Abstract
Altered high-density lipoprotein cholesterol (HDL-C) subclass distribution in hemodialysis (HD) patients is well documented. Aim of this study is to investigate the relationship between HDL-C subclass distribution and macrovascular events in patients undergoing HD. A total of 164 prevalent HD patients and 71 healthy individuals in one hospital-facilitated clinic were enrolled from May 2019 to July 2019 and individual HD patients was follow-up for one year. Macrovascular events (cerebral stroke, coronary heart disease) were recorded in the study period. The HDL-2b, HDL-3 proportions and biochemical parameters were measured. Pearson correlation test and logistic regression analysis were used to examine correlation and odds ratio (OR). 144 HD patients completed one-year follow-up. Cohort with macrovascular events revealed significantly lower HDL-2b and higher HDL-3 subclass proportions compared to those without events. By multivariable adjustment, HDL-3 subclass proportion revealed significantly increase risk for these events (OR 1.17, 95% CI 1.02–1.41, P = 0.044). HDL-2b subclass was significantly higher and HDL-3 subclass was significantly lower in the HD cohort under the hs-CRP level of < 3 mg/L compared to higher hs-CRP level. In conclusion, HDL-2b and HDL-3 subclasses distributions were associated with macrovascular events in HD patients. Proinflammatory status influences the distribution of HDL-2b and HDL-3 subclasses in HD patients.
Subject terms: Medical research, Nephrology
Introduction
Patients with chronic kidney disease (CKD) are at high risk for cardiovascular diseases (CVD) and cerebral stroke (CS)1,2. Various traditional risk factors, such as dyslipidemia, diabetes mellitus and cardiac arrhythmia, possibly contribute to these events1. However, nontraditional factors, such as chronic inflammation, mineral abnormality and uremia, also increased the risk of developing these ecvents3.
It is well recognized that high-density lipoprotein cholesterol (HDL-C) is an independent predictor of CVD in the general population4. Dyslipidemia also has been considered as a major risk factor for CVD in the CKD population5,6. Screening for dyslipidemia in patients with CKD is advocated by clinical guidelines4,7. A well- known in CKD population is that HDL-C alters its composition and function. However, the association of HDL-C with CVD risk is still controversial in CKD population8–11.
HDL is composed of varying subclass particles, different in chemical characteristics and physicochemical properities12,13. By analytic measures, HDL can be divided into the following subclasses: HDL-2a, HDL-2b, HDL-3a, HDL-3b, HDL-3c, pre-beta1-HDL, and pre-beta2-HDL13. In prior studies, HDL subclasses contribute to different effects on atherosclerosis. An increased in the proportion of large particle, such as HDL-2b, exerts an antiatherogenic effect. In contrast, an increased in the proportion of smaller particle, such as HDL-3 and pre-beta1-HDL, is positively associated with CVD12,14. Due to the complex technique and instrument requirement, the measurement of HDL subclasses is not widely applied in the past years. Recently, a microfluidic chip-based technique is developed. This fast, easy-to-operate technique is successfully applied to measure the HDL subclasses in the epidemiological studies15,16.
We hypothesized that patients with hemodialysis (HD) have altered HDL subclasses distribution compared with healthy individuals. Consequently, this effect leads to an increase risk for macrovascular events in HD patients. Therefore, this study aimed to investigate the impact of HDL-2b and HDL-3 subclass distributions on the incidence of macrovascular events in a cohort of HD patients.
Results
Characteristics of participants
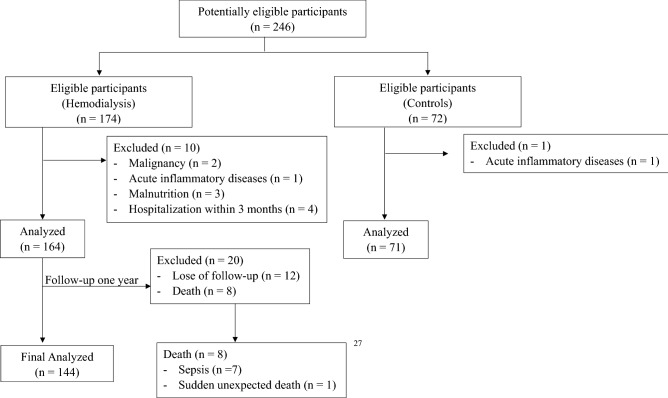
Participants were screening by inclusion and exclusion criteria. Finally, a total of 164 participants with HD and 71 healthy controls were included in the baseline analyses and 144 HD participants completed one-year follow-up (Fig. 1). The mean ages of participants with HD and healthy controls were 63.1 and 49.9 years, respectively. The proportions of male and female participants in the HD cohort and healthy controls were 48.8% and 51.2% and 31.0% and 69.0%, respectively.
Figure 1.
Participants flow diagram.
In the comparison of lipid profiles, HD patients showed a significantly lower HDL-2b subclass proportion compared with healthy controls as well as lower total cholesterol, HDL-C and LDL-C levels. The HDL-3 subclass proportion was not significantly different between the HD patients and healthy controls (Table 1).
Table 1.
Baseline characteristics in study cohort (N = 235).
| Variables | HD (n = 164) |
Control (n = 71) |
P |
|---|---|---|---|
| Age, years | 63.1 ± 12.3 | 49.9 ± 10.6 | < 0.001 |
| Sex | 0.017 | ||
| Female | 84 (51.2%) | 49 (69.0%) | |
| Male | 80 (48.8%) | 22 (31.0%) | |
| BMI, kg/m2 | 23.5 ± 12.5 | 23.3 ± 4.1 | 0.925 |
| Dialysis vintage, years | 10.48 ± 7.53 | – | – |
| Diabetes | 27 (16.5%) | N/A | – |
| Antihypertensive | 66 (40.2%) | – | |
| Lipid-lowering drugs | 24 (14.6%) | – | |
| Etiology of kidney failure | – | ||
| Primary kidney disease | 61 (37.2%) | N/A | |
| Systemic disease | 79 (48.2%) | N/A | |
| Unknown | 24 (14.6%) | N/A | |
| Laboratory measurements | |||
| Hemoglobin, g/dL | 10.7 ± 1.2 | 12.6 ± 1.7 | < 0.001 |
| Total leukocytes, 109/L | 6.3 ± 2.4 | 6.1 ± 1.7 | 0.567 |
| Albumin, g/dL | 3.9 ± 0.3 | 4.2 ± 0.8 | 0.075 |
| BUN, mg/dL | 69 (32–151) | 13 (7–46) | < 0.001 |
| Cr, mg/dL | 10.4 ± 2.3 | 0.7 ± 0.2 | < 0.001 |
| Total Cholesterol, mg/dL | 152 (94–314) | 189 (135–310) | < 0.001 |
| Triglyceride, mg/dL | 108 (30–994) | 80.5 (18–237) | < 0.001 |
| HDL-C, mg/dL | 44.5 ± 15.4 | 59.8 ± 14.0 | < 0.001 |
| HDL-2b, % | 23.6 ± 9.1 | 31.2 ± 6.7 | < 0.001 |
| HDL-3, % | 31.7 ± 11 | 33.6 ± 7.5 | 0.137 |
| LDL-C, mg/dL | 88.1 ± 35.0 | 112.1 ± 30.8 | < 0.001 |
| Intact-PTH, pg/ml | 268.2 (2.4–2819.9) | 53.6 (20.9–90.2) | < 0.001 |
| hs-CRP, mg/L | 6.9 ± 13.0 | 2.7 ± 5.0 | 0.016 |
BMI body mass index, HDL high-density lipoprotein, LDL low-density lipoprotein, C cholesterol, T total, PTH parathyroid hormone, BUN blood urea nitrogen, Cr creatinine, hs-CRP high-sensitivity C-reactive protein.
Comparison of macrovascular events in HD participants based on baseline characteristics.
In one-year follow-up in HD participants, cohort with macrovascular events (CHD/CS) revealed significantly lower HDL-2b subclass proportion and higher HDL-3 subclass proportion compared to those without events (Table 2). Table 3 performed the logistic regression analysis results that evaluated the association between CHD/CS and baseline characteristics in HD patients. All demographic characteristics and laboratory measurements in Table 2 were included in both univariate and fully adjusted multivariate regression analyses. In univariate analysis results, both HDL-2b and HDL-3 showed no significant association with the risk of CHD/CS. However, the fully adjusted multivariate regression results indicated HDL-3 subclass proportion revealed significantly increase risk for CHD/CS events (odds ratio 1.17, P = 0.044) (Table 3). Hence, the inclusion of other demographics characteristics and laboratory measurements were potentially adjusted the association between HDL-3 and CHD/CS.
Table 2.
Baseline characteristics in hemodialysis patients (N = 144).
| Variables | CHD/CS (n = 27) |
Non CHD/CS (n = 117) | P |
|---|---|---|---|
| Age, years | 65.8 ± 13.6 | 62.3 ± 12.3 | 0.226 |
| HD vintage, years | 11.2 ± 8.1 | 10.7 ± 7.6 | 0.784 |
| Sex | 0.122 | ||
| Female | 9 (33.3%) | 61 (52.1%) | |
| Male | 18 (66.7%) | 56 (47.9%) | |
| DM | 8 (29.6%) | 18 (15.4%) | 0.145 |
| Etiology | 0.862 | ||
| Primary kidney disease | 8 (29.6%) | 42 (35.9%) | |
| Systemic disease | 15 (55.6%) | 56 (47.9%) | |
| Others | 4 (14.8%) | 19 (16.2%) | |
| Laboratory measurements | |||
| Hemoglobin, g/dL | 11 ± 1.0 | 10.6 ± 1.3 | 0.139 |
| Total leukocytes, 109/L | 6.7 ± 2.5 | 6.1 ± 2.4 | 0.290 |
| Albumin, g/dL | 3.9 ± 0.4 | 3.9 ± 0.3 | 0.930 |
| BUN, mg/dL | 73 (37–111) | 68 (32–151) | 0.339 |
| Cr, mg/dL | 10.6 ± 2.5 | 10.3 ± 2.3 | 0.661 |
| Total cholesterol, mg/dL | 143 (102–195) | 151 (94–314) | 0.069 |
| Triglyceride, mg/dL | 110 (53–290) | 102 (30–300) | 0.664 |
| HDL-C, mg/dL | 42.6 ± 13.4 | 45.8 ± 15.4 | 0.285 |
| HDL-2b, % | 20.0 ± 7.7 | 24.9 ± 9.1 | 0.006 |
| HDL-3, % | 34.9 ± 11.0 | 29.6 ± 9.8 | 0.026 |
| LDL-C, mg/dL | 78.6 ± 26.7 | 88.1 ± 34.1 | 0.122 |
| Intact-PTH, pg/ml | 199.7 (17.2–2819.9) | 303.1 (2.4–2130) | 0.865 |
| hs-CRP, mg/L | 8.7 ± 12.4 | 6.8 ± 13.9 | 0.469 |
CHD coronary heart disease, CS cerebral stroke.
Table 3.
Logistic regression analysis for CHD/CS.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Dependent variables: CHD/CS vs. Non CHD/CS | ||||
| Age, years | 1.00 (0.95–1.06) | 0.986 | 0.95 (0.85–1.04) | 0.300 |
| HD vintage, years | 0.98 (0.89–1.07) | 0.700 | 0.97 (0.83–1.10) | 0.600 |
| Sex | ||||
| Female | – | – | ||
| Male | 0.74 (0.18–2.92) | 0.700 | 0.60 (0.06–5.27) | 0.600 |
| DM | 4.11 (0.95–16.8) | 0.047 | 1.07 (0.06–17.4) | 0.959 |
| Etiology | ||||
| Primary kidney disease/Others | – | – | ||
| Systemic disease | 3.88 (0.90–26.7) | 0.100 | 3.99 (0.26–113) | 0.300 |
| Laboratory measurements | ||||
| Hemoglobin, g/dL | 1.07 (0.62–1.83) | 0.800 | 1.08 (0.45–2.47) | 0.900 |
| Total leukocytes, 109/L | 1.18 (0.94–1.44) | 0.110 | 1.10 (0.68–1.58) | 0.700 |
| Albumin, g/dL | 0.22 (0.03–1.41) | 0.100 | 0.49 (0.01–28.4) | 0.700 |
| BUN, mg/dL | 0.98 (0.94–1.02) | 0.400 | 0.98 (0.92–1.05) | 0.600 |
| Cr, mg/dL | 0.72 (0.51–0.98) | 0.045 | 0.55 (0.19–1.10) | 0.200 |
| Total cholesterol, mg/dL | 1.00 (0.99–1.01) | 0.400 | 1.37 (0.70–3.08) | 0.400 |
| Triglyceride, mg/dL | 1.00 (0.98–1.01) | 0.800 | 0.19 (0.00–5.59) | 0.400 |
| HDL-C, mg/dL | 0.94 (0.87–0.99) | 0.060 | 4.63 (0.16–269) | 0.400 |
| HDL-2b, % | 0.99 (0.91–1.07) | 0.800 | 1.09 (0.93–1.33) | 0.300 |
| HDL-3, % | 1.06 (0.99–1.14) | 0.110 | 1.17 (1.02–1.41) | 0.044 |
| LDL-C, mg/dL | 1.00 (0.98–1.02) | 0.800 | 5.28 (0.18–322) | 0.400 |
| Intact-PTH, pg/ml | 1.00 (1.00–1.00) | 0.200 | 1.00 (0.99–1.00) | 0.500 |
| hs-CRP, mg/L | 1.02 (0.98–1.05) | 0.300 | 1.02 (0.93–1.08) | 0.700 |
Pearson correlation analysis on study parameters
The results from the Pearson correlation test showed that the HDL-2b subclass proportion was significantly negatively correlated with age, HDL-3 subclass proportion, triglyceride and total peripheral leukocyte count. On the other hand, it was significantly positively correlated with HDL-C, total cholesterol, and hemoglobin. The HDL-3 subclass proportion was significantly negatively correlated with the HDL-2b subclass proportion and HDL-C and positively correlated with triglyceride, hemoglobin, and total peripheral leukocyte count (Table 4).
Table 4.
Pearson correlation analysis.
| Variables | HDL-2b (%) | HDL-3 |
|---|---|---|
| Age (years) | − 0.26*** | 0.01 |
| HDL-2b (%) | 1.00 | − 0.54*** |
| HDL-3 (%) | − 0.54*** | 1.00 |
| HDL-C (mg/dL) | 0.68*** | − 0.52*** |
| Cholesterol (mg/dL) | 0.21** | − 0.03 |
| Triglyceride (mg/dL) | − 0.44*** | 0.51*** |
| LDL-C (mg/dL) | 0.10 | 0.02 |
| Intact-PTH (pg/ml) | < 0.001 | − 0.03 |
| Albumin (g/dL) | 0.05 | 0.13 |
| hs-CRP (mg/L) | − 0.11 | < 0.001 |
| Hemoglobin (g/dL) | 0.16* | 0.14* |
| Total leukocytes (109/L) | − 0.16* | 0.19** |
*p < 0.05, **p < 0.01, ***p < 0.001.
Associations of the hs-CRP levels with the HDL-2b and HDL-3 subclass proportions in HD participants.
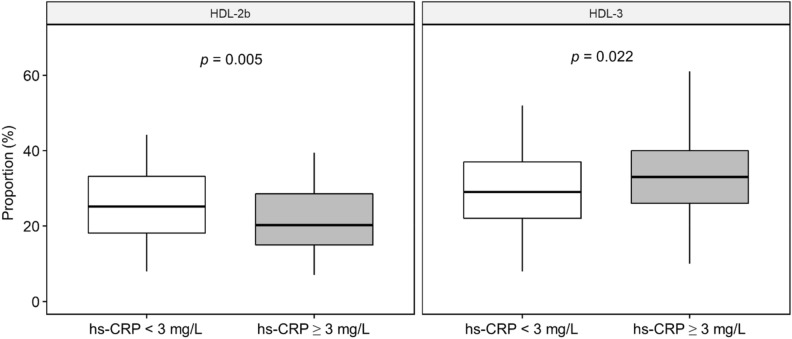
We stratified the entire HD cohort with the cutoff hs-CRP level of 3 mg/L (normal range < 3 mg/L in the laboratory) and examined the associations with the HDL-2b and HDL-3 subclass proportions in HD participants. The results showed that the HDL-2b subclass was significantly higher and HDL-3 subclass was significantly lower in the HD cohort under the hs-CRP level of < 3 mg/L compared to higher hs-CRP level (Fig. 2).
Figure 2.
Proportion of the HDL-2b and HDL-3 subclasses in patients with hemodialysis stratified by an hs-CRP concentration 3 mg/L.
Discussion
The key findings in our study are that HD participants presented lower HDL-2b and HDL-3 subclass proportions compared with healthy controls. Among HD participants, HDL-3 subclass proportion would increase risk for CHD/CS events. Moreover, HDL-2b and HDL-3 subclass distribution were influenced by proinflammatory status, stratified by hs-CRP levels. Our findings imply that potential utilization of the proportion of the HDL subclass analysis in clinical risk stratification for vascular events in HD patients. In this study, we prefer to use proportions of HDL subclass analysis instead of a simple subclass measurement to generalize across the whole spectrum of the HDL levels17,18. The main consideration is technique and cost-consuming in measurement of whole spectrum HDL proportion. Meanwhile, the microfluidic chip-based technique is easy to be applied in the clinical investigation and could forward to cause-effect relationship investigation.
CKD patients have different HDL particles that vary in function, composition, and metabolism19. A growing body of evidence has demonstrated that HDL in CKD decreased its function as anti-inflammatory, antioxidant, and endothelial protection20,21. The mechanisms are complex, but altered HDL composition in CKD is thought to be one of the significant points5,22. Acquired lecithin:cholesterol acyltransferase (LCAT) deficiency is a major reason for low blood HDL in CKD23,24. Moreover, pre-beta1-HDL metabolism is delayed in patients with CKD patients not on dialysis25,26. Result may progress residual renal function decline in CKD patients25. CKD patients initiating HD were reported greater abundance of HDL-associated proteins involving in atherosclerotic, lipid metabolism and inflammatory pathways27. The distribution of HDL subclasses in CKD is not yet uniform reported19. Two reports have described that HD patients have a lower HDL-3 cholesterol level and do not have a decrease in the HDL-2 cholesterol level compared with healthy individuals28. Another report observed contradictory results of increased HDL-3a and decreased HDL-2b subclass levels in patients with HD compared with healthy controls29. It is also reported that HD patients have decreased levels of both HDL-2 and HDL-3 cholesterol30 The discrepancies in the aforementioned studies may have resulted from either the different methods for HDL subclass measurement or statistical comparison in different dialysis populations and relatively small case number. In our study, we used the microfluidic chip-based technique more easily to examine the proportional distribution of the HDL subclasses in HD patients. This technique has been successfully used to measure the HDL subclasses in the Prospective Cardiovascular Munster study15. Our participants with HD showed decreased proportions of both HDL-2b and HDL-3 compared with healthy individuals. This result is in accordance with previous recognition regarding the alteration of HDL subclass distribution in patients with CKD8,22. Nevertheless, the cause-effect relationship of alteration in distributed proportions of the HDL-C subclasses and survival outcomes in patients with CKD still warrants further investigation in a population-based, longitudinal observational study.
CKD is in a proinflammatory status. In clinical practice, CRP is commonly used as a marker of proinflammatory status. In the prior report, hs-CRP level has been linked to malnutrition, inflammation, and atherosclerosis syndrome and is considered to be a risk factor for morbidity and mortality in patients with CKD31. In diabetic mouse model, CRP/oxLDL/β2GPI complex aggravated atherosclerosis by increasing lipid uptake32. In our study, we found that HDL-2b and HDL-3 subclass proportions were influenced by the hs-CRP levels in HD participants. Lower hs-CRP levels were significantly associated with higher HDL-2b and lower HDL-3 subclass proportions. Accordingly, proinflammatory status could suppress the HDL-2b and increase HDL-3 subclass proportions in patients with HD. However, the relationship between the severity of proinflammatory status and HDL subclass distribution still needs to be determined in a future investigation.
Several factors have been implicated in vascular injury in CKD. The factors involves age, obesity, anemia, diabetes, calcium/phosphorus imbalance, proinflammatory status and dyslipidemia33. In vessels of CKD population, there commonly exists atherosclerosis and vascular calcification. Although distinguish risk factors in individual pattern, these vascular phenomenon confer an untoward outcomes in CKD. Consequently, CKD and dialysis patients present a high risk for macrovascular events including CHD and CS2,34,35 HDL plays a major role to against the development and progression of atherosclerosis. Alteration of HDL composition and function have been documented in CKD36. Low lecithin:cholesterol acyltransferase (LCAT) and paraoxonase activity in CKD diminish the conversion of HDL 3 to HDL236,37. Cholesterol ester is transferred to low density lipoprotein (LDL) and very low density lipoprotein (VLDL) when HDL-2b is absent and results in increasing atherogenic particles, cholesterol ester. In addition, uremia-association inflammation causes oxidation of HDL and HDL subfraction abnormalities36. Consequently, CKD patients are predisposed to atherosclerosis. It is well recognized that a decrease content of HDL-2b are positively associated with the risk of CHD14,38. In present study, we found that HD participants with CHD/CS events had significantly lower HDL-2b subclass proportion and higher HDL-3 subclass proportion compared to those without CHS/CS event. This result is in line with reports in general population12,14 Moreover, we found that circulating triglyceride level was negatively correlated to HDL-2b subclass proportion and positively correlated to HDL-3 subclass proportion. We hypothesize that decreasing cholesterol ester donation from HDL-2 to VLDL remnants in exchange for triglyceride36. Taken together, abnormalities in HDL subclass distribution contribute to atherogenic burden in HD patients and increase a risk for CHD/CS events.
Our study has several limitations. First, this was a small-sized, single-center study on an Asian HD population. The results may not be extrapolated to other ethnic HD populations. Second, our study only measured the HDL subclass proportion in HD patients in one time point. The longitudinal effect of HD on HDL subclass distribution cannot be obtained in our study. Third, we did not measure whole HDL subclass proportion, therefore, impact of individual subclass cannot be obtained in this study. Finally, a subset of our cohort received lipid-lowering drugs during the study period. There may be a potential influence of the drug therapy on the HDL-C level or HDL particle distribution. Despite the aforementioned limitations, the strength of our study is that our results were comparable to those of previous studies19,28–30 that have examined the proportions of HDL subclasses, HDL-2b and HDL-3, in HD patients. Thus, our study provides cause-specific data to fill in knowledge gap on the reasons of HDL subclass distribution in these patients. The clinical utility of HDL subclass distribution analysis would be further facilitated by spanning a full-range HDL-C population and determine the subclass distribution on adverse macrovascular events.
In conclusion, HD patients have lower HDL-2b and HDL-3 subclass proportions compared with healthy individuals. The distribution of the HDL-2b and HDL-3 subclasses is influenced by proinflammatory status. Finally, these distribution contribute to the incidence of macrovascular events in HD patients.
Methods
Study design and participants
Adult patients (> 18 years) who underwent maintenance HD thrice weekly for at least 3 months in the outpatient clinic in Kaohsiung Chang Gung Memorial Hospital in Taiwan from May 2019 to July 2019 were enrolled in this study. The exclusion criteria were as follows: ongoing treatment for malignancy, acute inflammatory diseases, hospitalization within 3 months, malnutrition defined by serum albumin level < 3.5 g/dL, and pregnancy. Healthy controls were recruited voluntarily in the outpatient clinic by posted protocol notification. All HD participants were follow-up for one year. Informative patient data, including demographic profiles, laboratory parameters and macrovascular events [coronary heart disease (CHD), cerebral stroke (CS)] in the study period were collected.
The protocol for the study was approved by the Committee on Human Research at Kaohsiung Chang Gung Memorial Hospital (IRB document: 201801486B0) in Taiwan and conducted in accordance with the principles of the Declaration of Helsinki. All participants signed informed consent to approve study initiation.
All data supporting the study is presented in the manuscript or available upon request from the corresponding author of this manuscript, Jin-Bor Chen.
Analytic parameters
All blood samples from HD participants in the fasting status and in mid-week (Wednesday and Thursday) were obtained. Blood samples for biochemistry measurement were obtained using commercial kits and an autoanalyzer (Hitachi 7600-210, Hitachi Ltd., Tokyo, Japan). Albumin levels were measured using the bromocresol green method. Intact parathyroid hormone level was measured using a chemiluminescence immunoassay (Siemens Healthcare Diagnostics Inc., USA)39. The high-sensitivity C-reactive protein (hs-CRP) level was assayed using the immunoturbidimetric method (Spectra East Laboratories, Rockleigh, NJ, USA)40. The plasma total cholesterol, triglyceride, and HDL-C levels were determined enzymatically on the Eroset Hitachi 7600-210 analyzer. The low-density lipoprotein cholesterol (LDL-C) levels were calculated using to the Friedewald formula41, which provides reliable values up to a triglyceride level of 4.0 mmol/L.
HDL-C subclass profiles were measured by electrophoresis of a microfluidic chip system. Briefly, serum samples, calibrator, and QC materials were diluted 1:50 in sample buffer in the presence of a mixture of lipophilic fluorescent dyes and allowed to incubate for 5–15 min prior to loading on to chips. Separation was carried out in a microfluidic device (MICEP-30, Ardent BioMed). The entire procedure was performed in less than 1 h. The HDL-2b and HDL-3 subclasses were automatically calculated in line by a proprietary algorithm (Ardent BioMed LLC, Mt. View, California, USA)15.
Statistical analysis
The baseline demographic characteristics and laboratory measurements in HD patients and healthy controls are presented as frequency (percentage) and mean (standard deviation). The distribution difference was estimated using the independent two-sample t-test or chi-square test. Logistic regression analysis was performed to evaluate the association between CHD/CS and baseline characteristics in HD patients. The demographic characteristics including age, HD vintage, sex, DM, etiology and laboratory measurements were included in univariate logistic regression model. All included variables were retained in multivariate logistic regression model in order to further explore association between CHD/CS and HDL-2b, HDL-3 after adjusted common covariates in HD patients. The correlation between the HDL-2b and HDL-3 proportions and associated variables was estimated using the Pearson correlation test. All P values were two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the R 3.6.3 software (R Core Team, 2019)42.
Acknowledgements
This work was supported by the grant from Kaohsiung Chang Gung Memorial Hospital in Taiwan (document no: CMRPG8J0031).
Author contributions
J.B.C. conceptualized the study. J.B.C. and W.C.L. wrote the methodology. S.H.M. performed the formal analysis. J.B.C. wrote and prepared the original draft. W.C.L. and J.B.C. wrote, reviewed, and edited the manuscript. C.H.Y provided supervision.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sarnak MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American heart association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 2.Shinya Y, et al. Risk factors and outcomes of cerebral stroke in end-stage renal disease patients receiving hemodialysis. J. Stroke Cerebrovasc. Dis. 2020;29:104657. doi: 10.1016/j.jstrokecerebrovasdis.2020.104657. [DOI] [PubMed] [Google Scholar]
- 3.Sarnak MJ, Levey AS. Cardiovascular disease and chronic renal disease: A new paradigm. Am. J. Kidney Dis. 2000;35:S117–131. doi: 10.1016/S0272-6386(00)70239-3. [DOI] [PubMed] [Google Scholar]
- 4.Catapano AL, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 5.Silbernagel G, et al. HDL cholesterol, apolipoproteins, and cardiovascular risk in hemodialysis patients. J. Am. Soc. Nephrol. 2015;26:484–492. doi: 10.1681/ASN.2013080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gansevoort RT, et al. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 7.Li YH, et al. 2017 Taiwan lipid guidelines for high risk patients. J. Formos. Med. Assoc. 2017;116:217–248. doi: 10.1016/j.jfma.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Hager MR, Narla AD, Tannock LR. Dyslipidemia in patients with chronic kidney disease. Rev. Endocr. Metab. Disord. 2017;18:29–40. doi: 10.1007/s11154-016-9402-z. [DOI] [PubMed] [Google Scholar]
- 9.Chang TI, et al. Increments in serum high-density lipoprotein cholesterol over time are not associated with improved outcomes in incident hemodialysis patients. J. Clin. Lipidol. 2018;12:488–497. doi: 10.1016/j.jacl.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Kilpatrick RD, et al. Association between serum lipids and survival in hemodialysis patients and impact of race. J. Am. Soc. Nephrol. 2007;18:293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 11.Honda H, et al. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis. 2012;220:493–501. doi: 10.1016/j.atherosclerosis.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 12.Tian L, Li C, Liu Y, Chen Y, Fu M. The value and distribution of high-density lipoprotein subclass in patients with acute coronary syndrome. PLoS ONE. 2014;9:e85114. doi: 10.1371/journal.pone.0085114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Fu M. Alterations of HDL subclasses in hyperlipidemia. Clin. Chim. Acta. 2003;332:95–102. doi: 10.1016/S0009-8981(03)00138-4. [DOI] [PubMed] [Google Scholar]
- 14.Asztalos BF, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler. Thromb. Vasc. Biol. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 15.Mueller O, et al. PROCAM Study: Risk prediction for myocardial infarction using microfluidic high-density lipoprotein (HDL) subfractionation is independent of HDL cholesterol. Clin. Chem. Lab. Med. 2008;46:490–498. doi: 10.1515/CCLM.2008.117. [DOI] [PubMed] [Google Scholar]
- 16.Ma YH, et al. A case-control study on the relationship between HDL2b and non-alcoholic fatty liver disease in Chinese type 2 diabetic patients. Clin. Chem. Lab. Med. 2009;47:1067–1072. doi: 10.1515/CCLM.2009.257. [DOI] [PubMed] [Google Scholar]
- 17.Moriyama K, Negami M, Takahashi E. HDL2-cholesterol/HDL3-cholesterol ratio was associated with insulin resistance, high-molecular-weight adiponectin, and components for metabolic syndrome in Japanese. Diabetes Res. Clin. Pract. 2014;106:360–365. doi: 10.1016/j.diabres.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Yang HS, et al. HDL subclass analysis in predicting metabolic syndrome in Koreans with high HDL cholesterol levels. Ann. Lab. Med. 2020;40:297–305. doi: 10.3343/alm.2020.40.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsche G, Heine GH, Stadler JT, Holzer M. Current understanding of the relationship of HDL composition, structure and function to their cardioprotective properties in chronic kidney disease. Biomolecules. 2020;10:1348. doi: 10.3390/biom10091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007;72:1149–1156. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto S, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J. Am. Coll. Cardiol. 2012;60:2372–2379. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holzer M, et al. Uremia alters HDL composition and function. J. Am. Soc. Nephrol. 2011;22:1631–1641. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabresi L, et al. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J. Intern. Med. 2015;277:552–561. doi: 10.1111/joim.12290. [DOI] [PubMed] [Google Scholar]
- 24.Baragetti A, et al. Low plasma lecithin: Cholesterol acyltransferase (LCAT) concentration predicts chronic kidney disease. J. Clin. Med. 2020;9:2289. doi: 10.3390/jcm9072289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamatani K, et al. Preβ1-high-density lipoprotein metabolism is delayed in patients with chronic kidney disease not on hemodialysis. J. Clin. Lipidol. 2020;14:730–739. doi: 10.1016/j.jacl.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Kuchta A, et al. Plasma levels of preβ1-HDL are significantly elevated in non-dialyzed patients with advanced stages of chronic kidney disease. Int. J. Mol. Sci. 2019;20:1202. doi: 10.3390/ijms20051202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang K, et al. Alteration of HDL protein composition with hemodialysis initiation. Clin. J. Am. Soc. Nephrol. 2018;13:1225–1233. doi: 10.2215/CJN.11321017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiavon R, et al. HDL3-related decreased serum paraoxonase (PON) activity in uremic patients: Comparison with the PON1 allele polymorphism. Clin. Chim. Acta. 2002;324:39–44. doi: 10.1016/S0009-8981(02)00216-4. [DOI] [PubMed] [Google Scholar]
- 29.Alabakovska SB, Todorova BB, Labudovic DD, Tosheska KN. LDL and HDL subclass distribution in patients with end-stage renal diseases. Clin. Biochem. 2002;35:211–216. doi: 10.1016/S0009-9120(02)00300-4. [DOI] [PubMed] [Google Scholar]
- 30.Samouilidou E, Karpouza A, Grapsa E, Tzanatou-Exarchou H. Serum oxidized LDL is inversely associated with HDL2-cholesterol subclass in renal failure patients on hemodialysis. Nephron. Clin. Pract. 2010;115:c289–294. doi: 10.1159/000313488. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi AR, et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2002;13(Suppl 1):S28–36. [PubMed] [Google Scholar]
- 32.Zhang R, et al. C-reactive protein/oxidised low-density lipoprotein/beta2-glycoprotein I complex promotes atherosclerosis in diabetic BALB/c mice via p38mitogen-activated protein kinase signal pathway. Lipids Health Dis. 2013;12:42. doi: 10.1186/1476-511X-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoccali C. Traditional and emerging cardiovascular and renal risk factors: an epidemiologic perspective. Kidney Int. 2006;70:26–33. doi: 10.1038/sj.ki.5000417. [DOI] [PubMed] [Google Scholar]
- 34.Weiner DE, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J. Am. Soc. Nephrol. 2004;15:1307–1315. doi: 10.1097/01.ASN.0000123691.46138.E2. [DOI] [PubMed] [Google Scholar]
- 35.Sozio SM, et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: The choices for healthy outcomes in caring for ESRD (CHOICE) study. Am. J. Kidney Dis. 2009;54:468–477. doi: 10.1053/j.ajkd.2009.01.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat. Rev. Nephrol. 2010;6:287–296. doi: 10.1038/nrneph.2010.36. [DOI] [PubMed] [Google Scholar]
- 37.Vaziri ND, Liang K, Parks JS. Down-regulation of hepatic lecithin:cholesterol acyltransferase gene expression in chronic renal failure. Kidney Int. 2001;59:2192–2196. doi: 10.1046/j.1523-1755.2001.00734.x. [DOI] [PubMed] [Google Scholar]
- 38.Asztalos BF, et al. Distribution of ApoA-I-containing HDL subpopulations in patients with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2000;20:2670–2676. doi: 10.1161/01.ATV.20.12.2670. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, et al. Association between the achievement of target range CKD-MBD markers and mortality in prevalent hemodialysis patients in Taiwan by using the kidney disease: Improving global outcomes clinical guidelines. Biomed. Res. Int. 2016;2016:1523124. doi: 10.1155/2016/1523124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su YJ, Liao SC, Cheng BC, Hwang JC, Chen JB. Increasing high-sensitive C-reactive protein level predicts peritonitis risk in chronic peritoneal dialysis patients. BMC Nephrol. 2013;14:185. doi: 10.1186/1471-2369-14-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knopfholz J, et al. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterol. 2014;2014:261878. doi: 10.1155/2014/261878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ (2019).