Abstract
Precise allergy diagnosis and effective allergen specific immunotherapy are largely dependent on the quality of allergen extract. A new extract of Dermatophagoides farinae was commercially developed by Prolagen. The allergenic properties of the new extract were compared with those of other commercial products. The allergenic properties of the new extract were compared according to protein concentration, protein profiles, major allergen (Der f 1) contents, and allergenic potency to those for three commercially available extracts imported in Korea (Jubilant HollisterStier Allergy, Lofarma S.p.A., and Stallergenes Greer). Protein concentrations varied up to 2.62-fold (0.404 to 1.057 mg/mL), and Der f 1 contents varied up to 11.3-fold (3.597 to 40.688 µg/mL). Protein profiles of the extracts showed no major discrepancies, although there were some differences in SDS-PAGE band intensities, reflecting protein concentrations. Allergen potency ranged from 37038 to 60491 PAU/mL. The Prolagen product was highest in terms of protein concentration and allergen potency. The Lofarma product displayed Der f 1 content similar to that in Prolagen (19.4 µg/mg vs. 19.3 µg/mg). Endotoxin levels varied 8.9-fold (1020 to 8985 EU/mL). The newly developed house dust mite extract showed equal or better allergenic properties than available commercial extracts. This new product may be useful for better diagnostics and allergen-specific immunotherapeutics.
Keywords: Allergen, house dust mite, immunotherapeutics, standardization, potency
Allergy diagnosis and treatment depend on cross-reactivity between extracts from similar species, and allergenic properties may vary according to the environmental circumstances where an extract is produced. For the development of reliable allergy diagnostics and immunotherapeutics, standardization of allergen extract is a prerequisite.1 However, in Korea, all commercial allergen extracts in clinical use are imported from the United States of America or European countries. In addition, some commercial products are no longer available due to strengthened regulations.2,3 Thus, in Korea, efforts have been made to standardize locally produced allergens since 2009 with support from the Korea Center for Disease Control and Prevention.4 Previous studies have been performed for standardization of extracts for house dust mite (HDM),5 cockroach,6 pollens of regional importance,7 insect venom component allergen (Pac c 3 from Pachycondyla chinensis),8 and buckwheat.9 The stabilities of extracts for HDM,10 cockroach,11 and pollens12 have also been studied. Standardization of food allergens, including cereals and various regionally important fish species, is ongoing.
A company, ‘Prolagen (Seoul, Korea)’, established in 2017, obtained good manufacturing practice accreditation for the production of allergen extracts for allergy immunotherapeutics from the Ministry of Food and Drug Safety in 2019. Previously, eight skin prick test reagents produced from Prolagen were shown to be comparable to those of imported products.13
In this study, we compared the allergen extract for D. farinae produced by Prolagen with imported ones. Protein concentrations, major allergen contents, endotoxin levels, and potency among these commercially available products were compared by in vitro methods.
The Prolagen HDM allergen extract was prepared as described previously.5 For comparison, HDM extracts were obtained from Jubilant HollisterStier Allergy (Spokane, WA, USA), Lofarma S.p.A. (Milano, Italy), and Stallergenes Greer (Boston, MA, USA). All extracts investigated in this study are manufactured as immunotherapeutic reagents, except the Lofarma product, which is for skin testing. Also, Stallergenes Greer's product is for veterinary use, not human use.
Protein concentrations were determined using a Bradford assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Concentrations of major allergens Der f 1 and Der f 2 were quantified using two-site ELISA kits from Indoor Biotechnologies Inc. (Charlottesville, VA, USA). Protein concentrations were determined to be 404–1057 µg/mL. Among the D. farinae products, the Prolagen product had the highest concentrations of Der f 1 and Der f 2, while the Stallergenes Greer product licensed for veterinary use had the lowest concentrations (Table 1). Der f 1 and Der f 2 content varied about 5.6- to 14.0-fold, respectively. The sum of these two major allergen contents differed by up to 6.9-fold, and the sum of these major allergen concentrations differed up to approximately 3.4-fold even after conversion into µg/mg protein concentrations. The ratio of Der f 1 to Der f 2 varied from 1:0.404 to 1:1.396 (3.5-fold) (Table 1). Der f 1/2 ratio may be affected by diet14 and temperature.15 Extraction method may also affect Der f 1/2 ratio.5
Table 1. Contents of D. farinae Allergen Extracts Commercially Available in Korea.
| Product | Protein concentration | Major allergen content | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Der f 1 | Der f 2 | Sum (Der f 1 & 2) | |||||||
| Manufacture | Lot No. | Licensed use | µg/mL | µg/mL | µg/mg | µg/mL | µg/mg | µg/mL | µg/mg |
| H, HollisterStier | E1900466 | Human | 523 | 3.6 | 6.9 | 3.5 | 6.8 | 7.1 | 13.6 |
| L, Lofarma | 190702 | Human | 569 | 11.0 | 19.4 | 6.7 | 11.8 | 17.7 | 31.2 |
| G, Greer | 363113 | Veterinary | 404 | 5.0 | 12.4 | 2.0 | 5.0 | 7.1 | 17.5 |
| P, Prolagen | DF18001 | Human | 1057 | 20.3 | 19.3 | 28.4 | 26.9 | 48.8 | 46.1 |
| Average | 638.3 | 10.0 | 14.5 | 10.2 | 12.6 | 20.2 | 27.1 | ||
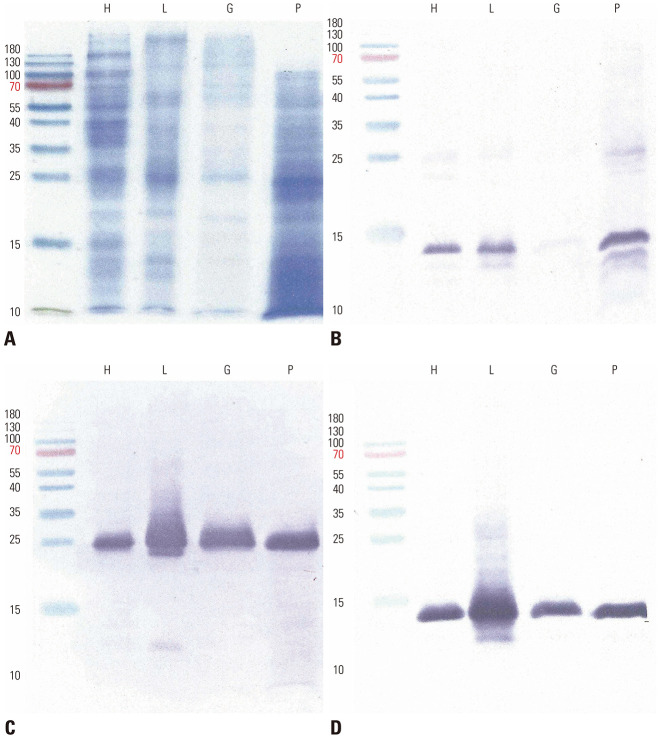
Protein profiles of the commercial extracts were compared by 15% SDS-PAGE (20 µL/well). Der f 1 and Der f 2 were detected with anti-recombinant Der f 1 monoclonal antibody (mAb) 3D716 and anti-recombinant Der f 2 mAb 2F3817 as described previously.5 IgE reactive components were probed with pooled serum samples (1:4 diluted). Serum samples were kindly provided by TRINA BIOREACTIVES (Naenikon, Switzerland). More intense bands of high molecular weight proteins were seen with the Hollister-Stier products, whereas additional strong bands were noted in the Prolagen product (Fig. 1A). The strongest IgE reactivity to a 14 kDa allergen, putative Der f 2, was shown by IgE immunoblot analysis in all examined extracts (Fig. 1B). Interestingly, relatively poor recognition of Der f 2 was observed for the Stallergenes Greer extract, even though similar recognition was shown with mAb 2F38. This is possibly due to polymorphism of Der f 2: mAb may recognize highly conserved regions, whereas human IgE antibodies recognize polymorphic residues. Weak IgE reaction to a 25 kDa component, putative Der f 1, was also displayed. MAbs strongly recognized Der f 1 (Fig. 1C) and Der f 2 (Fig. 1D) in the extracts. Degraded Der f 1 was detected in the Lofarma product (Fig. 1C).
Fig. 1. Protein and allergen profiles of commercial Dermatophagoides farinae extracts. (A) Proteins (20 µL each) were separated onto 15% SDS-PAGE. (B) IgE reactive components were then probed with pooled patient sera. Der f 1 (C) and Der f 2 (D) allergens were detected with the use of monoclonal antibodies.
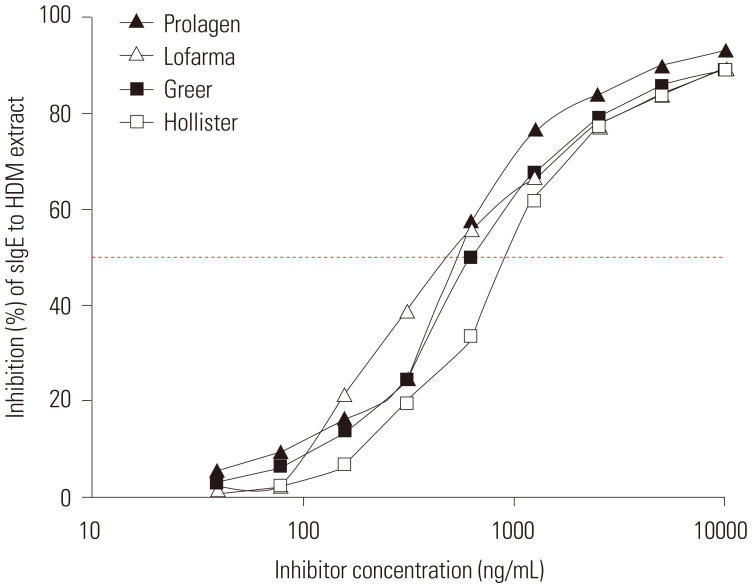
A previous study used intradermal skin tests to determine the potency of HDM extracts5 because this method is known to be more sensitive than skin prick tests.18 However, we produced a new in-house reference preparation and carried out skin prick tests to meet the European standard.19,20 A newly established potency was designated as Prolagen Allergy Unit (PAU). Competitive inhibition ELISA was performed, and allergenic potency was determined using 50% inhibitory concentrations of each allergen extract.21 Allergen potencies differed up to about 1.6-fold, 37038–60491 PAU/mL (Fig. 2 and Table 2), which is similar to the sum of major allergen contents.
Fig. 2. Allergen potencies of commercial Dermatophagoides farinae extracts. Prolagen product with defined allergen units was coated, and IgE reactivity was inhibited with different commercial extracts to compare potencies.
Table 2. Comparison of Allergen Potencies of Commercial D. farinae Extracts.
| Product | Potency (Allergy unit) | ||
|---|---|---|---|
| Manufacture | Provided | 50% inhibitory concentration (ng/mL) | PAU/mL (PAU/mg) |
| H, HollisterStier | 10000 AU/mL | 916.8 | 37038 (46125) |
| L, Lofarma | 100 DBU/mL | 561.3 | 60491 (75332) |
| G, Greer | 11000 PNU/mL | 703.9 | 48244 (60081) |
| P, Prolagen* | 150000 PAU/mL | 580.9 | 58453 (72794) |
| Average | 690.8 | 51057 (63583) | |
*Available as freeze-dried powder.
Endotoxin levels in extracts were measured using a kinetic Limulus amoebocyte lysate assay (Kinetic-QCL, Bio Whittaker, LONZA, Basel, Switzerland). Endotoxin levels in the extracts ranged from 1020–9110 EU/mL (up to 8.9-fold). Endotoxin levels per mg of protein differed by up to 6.9-fold (2524.8 to 17418.7) (Table 3). Endotoxin levels in commercial HDM extract vary up to 1000-fold (31 to 5200 EU).22 The facultative intracellular bacteria Bartonella may be a mite symbiont and is thought to be the major source of endotoxin in HDM extracts.23 Although difficult, endotoxin levels should be lowered to minimize possible side effects.
Table 3. Endotoxin Levels in D. farinae Allergen Extracts Commercially Available in Korea.
| Product | Endotoxin level | |
|---|---|---|
| Manufacture | EU/mL | EU/mg |
| H, HollisterStier | 9110 | 17418.7 |
| L, Lofarma | 6110 | 10738.1 |
| G, Greer | 1020 | 2524.8 |
| P, Prolagen | 8985 | 8500.0 |
| Average | 6306 | 9795.4 |
Varying composition and allergen content in commercial HDM allergen extracts commonly used for allergy diagnosis and immunotherapy have been described.24,25 Analysis of commercial allergen extracts of HDM may provide useful information for clinicians to select optimal products. Induction of blocking antibodies may vary according to the allergen products.26 The IgE reactivity of allergens may be affected by not only species, but also by polymorphism of individual allergens, especially for Der f 2 and Der p 2.27,28 Different sensitization patterns with different disease entities, respiratory or cutaneous allergies, also imply that careful product selection may influence the efficacy of immunotherapy.29 For patients with respiratory allergy sensitized to HDM, better efficacy is expected with products containing extracts from raw materials with abundant mite feces, which contain more group 1 and 2 allergens. However, patients with cutaneous allergy are more likely to benefit from products with whole body extracts that may contain more minor allergens.
We analyzed the various commercial products licensed for immunotherapeutic, skin test, and veterinary use. In fact, these products were the only products we could obtain at the moment in Korea, although we hope to compare more products. It should be mentioned that the major allergen concentrations and potency of the reagents should differ in relation to the purpose of clinical application.
In conclusion, the D. farinae extract newly manufactured by Prolagen is comparable to imported products. This product may be useful for the development of better diagnostics and allergen-specific immunotherapeutics.
ACKNOWLEDGEMENTS
This work was supported by the Technology Development Program (S2765576) funded by the Ministry of SMEs and Startups (MSS, Korea). This work was also supported by the Medical Device Technology Development Program (20006057, Highly sensitive three dimensional fluorescent chip for multiple allergy diagnosis) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea).
Footnotes
JD Kim, KY Jeong, KH Park, JH Lee, and JW Park share equivalents of Prolagen, Ltd. JW Park reports serving as an unpaid chief technology officer for Prolagen. KY Jeong is a Technical Advisor of Prolagen. JT Kim and JD Kim are co-chief executive officers. HH Kim, SH Kim, DJ Kim, and YJ Shin are employees of Prolagen. The interests of these authors did not influence the academic fairness in conducting this study, analyzing results, and writing a paper. Other authors have no potential conflicts of interest to disclose.
- Conceptualization: Ji Tae Kim, Kyoung Yong Jeong, and Jung-Won Park.
- Data curation: Ji Tae Kim, Kyoung Yong Jeong, and Jung-Won Park.
- Funding acquisition: Jung Dong Kim, Kyoung Yong Jeong, and Jung-Won Park.
- Investigation: Ji Tae Kim, Hyunho Kim, Sung Hyun Kim, Yeji Shin, Dong Jun Kim, Hangyeol Song, and Seok Woo Jang.
- Methodology: Kyoung Yong Jeong and Jung-Won Park.
- Project administration: Ji Tae Kim and Kyoung Yong Jeong.
- Resources: Jung Dong Kim, Kyung Hee Park, Jae-Hyun Lee, and Jung-Won Park.
- Supervision: Ji Tae Kim, Kyoung Yong Jeong, and Deug-chan Lee.
- Validation: Ji Tae Kim and Kyoung Yong Jeong.
- Writing—original draft: Ji Tae Kim, Kyoung Yong Jeong, and Jung-Won Park.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
References
- 1.Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011;52:393–400. doi: 10.3349/ymj.2011.52.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slater JE, Menzies SL, Bridgewater J, Mosquera A, Zinderman CE, Ou AC, et al. The US Food and Drug Administration review of the safety and effectiveness of nonstandardized allergen extracts. J Allergy Clin Immunol. 2012;129:1014–1019. doi: 10.1016/j.jaci.2012.01.066. [DOI] [PubMed] [Google Scholar]
- 3.Klimek L, Hoffmann HJ, Renz H, Demoly P, Werfel T, Matricardi PM, et al. Diagnostic test allergens used for in vivo diagnosis of allergic diseases are at risk: a European Perspective. Allergy. 2015;70:1329–1331. doi: 10.1111/all.12676. [DOI] [PubMed] [Google Scholar]
- 4.Jeong KY, Lee JH, Kim EJ, Lee JS, Cho SH, Hong SJ, et al. Current status of standardization of inhalant allergen extracts in Korea. Allergy Asthma Immunol Res. 2014;6:196–200. doi: 10.4168/aair.2014.6.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong KY, Choi SY, Lee JH, Lee IY, Yong TS, Lee JS, et al. Standardization of house dust mite extracts in Korea. Allergy Asthma Immunol Res. 2012;4:346–350. doi: 10.4168/aair.2012.4.6.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong KY, Choi SY, Lee JH, Lee JS, Yong TS, Hong CS, et al. Preparation and characterization of an extract of German cockroach from a Korean source. Allergy Asthma Immunol Res. 2013;5:102–105. doi: 10.4168/aair.2013.5.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong KY, Son M, Choi SY, Park KH, Park HJ, Hong CS, et al. Standardization of weed pollen extracts, Japanese hop and mugwort, in Korea. Yonsei Med J. 2016;57:399–406. doi: 10.3349/ymj.2016.57.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong KY, Yi MH, Son M, Lyu D, Lee JH, Yong TS, et al. IgE Reactivity of Recombinant Pac c 3 from the Asian Needle Ant (Pachycondyla chinensis) Int Arch Allergy Immunol. 2016;169:93–100. doi: 10.1159/000444364. [DOI] [PubMed] [Google Scholar]
- 9.Jeong KY, Park KH, Lee JH, Park JW. Monoclonal antibodies to recombinant Fag e 3 buckwheat allergen and development of a two-site ELISA for Its quantification. Allergy Asthma Immunol Res. 2017;9:417–422. doi: 10.4168/aair.2017.9.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong KY, Choi SY, Han IS, Lee JH, Lee JS, Hong CS, et al. The effects of storage conditions on the stability of house dust mite extracts. Allergy Asthma Immunol Res. 2013;5:397–401. doi: 10.4168/aair.2013.5.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong KY, Lee J, Yuk JE, Park KH, Lee JH, Kim JD, et al. Optimal conditions for the storage of German cockroach extract. Mol Med Rep. 2020;21:953–958. doi: 10.3892/mmr.2019.10854. [DOI] [PubMed] [Google Scholar]
- 12.Jeong KY, Yuk JE, Lee J, Jang SW, Park KH, Lee JH, et al. Stability of extracts from pollens of allergenic importance in Korea. Korean J Intern Med. 2020;35:222–230. doi: 10.3904/kjim.2018.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SC, Sim DW, Lee J, Jeong KY, Park KH, Lee JH, et al. Comparison between newly developed and commercial inhalant skin prick test reagents using in vivo and in vitro methods. J Korean Med Sci. 2018;33:e101. doi: 10.3346/jkms.2018.33.e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avula-Poola S, Morgan MS, Arlian LG. Diet influences growth rates and allergen and endotoxin contents of cultured Dermatophagoides farinae and Dermatophagoides pteronyssinus house dust mites. Int Arch Allergy Immunol. 2012;159:226–234. doi: 10.1159/000336026. [DOI] [PubMed] [Google Scholar]
- 15.Yella L, Morgan MS, Arlian LG. Population growth and allergen accumulation of Dermatophagoides farinae cultured at 20 and 25°C. Exp Appl Acarol. 2013;60:117–126. doi: 10.1007/s10493-012-9626-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim JT, Lee J, JE Yuk, Song H, Kim H, Kim SH, et al. Novel sensitive, two-site ELISA for the quantification of Der f 1 using monoclonal antibodies. Allergy Asthma Immunol Res. 2021 doi: 10.4168/aair.2021.13.4.665. (Forthcoming) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong KY, Jin HS, Oh SH, Hong CS, Lee IY, Ree HI, et al. Monoclonal antibodies to recombinant Der f 2 and development of a two-site ELISA sensitive to major Der f 2 isoallergen in Korea. Allergy. 2002;57:29–34. [PubMed] [Google Scholar]
- 18.Turkeltaub PC, Rastogi SC, Baer H, Anderson MC, Norman PS. A standardized quantitative skin-test assay of allergen potency and stability: studies on the allergen dose-response curve and effect of wheal, erythema, and patient selection on assay results. J Allergy Clin Immunol. 1982;70:343–352. doi: 10.1016/0091-6749(82)90023-9. [DOI] [PubMed] [Google Scholar]
- 19.European Directorate for the Quality of Medicines (EDQM) European pharmacopoeia. 6th ed. Council of Europe; 2010. Monograph: Allergen products–producta allergenica 01/2010:1063; pp. 679–680. [Google Scholar]
- 20.European Parliament and Council. Directive 2001/83/EC. The community code relating to medicinal products for human use. Official Journal of the European Union. 2004;L311:67–128. [Google Scholar]
- 21.Grier TJ. Laboratory methods for allergen extract analysis and quality control. Clin Rev Allergy Immunol. 2001;21:111–140. doi: 10.1385/CRIAI:21:2-3:111. [DOI] [PubMed] [Google Scholar]
- 22.Pascoe CD, Jha A, Basu S, Mahood T, Lee A, Hinshaw S, et al. The importance of reporting house dust mite endotoxin abundance: impact on the lung transcriptome. Am J Physiol Lung Cell Mol Physiol. 2020;318:L1229–L1236. doi: 10.1152/ajplung.00103.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valerio CR, Murray P, Arlian LG, Slater JE. Bacterial 16S ribosomal DNA in house dust mite cultures. J Allergy Clin Immunol. 2005;116:1296–1300. doi: 10.1016/j.jaci.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 24.Brunetto B, Tinghino R, Braschi MC, Antonicelli L, Pini C, Iacovacci P. Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy. 2010;65:184–190. doi: 10.1111/j.1398-9995.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 25.González-Pérez R, Poza-Guedes P, Barrios Del Pino Y, Matheu V, Sánchez-Machín I. Evaluation of major mite allergens from European standardized commercial extracts for in vivo diagnosis: addressing the need for precision medicine. Clin Transl Allergy. 2019;9:14. doi: 10.1186/s13601-019-0254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park KH, Lee SC, Son YW, Jeong KY, Shin YS, Shin JU, et al. Different responses in induction of allergen specific immunoglobulin G4 and IgE-blocking factors for three mite subcutaneous immunotherapy products. Yonsei Med J. 2016;57:1427–1434. doi: 10.3349/ymj.2016.57.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong KY, Lee IY, Yong TS, Lee JH, Kim EJ, Lee JS, et al. Sequence polymorphisms of Der f 1, Der p 1, Der f 2 and Der p 2 from Korean house dust mite isolates. Exp Appl Acarol. 2012;58:35–42. doi: 10.1007/s10493-012-9553-x. [DOI] [PubMed] [Google Scholar]
- 28.Park JW, Kim KS, Jin HS, Kim CW, Kang DB, Choi SY, et al. Der p 2 isoallergens have different allergenicity, and quantification with 2-site ELISA using monoclonal antibodies is influenced by the isoallergens. Clin Exp Allergy. 2002;32:1042–1047. doi: 10.1046/j.1365-2222.2002.01421.x. [DOI] [PubMed] [Google Scholar]
- 29.Park KH, Lee J, Lee JY, Lee SC, Sim DW, Shin JU, et al. Sensitization to various minor house dust mite allergens is greater in patients with atopic dermatitis than in those with respiratory allergic disease. Clin Exp Allergy. 2018;48:1050–1058. doi: 10.1111/cea.13164. [DOI] [PubMed] [Google Scholar]