Abstract
Purpose
To report a case of 2-month-old boy with Stevens-Johnson syndrome (SJS)/Toxic epidermal necrolysis (TEN) and ocular involvement that was successfully treated with cryopreserved amniotic membrane transplantation (AMT).
Observation
A 2-month-old otherwise healthy boy was referred to Boston Children's Hospital with extensive rash and desquamation concerning for SJS/TEN. A skin biopsy was performed which showed full-thickness epidermal necrosis. AMT was performed at the bedside under general anesthesia. A combination of tobramycin and dexamethasone ointment was prescribed four times per day. On reassessment two weeks following AMT, the entire ocular surface had healed with no signs of conjunctival and/or corneal inflammation or ulceration.
Conclusion and importance
To the best of our knowledge, our case represents the youngest patient with SJS/TEN to be managed by AMT and one of very few cases where acetaminophen is suspected to be the offending agent. This case highlights the efficacy of AMT at such a young age and feasibility of performing the procedure at bedside in these patients It also highlights that SJS/TEN can develop at such young age.
Keywords: Stevens-Johnson syndrome, Toxic epidermal necrolysis, Amniotic membrane transplantation
1. Introduction
Stevens-Johnson syndrome (SJS) and its more severe variant toxic epidermal necrolysis (TEN) are serious life-threatening conditions that involve wide spread skin and mucous membrane destruction.1,2 We present a case of 2 month old boy with Stevens-Johnson syndrome/Toxic epidermal necrolysis and ocular involvement that was successfully treated with cryopreserved amniotic membrane transplantation (AMT).
2. Case report
A 2-month-old otherwise healthy boy was referred to Boston Children's Hospital with extensive rash and desquamation concerning for SJS/TEN. One week prior to presentation, he developed non-specific viral prodromal symptoms including fever to102.6 F (39.2 °C), decreased urine output, emesis and decreased appetite. There was no history of any allergies. Family history was only significant for Sjögren's syndrome for his mother and recent pneumonia actively treated with azithromycin for the patient's older sister. A full sepsis work-up at outside hospital was performed and was negative. The patient was empirically started on acyclovir, vancomycin, ceftriaxone and acetaminophen. One day later, he developed a diffuse maculopapular rash with vesicles and desquamation involving head, trunk, upper and lower extremities (Fig. 1). The patient developed acute respiratory failure for which he was sedated and nasally intubated. At that time, he was transferred to Boston Children's Hospital with skin detachment involving more than 40% of his total body surface area (TBSA). A skin biopsy was performed which showed full-thickness epidermal necrosis. The adjacent epidermis demonstrated basal layer vacuolization and scattered dyskeratotic cells (Fig. 2). Special stains were performed including periodic acid Schiff (PAS), gram stain and Grocott-Gomori's (or Gömöri) methenamine silver which were all negative. No immunoreactivity was detected with IgG, IgA, IgM, C3 and fibrin. These findings were consistent with diagnosis of SJS/TEN. Systemic intravenous immunoglobulin (IVIG) was initiated.
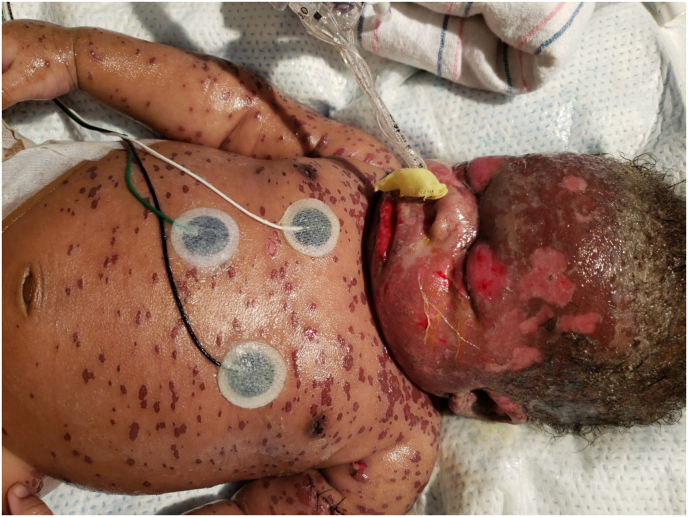
Fig. 1.
External photo showing diffuse maculopapular rash with skin desquamation and epidermal detachment involving head, trunk and upper extremities with bilateral macerated upper and lower eye lids.
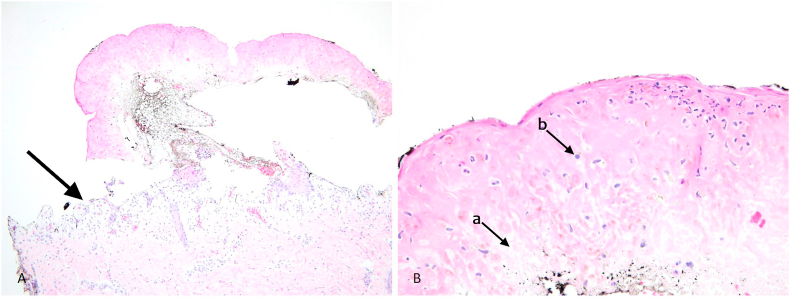
Fig. 2.
Histopathology showing A-full thickness epidermal necrosis and separation (black arrow). B- The adjacent epidermis demonstrated scattered dyskeratotic cells (a) and basal layer vacuolization (b).
Ocular evaluation by a senior faculty member at the time of initial presentation to our institution showed bilateral desquamated upper and lower eye lids (Fig. 1), mild conjunctival injection and bilateral corneal epithelial defects. No conjunctival staining was detected at that time. The anterior chamber was quiet bilaterally, the pupils were equal, round, regular and reactive to light. Dilated fundus examination disclosed normal disc, vessels, macula, and periphery. Erythromycin ointment and artificial tears were prescribed for the corneal epithelial defects which healed in two days. Five days later, diffuse perilimbal conjunctival staining was noted with early mild symblepharon formation nasally and temporally. AMT was performed at the bedside under general anesthesia using our previously described technique.3 In summary, intravenous tubing was used to create bolsters and a symblepharon ring, the diameter of which was estimated based on the distance between the superior and inferior orbital rims. A 5 × 5cm AM piece was then placed as a continuous sheet over the ocular surface. The symblepharon ring was inserted to push the AM into the fornices. Finally, the AM was anchored to the skin with 6–0 polypropylene sutures over the bolsters. A combination of tobramycin and dexamethasone ointment was prescribed four times per day. On reassessment two weeks following AMT, the entire ocular surface had healed with no signs of conjunctival and/or corneal inflammation or ulceration. There was no evidence of limbal stem cell deficiency, and the tobramycin/dexamethasone drops were gradually tapered. The general condition of the patient continued to improve with signs of skin re-epithelization (Fig. 3). At the last follow-up, 2 months later, ocular examination showed normal anterior segment with no evidence of lid margin keratinization, entropion, trichiasis or any other complication (Fig. 4). Further follow-up visits have been scheduled for early detection of late complications.
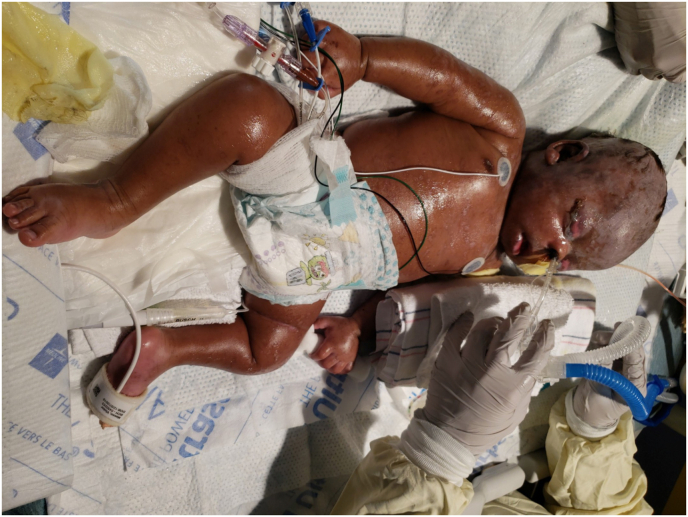
Fig. 3.
External photo taken 2 weeks after initial presentation showing marked improvement in the patient's general condition with continued skin re-epithelization.
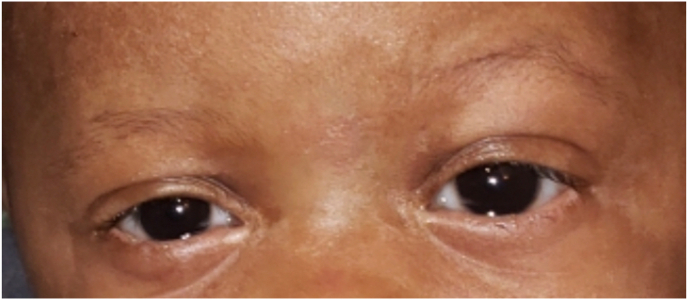
Fig. 4.
External photo taken after discharge showing complete healing of lid desquamation and normal anterior segment.
3. Discussion
Ocular involvement in SJS/TEN occurs in 84% of patients during the acute stage, especially those with epidermal detachment >10% of TBSA.4 Our patient had more than 40% of TBSA involved, therefore it is better classified as TEN rather than SJS. Atypical cases of SJS/TEN may initially present with ocular signs only.5 Prompt diagnosis of ocular surface inflammation and early intervention are essential to avoid cicatricial keratoconjunctivitis and limbal stem cell deficiency that may lead to vision threatening complications. AMT performed in the first week after ocular involvement has been reported to be an effective treatment modality due to anti-inflammatory and anti-scarring effects mediated by anti-inflammatory cytokines, including interleukin-10 and anti-inflammatory protease inhibitors such as alpha 1 antitrypsin inhibitor. This can subsequently facilitate rapid epithelial healing preventing long-term ocular morbidity during the chronic stage.3,6, 7, 8 We present a rare case of TEN in a very young patient (2 months) that was successfully managed with AMT. The triggering factor for SJS/TEN in our case remained unclear, however it may be multifactorial. It may be a viral infection based on the history of fever and sick contact with pneumonia (patient's older sister). Our patient received acetaminophen one day before development of maculopapular rash and desquamation which raise the possibility that it may be one of the factors that lead to TEN. It has been previously reported to be associated with SJS/TEN with few reports in the literature, however it was started together with vancomycin, ceftriaxone and acyclovir and any one of them may be the triggering agent.9,10 Neonatal lupus presenting as TEN-like syndrome was considered initially given mother's history of Sjögren's syndrome, however the skin biopsy and overall clinical picture was consistent with TEN. Long-term follow-up of these cases is essential for early detection of complications. Catt and colleagues retrospectively evaluated the ocular manifestations of pediatric SJS/TEN and reported that the median time for development of corneal opacification was 4 months, of limbal stem cell deficiency was 7 months and of corneal neovascularization was 10 months after admission.11
To the best of our knowledge our case represents the youngest patient with SJS/TEN to be managed by AMT and one of very few cases where acetaminophen is suspected to be the offending agent. This case highlights the efficacy of AMT at such a young age and feasibility of performing the procedure at bedside in these patients It also highlights that SJS/TEN can develop at such young age.
Patient consent
Written informed consent was obtained from the patient's legal guardian.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Funding
Children's Hospital Ophthalmology Foundation, Inc.
Declaration of competing interest
The following authors have no financial disclosures:
Abdelrahman M. Elhusseiny, Ryan Gise, Christina Scelfo, Iason Mantagos.
Acknowledgment
None.
References
- 1.Miliszewski M.A., Kirchhof M.G., Sikora S. Stevens-johnson syndrome and toxic epidermal necrolysis: an analysis of triggers and implications for improving prevention. Am J Med. 2016;129(11):1221–1225. doi: 10.1016/j.amjmed.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Kohanim S., Palioura S., Saeed H.N. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - a comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul Surf. 2016;14(2):168–188. doi: 10.1016/j.jtos.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Ma K.N., Thanos A., Chodosh J. A novel technique for amniotic membrane transplantation in patients with acute Stevens-Johnson syndrome. Ocul Surf. 2016;14(1):31–36. doi: 10.1016/j.jtos.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Morales M.E., Purdue G.F., Verity S.M. Ophthalmic manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis and relation to SCORTEN. Am J Ophthalmol. 2010;150(4):505–510.e1. doi: 10.1016/j.ajo.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Chan F., Benson M.D., Plemel D.J.A. A diagnosis of Stevens-Johnson Syndrome (SJS) in a patient presenting with superficial keratitis. Am J Ophthalmol Case Rep. 2018;11:167–169. doi: 10.1016/j.ajoc.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shay E., Kheirkhah A., Liang L. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol. 2009;54(6):686–696. doi: 10.1016/j.survophthal.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng S.C., Prabhasawat P., Barton K. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116(4):431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 8.Eleiwa T., Ozcan E., Abdelrahman S. Case series of perforated keratomycosis after laser-assisted in situ keratomileusis. Case Rep Ophthalmol Med. 2020;2020:7237903. doi: 10.1155/2020/7237903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajput R., Sagari S., Durgavanshi A., Kanwar A. Paracetamol induced Steven-Johnson syndrome: a rare case report. Contemp Clin Dent. 2015;6(Suppl 1):S278–S281. doi: 10.4103/0976-237X.166838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswal S., Sahoo S.S. Paracetamol induced Stevens-Johnson syndrome--toxic epidermal necrolysis overlap syndrome. Int J Dermatol. 2014;53(8):1042–1044. doi: 10.1111/ijd.12355. [DOI] [PubMed] [Google Scholar]
- 11.Catt C.J., Hamilton G.M., Fish J. Ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Am J Ophthalmol. 2016;166:68–75. doi: 10.1016/j.ajo.2016.03.020. [DOI] [PubMed] [Google Scholar]