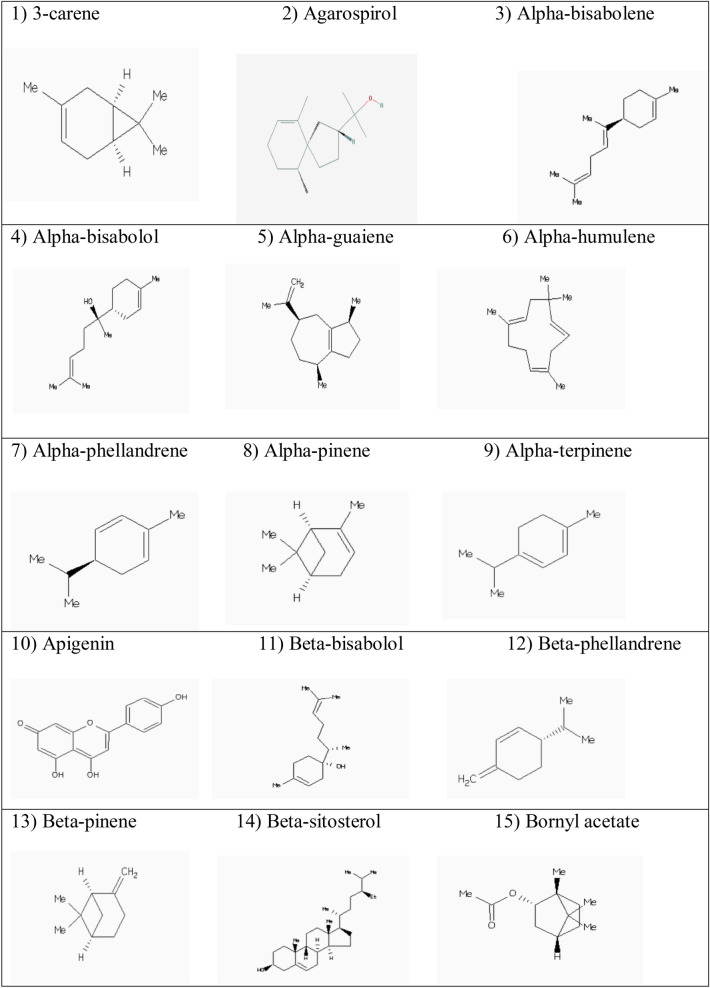
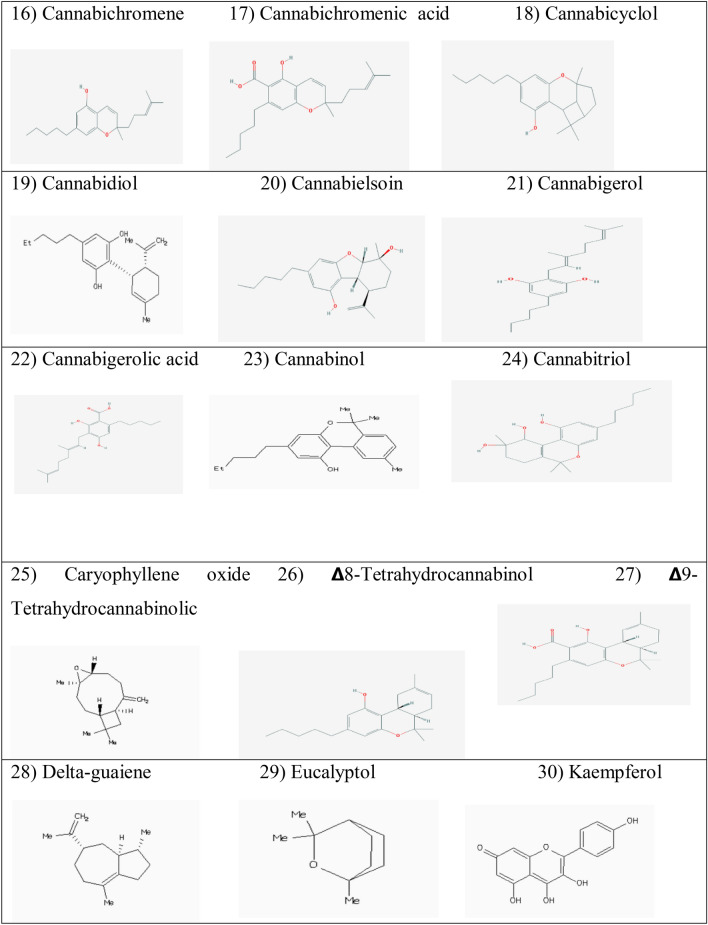
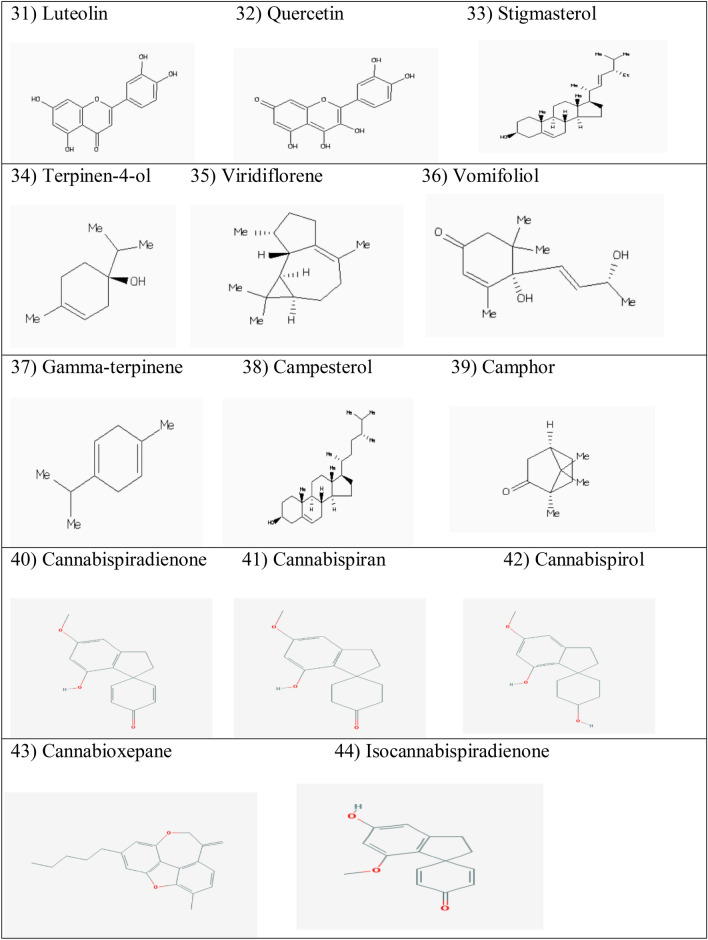
Abstract
Cannabis sativa L. Cannabaceae, used for psychoactive rituals in Mesopotamia. Here, we investigated in vitro inhibitory activity of methyl alcohol extract derived from leaves and resin of cannabis against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Moreover, the binding affinity (BA; kcal/mol) of selected phytochemicals of cannabis to AChE and BChE has been predicted in silico. Phytochemicals of cannabis had acceptable BA towards AChE ranging from – 6.4 (beta-pinene) to – 11.4 (campesterol) and BChE ranging from – 5.5 (alpha-pinene) to – 9.8 (cannabioxepane). All cannabinoids, flavonoids (apigenin), terpenes, and phytosterols of cannabis were double inhibitors due they utilized hydrogen bonds and hydrophobically interacted with both catalytic triad and peripheral anionic site (PAS) of AChE and BChE. Campesterol is phytosterol docked with AChE and BChE via hydrogen bond and it will be a lead-like molecule for further drug design. Delta-9-Tetrahydrocannabinolic acid has been docked with AChE and BChE and it can be a candidate molecule for further drug design. To sum up, this study not only approved cholinesterase inhibitory effects of cannabis but also suggested an array of phytocompounds as hit small molecules for discovery or design of ecofriendly botanical antiinsectants or phytonootropic drugs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-021-00075-0.
Keywords: Cannabis sativa, Phytonootropics; insecticides, Tetrahydrocannabinolic acid, Terpenes, Phytosterols; cannabinoids
Introduction
Cannabis sativa L. Cannabaceae, hemp, has been used as medicinal herb dates back to 3000 BC (Turner et al. 1980). The seeds and leaves of the plant have therapeutic effects and its psychotropic resins have been used for medical, ritual, or spiritual purposes (Carod-Artal 2013). In Mesopotamia, cradle of civilization, cannabis was utilized for psychoactive or other pharmacological properties (Russo 2007; Teall 2014). It also utilized as insecticide administered orally and topically (McPartland 1997).
Cholinesterase (ChE) terminates acetylcholine (ACh) efficacy in synapses (Wang 2013). Two types of ChE reported in vertebrates are known as acetylcholinesterase (AChE; EC 3.1.1.7), and butyrylcholinesterase (BChE; EC 3.1.1.8; Darvesh et al. 2003), found in the brain and in neurofibrillary tangles and neuritic plaques (Beard et al. 1995). Previous studies have shown that the major cause of the cognitive dysfunction in patients with Alzheimer’s disease (AD) is a decrease of cholinergic neurotransmission in the cortical regions of the human brain (Schuster et al. 2010). Therefore, these enzymes have been considered as important therapeutic targets for improving the cholinergic deficit which is the main reason for the decline of cognitive and behavioral impairments in AD (Greig et al. 2002).
This study was aimed to investigate the nootropic and/or insecticidal effects of cannabis with a focus on ChE (AChE and BChE) inhibitory effects. In silico molecular docking has been also implemented to elucidate possible mechanisms mediated by main components of cannabis that interact with ChE.
Materials and methods
Plant extraction
The top flowers of Cannabis sativa L. have been prepared from local gardens of Iraq in autumn 2018 and authentitized by a botanist in our department while resin (charas = hashish = marijuana concentrate) has been bought from herbal store. Specimens were dried for 12 h in a ventilation oven, disassembled, and blended. Then, fine powder (6 g) weighed, homogenized in a glass vial, and extracted with 600 ml of methanol: chloroform (9:1) mixture. After that, the resulting mixture was settled down and centrifuged at 3000 rpm to separate the sediment. The resulting extract has been filtered, diluted, and preserved in a dark glass flask until use.
In vitro ChE inhibitory effect
All chemicals were obtained from Sigma-Aldrich (St. Louis, MA, USA) and used as received. For measuring ChE activity (Ellman et al. 1961), the reaction mixture contained 1000 μL Na2HPO4 buffer (50 mM, pH 7.7), 100 μL of test compound followed by the addition 100 μL of enzyme pre-read at 412 nm using UV–VIS spectrophotometer. Then, contents were pre-incubated for 10 min at 37 °C. The reaction was initiated by the addition 100 μL of substrate (acetylthiocholine iodide and butyrylthiocholine chloride for evaluation of AChE and BChE activities, respectively) and 100 μL of Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB). After 30 min of incubation at 37 °C, absorbance was assayed at 412 nm. All tests were carried out thrice with their respective controls. The percent inhibition was calculated by the help of following equation: Inhibition (%) = (absorbance of negative control—absorbance of test compound)/ (absorbance of negative control) × 100; where, control = total enzyme activity without inhibitor; test = activity in the presence of test extract. The inhibition percentage of extract has been reported in proportion to eserine as a canonical reversible ChE inhibitor.
Molecular docking studies
For docking, crystal structure of protein target was retrieved from Protein Data Bank (PDB) (http://www.RCSB.org; Berman et al. 2002). The PDB format of target proteins, AChE (1EVE: Pacific electric ray (Torpedo californica); [Tax Id: 7787] and BChE (1P0I: Homo sapiens); [Tax Id:9606] have been edited, optimized and trimmed in Molegro Virtual Docker (Thomsen and Christensen 2006) and Chimera 1.8.1 (http://www.rbvi.ucsf.edu/chimera) before submission to PyRx software version 0.8 (Dallakyan and Olson 2015). The number of each amino acid has been reported in edited 1EVE is Torpedo’s number minus one and in edited 1P0I is Homo sapiens’s number minus three. The structures of the major known bioactive compounds of selected plants were retrieved from PubChem databases (https://pubchem.ncbi.nlm.nih.gov) and ZINC database ver. 12.0 (http://zinc.docking.org/) and ChemSpider databases (http://www.chemspider.com). After accomplishment of in silico molecular docking, the results have been presented as binding affinity (BA; kcal/mol) values. The more negative BA reflects the best pose and binding of ligand to target protein in its binding sites. The selected structure of ligand and target protein were presented here due to limitation of space (vide infra).
Pharmacokinetic parameters
The prediction of ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties is an initial phase of drug discovery. The structures of the major phytocompounds were retrieved from aforementioned databases (vide supra) and their canonical SMILES formats submitted to AdmetSAR Database https://lmmd.ecust.edu.Cn/admetsar1 and parameters including blood–brain barrier (BBB) penetration, human gastrointestinal tract (GIT) absorption, distribution, subcellular localization, metabolism via CYP450 2C9, and cardiotoxicity index of human ether-a-go-go-related gene (HERG) inhibition have been considered as drug-likeness or lead-likeness fitness (Cheng et al. 2012).
Results and discussion
Alzheimer's disease causes loss of intellectual and behavioral abilities and is the most common cause of dementia (Loizzo et al. 2008). Many therapeutic strategies have been developed to treat AD, however ChE inhibitors are forefront. Drugs such as eserine, tacrine, donepezil, rivastigmine, and galanthamine were approved for the treatment of AD, although the search for new inhibitors of AChE and BChE is still continuous (Shoaib et al. 2015).
Enzymologically, AChE and BChE employ an amino acid sequence homology of 65% while they are encoded via various genes located on human chromosomes 7 and 3 (AChE (7q22) and BChE (3q26); Soreq and Zakut 1993). Both AChE and BChE have the same structure (Vellom et al. 1993), where they possess hydrophobic active gorge (Sussman et al. 1991). Upon entering ACh into active site of these enzymes, it binds to two sites including catalytic region adjacent to the base of the gorge and a choline-binding site midway up gorge. In this line, AChE is specific to hydrolyze ACh whilst BChE has the ability to break many various molecules (Taylor and Radic 1994). The acylation or catalytic site (CS) of AChE composed of Ser200, His440, and Glu327 residues buried at the base of a narrow 20 Å deep gorge, lined predominantly with aromatic residues, whilst catalytic triad in BChE consists of Ser198, His438, and Glu325 (Suárez and Field 2005). The major subsites of AChE are the oxyanion hole (OH) consists of Gly118, Gly119, and Ala201 residues (Ordentlich et al. 1998), while OH site located near of the choline-binding site and contains Gly116, Gly117 and Ala199 residues in BChE (Bajda et al. 2013). Another site of AChE is anionic subsite (AS) contains aromatic residues (Trp84 and Phe330) and Glu199 in AChE (Kua et al. 2003) whilst AS in BChE contains Trp82, Tyr128, Phe329, and Ala328 residues (Ali et al. 2017). The difference between AChE and BChE lies in the acyl pocket (AP) and the peripheral anionic site (PAS; Houghton et al. 2006). The AP of AChE includes Trp233, Phe288, Phe290, and Phe331 residues (Radic et al. 1992) while AP of BChE includes Leu286 and Val288 residues and PAS of AChE including Tyr70, Asp72, Tyr121, Trp279, and Tyr334 residues (Eichler et al. 1994). The PAS of BChE contains Tyr332 and Asp70 residues (Bajda et al. 2013).
We found that the fraction of resin of Cannabis sativa L. (hashish) showed inhibitory effects of 80.00% and 68.00% for AChE and BCHE, respectively (p = 0.001). While fraction of leaves of Cannabis sativa L. (marihuana) showed similar inhibitory effects of 52.33% and 49.00% for AChE and BCHE, respectively (p = 0.226). Moreover, phytocompounds of Cannabis sativa L. that showed acceptable BA with AChE and BChE have been reported at this juncture. In this continuum, delta-3-carene or 3-carene (Fig. 1) is a bicyclic monoterpenoid alkene found naturally in cannabis and white pepper (Kendall and Alexander 2017), has AChE inhibitory effects (Lomarat et al. 2015). The 3-carene has been docked with AChE with BA of – 7.3 kcal/mol. The 3-carene has hydrophobic interactions with amino acid residues in the catalytic triad (Trp83, Phe329 and Tyr333) and PAS (Tyr120) and AS (Phe330; Supplementary material (SM)). In this line, it has appropriate BA ( – 6.1 kcal/mol) with BChE via hydrophobic interactions with amino acid residues of PAS (Tyr329) and AS (Ala325, Trp79 and Phe326) and the CS (His433; SM). ADMET properties of 3-carene showed that it can go through BBB and absorbed from GIT whilst it is a weak inhibitor of HERG (Table 3).
Fig. 1.
The selected phytochemicals of Cannabis sativa L
Table 3.
In silico ADMET properties of phytochemicals of Cannabis sativa L. by admetSAR
| Compound | BBB | HIA | CYP inhibition/substrate | Subcellular localization | HERG Inhibition |
|---|---|---|---|---|---|
| 3-Carene | 0.9473 | 0.9943 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Agarospirol | 0.9669 | 0.9954 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Alpha-bisabolene | 0.9399 | 0.9934 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Alpha-bisabolol | 0.9667 | 0.9900 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Alpha-guaiene | 0.9723 | 0.9940 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Alpha-humulene | 0.9733 | 0.9972 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Alpha-phellandrene | 0.9049 | 0.9965 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Alpha-pinene | 0.8959 | 0.9964 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Alpha-terpinene | 0.9415 | 0.9953 | Non-substrate/Non-inhibitor | Nucleus | Weak |
| Apigenin | 0.6364 | 0.9887 | Non-substrate/inhibitor | Mitochondria | Weak |
| Beta-bisabolol | 0.9462 | 0.9899 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Beta-phellandrene | 0.9261 | 0.9830 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Beta-pinene | 0.9229 | 0.9834 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Beta-sitosterol | 0.9743 | 1.0000 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Bornyl acetate | 0.9719 | 0.9969 | Non-substrate/ Non-inhibitor | Mitochondria | Weak |
| Campesterol | 0.9749 | 1.0000 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Camphor | 0.9836 | 0.9971 | Non-substrate/inhibitor | Mitochondria | Weak |
| Cannabichromene | 0.9672 | 0.9937 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabichromenic acid | 0.5785 | 0.8983 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabicyclol | 0.9791 | 0.9926 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabidiol | 0.6597 | 0.9937 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabielsoin | 0.9379 | 0.9964 | Non-substrate/inhibitor | Mitochondria | Weak |
| Cannabigerol | 0.5888 | 0.9960 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabigerolic acid | 0.6891 | 0.9773 | Non-substrate/inhibitor | Mitochondria | Weak |
| Cannabinol | 0.9856 | 0.9922 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabitriol | 0.7859 | 0.9707 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Caryophyllene oxide | 0.9474 | 0.9950 | Non-substrate/inhibitor | Lysosome | Weak |
| Δ8-THC | 0.9685 | 0.9949 | Non-substrate/ inhibitor | Mitochondria | Weak |
| Δ9-THC | 0.5953 | 0.9164 | Non-substrate/inhibitor | Mitochondria | Weak |
| Delta-guaiene | 0.9723 | 0.9940 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Eucalyptol | 0.9842 | 0.9880 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Gamma-terpinene | 0.9431 | 0.9972 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Kaempferol | 0.6286 | 0.9855 | Non-substrate/inhibitor | Mitochondria | Weak |
| Luteolin | 0.5711 | 0.9650 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Quercetin | 0.5711 | 0.9650 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Stigmasterol | 0.9743 | 1.0000 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Terpinene-4-Ol | 0.9104 | 0.9969 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Viridiflorene | 0.9790 | 1.0000 | Non-substrate/Non-inhibitor | Lysosome | Weak |
| Vomifoliol | 0.5351 | 1.0000 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabispiradienone | 0.9259 | 1.0000 | Non-substrate/inhibitor | Mitochondria | Weak |
| Cannabispiran | 0.9225 | 0.9967 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabispirol | 0.8235 | 0.9957 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
| Cannabioxepane | 0.9709 | 1.0000 | Non-substrate/Non-inhibitor | Plasma membrane | Weak |
| Isocannabispiradie- | 0.9259 | 1.0000 | Non-substrate/Non-inhibitor | Mitochondria | Weak |
ADMET absorption, distribution, metabolism, and excretion-toxicity, BBB blood–brain barrier penetration, HIA human intestinal absorption, CYP cytochrome P, HERG human ether-a-go-go-related genes inhibition
Agarospirol is a sesquiterpene found in cannabis (Rothschild et al. 2005). This study showed that agarospirol has been docked with AChE (BA of – 8 kcal/mol) via hydrogen bonds with hydroxyl group of Ser121 and through hydrophobic interactions with PAS (Tyr333, Tyr120 and Asp71) and AS (Trp83 and Phe329) and AP (Phe330) and with His439 residue of CS (SM). It also docked with BChE through hydrophobic interactions with amino acid residues in AS (Trp79) and PAS (Asp67 and Tyr329) and His433 residue of the CS (SM). According to study ADMET properties, it can be absorbed from BBB and GIT, while localized in lysosome (Table 3).
Alpha-bisabolene is a sesquiterpene found in cannabis (Gulluni et al. 2018). In this study, it has been docked with AChE with BA of – 9.2 kcal/mol via hydrophobic interactions with amino acid residues in AP (Phe330) and PAS (Tyr333 and Tyr120) and AS (Trp83 and Phe329) and OH (Gly117) and CS (His439; SM). In this regard, alpha-bisabolene has been docked with BChE with appropriate BA (Table 2) through hydrophobic interactions with amino acid residues in PAS (Tyr329) and AS (Ala325, Phe326, Trp79 and Tyr125; SM). Based on ADMET results, it can be absorbed from GIT and crossed BBB (Table 3).
Table 2.
In silico molecular docking of major phytochemicals of Cannabis sativa L. against BChE (PDB code 1P0I)
| Ligand | Binding affinity | RMSD/UB | RMSD/LB | |
|---|---|---|---|---|
| 3-carene | – 6.1 | 2.671 | 1.536 | |
| Agarospirol | – 7.4 | 2.667 | 1.513 | |
| Alpha-bisabolene | – 7.1 | 6.57 | 1.11 | |
| Alpha-bisabolol | – 7.5 | 1.891 | 1.193 | |
| Alpha-guaiene | – 7.3 | 5.44 | 2.671 | |
| Alpha-humulene | – 7.7 | 4.411 | 1.365 | |
| Alpha-phellandrene | – 5.8 | 21.44 | 20.187 | |
| Alpha-pinene | – 5.5 | 3.404 | 0.963 | |
| Alpha-terpinene | – 6.1 | 2.279 | 1.131 | |
| Apigenin | – 9.1 | 5.312 | 2.857 | |
| Beta-bisabolol | – 6.9 | 6.161 | 1.235 | |
| Beta-phellandrene | – 6.0 | 2.42 | 1.438 | |
| Beta-pinene | – 5.6 | 20.444 | 19.181 | |
| Beta-sitosterol | – 9.3 | 8.016 | 3.152 | |
| Bornyl acetate | – 6.3 | 4.046 | 2.144 | |
| Campesterol | – 9.3 | 8.235 | 3.201 | |
| Camphor | – 6.6 | 2.695 | 1.505 | |
| Cannabichromene | – 7.7 | 21.782 | 19.152 | |
| Cannabichromenic | – 7.7 | 4.05 | 3.681 | |
| Cannabicyclol | – 8.4 | 4.604 | 3.004 | |
| Cannabidiol | – 8.2 | 3.316 | 1.856 | |
| Cannabielsoin | – 8.9 | 3.631 | 1.341 | |
| Cannabigerol | – 7.3 | 5.47 | 3.775 | |
| Cannabigerolic acid | – 7.5 | 19.972 | 17.886 | |
| Cannabinol | – 8.8 | 4.942 | 3.402 | |
| Cannabitriol | – 8.7 | 7.986 | 4.66 | |
| Caryophyllene oxide | – 7.4 | 4.192 | 2.067 | |
| Delta8-Tetrahydrocannabinol | – 8.7 | 5.913 | 2.114 | |
| Delta9-Tetrahydrocannabinolic acid | – 8.1 | 5.861 | 2.358 | |
| Delta-Guaiene-85598968 | – 7.5 | 4.023 | 1.143 | |
| Eucalyptol | – 5.7 | 21.312 | 19.967 | |
| Gamma-terpinene | – 5.7 | 2.965 | 1.755 | |
| Kaempferol | – 8.5 | 5.679 | 4.208 | |
| Luteolin-18185774-mh | – 9.4 | 2.76 | 1.074 | |
| Quercetin | – 9 | 6.803 | 5.141 | |
| Stigmasterol | – 9.7 | 2.594 | 1.798 | |
| Terpinene-4-ol | – 5.9 | 4.512 | 2.743 | |
| Viridiflorene | – 8.0 | 3.884 | 1.355 | |
| Vomifoliol | – 6.7 | 20.743 | 19.476 | |
| Cannabispiradienone | – 8.2 | 5.875 | 2.186 | |
| Cannabispiran | – 8.2 | 5.942 | 2.23 | |
| Cannabispirol | – 8.1 | 6.517 | 3.638 | |
| Cannabioxepane | – 9.8 | 3.235 | 0.658 | |
| Isocannabispiradienone | – 8.3 | 4.914 | 2.007 | |
RMSD root mean-square deviation is the measure of the average distance between the atoms,UB upper bound, LB lower bound
Alpha-bisabolol is a sesquiterpene found in cannabis (Rothschild et al. 2005) and several plants. It is a strong inhibitor of AChE (Shanmuganathan et al. 2015). In this study, alpha-bisabolol has been docked with AChE with BA ( – 8.7 kcal/mol; Table1) through hydrogen bond with Asp71 residue of PAS and through hydrophobic interactions with Trp83, Phe329 and Glu198 residues of AS and Tyr120 and Tyr333 residues of PAS and Phe330 residues of AP and His residues of CS. Alpha-bisabolol has been docked with BChE (BA – 7.5 kcal/mol; Table 2) through hydrophobic interactions with amino acid residues in PAS (Tyr329 and Asp67) and AS (Ala325, Phe326, Trp79 and Tyr125) and the CS (His433; SM). The ADMET assays showed that it can be absorbed orally and transferred through BBB while it is a weak inhibitor of HERG (Table 3).
Table 1.
In silico molecular docking of major phytochemicals of Cannabis sativa L. against AChE (PDB code 1EVE)
| Ligand | Binding affinity | RMSD/UB | RMSD/LB |
|---|---|---|---|
| 3-Carene | – 7.3 | 5.115 | 2.521 |
| Agarospirol | – 8 | 3.392 | 1.753 |
| Alpha-bisabolene | – 9.2 | 6.7 | 1.296 |
| Alpha-bisabolol | – 8.7 | 2.305 | 1.905 |
| Alpha-guaiene | – 8.9 | 5.216 | 1.658 |
| Alpha-humulene | – 8.9 | 4.833 | 1.533 |
| Alpha-phellandrene | – 6.6 | 5.105 | 2.371 |
| Alpha-pinene | – 6.6 | 4.202 | 1.947 |
| Alpha-terpinene | – 7.4 | 4.25 | 0.964 |
| Apigenin | – 9.9 | 5.793 | 2.522 |
| Beta-bisabolol | – 8.3 | 2.712 | 1.55 |
| Beta-phellandrene | – 7 | 4.116 | 1.507 |
| Beta-pinene | – 6.4 | 3.376 | 1.016 |
| Beta-Sitosterol | – 10.9 | 2.504 | 1.353 |
| Bornyl Acetate | – 7.1 | 2.89 | 1.928 |
| Campesterol | – 11.4 | 3.222 | 1.703 |
| Camphor | – 6.6 | 2.695 | 1.505 |
| Cannabichromene | – 9.4 | 7.731 | 1.913 |
| Cannabichromenic | – 9.3 | 9.687 | 3.726 |
| Cannabicyclol | – 9.6 | 8.896 | 3.467 |
| Cannabidiol | – 9.8 | 1.778 | 0.315 |
| Cannabielsoin | – 8.9 | 4.138 | 2.003 |
| Cannabigerol | – 8.9 | 2.51 | 0.907 |
| Cannabigerolic acid | – 9.2 | 9.104 | 2.069 |
| Cannabinol | – 10.1 | 3.461 | 1.348 |
| Cannabitriol | – 9.8 | 7.238 | 2.23 |
| Caryophyllene oxide | – 9.0 | 4.5 | 2.045 |
| Delta8-Tetrahydrocannabinol | – 10.1 | 6.03 | 1.86 |
| Delta9-Tetrahydrocannabinolic acid | – 10.3 | 8.8 | 3.978 |
| Delta-guaiene-85598968 | – 8.8 | 4.915 | 1.32 |
| Eucalyptol | – 6.7 | 2.22 | 1.432 |
| Gamma-Terpinene | – 7.4 | 4.314 | 0.922 |
| Kaempferol | – 10.1 | 5.646 | 2.428 |
| Luteolin-18185774-Mh | – 9.9 | 2.662 | 1.143 |
| Quercetin | – 9.8 | 4.156 | 2.069 |
| Stigmasterol | – 11.3 | 1.196 | 0.986 |
| Terpinene-4-Ol | – 7.1 | 4.513 | 1.104 |
| Viridiflorene | – 8.7 | 6.398 | 3.745 |
| Vomifoliol | – 7.8 | 5.191 | 3.09 |
| Cannabispiradienone | – 9.2 | 6.772 | 2.527 |
| Cannabispiran | – 8.9 | 3.009 | 2.019 |
| Cannabispirol | – 9.2 | 1.595 | 1.375 |
| Cannabioxepane | – 10.4 | 7.894 | 2.685 |
| Isocannabispiradienone | – 9.4 | 7.727 | 5.157 |
RMSD root mean-square deviation is the measure of the average distance between the atoms, UB upper bound, LB lower bound
Alpha-guaiene is a sesquiterpene found in many plants including cannabis (Fischedick et al. 2010) and works as an insect repellent (Murugesan et al. 2012). This study showed, alpha-guaiene has been docked with AChE ( – 8.9 kcal/mol; Table1) via hydrophobic interactions with amino acid residues of PAS (Tyr333, Tyr120 and Asp71) and AP (Phe330) and catalytic AS (Trp83 and phe330; SM). In addition, it hydrophobically interacts with amino acid residues in PAS (Tyr329 and Asp67) and AS (Phe326 and Trp79) and CS (His433) of BChE (SM) with BA -7.3 kcal/mol (Table 2). The ADMET properties results of alpha-guaiene showed that it can be transferred via BBB and absorbed from GIT; it is a weak inhibitor of HERG (Table 3).
Alpha-humulene is a sesquiterpene found in several medicinal plants like cannabis (Fischedick et al. 2010), Commiphora myrrha (Nees), Engler and ginseng (Gitau 2015). Alpha-humulene contained in herbal extracts have insecticidal effects through inhibition of AChE (Lee and Ahn 2013). In this study, alpha-humulene has been docked with AChE with acceptable BA ( – 9.0 kcal/mol) through hydrophobic interactions with amino acid residues in PAS (Tyr120 and Asp71) and catalytic triad (Trp83; SM). Alpha-humulene has been hydrophobically docked with BA of – 7.7 kcal/mol. In this regard, alpha-humulene interacted with amino acid residue in catalytic triad (His433) and PAS (Tyr329 and Asp67) and AS (Phe326, Trp79 and Ala325) of BChE. Based on ADMET properties, it can pass BBB and GIT (Table 3).
Alpha and beta-phellandrene are monoterpenes found in cannabis (Fischedick et al. 2010). Alpha-phellandrene has insecticidal and pesticidal activity (Onyenekwe et al. 2012). In this study, alpha-phellandrene interacted hydrophobically with amino acid residues in PAS (Tyr120, Tyr333 and Asp71), AS (Phe329), AP (Phe330) and CS (His439; SM) of AChE (BA of – 6.6 kcal/mol). Beta-phellandrene also interacted hydrophobically with amino acid residues in PAS (Tyr333), AS (Trp83 and Phe329), AP (Phe330) and CS (His439; SMSM) of AChE (BA of – 7 kcal/mol). Alpha-phellandrene has been loosely docked with BChE (BA – 5.8 kcal/mol; Table 2). While beta-phellandrene has been docked with BChE (BA – 6 kcal/mol; Table 2) via hydrophobic interactions with amino acid residues of AS (Tyr125, Trp79 and Ala325) and CS (His433; SM). The ADMET assays showed that alpha and beta-phellandrene can be absorbed orally and crossed BBB while they are week inhibitor of HERG (Table 3).
Alpha and beta-pinene are monoterpenes. Pinene has capability to cross the BBB readily where it can inhibit activity of ACHE, leading to better memory results (Owokotomo et al. 2015). Alpha-pinene has insecticidal and pesticidal activities (Onyenekwe et al. 2012). This study showed that alpha and beta-pinene have been docked with AChE with BA of – 6.6 and – 6.4 kcal/mol, respectively. In this line, alpha-pinene interacted hydrophobically with amino acid residues in PAS (Tyr333, Tyr120) and AP (Phe330) and CS (His439) of AChE while beta-pinene interacted hydrophobically with amino acid residues of AS (Trp83 and Phe329) and AP (Phe330) solely with His439 residues in CS. Alpha and beta-pinene have been hydrophobically docked with BChE with BA of -5.5 and -5.6 kcal/mol, respectively. In this line, beta-pinene has been docked with BChE through hydrophobic interactions with amino acid residues of PAS (Tyr329) and AS (Phe326, Trp79 and Ala325; SM). Based on ADMET properties, they can be absorbed orally and passed through BBB without considerable side effects (Table 3).
Alpha and gamma-terpinene are monoterpenes found in an array of medicinal plants like cannabis and tea tree oil (Miyazawa and Yamafuji 2006; Hartsel et al. 2016). In the pioneered work, it has been reported that herbal extracts containing terpinene possess inhibitory effects of AChE (Miyazawa and Yamafuji 2006). Alpha-terpinene has hydrophobic interactions with amino acid residues in PAS (Tyr333) and AS (Trp83 and Phe329) and AP (Phe330). Gamma-terpinene has also hydrophobic interactions with amino acid residues in PAS (Tyr333), AS (Trp83 and Phe329), CS (His439) and AP (Phe330; SM). Alpha and gamma-terpinene have been hydrophobically docked with BChE with BA of – 6.1 and – 5.7 kcal/mol, respectively. In this line, alpha-terpinene has been docked with BChE through hydrophobic interactions with amino acid residues of PAS (Tyr329) and AS (Phe326, Tyr125, Trp79 and Ala325) and CS (His433). Gamma-terpinene has hydrophobic interactions with amino acid residues in AS (Tyr125, Trp79 and Ala325) and CS (His433; SM). The ADMET prediction showed that they can be crossed from GIT and BBB (Table 3).
Apigenin is a flavonoid compound found in cannabis (Hartsel et al. 2016). Apigenin has ability to inhibit AChE activity (Balkis et al. 2015). Apigenin has been docked with AChE with BA – 9.9 kcal/mol via hydrogen bonds with amino acid residues in AS (Glu198) and with Tyr69 in PAS and with hydroxyl group of Gln68. It also has hydrophobic interactions with amino acid residues of PAS (Asp71 and Tyr120), AS (Trp83 and Phe329) and CS (His439) of AChE. The Tyr125 residue of PAS and Ala325 residue of AS interacted with apigenin with hydrogen bond while Trp79 residue of AS interacted hydrophobically with apigenin (SM). Based on ADMET, it can be absorbed from GIT, crossed BBB, and localized in mitochondria while it is a week inhibitor of HERG (Table 3).
Beta-bisabolol is a sesquiterpene found in cannabis (Hartsel et al. 2016). In present study, beta-bisabolol has been docked with AChE with BA ( – 8.3 kcal/mol; Table) via hydrogen bonding with Tyr120 and several hydrophobic interactions with amino acid residues of PAS (Tyr333 and Asp71), AS (Trp83 and Phe329), AP (Phe289 and Phe330) and CS (His439). Beta-bisabolol docked with BChE with BA – 6.9 kcal/mol (Table 2) through hydrophobic interactions with amino acid residues in PAS (Tyr329 and Asp67) and AS (Ala325, Phe326, Trp79 and Tyr125) and CS (His433; SM). Based on ADMET properties, it has ability to penetrate BBB and cross GIT while it weak HERG inhibitor (Table 3).
Beta-sitosterol is a phytosterol found in cannabis (Hartsel et al. 2016). Beta-sitosterol has been docked with AChE (BA – 10.9 kcal/mol) via hydrophobic interactions with amino acid residues in PAS (Tyr333, Asp71, Tyr120 and Trp278), AS (Trp83 and Phe329) and AP (Phe289, Phe330 and Phe289; SM). It has been docked with BChE with BA of – 9.3 kcal/mol through hydrophobic interactions with amino acid residues in AS (Trp79, Phe326 and Ala325) and PAS (Tyr329 and Asp67) and CS (His 433; SM). Beta-sitosterol can be absorbed from GIT, crossed BBB, and localized in lysosome while it is a week inhibitor of HERG (Table 3).
Bornyl acetate is a monoterpene found in cannabis (Hartsel et al. 2016). Bornyl acetate has insecticidal activities against several warehouse insects and mites (Li et al. 2015). In this study, bornyl acetate has a BA of – 7.1 kcal/mol with AChE through two hydrogen bonds with amino acid Gly117 and Gly118 in OH and with amino acid Ser199 in CS (SM) and via hydrophobic interactions with Tyr120 residues of PAS and with amino acid residues of AS (Phe329 and Trp83) and AP (Phe330; SM). Bornyl acetate has been docked with BChE (BA of – 6.3 kcal/mol) through hydrophobic interactions with amino acid residues in AS (Trp79, Phe326 and Ala325) and PAS (Tyr329) and CS (His 433; SM). Based on ADMET results, it can be absorbed from GIT and crossed BBB (Table 3).
Campesterol is another phytosterol found in cannabis (Montserrat-de la Paz et al. 2014). Campesterol has cholinesterase inhibitory activity (Elufioye et al. 2017). In this study, campesterol has been docked with AChE (BA of – 11.4 kcal/mol) via hydrogen bonds with hydroxyl group of Ser285 and through hydrophobic interactions with OH (Gly 117), AP (Phe330 and Phe 289), AS (Trp83, Phe329 and Glu198) and PAS (Tyr333, Tyr120 and Asp71). Campesterol has been docked with BChE (BA of – 9.3 kcal/mol) through hydrogen bonds with hydroxyl group of Ser284 and through hydrophobic interactions with PAS (Asp67 and Tyr329) and AS (Ala325 and Trp79) and CS (His433; SM). Campesterol can be absorbed from GIT, crossed BBB, and localized in mitochondria while it is a week inhibitor of HERG (Table 3).
Camphor is a monoterpene found in cannabis (Fischedick et al. 2010). In this study, Ser199 as crucial amino acid residue in CS of AChE interacted with camphor through hydrogen binding with length 3.33 Å while Gly117, His439, Phe 330 and Trp83 residues in catalytic triad interacted hydrophobically with camphor. Camphor has a BA of – 5.9 kcal/mol with BChE through hydrogen bond with amino acid Tyr329 in PAS with a bond length of 2.75 Å (SM) and through hydrophobic interactions with (Ala325, Phe326and Trp79) residues of AS. Camphor can be absorbed from GIT, crossed BBB, and localized in mitochondria while it is a week inhibitor of HERG (Table 3).
Phytocannabinoids found in cannabis including cannabidiol, cannabielsoin, cannabigerol, cannabigerolic acid, cannabinol and cannabitriol (Hartsel et al. 2016) have been docked with AChE and BChE with satisfactory BA. In this line, cannabidiol docked with AChE via hydrophobic interactions with PAS (Tyr333, Trp278, Asp71 and Tyr120) and AS (Trp83 and Phe329) AP (Phe289 and Phe330). Cannabidiol has been docked with BChE with BA of – 8.2 kcal/mol through hydrogen bonds with hydroxyl group of Thr117 and through hydrophobic interactions with AS (Trp79 and Phe 326) and AP (Leu283) and CS (His433 and Ser195; SM).
The Asp71 residue of PAS interacted with cannabielsoin with hydrogen bond while Tyr333, Tyr120, Trp69 and Trp278 residues of PAS and Phe330, Phe287 residues of AP and Phe329 and Trp83 residues of AS interacted hydrophobically with cannabielsoin (SM). Cannabielsoin docked with BChE via hydrogen bonding with Trp79 and hydroxyl group of Gly75 and with several hydrophobic interactions with amino acid residues of PAS (Tyr329 and Asp67) and AS (Ala325) and CS (His433; SM).
Cannabigerol interacted with AChE via hydrogen bond with Tyr120 and with several hydrophobic interactions with amino acid residues of PAS (Asp71, Tyr333 and Trp278) and AS (Phe329, Glu198 and Trp83) and AP (Phe330; SM). Cannabigerol has a BA of -7.3 kcal/mol with BChE through hydrogen bonds with Trp79 of AS and hydroxyl group of Asn80 (SM) and through hydrophobic interactions with Asp67 of PAS and Tyr125 of AS and His433 of CS.
Cannabigerolic docked with AChE through two hydrogen bonds with amino acid Tyr120 and one with Asp71 in PAS (SM) and through hydrophobic interactions with (Tyr333, Trp69 and Trp278) and AS (Trp83 and Phe329) and AP (Phe287 and Phe330; Figure). In this regard, cannabigerol has been docked with BChE (BA – 7.5 kcal/mol) through hydrogen bonds with hydroxyl group of Ser284 and through hydrophobic interactions with AS (Ala325, Trp79 and Phe326) and PAS (Tyr329 and Asp67) and AP (Leu283) and CS (His433; SM).
Cannabinol also docked with AChE through hydrogen bond with amino acid Tyr120 in PAS with a bond length of 2.75 (SM) and through hydrophobic interactions with several amino acid residues of activity site of AChE. Cannabinol docked with BChE (BA of -8.8 kcal/mol) through hydrogen bonds with hydroxyl group of Thr117 and through hydrophobic interactions with PAS (Asp67 and Tyr329) and AS (Ala325, Tyr125, Trp79 and Phe326) and CS (His433; SM).
Cannabitriol docked with AChE through hydrogen bond with amino acid Tyr120 and with through hydrophobic interactions with several amino acid residues in PAS (Tyr333, Trp69 and Trp278) and AS (Phe329 and Trp83) and AP (Phe330; SM). Cannabitriol has been docked with BChE (BA of – 8.7 kcal/mol) through hydrogen bonds with His433 and through hydrophobic interactions with CS (Ser195) and PAS (Tyr329) and AS (Ala325, Trp79 and Phe326). All phytocannabinoids have accepted ADMET properties to be considered as lead-like compounds.
Cannabichromene is a cannabinoid found in cannabis (Hartsel et al. 2016). In present study, cannabichromene has been docked with AChE with BA of – 9.4 kcal/mol via hydrogen bonds with Asp71 and Tyr120 residues and hydrophobic interactions with amino acid residue of PAS (Tyr333) and AS (Trp83 and Phe329) and AP (Phe330) and OH (Gly117; SM). Cannabichromene has been docked with BChE via hydrogen bonds with hydroxyl group of Thr117 and through hydrophobic interactions with AS (Ala325, Trp79 and Phe326) and PAS (Asp67 and Tyr329) and AP (Leu283) and CS (His433 and Ser195; SM). The ADMET properties assay showed that cannabichromene can be absorbed orally and passed through BBB without considerable side effects.
Cannabichromenic acid is a cannabinoid found in cannabis (Hartsel et al. 2016). In present study, cannabichromenic acid has BA – 9.3 kcal/mol with AChE through hydrogen bonds with amino acid residue of AP (Phe287) and with hydroxyl group of Arg288 and through hydrophobic interactions with PAS (Tyr333, Asp71, Trp278 and Tyr120) and AP (Phe330) and AS (Trp83 and Phe329; SM). Cannabichromenic acid has been docked with BChE with BA -7.7 kcal/mol via hydrophobic interactions with amino acid residues of PAS (Asp67 and Tyr329) and AS (Ala325, Trp79 and Phe326) and CS (His433; SM). The ADMET properties showed cannabichromenic acid can be crossed BBB and GIT and distributed into mitochondria (Table 3).
Cannabicyclol is other cannabinoid found in cannabis (Hartsel et al. 2016). In this study, it has been docked with AChE with BA of – 9.6 kcal/mol through hydrophobic interactions with amino acid residues in AP (Phe289 and Phe330) and PAS (Tyr333, Asp71 and Tyr120) and CS (His439) and AS (Phe329 and Trp83; SM). Cannabicyclol has a BA of – 8.4 kcal/mol with BChE through two hydrogen bonds with hydroxyl group of Thr117 and through hydrophobic interactions with PAS (Asp67 and Tyr329) and AP (Leu283 and Val285) and AS (Ala325, Tyr125, Trp79 and Phe326) and CS (His433; SM). The ADMET properties of cannabicyclol showed that it can be transferred through BBB and orally absorbed from GIT while it is a weak inhibitor of HERG (Table 3).
Caryophyllene oxide is a sesquiterpene found in cannabis (Hartsel et al. 2016). Caryophyllene oxide has been docked with BA of – 9 kcal/mol via several hydrophobic interactions with PAS (Tyr120, Tyr278, Tyr 333, Tyr69 and Asp71) and AS (Trp83 and Phe329) and AP (Phe330) and CS (His439; SM). Asp67 and Tyr329 residues of PAS and Ala325, Trp79 and Phe326 residues of AS interacted hydrophobically with caryophyllene oxide (SM). Based on ADMET properties, caryophyllene oxide can be absorbed from GIT and crossed BBB (Table 3).
Δ8-Tetrahydrocannabinol (Δ8–THC) and Δ9-Tetrahydrocannabinol (Δ9–THC) are phytocannabinoids (Hartsel et al. 2016). In this study, Δ8-THC has been hydrophobically docked with amino acid residues in PAS (Tyr120, Asp71 and Tyr333) and AS (Trp83 and Phe329) and AP (Phe330, Phe289) and OH (Gly117) of AChE (SM) with appropriate BA of – 10.1 kcal/mol, while Δ9-THC has been docked with AChE with BA – 10.3 kcal/mol via hydrogen bonding with Tyr120 and several hydrophobic interactions with amino acid residues of PAS (Tyr333, Tyr69, Trp278 and Asp71) and AP (Phe330 and Phe287) and AS (Trp83 and Phe329; SM). In this line, Δ8-THC has been docked with BChE ( – 8.7 kcal/mol) through hydrophobic interactions with AS (Ala325, Tyr125, Trp79 and Phe326) and PAS (Tyr329), while Δ9-THC hydrophobically interaction with PAS (Asp67) and AP (Leu283) and AS (Ala325, Tyr125, Trp79 and Phe326) and CS (His433 and Ser195) of BChE (SM). Based on ADMET results, THC can be absorbed from GIT and crossed BBB, localized in mitochondria, while it is a weak inhibition of HERG (Table 3).
Delta-guaiene is a sesquiterpene found in cannabis (Romano and Hazekamp 2013). In present study, delta-guaiene has been docked with AChE (BA -8.8 kcal/mol) via hydrophobic interactions by Asp71, Tyr120 and Tyr333 in PAS and by Trp83 and Phe329 in AS and by Phe330 in AP (SM). In addition, it docked with BChE with BA – 7.5 kcal/mol through hydrophobic interactions with AS (Ala325, Trp79 and Phe326) and PAS (Tyr329) and CS (His433; SM). Delta-guaiene can be absorbed from GIT, crossed BBB, and localized in lysosome while it is a week inhibitor of HERG (Table 3).
Eucalyptol is a monoterpene found in cannabis (Hartsel et al. 2016). It used as insecticide and insect repellent (Klocke et al. 1987; Sfara et al. 2009). In this study, eucalyptol has been docked with AChE via hydrogen bonds with Tyr120 and through hydrophobic interactions with Tyr333 in PAS and Phe329 in AS and Phe330 in AP and Gly117 in OH (SM). In this regard, eucalyptol has been docked with BChE ( – 5.7 kcal/mol) through hydrophobic interactions (SM). The ADMET properties of eucalyptol showed that it can be transferred via BBB and absorbed from GIT while it tends to be localized in lysosome (Table 3).
Kaempferol is a flavonoid found in cannabis (Flores-Sanchez and Verpoorte, 2008). In a seminal work, kaempferol has showed AChE inhibitory potential (Ding et al. 2013). In this study, kaempferol has been docked with AChE (BA – 10.1 kcal/mol; Table). The Tyr69 residue of PAS and Glu198 residue of AS interacted with kaempferol with hydrogen bonds while Tyr120 and Asp71 residues of PAS and Phe329 and Trp83 residues of AS interacted hydrophobically with kaempferol (SM). Kaempferol has been docked with BChE with BA – 8.5 kcal/mol via hydrogen bonding with Tyr329 and Asp67 residues of PAS and with His433 residue of CS and with hydroxyl group of Asn65 (SM) and through hydrophobic interactions with amino acid residues of AS (Trp79). The ADMET properties showed kaempferol can be crossed BBB and GIT and distributed into mitochondria (Table 3).
Luteolin is a flavonoid found in cannabis (Flores-Sanchez and Verpoorte, 2008). Luteolin induces neurite outgrowth and enhanced cholinergic activities (Omri et al. 2012). In this study, luteolin has a BA of – 9.9 kcal/mol with AChE through two hydrogen bonds with Glu198 in AS and Tyr69 in PAS and with hydroxyl group of Gln68 (SM) and through hydrophobic interactions with amino acid residues in PAS (Tyr120 and Asp71) and AS (Trp83 and Phe329). In this line, luteolin has a BA – 9.4 kcal/mol with BChE via hydrogen bonding with PAS (Asp67) and CS (His433) and two hydroxyl group of Asn65 and Asn80 (SM) and through hydrophobic interactions with AS (Trp79). Based on ADMET properties, luteolin has ability to penetrate BBB and cross GIT while it weak HERG inhibitor (Table 3).
Quercetin is another flavonoid found in cannabis (Flores-Sanchez and Verpoorte 2008). Quercetin exerts the cognitive enhancing effect in rat model of Parkinson’s disease via its antioxidative effect resulting in the promotion of neuron survival (Sriraksa et al. 2012). Quercetin has been docked with AChE ( – 9.8 kcal/mol) through hydrogen bonds with Glu198 in AS and Tyr69 in PAS and with hydroxyl group of Gli68 (SM) and through several hydrophobic interactions with amino acid residues in PAS (Tyr120 and Asp71) and AS (Trp83 and Phe329). Quercetin has been docked with BChE with BA – 9 kcal/mol via hydrogen bonding with PAS (Tyr329 and Asp67) and CS (His433) and with two hydroxyl group of Asn65 and Glu194 (SM) and through hydrophobic interactions with Trp79 residue of AS. Based on ADMET properties, quercetin has ability to pass BBB and GIT (Table 3).
Stigmasterol is a phytosterol found in cannabis (Ryz et al. 2017). In this study, stigmasterol has been docked with AChE with BA of – 9.7 kcal/mol through hydrophobic interactions with AP (Phe289) and PAS (Tyr333, Trp278 and Tyr120) and AS (Phe329 and Trp83; SM). Stigmasterol has been docked with BChE (BA of – 11.3 kcal/mol) through hydrogen bonds with two hydroxyl groups of Asn286 and Ser284 and through hydrophobic interactions with PAS (Tyr329 and Asp67) and AS (Trp79 and Phe326) and CS (His433; SM). The ADMET properties of stigmasterol showed that it can be absorbed from GIT and transferred through BBB while it tends to be localized in lysosome (Table 3).
Terpinen-4-ol is a terpene found in cannabis (Grotenhermen and Russo 2006). It has the ability to inhibit AChE (Dohi et al. 2009). In this study, terpinen-4-ol has a BA of – 7.1 kcal/mol with AChE through hydrogen bonds with Tyr120 residue in PAS and through hydrophobic interactions with Tyr333 residue of PAS and Phe330 of AP and Trp83 of AS (SM). In addition, it has been docked with BChE with BA of – 5.9 kcal/mol through hydrophobic interactions with PAS (Tyr329) and AS (Trp79) and CS (His433; SM). Based on ADMET properties, terpinen-4-ol has ability to penetrate BBB and cross GIT while it weak HERG inhibitor (Table 3).
Viridiflorene is a sesquiterpene found in cannabis (Hartsel et al. 2016). This study showed that viridiflorene has been docked with AChE with BA of -8.7 kcal/mol through hydrophobic interactions with PAS (Tyr333, Tyr120 and Asp71) and AS (Trp83 and Phe329) and AP (Phe330; SM). Viridiflorene has a BA of – 8 kcal/mol with BChE through hydrophobic interactions with AS (Ala325, Trp79 and Phe326) and PAS (Tyr329) and CS (His433). Based on ADMET properties, viridiflorene has ability to penetrate BBB and cross GIT while it weak HERG inhibitor (Table 3).
Vomifoliol has anti-AChE activity (Mogana et al. 2014). Vomifoliol has been docked with AChE with BA – 7.8 kcal/mol via hydrogen bonds with Tyr120 in PAS and Gly117 and Gly117 in OH and Ser199 in CS and with several hydrophobic interactions with amino acid residues of PAS (Tyr333 and Asp71) and catalytic triad (Trp83 and His439; SM). Vomifoliol has been docked with BChE (BA of – 6.7 kcal/mol) through hydrogen bonds with three hydroxyl groups of Tyr391 and Thr517 and Asn225 (SM). Based on ADMET results, vomifoliol can be absorbed from GIT and crossed BBB (Table 3).
Cannabispiradienone is a cannabinoid found in cannabis (Pagani et al. 2011). Cannabispiradienone has been docked with AChE (BA – 9.2 kcal/mol) via hydrogen bond with Tyr69 residue in PAS and through several hydrophobic interactions with Trp278, Tyr333, Tyr120 and Asp71 in PAS and Phe329 in AS and Phe330 in AP (SM). It has been docked with BChE with BA – 8.2 kcal/mol via hydrogen bonding with Asp67 residues of PAS and with hydroxyl group of Thr117 (SM) and through hydrophobic interactions with amino acid residues of AS (Trp79) and CS (His433). The ADMET properties showed it can be crossed BBB and GIT and distributed into mitochondria (Table 3).
Cannabispiran is a cannabinoid found in cannabis (Pagani et al. 2011). In present study, it docked with AChE through hydrogen bond with Asp71 and Tyr69 and through hydrophobic interactions with Tyr333, Trp278 and Tyr120 residues of PAS and Phe329 residues of AS and Phe330 residues of AP (SM). Cannabispiran has a BA – 8.2 kcal/mol with BChE via hydrogen bonding with Asp67 residues of PAS and with hydroxyl group of Thr117 (SM) and through hydrophobic interactions with amino acid residues of AS (Trp79) and CS (His433). The ADMET properties showed cannabispiran can be crossed BBB and GIT and distributed into mitochondria (Table 3).
Cannabispirol is another cannabinoid found in cannabis (Pagani et al. 2011). In this study, cannabispirol has been docked with AChE with BA ( – 9.2 kcal/mol) through hydrogen bonding with Tyr120 and several hydrophobic interactions with amino acid residues of PAS (Tyr333), AS (Trp83 and Phe329), AP (Phe330) and CS (His439) and OH (Gly117; Fig. 2). In this line, cannabispirol docked with BChE with BA – 8.1 kcal/mol (Table 2) through hydrogen bonds with Trp79 of AS and through hydrophobic interactions with amino acid residues in PAS (Tyr329) and AS (Ala325 and Tyr125) and CS (His433; SM). Based on ADMET properties, it has ability to penetrate BBB and cross GIT while it weak HERG inhibitor (Table 3).
Cannabioxepane is a cannabinoid found in cannabis (Pagani et al. 2011). The present study showed cannabioxepane has been hydrophobically docked with AChE with BA of – 10.4 kcal/mol through Tyr333, Tyr69, Tyr120, Asp71 and Trp278 residues of PAS and Phe330, Phe287 residues of AP and Trp83 and Phe329 of AS and His439 of CS (SM). Cannabioxepane has BA ( – 9.8 kcal/mol) with BChE via hydrophobic interactions with amino acid residues of PAS (Tyr329 and Asp67) and AS (Ala325 and Trp79) and CS (His433; SM). ADMET properties of cannabioxepane showed that it can go through BBB and absorbed from GIT whilst it is a weak inhibitor of HERG (Table 3).
Isocannabispiradienone is a cannabinoid found in cannabis (Pagani et al. 2011). In this study, isocannabispiradienone docked with AChE with BA – 9.4 kcal/mol via hydrogen bonds with AS (Glu198) and with hydroxyl group of Asn84 and via hydrophobic interactions with PAS (Asp71) and CS (His439) and AS (Trp83 and Phe329). It has been docked with BChE (BA – 8.3 kcal/mol) via hydrogen bonds with PAS (Asp67 and Tyr329) and AS (Tyr125) and with two hydroxyl groups of Glu194 and Gly112 residues (SM). It can be crossed BBB and GIT and distributed into mitochondria (Table 3).
Conclusions
Resin of Cannabis sativa L. showed an inhibitory effect of 80.00 and 68.00% for AChE and BChE, respectively. The fraction of leaves of C. sativa L. showed an inhibitory effect of 52.33 and 49.00% for AChE and BCHE, respectively. In silico results comparable with experimental findings showed that and an array of phytochemicals found in C. sativa L. including all cannabinoids, beta-sitosterol, campesterol, apigenin, alpha-bisabolene, Δ8–THC and Δ9–THC, kaempferol, luteolin, stigmasterol, quercetin, alpha-guaiene, alpha-humulene and caryophyllene oxide have experimentally inhibitory effects for AChE and BChE and would be considered as candidates for designing antiinsectants and/or anti-AD drugs. In sum, more studies are welcomed to dig deeper mechanisms of anti-ChE properties of C. sativa L. and its byproducts like hashish.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgement
IK and NY conceptualized the idea of this paper and BAH and IK conceived experimental and computational works and wrote the initial draft, and IK and NY critically revised and prepared the final manuscript. This paper originated from MSc thesis of third author submitted to Department of Biology, Faculty of Science, Razi University 67149-67346, Kermanshah, Iran. This study was supported by intramural fund.
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali M, Muhammad S, Shah MR, Khan A, Rashid U, Farooq U, Ullah F, Sadiq A, Ayaz M, Ali M, Latif A, Ahmad M. Neurologically potent molecules from Crataegus oxyacantha; isolation, anticholinesterase inhibition, and molecular docking. Front Pharmacol. 2017;8:327. doi: 10.3389/fphar.2017.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajda M, Więckowska A, Hebda M, Guzior N, Sotriffer CA, Malawska B. Structure-based search for new inhibitors of cholinesterases. Int J Mol Sci. 2013;14(3):5608–5632. doi: 10.3390/ijms14035608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkis A, Tran K, Lee YZ, Ng K. Screening flavonoids for inhibition of acetylcholinesterase identified baicalein as the most potent inhibitor. J Agric Sci. 2015;7(9):26. [Google Scholar]
- Beard CM, Kokmen E, O’brien PC, Kurland LT. The prevalence of dementia is changing over time in Rochester, Minnesota. Neurology. 1995;45(1):75–79. doi: 10.1212/WNL.45.1.75. [DOI] [PubMed] [Google Scholar]
- Berman HM, Battistuz T, Bhat TN, Bluhm WF, Bourne PE, Burkhardt K, Fagan P. The protein data bank. Acta Crystallogr D Biol Crystallogr. 2002;58:899–907. doi: 10.1107/S0907444902003451. [DOI] [PubMed] [Google Scholar]
- Carod-Artal FJ. Psychoactive plants in ancient Greece. Neurosci History. 2013;1(1):28–38. [Google Scholar]
- Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Lee PW, Tang Y. Admetsar: a comprehensive source and free tool for assessment of chemical admet properties. J Chem Inf Model. 2012;52:3099–3105. doi: 10.1021/ci300367a. [DOI] [PubMed] [Google Scholar]
- Dallakyan S, Olson AJ. Small–molecule library screening by docking with PyRx. Methods Mol Biol. 2015;1263:243–450. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4(2):131. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- Ding X, Ouyang MA, Liu X, Wang RZ. Acetylcholinesterase inhibitory activities of flavonoids from the leaves of Ginkgo biloba against brown planthopper. J Chem. 2013 doi: 10.1155/2013/645086. [DOI] [Google Scholar]
- Dohi S, Terasaki M, Makino M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J Agric Food Chem. 2009;57(10):4313–4318. doi: 10.1021/jf804013j. [DOI] [PubMed] [Google Scholar]
- Eichler JERRY, Anselment A, Sussman JL, Massoulié JEAN, Silman ISRAEL. Differential effects of" peripheral" site ligands on Torpedo and chicken acetylcholinesterase. Mol Pharmacol. 1994;45(2):335–340. [PubMed] [Google Scholar]
- Ellman GL, Courtney DK, Andreas V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Elufioye TO, Obuotor EM, Agbedahunsi JM, Adesanya SA. Anticholinesterase constituents from the leaves of Spondias mombin L. (Anacardiaceae) Biol Targets Therapy. 2017;11:107. doi: 10.2147/BTT.S136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischedick JT, Hazekamp A, Erkelens T, Choi YH, Verpoorte R. Metabolic fingerprinting of Cannabis sativa L. cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 2010;71(17–18):2058–2073. doi: 10.1016/j.phytochem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Flores-Sanchez IJ, Verpoorte R. Secondary metabolism in cannabis. Phytochem Rev. 2008;7(3):615–639. doi: 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- Gitau WJ (2015) Evaluation of the composition, physico-chemical characteristics, surfactant and anti-microbial potential of Commiphora abyssinica gum resin (doctoral dissertation, University of Nairobi)
- Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: an important new target in Alzheimer's disease therapy. Int Psychogeriatr. 2002;14(S1):77–91. doi: 10.1017/S1041610203008676. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F, Russo EB (2006) Handbook of Cannabis Therapeutics: From Bench to Bedside; [a Compilation of Selected Articles from the Journal of Cannabis Therapeutics... from 2001 to 2004]. Haworth Press
- Gulluni N, Re T, Loiacono I, Lanzo G, Gori L, Macchi C, Epifani F, Bragazzi N, Firenzuoli F. Cannabis essential oil: a preliminary study for the evaluation of the brain effects. eCAM. 2018 doi: 10.1155/2018/1709182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsel, J. A., Eades, J., Hickory, B., Makriyannis, A. 2016. Cannabis sativa and Hemp. In Nutraceuticals (pp. 735–754).
- Houghton PJ, Ren Y, Howes MJ. Acetylcholinesterase inhibitors from plants and fungi. Natural Prod Rep. 2006;23(2):181–199. doi: 10.1039/b508966m. [DOI] [PubMed] [Google Scholar]
- Kendall D, Alexander S (2017) Cannabinoid Pharmacology (Vol. 80). Academic Press
- Klocke JA, Darlington MV, Balandrin MF. 1, 8-Cineole (Eucalyptol), a mosquito feeding and ovipositional repellent from volatile oil of Hemizonia fitchii (Asteraceae) J Chem Ecol. 1987;13(12):2131–2141. doi: 10.1007/BF01012562. [DOI] [PubMed] [Google Scholar]
- Kua J, Zhang Y, Eslami AC, Butler JR, McCammon JA. Studying the roles of W86, E202, and Y337 in binding of acetylcholine to acetylcholinesterase using a combined molecular dynamics and multiple docking approach. Protein Sci. 2003;12(12):2675–2684. doi: 10.1110/ps.03318603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Ahn YJ. Laboratory and simulated field bioassays to evaluate larvicidal activity of Pinus densiflora hydrodistillate, its constituents and structurally related compounds against Aedes albopictus, Aedes aegypti and Culex pipiens pallens in relation to their inhibitory effects on acetylcholinesterase activity. Insects. 2013;4:217–229. doi: 10.3390/insects4020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Liu XC, Chen XB, Liu QZ, Liu ZL. Chemical composition and insecticidal activities of the essential oil of Clinopodium chinense (Benth) Kuntze aerial parts against Liposcelis bostrychophila badonnel. J Food Prot. 2015;78(10):1870–1874. doi: 10.4315/0362-028X.JFP-15-089. [DOI] [PubMed] [Google Scholar]
- Loizzo MR, Tundis R, Menichini F, Menichini F. Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: an update. Curr Med Chem. 2008;15(12):1209–1228. doi: 10.2174/092986708784310422. [DOI] [PubMed] [Google Scholar]
- Lomarat P, Sripha K, Phanthong P, Kitphati W, Thirapanmethee K, Bunyapraphatsara N (2015) In vitro biological activities of black pepper essential oil and its major components relevant to the prevention of Alzheimer’s disease. Thai J Pharm Sci 39(3)
- McPartland JM. Cannabis as repellent and pesticide. J Int Hemp Assoc. 1997;4(2):89–94. [Google Scholar]
- Miyazawa M, Yamafuji C. Inhibition of acetylcholinesterase activity by tea tree oil and constituent terpenoids. Flavour Fragrance J. 2006;21(2):198–201. doi: 10.1002/ffj.1580. [DOI] [Google Scholar]
- Mogana R, Adhikari A, Debnath S, Hazra S, Hazra B, Teng-Jin K, Wiart C. 2014. Biomed Research International: The antiacetylcholinesterase and antileishmanial activities of Canarium patentinervium Miq; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montserrat-de la Paz S, Marín-Aguilar F, García-Gimenez MD, Fernández-Arche MA. Hemp (Cannabis sativa L.) seed oil: analytical and phytochemical characterization of the unsaponifiable fraction. J Agric Food Chem. 2014;62(5):1105–1110. doi: 10.1021/jf404278q. [DOI] [PubMed] [Google Scholar]
- Murugesan S, Rajeshkannan C, Suresh Babu D, Sumathi R, Manivachakam P. Identification insecticidal properties in common weed-Lantana camara Linn. by gas chromatography and mass spectrum (GC-MS-MS) Adv Appl Sci Res. 2012;3(5):2754–2759. [Google Scholar]
- Omri AE, Han J, Kawada K, Abdrabbah MB, Isoda H. Luteolin enhances cholinergic activities in PC12 cells through ERK1/2 and PI3K/Akt pathways. Brain Res. 2012;1437:16–25. doi: 10.1016/j.brainres.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Onyenekwe PC, Stahl M, Adejo G. Post-irradiation changes of the volatile oil constituents of Monodora myristica (Gaertn) Dunal. Nat Prod Res. 2012;26(21):2030–2034. doi: 10.1080/14786419.2011.631137. [DOI] [PubMed] [Google Scholar]
- Ordentlich A, Barak D, Kronman C, Ariel N, Segall Y, Velan B, Shafferman A. Functional characteristics of the oxyanion hole in human acetylcholinesterase. J Biol Chem. 1998;273(31):19509–19517. doi: 10.1074/jbc.273.31.19509. [DOI] [PubMed] [Google Scholar]
- Owokotomo IA, Ekundayo O, Abayomi TG, Chukwuka AV. In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol Rep. 2015;2:850–857. doi: 10.1016/j.toxrep.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani A, Scala F, Chianese G, Grassi G, Appendino G, Taglialatela-Scafati O. Cannabioxepane, a novel tetracyclic cannabinoid from hemp, Cannabis sativa L. Tetrahedron. 2011;67(19):3369–3373. doi: 10.1016/j.tet.2011.03.062. [DOI] [Google Scholar]
- Radic Z, Gibney G, Kawamoto S, MacPhee-Quigley K, Bongiorno C, Taylor P. Expression of recombinant acetylcholinesterase in a baculovirus system: kinetic properties of glutamate 199 mutants. Biochemistry. 1992;31(40):9760–9767. doi: 10.1021/bi00155a032. [DOI] [PubMed] [Google Scholar]
- Rothschild M, Bergström G, Wängberg SÅ. Cannabis sativa: volatile compounds from pollen and entire male and female plants of two variants, Northern Lights and Hawaian Indica. Bot J Linn Soc. 2005;147(4):387–397. doi: 10.1111/j.1095-8339.2005.00417.x. [DOI] [Google Scholar]
- Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers. 2007;4(8):1614–1648. doi: 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- Ryz NR, Remillard DJ, Russo EB. Cannabis roots: a traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017;2(1):210–216. doi: 10.1089/can.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster D, Spetea M, Music M, Rief S, Fink M, Kirchmair J, Schütz J, Wolber G, Langer T, Stuppner H, Rollinger JM, Schmidhammer H. Morphinans and isoquinolines: acetylcholinesterase inhibition, pharmacophore modeling, and interaction with opioid receptors. Bioorg Med Chem. 2010;18(14):5071–5080. doi: 10.1016/j.bmc.2010.05.071. [DOI] [PubMed] [Google Scholar]
- Sfara V, Zerba EN, Alzogaray RA. Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus. J Med Entomol. 2009;46(3):511–515. doi: 10.1603/033.046.0315. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan B, Malar DS, Sathya S, Devi KP. Antiaggregation potential of Padina gymnospora against the toxic Alzheimer’s beta-amyloid peptide 25–35 and cholinesterase inhibitory property of its bioactive compounds. PLoS ONE. 2015;10(11):e0141708. doi: 10.1371/journal.pone.0141708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Shah I, Ali N, Shah SWA. In vitro acetylcholinesterase and butyrylcholinesterase inhibitory potentials of essential oil of Artemisia macrocephala. Bangladesh J Pharmacol. 2015;10:87–91. [Google Scholar]
- Soreq H, Zakut H. Human Cholinesterases and Anticholinesterases. New York: Academic Press; 1993. [Google Scholar]
- Sriraksa N, Wattanathorn J, Muchimapura S, Tiamkao S, Brown K, Chaisiwamongkol K (2012) Cognitive-enhancing effect of quercetin in a rat model of Parkinson's disease induced by 6-hydroxydopamine. Evidence Based Complementary and Alternative Medicine
- Suárez D, Field MJ. Molecular dynamics simulations of human butyrylcholinesterase. Proteins Struct Func Bioinform. 2005;59(1):104–117. doi: 10.1002/prot.20398. [DOI] [PubMed] [Google Scholar]
- Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Taylor P, Radic Z. The cholinesterases: from genes to proteins. Ann Rev Pharmacol Toxicol. 1994;34(1):281–320. doi: 10.1146/annurev.pa.34.040194.001433. [DOI] [PubMed] [Google Scholar]
- Teall EK. Medicine and doctoring in ancient Mesopotamia. Grand Valley J History. 2014;3(1):2. [Google Scholar]
- Thomsen R, Christensen MH. Mol Dock: a new technique for high–accuracy molecular docking. J Med Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L XVII A review of the natural constituents. J Nat Prod. 1980;43(2):169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- Vellom DC, Radic Z, Li Y, Pickering NA, Camp S, Taylor P. Amino acid residues controlling acetylcholinesterase and butyrylcholinesterase specificity. Biochemistry. 1993;32(1):12–17. doi: 10.1021/bi00052a003. [DOI] [PubMed] [Google Scholar]
- Wang ZT. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of β-carboline and quinoline alkaloids derivatives from the plants of genus Peganum. J Chem. 2013 doi: 10.1155/2013/717232. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.