Highlights
-
•
Complicated hiatal hernia following gastrectomy for carcinoma is a rare surgical entity.
-
•
The early diagnosis is often challenging, therefore, a high index of suspicion and an appropriate imaging diagnosis are paramount.
-
•
Crural exploration and repair during primary surgery are recommended to avoid future revisional surgery.
Keywords: Hiatal hernia, Gastrectomy, Complicated hiatal hernia, Gastric cancer, Case report
Abstract
Introduction
Diaphragmatic complications following gastrostomies for gastric malignancies are extremely rare. The incidence of hiatal hernias after total gastrectomy for carcinoma is not well documented because of the poor prognosis associated with gastric cancer and the short life expectancy.
Presentation of case
This case report presents a 66-year-old male patient who developed an acute incarcerated hiatal hernia 8 month after total gastrectomy for gastric adenocarcinoma. The patient was found to have a herniated alimentary limb and dilated, incarcerated loops of the bowel through the 3.5-cm hiatal defect. The hernia was gently reduced. Posterior cruroplasty without mesh augmentation was performed with nonabsorbable sutures. The patient was discharged in good general condition. His history highlights an important and potentially morbid complication following gastrectomy.
Discussion
To our knowledge, only 5 cases have been reported in the literature. The incidence of symptomatic hiatal hernias following esophageal and gastric resection for carcinoma is 2.8%, and the median time between primary surgery and the diagnosis of hiatal hernias is 15 months.
Conclusion
During primary surgery, it is recommended, in the cases of pre-existing hiatal hernias or a crural dissection, to perform cruroplasty after adequate mobilization of the lower thoracic esophagus and a tension-free subdiaphragmatic anastomosis.
1. Introduction
Many advances in oncology and chemotherapy regimens have shown promising results in the treatment of gastric cancer, but surgery remains the mainstay of treatment [1].
Globally, over 1 million new cases of gastric cancer were diagnosed in 2018, making it the fifth most common cancer worldwide. Gastric cancer was estimated to be responsible for approximately 783,000 deaths worldwide in 2018, making it the third most lethal cancer type [2].
The incidence of symptomatic hiatal hernias following esophageal and gastric resection for carcinoma is 2.8% (total/subtotal gastrectomy, 0.7%; transthoracic esophagectomy, 2.7%; extended gastrectomy, 6.1%), and the median time between primary surgery and the diagnosis of hiatal hernias is 15 months [3].
Studies have suggested that the BMI of patients, pre-existing hiatal hernias, transhiatal dissection and a minimally invasive approach are risk factors. Another recent study has suggested that the increasing incidence is due to an improved survival rate, secondary to neoadjuvant therapies [4,5].
Postgastrectomy hiatal hernias are divided into three types, as follows: 1- conventional type, which includes herniated contents other than the alimentary limb; 2- migration type, which involves mediastinal migration of the esophagojejunostomy through the esophageal hiatus; 3- combination of both [6].
To our knowledge, only 5 cases have been reported in the literature [[7], [8], [9], [10], [11]].
2. Case presentation
A 66-year-old male patient with a BMI of 18 kg/m2, a known case of arterial hypertension and type 2 diabetes mellitus, ASA grade 2, who was diagnosed with cancer in the stomach body, underwent neoadjuvant chemotherapy with FLOT + ramucirumab, followed by conventional total gastrectomy and D2 lymphadenectomy with retrocolic Roux-en-Y reconstruction at our hospital on September 14, 2019. The histopathology returned as poorly differentiated gastric adenocarcinoma G3, and the tumor stages were ypT3, ypN1(2\32), L0, V0, R0, cM0. The patient received adjuvant chemotherapy postoperatively.
The patient presented to our emergency department on May 10, 2020, with a sudden acute onset of severe epigastric pain and nausea, and he denied a history of vomiting or dyspnea. The patient has no history of allergies and has no history of taking any routine medications. No positive family history gastrointestinal malignancy. He denied psychosocial related issues.
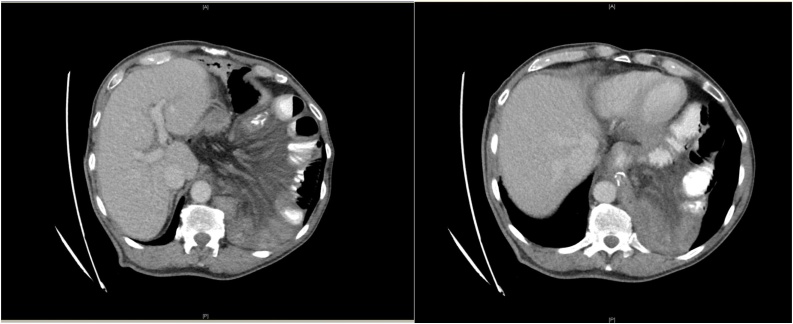
Physical examination revealed normal vital signs with severe tenderness in the left upper quadrant and epigastrium with normoactive bowel sounds. The initial laboratory results, including cardiac enzymes and ECG, showed no abnormalities. Abdominal sonography triggered concerns about an internal hernia. CT of the abdomen with intravenous contrast demonstrated an incarcerated hiatal hernia through a 3.5-cm hiatal defect, through which the esophagojejunostomy and dilated jejunal loops had herniated into the left hemithorax (Figs. 1 and 2). There was no free fluid or air. Emergency relaparotomy was performed. There was no evidence of local recurrence, peritoneal disease or liver metastasis. The patient was found to have a herniated alimentary limb and dilated, incarcerated loops of the bowel through the 3.5-cm hiatal defect. The hernia was gently reduced, and there were no signs of ischemia. Posterior cruroplasty without mesh augmentation was performed with nonabsorbable sutures. The surgery was performed by consultant gastrointestinal surgeon.
Figs. 1 and 2.
Incarcerated hiatal hernia in 66-year-old man postgastrectomy.
Axial CT Abdomen with intravenous contrast shows incarcerated hiatal hernia into the left pleural cavity containing esophagojejunostomy and dilated jejunal loops
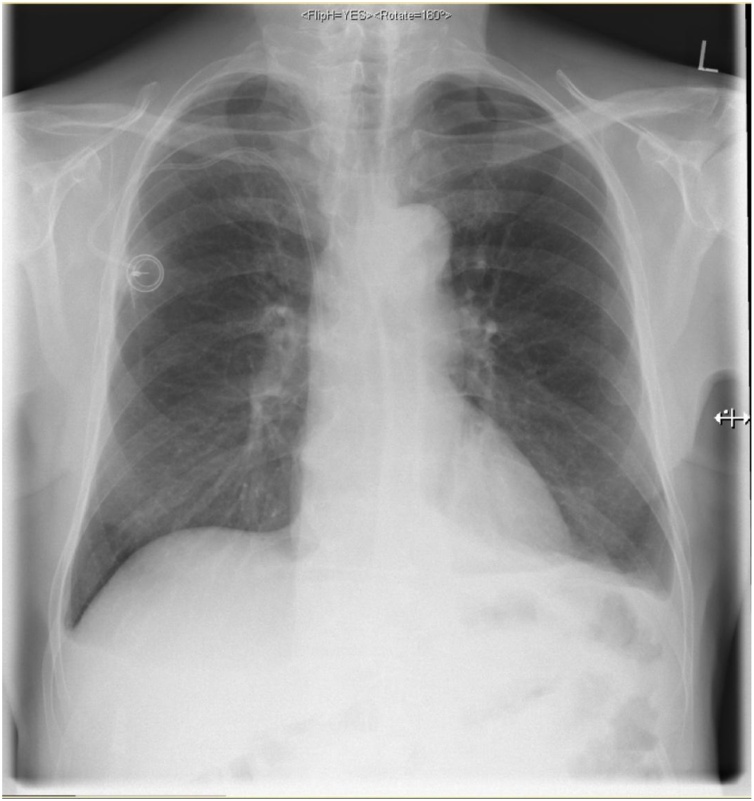
The postoperative course was uneventful. Oral intake was gradually increased as tolerated. The postoperative follow-up chest X-ray showed no recurrence (Fig. 3). The patient was discharged on postoperative day 6 in good general condition.
Fig. 3.
The postoperative follow up chest X-ray showed no recurrence.
The follow up surveillance, one month and three months after the surgery, the patient had recovered well and had no abdominal complaints.
3. Discussion
The 3-year incidence rate of the internal hernia after gastrectomy was 0.19%, which was significantly higher after laparoscopy-assisted than open gastrectomy (0.53 vs. 0.15%, p = 0.03) [12].
Complicated hiatal hernias can occur within 1 year postsurgery as in our case but may be observed up to 5 years after initial surgery [7].
Our patient had a pre-existing asymptomatic hiatal hernia, which was detected during his previous follow-up and surveillance. His low BMI and a redundant, freely mobile small bowel mesentery were the major risk factors.
Acute hiatal hernias could be explained by a secondary sudden rise in intra-abdominal pressure following a large portion meal, which relaxes the hiatus or induces flatulence, causing dilated jejunal loops. Therefore, an antidumping postgastrectomy diet is strongly advised.
The early diagnosis of a complicated hiatal hernia after gastrectomy is often challenging because of nonspecific symptoms and a variety of differential diagnoses. Therefore, a high index of suspicion and an appropriate imaging diagnosis are paramount [6].
Management of asymptomatic hiatal hernias following gastrectomy remains controversial. Although small hernias may be followed, once they increase in size or become symptomatic, they require surgical intervention [6].
During total gastrectomy, it is recommended, in the cases of pre-existing hiatal hernias or a crural dissection, to perform cruroplasty after adequate mobilization of the lower thoracic esophagus and a tension-free subdiaphragmatic anastomosis. This case has been reported in line with the SCARE Guideline [13].
4. Conclusion
Complicated hiatal hernias following gastrectomy are a rare surgical entity. Therefore, crural exploration and repair during primary surgery are recommended to avoid future revisional surgery, which may add to morbidity and mortality.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Funding
None.
Ethical approval
Not applicable. This are a case report based on the clinical notes of an individual patient where written consent for publication has been obtained from the patient.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal on request.
Author contribution
Dr. Mohsen Ezzy: study concept, literature review, data collection and writing the manuscript.
DR. Mostafa Elshafei: final draft correction, review and editing.
DR. Peter Heinz: primary surgeon.
DR. Thomas Kraus: final review.
All authors read and approved the final manuscript.
Registration of research studies
Not Applicable.
Guarantor
Dr. Mohsen Ezzy is the person in charge for the publication of our manuscript.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Contributor Information
Mohsen Ezzy, Email: Dr.Ezzy@hotmail.com.
Peter Heinz, Email: heinz.peter@khnw.de.
Thomas W. Kraus, Email: Kraus.thomas@khnw.de.
Mostafa Elshafei, Email: Elshafei.Moustafa@gmail.com.
References
- 1.Dikken J.L., van Sandick J.W., Allum W.H. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br. J. Surg. 2013;100:83–94. doi: 10.1002/bjs.8966. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Andreou A., Pesthy S., Struecker B. Incidence and risk factors of symptomatic hiatal hernia following resection for gastric and esophageal cancer. Anticancer Res. 2017;37:7031–7036. doi: 10.21873/anticanres.12173. [DOI] [PubMed] [Google Scholar]
- 4.Ulloa Severino B., Fuks D., Christidis C. Laparoscopic repair of hiatal hernia after minimally invasive esophagectomy. Surg. Endosc. 2016:1068–1072. doi: 10.1007/s00464-015-4299-2. [DOI] [PubMed] [Google Scholar]
- 5.Matthews J., Bhanderi S., Mitchell H. Diaphragmatic herniation following esophagogastric resectional surgery: an increasing problem with minimally invasive techniques? Post-operative diaphragmatic hernias. Surg. Endosc. 2016;30(December (12)):5419–5427. doi: 10.1007/s00464-016-4899-5. [DOI] [PubMed] [Google Scholar]
- 6.Ito E., Ohdaira H., Nakashima K. Crus incision without repair is a risk factor for esophageal hiatal hernia after laparoscopic total gastrectomy: a retrospective cohort study. Surg. Endosc. 2017;31(January (1)):237–244. doi: 10.1007/s00464-016-4962-2. [DOI] [PubMed] [Google Scholar]
- 7.Svoronos C., Dannenberg S., Eder F.R., Meyer F.R. Hiatal hernia as a late complication after gastrectomy. Asp. Biomed. Clin. Case Rep. 2019;2(October (2)):74–76. [Google Scholar]
- 8.Murata S., Yamazaki M., Kosugi C. Hiatal hernia following total gastrectomy with Roux-en-Y reconstruction. Hernia. 2014;18:889–891. doi: 10.1007/s10029-013-1142-3. [DOI] [PubMed] [Google Scholar]
- 9.Piciucchi S., Milandri C., Verdecchia G.M. Acute hiatal hernia: a late complication following gastrectomy. Int. Arch. Med. 2010;3:23. doi: 10.1186/1755-7682-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.do Nascimento Santos B., Belotto de Oliveira M., D’Alpino Peixoto R. Hiatal hernia as a total gastrectomy complication. Case Rep. Oncol. 2016;9:100–105. doi: 10.1159/000443633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalo M.A., Almeida H., Güemes A. Hiatal herniation following total gastrectomy. Rev. Esp. Enferm. Dig. 2016;108(April (4)):234. doi: 10.17235/reed.2016.4009/2015. [DOI] [PubMed] [Google Scholar]
- 12.Miyagaki H., Takiguchi S., Kurokawa Y., Hirao M., Tamura S., Nishida T., Kimura Y., Fujiwara Y., Mori M., Doki Y. Recent trend of internal hernia occurrence after gastrectomy for gastric cancer. World J. Surg. 2012;36(April (4)):851–857. doi: 10.1007/s00268-012-1479-2. [DOI] [PubMed] [Google Scholar]
- 13.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]