared to non-European American participants. Children from higher SES backgrounds showed longer actigraph and parent-reported sleep duration, higher quality, but higher daytime sleepiness, compared to children from lower SES backgrounds. Finally, children who completed the study during a school break or vacation showed later sleep midpoint time and greater sleep midpoint variability compared to children who completed the study while in school. Finally, twin intraclass correlations (ICCs) indicated that MZ twins were more similar particularly on objective sleep duration and sleep efficiency compared to DZ twins, indicating these sleep parameters are heritable.
Univariate ACE models
Table 2 gives the fit statistics and parameter estimates for full and reduced univariate ACE models that estimate genetic and environmental influences on sleep parameter variances. The reduced AE model fit the data best for objective nighttime sleep duration and objective sleep efficiency, with the greatest proportion accounted for by additive genetics, and the remaining variance accounted for by the nonshared environment. In contrast, the full ACE model was the best fit for objective SOL. The reduced CE model fit the data well for objective sleep midpoint time and midpoint variability, such that the greatest proportion of the variance for both sleep indicators was accounted for by the shared environment and remaining variance accounted for by the nonshared environment. Regarding subjective sleep indicators, the full univariate ACE model was the best fit for parent-reported sleep duration and daytime sleepiness, with the shared environment accounted for most of the variance in daytime sleepiness and the remaining variance divided between additive genetic and nonshared environmental factors.
Table 2.
Full and best-fitting univariate ACE fit statistics and parameter estimates
| Scale | Model | −2LL | df | AIC | ∆ df | ∆χ2 | P | A | C | E |
|---|---|---|---|---|---|---|---|---|---|---|
| Objective nighttime sleep | ACE | 889.95 | 455 | −20.05 | – | – | – | 0.69 (0.45–1.00) | 0.12 (0.00–0.50) | 0.19 (0.13–0.27) |
| duration | AE | 890.56 | 456 | −21.44 | 1 | 0.60 | 0.44 | 0.81 (0.67–0.97) | – | 0.19 (0.13–0.26) |
| CE | 910.98 | 456 | −1.02 | 1 | 21.02 | <0.001 | – | 0.56 (0.42–0.71) | 0.44 (0.36–0.53) | |
| E | 991.87 | 457 | 77.87 | 2 | 101.91 | <0.001 | – | – | 1.00 | |
| Objective sleep efficiency | ACE | 2,819.34 | 455 | 1,909.22 | – | – | – | 0.58 (0.35–0.88) | 0.20 (0.03–0.53) | 0.22 (0.15–0.30) |
| AE | 2,821.43 | 456 | 1,909.43 | 1 | 2.21 | 0.14 | 0.79 (0.65–0.95) | – | 0.21 (0.15–0.28) | |
| CE | 2,835.29 | 456 | 1,923.29 | 1 | 16.07 | <0.001 | – | 0.59 (0.45–0.75) | 0.41 (0.33–0.49) | |
| E | 2,932.92 | 457 | 2,018.92 | 2 | 113.71 | <0.001 | – | – | 1.00 | |
| Objective SOL | ACE | 753.33 | 456 | −158.67 | – | – | – | 0.30 (0.11–0.58) | 0.47 (0.20–0.71) | 0.23 (0.16–0.32) |
| AE | 769.19 | 457 | −144.81 | 1 | 15.86 | <0.001 | 0.79 (0.66–0.94) | – | 0.21 (0.15–0.27) | |
| CE | 758.54 | 457 | −155.46 | 1 | 5.22 | 0.02 | – | 0.66 (0.51–0.82) | 0.34 (0.28–0.41) | |
| E | 885.10 | 458 | −30.90 | 2 | 131.77 | <0.001 | – | – | 1.00 | |
| Objective sleep midpoint time | ACE | 555.67 | 456 | −356.33 | – | – | – | 0.03 (0.03–0.04) | 0.91 (0.90–0.91) | 0.06 (0.05–0.06) |
| AE | 730.33 | 457 | −183.67 | 1 | 174.67 | <0.001 | 0.94 (0.93–0.94) | – | 0.06 (0.05–0.06) | |
| CE | 557.23 | 457 | −356.77 | 1 | 1.56 | 0.21 | – | 0.93 (0.92–0.94) | 0.07 (0.06–0.08) | |
| E | 1,008.97 | 458 | 92.97 | 2 | 453.31 | <0.001 | – | – | 1.00 | |
| Objective sleep midpoint time | ACE | −41.80 | 455 | −951.80 | – | – | – | 0.10 (0.00–0.34) | 0.71 (0.53–0.92) | 0.19 (0.13–0.27) |
| variability | AE | 6.92 | 456 | −905.08 | 1 | 48.73 | <0.001 | 0.83 (0.70–0.98) | – | 0.17 (0.12–0.22) |
| CE | −40.61 | 456 | −952.61 | 1 | 1.19 | 0.27 | – | 0.77 (0.62–0.94) | 0.23 (0.19–0.27) | |
| E | 157.37 | 457 | −756.63 | 2 | 199.17 | <0.001 | – | – | 1.00 | |
| Parent-reported sleep duration | ACE | 1,112.35 | 565 | −17.65 | – | – | – | 0.21 (0.16–0.39) | 0.66 (0.47–0.83) | 0.13 (0.07–0.13) |
| AE | 1,171.48 | 566 | 39.48 | 1 | 59.13 | <0.001 | 0.88 (0.76–1.00) | – | 0.12 (0.01–0.56) | |
| CE | 1,121.81 | 566 | −10.19 | 1 | 9.46 | <0.001 | – | 0.79 (0.64–0.94) | 0.23 (0.17–0.24) | |
| E | 1,403.56 | 567 | 269.56 | 2 | 291.20 | <0.001 | – | – | 1.00 | |
| Parent-reported daytime | ACE | −72.73 | 575 | −1,222.73 | – | – | – | 0.27 (0.02–1.00) | 0.66 (0.55–0.83) | 0.07 (0.05–0.09) |
| sleepiness | AE | −3.09 | 576 | −1,155.09 | 1 | 69.64 | <0.001 | 0.93 (0.81–1.00) | – | 0.07 (0.06–0.09) |
| CE | −43.51 | 576 | −1195.51 | 1 | 29.21 | <0.001 | – | 0.83 (0.69–0.99) | 0.17 (0.14–0.19) | |
| E | 300.51 | 577 | −853.49 | 2 | 373.24 | <0.001 | – | – | 1.00 |
Bolded models denote the best-fitting models for each variable. The −2LL is the chi-squared measure of model fit, and the AIC is the Akaike’s Information Criterion, which is an additional measure of model fit. ∆df shows the change in the degrees of freedom, which occurs when model parameters are dropped. ∆χ2 is the change in −2 log likelihood values when dropping model parameters. p denotes the p-value level of significance for the chi-squared test.
Bivariate ACE models for correlated objective sleep parameters
Tables 3 and 4 provide fit statistics and standardized path estimates (including variance-based confidence intervals) for full and best-fitting (final) bivariate twin models. Figures 1, A–D and 2, A–C show standardized variance components and variance-based confidence intervals for each full bivariate twin model (Full Cholesky decomposition models shown), with paths that could be dropped without significant loss of model fit indicated by dashed lines. Supplementary Tables S1–S7) also show model fit statistics and genetic and environmental correlations for all bivariate twin models tested in the study, including the full and best-fitting bivariate models of interest.
Table 3.
Full and best-fitting bivariate Cholesky decomposition fit statistics for associations between objective and subjective sleep indicators
| Variables | Model | −2LL | df | AIC | ∆df | ∆χ 2 | P | rG | rC | rE |
|---|---|---|---|---|---|---|---|---|---|---|
| Objective sleep duration and sleep efficiency | ACE-ACE-ACE | 3,335.25 | 909 | 1,517.25 | – | – | – | 0.79 | 0.00 | 0.00 |
| AE-AE-ACE | 3,331.78 | 911 | 1,509.78 | 2 | 7.47 | 0.99 | 0.85 | – | 0.81 | |
| Objective sleep duration and SOL | ACE-ACE-ACE | 3,279.07 | 909 | 1,461.07 | – | – | – | 0.94 | 1.00 | 0.26 |
| AE-AE-ACE | 3,280.50 | 911 | 1,458.50 | 2 | 1.43 | 0.49 | 0.97 | – | 0.27 | |
| Objective sleep duration and sleep midpoint time variability | ACE-ACE-ACE | 839.09 | 909 | −978.91 | – | – | – | 0.00 | 0.61 | 0.08 |
| ACE-CE-CE | 840.53 | 911 | −981.47 | 2 | 1.44 | 0.49 | – | 0.58 | 0.07 | |
| Objective sleep midpoint time and sleep midpoint time variability | ACE-ACE-ACE | 437.05 | 908 | −1,378.95 | – | – | – | 1.00 | 0.35 | 0.28 |
| CE-CE-CE | 440.52 | 911 | −1,381.48 | 3 | 3.47 | 0.33 | – | 0.37 | 0.41 | |
| Objective sleep duration and parent- reported sleep duration | ACE-ACE-ACE | 1,973.29 | 1,019 | −64.71 | – | – | – | 0.02 | 0.89 | 0.09 |
| ACE-CE-ACE | 1,973.29 | 1,020 | −66.71 | 1 | 0.00 | 0.94 | – | 1.00 | 0.16 | |
| Objective sleep midpoint time and parent-reported sleep duration | ACE-ACE-ACE | 1,667.91 | 1,018 | −368.09 | – | – | – | 0.00 | 0.00 | 0.07 |
| ACE-E-ACE | 1,667.91 | 1,020 | −372.09 | 2 | 0.00 | 0.99 | – | – | 0.07 | |
| Objective sleep midpoint time variability and parent-reported sleep duration | ACE-ACE-ACE | 1,070.58 | 1,019 | −967.42 | – | – | – | 0.00 | 0.00 | 0.00 |
| ACE-E-ACE | 1,070.58 | 1,021 | −971.42 | 2 | 0.00 | 0.99 | – | – | 0.14 |
Sex and age were regressed out of variables prior to conducting models. Bolded models denote the best-fitting models. Models (e.g. ACE-ACE-ACE) are denoted to represent the genetic and environmental variance paths (retained) in the first phenotype, followed by the covariance paths shared (retained) between the first and second phenotype, and finally the genetic and environmental variance paths (retained) in the second phenotype after accounting for variance in the first phenotype. The −2LL is the −2 log likelihood model fit, and the AIC is the Akaike’s Information Criterion, which is an additional measure of model fit. ∆df shows the change in the degrees of freedom. ∆χ2 is the change in chi-squared values when dropping model parameters. p denotes the p-value level of significance for the chi-squared test. rG, genetic correlation between first and second phenotype in each model. rC, shared environmental correlation between first and second phenotype in each model. rE, nonshared environmental correlation between first and second phenotype in each model.
Table 4.
Parameter estimates for full and best-fitting bivariate Cholesky decompositions for associations between objective and subjective sleep indicators
| Model | A11 | C11 | E11 | A21 | C21 | E21 | A22 | C22 | E22 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Objective sleep duration and objective sleep efficiency | ACE-ACE-ACE | 0.72 (0.57–1.00) | 0.09 (0.02–.53) | 0.19 (0.13–0.27) | 0.00 (0.00–0.03) | 0.26 (0.15–1.00) | 0.01 (0.001–0.03) | 0.11 (0.01–0.35) | 0.44 (0.01–1.00) | 0.18 (0.13–1.00) |
| AE-AE-ACE | 0.80 (0.59–0.97) | – | 0.20 (0.16–0.32) | 0.37 (0.28–0.57) | – | 0.14 (0.08–0.21) | 0.14 (0.07–0.25) | 0.26 (0.12–0.34) | 0.09 (0.05–0.11) | |
| Objective sleep duration and objective SOL | ACE-ACE-ACE | 0.98 (0.86–1.00) | 0.01 (0.00–0.04) | 0.01 (0.00–0.01) | 0.54 (0.29–1.00) | 0.14 (0.06–0.46) | 0.00 (0.00–0.00) | 0.27 (0.02–0.31) | 0.00 (0.00–0.00) | 0.05 (0.04–0.06) |
| AE-AE-ACE | 0.99 (0.86–1.00) | – | 0.01 (0.01–0.04) | 0.80 (0.31–1.00) | – | 0.01 (0.001–0.02) | 0.06 (0.02–0.11) | 0.09 (0.05–0.14) | 0.05 (0.03–0.06) | |
| Objective sleep duration and objective sleep midpoint variability | ACE-ACE-ACE | 0.72 (0.47–1.00) | 0.08 (0.02–0.54) | 0.19 (0.13–0.26) | 0.00 (0.00–0.03) | 0.26 (0.14–1.00) | 0.01 (0.002–0.02) | 0.11 (0.01–0.36) | 0.44 (0.002–1.00) | 0.18 (0.11–0.24) |
| ACE-CE-CE | 0.72 (0.38–1.00) | 0.08 (0.02–0.54) | 0.19 (0.14–0.26) | – | 0.26 (0.12–1.00) | 0.01 (0.001–0.02) | – | 0.51 (0.02–1.00) | 0.21 (0.20–0.26) | |
| Objective sleep midpoint time and sleep midpoint variability | ACE-ACE-ACE | 0.04 (0.001–0.10) | 0.90 (0.73–1.00) | 0.06 (0.04–0.08) | 0.11 (0.01–0.37) | 0.08 (0.001–0.12) | 0.01 (0.001–0.05) | 0.00 (1.00–1.00) | 0.62 (0.49–0.86) | 0.18 (0.12–0.24) |
| CE-CE-CE | – | 0.93 (0.76–1.00) | 0.07 (0.06–0.08) | – | 0.11 (0.03–0.15) | 0.04 (0.03–0.08) | – | 0.66 (0.48–0.72) | 0.19 (0.12–0.24) | |
| Objective sleep duration and parent-reported sleep duration | ACE-ACE-ACE | 0.71 (0.55–0.88) | 0.09 (0.02–0.24) | 0.20 (0.14–0.43) | 0.00 (0.00–0.03) | 0.66 (0.51–0.82) | 0.01 (0.001–0.03) | 0.21 (0.10–0.35) | 0.00 (1.00–1.00) | 0.12 (0.09–0.18) |
| ACE-CE-ACE | 0.71 (0.56–0.84) | 0.09 (0.04–0.20) | 0.20 (0.14–0.26) | – | 0.66 (0.50–0.85) | 0.01 (0.001–0.02) | 0.21 (0.10–0.35) | 0.00 (1.00–1.00) | 0.12 (0.09–0.18) | |
| Objective sleep midpoint time and parent-reported sleep duration | ACE-ACE-ACE | 0.04 (0.01–0.12) | 0.91 (0.73–1.00) | 0.05 (0.04–0.08) | 0.00 (0.10–0.10) | 0.00 (0.01–0.01) | 0.01 (0.001–0.02) | 0.21 (0.10–0.35) | 0.66 (0.51–0.85) | 0.12 (0.09–0.17) |
| ACE-E-ACE | 0.04 (0.01–0.12) | 0.91 (0.73–1.00) | 0.05 (0.04–0.08) | – | – | 0.01 (0.001–0.02) | 0.21 (0.10–0.35) | 0.66 (0.51–0.85) | 0.12 (0.09–0.17) | |
| Objective sleep midpoint time variability and parent-reported sleep duration | ACE-ACE-ACE | 0.10 (0.001–0.33) | 0.71 (0.44–0.78) | 0.19 (0.11–0.22) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.21 (0.10–0.35) | 0.66 (0.49–0.84) | 0.13 (0.09–0.17) |
| ACE-E-ACE | 0.10 (0.001–0.24) | 0.71 (0.49–0.82) | 0.19 (0.12–0.27) | – | – | 0.00 (0.00–0.00) | 0.21 (0.10–0.35) | 0.66 (0.48–0.83) | 0.13 (0.09–0.18) |
Sex and age were regressed out of variables prior to conducting models. Bolded models denote the best-fitting models. Models (e.g. ACE-ACE-ACE) are denoted to represent the genetic and environmental variance paths (retained) in the first phenotype, followed by the covariance paths shared (retained) between the first and second phenotype, and finally the genetic and environmental variance paths (retained) in the second phenotype after accounting for variance in the first phenotype. Estimates are provided with variance-based confidence intervals provided in parentheses and are based on standardized path estimates. Standardized path estimates for the second phenotype in each model are adjusted after accounting for the covariance between phenotypes. A11, additive genetic components for first phenotype; C11, shared environment component for first phenotype; E11, nonshared environment component for first phenotype. A22, C22, E22, residual additive genetic, shared environmental, and nonshared environmental components for second phenotype not shared with first phenotype, respectively.
The bivariate Cholesky Decomposition of objective sleep duration and efficiency revealed the AE-AE-ACE model to be the best-fitting model, with the covariance between objective sleep duration and efficiency accounted for by additive genetics and the nonshared environment and explaining 37% and 14% of the total variance in sleep efficiency, respectively (Figure 1, A, Supplementary Table S1). The genetic correlation in the best-fitting model was high at 0.85, as was the nonshared environmental correlation (0.81).
The bivariate model for objective sleep duration and SOL revealed the AE-AE-ACE model to be the best-fitting model, with the covariance between objective sleep duration and midpoint variability accounted for entirely by additive genetics and explaining 80% of the total variance in SOL (Figure 1, B, Supplementary Table S2). Notably, the additive genetic covariance on objective sleep duration almost entirely accounted for genetic influence on SOL (rG = 0.97), and nonshared environmental influences were correlated at 0.27 in the best-fitting model.
The bivariate model for objective sleep duration and midpoint variability revealed the full ACE-CE-CE model to be the best-fitting model, with the covariance between objective sleep duration and midpoint variability accounted for by the shared and nonshared environment which explained 26% and 1% of the total variance in midpoint variability, respectively (Figure 1, C, Supplementary Table S3). Shared environmental influences were correlated at 0.58, and nonshared environmental influences were correlated at 0.07 in the best-fitting model.
The bivariate model for objective sleep midpoint time and midpoint variability revealed the CE-CE-CE model was the best-fitting model, with the covariance between objective sleep midpoint time and midpoint variability accounted for entirely by shared and nonshared environmental factors which explained 11% and 4% of the total variance in midpoint variability, respectively (Figure 1, D, Supplementary Table S4). Genetic and environmental correlations showed that shared environmental influences were correlated at 0.37, and nonshared environmental influences were correlated at 0.41.
Bivariate ACE models between subjective and objective sleep parameters
The bivariate model for objective sleep duration and parent-reported sleep duration at 8 years revealed the full ACE-CE-ACE model to be the best-fitting model, with the covariance between objective and parent-reported sleep duration accounted for by the shared and nonshared environment and explaining 66% and 1% of the total variance in parent-reported sleep duration, respectively (Figure 2, A, Supplementary Table S5). Shared environmental correlations showed that shared environmental influences were correlated at 1.00, and nonshared environmental influences were correlated at 0.16 in the best-fitting model.
The bivariate model for objective sleep midpoint time and parent-reported sleep duration revealed the ACE-E-ACE model to be the best-fitting model (Figure 2, B, Supplementary Table S6), with the covariance between objective sleep midpoint time and parent-reported sleep duration entirely accounted for by the nonshared environment (1% of the total variance in parent-reported sleep duration). Nonshared environmental influences were correlated at 0.07.
Finally, the bivariate model for objective sleep midpoint variability and parent-reported sleep duration revealed the ACE-E-ACE model to be the best-fitting model (Figure 2, C, Supplementary Table S7), with the covariance between objective sleep midpoint variability and parent-reported sleep duration accounted for entirely by shared environmental factors. Notably, the ACE-C-ACE fit the data similarly to the ACE-E-ACE model compared to the full ACE-ACE-ACE model (fit statistics, standardized estimates, and genetic and environmental correlations were consistent across models). Although the best-fitting model includes the covariance path for nonshared environmental factors between objective sleep midpoint variability and parent-reported sleep duration, the covariance estimate was 0.00 and not significant. Environmental correlations showed that only nonshared environmental influences were correlated at 0.14.
Sensitivity analyses
We conducted sensitivity analyses to test whether genetic and environmental influences in weekday nights of sleep (most participants had five nights of weekday sleep which provides a reliable estimate) [25] differed from estimates for all nights of sleep. Full and best-fitting model estimates (with 95% confidence intervals) for sleep indicators are provided in Supplementary Table S8. Additive genetic, shared environmental, and nonshared environmental influences for objective sleep duration, efficiency, sleep midpoint time, and sleep midpoint variability were highly similar when comparing weekday only nights to all available nights of sleep (see Supplementary Table S8). Specifically, the best-fitting models for objective sleep duration and efficiency across all nights compared to weekday nights only were almost identical, while the best-fitting models for objective sleep midpoint time showed slight increases in additive genetic influence on weekday nights only, and sleep midpoint variability showed small increases in nonshared environmental influence on weekday nights only. In contrast, full and best-fitting models for objective SOL looked different across all nights of sleep compared to weekday only nights of sleep, with weekday nights of SOL showing lower genetic and higher nonshared environmental influence compared to all nights of sleep examined together.
Discussion
Our findings contribute to the growing body of literature showing strong genetic contributions to objectively-measured sleep indicators, as well as at least one study demonstrating that the covariance between different indicators of subjective sleep quality is explained primarily by shared additive genetic factors [10, 11, 13]. Importantly, we extend the current literature by showing divergence in genetic and environmental contributions to objective versus subjective sleep indicators. Additionally, our findings demonstrate different patterns of genetic and environmental correlations between objective and subjective sleep indicators, such that the covariance between objective sleep indicators is often explained by additive genetics, whereas links between objective and subjective sleep indicators are primarily explained by shared and nonshared environmental influences, which significantly adds to the prior literature [10, 11, 13].
Genetic and environmental influences on unique sleep indicators
Consistent with prior research and our hypotheses, the greatest proportion of the variance in actigraphy-based sleep duration and efficiency was accounted for by additive genetic factors (0.81 and 0.79, respectively). These findings suggest that objective sleep quantity and quality are highly heritable during middle childhood and that individual differences between children on sleep quantity and quality can be primarily attributed to genetic differences. In contrast, we found that the greatest proportion of the variance in parent-reported sleep duration was accounted for by shared environmental factors, and only about 20% of the reason why children differ from one another on parent-reported sleep duration could be explained by additive genetics. Importantly, these findings indicate that estimates for objective sleep duration (0.81) and efficiency (0.79) were higher than those reported in prior studies with objectively measured sleep [10, 11], and higher than parent-reported sleep duration estimates. Genetic estimates specifically for parent-reported sleep duration in the current study are similar to those reported in other studies (e.g. 30%–46%) [13, 14, 34]. Regarding differences between our findings and those of other studies in heritability estimates for objective and subjective sleep duration and sleep efficiency, prior research with objective sleep measures had samples with slightly older children [11, 12] and almost all prior studies [e.g. 6, 35] examining genetic and environmental contributions to child sleep relied on parent and child reports rather than objective reports of sleep. These factors likely explain differences in genetic estimates. Overall, these findings highlight two key points: (1) objective assessment of sleep quantity and quality demonstrate greater additive genetic influence than parent reports of sleep indicators and (2) multimethod assessment of sleep is critical in child sleep research, as estimates of genetic influences on various sleep indicators differ by the method.
In contrast with objective sleep duration and quality, objective sleep midpoint time, and midpoint variability demonstrated little additive genetic influence in the full and best-fitting models, which supports our hypothesis and fits with prior literature showing the lower additive genetic influence on sleep timing indicators during middle childhood (full models: 0.03 and 0.10, respectively; no additive genetic influence in best-fitting models) [11, 12]. Yet, this is the first study to our knowledge to test the heritability of sleep midpoint variability, which contributes to a growing body of literature that calls for the examination of other sleep indicators beyond sleep duration [35]. Specifically, we found that the greatest proportion of the variance in sleep midpoint variability was accounted for by shared environmental factors (0.77). Notably, sleep midpoint time and variability account for the timing and variability in both bedtime and waketime from night to night across a week.
During this developmental period, child bedtimes and waketimes are heavily influenced and restricted by school start times, parent work schedules, and family routines and bedtime schedules more broadly [36–38]. Room or bed-sharing and qualities of the home environment (e.g. household chaos and overcrowding) may also strongly influence sleep timing and variability, with most of the literature showing that room and bed-sharing, household chaos and conflict, and low-quality home environments are associated with reduced sleep quantity and quality in children [39–42]. Links between these family or household factors and child sleep are often confounded by and depend on factors such as ethnicity or SES making it difficult to tease apart which specific environmental factors may be directly contributing to similarities or individual differences in child sleep [40, 42, 43]. Continued research is necessary to more clearly understand the extent to which family or household factors explain similarities or differences in child sleep.
In addition, genetic influence on sleep midpoint time and variability may differ by weekday and weekend days [44], but our sensitivity analyses found that only SOL had a less genetic influence on weekdays compared to all nights of the week (both weekday and weekend nights of sleep). Further, while we did not find significant changes in genetic influence on objective sleep duration, efficiency, timing, and variability when comparing weekday nights of sleep to all available nights of sleep in our sensitivity analyses (Supplementary Table S8), it is possible that we may observe increased genetic influence specifically on sleep timing and variability, for example, on weekends or with schedules that better match individuals’ preferred sleep–wake patterns. Overall, parents and various external factors may serve as common environmental factors that restrict the genetic influence on sleep midpoint time and variability.
Contrary to our hypothesis and prior literature [11, 12], SOL demonstrated greater environmental influences (0.70 across shared and nonshared environmental factors) than prior studies. However, previous studies examining genetic and environmental influences on objective SOL established high heritability for slightly older samples (i.e. late childhood and adolescence), which may explain differences. Additionally, various environmental factors likely contribute to SOL, particularly during middle childhood. Greater family disorganization, less healthy behaviors before bedtime (poor sleep hygiene), and lower quality home environments have all been associated with longer sleep latencies in middle childhood and adolescence [40, 45], just as with sleep quantity and quality. Additionally, a large review found that a greater amount of television time during the day and more screen time exposure during early and middle childhood were consistently associated with longer sleep latencies [46]. More specifically, these environmental factors may contribute to longer sleep latencies in children through increased vigilance and arousal before sleep (e.g. emotional and cognitive) [47], such that characteristics of family chaos, lower-quality home environment, and pre-bedtime activities contribute to longer sleep latencies and more sleep disturbances. Indeed, at least one study has shown that increased emotional intensity and lower emotional regulation before bedtime predicted shorter sleep duration and greater sleep disturbances in middle childhood (including sleep duration) [48].
Finally, high shared environmental influences on parent-reported sleep duration (0.66) and daytime sleepiness (0.66) may capture parent-report bias, with parents tending to report similar sleep durations for both children despite their differences in sleep quantity and daytime functioning. However, high shared environmental contributions could also indicate that numerous factors in children’s homes or sleep environments, such as having similar daily and bedtime routines or sharing a room or bed, may explain individual differences in sleep quantity and daytime functioning during middle childhood. Sharing a room or bed may lead to more similarity in bedtime routines for children in the same home as well as similar levels of sleep on multiple indicators. Alternatively, strong shared environmental contribution to parent-reported sleep quantity and daytime sleepiness suggest that parents may not be strong reporters of these characteristics because they are unaware of when children are falling asleep at night and children’s sleepiness throughout the day. Children also gain increased autonomy and decision making during middle childhood [49] and may have a greater opportunity to make independent decisions about sleep–wake patterns that match their own preferences. Indeed, findings from the current study and others demonstrate large discrepancies between parent-reported and actigraphy-based sleep duration times with parent reports of sleep duration being consistently longer by as much as an hour [6–8]. Parents, however, may be better reporters of bedtime, waketime and total time spent in bed (instead of true sleep time). This supports and highlights the importance of using multiple methods to assess behaviors like sleep.
Genetic and environmental influence on associations between sleep indicators
Our hypothesis that additive genetics would primarily explain links between objective sleep duration and efficiency was supported, as additive genetic influences on sleep duration were highly correlated with additive genetic influences on sleep efficiency (rG = 0.85), suggesting that a shared genetic factor influences both sleep quantity and quality. However, this association is likely inflated because sleep duration contributes to the measure of sleep efficiency, so these commonly used assessments are not entirely independent. Nevertheless, they tap two important aspects of sleep, and this is the first study to our knowledge that considers covariances between objective sleep quantity and quality.
Similarly, our hypothesis that additive genetics would primarily explain links between objective sleep duration and SOL was supported, with about 80% of the covariance in SOL accounted for by objective sleep duration. This finding also supports a shared genetic factor contributing to both sleep quantity and latency. Research indicates that specific genes such as the clock gene are responsible for maintaining circadian rhythms and sleep patterns as well as alterations in metabolism [50, 51]. Thus, examining candidate genes (and functional gene networks) that regulate multiple aspects of sleep may be a future direction that further clarifies the extent of the association between objective sleep duration and latency.
Our hypothesis that most of the covariance between objective sleep midpoint time and midpoint variability would be accounted for by additive genetic factors was not supported (0% in the best-fitting model; see Table 4); rather, shared and nonshared environmental influences accounted for a significant proportion of the covariance (11% and 4%, respectively). It is notable that our hypothesis was based on prior findings with other objective indicators of sleep, as no studies to date have examined genetic and environmental associations or overlap between any sleep timing or variability indicators. Similar to objective sleep quantity and quality, both genetic and environmental links between sleep midpoint time and midpoint variability are likely inflated because the measures are not independent of one another. Yet, our findings suggest that shared genetic influences and common environmental factors like qualities of the home environment, family routines, schedules, and school start times contribute to overlapping between sleep midpoint time and midpoint variability.
Contrary to our hypothesis, almost all of the covariance between longer objective sleep duration and lower sleep midpoint variability was accounted for by the common environment in middle childhood (26%), with a high shared environmental correlation (0.58). The inverse association suggests that regularity of daily schedules (or sleep schedules) in particular may explain associations between objective sleep duration and midpoint variability.
We found that shared environmental factors primarily explained links between objective and parent-reported sleep duration (0.58; 66%), with some of the covariance explained by nonshared environmental factors (0.07; 1%). Thus, objective and subjective sleep duration do not share genetic etiology during middle childhood, indicating that these measures are assessing or capturing different aspects of sleep. As previously noted, subjective sleep duration may be more akin to total time in bed while objective sleep captures true sleep time or duration.
However, common environmental links between objective and subjective sleep duration may be stronger and specific to development in middle childhood. Research shows that heritability of numerous characteristics and traits tend to increase with age and development, in part due to niche picking and selection of environments that better match underlying genetic tendencies [29]. For example, as children move into early adolescence and have increased autonomy and decision-making responsibilities, youth may play a larger role in decisions regarding sleep–wake patterns and daily schedule, and they may choose schedules that match their own (genetic) preferences, thus allowing for greater genetic influence on sleep quantity. As such, both objective and parent-reported sleep duration may demonstrate increased additive genetic influence in older children and show shared additive genetic covariance later in development. Additionally, studies have suggested large discrepancies not only between objective and parent-reported sleep, but between child- and parent-reported sleep [52]. Specifically, parents significantly overestimate adolescent sleep duration and wake time and underestimate bedtimes when comparing to actigraphy as well as adolescent reports on questionnaires and daily sleep diaries [52]. As such, self-reported sleep during childhood and adolescence may have greater additive genetic influence than parent-reported sleep or be more comparable to objective sleep. Indeed, adolescent twin studies show higher genetic [e.g. 11] and nonshared environmental contributions [13] to self-reported sleep quantity.
Alternately, the nonshared environment may begin to play a more prominent role in associations between objective and subjective sleep durations as children move from middle childhood to adolescence, given the large number of different environmental and social changes occurring with the transition to adolescence [53], which may explain individual differences in sleep duration. However, effect sizes for nonshared environmental influences on traits are typically quite small and there are objective (e.g. observer-reported factors) and subjective factors (e.g. perceived uniqueness or differences between individuals), as well as measurement error included in the interpretation of nonshared environmental influences on traits [54]. It is difficult to estimate or hypothesize which particular unique experiences may explain or contribute to nonshared environmental influences. Rather, future research is needed to better understand and elucidate the extent to which different unique environmental influences may contribute to sleep indicators in childhood. Overall, we found that associations between objective and subjective sleep duration were solely explained by shared environmental factors during middle childhood, which highlights that different measures of the same construct do not share genetic etiology and that multimethod assessment of childhood sleep is critical.
Finally, we found that links between objective sleep midpoint time and parent-reported sleep duration were entirely accounted for by overlapping nonshared environmental influences (1%). Factors in the home environment that make individuals different from one another may explain these associations, such as children having different bedtimes and wake times or differing routines and schedules. Additionally, lack of covariance between objective sleep midpoint time and sleep midpoint variability with parent-reported sleep duration, particularly as compared to genetic and environmental overlap between objective sleep quantity and sleep midpoint time and variability, suggests the importance of reporter and measurement when collecting sleep measures, and that objective and subjective reports of facets like sleep duration are not always similarly associated with other sleep indicators or developmental outcomes.
Strengths, limitations, and conclusions
The present study is characterized by a number of strengths both conceptually and methodologically. We employed multimethod assessments of multiple aspects of sleep, which is critical given differential phenotypic and genetic and environmental associations shown for objective and subjective sleep indicators. Additionally, we used a large (over 500 participants), longitudinal sample of twins recruited through state birth records in the United States, making this a substantial community sample of socioeconomically and ethnically diverse families. Furthermore, we focused on middle childhood, capitalizing on a unique developmental period in which little sleep research has been conducted. These characteristics of our sample are valuable as many twin studies have been conducted with ethnically homogeneous samples of young children or adults of Northern European descent and estimates of genetic and environmental influences on traits may vary according to population or sample composition [29]. Furthermore, the use of a twin sample allows for elucidating genetic and environmental influences on trait variance and covariance, which can help identify the etiology of various sleep difficulties as well as where to best direct intervention efforts for health problems related to sleep. Using objective assessments, individual differences in sleep quantity and quality in middle childhood were largely due to individual differences in genetics. However, other objective sleep indicators such as latency, timing, and variability suggest individual differences may be accounted for by shared and nonshared environmental factors. Thus, changing family and sleep environments may improve certain facets of sleep, while these interventions may have less strong impacts on quantity and quality of children’s sleep and other measures should be taken to improve health.
However, the present study also has a number of limitations. Genetic and environmental variance and covariances between sleep indicators do not account for potential gene × environment interactions. In adult samples, negative life events increase both additive genetic and nonshared environmental influences on sleep quantity and quality [55, 56]. However, moderated heritability and gene-environment interactions related to child sleep have not been sufficiently tested in large, community samples of children. Thus, future studies should test whether environmental factors like family routines and schedules may increase or decrease additive genetic influences on children’s sleep, as a way to understanding possible points of intervention for sleep. Future studies can further uncover genetic and environmental underpinnings of sleep difficulties and better understand how genetic and environmental contributions to sleep problems in childhood may predict changes in other indicators of health and well-being later in development. Finally, genetic and environmental influences on phenotypes may change with age and across development [29]. Indeed, we found that age was correlated with multiple objective and subjective sleep indicators. Future work should examine age-related changes in genetic and environmental influences on sleep particularly as children move into adolescence and adulthood and experience puberty and lifestyle changes.
Overall, our findings suggest that individual differences in objective sleep quantity, quality, and to some extent sleep latency, can be attributed to underlying genetics. However, there were significant shared environmental contributions to objective sleep timing and variability as well as parent-reported sleep duration and daytime sleepiness, which suggests that using multiple methods and reporters to assess sleep duration middle childhood is critically important, as estimates of genetic variance (and covariance) on various sleep indicators differ by a reporter. Additionally, the extent to which objective sleep indicators and objective and subjective sleep indicators share genetic and environmental influences varies greatly, demonstrating that different mechanisms may be at play when linking facets of sleep. Finally, objective and parent-reported measures of sleep did not share genetic etiology during middle childhood, suggesting that objective and parent-reported measures of sleep are capturing different components of sleep and should not be used interchangeably in sleep and developmental research.
Supplementary Material
Acknowledgments
The authors would like to thank all of the participants, study staff, graduate students, and research assistants of the Arizona Twin Project, without whom this paper would not be possible. We also thank Drs Marisol Perez and Kevin Grimm for their feedback on the manuscript concept and statistical analyses. All work was performed at Arizona State University, Tempe, AZ 85287-1104.
Funding
This research was supported by grants from the Institute for Mental Health Research and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD079520).
Disclosure Statements
Financial disclosure: There are no conflicts of interest for any of the authors. This was not an industry-supported study and does not include off-label or investigational use. The authors have no financial conflicts of interest. This study is not linked to any clinical trials.
Non-financial disclosure: none.
References
- 1. National Sleep Foundation. 2014 Sleep in America Poll—sleep in the modern family: summary of findings.March 3, 2014. http://sleepfoundation.org/sleep-polls-data/2014-sleep-the-modern-family. Accessed August 1, 2019.
- 2. American Academy of Sleep Medicine. Recharge with sleep: pediatric sleep recommendations promoting optimal health.June 13, 2016. https://aasm.org/recharge-with-sleep-pediatric-sleep-recommendations-promoting-optimal-health/. Accessed August 1, 2019.
- 3. American Academy of Pediatrics. American Academy of Pediatrics supports childhood sleep guidelines. 2016. https://www.aap.org/en-us/about-the-aap/aap-press-room/Pages/American-Academy-of-Pediatrics-Supports-Childhood-Sleep-Guidelines.aspx. Accessed August 1, 2019. [Google Scholar]
- 4. Sadeh A, et al. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36(3):291–301. [DOI] [PubMed] [Google Scholar]
- 5. Smaldone A, et al. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007;119(Suppl 1):S29–S37. [DOI] [PubMed] [Google Scholar]
- 6. Gregory AM, et al. A twin-study of sleep difficulties in school-aged children. Child Dev. 2006;77(6):1668–1679. [DOI] [PubMed] [Google Scholar]
- 7. Martinez SM, et al. Mother-reported sleep, accelerometer-estimated sleep and weight status in Mexican American children: sleep duration is associated with increased adiposity and risk for overweight/obese status. J Sleep Res. 2014;23(3):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nixon GM, et al. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tremaine RB, et al. Subjective and objective sleep in children and adolescents: measurement, age, and gender differences. Sleep Biol Rhythms. 2010;8(4):229–238. doi: 10.1111/j.1479-8425.2010.00452x [DOI] [Google Scholar]
- 10. Inderkum AP, et al. High heritability of adolescent sleep-wake behavior on free, but not school days: a long-term twin study. Sleep. 2018;41(3). doi: 10.1093/sleep/zsy004 [DOI] [PubMed] [Google Scholar]
- 11. Sletten TL, et al. Genetic and environmental contributions to sleep-wake behavior in 12-year-old twins. Sleep. 2013;36(11):1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gregory AM, et al. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep. 2009;32(2):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barclay NL, et al. Genetic and environmental influences on different components of the Pittsburgh Sleep Quality Index and their overlap. Sleep. 2010;33(5):659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breitenstein RS, et al. Children’s sleep and daytime functioning: increasing heritability and environmental associations with sibling conflict. Soc Dev. 2018;44(2):389–404. doi: 10.1111/sode.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neale MCCL, et al. The scope of genetic analyses. In: Neale MC, et al. , eds. Methodology for genetic studies of twins and families. Vol. 67 Switzerland: Springer Science & Business Media; 2013. [Google Scholar]
- 16. Posthuma D, et al. A note on the statistical power in extended twin designs. Behav Genet. 2000;30(2):147–158. doi: 10.1023/A:1001195930625 [DOI] [PubMed] [Google Scholar]
- 17. Verhulst B. A power calculator for the classical twin design. Behav Genet. 2017;47:255–261. doi: 10.1007/s10519-016-9828-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shochat T, et al. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev. 2014;18(1):75–87. [DOI] [PubMed] [Google Scholar]
- 19. Lemery-Chalfant K, et al. Arizona Twin Project: a focus on early resilience. Twin Res Hum Genet. 2013;16(1):404–411. [DOI] [PubMed] [Google Scholar]
- 20. U.S. Census Bureau. Quick Facts: Arizona. July 1, 2018. https://www.census.gov/quickfacts/fact/table/US/PST045219. Accessed January 15, 2020. [Google Scholar]
- 21. Adam EK, et al. Sleep timing and quantity in ecological and family context: a nationally representative time-diary study. J Fam Psychol. 2007;211:4– 19. doi: 10.1037/0893-3200.21.1 [DOI] [PubMed] [Google Scholar]
- 22. Oakley NR. Validation with polysomnography of the Sleepwatch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. Bend: Mini Mitter, Cambridge Neurotechnology; 1997. [Google Scholar]
- 23. Sadeh A, et al. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. [DOI] [PubMed] [Google Scholar]
- 24. Sadeh A, et al. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18(4):288–302. [DOI] [PubMed] [Google Scholar]
- 25. Acebo C, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. [DOI] [PubMed] [Google Scholar]
- 26. Owens JA, et al. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- 27. Goldsmith HH. A zygosity questionnaire for young twins: a research note. Behav Genet. 1991;21(3):257–269. doi: 10.1007/BF01065819 [DOI] [PubMed] [Google Scholar]
- 28. Forget-Dubois N, et al. Diagnosing zygosity in infant twins: physical similarity, genotyping, and chorionicity. Twin Res. 2003;6(6):479–485. [DOI] [PubMed] [Google Scholar]
- 29. Plomin R, et al. Nature, nurture and human behavior. In: Woods, ed. Behavioral genetics. 6th ed. New York, NY: Worth Publishers; 2013: 73–85. [Google Scholar]
- 30. Boker S, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGue M, et al. Adjustment of twin data for the effects of age and sex. Behav Genet. 1984;14(4):325–343. [DOI] [PubMed] [Google Scholar]
- 32. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–723. [Google Scholar]
- 33. Neale MC, et al. Univariate analysis. Dordrecht: Kluwer Academic Publishers B.V; 2004: 109–136. [Google Scholar]
- 34. Brescianini S, et al. Genetic and environmental factors shape infant sleep patterns: a study of 18-month-old twins. Pediatrics. 2011;127(5):e1296–e1302. [DOI] [PubMed] [Google Scholar]
- 35. Patel SR, et al. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16(3):643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson PM. Parental employment, family routines and childhood obesity. Econ Hum Biol. 2012;10(4):340–351. [DOI] [PubMed] [Google Scholar]
- 37. Crowley SJ, et al. Sleep during adolescence. In: Sheldon SH, et al. , eds. Principles and practice of pediatric sleep medicine. 2nd ed. London: Elseviers Saunders; 2014:45–51. [Google Scholar]
- 38. Fiese BH, et al. Family mealtimes: a contextual approach to understanding childhood obesity. Econ Hum Biol. 2012;10(4):365–374. [DOI] [PubMed] [Google Scholar]
- 39. Bagley EJ, et al. What keeps low-SES children from sleeping well: the role of presleep worries and sleep environment. Sleep Med. 2015;16(4):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doane LD, et al. Early life socioeconomic disparities in children’s sleep: the mediating role of the current home environment. J Youth Adolesc. 2019;48(1):56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kelly RJ, et al. Marital conflict and children’s sleep: reciprocal relations and socioeconomic effects. J Fam Psychol. 2011;25(3):412–422. [DOI] [PubMed] [Google Scholar]
- 42. Milan S, et al. The context of preschool children’s sleep: racial/ethnic differences in sleep locations, routines, and concerns. J Fam Psychol. 2007;21(1):20–28. [DOI] [PubMed] [Google Scholar]
- 43. Spilsbury JC, et al. Effects of the home environment on school-aged children’s sleep. Sleep. 2005;28(11):1419–1427. [DOI] [PubMed] [Google Scholar]
- 44. Benca-Bachman C, et al. Heritability of sleep duration from early adolescence to young adulthood. Behav Genet. 2018;48(6):457. [Google Scholar]
- 45. Billows M, et al. Family disorganization, sleep hygiene, and adolescent sleep disturbance. J Clin Child Adolesc Psychol. 2009;38(5):745–752. [DOI] [PubMed] [Google Scholar]
- 46. Cain N, et al. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11(8):735–742. [DOI] [PubMed] [Google Scholar]
- 47. Dahl RE. The regulation of sleep and arousal: development and psychopathology. Dev Psychopathol. 1996;8(1):3–27. doi: 10.1017/S0954579400006945 [DOI] [Google Scholar]
- 48. El-Sheikh M, et al. Vagal regulation and emotional intensity predict children’s sleep problems. Dev Psychobiol. 2005;46(4):307–317. [DOI] [PubMed] [Google Scholar]
- 49. Collins WA. Development during middle childhood: the years from six to twelve. Washington, DC: National Academy Press; 1984. [PubMed] [Google Scholar]
- 50. Laposky AD, et al. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 2008;582(1):142–151. [DOI] [PubMed] [Google Scholar]
- 51. Vitaterna MH, et al. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264(5159):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Short MA, et al. Estimating adolescent sleep patterns: parent reports versus adolescent self-report surveys, sleep diaries, and actigraphy. Nat Sci Sleep. 2013;5:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eccles JS, et al. Development during adolescence: the impact of stage-environment fit on young adolescents’ experiences in schools and in families. In: Notterman JM, ed. The evolution of psychology: Fifty Years of the American Psychologist. Washington, DC: American Psychological Association; 1993: 475–501. doi: 10.1037/10254-034 [DOI] [PubMed] [Google Scholar]
- 54. Turkheimer E, et al. Nonshared environment: a theoretical, methodological, and quantitative review. Psychol Bull. 2000;126(1):78–108. [DOI] [PubMed] [Google Scholar]
- 55. Barclay NL, et al. Nonshared environmental influences on sleep quality: a study of monozygotic twin differences. Behav Genet. 2012;42(2):234–244. [DOI] [PubMed] [Google Scholar]
- 56. Barclay NL, et al. Quantitative genetic research on sleep: a review of normal sleep, sleep disturbances and associated emotional, behavioural, and health-related difficulties. Sleep Med Rev. 2013;17(1):29–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.