Abstract
Background
Adverse mental health consequences of COVID-19, including anxiety and depression, have been widely predicted but not yet accurately measured. There are a range of physical health risk factors for COVID-19, but it is not known if there are also psychiatric risk factors. In this electronic health record network cohort study using data from 69 million individuals, 62 354 of whom had a diagnosis of COVID-19, we assessed whether a diagnosis of COVID-19 (compared with other health events) was associated with increased rates of subsequent psychiatric diagnoses, and whether patients with a history of psychiatric illness are at a higher risk of being diagnosed with COVID-19.
Methods
We used the TriNetX Analytics Network, a global federated network that captures anonymised data from electronic health records in 54 health-care organisations in the USA, totalling 69·8 million patients. TriNetX included 62 354 patients diagnosed with COVID-19 between Jan 20, and Aug 1, 2020. We created cohorts of patients who had been diagnosed with COVID-19 or a range of other health events. We used propensity score matching to control for confounding by risk factors for COVID-19 and for severity of illness. We measured the incidence of and hazard ratios (HRs) for psychiatric disorders, dementia, and insomnia, during the first 14 to 90 days after a diagnosis of COVID-19.
Findings
In patients with no previous psychiatric history, a diagnosis of COVID-19 was associated with increased incidence of a first psychiatric diagnosis in the following 14 to 90 days compared with six other health events (HR 2·1, 95% CI 1·8–2·5 vs influenza; 1·7, 1·5–1·9 vs other respiratory tract infections; 1·6, 1·4–1·9 vs skin infection; 1·6, 1·3–1·9 vs cholelithiasis; 2·2, 1·9–2·6 vs urolithiasis, and 2·1, 1·9–2·5 vs fracture of a large bone; all p<0·0001). The HR was greatest for anxiety disorders, insomnia, and dementia. We observed similar findings, although with smaller HRs, when relapses and new diagnoses were measured. The incidence of any psychiatric diagnosis in the 14 to 90 days after COVID-19 diagnosis was 18·1% (95% CI 17·6–18·6), including 5·8% (5·2–6·4) that were a first diagnosis. The incidence of a first diagnosis of dementia in the 14 to 90 days after COVID-19 diagnosis was 1·6% (95% CI 1·2–2·1) in people older than 65 years. A psychiatric diagnosis in the previous year was associated with a higher incidence of COVID-19 diagnosis (relative risk 1·65, 95% CI 1·59–1·71; p<0·0001). This risk was independent of known physical health risk factors for COVID-19, but we cannot exclude possible residual confounding by socioeconomic factors.
Interpretation
Survivors of COVID-19 appear to be at increased risk of psychiatric sequelae, and a psychiatric diagnosis might be an independent risk factor for COVID-19. Although preliminary, our findings have implications for clinical services, and prospective cohort studies are warranted.
Funding
National Institute for Health Research.
Introduction
From the early stages of the COVID-19 pandemic, concerns have been raised about its effect on mental health1, 2, 3 and on patients with mental illness.4 Yet several months later, we still know little about the mental health consequences of COVID-19 (its psychiatric sequelae) and the susceptibility of patients with mental illness to COVID-19 (its psychiatric antecedents).
Several surveys have suggested that patients with COVID-19 have symptoms of anxiety5, 6, 7, 8 (including post-traumatic stress disorder7, 8), depression,5, 6, 9 and insomnia.6 Cross-sectionally, 22·5% of patients with COVID-19 had a concurrent neuropsychiatric diagnosis.10 CORONERVE, a UK-wide surveillance programme, identified 23 patients with a psychiatric diagnosis following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).11 A meta-analysis of pooled data from studies that estimated the incidence of psychiatric disorders after the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome outbreaks suggested that coronavirus infections can lead to delirium, anxiety, depression, manic symptoms, poor memory, and insomnia.12 However, cohort studies of patients with COVID-19 with adequate control groups and follow-up are urgently needed to quantify the incidence and relative risks of psychiatric sequelae after infection.
Research in context.
Evidence before this study
From Jan 1, to Aug 1, 2020, we searched PubMed with the terms (COVID-19 OR SARS-CoV2 OR SARS-CoV-2) AND (psych* OR cognit* OR mental) and medRxiv with the terms COVID-19 OR SARS-CoV2 OR SARS-CoV-2 in the neurology and/or psychiatry categories, for studies published in English. We also manually reviewed the reference lists in the identified papers. In general, studies investigating the psychiatric consequences of COVID-19 did not have a control condition, consisted mostly of surveys, and used self-reported symptoms (rather than diagnoses) as an outcome. To our knowledge, no study has assessed the risk of developing psychiatric sequelae over time and only anecdotal evidence exists for the risk of dementia as a potential consequence of COVID-19. In terms of psychiatric risk factors for COVID-19, we identified two case-control studies. One study investigated risk factors for admission to hospital with (rather than diagnosis of) COVID-19. The other study used historical data (not acquired during the same period as COVID-19) as a control group. As these were case-control studies, only odds ratios could be estimated rather than relative or absolute risks. Additionally, in both studies, controls were not well matched to cases. Other surveys (such as the Australian COLLATE and the UK Household Longitudinal Study) have investigated the mental health challenges resulting from the COVID-19 pandemic rather than the COVID-19 illness.
Added value of this study
To our knowledge, this is the first dataset allowing the psychiatric sequelae and antecedents of COVID-19 to be measured reliably in terms of clinical diagnoses. The study cohorts are substantially larger than previous studies, producing more precise, representative estimates of even small but important effects, such as the incidence of dementia. The study uses propensity score matching to control for many variables, including established physical risk factors for COVID-19 and for more severe COVID-19 illness, and uses large-scale real-world data, thus providing more clinically relevant findings. We used time-to-event data for analysis of psychiatric sequelae, thus providing evidence for their temporal evolution. Our findings show that COVID-19 survivors have significantly higher rates of psychiatric diagnoses and psychiatric history is a potential risk factor for being diagnosed with COVID-19, independent of known physical risk factors.
Implications of all the available evidence
Prospective cohort studies and longer-term follow-up studies are urgently needed to support and extend the findings of our study. Furthermore, enhanced psychiatric follow-up should be considered for patients who survive COVID-19. Finally, psychiatric history should be queried during assessment of a patient presenting with COVID-19 symptoms to adjust pre-test probability.
A separate question is whether pre-existing psychiatric disorder affects susceptibility to SARS-CoV-2 infection, as has been reported for some other infections including pneumonia.13 A large case-control study based on electronic health records of patients in the USA found that the odds of being diagnosed with COVID-19 were higher for patients with attention deficit hyperactivity disorder, bipolar disorder, depression, and schizophrenia.14 However, a Korean study found no such associations, except for patients with schizophrenia.15 Reliable estimation of a possible increased risk of COVID-19 among patients with psychiatric illness requires large, well-controlled cohort studies.
In this electronic health record network cohort study using data from 69 million individuals, 62 354 of whom have had a diagnosis of COVID-19, we assessed whether a diagnosis of COVID-19 was associated with increased rates of subsequent psychiatric diagnoses, and whether patients with a history of psychiatric illness are at a higher risk of being diagnosed with COVID-19.
Methods
Data and study design
We used the TriNetX Analytics Network, a global federated network that captures anonymised data from electronic health records in 54 health-care organisations in the USA, totalling 69·8 million patients. The TriNetX platform and its functionalities have been described elsewhere,16 and more details are provided in the appendix (pp 1–2). Available data include demographics, diagnoses (using ICD-10 codes), procedures, medications, and measurements (eg, laboratory test results and body-mass index). The health-care organisations are a mixture of hospitals, primary care, and specialist providers and contribute data from insured and uninsured patients alike. 41 (60%) of the health-care organisations have both inpatient and outpatient data. The data from a typical health-care organisation generally go back around 7 years, with some going back 13 years. The data are continuously updated; health-care organisations update their data at various times, with 51 (94%) of 54 health-care organisations refreshing every 1, 2, or 4 weeks. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating health-care organisations and their individual contribution to each dataset are not disclosed.
By use of the TriNetX user interface, cohorts can be created based on specified inclusion and exclusion criteria and matched for confounding variables using the built-in propensity score matching capability. Outcomes of interest are then compared between cohorts over defined time periods. This study followed the RECORD reporting guidelines.
Variables of interest and their coding
We defined a diagnosis of COVID-19 as one of the following, recorded on or after Jan 20, 2020 (date of the first recorded COVID-19 case in the USA): COVID-19 (U07.1 and U07.2); pneumonia due to SARS-associated coronavirus (J12.81); other coronavirus as the cause of disease classified elsewhere (B97.29); or coronavirus infection unspecified (B34.2). The latter three definitions (which make up 4533 [7·3%] of the total COVID-19 sample) were included to capture the early stage of the pandemic when the ICD code for COVID-19 (U07) was not yet defined. We defined a psychiatric illness as any of the ICD-10 codes F20–F48, comprising psychotic (F20–F29), mood (F30–F39), and anxiety (F40–F48) disorders.
We identified a set of established and suspected risk factors for COVID-19, as follows:17, 18, 19 age, sex, race, obesity, hypertension, diabetes, chronic kidney disease, asthma, chronic lower respiratory diseases, nicotine dependence, ischaemic heart disease, and other forms of heart disease. To capture these risk factors in patient electronic health records, we used 28 variables (eg, diabetes was separated into type 1 and type 2 and hypertension was represented both as a diagnosis and a measurement of systolic and diastolic blood pressure). We also identified an additional set of established risk factors for death due to COVID-1920 (which we take to be risk factors for severe forms of COVID-19 illness), as follows: cancer (particularly haematological cancer), chronic liver disease, stroke, dementia, organ transplantation, rheumatoid arthritis, lupus, psoriasis, and other immunosuppression. These risk factors were captured using 22 variables from patient electronic health records. Further details are provided in the appendix (pp 2–3).
Analysis of psychiatric sequelae
To assess the psychiatric sequelae of COVID-19, we produced matched cohorts of patients who had been diagnosed with another health event. The other health events were selected to represent a range of common acute presentations (some clinically similar to COVID-19 and others very different). These control health events comprised influenza, another respiratory tract infection, skin infection, cholelithiasis, urolithiasis, and fracture of a large bone (appendix p 3).
All seven cohorts (COVID-19 and six control health events) included all patients older than 10 years who had the corresponding health event on or after Jan 20, 2020. This age threshold was recommended by TriNetX for COVID-19 cohorts defined within the network to make results consistent across studies. We excluded patients who had died by the time of the analyses (Aug 1, 2020). In the primary analysis, we also excluded patients who had a psychiatric diagnosis recorded before the health event (COVID-19 or control health event). Cohorts were matched for 50 variables—28 variables capturing risk factors for COVID-19 and 22 variables capturing risk factors for more severe COVID-19 illness.
The primary outcome was the incidence of a first psychiatric diagnosis, over a period from 14 days to 90 days after a diagnosis of COVID-19, represented by hazard ratio (HR) and the estimated probability of outcome over that period. We also assessed dementia and insomnia (appendix p 4), as they are potential sequelae of COVID-19.6, 12 For dementia, the analysis was repeated among patients older than 65 years. Finally, we measured the incidence of all F20–F48 diagnoses over the same period (ie, recurrences as well as first episodes).
We did a range of sensitivity analyses to test the robustness of the findings, and to aid their interpretation (appendix pp 4–7). We repeated the analysis in seven scenarios, as follows: excluding individuals whose race was unknown (in case this differentially affected cohorts), adjusting for the ICD-10 code Z59 (problems related to housing and economic circumstances; as a proxy for extreme socioeconomic deprivation), restricting the diagnosis of COVID-19 to confirmed diagnoses (ICD-10 code U07.1), further restricting the diagnosis of COVID-19 to cases confirmed using RNA or antigen testing, focusing on patients who made at least one health-care visit between 14 and 90 days after the health event (in case of differential dropout rates between cohorts), comparing the rate of psychiatric sequelae of control health conditions to the rate observed before the COVID-19 pandemic, and using unmatched cohorts.
Besides the explanation that COVID-19 itself leads to increased rates of psychiatric sequelae, we tested two alternative hypotheses that could explain differences in outcomes between cohorts. The severity hypothesis posits that differences in rates of psychiatric sequelae are due to differences in the severity of the health event (eg, COVID-19 might lead to more severe presentations than the control health events). We tested this hypothesis by limiting the cohorts to patients with the least severe presentations (taken to be those not requiring inpatient admission). If the hypothesis was correct, the difference in rates of psychiatric sequelae between these cohorts would be substantially smaller than in the original cohorts. The contextual factors hypothesis posits that COVID-19 was mostly diagnosed at a time when having any health event would have increased the risk of psychiatric sequelae (eg, because of overwhelmed health services, fear of COVID-19, and little social support). Assuming that these contextual factors might have changed substantially between January, and April 2020, we tested this hypothesis by comparing the rate of psychiatric sequelae of health events before versus after April 1, 2020, and by comparing the rate of psychiatric sequelae between COVID-19 and control health events after April 1, 2020 (appendix p 6).
Analysis of psychiatric antecedents
We tested whether patients with a psychiatric diagnosis were at a higher risk of developing COVID-19 compared with a matched cohort of patients with otherwise similar risk factors for COVID-19. We defined two cohorts. The first cohort included all patients older than 18 years who had a diagnosis of a psychiatric illness recorded in their electronic health records in the previous year (from Jan 21, 2019, to Jan 20, 2020). The second cohort had no psychiatric illness recorded in their electronic health records but did make a health-care visit in the same period (thus excluding patients who made no contact with the participating health-care organisations). We also defined separate cohorts for the three main classes of psychiatric illness (psychotic disorders [F20–F29], mood disorders [F30–F39], and anxiety disorders [F40–F48]). Patients who died before Jan 20, 2020, were excluded from both cohorts.
Cohorts were matched for the 28 variables capturing risk factors for COVID-19. The primary outcome was the relative risk (RR) of being diagnosed with COVID-19 between matched cohorts. The robustness of the findings was tested by repeating the analysis in the following six scenarios: limiting the cohorts to those with none of the physical risk factors for COVID-19; extending the window for a psychiatric diagnosis from 1 to 3 years before Jan 20, 2020; limiting the cohort to patients with a first diagnosis of psychiatric illness (ie, with no diagnosis present before Jan 21, 2019; excluding patients with unknown race; adjusting for problems related to housing and economic circumstances using the ICD-10 code Z59; and redefining the primary outcome as a confirmed (U07.1) COVID-19 diagnosis.
Further details on the sensitivity analyses are provided in the appendix (pp 4–7).
Statistical analysis
We used propensity score matching to create cohorts with matched baseline characteristics.21 The propensity score was calculated using logistic regression implemented by the function LogisticRegression of the scikit-learn package in Python version 3.7. Propensity score 1:1 matching used a greedy nearest neighbour matching approach, with a caliper distance of 0·1 pooled SDs of the logit of the propensity score (appendix p 7). To eliminate the influence of ordering of records, the order of the records in the covariate matrix was randomised before matching. Any baseline characteristic with a standardised mean difference between cohorts lower than 0·1 is considered well matched.22 For analysis of psychiatric sequelae, propensity score matching was directly applied to each cohort pair. For analysis of psychiatric antecedents, given their much larger sample sizes (which exceeded the maximum number of 1·5 million patients possible per matched cohort), cohorts were first stratified by sex and age (18–30 years, 31–45 years, 46–60 years, 61–75 years, and ≥76 years) and propensity score matching (including for age) was achieved within each stratum separately.
For psychiatric sequelae, Kaplan-Meier analysis was done to estimate the probability of outcomes from 14 days to 90 days. Comparisons between cohorts were made using a log-rank test. The HR was calculated using a proportional hazard model (with the survival package 3.2.3 in R), wherein the cohort to which the patient belonged was used as the independent variable. The proportional hazard assumption was tested using the generalized Schoenfeld approach.23 If the assumption was violated, a piecewise constant HR was estimated by calculating a separate HR for the early and late phases of the follow-up period and the assumption was tested again in each subperiod (appendix p 7).
In the analysis of psychiatric antecedents, the RR of being diagnosed with COVID-19 was calculated for each stratum and for the whole cohort. The null hypothesis that the outcome rate was equal in the two cohorts was tested using a χ2 test. Logistic regression was used to test for a potential association between age and RR (appendix p 8).
Statistical analyses were done in R version 3.4.3 except for the log-rank tests, which were done within TriNetX. Statistical significance was set at two-sided p<0·05.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. MT and PJH had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
62 354 patients had a diagnosis of COVID-19 (table 1 ; appendix p 9). For the analysis of psychiatric sequelae, a subset of 44 779 patients who had no previous psychiatric illness and who were alive was used as the COVID-19 cohort. Successful matching was achieved between this cohort and cohorts with other acute health events (appendix pp 9–20). For the analysis of psychiatric antecedents, a cohort of 1 729 837 patients with a psychiatric diagnosis between Jan 21, 2019, and Jan 20, 2020, was defined and successfully matched with a cohort of 1 729 837 patients who never had a psychiatric diagnosis (appendix p 21).
Table 1.
Baseline characteristics of the 62 354 COVID-19 cases
| Patients | |||
|---|---|---|---|
| Diagnosis of COVID-19 | 62 354 (100%) | ||
| Of which confirmed diagnosis | 57 476 (92·2%) | ||
| Age, years | 49·3 (19·7) | ||
| Sex | |||
| Female | 34 564 (55·4%) | ||
| Male | 27 525 (45·1%) | ||
| Other | 265 (0·4%) | ||
| Race | |||
| White | 31 789 (51·0%) | ||
| Black or African American | 14 700 (23·6%) | ||
| Asian | 1554 (2·5%) | ||
| American Indian or Alaska Native | 329 (0·5%) | ||
| Native Hawaiian or Other Pacific Islander | 107 (0·2%) | ||
| Unknown | 13 875 (22·3%) | ||
| Geographical region of the USA | |||
| Northeast | 22 817 (36·6%) | ||
| Midwest | 7908 (12·6%) | ||
| South | 19 643 (31·%) | ||
| West | 9719 (15·6) | ||
| Other or unknown | 2267 (3·6%) | ||
| Obesity | |||
| Overweight and obesity | 12 249 (19·6%) | ||
| Body-mass index, kg/m2 | |||
| Participants with data | 23 728 (38·1%) | ||
| Mean (SD) | 28·1 (8·2) | ||
| Hypertension | |||
| Systolic blood pressure, mm Hg | |||
| Participants with data | 41 011 (65·8%) | ||
| Mean (SD) | 128 (20·6) | ||
| Diastolic blood pressure, mm Hg | |||
| Participants with data | 41 009 (65·8%) | ||
| Mean (SD) | 76·9 (13·1) | ||
| Hypertensive diseases | 21 228 (34·0%) | ||
| Diabetes | |||
| Type 1 | 1535 (2·5%) | ||
| Type 2 | 10 998 (17·6%) | ||
| Chronic lower respiratory diseases | |||
| Bronchitis; not specified as acute or chronic | 3125 (5·0%) | ||
| Simple and mucopurulent chronic bronchitis | 329 (0·5%) | ||
| Unspecified chronic bronchitis | 388 (0·6%) | ||
| Emphysema | 1211 (1·9%) | ||
| Other chronic obstructive pulmonary disease | 3582 (5·7%) | ||
| Asthma | 7101 (11·4%) | ||
| Bronchiectasis | 384 (0·6%) | ||
| Nicotine dependence | 4579 (7·3%) | ||
| Heart diseases | |||
| Ischaemic heart diseases | 6579 (10·6%) | ||
| Other forms of heart disease | 12 633 (20·3%) | ||
| Chronic kidney diseases | |||
| Chronic kidney disease | 5554 (8·9%) | ||
| Hypertensive chronic kidney disease | 2890 (4·6%) | ||
| Chronic liver diseases | |||
| Alcoholic liver disease | 351 (0·6%) | ||
| Hepatic failure; not elsewhere classified | 502 (0·8%) | ||
| Chronic hepatitis; not elsewhere classified | 83 (0·1%) | ||
| Fibrosis and cirrhosis of liver | 775 (1·2%) | ||
| Fatty (change of) liver; not elsewhere classified | 2152 (3·5%) | ||
| Chronic passive congestion of liver | 388 (0·6%) | ||
| Portal hypertension | 322 (0·5%) | ||
| Other specified diseases of liver | 1502 (2·4%) | ||
| Cerebral infarction | 1910 (3·1%) | ||
| Dementia | |||
| Vascular dementia | 558 (0·9%) | ||
| Dementia in other diseases classified elsewhere | 740 (1·2%) | ||
| Unspecified dementia | 1794 (2·9%) | ||
| Alzheimer's disease | 672 (1·1%) | ||
| Neoplasms | |||
| Neoplasms | 12 655 (20·3%) | ||
| Malignant neoplasms of lymphoid; haematopoietic and related tissue | 836 (1·3%) | ||
| Organ transplant | |||
| Renal transplantation procedures | 137 (0·2%) | ||
| Liver transplantation procedures | 44 (0·1%) | ||
| Psoriasis | 669 (1·1%) | ||
| Rheumatoid arthritis | |||
| Rheumatoid arthritis with rheumatoid factor | 301 (0·5%) | ||
| Other rheumatoid arthritis | 982 (1·6%) | ||
| Systemic lupus erythematosus | 414 (0·7%) | ||
| Disorders involving the immune mechanism | 1532 (2·5%) | ||
| Psychiatric diagnoses | |||
| Psychiatric illness (F20–F48) | 15 980 (25·6%) | ||
| Psychotic disorder (F20–F29) | 1219 (2·0%) | ||
| Mood disorder (F30–F39) | 9921 (15·9%) | ||
| Anxiety disorder (F40–F48) | 12 145 (19·5%) | ||
Data are n (%) or mean (SD).
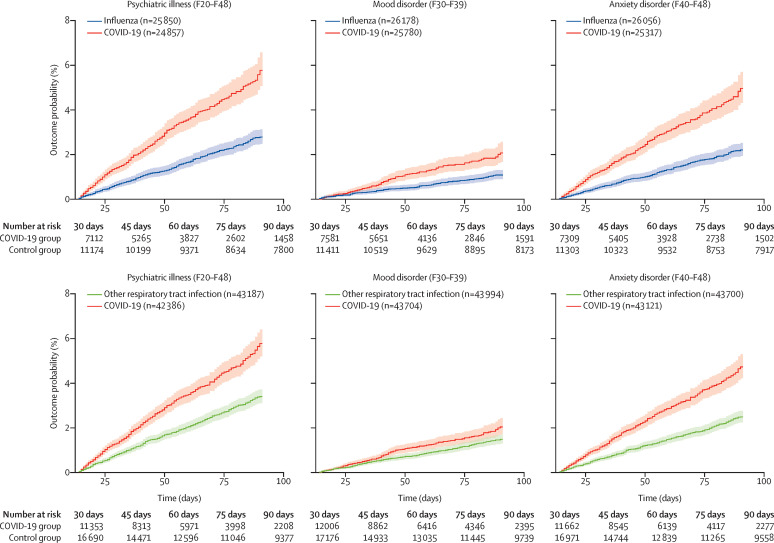
The estimated probabilities of psychiatric sequelae during the first 14 to 90 days after COVID-19 diagnosis and other control health events are presented in table 2 (corresponding HRs are reported in the appendix p 22). A diagnosis of COVID-19 led to more first diagnoses of psychiatric illness compared with all six control health events (HRs between 1·58 and 2·24, all p<0·0001; figure 1 ; appendix p 22). At 90 days, the estimated probability of having been newly diagnosed with a psychiatric illness after COVID-19 diagnosis was 5·8% (95% CI 5·2–6·4). The proportional hazard assumption was valid for three of six control health events (influenza, other respiratory tract infection, and urolithiasis). For the other three events (skin infection, cholelithiasis, and fracture), we observed evidence of non-proportionality and the HR tended to increase over time (appendix p 24). However, the HR remained significantly greater than 1 for both the early and late phases of the follow-up period (all p<0·0001, except for cholelithiasis in the early phase p=0·0044). The most frequent psychiatric diagnosis after COVID-19 diagnosis was anxiety disorder (HRs 1·59–2·62, all p<0·0001), with a probability of outcome within 90 days of 4·7% (95% CI 4·2–5·3). Among anxiety disorders, adjustment disorder, generalised anxiety disorder, and, to a lesser extent, post-traumatic stress disorder and panic disorder were the most frequent (appendix pp 25–26).
Table 2.
Estimated incidence of first psychiatric diagnoses during the first 14 to 90 days after a diagnosis of COVID-19 compared with other health events
|
COVID-19 |
Influenza in matched cohort (n=26 497) |
Other respiratory tract infection in matched cohort (n=44 775) |
Skin infection in matched cohort (n=38 977) |
Cholelithiasis in matched cohort (n=19 733) |
Urolithiasis in matched cohort (n=28 827) |
Fracture in matched cohort (n=37 841) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | p value | % (95% CI) | p value | % (95% CI) | p value | % (95% CI) | p value | % (95% CI) | p value | % (95% CI) | p value | ||
| Psychiatric illness | 5·8 (5·2–6·4) | 2·8 (2·5–3·1) | <0·0001 | 3·4 (3·1–3·7) | <0·0001 | 3·3 (3–3·7) | <0·0001 | 3·2 (2·8–3·7) | <0·0001 | 2·5 (2·2–2·8) | <0·0001 | 2·5 (2·2–2·7) | <0·0001 | |
| Psychotic disorder | 0·1 (0·08–0·2) | 0·04 (0·01–0·10) | 0·019 | 0·1 (0·06–0·16) | 0·23 | 0·15 (0·096–0·24) | 0·83 | 0·11 (0·054–0·24) | 0·21 | 0·044 (0·016–0·12) | 0·0051 | 0·16 (0·11–0·24) | 0·77 | |
| Mood disorder | 2·0 (1·7–2·4) | 1·1 (0·9–1·3) | <0·0001 | 1·5 (1·3–1·7) | 0·0054 | 1·7 (1·5–1·9) | 0·55 | 1·6 (1·3–1·9) | 0·14 | 1·2 (1–1·4) | 0·00011 | 1·4 (1·2–1·6) | 0·0050 | |
| Anxiety disorder | 4·7 (4·2–5·3) | 2·2 (1·9–2·5) | <0·0001 | 2·5 (2·2–2·8) | <0·0001 | 2·4 (2·1–2·7) | <0·0001 | 2·6 (2·2–3) | <0·0001 | 1·8 (1·6–2·1) | <0·0001 | 1·6 (1·4–1·8) | <0·0001 | |
| Insomnia | 1·9 (1·6–2·2) | 0·6 (0·5–0·8) | <0·0001 | 0·8 (0·7–1·0) | <0·0001 | 0·89 (0·73–1·1) | <0·0001 | 1·1 (0·88–1·4) | <0·0001 | 0·57 (0·43–0·74) | <0·0001 | 0·7 (0·57–0·85) | <0·0001 | |
| Dementia in all participants | 0·44 (0·33–0·60) | 0·11 (0·06–0·20) | 0·00044 | 0·25 (0·18–0·35) | 0·00063 | 0·28 (0·20–0·39) | 0·13 | 0·24 (0·14–0·38) | <0·0001 | 0·16 (0·09–0·28) | <0·0001 | 0·34 (0·25–0·44) | 0·14 | |
| Dementia (among those ≥65 years) | 1·6 (1·2–2·1) | 0·66 (0·41–1·1) | 0·0043 | 0·84 (0·61–1·1) | 0·00071 | 0·70 (0·49–1·0) | 0·00069 | 0·58 (0·36–0·94) | <0·0001 | 0·60 (0·38–0·95) | <0·0001 | 0·94 (0·68–1·3) | 0·0036 | |
p values obtained using a log-rank test. A breakdown of the results for different diagnoses of the anxiety disorders and mood disorders categories is provided in the appendix (pp 26–27).
Figure 1.
Kaplan-Meier curves for onset of first psychiatric diagnoses after COVID-19 diagnosis compared with influenza and other respiratory tract infections
Curves for the other control health events are presented in the appendix (p 23)). Shaded areas represent 95% CIs. The number of subjects within each cohort corresponds to all those who did not have the outcome before the follow-up period.
The probability of a first diagnosis of mood disorder within 14 to 90 days after COVID-19 diagnosis was 2% (95% CI 1·7–2·4). The corresponding hazard rate was significantly higher than that after a diagnosis of influenza (HR 1·79, 95% CI 1·37–2·33; p<0·0001), another respiratory tract infection (1·33, 1·09–1·63; p=0·0054), urolithiasis (1·62, 95% CI 1·26–2·07; p=0·00011), or a fracture (1·35, 1·094–1·67; p=0·0050), but similar to that after a diagnosis of skin infection (1·07, 0·87–1·31; p=0·55) or cholelithiasis (1·22, 0·93–1·59; p=0·14). Depressive episode was the most common first diagnosis of mood disorder (1·7%, 95% CI 1·4–2·1; appendix p 27).
We found a low probability of being newly diagnosed with a psychotic disorder in the 14 to 90 days after COVID-19 diagnosis (0·1%, 95% CI 0·08–0·2), broadly similar to the probability after control health events (table 2). The probability of a first diagnosis of insomnia in the 14 to 90 days after COVID-19 diagnosis was 1·9% (95% CI 1·6–2·2), more common than after control health events (HRs 1·85–3·29, all p<0·0001). Around 60% of the insomnia diagnoses were not accompanied by a concurrent diagnosis of an anxiety disorder (appendix p 27). The probability of being diagnosed with dementia was increased after a diagnosis of COVID-19 compared with all control health events (table 2; appendix p 28); among patients older than 65 years the risk was 1·6% (95% CI 1·2–2·1), with an HR between 1·89 and 3·18 (table 2).
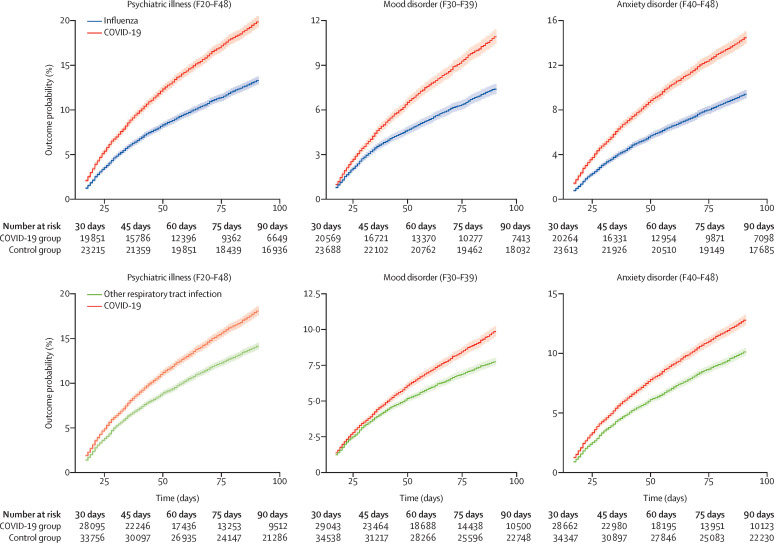
We found that the rate of all diagnoses of psychiatric disorders (ie, including relapses) was higher after COVID-19 diagnosis than after control health events (figure 2 ; table 3 ; appendix p 29). The estimated probability of having been diagnosed with any psychiatric illness in the 14 to 90 days after COVID-19 diagnosis was 18·1% (95% CI 17·6–18·6), significantly higher than for all control health events (HRs 1·24–1·49, all p<0·0001). The most common psychiatric diagnosis after COVID-19 diagnosis was anxiety disorder (12·8%, 95% CI 12·4–13·3), followed by mood disorders (9·9%, 9·5–10·3). Both these rates were higher than those for all control health events (HRs 1·24–1·60 for anxiety disorders, and HRs 1·12–1·44 for mood disorders, all p<0·0001). The rate of first or relapsed psychotic disorder diagnosis after COVID-19 diagnosis was 0·9% (95% CI 0·8–1·1), substantially higher than that for all control health events (HRs 1·20–2·16, all p<0·05, except for skin infection p=0·44).
Figure 2.
Kaplan-Meier curves for any (first or recurrent) psychiatric diagnoses after COVID-19 compared with influenza and other respiratory tract infections
Curves for the other control health events are presented in the appendix (p 29)). Shaded areas represent 95% CIs. The number of subjects within each cohort corresponds to all those who did not have the outcome before the follow-up period.
Table 3.
Estimated incidence of all (first and recurrent) psychiatric diagnoses during the first 14 to 90 days after a diagnosis of COVID-19 compared with other health events
|
COVID-19 |
Influenza |
Other respiratory tract infection |
Skin infection |
Cholelithiasis |
Urolithiasis |
Fracture |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | p value | % (95% CI) | p value | % (95% CI) | p value | % (95% CI) | p value | % (95% CI) | p value | % (95% CI) | p value | ||
| Psychiatric illness | 18·1 (17·6–18·6) | 13·3 (12·8–13·7) | <0·0001 | 14·1 (13·8–14·5) | <0·0001 | 14·8 (14·4–15·2) | <0·0001 | 15·1 (14·6–15·6) | <0·0001 | 13·7 (13·3–14·1) | <0·0001 | 12·7 (12·4–13·1) | <0·0001 | |
| Psychotic disorder | 0·94 (0·82–1·1) | 0·49 (0·41–0·59) | <0·0001 | 0·60 (0·53–0·70) | <0·0001 | 0·92 (0·82–1·0) | 0·44 | 0·72 (0·61–0·86) | 0·045 | 0·44 (0·37–0·53) | <0·0001 | 0·74 (0·65–0·84) | 0·034 | |
| Mood disorder | 9·9 (9·5–10·3) | 7·4 (7·1–7·8) | <0·0001 | 7·6 (7·3–7·9) | <0·0001 | 8·6 (8·3–9·0) | <0·0001 | 9·2 (8·8–9·7) | <0·0001 | 8·3 (8·0–8·6) | <0·0001 | 8·1 (7·8–8·4) | <0·0001 | |
| Anxiety disorder | 12·8 (12·4–13·3) | 9·4 (9·0–9·8) | <0·0001 | 10·1 (9·8–10·5) | <0·0001 | 10·0 (9·6–10·4) | <0·0001 | 10·0 (9·6–10·5) | <0·0001 | 9·5 (9·2–9·9) | <0·0001 | 7·9 (7·6–8·3) | <0·0001 | |
The increased risk of psychiatric sequelae after COVID-19 diagnosis remained unchanged in all sensitivity analyses: when the cohorts were limited to patients with known race (HRs between 1·52 and 2·19, all p<0·0001; appendix p 30), when controlling for problems related to housing and economic circumstances (HRs between 1·53 and 2·09, all p<0·0001; appendix p 31), when cohorts were limited to patients with confirmed COVID-19 (HRs between 1·63 and 2·28, all p<0·0001; appendix p 32), for cohorts in which COVID-19 was diagnosed by RNA or antigen test (HRs between 1·53 and 2·04, all p<0·0001; appendix p 33), for patients who made at least one health-care visit between 14 and 90 days after their health event (HRs between 1·66 and 1·77, all p<0·0001; appendix p 34), when comparing with the psychiatric sequelae of control health events before the COVID-19 pandemic (HRs between 1·89 and 2·56, all p<0·0001; appendix p 35), and when comparing unmatched cohorts (HRs between 1·58 and 2·36, all p<0·000; appendix p 36).
The elevated risk of psychiatric sequelae after COVID-19 diagnosis compared with control health events could not be readily explained by differences in illness severity. Patients with COVID-19 who required inpatient admission were at a higher risk of psychiatric sequelae than patients not requiring admission (HR 1·40, 95% CI 1·06–1·85; p=0·019). However, when limiting cohorts to those not requiring inpatient admission, large differences in psychiatric sequelae remained between those with COVID-19 and the other cohorts (HRs between 1·54 and 2·23, all p<0·0001; appendix p 37).
Contextual factors provide part of the explanation for the difference in psychiatric sequelae between COVID-19 and control health events. All health events had higher rates of psychiatric sequelae when they occurred after (vs before) April 1, 2020 (HR comparing the period before April 1, with the period after April 1, between 1·32 and 1·79, all p<0·05; appendix p 38), and the HRs between COVID-19 and control health events were lower when these events occurred after April 1, 2020 (HRs between 1·31 and 1·83 vs between 1·58 and 2·24 when considering the whole study period; appendix p 39). However, these HRs all remained larger than 1, indicating that contextual factors alone are insufficient to explain differences in psychiatric sequelae. In other words, having any health event after (vs before) April 1, 2020, led to a higher rate of psychiatric sequelae, but this rate was higher still after having COVID-19.
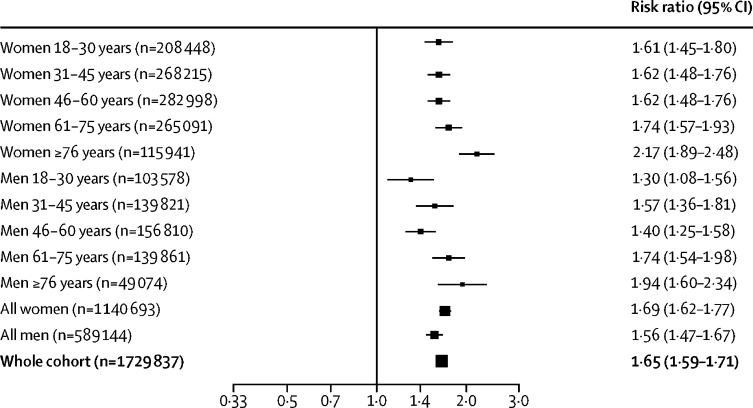
Having a diagnosis of psychiatric disorder in the year before the COVID-19 outbreak was associated with a 65% increased risk of COVID-19 (RR 1·65, 95% CI 1·59–1·71; p<0·0001) compared with a cohort matched for established physical risk factors for COVID-19 but without a psychiatric diagnosis (figure 3 ). The RR was higher in older patients (odds ratio 1·25, 95% CI 1·14–1·38; p<0·0001). These results were robust in all sensitivity analyses if this was a first psychiatric diagnosis (RR 1·67, 95% CI 1·57–1·79; p<0·0001; appendix p 40), among patients with a psychiatric diagnosis in the past 3 years (1·80, 1·74–1·86; p<0·0001; appendix p 40), among patients whose race was known (1·64, 1·58–1·70; p<0·0001; appendix p 41), if the cohorts were limited to patients without any of the physical comorbidities that are risk factors for COVID-19 (1·57, 1·39–1·76; p<0·0001; appendix p 42), if the outcome was limited to a confirmed diagnosis of COVID-19 (1·57, 1·51–1·64; p<0·0001; appendix p 43), and if cohorts were matched for problems related to housing and economic circumstances (1·57, 1·52–1·61; p<0·0001; appendix p 44).
Figure 3.
Relative risks of COVID-19 among patients with a psychiatric illness recorded in the past year compared with a matched cohort of patients with no history of psychiatric illness
RR=risk ratio.
Only small differences in the RR of COVID-19 were observed when comparing classes of psychiatric diagnoses against each other; the RR among patients with a psychotic disorder was 1·17 (95% CI 1·02–1·33; p=0·022) when compared with mood disorder and 1·08 (0·95–1·23; p=0·22) when compared with anxiety disorder. When compared with anxiety disorder, the RR among patients with mood disorder was 0·95 (0·92–0·99; p=0·020).
Discussion
Using a large federated electronic health record network in the USA to create propensity score matched cohorts of patients, we found that COVID-19 survivors have a significantly higher rate of psychiatric disorders, dementia, and insomnia. We also showed that a previous psychiatric illness is independently associated with an increased risk of being diagnosed with COVID-19.
In the period between 14 and 90 days after COVID-19 diagnosis, 5·8% COVID-19 survivors had their first recorded diagnosis of psychiatric illness (F20–F48), compared with 2·5–3·4% of patients in the comparison cohorts. Thus, adults have an approximately doubled risk of being newly diagnosed with a psychiatric disorder after COVID-19 diagnosis. The comparable figures when recurrences of previous diagnoses are included are indicative of the rates of psychiatric disorder that might be anticipated in practice. These incidence figures are minimum estimates for three reasons. First, there will be patients who have not yet presented or received a diagnosis. Second, patients might seek health care from organisations not included in the network. Third, diagnostic rates overall in the network are about 30% lower for both psychiatric and physical disorders since the onset of COVID-19 (see appendix p 45), consistent with other evidence for reduced presentations in the USA.24
The psychiatric effects of COVID-19 were broad but not uniform. The HR was greater for anxiety disorders than for mood disorders. The impact of COVID-19 on anxiety is in line with expectations and highlights the need for effective and accessible interventions. Our data show increased diagnoses in all major anxiety disorder categories, and it remains unclear whether post-COVID-19 anxiety will have a particular post-traumatic stress disorder-like picture. Rates of insomnia diagnosis were also markedly elevated, in agreement with predictions that circadian disturbances will follow COVID-19 infection. By contrast, we did not find a clear signal for newly diagnosed psychotic disorders, despite case reports suggesting that this might occur.11, 25 The two to three times increased risk of dementia after COVID-19 infection extends findings from previous case series11, 26 and is concerning. Some of the excess might reflect misdiagnosed cases of delirium, or transient cognitive impairments due to reversible cerebral events. However, our exclusion of the first 14 days after COVID-19 diagnosis reduces this likelihood, and the incidence of dementia was not higher among inpatients (who are more prone to having delirium) than outpatients (appendix p 45), further suggesting that delirium misdiagnosis does not explain this finding. Detailed follow-up and investigation of this group should be a research priority, as should evaluation of other severe neuropsychiatric phenotypes that become apparent.
The HRs from COVID-19 were higher compared with all other cohorts, indicating that COVID-19 has an impact on psychiatric health above and beyond that which occurs after other acute health events. Since our severity and contextual factors hypotheses cannot explain most of the associations, it is necessary to explore the cause of the particular effect of COVID-19 on the risk of psychiatric disorder. Despite various speculations, the underlying mechanisms are unknown and require urgent investigation. The relationship between the severity of illness (as proxied by inpatient admission) and psychiatric outcomes, albeit modest, might represent a dose–response relationship, suggesting that the association might at least partly be mediated by biological factors directly related to COVID-19 (eg, viral load, breathlessness, or the nature of the immune response).6, 27, 28
We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial—a 1·65 times excess. This result was not related to any specific psychiatric diagnostic category, and was similar regardless of whether the diagnosis was made within 1 or 3 years, and whether or not the known physical risk factors for COVID-19 were present. The risk persisted when problems related to housing and economic circumstances were controlled for. This result is consistent with a recent case-control study using a different US electronic health record network, although the previous study found much higher relative risks.14 Nevertheless, we interpret this finding cautiously, as a Korean study found no association between psychiatric diagnosis and COVID-19 diagnosis, albeit in a much smaller sample and with less matching.15 Possible explanations for the association include behavioural factors (eg, less adherence to social distancing recommendations) and residual socioeconomic and lifestyle factors (eg, smoking) that are not sufficiently captured by the available data in any of the studies. It could also be that vulnerability to COVID-19 is increased by the pro-inflammatory state postulated to occur in some forms of psychiatric disorder or be related to psychotropic medication.
The strengths of this study are the sample size, the amount of data available, the use of propensity score matching, the range of sensitivity analyses, and the real-world nature of the data. The study also has limitations. First, despite the matching and use of various comparison cohorts, there might well be residual confounding, particularly related to social and economic factors, which are not captured in the network and which could influence outcomes. Second, we do not know whether diagnoses were made in primary or secondary care, nor by whom. It is possible that some health-care centres were closed as a result of lockdowns and this might influence where and how patients were diagnosed. The study can provide no information about undiagnosed patients with COVID-19. Third, clinicians might be more likely to diagnose a psychiatric illness after a COVID-19 diagnosis than after the comparison events because of a difference in the nature or extent of assessments; this could also lead to improved detection of conditions (eg, dementia), which had been present but undiagnosed before COVID-19 diagnosis. Fourth, some patients might receive additional care, especially for mental health, at locations not included in the network; this would reduce the absolute incidence figures but is unlikely to confound the relative risks associated with COVID-19. Fifth, propensity score matching raises some statistical issues, but these should not affect the results to any extent (appendix p 7). Moreover, similar results were seen in the unmatched analyses (appendix p 36). Sixth, we did not control statistically for multiple comparisons, although most results were significant at the p<0·0001 level or lower. Finally, our results cannot necessarily be generalised to other populations or health-care settings.
In conclusion, our findings are of sufficient robustness and magnitude to have some immediate implications. The figures provide minimum estimates of the excess in psychiatric morbidity to be anticipated in survivors of COVID-19 and for which services need to plan.29 As COVID-19 sample sizes and survival times increase, it will be possible to refine these findings and to identify rarer and delayed psychiatric presentations. Prospective cohort studies and inclusive case registers will be valuable to complement electronic health record analyses. It will also be important to explore additional risk factors for contracting COVID-19, and for developing psychiatric disorders thereafter, as some elements might prove to be modifiable.
This online publication has been corrected. The corrected version first appeared at thelancet.com/psychiatry on November 12, 2020
Data sharing
The TriNetX system returned the results of these analyses as csv files, which were downloaded and archived. The data presented in this paper and the appendix can be freely accessed at https://osf.io/fjnw8. Additionally, TriNetX will grant access to researchers if they have a specific concern (via the third party agreement option).
Acknowledgments
Acknowledgments
MT is a National Institute for Health Research (NIHR) Academic Clinical Fellow. JRG and PJH are supported by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005). The views expressed are those of the authors and not necessarily those of the UK National Health Service, NIHR, or the UK Department of Health. PJH and MT were granted unrestricted and free access to the TriNetX Analytics network for the purposes of research relevant to psychiatry, and with no constraints on the analyses done nor the decision to publish.
Contributors
PJH and MT designed the study and directly accessed the TriNetX Analytics web interface to carry it out. PJH and MT defined the inclusion and exclusion criteria for each cohort, checked all characteristics of the cohorts, and defined the outcome criteria and analytic approaches. MT did the data analyses. SL and JRG assisted with data analysis and interpretation. MT and PJH wrote the paper with input from JRG and SL. PJH is the guarantor.
Declaration of interests
SL is an employee of TriNetX. MT, JRG, and PJH declare no competing interests.
Supplementary Material
References
- 1.Xiang Y-T, Yang Y, Li W, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7:228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Mental health and psychosocial considerations during the COVID-19 outbreak. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/mental-health-considerations.pdf?sfvrsn=6d3578af_22020
- 3.Holmes EA, O'Connor RC, Perry VH, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao H, Chen J-H, Xu Y-F. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7:e21. doi: 10.1016/S2215-0366(20)30090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paz C, Mascialino G, Adana-Díaz L, et al. Anxiety and depression in patients with confirmed and suspected COVID-19 in Ecuador. Psychiatry Clin Neurosci. 2020;74:554–555. doi: 10.1111/pcn.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gennaro Mazza M, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2020 doi: 10.1002/jmv.26368. published online July 18. [DOI] [PubMed] [Google Scholar]
- 8.Bo H-X, Li W, Yang Y, et al. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol Med. 2020 doi: 10.1017/S0033291720000999. published online March 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Lu H, Zeng H, et al. The differential psychological distress of populations affected by the COVID-19 pandemic. Brain Behav Immun. 2020;87:49–50. doi: 10.1016/j.bbi.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalleballe K, Reddy Onteddu S, Sharma R, et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav Immun. 2020;88:71–74. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seminog OO, Goldacre MJ. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax. 2013;68:171–176. doi: 10.1136/thoraxjnl-2012-202480. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2020 doi: 10.1002/wps.20806. published online Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30421-1. published online Sept 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison PJ, Luciano S, Colbourne L. Rates of delirium associated with calcium channel blockers compared to diuretics, renin-angiotensin system agents and beta-blockers: an electronic health records network study. J Psychopharmacol. 2020;34:848–855. doi: 10.1177/0269881120936501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. published online Feb 19. [DOI] [PubMed] [Google Scholar]
- 19.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19 deaths using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314:1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 24.Jeffery MM, D'Onofrio G, Paek H, et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med. 2020;180:1328–1333. doi: 10.1001/jamainternmed.2020.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parra A, Juanes A, Losada CP, et al. Psychotic symptoms in COVID-19 patients. A retrospective descriptive study. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinna P, Grewal P, Hall JP, et al. Neurological manifestations and COVID-19: experiences from a tertiary care center at the frontline. J Neurol Sci. 2020;415 doi: 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postolache TT, Benros ME, Brenner LA. Targetable biological mechanisms implicated in emergent psychiatric conditions associated with SARS-CoV-2 infection. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2020.2795. published online July 31. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370 doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The TriNetX system returned the results of these analyses as csv files, which were downloaded and archived. The data presented in this paper and the appendix can be freely accessed at https://osf.io/fjnw8. Additionally, TriNetX will grant access to researchers if they have a specific concern (via the third party agreement option).