Abstract
Background
Granulomatous Lung Diseases (GLD) encompasses a wide range of infectious and non-infectious conditions characterized by chronic inflammatory response. However, different GLD may share similar imaging findings. In this context, the purpose of this study was to outline the etiological profile and their imaging features in patients with GLD who underwent lung biopsy.
Methods
Patients with granulomatous lesions in lung biopsies and previous chest CT performed from 2014 to 2017 at our institution had imaging data reviewed by three blinded radiologists. The imaging features were analyzed according to the Fleischner Society glossary. Categorical data were represented by absolute (n) and relative (%) frequency. The contingency matrices were analyzed by Pearson's Chi-square test. Interreader agreement was assessed by calculating the intraclass correlation coefficient, using kappa (κ) statistic.
Results
Thirty-eight of 75 (50.7%) patients were women with a mean age of 59 ± 39 years. Infection was the most common cause of GLD (47/75, 62.7%) and Histoplasma capsulatum (27/75, 36%) was the most prevalent etiology. Nodular pattern was the most common imaging feature in histoplasmosis cases (25/27, 92.6%), whereas it occurred in half of cases (24/48) of GLD of other causes (p < 0.05). Among patients with tuberculosis, the second etiology of GLD in our study population, the most common imaging pattern was centrilobular micronodules (3/7, 42.9%), significantly more frequent than in other causes of GLD (6/68, 8.8%). Interreader agreement in detecting imaging features was almost perfect (κ = 0.88-1.00), except the nodular pattern, which had substantial agreement (κ = 0.73).
Conclusions
In our study population, the main etiologies found in patients with granulomatous disease who underwent lung biopsy were fungal or mycobacterial disease, specially histoplasmosis and tuberculosis, and nodular pattern with focal distribution was the most common imaging finding which was detected with substantial interreader agreement.
Abbreviations: CT, Computed Tomography; FOV, Field of View; GLD, Granulomatous lung diseases; GLUS, Granulomatous Lesions of Unknown Significance; JR, Junior Radiologists; κ, Kappa; TB, Tuberculosis
Keywords: Granuloma, lung biopsy, solitary pulmonary nodule, histoplasmosis, pulmonary tuberculosis
1. Introduction
Granulomatous Lung Diseases (GLD) encompasses a wide range of infectious and non-infectious conditions characterized by chronic inflammatory response [1]. Generally, the etiology is defined combining clinical symptoms, laboratorial findings, and imaging features. Patients undergo lung biopsy when the manifestation is atypical or when excluding lung cancer is needed [2].
Mycobacterial and fungal infections are the most common infectious causes of GLD, including tuberculosis (TB), nontuberculous mycobacteriosis, histoplasmosis, cryptococcosis, coccidioidomycosis, blastomycosis, and aspergillosis [3]. TB is a public health problem worldwide, mainly in underdeveloped countries, and was responsible for 1.5 million deaths in 2018 [[4], [5], [6]]. With the development of the HIV epidemic, these infections increased in incidence and lethality. Non-infectious GLD might be the result of several different physiopathological conditions [[7], [8], [9]] and are summarized in Table 1.
Table 1.
Main differential diagnosis of granulomatous lung diseases.
| Infectious | Non-Infectious |
|---|---|
| Fungal | |
| Histoplasmosis | |
| Paracoccidioidomycosis | |
| Coccidioidomycosis | Sarcoidosis |
| Aspergillosis | Bronchocentric Granulomatosis |
| Cryptococcosis | Inflammatory Bowel Disease |
| Blastomycosis | Hypersensitivity Pneumonitis |
| Pneumocystosis | Chronic Beryllium disease |
| Actinomycosis | Silicosis |
| Mycobacterial | Foreign body reaction |
| Tuberculosis | Granulomatosis with Polyangiitis |
| Nontuberculous mycobacteriosis | Eosinophilic Granulomatosis with Polyangiitis |
| Hansen’s Disease | Wegener Granulomatosis |
| Parasitic | Churg-Strauss Syndrome |
| Paragonimiasis | Lymphoid Interstitial Pneumonia |
| Bacterial | Cancer associated with GI |
| Syphilis | Rheumatoid Nodule |
| Tularaemia | Pulmonary Langerhans Cell Histiocytosis |
| Cat-scratch Disease | |
| Whipple’s Disease | |
| Aspiration Pneumonia |
GI = Granulomatous Inflammation
Finally, there are granulomatous lesions of uncertain etiology, comprising a group known as Granulomatous Lesions of Unknown Significance (GLUS). This term was used for the first time in 1990 to designate biopsies results which contained epithelioid granulomas without a definitive cause, even after subsequent investigation [10].
Computed tomography (CT) is the most common imaging modality used to evaluate GLD. However, different GLD may share similar imaging findings. In this context, the purpose of this study was to outline the etiological profile and their imaging findings in patients with granulomatous diseases who underwent lung biopsy. This information can guide further clinical investigation in atypical granulomatous lung cases to possibly avoid invasive procedures.
2. Material and Methods
2.1. Study population
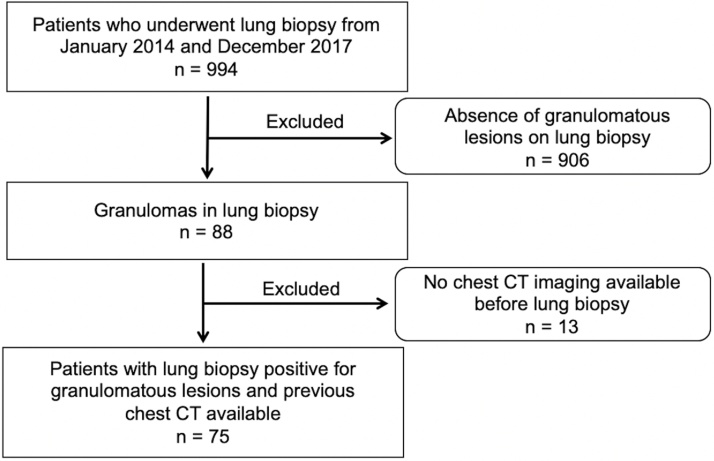
The institutional and national review boards approved our retrospective study and waived the requirement for informed consent. We searched our hospital database for consecutive lung biopsies performed between January 2014 and December 2017 at our institution. The following types of lung biopsies were included: intraoperative biopsy, bronchoscopy with transbronchial biopsy and percutaneous CT-guided needle biopsy. The inclusion criterion was the existence of granulomatous lesions in the pathology report (88 of 994 patients). Patients without chest CT previous to lung biopsy were excluded and the final study population was 75 patients. The patient accrual is summarized in Fig. 1.
Fig. 1.
Flowchart of patient selection.
2.2. Demographic and Pathologic Data
Pathological reports of all patients who underwent lung biopsy between January 2014 and December 2017 were obtained from the hospital database. A keyword search was performed and reports containing “granuloma(s)” and/or “granulomatous” were selected. The following data were assessed: gender, age and the etiology of the GLD.
All cases were analyzed by two pathologists with subspecialty training in pulmonary pathology and with at least 8 years of training. Each pathological specimen with diagnosis of GLD underwent specific tests for infection, besides the usual Hematoxylin-Eosin stain.
2.3. Computed Tomography Exams
CT scans were performed on one of the following devices: a dual-source 256 row detector CT system (SOMATOM® Definition Flash; Siemens Healthcare, Erlangen, Germany) or a 128-row detector CT system (SOMATOM® Definition AS; Siemens Healthcare, Erlangen, Germany). The acquisition parameters were the same for both devices as follows: 115–160 mAs; 120 kVp; tube rotation time, 0.5 s; pitch, 1.2; and field of view (FOV), 400–430 mm. When contrast was required, the timing was determined with a test bolus that consisted of 10–15 ml of iohexol solution (OmnipaqueTM 300; GE Healthcare, Milwaukee, WI) with an iodine concentration of 300 mg ml-1 injected at a flow rate of 4.0–5.5 ml/s-1. Contrast imaging was performed with 1.0 - 1.5 ml/kg injected at the same flow rate. Images were reconstructed in the axial view using a slice thickness of 1 mm and an increment of 0.7 mm.
2.4. Image review
Two junior thoracic radiologists with 1 year experience in chest radiology, blinded to the pathological data, reviewed the radiological exams. The imaging features were analyzed according to the Fleischner Society glossary [11]. A senior thoracic radiologist with 10 years of experience reviewed all cases of disagreement to reach a consensus.
The CT findings were categorized according the main radiological pattern: consolidation, ground-glass opacity, mass, nodular or micronodular. Micronodular pattern was subcategorized into random, perilymphatic or centrilobular. The location of the imaging findings was divided into right, left or bilateral; the distribution was focal, multifocal or diffuse; and the associated findings were cavity, mediastinal/hilar lymphadenopathy, and pleural effusion.
Consolidation was defined as a homogeneous increase in pulmonary parenchymal attenuation that obscured the margins of the vessels and airway walls. Ground-glass opacity was noted as a hazy increased opacity of lung, with preservation of the bronchial and vascular margins. Nodule corresponded to a rounded or irregular opacity, measuring up to 3 cm in diameter, while mass corresponded to a solid or partly solid lesion greater than 3 cm. Micronodular pattern was defined by the presence of innumerable small rounded opacities that ranged from 2 to 10 mm [11].
Cavity was a gas-filled space within pulmonary consolidation, mass or nodule. Lymphadenopathy consisted of mediastinal lymph nodes greater than 1 cm in short-axis diameter and hilar nodes bigger than 3 mm. Pleural effusion was noted when there were free liquid in the pleural space [11].
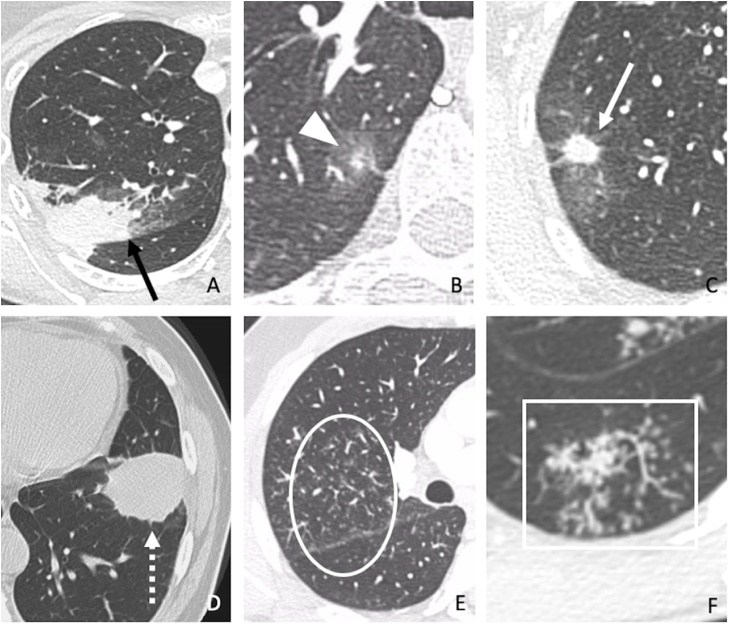
Fig. 2, Fig. 3 demonstrate the main radiological findings evaluated in our study.
Fig. 2.
Main radiological findings assessed in our study. (A) Consolidation (black arrow) in a 54-year-old patient with sarcoidosis. (B) Ground-glass opacity (arrowhead) in a 61-year-old patient with paracoccidioidomycosis. (C) Nodule (white arrow) in a 77-year-old patient with histoplasmosis. (D) Mass (dashed arrow) in a 51-year-old patient with cryptococcosis. (E) Perilymphatic micronodules in a 41-year-old patient with granulomatous lesions of unknown significance (GLUS) (circle). (F) Centrilobular micronodules (rectangle) in a 30-year-old patient with tuberculosis.
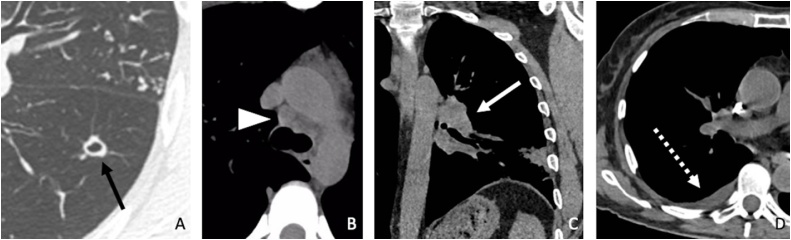
Fig. 3.
Main associated radiological findings evaluated in our study. (A) Cavitated nodule (black arrow) in a 34-year-old patient with tuberculosis. (B) Mediastinal lymphadenopathy (arrowhead) in a 39-year-old patient with sarcoidosis. (C) Hilar lymphadenopathy (white arrow) in a 75-year-old patient with histoplasmosis. (D) Pleural effusion (dashed arrow) in a 76-year-old patient with unclassified mycobacteriosis.
2.5. Statistical Analysis
Categorical data were represented by absolute (n) and relative (%) frequency. Two-tailed p-values ≤ 0.05 were considered significant. The contingency matrices were analyzed by Pearson's Chi-square test. Interreader agreement was assessed by calculating the intraclass correlation coefficient. On categorical features, it was assessed using kappa (κ) statistic or weight kappa statistic with squared weights (for more than two levels) between the two junior radiologists (JR). Kappa (κ) values were interpreted as follows: 0.00-0.20, slight agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderate agreement; 0.61-0.80, substantial agreement; and 0.81-1.00, almost perfect agreement [12]. Statistical analyses were done using SPSS for Windows version 19.0 (Chicago, IL, USA).
3. Results
3.1. Population Data
The study population consisted of 75 patients, 49,3% men (37/75) and 50,7% women (38/75). The mean age was 57 (range 18-96). The majority of the samples were obtained by CT-guided biopsy (47/75, 62.7%), 23/75 (30.7%) by intra-operative biopsy and 5/75 (6.7%) were acquired by bronchoscopy.
Infection was the most common cause of GLD (47/75, 62.7%) and GLUS corresponded to 24% (18/75) of the cases. Among infectious causes of GLD, the great majority was fungal or mycobacterial, caused by Histoplasma capsulatum (27/75, 36%) and Mycobacterium tuberculosis (7/75, 9.3%). Unclassified mycobacterial infection represented 6.7% of cases (5/75). Other fungal infections included Paracoccidioidomycosis (3/75, 4.0%), Aspergillosis (3/75, 4.0%) and Cryptococosis (1/75, 1.3%). Only one case of parasitic infection occurred, represented by Paragominiasis.
Among non-infectious causes, sarcoidosis occurred in 4 cases (5.4%) and granulomatous inflammation associated with cancer occurred in 6 cases (8.0%). Other non-infectious causes did not occur in our study population. Table 2 summarizes the etiological profile of the included patients.
Table 2.
Imaging features of granulomatous lung diseases.
| Histoplasmosis | Tuberculosis | Cancer + GI |
Unclassified Mycobacteriosis |
Sarcoidosis | Aspergillosis | Paracoccidioido-mycosis | Cryptococcosis | Paragonimiasis | GLUS | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiological Pattern | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| Consolidation | 1 | (3.7) | 2 | (28.6) | 0 | 1 | (20.0) | 2 | (50.0) | 1 | (33.3) | 0 | 0 | 0 | 0 | 7 | (9.3) | |||||
| Ground-glass opacity | 0 | 0 | 1 | (16.7) | 0 | 0 | 0 | 1 | (33.3) | 0 | 0 | 0 | 2 | (2.7) | ||||||||
| Nodule | 25 | (92.6) | 2 | (28.6) | 3 | (50.0) | 1 | (20.0) | 1 | (25.0) | 0 | 2 | (66.7) | 0 | 0 | 15 | (83.3) | 49 | (65.3) | |||
| Mass | 1 | (3.7) | 0 | 2 | (33.3) | 1 | (20.0) | 0 | 1 | (33.3) | 0 | 1 | (100.0) | 0 | 0 | 6 | (8.0) | |||||
| Random micronodules | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| Perilymphatic micronodules | 0 | 0 | 0 | 0 | 1 | (25.0) | 0 | 0 | 0 | 0 | 1 | (5.6) | 2 | (2.7) | ||||||||
| Centrilobular micronodules | 0 | 3 | (42.9) | 0 | 2 | (40.0) | 0 | 1 | (33.3) | 0 | 0 | 1 | (100.0) | 2 | (11.1) | 9 | (12.0) | |||||
| Associated Findings | ||||||||||||||||||||||
| Cavity | 0 | 4 | (57.1) | 1 | (16.7) | 1 | (20.0) | 0 | 0 | 0 | 0 | 0 | 0 | 6 | (8.0) | |||||||
| Mediastinal lymphadenopathy | 3 | (11.1) | 1 | (14.3) | 1 | (16.7) | 0 | 1 | (25.0) | 1 | (33.3) | 0 | 0 | 0 | 1 | (5.5) | 8 | (10.7) | ||||
| Hilar lymphadenopathy | 5 | (18.5) | 0 | 1 | (16.7) | 1 | (20.0) | 2 | (50.0) | 1 | (33.3) | 0 | 0 | 0 | 2 | (11.1) | 12 | (16.0) | ||||
| Pleural effusion | 2 | (7.4) | 1 | (14.3) | 0 | 2 | (40.0) | 0 | 2 | (66.7) | 0 | 0 | 0 | 1 | (5.6) | 8 | (10.7) | |||||
| Location and Distribution | ||||||||||||||||||||||
| Unilateral (Right) | 11 | (40.7) | 2 | (28.6) | 3 | (50.0) | 2 | (40.0) | 1 | (25.0) | 1 | (33.3) | 2 | (66.7) | 0 | 0 | 6 | (33.3) | 28 | (37.3) | ||
| Unilateral (Left) | 16 | (59.3) | 2 | (28.6) | 2 | (33.3) | 1 | (20.0) | 0 | 1 | (33.3) | 1 | (33.3) | 1 | (100.0) | 0 | 7 | (38.9) | 31 | (41.3) | ||
| Bilateral | 0 | 3 | (42.9) | 1 | (16.7) | 2 | (40.0) | 3 | (75.0) | 1 | (33.3) | 0 | 0 | 1 | (100.0) | 5 | (27.8) | 16 | (21.3) | |||
| Focal | 26 | (96.3) | 3 | (42.9) | 5 | (83.3) | 2 | (40.0) | 0 | 2 | (66.7) | 3 | (100.0) | 0 | 0 | 9 | (50.0) | 50 | (66.7) | |||
| Multifocal | 1 | (3.7) | 3 | (42.9) | 1 | (16.7) | 1 | (20.0) | 4 | (100.0) | 0 | 0 | 1 | (100.0) | 1 | (100.0) | 8 | (44.4) | 20 | (26.7) | ||
| Diffuse | 0 | 1 | (14.3) | 0 | 2 | (40.0) | 0 | 1 | (33.3) | 0 | 0 | 0 | 1 | (5.6) | 5 | (6.7) | ||||||
| Right upper lobe | 5 | (18.5) | 4 | (57.1) | 3 | (50.0) | 3 | (60.0) | 3 | (75.0) | 1 | (33.3) | 1 | (33.3) | 0 | 1 | (100.0) | 6 | (33.3) | 27 | (36.0) | |
| Right middle lobe | 4 | (14.8) | 4 | (57.1) | 0 | 2 | (40.0) | 4 | (100.0) | 1 | (33.3) | 0 | 0 | 1 | (100.0) | 5 | (27.8) | 21 | (28.0) | |||
| Right lower lobe | 5 | (18.5) | 3 | (42.9) | 2 | (33.3) | 3 | (60.0) | 3 | (75.0) | 2 | (66.7) | 1 | (33.3) | 0 | 1 | (100.0) | 6 | (33.3) | 26 | (34.7) | |
| Left upper lobe | 6 | (22.2) | 5 | (71.4) | 2 | (33.3) | 3 | (60.0) | 3 | (75.0) | 1 | (33.3) | 0 | 1 | (100.0) | 1 | (100.0) | 5 | (27.8) | 27 | (36.0) | |
| Left lower lobe | 10 | (37.0) | 2 | (28.6) | 1 | (16.7) | 3 | (60.0) | 2 | (50.0) | 2 | (66.7) | 1 | (33.3) | 1 | (100.0) | 1 | (100.0) | 10 | (55.6) | 33 | (44.0) |
| Total | 27 | (36.0) | 7 | (9.3) | 6 | (8.0) | 5 | (6.7) | 4 | (5.3) | 3 | (4.0) | 3 | (4.0) | 1 | (1.3) | 1 | (1.3) | 18 | (24.0) | 75 | (100.0) |
GI: Granulomatous Inflammation; GLUS: Granulomatous Lesions of Unknown Significance.
3.2. Chest CT findings
The main radiological pattern, location and distribution, as well as associated imaging findings are summarized in Table 2.
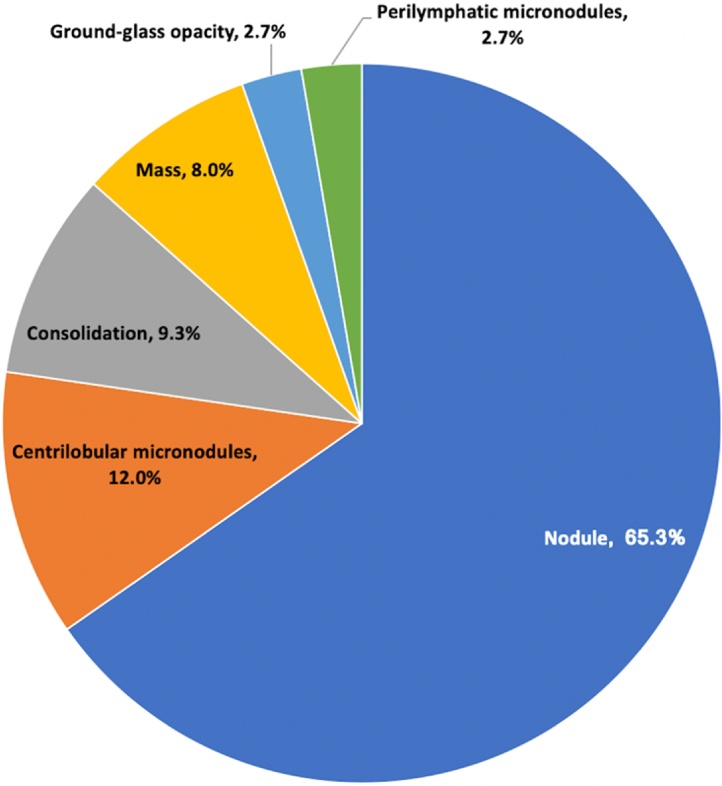
Overall, nodular pattern was the most common CT finding (49/75, 65.3%), followed by centrilobular micronodules (9/75, 12%), as seen in Fig. 4. The majority of cases had unilateral pulmonary findings (59/75, 78.7%), slightly more common in the left lung (31/59, 52.5%). Focal distribution was the most frequent (50/75, 66.7%).
Fig. 4.
Distribution of the radiological patterns in our study population.
Among associated findings, hilar or mediastinal lymphadenopathy occurred respectively in 16% (12/75) and 10.7% (8/75) of the cases; pleural effusion (8/75) and cavitation (8/75) occurred in 10.7% of cases.
Nodular pattern was the most common imaging feature in histoplasmosis cases (25/27, 92.6%), whereas it occurred in half of cases (24/48) of GLD of other causes (p < 0.05). Regarding distribution of the lung lesions, 26/27 (96.3%), were focal in histoplasmosis, while in other causes of GLD focal distribution occurred in 50% (24/48) of cases (p < 0.05) (Table 3).
Table 3.
Comparison of imaging features on chest computed tomography of patients with pulmonary histoplasmosis and tuberculosis.
| Histoplasmosis | Other etiologies | P-value | Tuberculosis | Other etiologies | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Radiological Pattern | n | % | n | % | p | n | % | n | % | p |
| Consolidation | 1 | (3.7) | 6 | (12.5) | 0.208 | 2 | (28.6) | 5 | (7.4) | 0.066 |
| Ground-glass opacity | 0 | 2 | (4.2) | 0.282 | 0 | 2 | (2.9) | 0.646 | ||
| Nodule | 25 | (92.6) | 24 | (50.0) | < 0.001 | 2 | (28.6) | 47 | (69.1) | 0.032 |
| Mass | 1 | (3.7) | 5 | (10.4) | 0.304 | 0 | 6 | (8.8) | 0.413 | |
| Random micronodules | 0 | 0 | 0 | 0 | ||||||
| Perilymphatic micronodules | 0 | 2 | (4.2) | 0.282 | 0 | 2 | (2.9) | 0.646 | ||
| Centrilobular micronodules | 0 | 9 | (18.8) | 0.016 | 3 | (42.9) | 6 | (8.8) | 0.008 | |
| Associated Findings | ||||||||||
| Cavity | 0 | 6 | (12.5) | 0.055 | 4 | (57.1) | 2 | (2.9) | < 0.001 | |
| Mediastinal lymphadenopathy | 3 | (11.1) | 5 | (10.4) | 0.925 | 1 | (14.3) | 7 | (10.3) | 0.745 |
| Hilar lymphadenopathy | 5 | (18.5) | 7 | (14.6) | 0.655 | 0 | 12 | (17.6) | 0.225 | |
| Pleural effusion | 2 | (7.4) | 6 | (12.5) | 0.493 | 1 | (14.3) | 7 | (10.3) | 0.745 |
| Location and Distribution | ||||||||||
| Unilateral (Right) | 11 | (40.7) | 17 | (35.4) | 0.647 | 2 | (28.6) | 26 | (38.2) | 0.615 |
| Unilateral (Left) | 16 | (59.3) | 15 | (31.3) | 0.018 | 2 | (28.6) | 29 | (42.6) | 0.471 |
| Bilateral | 0 | 16 | (33.3) | < 0.001 | 3 | (42.9) | 13 | (19.1) | 0.144 | |
| Focal | 26 | (96.3) | 24 | (50.0) | < 0.001 | 3 | (42.9) | 47 | (69.1) | 0.161 |
| Multifocal | 1 | (3.7) | 19 | (39.6) | < 0.001 | 3 | (42.9) | 17 | (25.0) | 0.309 |
| Diffuse | 0 | 5 | (10.4) | 0.083 | 1 | (14.3) | 4 | (5.9) | 0.396 | |
Among patients with tuberculosis, the second etiology of GLD in our study population, the most common imaging pattern was centrilobular micronodules (3/7, 42.9%), significantly more frequent than in other causes of GLD (6/68, 8.8%). Cavitation also was highly suggestive of mycobacterial infection, occurring in 57.1% (4/7) of cases of tuberculosis, compared to only 2 cases (2.9%) of all other causes of GLD, considering that one of these 2 cases occurred in a patient with unclassified mycobacteriosis.
All 4 cases of sarcoidosis had multifocal distribution and patterns varied between consolidation, nodular and perilymphatic micronodules. Mediastinal or hilar lymphadenopathy weren’t detected in just one case.
Among the 3 cases of aspergillosis, each one presented with a different main radiological pattern, which were consolidation, mass and centrilobular micronodules. The majority of paracoccidioidomycosis cases exhibited nodular pattern (2/3, 66.7%).
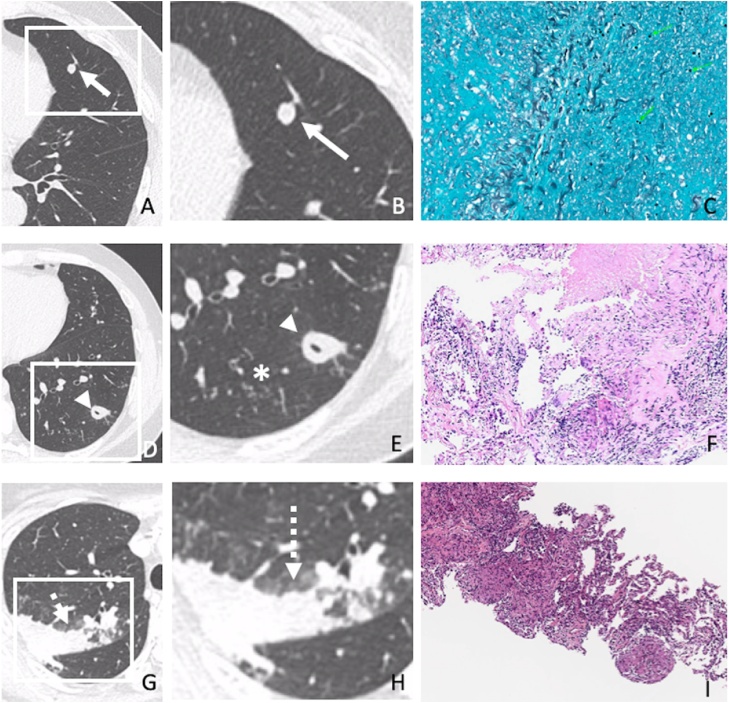
Fig. 5 demonstrates radiologic-pathologic correlation of three sample cases of our population.
Fig. 5.
Radiologic-pathologic correlation of three sample cases of our population. (A-C) 52-year-old patient with histoplasmosis. Chest CT (A) and enlarged image (B) demonstrating a solitary pulmonary nodule (arrows). (C) Lung biopsy showed necrotizing granulomatous inflammation with Histoplasma capsulatum yeasts (GMS 400x). (D-F) 34-year-old patient with tuberculosis. Chest CT (D) and enlarged image (E) demonstrating cavitated nodule (arrowheads) and centrilobular micronodules (asterisk). (F) Lung biopsy showed necrotizing granulomatous inflammation (HE 50x) consistent with tuberculosis. (G-I) 54-year-old patient with sarcoidosis. Chest CT (G) and enlarged image (H) showing consolidation (dashed arrows). (I) Lung biopsy depicted non-necrotizing granulomatous inflammation (HE 50x) consistent with sarcoidosis.
3.3. Interreader agreement
Interreader agreement in detecting the associated findings and radiological patterns was almost perfect (κ = 0.88-1.00), except the nodular pattern, which had substantial agreement (κ = 0.73) (Supplementary table).
4. Discussion
In most studies, non-infectious GLD are the most prevalent among patients submitted to lung biopsy [1,13]. This difference is probably due to the high prevalence of fungal diseases, such as histoplasmosis and tuberculosis in our population. The etiological profile found in our study was similar to that presented by Nazarullah et al. [14], where infectious GLD were more prevalent than non-infectious.
Among the patients with biopsy proven granulomatous disease, as in the studies of Sakakibara et al. [15] and Zhu et al. [1], the most commonly found radiological pattern was solitary pulmonary nodule, which can be explained by the crescent indication of lung biopsies for investigation of indeterminate solitary pulmonary nodules suspected for malignancy [16]. This pattern was significantly associated with histoplasmosis (p < 0.05). Centrilobular micronodules and cavity were also frequent and significantly associated with tuberculosis (p < 0.05). Sarcoidosis and other infectious causes (such as aspergillosis, paracoccidiodomycosis, cryptococcosis and paragonimiasis) did not present statistically significant association with a specific radiological pattern.
The clinical relevance of our results relies on the approach of the differential diagnosis in cases of patients with solitary pulmonary nodules in countries with high prevalence of GLD. Histoplasmosis should be considered a possible differential diagnosis in cases of solitary pulmonary nodule in patients with low risk for lung cancer [17]. Besides, the recognition of radiological patterns strongly associated with granulomatous diseases can be useful for clinicians to guide further investigations.
Being a single-center retrospective study, it is subjected to selection bias. Another limitation is that clinical and laboratorial data weren’t available. A larger sample would have resulted in a greater generalizability of our imaging findings. Consequently, further multicentric studies, particularly prospective cohorts, would be necessary to overcome these limitations and provide a better generalization of our results.
5. Conclusions
In our study population, the main etiologies found in patients with granulomatous disease who underwent lung biopsy were fungal or mycobacterial disease, specially histoplasmosis and tuberculosis, and nodular pattern with focal distribution was the most common imaging finding, detected with substantial interreader agreement.
Funding
No funding.
Ethics approval and consent to participate
The institutional (“Comitê de Ética em Pesquisa do Hospital Sírio-Libanês” – Research Ethics Committee of the Hospital Sírio-Libanês) and national (“Comissão Nacional de Ética em Pesquisa do Conselho Nacional de Saúde CEP/CONEP” - National Research Ethics Commission of the National Health Council) review boards approved our retrospective study.
Research Ethics Committee of the Hospital Sírio-Libanês Registration Number 844
CEP/CONEP’s Registration Number 03433918.9.0000.5461.
Consent for publication
The institutional and national review boards approved our retrospective study and waived the requirement for informed consent.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Camila Vilela de Oliveira: Study concepts; literature search; patient selection; interpretation of data; manuscript drafting and edition; approval of final version of submitted manuscript.
Natally Horvat: Study concepts; study design; interpretation of data; manuscript drafting and edition; approval of final version of submitted manuscript.
Leonardo de Abreu Testagrossa: Performed the histological review; approval of final version of submitted manuscript.
Davi dos Santos Romão and Marina Bastos Rassi: Junior radiologists who performed the image review; approval of final version of submitted manuscript.
Hye Ju Lee: Conception; senior radiologist who performed the image review; review of the manuscript; approval of final version of submitted manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ejro.2021.100325.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Zhu Q., Xu X., Li M., Wang X. Analysis of chest computed tomography manifestations of non-Mycobacterium tuberculosis induced granulomatous lung diseases. Radiology of Infectious Diseases. 2017:157–163. [Google Scholar]
- 2.Yang X., He J., Wang J., Li W., Liu C., Gao D. CT-based radiomics signature for differentiating solitary granulomatous nodules from solid lung adenocarcinoma. Lung Cancer. 2018;125:109–114. doi: 10.1016/j.lungcan.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Ohshimo S., Guzman J., Costabel U., Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series "Pathology for the clinician" Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145) doi: 10.1183/16000617.0012-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachiappan A.C., Rahbar K., Shi X., Guy E.S., Mortani Barbosa E.J., Shroff G.S. Pulmonary Tuberculosis: Role of Radiology in Diagnosis and Management. Radiographics. 2017;37(1):52–72. doi: 10.1148/rg.2017160032. [DOI] [PubMed] [Google Scholar]
- 5.WHO . World Health Organization; Geneva: 2019. Global tuberculosis report 2019. [Google Scholar]
- 6.Aljadani R., Ahmed A.E., Al-Jahdali H. Tuberculosis mortality and associated factors at King Abdulaziz Medical City Hospital. BMC Infect Dis. 2019;19(1):427. doi: 10.1186/s12879-019-4063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukhopadhyay S., Gal A.A. Granulomatous lung disease: an approach to the differential diagnosis. Arch Pathol Lab Med. 2010;134(5):667–690. doi: 10.5858/134.5.667. [DOI] [PubMed] [Google Scholar]
- 8.El-Zammar O.A., Katzenstein A.L. Pathological diagnosis of granulomatous lung disease: a review. Histopathology. 2007;50(3):289–310. doi: 10.1111/j.1365-2559.2006.02546.x. [DOI] [PubMed] [Google Scholar]
- 9.Dagaonkar R.S., Choong C.V., Asmat A.B., Ahmed D.B., Chopra A., Lim A.Y. Significance of coexistent granulomatous inflammation and lung cancer. J Clin Pathol. 2017;70(4):337–341. doi: 10.1136/jclinpath-2016-203868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese C., Cirene M., Colotto M. A singular case of granulomatous lesions of unknown significance. Minerva Med. 2008;99(2):213–218. [PubMed] [Google Scholar]
- 11.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Fleischner Society Remy J. glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 12.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 13.Mukhopadhyay S., Farver C.F., Vaszar L.T., Dempsey O.J., Popper H.H., Mani H. Causes of pulmonary granulomas: a retrospective study of 500 cases from seven countries. J Clin Pathol. 2012;65(1):51–57. doi: 10.1136/jclinpath-2011-200336. [DOI] [PubMed] [Google Scholar]
- 14.Nazarullah A., Nilson R., Maselli D.J., Jagirdar J. Incidence and aetiologies of pulmonary granulomatous inflammation: a decade of experience. Respirology. 2015;20(1):115–121. doi: 10.1111/resp.12410. [DOI] [PubMed] [Google Scholar]
- 15.Sakakibara Y., Suzuki Y., Fujie T., Akashi T., Iida T., Miyazaki Y. Radiopathological Features and Identification of Mycobacterial Infections in Granulomatous Nodules Resected from the Lung. Respiration. 2017;93(4):264–270. doi: 10.1159/000456550. [DOI] [PubMed] [Google Scholar]
- 16.Gazzoni F.F., Severo L.C., Marchiori E., Irion K.L., Guimarães M.D., Godoy M.C. Fungal diseases mimicking primary lung cancer: radiologic-pathologic correlation. Mycoses. 2014;57(4):197–208. doi: 10.1111/myc.12150. [DOI] [PubMed] [Google Scholar]
- 17.Chong S., Lee K.S., Yi C.A., Chung M.J., Kim T.S., Han J. Pulmonary fungal infection: imaging findings in immunocompetent and immunocompromised patients. Eur J Radiol. 2006;59(3):371–383. doi: 10.1016/j.ejrad.2006.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.