Abstract
Purpose
Painful muscle cramps are a common complication in liver cirrhosis patients, and no effective treatment is available. This pilot study aimed to evaluate whether taurine supplementation improves muscle cramps in Korean cirrhotic patients.
Materials and Methods
Ten cirrhotic patients who experienced muscle cramps one or more times/week were enrolled in this prospective single-arm study and administered with an oral taurine solution (1 g/50 mL) thrice a day for 4 weeks. Taurine was discontinued for the subsequent 4 weeks. The frequency and intensity of muscle cramps were evaluated using a questionnaire at weeks 0, 2, 4, 6, and 8 after the start of treatment.
Results
At baseline, the median frequency of muscle cramps was six times/week, and all patients had severe pain. Muscle cramp scores (frequency×intensity) decreased in seven patients by weeks 4 and 8 after treatment initiation. Compared to baseline muscle cramp scores [median 21, interquartile range (IQR): 8–84], median muscle cramp scores were lower at week 4 (6.5, IQR: 3–12, p=0.126) and week 8 (5, IQR: 1.5–56, p=0.066). All five patients whose baseline plasma taurine levels were below the normal limit showed increased taurine levels at week 4; 60% of them experienced improvements in their muscle cramps. Of the five patients with normal or higher taurine levels, 80% experienced an improvement in symptoms at week 4. The safety and tolerability of the 4-week taurine therapy were excellent.
Conclusion
Oral taurine therapy for 4 weeks improved muscle cramps safely in cirrhotic patients.
Keywords: Liver cirrhosis, muscle cramp, taurine
INTRODUCTION
Muscle cramps are painful asymmetrical muscle contractions that can last from several seconds to minutes.1 Occurring in only about one-third of healthy adults, muscle cramping is common in hemodialysis patients; patients with severe dehydration and chronic illnesses, such as motor neuron and thyroid diseases; and patients taking medications, such as diuretics. Liver cirrhosis (LC) patients are especially vulnerable to painful muscle cramps and constitute 51–88% of the overall prevalence of muscle cramps.2,3 Interestingly, research has shown that between cirrhotic patients and patients with other diseases (e.g., heart failure) necessitating the consumption of the same doses of diuretics, the frequency of muscle cramps was higher in cirrhotic patients than in the other patients. Accordingly, muscle cramps are considered as cirrhotic complications.4,5,6
In cirrhotic patients, although muscle cramping is significantly associated with a lower quality of life,7,8 it is usually overlooked by physicians, as these patients face more life-threatening complications, such as ascites, variceal bleeding, or encephalopathy. As such, the pathophysiology of muscle cramps remains not well-established. Early studies have revealed that the frequency of muscle cramps increases as a patient's hepatic function deteriorates (i.e., as serum albumin levels decrease or bilirubin levels increase).4,5 It has been recently suggested that the mechanism of muscle cramp could include an electrolyte imbalance involving magnesium (Mg) or zinc (Zn) deficiencies, nerve dysfunction due to oxidative stress, and impaired energy metabolism in the skeletal muscle cells.9
Several agents have been investigated as therapeutic options for muscle cramps in cirrhotic patients. These included quinine,10 baclofen,11,12 Zn,13 vitamin E,14,15 L-carnitine,16,17 and branchedchain amino acids.18,19 While previous studies have reported favorable responses, most of these studies were small case series. Although several randomized controlled trials have been performed, none have demonstrated a significant improvement in muscle cramp frequency with vitamin E,15 while some have also reported more frequent adverse events, such as dizziness and diarrhea, for quinidine,20 compared to a placebo.
Taurine has been suggested as a therapeutic agent for muscle cramps due to its stabilizing effect on the skeletal muscle cell membrane.21 Though several studies have investigated its effect on muscle cramps in patients with chronic liver disease, these studies were small and involved heterogeneous liver disease patients. Furthermore, the dose of taurine administered also differed. No study has investigated the effect of taurine in Korean LC patients with muscle cramps, as taurine is not readily available in South Korea. Therefore, in this study, we investigated whether a drinking formula containing taurine 3 g/day safely improves muscle cramps in Korean LC patients. Additionally, the blood levels of taurine and other electrolytes were measured to analyze their correlations with muscle cramps.
MATERIALS AND METHODS
Subjects
In this prospective single-arm study, 10 LC patients above 18 years of age who had experienced muscle cramps one or more times per week in the recent 3 months were consecutively enrolled in a tertiary hospital from March to October 2017. LC was diagnosed if the patients presented with at least two of the following: 1) thrombocytopenia (<120000/mm3), 2) ascites, 3) esophageal or gastric varices, 4) nodular appearance during abdominal ultrasound or computed tomography, and 5) pathologically proven cirrhosis. Patients were excluded if they were pregnant or lactating or if they presented with chronic kidney disease, creatinine clearance <30 mL/min/1.73 m2, uncontrolled hepatic encephalopathy, or pre-existing neuromuscular diseases, including motor neuron diseases or peripheral neuropathy.
All participants provided informed consent voluntarily, and the study was conducted in accordance with the guidelines of the Helsinki Declaration. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1605/346-006).
Study drug and design
All enrolled participants consumed 3 g of taurine per day (1 g, thrice) for 4 weeks. The study drug was a solution of 1 g taurine in 50 mL water (Dona-A ST, Youngin, Korea) that was contained in a bottle. At every visit, all drug bottles prescribed at the last visit were returned, and the number of empty bottles was counted to check compliance strictly. After 4 weeks of active treatment, all subjects were monitored for an additional 4 weeks to determine whether the status of muscle cramps changed after discontinuation of taurine.
A structured questionnaire8 was used to assess changes in the frequency, intensity, and duration of muscle cramps with respect to aggravating or relieving factors at baseline and at 1, 2, 4, 6, and 8 weeks after treatment initiation (Supplementary Material, only online). A muscle cramp score was calculated by multiplying the weekly frequency and intensity of cramps. Comorbidities and comedications were recorded.
Blood was sampled at baseline and weeks 4 and 8 for determining plasma taurine levels, serum Mg and Zn levels, complete blood count, and liver function. The plasma taurine levels were measured by high-performance liquid chromatography using the Agilent Series 1100 HPLC system, and the ZORBAX Eclipse Plus C18 columns; measurement was performed as per the manufacturer's instruction (Agilent, Santa Clara, CA, USA).
Statistical analysis
All statistical analyses were performed using Stata version 14.0 (StataCorp LP, College Station, TX, USA). Descriptive statistics were used for demographic data. Changes in the frequency and intensity of muscle cramps at weeks 4 and 8 with respect to baseline were assessed using the Wilcoxon signed-rank test or the Friedman test to detect differences across multiple response measurements as a non-parametric analysis. The Mann-Whitney U test was used to compare muscle cramp scores between subgroups categorized according to baseline plasma taurine levels. Associations among plasma taurine, serum Zn or Mg, and muscle cramp scores were estimated by Spearman correlation analysis. A p value of <0.05 was considered statistically significant.
RESULTS
Baseline patient characteristics
The median age of the enrolled patients was 66 years [interquartile range (IQR): 58–75 years] and 6 patients (60%) were males (Table 1). Ascites were observed in 7 (70%). 6 (60%) patients had taken diuretics. The etiologies of LC included hepatitis B virus infection (n=4), hepatitis C virus infection (n=4), and alcohol (n=2). Regarding comorbidities, three patients had diabetes mellitus and four had hypertension. The median serum sodium, potassium, and calcium levels were within the normal ranges (Table 1); however, the median serum Zn level was lower (57 µg/dL) than the normal limit (80–120 µg/dL). The median study drug compliance was 98.5% (range: 90.5–100%, data not shown). One subject experienced mild dyspepsia as an adverse event associated with the study drug.
Table 1. Baseline Characteristics of Enrolled Patients.
| Variables | n=10 |
|---|---|
| Age, yr | 66 (58–75) |
| Sex | |
| Male/female | 6 (60)/4 (40) |
| Body mass index, kg/m2 | 25.7 (23.9–26.8) |
| Alcohol | |
| Never/quit | 6 (60)/4 (40) |
| Child-Pugh class | |
| A/B | 2 (20)/8 (80) |
| MELD score | 12.5 (10–14) |
| Ascites, yes | 7 (70) |
| Comorbidities | 4 (40) |
| Diabetes | 3 (30) |
| Hypertension | 4 (40) |
| Comedications | |
| Diuretics | 6 (60) |
| Anti-hypertensive | 4 (40) |
| Oral hypoglycemic agents | 3 (30) |
| Propranolol | 3 (30) |
| Pregabalin | 1 (10) |
| Branched-chain amino acid | 4 (40) |
| Laboratory findings | |
| WBC, /mm3 | 3870 (2710–4970) |
| Hb, g/dL | 12.7 (11.7–13.4) |
| PLT, ×1000/mm3 | 79 (63–91) |
| Cholesterol, mg/dL | 125.5 (119–147) |
| Bilirubin, mg/dL | 1.85 (1.1–2.3) |
| Albumin, g/dL | 3.2 (3.1–3.4) |
| AST, IU/L | 57 (42–65) |
| ALT, IU/L | 37 (25–43) |
| GGT, IU/L | 38 (29–63) |
| PT (INR) | 1.31 (1.24–1.35) |
| BUN, mg/dL | 16.5 (14–19) |
| Cr, mg/dL | 0.84 (0.77–1.23) |
| Fasting glucose, mg/dL | 109 (97–122) |
| Na, mM/L | 137.5 (134–140) |
| K, mM/L | 4.3 (3.8–4.6) |
| Ca, mg/dL | 8.7 (8.3–8.9) |
| Zn, ug/dL* | 57 (49.5–61) |
| Mg, mg/dL* | 2 (1.95–2.1) |
| Taurine, μM/L* | 73.5 (40.7–401.9) |
MELD, Model For End-Stage Liver Disease; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl-transferase; PT, prothrombin time; INR, international normalized ratio; BUN, blood urea nitrogen; Cr, creatinine; Na, sodium; K, potassium; Ca, calcium; Zn, zinc; Mg, magnesium.
Median (interquartile range) or number (%).
*Reference levels: taurine, 65–179 µM/L; Zn, 66–110 ug/dL; Mg, 1.5–2.5 mg/dL.
Characteristics of muscle cramps in LC patients at baseline
The median frequency of muscle cramps was 4.5 times/week (IQR: 2–21 times/week) (Table 2). All subjects reported experiencing severe pain (score ≥4 on a pain scale of 5). The most common site of muscle cramping was the calf (100%), followed by the foot (40%), hand (40%), and thigh (20%). 7 patients (70%) experienced pain at multiple sites. In all patients, the pain developed at night (100%), and in 50% of the patients, it lasted for 1–5 minutes. Exercise aggravated muscle cramps in 3 patients (30%). 6 patients (60%) developed cramps during sleep. 6 (60%), 2 (20%), and 1 (10%) patients used massage, stretching, and herbal supplements to relieve pain, respectively; however, 2 patients (20%) recovered spontaneously. Muscle cramps limited labored work in 8 patients (80%) (Table 2).
Table 2. Baseline Characteristics of Muscle Cramps in Liver Cirrhosis Patients.
| Characteristics of muscle cramps | No (%) |
|---|---|
| Frequency | |
| ≥7 times/week | 4 (40) |
| 1–6 times/week | 6 (60) |
| Intensity | |
| Severe | 10 (100) |
| Mild | 0 (0) |
| Site | |
| Calf | 10 (100) |
| Thigh | 2 (20) |
| Foot | 4 (40) |
| Hand | 4 (40) |
| No. of painful sites | |
| 1 | 3 (30) |
| 2 | 5 (50) |
| ≥3 | 2 (20) |
| Time | |
| Day | 0 (0) |
| Night | 7 (70) |
| Both | 3 (30) |
| Duration (min) | |
| <1 | 1 (10) |
| 1–5 | 5 (50) |
| 5–10 | 2 (20) |
| 10–30 | 2 (20) |
| Aggravating factor | |
| Exercise | 3 (30) |
| Post-exercise | 1 (10) |
| Cold | 2 (20) |
| Rest | 5 (50) |
| Sleeping | 6 (60) |
| Relieving factor | |
| Spontaneously | 2 (20) |
| Herbal supplements | 1 (10) |
| Massage | 6 (60) |
| Stretching | 2 (20) |
| Difficulties in life | |
| Often limited daily life | 2 (20) |
| Limited labored work | 3 (30) |
| Often limited labored work | 5 (50) |
Changes in muscle cramps after taurine supplementation in LC patients
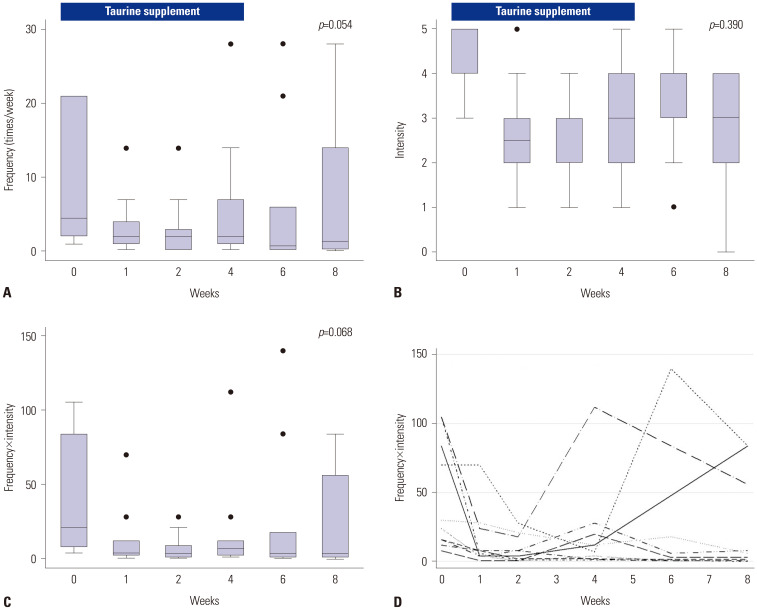
The frequency (Fig. 1A), intensity (Fig. 1B), and scores (frequency ×intensity, Fig. 1C) of muscle cramps during and after four weeks of taurine supplementation (3 g/day) are shown in Fig. 1. Improvement in muscle cramps through weeks 4 and 8 was not statistically significant (frequency, p=0.054; intensity, p=0.390; and frequency×intensity, p=0.068). Compared to baseline, the intensity of muscle cramps significantly decreased at week 4 (p=0.023, Fig. 1B). Fig. 1D indicates the individual changes in the muscle cramp scores of each subject. While muscle cramp scores reduced in 7 patients (70%) by week 4, it improved by week 8 in 2 patients (20%) in whom the score did not decrease by week 4 (Cases #4 and #6 in Table 3). Seven patients (70%) showed a durable response after stopping taurine therapy (until week 8), although three patients showed early or late recurrence (Cases #1, #8, and #9 in Table 3).
Fig. 1. Changes in the frequency of muscle cramps (A), intensity of muscle cramps (B), and muscle cramp scores (C, overall; D, individually) in Korean liver cirrhosis patients before and after taurine replacement.
Table 3. Changes in Plasma Taurine Levels and Muscle Cramp Scores in Korean Liver Cirrhosis Patients at Baseline and at 4 and 8 Weeks after Initiation of Taurine Supplementation.
| No | Age | Sex | Plasma taurine level (µM/L)* | Muscle cramp score (frequency×intensity) | Muscle cramp frequency (times/week) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | |||
| 1 | 75 | F | 18.4 | 147.8† | 329.2 | 4 | 10 | 6 | 1 | 2 | 1.5 |
| 2 | 43 | M | 36.0 | 75.9† | 25.6 | 12 | 4† | 2† | 3 | 1† | 0.5† |
| 3 | 58 | M | 40.8 | 67.9† | 43.1 | 105 | 2† | 0† | 21 | 0.5† | 0† |
| 4 | 72 | M | 53.5 | 476.2† | 33.6 | 8 | 28 | 4† | 2 | 14 | 2† |
| 5 | 62 | M | 55.9 | 71.1† | 162.2 | 6 | 3† | 1† | 2 | 1† | 0.5† |
| 6 | 80 | F | 90.3 | 63.1 | 151.8 | 105 | 112 | 56† | 21 | 28 | 14† |
| 7 | 62 | M | 135.8 | 69.5 | 43.1 | 30 | 6† | 6† | 6 | 2† | 2† |
| 8 | 79 | M | 401.9 | 346.8 | 67.9 | 84 | 12† | 84 | 21 | 6† | 21 |
| 9 | 52 | F | 629.6 | 976.4† | 205.4 | 70 | 7† | 84 | 14 | 7† | 28 |
| 10 | 70 | F | 660.0 | 539.4 | 4.0 | 8 | 2† | 1.5† | 2 | 1† | 0.5† |
*Reference level: 65–179 µM/L, †Improvement from baseline.
Associations of plasma taurine and serum magnesium and zinc levels with muscle cramps in LC patients
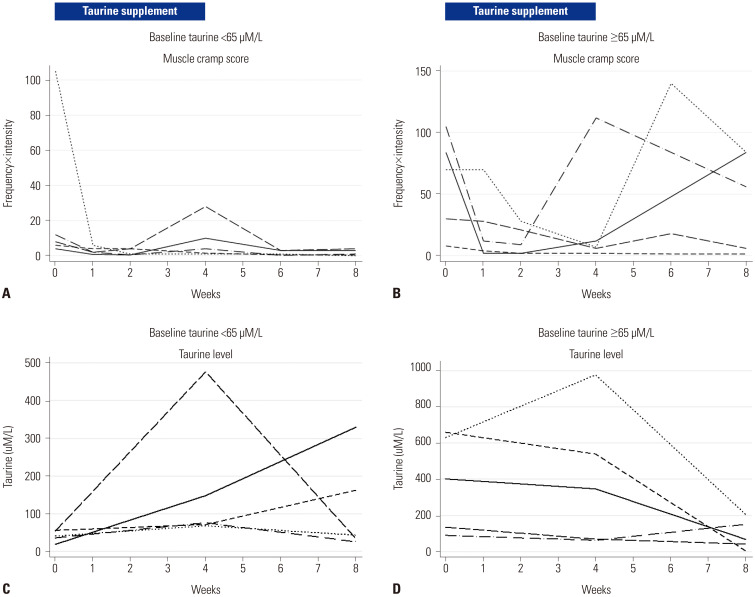
At baseline, the median plasma taurine level was 73.5 µM/L (IQR: 40.7–401.9 µM/L) and was within the reference range (65–179 µM/L);22 however, 5 patients (50%) had taurine levels below the reference limit (<65 µM/L). After 4 weeks of taurine supplementation, the median plasma taurine level increased to 111.9 µM/L (IQR: 69.5–476.2 µM/L; p=0.475). After discontinuation of taurine for 4 weeks, the median plasma taurine level at week 8 decreased to 55.5 µM/L (IQR: 33.6–162.2 µM/L); however, this was not significantly different from the levels at week 4 (p=0.203) or at baseline (p=0.328). Nonetheless, all five patients with low baseline plasma taurine levels showed an increased taurine level after 4 weeks of taurine supplementation; among these, 3 (60%) and 4 (80%) patients experienced an improvement in the muscle cramps at weeks 4 and 8, respectively. Among the remaining five patients with normal or higher taurine levels, 4 (80%) and 3 (60%) patients had an improved muscle cramp score at weeks 4 and 8, respectively (Fig. 2).
Fig. 2. Change in muscle cramp scores (A and B) and plasma taurine levels (C and D) according to baseline taurine levels in Korean liver cirrhosis patients before and after taurine replacement.
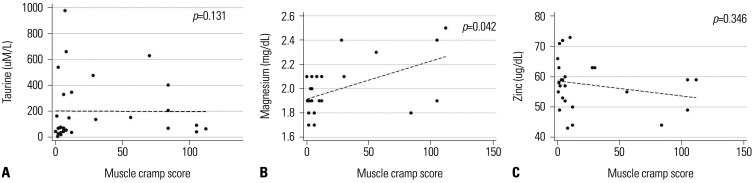
Muscle cramp scores tended to be lower in patients with low taurine levels (median score: 8, IQR: 6–12) than in patients with normal or higher taurine levels (median score: 70, IQR: 30–84, p=0.058). This suggested that taurine level is not a major determinant of muscle cramps. As shown in Fig. 3A, plasma taurine levels were not significantly correlated with muscle cramp scores (ρ=−0.02, p=0.306). After 4 weeks of taurine therapy, plasma taurine levels increased in six patients (60%; Cases #1–5 and #9 in Table 3); among these, four patients experienced an improvement in symptoms at week 4 (Cases #2, #3, #5, and #9), while one patient (16.7%) experienced recurrence after stopping the therapy (Case #9, Table 3) in spite of a remarkably high drug compliance (98.5%).
Fig. 3. Associations among plasma taurine (A), serum magnesium (B), and serum zinc (C) levels, and muscle cramp scores in liver cirrhosis patients.
As electrolytes potentially correlated with muscle cramps, serum Mg and Zn levels were analyzed in relation to muscle cramp scores. Median Mg levels at baseline, week 4, and week 8 were 2.1 (IQR: 1.9–2.1), 1.9 (IQR: 1.9–1.9), and 1.9 (IQR: 1.8–2.1) mg/dL, respectively (p=0.056). The median Zn levels at baseline, week 4, and week 8 were 57 (IQR: 49–63), 60 (IQR: 57–63), and 55 (IQR: 52–59) µg/dL, respectively (p=0.166). Serum Mg levels were positively correlated with muscle cramp score (ρ=0.410, p=0.042, Fig. 3B), while serum Zn levels were not (ρ=−0.197, p=0.346, Fig. 3C).
DISCUSSION
In this study, we noted that daily supplementation of an oral solution of 3 g taurine for 4 weeks improved muscle cramps in 70% and 80% of LC patients by weeks 4 and 8, respectively. Around 50% of the patients exhibited lower taurine levels at baseline, and taurine supplementation in these individuals tended to provide a durable benefit. During the 4-week taurine therapy, no serious adverse events were observed.
Matsuzaki, et al.23,24 reported that muscle cramps disappeared with taurine supplementation (3 g thrice daily for 5–24 months, n=11) in 72.7% of their patients and with taurine supplementation (6 g thrice daily for 6 months, n=12) in 67% of cirrhotic patients. Yamamoto21 indicated that a lower dose of taurine (3 g/day for 4 weeks, n=35) resulted in the disappearance and improvement of muscle cramps in 37.1% and 71.4% of the patients, respectively, which is similar to the present study. However, these studies were published as letters; therefore, the methods for assessing compliance to taurine replacement and measuring muscle cramps in the study populations were unclear. According to a meta-analysis25 on 77 patients (from five prospective treatment reports and one case study), the use of 3–18 g of taurine daily for 4–24 weeks resolved or improved muscle cramps in all subjects. Moreover, serious adverse events were not reported. Furthermore, a recent placebo-controlled, cross-over designed trial26 in Australian patients using taurine capsules (1 g/day for 2 weeks and 2 g/day for the subsequent 2 weeks) showed that 2 g/day of taurine significantly reduced the frequency, duration, and severity of muscle cramps. In that study, the subjects' mean age was 54 years, and 61.2% (30/49) had completed the study protocol taking taurine. Compared to these previous studies, our study clearly demonstrated high adherence to an oral taurine solution (98.5% of compliance), which improved muscle cramps in 70% of the subjects.
The serial measurement of blood taurine, Mg, and Zn levels in the study population was a strength of this study. At baseline, the plasma taurine level was below normal in five of the 10 cirrhotic patients in this study. A 4-week taurine replacement achieved normal plasma taurine levels in all patients by week 4; however, neither the plasma taurine level nor its increase were individually correlated with muscle cramp scores. Notably, two patients (Cases #8 and #9 in Table 3) who showed rebound aggravation of muscle cramps after taurine supplementation had very high plasma taurine levels at baseline, which may have attenuated the effect of oral taurine supplementation. Taurine concentrations can change in stress states, such as osmotic changes or anoxia,27 and with the intake of taurine-rich foods, such as seafood, fish, meat, and dairy.28 A randomized controlled study26 reported that in response to oral taurine replacement with a titrated dose of 1–2 g/day for 4 weeks, the mean serum taurine level in the taurine-treated group increased by 2.5 times (178±19 µmol/L) from baseline (59±3 µmol/L), while that in the placebo group (67±6 µmol/L) remained similar to baseline (reference range: 50–100 µmol/L). However, correlation between muscle cramps and serum taurine level was not investigated in that study. The present study showed that subjects with very low baseline taurine levels benefitted from taurine supplementation, as most of these patients experienced an improvement in muscle cramps after taurine therapy.
Recently, two hypotheses have been suggested for the mechanism of muscle cramp development. The first is the electrolyte depletion/dehydration hypothesis that focuses on the shift in interstitial fluid due to electrolyte deficiency, which results in the alteration of nerve excitability. The second is the neuromuscular hypothesis that emphasizes muscle fatigue resulting from an imbalance between excitation from the muscle spindle and inhibition from the Golgi system.29 Theoretically, taurine replacement is expected to enhance neuromuscular stability in cirrhotic patients, consequently improving muscle cramps therein; however, there are various factors that affect both electrolyte balance and muscle cell stability. Because plasma taurine and serum Mg and Zn deficiencies were not significantly related with muscle cramp scores in the present study, the mechanism of symptom improvement with taurine supplementation remains unclear. This study had a limitation as a single-arm pilot study and enrolled a limited number of subjects. Nonetheless, our results stress the need for assessment of and interventions for muscle cramps in cirrhotic patients. Further studies considering various determinants of neuronal activity and the effect of hepatic impairment on them are warranted to understand the entire mechanism of muscle cramps in cirrhotic patients.
In conclusion, muscle cramps are s a significantly problematic symptom that lowers quality of life in cirrhotic patients. These patients could benefit from an attempt to address and control the symptoms. A 4-week oral taurine solution therapy showed excellent compliance and safety, with improvement in muscle cramps in most study subjects, regardless of an increase in blood taurine. The mechanism of the symptom improvement needs to be elucidated in a larger-scale study.
ACKNOWLEDGEMENTS
This study was funded by Dong-A Pharmaceutical. The authors are grateful to the devoted research coordinator Dawoon Jeong.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Eun Sun Jang and Sook-Hyang Jeong.
- Data curation: all authors.
- Formal analysis: Eun Sun Jang.
- Funding acquisition: Sook-Hyang Jeong.
- Investigation: all authors.
- Methodology: all authors.
- Project administration: Sook-Hyang Jeong.
- Resources: all authors.
- Software: Eun Sun Jang.
- Supervision: Sook-Hyang Jeong.
- Validation: all authors.
- Visualization: Eun Sun Jang.
- Writing—original draft: Eun Sun Jang.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
References
- 1.Miller TM, Layzer RB. Muscle cramps. Muscle Nerve. 2005;32:431–442. doi: 10.1002/mus.20341. [DOI] [PubMed] [Google Scholar]
- 2.Chatrath H, Liangpunsakul S, Ghabril M, Otte J, Chalasani N, Vuppalanchi R. Prevalence and morbidity associated with muscle cramps in patients with cirrhosis. Am J Med. 2012;125:1019–1025. doi: 10.1016/j.amjmed.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murata A, Hyogo H, Nonaka M, Sumioka A, Suehiro Y, Furudoi A, et al. Overlooked muscle cramps in patients with chronic liver disease: in relation to the prevalence of muscle cramps. Eur J Gastroenterol Hepatol. 2019;31:375–381. doi: 10.1097/MEG.0000000000001294. [DOI] [PubMed] [Google Scholar]
- 4.Konikoff F, Theodor E. Painful muscle cramps. A symptom of liver cirrhosis? J Clin Gastroenterol. 1986;8:669–672. doi: 10.1097/00004836-198612000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Abrams GA, Concato J, Fallon MB. Muscle cramps in patients with cirrhosis. Am J Gastroenterol. 1996;91:1363–1366. [PubMed] [Google Scholar]
- 6.Marotta PJ, Graziadei IW, Ghent CN. Muscle cramps: a ‘complication’ of cirrhosis. Can J Gastroenterol. 2000;14 Suppl D:21D–25D. doi: 10.1155/2000/214916. [DOI] [PubMed] [Google Scholar]
- 7.Marchesini G, Bianchi G, Amodio P, Salerno F, Merli M, Panella C, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 8.Iwasa M, Karino Y, Kawaguchi T, Nakanishi H, Miyaaki H, Shiraki M, et al. Relationship of muscle cramps to quality of life and sleep disturbance in patients with chronic liver diseases: a nationwide study. Liver Int. 2018;38:2309–2316. doi: 10.1111/liv.13745. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SS, Fallon MB. Muscle cramps in cirrhosis: a moving target. Clin Gastroenterol Hepatol. 2015;13:1544–1546. doi: 10.1016/j.cgh.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Corbani A, Manousou P, Calvaruso V, Xirouchakis I, Burroughs AK. Muscle cramps in cirrhosis: the therapeutic value of quinine. Is it underused? Dig Liver Dis. 2008;40:794–799. doi: 10.1016/j.dld.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Elfert AA, Abo Ali L, Soliman S, Zakaria S, Shehab El-Din I, Elkhalawany W, et al. Randomized placebo-controlled study of baclofen in the treatment of muscle cramps in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:1280–1284. doi: 10.1097/MEG.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 12.Henry ZH, Northup PG. Baclofen for the treatment of muscle cramps in patients with cirrhosis: a new alternative. Hepatology. 2016;64:695–696. doi: 10.1002/hep.27988. [DOI] [PubMed] [Google Scholar]
- 13.Kugelmas M. Preliminary observation: oral zinc sulfate replacement is effective in treating muscle cramps in cirrhotic patients. J Am Coll Nutr. 2000;19:13–15. doi: 10.1080/07315724.2000.10718908. [DOI] [PubMed] [Google Scholar]
- 14.Konikoff F. The therapeutic benefit of vitamin E in patients with liver disease. J Hepatol. 1994;21:687–688. doi: 10.1016/s0168-8278(94)80124-x. [DOI] [PubMed] [Google Scholar]
- 15.Chandok N, Tan P, Uhanova J, Shankar N, Marotta P. A pilot study of vitamin E for the treatment of cirrhotic muscle cramps. Liver Int. 2011;31:586–587. doi: 10.1111/j.1478-3231.2011.02464.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi H, Kurosaki M, Tsuchiya K, Nakakuki N, Takada H, Matsuda S, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540–1543. doi: 10.1016/j.cgh.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Imbe A, Tanimoto K, Inaba Y, Sakai S, Shishikura K, Imbe H, et al. Effects of L-carnitine supplementation on the quality of life in diabetic patients with muscle cramps. Endocr J. 2018;65:521–526. doi: 10.1507/endocrj.EJ17-0431. [DOI] [PubMed] [Google Scholar]
- 18.Sako K, Imamura Y, Nishimata H, Tahara K, Kubozono O, Tsubouchi H. Branched-chain amino acids supplements in the late evening decrease the frequency of muscle cramps with advanced hepatic cirrhosis. Hepatol Res. 2003;26:327–329. doi: 10.1016/s1386-6346(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 19.Hidaka H, Nakazawa T, Kutsukake S, Yamazaki Y, Aoki I, Nakano S, et al. The efficacy of nocturnal administration of branched-chain amino acid granules to improve quality of life in patients with cirrhosis. J Gastroenterol. 2013;48:269–276. doi: 10.1007/s00535-012-0632-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee FY, Lee SD, Tsai YT, Lai KH, Chao Y, Lin HC, et al. A randomized controlled trial of quinidine in the treatment of cirrhotic patients with muscle cramps. J Hepatol. 1991;12:236–240. doi: 10.1016/0168-8278(91)90944-7. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto S. Oral taurine therapy for painful muscle cramp in liver cirrhosis. Am J Gastroenterol. 1994;89:457–458. [PubMed] [Google Scholar]
- 22.Ghandforoush-Sattari M, Mashayekhi S, Nemati M, Routledge PA. A rapid determination of taurine in human plasma by LC. Chromatographia. 2009;69:1427–1430. [Google Scholar]
- 23.Matsuzaki Y, Tanaka N, Yamaguchi T, Chuganji Y, Nishi M, Chiba T, et al. Effect of taurine administration for muscle cramp in chronic liver disease: a case of decompensated liver cirrhosis in which muscle cramp disappeared after oral administration of taurine. Kanzo. 1990;31:1464–1469. [Google Scholar]
- 24.Matsuzaki Y, Tanaka N, Osuga T. Is taurine effective for treatment of painful muscle cramps in liver cirrhosis? Am J Gastroenterol. 1993;88:1466–1467. [PubMed] [Google Scholar]
- 25.Vidot H, Carey S, Allman-Farinelli M, Shackel N. Systematic review: the treatment of muscle cramps in patients with cirrhosis. Aliment Pharmacol Ther. 2014;40:221–232. doi: 10.1111/apt.12827. [DOI] [PubMed] [Google Scholar]
- 26.Vidot H, Cvejic E, Carey S, Strasser SI, McCaughan GW, Allman-Farinelli M, et al. Randomised clinical trial: oral taurine supplementation versus placebo reduces muscle cramps in patients with chronic liver disease. Aliment Pharmacol Ther. 2018;48:704–712. doi: 10.1111/apt.14950. [DOI] [PubMed] [Google Scholar]
- 27.Ghandforoush-Sattari M, Mashayekhi S, Krishna CV, Thompson JP, Routledge PA. Pharmacokinetics of oral taurine in healthy volunteers. J Amino Acids. 2010;2010:346237. doi: 10.4061/2010/346237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purchas RW, Rutherfurd SM, Pearce PD, Vather R, Wilkinson BH. Concentrations in beef and lamb of taurine, carnosine, coenzyme Q(10), and creatine. Meat Sci. 2004;66:629–637. doi: 10.1016/S0309-1740(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 29.Giuriato G, Pedrinolla A, Schena F, Venturelli M. Muscle cramps: a comparison of the two-leading hypothesis. J Electromyogr Kinesiol. 2018;41:89–95. doi: 10.1016/j.jelekin.2018.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.