Abstract
Background/Aims
Compared with Western countries, chronic lymphocytic leukemia (CLL) rarely occurs in Asia and has different clinical characteristics. Thus, we aimed to evaluate the clinical characteristics, treatment outcomes, and prognostic significance of Korean patients with CLL.
Methods
We retrospectively analyzed 90 patients with CLL who had received chemotherapy at 6 centers in Korea between 2000 and 2012.
Results
Compared with Western patients with CLL, Korean patients with CLL express lambda (42.0%) and atypical markers such as CD22 and FMC7 (76.7% and 40.0%, respectively) more frequently. First-line chemotherapy regimens included chlorambucil (n = 43), fludarabine and cyclophosphamide (FC) (n = 20), fludarabine (n = 13), rituximab-FC (n = 4). The remaining patients were treated with other various regimens (n = 10). The 5-year overall survival (OS) and progression-free survival (PFS) rates were 79.3% and 28.1%, respectively. Multivariate analyses showed that hyperleukocytosis (≥ 100 × 103/μL), extranodal involvement, and the Binet C stage were significant negative prognostic factors for OS (hazard ratio [HR] 4.75, p = 0.039; HR 21.6, p = 0.002; and HR 4.35, p = 0.034, respectively). Cytogenetic abnormalities including complex karyotypes (≥ 3), del(11q), and del(17) had a significantly adverse impact on both OS and PFS (p < 0.001 and p = 0.010, respectively).
Conclusions
Initial hyperleukocytosis, extranodal involvement, complex karyotype, del(17) and del(11q) need to be considered in the risk stratification system for CLL.
Keywords: Leukemia, lymphocytic, chronic, B-cell; Treatment outcome; Prognosis; Korean
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is a mature B cell neoplasm, usually with coexpressed cluster of differentiation 5 (CD5) and CD23 [1]. The diagnostic criteria of CLL requires the presence of at least 5 × 109/L monoclonal B lymphocytes with the CLL phenotype in peripheral blood [2]. To determine the disease extent and prognosis, Rai and Binet clinical staging systems have been used to predict the prognostic risks of CLL for > 3 decades [2-4]. With the advancement of genetic analysis techniques, genetic abnormalities were found to have important clinical implications. Immunoglobulin heavy-chain variable (IGHV) gene somatic hypermutations are found in 50% to 60% of CLL cases and are associated with more favorable outcomes [5,6]. About 80% of the patients have cytogenetic abnormalities detected by fluorescence in situ hybridization (FISH) [7]. Del(11q), del(17p), and del(6q) were associated with chemotherapy resistance and worse outcome [6]. Del(13q), however, has been known to predict a more favorable clinical course [7].
CLL is the most common type of leukemia in Western countries; however, CLL rarely occurs in Asian populations [8-10]. In Korea, the annual incidence of CLL is only 0.11 per 100,000 people, while that in the Western countries is 2.8 to 3.9 per 100,000 people, which is 25 to 35 times higher than that in Korea [10,11]. Asian CLL has been reported to have different immunophenotypes and clinical characteristics [8,12]. Therefore, the clinical outcomes and prognostic factors after chemotherapy in Asian patients with CLL might be different from those of Western patients with CLL. However, only a few studies have been reported on CLL in Asia because of the limited patient population. Therefore, we aimed to investigate the clinical characteristics, treatment outcomes, and prognostic significances of Korean patients with CLL.
METHODS
We retrospectively analyzed 90 newly diagnosed patients with CLL who received chemotherapy at one of six centers in Korea between 2000 and 2012. CLL diagnosis and response evaluation were based on the International Workshop on CLL criteria, updated in 2008. Patients with small lymphocytic lymphoma were excluded in this study. Data on baseline clinicopathological features, treatment modalities, and outcomes were obtained from the medical records of each patient. Cytogenetics and FISH were performed using the bone marrow samples of 73 patients and 27 patients, respectively, at the time of CLL diagnosis. Immunophenotypic data at CLL diagnosis were available in 66 patients. Surface antigen expression on leukemic blasts was determined by 4-color flow cytometric analysis using either the FACScan system and Cell Quest software or the FACSCanto system and FACS Diva software (all from Becton Dickinson, San Jose, CA, USA). A CD45 blast gating method was used to distinguish leukemic blasts from other cells. The following antibodies directed to membrane antigens were used for standard immunophenotypic analyses of CLL cells: anti-CD3, anti-CD5, anti-CD7, anti-CD10, anti-CD19, anti-CD20, anti-CD22, anti-CD23, FMC7, surface membrane immunoglobulin (SmIg; kappa and lambda), HLA-DR (Becton Dickinson, Franklin Lakes, NJ, USA), and terminal deoxynucleotidyl transferase (TdT; Dako, Glostrup, Denmark). Positivity for each surface antigen was defined as expression on ≥ 20% leukemic cells, except for TdT positivity, which was defined as expression on ≥ 10% leukemic cells.
This study was approved by the Institutional Review Boards (Konkuk University Medical Center, KUH 1010527; and Ulsan University Hospital, 2015-04-008) of all the participating hospitals, Korea. Written informed consent by the patients was waived due to a retrospective nature of our study.
Statistical analysis
Continuous variables were compared using Student’s t test, whereas categorical variables were analyzed using either the Pearson’s chi-square test, or Fisher’s exact test. Survival probabilities were estimated using the Kaplan-Meier, method and differences in survival distributions were compared using the log-rank test. Overall survival (OS) was calculated from the date of CLL diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was measured from the time of initial chemotherapy to the date of progression or death from any cause. Among patients attaining complete remission (CR) after induction chemotherapy, relapse-free survival (RFS) was defined from the time of CR to the date of relapse, or death from any cause. After chemotherapy, the overall response rate was defined as CR plus the partial remission (PR) rates. Factors affecting CR achievement were determined by either Pearson’s chi-square test, or Fisher’s exact test. Factors affecting OS, PFS, and RFS were analyzed using the log-rank test in univariate analyses, and the Cox proportional hazard model in multivariate analyses. The following variables were included in the analysis of prognostic factors: age, white blood cell (WBC) and platelet counts, hemoglobin level, presence of constitutional symptoms, lymphadenopathy or splenomegaly at CLL diagnosis, expression of each surface antigen on leukemic blasts, cytogenetics, Rai stage, Binet system, chemotherapeutic regimens, and responses following chemotherapy. All statistical analyses were performed using the SPSS version 21.0 software (IBM Corp., Armonk, NY, USA). Variables with p < 0.1 in univariate analyses were included in the multivariate analyses. For all analyses, p values were 2 sided, and p < 0.05 was considered statistically significant.
RESULTS
Patients
The baseline characteristics of the patients with CLL at diagnosis are listed in Table 1. The study population comprised 53 male and 37 female patients; the median age was 61.5 years (range, 25 to 93), and all included patients received systemic chemotherapy. The median time from diagnosis to treatment was 1.0 months (range, 0 to 128.6). The clinical characteristics and chemotherapeutic regimens at the time of chemotherapy are summarized in Supplementary Table 1. Approximately 43.3% of all treated patients were treated in Rai stages III or IV. Patients having either symptoms related to CLL including lymphadenopathy, splenomegaly, and constitutional symptoms or significantly high leukocyte counts were treated in Rai stage 0 (25.6%), I (14.7%), and II (15.6%). First-line chemotherapy comprised oral chlorambucil (n = 43, 47.8%), fludarabine and cyclophosphamide (FC) (n = 20, 22.2%); fludarabine alone (n = 13, 14.4%); fludarabine, cyclophosphamide, and rituximab (FCR) (n = 4, 4.4%); fludarabine and rituximab (FR) (n = 1 [1.1%]); and cyclophosphamide, vincristine, and prednisolone (CVP) (n = 4, 4.4%). The remaining patients (n = 5) received other various regimen including vincristine plus prednisolone (VP) or cyclophosphamide plus prednisolone. The second-line chemotherapies were administered in 52 patients, of whom 16 received chlorambucil, and 12 received fludarabine. In addition, FCR (n = 5), FC (n = 3), FR (n = 3), and other various regimens (n = 13) including CVP; VP plus cyclophosphamide, adriamycin, vincristine, and prednisolone (CHOP); rituximab plus CHOP (R-CHOP); and fludarabine, cyclophosphamide, and mitoxantrone (FCM) were administered as second-line chemotherapy. Third-line chemotherapy was performed in 26 patients. Only two patients who achieved PR, and had stable disease after chemotherapy underwent allogeneic hematopoietic stem cell transplantation (allo-HCT).
Table 1.
Baseline characteristics at diagnosis
| Characteristic | Total (n = 90) |
|---|---|
| Sex, male/female (n = 90) | 53 (58.9)/37 (41.1) |
| Age, yr (n = 950) | 61.5 (25–93) |
| Hypertension (n = 89) | 22 (24.4) |
| Diabetes mellitus (n = 89) | 11 (12.2) |
| History of cancer (n = 90) | 4 (4.4) |
| Comorbidity (n = 89)a | 16 (17.8) |
| Constitutional symptom (n = 89) | 14 (15.6) |
| Lymphadenopathy (n = 84) | 45 (50.0) |
| Bulky lymph node (> 5 cm) (n = 90) | 2 (2.2) |
| Hepatomegaly (n = 82) | 4 (4.4) |
| Splenomegaly (n = 83) | 28 (31.1) |
| Extranodal involvement (n = 90) | 4 (4.4) |
| WBC count, × 103/μL (n = 90) | 27.4 (3.83–447.4) |
| Hemoglobin, g/dL (n = 90) | 12.6 (2.1–19.9) |
| Platelet, × 103/μL (n = 90) | 175.5 (5–308) |
| Lymphocyte count, × 103/μL (n = 88) | 22.8 (1.4–438.5) |
| Bone marrow cellularity, % (n = 68) | 60 (10–100) |
| Bone marrow neoplastic cells, % (n = 63) | 77.0 (9.0–96.1) |
| LDH (n = 73) | 285 (112–1112) |
| Stage (Binet system) (n = 90) | |
| A | 54 (60.0) |
| B | 9 (10.0) |
| C | 27 (30.0) |
| Cytogenetics (n = 73)b | |
| Normal karyotype | 60 (66.7) |
| Inv(9)/dup(9) | 3 (3.3) |
| Del(13q) | 1 (1.1) |
| Del(11q) | 2 (2.2) |
| Trisomy 12 | 2 (2.2) |
| Complex karyotype (≥ 3) | 3 (3.3) |
| Others | 2 (2.2) |
| FISH | |
| IgH gene rearrangement (n = 20) | 6 |
| Del(13q) (n = 18) | 7 |
| Trisomy 12 (n = 21) | 6 |
| Del(11q) (n = 20) | 2 |
| Del(17p) (n = 25) | 2 |
Values are presented as number (%) or median (range).
WBC, white blood cell; LDH, lactate dehydrogenase; FISH, f luorescence in situ hybridization; IgH, immunoglobulin heavy locus.
Comorbidity includes cerebrovascular disease, heart disease, liver disease, and lung disease other than hypertension, diabetes and prior cancers.
At the time of diagnosis.
Immunophenotypic characteristics
Immunophenotypic findings of CLL patients are summarized in Table 2. CD19 expression was observed in all tested patients with B cell CLL, except three with T cell-type CLL. Approximately 78.1% of all tested patients showed CD5 positivity, while 14 patients presented with atypical CLL (CD5 negative). Interestingly, CD22 and FMC7 positivity was frequently found in 76.7% (33/43) and 40.0% (12/30) of the patients, respectively. Expression of kappa and lambda SmIg were observed in 38.0% and 42.0% of the patients, respectively.
Table 2.
Immunophenotypic characteristics
| Immunophenotype (patient no. analyzed for the immunophenotype) | No. of positive patient (%) |
|---|---|
| CD3 (+) (n = 40) | 3 (7.5) |
| CD5 (+) (n = 64) | 50 (78.1) |
| CD7 (+) (n = 55) | 4 (7.3) |
| CD19 (+) (n = 66) | 63 (95.5) |
| CD20 (+) (n = 57) | 54 (94.7) |
| CD22 (+) (n = 43) | 33 (76.7) |
| CD23 (+) (n = 41) | 37 (90.2) |
| CD45 (+) (n = 22) | 22 (100) |
| CD56 (+) (n = 16) | 0 |
| FMC7 (+) (n = 30) | 12 (40.0) |
| HLA-DR (+) (n = 24) | 24 (100) |
| TdT (+) (n = 29) | 3 (10.3) |
| SmIg (n = 50) | |
| Kappa (+) | 19 (38.0) |
| Lambda (+) | 21 (42.0) |
| Negative | 10 (20.0) |
TdT, terminal deoxynucleotidyl transferase; SmIg, surface membrane immunoglobulin.
Chemotherapy responses
The clinical characteristics of patients at the time of chemotherapy are summarized in Supplementary Table 1. Twelve patients (12.6%) presented with constitutional symptoms such as fever, night sweats, or significant weight loss (≥ 10% for 6 months). Among 45 patients who received chemotherapy at Binet A, 40.0% had progressive higher WBC counts > 40.0 × 103/μL, 52.5% had symptomatic lymphadenopathy or splenomegaly, and 11.4% had constitutional symptoms. After administering first-line chemotherapy, 18.9% of the patients achieved CR. The CR rate after the maximum third-line chemotherapy was 27.8%. Only two patients achieved a CR after third-line chemotherapy. The overall response rates were 70.0%, and 32.2% after first-, and second-line chemotherapy, respectively. Among the 25 patients who achieved CR, 12 (48.0%) experienced relapse. Two patients with refractory CLL who underwent allo-HCT survived after achieving CR status. The CR rates according to initial chemotherapeutic regimen are summarized in Table 3. The CR rates of the FCR, and FC regimens were 25%, and 35%, respectively (Table 3). There were, however, significant differences in the CR rate regarding cytogenetic abnormalities (p = 0.011) (Table 3). Patients with immunoglobulin heavy locus (IgH) rearrangement showed a significantly higher CR rate than those with no IgH rearrangement (66.7% vs. 17.2%, respectively; p = 0.011). However, patients with del(11q), del(17), or complex karyotype (≥ 3 chromosomal abnormalities) did not achieve CR.
Table 3.
Treatment outcomes after chemotherapy
| Variable | CR rate | Response rate (CR + PR) |
|---|---|---|
| 1st line chemotherapy regimen (n = 90) | ||
| Chlorambucil (n = 43) | 7 (16.3) | 29 (67.5) |
| Fludarabine (n = 13) | 1 (7.7) | 7 (63.6) |
| FCR (n = 4) | 1 (25) | 3 (75.0) |
| FC (n = 20) | 1 (35.0) | 18 (90) |
| CVP (n = 4) | 0 | 3 (75.0) |
| RF (n = 1) | 0 | 1 (100) |
| Others (hydroxyurea, VP, Cy + PD) (n = 5) | 0 | 2 (40.0) |
| Cytogenetics (n = 73)a | p = 0.011 | |
| IgH rearrangement (n = 6) | 4 (66.7) | |
| Normal karyotype/others (n = 62) | 11 (17.7) | |
| Del(11q)/del(17)/CK (n = 5) | 0 |
Values are presented as number (%).
CR, complete remission; PR, partial remission; FCR, fludarabine, cyclophosphamide, and rituximab; FC, fludarabine and cyclophosphamide; CVP, cyclophosphadmie, vincristine and prednisolone; RF, rituximab and fludarabine; VP, vincristine and prednisolone; Cy + PD, cyclophosphadmie and prednisolone; IgH, immunoglobulin heavy locus; CK, complex karyotype.
At the initiation of first-line chemotherapy.
Univariate analysis for survival outcomes
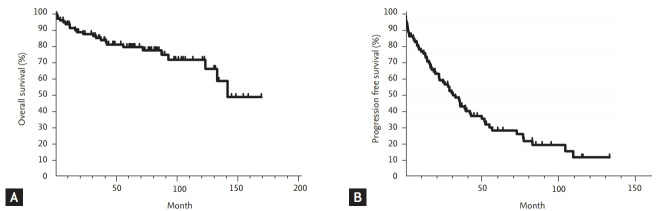
The median follow-up duration for surviving patients was 80.5 months (range, 2.0 to 167.8). The 5-year survival rate for all included patients was 79.3%, and the median OS was not reached (Fig. 1A). During the follow-up period, 23 patients with persistent disease died, either from infection (n = 12), or disease progression (n = 11). After the initial chemotherapy regimen, the median PFS was 31.0 months (95% confidence interval [CI], 23.1 to 39.1) and the 5-year PFS rate was 28.1% (Fig. 1B). The median RFS of the 25 patients who achieved CR after chemotherapy was 50.3 months (95% CI, 8.6 to 91.9), while 12 patients experienced relapse. The 5-year RFS rate for the patients who achieved CR was 47.3%. Univariate analyses for OS and PFS according to the clinical parameters are summarized in Table 4. The traditional staging criteria according to Rai system and Binet system had significant effects on the OS (p = 0.001, and p = 0.006, respectively). Lymphadenopathy, splenomegaly, and extranodal involvement were significantly associated with lower OS rates (p = 0.029, p = 0.003, and p = 0.008, respectively). Initial hyperleukocytosis (WBC ≥ 100 × 103/μL), anemia (hemoglobin < 10 g/dL), and thrombocytopenia (platelet count < 80 × 103/μL) were significantly associated with worse OS (p < 0.001, p = 0.004, and p = 0.032, respectively). In contrast to the risk classification of the Rai and Binet systems, in which platelet count less than 100 × 103/μL was associated with worst prognosis, our data showed that patients with a platelet count between 80 × 103 and 110 × 103/μL had survival rates comparable to those with higher platelet counts (> 110 × 103/μL) (5-year OS rate: 92.9% vs. 82.0%, respectively), whereas those with a lower platelet count (< 80 × 103/μL) had a significantly lower OS rate (46.7%, p = 0.032). In addition, the univariate analyses revealed that higher proportions of neoplastic cells in the bone marrow (≥ 80%), and higher lactate dehydrogenase (LDH) (≥ 500 IU/L) levels were significantly associated with worse OS rates (p = 0.021, and p = 0.003, respectively). In particular, higher LDH levels (> 500 IU/L) were also an adverse factor for predicting PFS (p = 0.016). Lymphadenopathy tended to be associated with worse PFS (p = 0.067). Cytogenetic abnormalities had a significant impact on OS and PFS (p = 0.002 and p = 0.006, respectively). IgH gene rearrangement was a significant favorable factor for OS (5-year OS and PFS: 100% and 50%, respectively). Compared with normal karyotypes and other karyotypes, del(11q), del(17), and complex karyotypes (≥ 3 chromosomal abnormalities) were significant negative prognosticators (5-year OS and PFS rates: 82.9% vs. 26.7%, p = 0.002; and 29.7% vs. 0%, p = 0.006, respectively).
Figure 1.
Survivals of Korean patients with chronic lymphocytic leukemia. (A) Overall survival and (B) progression-free survival.
Table 4.
Univariate analysis for survivals
| Variable | 5-year OS, % | p value | 5-year PFS, % | p value |
|---|---|---|---|---|
| Sex, male/female (n = 90) | 76.4/84.3 | 0.544 | 24.3/32.8 | 0.701 |
| Age, < 65/≥ 65 yr (n = 90) | 85.0/68.8 | 0.224 | 30.6/21.0 | 0.235 |
| Stage (Rai system)a (n = 90) | 0.001 | 0.038 | ||
| 0 | 100 | 26.0 | ||
| I | 75.5 | 17.8 | ||
| II | 73.3 | 64.8 | ||
| III | 66.1 | 21.4 | ||
| IV | 68.2 | 25.0 | ||
| Stage (Binet) (n = 90), % a | 0.006 | 0.239 | ||
| A | 88.0 | 27.3 | ||
| B | 72.9 | 53.3 | ||
| C | 62.2 | 20.0 | ||
| Constitutional symptom (+/–) (n = 89), % | 76.4/84.3 | 0.266 | 25.4/26.9 | 0.869 |
| Lymphadenopathya (+/–) (n = 84) | 68.5/86.9 | 0.029 | 24.2/36.3 | 0.127 |
| Splenomegaly a (+/–) (n = 83), % | 63.4/84.0 | 0.003 | 33.2/25.7 | 0.340 |
| Extranodal involmenta (+/–) (n = 90), % | 37.5/81.0 | 0.008 | 50.0/27.9 | 0.639 |
| WBC count a, < 100/≥ 100 × 103/μL (n = 90) | 83.9/28.6 | < 0.001 | 29.3/14.3 | 0.049 |
| Hemoglobin b, < 10/≥ 10 g/dL (n = 89) | 55.9/85.7 | 0.004 | 11.8/30.6 | 0.056 |
| Platelet b, < 80/80 to < 110/≥ 110 × 103/μL (n = 90) | 46.7/92.3/81.7 | 0.032 | 13.3/0/33.3 | 0.174 |
| Bone marrow neoplastic cells, < 80%/≥ 80% (n = 63) | 88.0/73.1 | 0.021 | 28.7/41.4 | 0.296 |
| LDH, < 500/≥ 500 IU/L (n = 70) | 84.3/34.3 | 0.003 | 28.8/0 | 0.016 |
| Cytogenetics (n = 75)a | 0.002 | 0.006 | ||
| IgH mutation | 100 | 50.0 | ||
| Normal karyotype/other chromosomes | 82.3 | 29.7 | ||
| Del(11q) or del(17) or CK | 26.7 | 0 | ||
| Immunophenotyping, % | ||||
| SmIg (n = 50) | 0.623 | 0.572 | ||
| Kappa (+) | 83.1 | 35.6 | ||
| Lambda (+) | 76.0 | 13.6 | ||
| Negative | 87.5 | 21.1 | ||
| CD5 (n = 37) | 0.856 | 0.018 | ||
| Bright | 100 | 0 | ||
| Intermediate | 88.4 | 21.6 | ||
| Dim/Negative | 82.5 | 29.3 | ||
| CD20 (n = 30) | 0.138 | 0.058 | ||
| Bright | 90.0 | 53.5 | ||
| Intermediate/dim/negative | 76.9 | 12.7 | ||
| Initial chemotherapy regimen (n = 90) | 0.255 | 0.007 | ||
| FCR or RF | 75.0 | 53.3 | ||
| FC | 75.6 | 46.6 | ||
| Chlorambucil | 85.1 | 23.0 | ||
| Fludarabine | 59.8 | 15.4 | ||
| Others | 88.9 | 12.5 | ||
| 1st line chemotherapy response (n = 87) | < 0.001 | < 0.001 | ||
| CR | 100 | 66.2 | ||
| PR | 83.3 | 26.9 | ||
| SD | 66.9 | 0 | ||
| PD | 37.5 | 0 |
OS, overall survival; PFS, progression-free survival; WBC, white blood cell; LDH, lactate dehydrogenase; IgH, immunoglobulin heavy locus; CK, complex karyotype; CD, cluster of differentiation; FCR, fludarabine, cyclophosphamide, and rituximab; RF, rituximab and fludarabine; FC, fludarabine and cyclophosphamide; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
At diagnosis.
At the initiation of first-line chemotherapy.
With regard to immunophenotypic markers, expression of lambda SmIg showed a significantly worse PFS compared to expression of kappa SmIg, or the absence of both (5-year PFS rate: 6.3% vs. 40.4%, and 21.1%, respectively, p = 0.040). The bright expression of CD5 was significantly associated with a worse PFS, compared to intermediate or dim/negative expression of CD5 (0% vs. 21.6, and 29.3%, respectively, p = 0.018). On the contrary, patients with bright expression of CD20 tended to show higher PFS rates than those with intermediate or dim expression of CD20 (53.5% vs. 12.7%, p = 0.058). Apart from light chain, CD5, and CD20 expression, there were no significant differences in the survival outcomes among patients in terms of other immunophenotypic markers.
The initial chemotherapy regimens including FCR or RF, FC, chlorambucil, fludarabine, and other various regimens, significantly affected the 5-year PFS (53.3%, 46.6%, 23.0%, 15.4%, and 12.5%, respectively, p = 0.007) and RFS rates (100%, 87.5%, 18.2%, 0%, and 0%, respectively, p = 0.004), although there was no significant difference in OS (p = 0.255). The response after initial chemotherapy was a significant predictor of OS (p < 0.001) and PFS (p < 0.001) (Table 4). All patients who achieved a CR even after second-, or third-line chemotherapy, or allogeneic stem cell transplantation survived more than 5 years.
Multivariate analysis
Multivariate analysis showed that initial hyperleukocytosis (WBC count ≥ 100 × 103/μL) (HR, 4.748; p = 0.039) and extranodal involvement (HR, 21.639; p = 0.002), Binet stage C (HR, 4.354; p = 0.034), and chromosomal abnormalities including del(11q), del(17), or complex karyotype (≥ 3 chromosomal abnormalities) (HR, 17.022; p < 0.001) were powerful adverse prognostic factors for OS and PFS (HR 17.022; p < 0.001; and HR 9.620; p = 0.010; respectively) (Table 5). Moreover, the light chain types of SmIg had a significant impact on PFS.
Table 5.
Multivariate analysis
| Variable | Overall survival |
Progression-free survival |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| WBC count, ≥ 100, × 103/μLa | 4.748 (1.084–20.792) | 0.039 | - | - |
| Extranodal involvementa | 21.639 (2.972–157.553) | 0.002 | - | |
| Binet systema | ||||
| A | Reference | - | ||
| B | 0.455 (0.040–5.108) | 0.523 | - | |
| C | 4.354 (1.117–16.975) | 0.034 | - | |
| Cytogeneticsa | ||||
| Normal karyotype/other chromosomes | Reference | Reference | ||
| Del(11q) or del(17) or CK | 17.022 (3.674–78.864) | < 0.001 | 9.620 (1.732–53.444) | 0.010 |
| IgH mutation | - | 0.988 | 0.503 (0.114–2.215) | 0.364 |
HR, hazard ratio; CI, confidence interval; WBC, white blood cell; CK, complex karyotype (≥ 3); IgH, immunoglobulin heavy locus.
At the time of diagnosis.
DISCUSSION
Among hematologic malignancies, CLL is a disease that demonstrates the greatest genetic differences between Western and Asian people [9]. CLL rarely occurs in Korean people, and only 56 patients are newly diagnosed with CLL annually [8]. The number of Korean patients with CLL has been slowly increasing [13]. The crude incidence rate of CLL or small lymphocytic lymphoma has increased from 0.19, to 0.31 between 1999 and 2012 according to the national cancer statistics [13]. Korean patients with CLL in our study were relatively young, with a median age at diagnosis of 61 years. In contrast, the median age at diagnosis in the United States is 70 years [9,14]. In general, younger age may favorably affect the treatment outcomes of hematologic malignances. However, CLL occurring in Asian people showed a more aggressive progression than that occurring in Caucasian people [14]. Gunawardana et al. [14] reported that Asian patients required treatment almost twice as often as Caucasian patients (HR, 1.94) and had a shorter time to first treatment (p = 0.0063). A previous study from India suggested that a higher proportion of patients with CLL presented with an advanced clinical stage [15].
In the present study, Korean patients with CLL expressed a unique immunophenotypic pattern on the CLL cells. Compared with Western data, expression of atypical immunophenotypic markers such as CD22 and FMC7 were frequently observed among Korean CLL patients. Similarly, these findings were previously reported in a small retrospective study conducted by Jang et al. [12]. In addition, expression of lambda SmIg was more frequent among Asian patients with CLL than among Western patients. Even though there were no significant differences in univariate and multivariate analysis, lambda SmIg expression tended to be associated with a worse PFS compared with kappa SmIg expression. Therefore, compared to CLL observed among Western patients, a relatively greater proportion of patients with lambda SmIg expression in Korean patients with CLL might influence the treatment outcome, which was worse than that of Western patients with CLL. In terms of the clinical implication of immunophenotypic markers, we found that the intensity of the CD5 and CD20 expression was more important than the positivity thereof. Bright CD5 expressions was a significant predictor for worse PFS, compared to both intermediate-, and dim/negative expression.
Among the first-line chemotherapeutic regimens, the FCR and FC regimens achieved a CR rate of 25%, and response rate of 75%. The FCR regimen has been recommended as first-line chemotherapy in patients younger than 65 years without significant comorbidities according to the 2018 National Comprehensive Cancer Network guidelines [16]. The CR and response rates of Korean patients with CLL treated with FCR seems to be lower than that reported in the CLL10 study [16]. Relatively smaller numbers of patients received rituximab-containing chemotherapy as first-, or second-line chemotherapy in our data. Nowadays, several novel agents including the Bruton’s tyrosine kinase inhibitor (ibrutinib), anti-CD20 monoclonal antibody (obinutuzumab), bendamustine, and ofatumumab have been introduced as first-line therapy for CLL [8,17-19]. Unfortunately, the national medical insurance reimbursement system in Korea does not cover the expenses for the procurement of those novel agents as first-line chemotherapy for CLL. Therefore, our data have some limitations, as we were unable to include the therapeutic experiences of patients who were treated using newer agents.
OS of Korean patients with CLL who received chemotherapy was not reached, and the 5-year PFS rate was only 28.1%. Although the Rai, and Binet staging systems were introduced more than 37 years ago, these two prognostic systems have been widely used to stratify the prognosis of patients with CLL [3,4]. With regard to the therapeutic outcome prediction in CLL, genetic abnormalities have important clinical implications. There might be ethnic differences in the clinical implication of genetic aberrations, including IgH mutations. In our study, IgH gene rearrangement was associated with a higher CR rate. In the CLL10 study, however, IgH mutations had no influence on either CR rate, or overall response rate in both treatment arms (FCR vs. bendamustine and rituximab) [16]. Del(11q), del(17p), and del(6q) have been reported to be associated with a worse outcome [6,7]. Previous large-scale studies reported that patients with del(17p) and TP53 mutations had poorer chemotherapy responses and survival rates [20-22]. We also demonstrated that patients with a complex karyotype (≥ 3 cytogenetic abnormalities) as well as del(11q) and del(17p) were important adverse factors in terms of CR achievement, OS, and PFS in patients with CLL. None of the patients with a complex karyotype (≥ 3) as well as del(11q) and del(17p) attained a CR after chemotherapy. In CLL, the prognostic significance of a complex karyotype had also been reported in other recent studies [23-25].
For the prediction of CLL prognosis, Wierda et al. [26] suggested that age, absolute lymphocyte count, beta 2-microglobulin, sex, and nodal involvement were significant prognostic factors in addition to Rai staging. Similarly, we also demonstrated that hyperleukocytosis (≥ 100 × 103/μL) was an independently significant poor prognostic factor for Korean patients with CLL. Whereas traditional prognostic stratification systems such as the Rai and Binet systems cannot reflect tumor burdens, hyperleukocytosis (≥ 100 × 103/μL) as well as the proportion of neoplastic cells in the bone marrow (≥ 80%), and higher LDH (≥ 500 IU/L) levels, which might reflect tumor burden, were also important factors affecting treatment outcomes in Korean patients with CLL, as shown in our univariate analysis of the survival rates. In addition, extranodal involvement was a poor prognostic factor, although extranodal involvement was only found in 4.2% of the patients in our study. The extranodal sites involved were pleura, cerebrospinal fluid (CSF), brain, parotid gland, and nasopharynx.
We also found that mild thrombocytopenia (platelet count between 80 × 103/μL and 110 × 103/μL) did not have a poor prognostic significance in our data. The survival of patients with platelet counts between 80 × 103/μL and 110 × 103/μL was not different from that of patients with a platelet count > 110 × 103/μL. Traditionally, according to the Rai and Binet staging systems, patients with thrombocytopenia (platelet count: < 100 × 103/μL, irrespective of organomegaly) were categorized into the worst prognostic group, stage IV and stage C, respectively [3]. In contrast to the results of previous studies using clinical staging systems such as Rai and Binet systems, the German CLL Study Group (GCLLSG) data, or the CLL international prognostic index (CLL-IPI) [3,4,6,22], our data showed that a platelet count < 80 × 103/μL had poor prognostic significance. Therefore, we suggest that thrombocytopenia needs to be more stratified in Korean patients with CLL. If patients have a platelet count > 80 × 103/μL without any other therapeutic indications for CLL such as progressive organomegaly, severe anemia, and constitutional symptoms, a more conservative approach might be acceptable, and chemotherapy might be deferred for these patients.
Our data have several limitations. This study is retrospective in nature, included a small number of patients, and had limited FISH data. Our study population had limited exposure to newer therapeutic agents such as rituximab. However, our results showed the unique clinical characteristics and implications of CLL among Korean patients, including its clinical factors and immunophenotypic features and more stratified additional prognostic factors.
Although a diagnosis of CLL requires the presence of ≥ 5 × 109/L B lymphocytes in the peripheral blood, sustained for at least 3 months [27], some patients with CLL in the present study have less than 5 × 109/L B lymphocytes. According to the 2008 International Workshop on Chronic Lymphocytic Leukemia, the presence of a cytopenia caused by a typical marrow infiltrate establishes the diagnosis of CLL regardless of the number of peripheral blood B lymphocytes, or the presence of lymph node involvement [27].
To the best of our knowledge, this is the first multicenter retrospective study to analyze the treatment outcomes and prognostic factors of Korean patients with CLL who received first-line chemotherapy. Considering the rarity of CLL in Asia, our sample size is not really small.
In conclusion, Korean patients with CLL have different characteristics in terms of the frequency of lambda SmIg expression and atypical CD22 and FMC7 marker positivity. Hyperleukocytosis, extranodal involvement, Binet stage C, a complex karyotype, del(17) and del(11q) were independent significant negative predictors. We suggest that our results might be incorporated in a better risk stratification system of CLL in the future.
KEY MESSAGE
1. Korean patients with, chronic lymphocytic leukemia (CLL) had higher lambda surface membrane immunoglobulin (SmIg) expression and atypical CD22 and FMC7 marker positivity than Western patients.
2. Hyperleukocytosis, extranodal involvement, and complex karyotype as well as del(17) and del(11q) and traditional Rai/Binet system are important adverse prognostic factors for CLL among Korean patients.
Mild thrombocytopenia (80,000/mm3 ≤ platelet count < 110,000/mm3) had no negative effects on the treatment outcomes.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (NRF-2017R1C1B5015107) (to Yunsuk Choi).
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Clinical characteristics at the time of chemotherapy and treatment outcomes
REFERENCES
- 1.Matutes E, Owusu-Ankomah K, Morilla R, et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia. 1994;8:1640–1645. [PubMed] [Google Scholar]
- 2.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 5.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 6.Pflug N, Bahlo J, Shanafelt TD, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124:49–62. doi: 10.1182/blood-2014-02-556399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 8.Jeon YW, Cho SG. Chronic lymphocytic leukemia: a clinical review including Korean cohorts. Korean J Intern Med. 2016;31:433–443. doi: 10.3904/kjim.2015.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang SM, Li JY, Gale RP, Huang XJ. The mystery of chronic lymphocytic leukemia (CLL): why is it absent in Asians and what does this tell us about etiology, pathogenesis and biology? Blood Rev. 2015;29:205–213. doi: 10.1016/j.blre.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Yoon SO, Suh C, Lee DH, et al. Distribution of lymphoid neoplasms in the Republic of Korea: analysis of 5318 cases according to the World Health Organization classification. Am J Hematol. 2010;85:760–764. doi: 10.1002/ajh.21824. [DOI] [PubMed] [Google Scholar]
- 11.Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536545. doi: 10.1111/bjh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang MA, Yoo EH, Kim K, et al. Chronic lymphocytic leukemia in Korean patients: frequent atypical immunophenotype and relatively aggressive clinical behavior. Int J Hematol. 2013;97:403–408. doi: 10.1007/s12185-013-1286-z. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Park HJ, Park EH, et al. Nationwide statistical analysis of lymphoid malignancies in Korea. Cancer Res Treat. 2018;50:222–238. doi: 10.4143/crt.2017.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunawardana C, Austen B, Powell JE, et al. South Asian chronic lymphocytic leukaemia patients have more rapid disease progression in comparison to White patients. Br J Haematol. 2008;142:606–609. doi: 10.1111/j.1365-2141.2008.07226.x. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal N, Naithani R, Mahapatra M, et al. Chronic lymphocytic leukemia in India: a clinico-hematological profile. Hematology. 2007;12:229–233. doi: 10.1080/10245330701255064. [DOI] [PubMed] [Google Scholar]
- 16.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942. doi: 10.1016/S1470-2045(16)30051-1. [DOI] [PubMed] [Google Scholar]
- 17.Woyach JA, Johnson AJ, Byrd JC. Chronic lymphocytic leukemia: recent progress and current challenges. Semin Oncol. 2016;43:199–200. doi: 10.1053/j.seminoncol.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Walter HS, Salles GA, Dyer MJ. New agents to treat chronic lymphocytic leukemia. N Engl J Med. 2016;374:21852186. doi: 10.1056/NEJMc1602674. [DOI] [PubMed] [Google Scholar]
- 19.Vela CM, McBride A, Jaglowski SM, Andritsos LA. Ibrutinib for treatment of chronic lymphocytic leukemia. Am J Health Syst Pharm. 2016;73:367–375. doi: 10.2146/ajhp140760. [DOI] [PubMed] [Google Scholar]
- 20.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 21.Rossi D, Cerri M, Deambrogi C, et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of Del17p13: implications for overall survival and chemorefractoriness. Clin Cancer Res. 2009;15:9951004. doi: 10.1158/1078-0432.CCR-08-1630. [DOI] [PubMed] [Google Scholar]
- 22.International CLL-IPI working group An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;17:779–790. doi: 10.1016/S1470-2045(16)30029-8. [DOI] [PubMed] [Google Scholar]
- 23.Le Bris Y, Struski S, Guieze R, et al. Major prognostic value of complex karyotype in addition to TP53 and IGHV mutational status in first-line chronic lymphocytic leukemia. Hematol Oncol. 2017;35:664–670. doi: 10.1002/hon.2349. [DOI] [PubMed] [Google Scholar]
- 24.Rigolin GM, Saccenti E, Guardalben E, et al. In chronic lymphocytic leukaemia with complex karyotype, major structural abnormalities identify a subset of patients with inferior outcome and distinct biological characteristics. Br J Haematol. 2018;181:229–233. doi: 10.1111/bjh.15174. [DOI] [PubMed] [Google Scholar]
- 25.Herling CD, Klaumunzer M, Rocha CK, et al. Complex karyotypes and KRAS and POT1 mutations impact outcome in CLL after chlorambucil-based chemotherapy or chemoimmunotherapy. Blood. 2016;128:395–404. doi: 10.1182/blood-2016-01-691550. [DOI] [PubMed] [Google Scholar]
- 26.Wierda WG, O’Brien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–4685. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 27.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics at the time of chemotherapy and treatment outcomes