Abstract
Endothelial progenitor cell transplantation is a potential therapeutic approach in brain ischemia. However, whether the therapeutic effect of endothelial progenitor cells is via affecting complement activation is unknown. We established a mouse focal ischemia model (n = 111) and transplanted endothelial progenitor cells into the peri-infarct region immediately after brain ischemia. Neurological outcomes and brain infarct/atrophy volume were examined after ischemia. Expression of C3, C3aR and pro-inflammatory factors were further examined to explore the role of endothelial progenitor cells in ischemic brain. We found that endothelial progenitor cells improved neurological outcomes and reduced brain infarct/atrophy volume after 1 to 14 days of ischemia compared to the control (p < 0.05). C3 and C3aR expression in the brain was up-regulated at 1 day up to 14 days (p < 0.05). Endothelial progenitor cells reduced astrocyte-derived C3 (p < 0.05) and C3aR expression (p < 0.05) after ischemia. Endothelial progenitor cells also reduced inflammatory response after ischemia (p < 0.05). Endothelial progenitor cell transplantation reduced astrocyte-derived C3 expression in the brain after ischemic stroke, together with decreased C3aR and inflammatory response contributing to neurological function recovery. Our results indicate that modulating complement C3/C3aR pathway is a novel therapeutic target for the ischemic stroke.
Keywords: Astrocyte, C3, endothelial progenitor cell, ischemia, stroke
Introduction
Ischemic stroke constitutes 80% of stroke, which is a leading cause of permanent disability and mortality worldwide.1 However, few effective treatments are currently available to the majority of stroke patients except for thrombolysis and endovascular thrombectomy, which are for accessible to less than 10% of the patients.2 Increasing evidence showed that stem cell therapy could provide extended therapeutic time window and is highly convergent with rehabilitation of ischemic stroke.3,4
Endothelial progenitor cells (EPCs) hold particular promise for ischemic therapy due to its wide variety of sources and with fewer ethical constraints.5 Previous studies showed that EPC transplantation could improve neurological outcomes after ischemic brain injury mainly via promoting angiogenesis and increasing focal blood flow.6–9 A recent study found that mesenchymal stem cell transplantation reduced C3 expression in the brain within three days after ischemia.10 Similar with mesenchymal stem cells (MSCs), EPCs are also originated from the hematopoietic stem cells in the bone marrow.5 Evidence intrigue us to explore whether EPCs modulate immune response, especially affecting complement activation during ischemic stroke.
Complement system constitutes the key component mediating immune response and plays a crucial role in the central nervous system.11–14 After ischemic stroke, complement system is activated and can exacerbate ischemic brain injury.15–17 C3 is the core component in complement activation pathways.18,19 Clinical studies found that the level of C3 in the plasma of stroke patients was up-regulated at 1 day and 28 days after ischemia, and associated with the prognosis at 3 months.20,21 The higher level of C3 in the plasma, the worse neurological disability.22 On the other hand, animal studies showed that C3 was up-regulated in one to seven days in the brain after cerebral ischemia. Depletion of C3 in the brain reduced ischemic brain injury and improved neurological function.22–25 However, these studies were mainly focusing on the early stage of ischemic stroke, mostly within three days. The exact origination of up-regulated C3 and its effect on tissue repair and neurological recovery at the late stage of stroke remains obscure.
In this study, we established transient middle cerebral artery occlusion (tMCAO) model in mice and transplanted EPCs into ischemic brain via stereotaxic injection immediately after reperfusion. We found the novel mechanism of EPC treatment for ischemic stroke and clarified that up-regulated C3 and C3aR at the late stage of ischemic stroke were related to brain injury and neurological function deficiency. Inhibiting astrocyte-derived C3 in the brain is a potential therapeutic strategy for promoting tissue repair and functional recovery after cerebral ischemia.
Materials and methods
EPC isolation and identification
The procedure for EPC isolation was approved by the Ethics Committee of Shanghai Jiao Tong University (Shanghai, China). The ethical standards were consistent with the Helsinki Declaration of 1975 (and as revised in 1983) and confirmation that written, informed consent was obtained from all donors. Human umbilical cord blood was obtained from the International Peace Maternity & Child Health Hospital of China (IPMCH, Shanghai, China). The purification of EPCs was performed as previously described.26 Briefly, monocytes were isolated and cultured in a 6-well plate coated with collagen I (Corning, Bedford, MA) with a density of 1 × 107 per well. EPCs within five to eight passages were used in the following experiments. EPCs were identified using immunofluorescent staining and flow cytometry methods. For immunofluorescent staining, EPCs were fixed with 4% paraformaldehyde, followed by 0.3% Triton-X 100 for 10 min and 10% donkey serum for 1 h at room temperature. Then EPCs were incubated with primary antibodies of CD34 (1:50 dilution, BD Biosciences, Franklin Lakes, NJ) and KDR (1:50 dilution, R&D, Minneapolis, MN), CD133 (1:50 dilution, Abnova, Taipei, China) and KDR (1:50 dilution, R&D), and vWF (1:400 dilution, Abcam, Cambridge, MA) at 4°C overnight. After three times washing, EPCs were incubated with the secondary antibodies: Alexa Fluor 594-conjugated donkey anti-mouse and Alexa Fluor 488-conjugated donkey anti-goat, or Alexa Fluor 488-conjugated donkey anti-rabbit (1:500 dilution, Invitrogen, Carlsbad, CA) for 1 h at room temperature. Images were taken under a confocal microscope (Leica, Solms, Germany).
For flow cytometry analysis, EPCs were incubated with fluorescence-conjugated mouse anti-human monoclonal antibodies of CD34-PE, KDR-APC, CD31-PE, IgG isotype-APC and IgG isotype-PE (BD Biosciences), and CD133-PE (Miltenyi Biotec, Cologne, Germany) for 30 min at 4°C. Then the cells were washed with PBS and analyzed by flow cytometry (BD Biosciences). For each test, 3 × 105 events were analyzed.
EPC-conditioned medium preparation
To produce EPC-conditioned medium (EPCCM), EPCs were cultured in a 10-cm dish (Corning Incorporated, Corning, NY) at about a density of 1.5 × 106 cells. Fresh medium (EGM-2) was added and followed by 24 h of incubation at 37°C. The medium was collected and centrifuged at 1000 r/min at 4 °C, the supernatant was in −20°C until use. The EBM-2 was served as a control medium (un-CM: unconditioned medium). Throughout all the experiments, EPCCM and un-CM were processed simultaneously with the same treatments.
Microglia culture and C3a treatment
BV2, immortalized mouse microglial cells, were plated in the 12-well (Corning Incorporated) with a density of 7 × 104 per well and cultured in DMEM with 5% heat-inactivated FBS. When the density of microglia reached about 50% confluence (about one day), recombinant mouse C3a (R&D) with a final concentration of 100 Mm was added to the culture medium. The groups during C3a treatment were as follows: control (untreated cells), C3a, C3a plus un-CM (culture medium was control medium mixed with equal volume of basic BV2 culture medium), and C3a plus EPCCM (culture medium was EPCCM mixed with equal volume of basic BV2 culture medium). After incubated for 24 h, Trizol reagent (Invitrogen) was added to each well and cells were collected for the extraction of total RNA.
Transient middle cerebral artery occlusion in mice
Animal studies were performed in accordance with ARRIVE guidelines and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Procedure for using laboratory animals was approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University (Shanghai, China). The surgery of tMCAO was performed as described previously.6,27 Briefly, a 6-0 silicone-coated nylon suture was inserted into the external carotid artery, went along the internal carotid artery, and stopped at the origin of the middle cerebral artery. After 90 min, the suture was withdrawn. The success of occlusion and the reperfusion of cerebral blood flow were confirmed by a laser Doppler flowmetry (Moor Instruments, Axminster, Devon, UK). Mice in which cerebral blood flow was not reduced by more than 80% were excluded from the study. Sham-operated animals underwent identical procedures except for the occlusion of the middle cerebral artery occlusion. Male ICR mice weighing 25–30 g (n = 111) were used in the study.
EPC transplantation
Mice were fixed on a stereotaxic frame (RWD life science, Shenzhen, China) and a small skull hole was made using a microsurgical drill. EPC suspension with 3 × 105 cells was injected through the skull hole at a rate of 1000 nl/min under the stereotactic instrument (RWD life science). The injection location was 2 mm lateral to the bregma and 2.5 mm under the dura. The needle was maintained for 10 min after completing the injection in case of liquid leakage during withdrawing the needle. After the needle was withdrawn, the skull hole was sealed with bone wax and the skin was sutured.
Statistical analysis
Data are represented as mean ± SD. Both parametric and nonparametric comparisons were analyzed using the SPSS18.0 software (SPSS Inc., Chicago, IL). For parametric analysis, multiple comparisons were evaluated by one-way ANOVA following Bonferroni (homogeneity of variance) or the Tamhane test (heterogeneity of variance). For comparisons between two groups, statistical significance was determined using an unpaired Student t-test. For nonparametric analysis, the Kruskal–Wallis test and Mann–Whitney U test were applied. p <0.05 was considered to be statistical significance.
Results
EPC isolation, identification, and transplantation
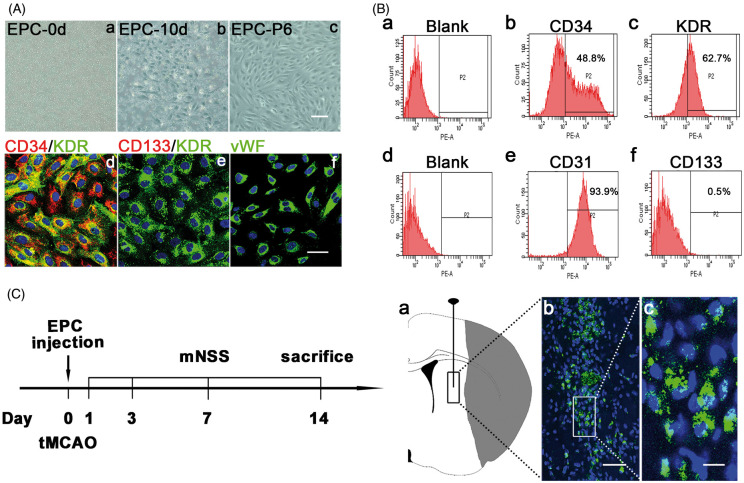
EPCs were isolated from human umbilical cord blood cells and seeded in a 6-well plate with a density of 1 × 107/well (Figure 1(Aa)). At 10 days, the cobblestone-like EPCs appeared (Figure 1(Ab)). EPCs remained cobblestone-like shape and held high proliferation ability at passage six (Figure 1(Ac)). The result of immunofluorescent staining showed that EPCs expressed both CD34 and KDR (Figure 1(Ad)), which were the characteristic markers of EPCs.28,29 In addition, EPCs also expressed endothelial cells marker vWF (Figure 1(Af)). EPCs did not express the other stem cell marker CD133 (Figure 1(Ae)). The result of flow cytometry (Figure 1(b)) also showed that cultured EPCs expressed CD34 (48.8%) and KDR (62.7%). In addition, EPCs highly expressed CD31 (93.9%), but weakly expressed CD133 (0.5%).
Figure 1.
EPC isolation, identification, and transplantation. (A) Images of plated monocytes on the 6-well plate after isolation (a); EPCs with cobblestone shape appeared at 10 days after isolation (b); EPCs maintained cobblestone shape at passage six (c). Scale bar=100 μm. Representative images of EPCs labeled by CD34 (red) and KDR (green) (d), CD133 (red) and KDR (green) (e), and vWF (green) (f). Scale bar=50 μm. (B) Results of flow cytometry analysis of cultured EPCs. Blank (a); CD34 (b); KDR (c); Blank (d); CD31 (e); CD133 (f). (C) The left flow chart showed the experimental design. Mice underwent tMCAO and were randomly assigned to groups. EPCs were injected into the peri-infarct region of striatum in ischemic brain within 120 min after tMCAO. The control group was injected the same volume of PBS with the same procedure. At 1, 3, 7 and 14 days after tMCAO, neurological function deficits in mice were evaluated by two blind investigators using mNSS, which included the examination of motor, sensory, and balance functions. The total score was 14. The higher the score, the more severity of neurological function.56 At 1, 3 and 14 days, mice were sacrificed and brains were collected for mRNA, protein and immunostaining assay. The right schematic diagram of ischemic brain showed the injection region of EPCs (a); Representative images of injected EPCs labeled by GFP in the peri-infarct region of striatum at 14 days after tMCAO (b); Scale bar=50 μm. Zoom of the cells in the white box of picture b (c). The nuclear (blue) of GFP labeled EPCs was positive and the morphology was round. Scale bar=10 μm.
We injected EPCs into the peri-infarct region of the striatum in ischemic brain (Figure 1(Ca)). To track transplanted cells in the ischemic brain, we transfected GFP gene into EPCs (Figure 1(Cb)). The result showed that GFP fluorescence was positive in injected cells, suggesting that the grafted cells were still alive at 14 days after tMCAO (Figure 1(Cc)).
EPC transplantation reduced brain infarct and atrophy volume in ischemic brain
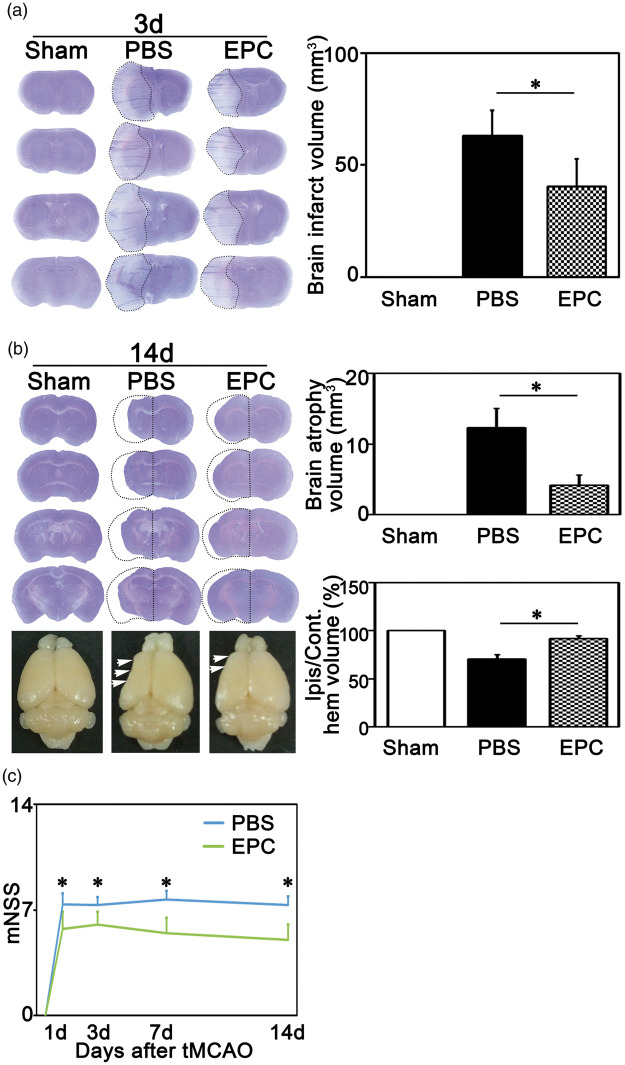
To evaluate the therapeutic effects of EPC transplantation, we examined brain injury and neurological function deficiency in mice following tMCAO. The results showed that the total infarct volume decreased in EPC group compared with PBS group at three days after tMCAO (Figure 2(a), p < 0.05). To clarify whether EPC transplantation maintained the therapeutic effect in the late stage of ischemic stroke, we also evaluated brain injury at 14 days after tMCAO. The results showed that the total brain atrophy volume was smaller in EPC group compared with the PBS group (Figure 2(b), p < 0.01), and the ratio of the ischemic brain volume to the non-ischemic brain volume was larger in EPC group (Figure 2(b), p < 0.01). To evaluate neurological function deficiency in mice after tMCAO, we assessed motor, balance, and reflex functions in mice at 1, 3, 7, and 14 days using modified neurological severity scores (mNSS). The mNSS was lower in whole examination period in EPC group compared with the PBS group (Figure 2(c), p < 0.01). These results indicated that EPC transplantation attenuated brain injury and neurological function deficiency after cerebral ischemia.
Figure 2.
EPC transplantation attenuated ischemic brain injury and neurological function deficiency. (a) Cresyl violet stained brain tissue in Sham, PBS and EPC groups at three days after tMCAO; Dotted lines represented the infarct region. The bar graph showed the quantifications of brain infarct volume among groups. Data are represented as mean±SD. n=5–6 per group. *, p<0.05. (b) Cresyl violet stained brain tissue in Sham, PBS and EPC groups at 14 days after tMCAO; The upper bar graph showed the quantification of total brain atrophy volume among groups and the lower bar graph showed the quantification of the percentage of ischemic ipsilateral hemisphere volume/contralateral hemisphere volume among groups. Data are represented as mean±SD. n=7 per group. *p<0.01. (c) mNSS in EPC and PBS groups at 1, 3, 7, and 14 days after tMCAO. Data are represented as mean±SD. n=7–12 per group. *p<0.01. mNSS: modified neurological severity scores.
EPC transplantation reduced brain C3 expression after tMCAO
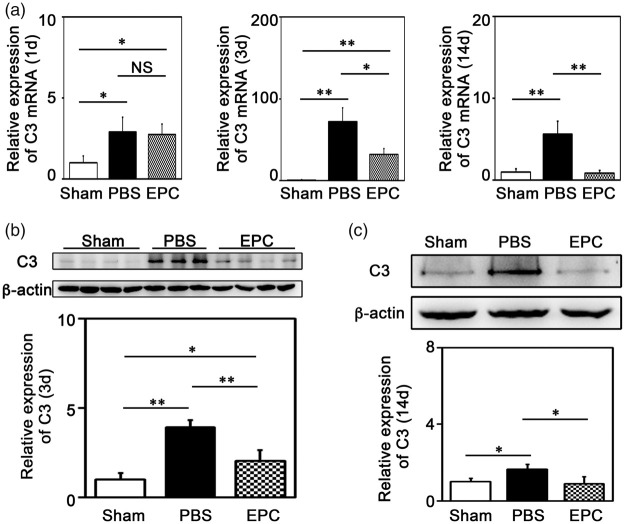
C3 is the core component in complement activation pathway and exacerbates ischemic brain injury at the early stage of ischemia.16,23 To clarify whether EPC transplantation affected C3, we examined C3 expression at 1, 3, and 14 days. We found that C3 increased after tMCAO (Table 1 and Figure 3(a), p < 0.05). Western blot analysis confirmed that C3 was up-regulated at 3 (3.9 folds vs. the sham) and 14 days (1.6 folds) after tMCAO, and EPC transplantation reduced ischemia-induced C3 expression in the brain to 2.0 and 0.9 folds, respectively (Figure 3(b) and (c), p < 0.05).
Table 1.
mRNA expression in EPC-treated mice with tMCAO.
| PBS |
EPC |
||||||
|---|---|---|---|---|---|---|---|
| Sham | 1d | 3d | 14d | 1d | 3d | 14d | |
| TNF-αmRNA | 1 | 1.7 | 13.8 | 4.4 | 1.0 | 4.0 | 1.6 |
| IL-1βmRNA | 1 | 15.1 | 6.0 | 4.7 | 6.3 | 6.9 | 2.4 |
| IL-6 mRNA | 1 | 42.8 | 7.0 | 4.6 | 26.9 | 1.43 | 1.4 |
| C3 mRNA | 1 | 2.9 | 72.3 | 5.6 | 2.8 | 32.4 | 0.9 |
| C3aR mRNA | 1 | 3.2 | 3.4 | 2.0 | 2.3 | 4.2 | 0.7 |
| C3+ cells | 1 | – | 14.8 | 44.4 | – | 2.9 | 6.3 |
| GFAP+ cells | 1 | – | 4.1 | 2.8 | – | 2.3 | 1.4 |
| C3+/GFAP+ cells | 1 | – | 5.2 | 31.5 | – | 0.9 | 3.3 |
Figure 3.
EPC transplantation reduced C3 expression in the mouse brain. (a) Quantification of the expression of C3 mRNA in ischemic brain at 1 day, 3 days and 14 days after tMCAO, respectively. (b–c) Detection of C3 expression using Western blot in ischemic brain at 3 and 14 days after tMCAO, respectively. Bar graphs showed the quantification of the expression of C3. Data are represented as mean±SD. n=3–4 per group. *p<0.05, **p<0.01.
EPC transplantation attenuated astrocyte proliferation and reduced astrocyte-derived C3 expression after tMCAO
Many cells in the brain expressed C3.11,30–32 We demonstrated that astrocyte was the main cell type expressing C3 in ischemic brain at three days after tMCAO, a small number of endothelial cells and neurons also expressed C3, and few microglia expressed C3 (Figure 4(a)). At 14 days after tMCAO, C3 was mainly expressed on astrocyte and less expressed on endothelial cells. No C3+ neurons and microglia were detected (Figure 4(c)). To further analyze whether EPC transplantation attenuated astrocyte proliferation and reduced astrocyte-derived C3 expression, we quantified the number of C3+, GFAP+ and C3+/GFAP+ cells among groups. The results demonstrated that the number of C3+, GFAP+, and C3+/GFAP+ cells was increased after cerebral ischemia. EPC transplantation reduced the number of C3+, GFAP+, and C3+/GFAP+ cells (Table 1 and Figure 4(b) and (d), p < 0.05), suggesting that EPCs could inhibit ischemia-induced astrocyte proliferation and astrocyte-derived C3 expression.
Figure 4.
EPC transplantation attenuated astrocyte proliferation and reduced astrocyte-derived C3 expression in the mouse brain. (a) Representative images of double fluorescence immunostaining of C3 (green) with CD31 (red; a, e, i), MAP2 (red; b, f, j), GFAP (red; c, g, k) and Iba1 (red; d, h, i) in mice brain at 3 days after tMCAO. Scale bar=50 μm. (b) Quantifications of the numbers of C3+, GFAP+ and C3+/GFAP+ cells among groups. The data in Sham group were normalized to 1. Data are represented as mean ± SD. n=3–4 per group. *p<0.05. (c) Representative images of double fluorescence immunostaining of C3 (green) with CD31 (red; a, e, i), MAP2 (red; b, f, j), GFAP (red; c, g, k) and Iba1 (red; d, h, i) in mice brain at 14 days after tMCAO. Scale bar=50 μm. (d) Quantifications of the numbers of C3+, GFAP+ and C3+/GFAP+ cells among groups. The data in Sham group were normalized to 1. Data are represented as mean±SD. n=3–4 per group. *p<0.05.
EPC transplantation reduced C3aR expression in the brain after ischemic stroke
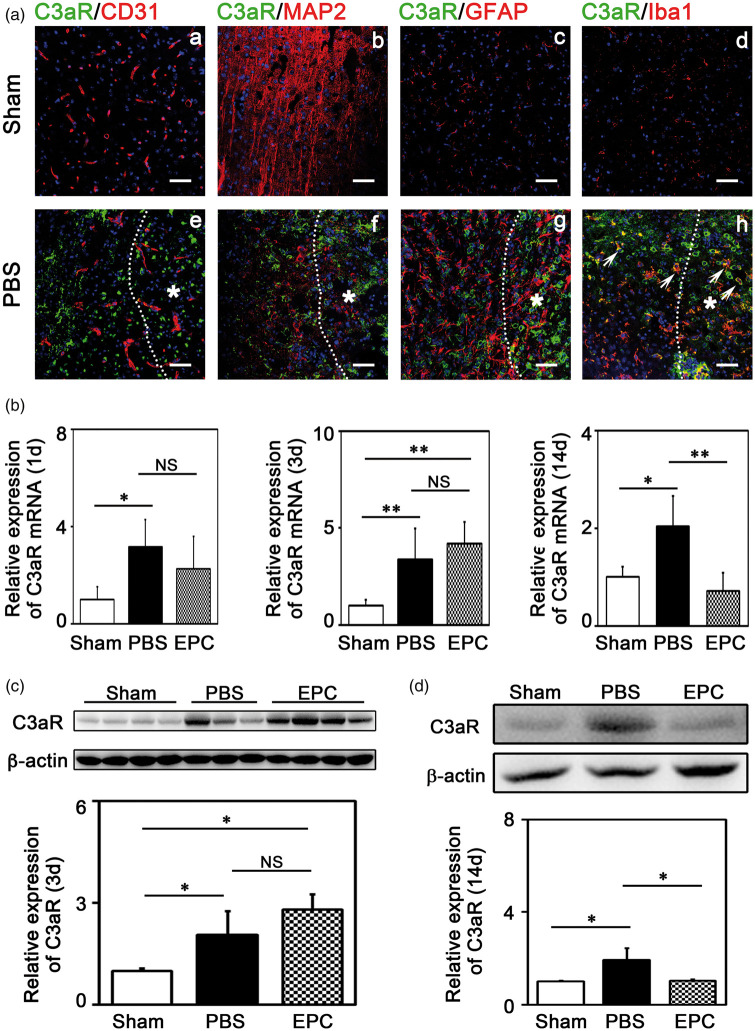
C3aR is the receptor of C3 and plays an important role in the pathophysiological function of C3.33 To determine the types of cells expressing C3aR, we examined C3aR expression using double fluorescence immunostaining. The results showed that C3aR was mainly expressed on microglia in the brain after tMCAO (Figure 5(a)). We further examined C3aR expression using real-time PCR. The results showed that C3aR increased after cerebral ischemia (Table 1 and Figure 5(b), p < 0.05). Western blot analysis confirmed that C3aR was up-regulated at 3 (2.1 folds vs. the sham) and 14 days (1.9 folds) after tMCAO, and EPC transplantation reduced C3aR expression to the basic level close to that in the sham group after 14 days of tMCAO (Figure 5(d), p < 0.05).
Figure 5.
EPC transplantation reduced C3aR expression in the mouse brain. (A) Representative images of double fluorescence immunostaining of C3aR (green) with CD31 (red; a, e), MAP2 (red; b, f), GFAP (red; c, g) and Iba1 (red; d, h) in mice brains of Sham (a–d) and PBS (e–h) groups at 3 days after tMCAO. Scale bar=50 μm. *Represented the infarct zone in mice brains after cerebral ischemia (e–h). White arrows showed the microglia expressing C3aR (h). (B) Quantification of the expression of C3aR mRNA in ischemic brain at 1 day, 3 days and 14 days after tMCAO, respectively. (C–D) Detection of C3aR expression using Western blot in ischemic brain at 3 and 14 days after tMCAO, respectively. Bar graphs showed the quantifications of the expression of C3aR. The data in Sham group were normalized to 1. Data are represented as mean±SD. n=3–4 per group. *p<0.05, **p<0.01.
EPC transplantation reduced inflammatory response in the brain after ischemic stroke
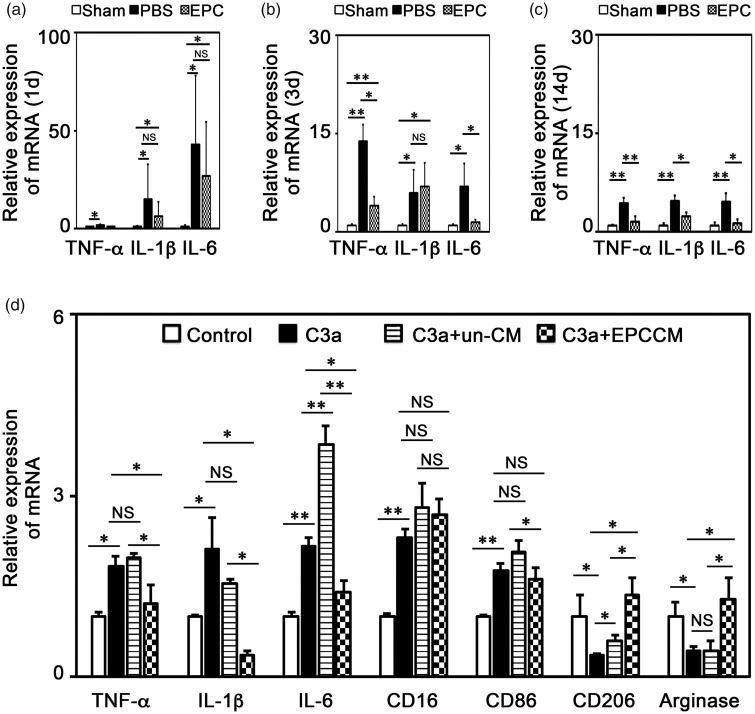
The interaction between C3 and C3aR mainly mediated inflammatory response during pathological conditions and exacerbated brain injury at the early stage of ischemic stroke.34 Since EPC transplantation reduced C3 and C3aR expression after cerebral ischemia, we clarified whether the inflammatory response was reduced. We demonstrated that TNF-α, IL-1β and IL-6 mRNA expression increased after tMCAO from 1 day to 14 days. EPC transplantation reduced TNF-α, IL-1β and IL-6 expression (Table 1 and Figure 6(a) to (c), p < 0.05).
Figure 6.
EPC transplantation reduced inflammatory response in vivo and in vitro. (a–c) Quantifications of the expressions of TNF-α, IL-1β and IL-6 mRNA in ischemic brain at 1, 3 and 14 days after tMCAO, respectively. The data in Sham group were normalized to 1. Data are represented as mean±SD. n=3–4 per group. *, p<0.05, **, p<0.01. (d) Quantifications of the expressions of TNF-α, IL-1β, IL-6, CD16, CD86, CD206 and Arginase mRNA in cultured microglia under C3a treatment among groups. The data in Control group were normalized to 1. Data are represented as mean±SD. n=3 per group. *, p<0.05, **, p<0.01. Control: untreated group; C3a: C3a treated group; C3a+un-CM: control medium treated under C3a treatment; C3a+EPCCM: EPC-conditional medium treated under C3a treatment.
In order to further clarify the correlation between EPC transplantation and C3 reduction, we performed experiments. The real-time PCR results showed that C3a treatment resulted in increased expressions of TNF-α (1.82 folds), IL-1β (2.12 folds), and IL-6 (2.15 folds, Figure 6(d), p < 0.05). In addition, C3a treatment increased the CD16 (2.3 folds) and CD86 (1.76 folds) expression and reduced CD206 (0.34 folds) and Arginase (0.44 folds, Figure 6(d), p < 0.05) expression, which are the markers of pro-inflammatory (M1) and anti-inflammatory (M2) phenotype of microglia.35 EPC-conditioned medium reversed the up-regulation of TNF-α, IL-1β, IL-6, CD16 and CD86 and enhanced the expression of CD206 and Arginase during C3a treatment (Figure 6(d), p < 0.05), suggesting that EPCs could inhibit pro-inflammatory response and reverse pro-inflammatory phenotype transformation of microglia induced by C3a/C3aR pathway.
Discussion
In the present study, we aimed to clarify whether EPC transplantation affected complement activation to reduce ischemic brain injury. We found that C3 and C3aR were up-regulated in the brain not only at the early stage but also at the late stage of ischemic stroke. The up-regulation of C3 and C3aR in the brain was associated with severe brain injury and impaired neurological function. Astrocyte was the main cell type expressing C3. EPC transplantation attenuated astrocyte proliferation and reduced astrocyte-derived C3 expression. Furthermore, we demonstrated that EPC transplantation reduced C3aR expression in the brain at 14 days after tMCAO, contributing to attenuated inflammatory response, brain injury and neurological function deficiency.
EPCs were injected into the brain within 120 min after tMCAO. We counted 105 brain sections in which all the grafted cells were included (5 sections were counted and the next 20 sections were discarded). We continued counting in this fashion five times throughout the 105 sections. We then counted the number of surviving cells, which averaged to 53 cells in each brain section. Therefore, survival cells were 5565 (≈2%) of the total injected cells (3 × 105) in the brain after 14 days of transplantation. Nevertheless, these survival cells functionally reduced ischemic brain injury and improved neurological function. A recent study showed that there were 100 EPCs per brain section at seven days after ischemic stroke and displayed reduced neurological deficiency in rats.36 Our previous study demonstrated that there were only few EPCs per microscope field left after five weeks of brain ischemia. However, it could promote angiogenesis and neurogenesis and improve neurological function in ischemic mice.37 Therefore, we assume that the number of surviving cells would decline over time. Future study is required to establish better label techniques that help to determine the portion of grafted cells alive in the brain after transplantation over time.
It is noted that the infarct area varies with individual animal. Our lab has done much work on establishing relative stable tMCAO model in mice.38–40 The injected location was determined to be in the peri-infarct area and not in the infarct core based on multiple preliminary experiences.41–43 We could see EPCs alive in the ipsilateral hemisphere of ischemic brain (Figure 1), suggesting that injected EPCs were not in the infarct core, where there was no blood supply. Lastly, brain injury was attenuated and neurological function was improved in mice at 14 days after EPC transplantation, suggesting that at least there were still survived EPCs in the brain and exerted therapeutic function.
Our previous data had already shown that EPC transplantation could reduce ischemic brain injury both during the acute phase7,26 and during the chronic phase of ischemic stroke.6,37 A recent study using the 3 × 105 of EPCs to inject into the brain also showed improved neurological function in mice during the acute phase of ischemic stroke.36 Thus, we supposed that EPC transplantation could protect brain in the whole period of ischemia. In addition, we believed that EPC transplantation via direct brain injection was better than other approaches since more EPCs were present in the injured brain.
C3 is the core component of complement activation pathways and plays a vital role in the pathophysiological process of ischemic stroke.14,19 Previous studies showed that C3 was up-regulated after ischemic stroke and depletion of C3 or alleviating C3 activation reduced neuronal death, brain infarct volume and neurological score.16,23,44 However, these studies mainly focused on the early stage of cerebral ischemia and the exact role of up-regulated C3 in the neurological function recovery of late stage of ischemic stroke is obscure. In addition, whether depletion of C3 using Cobra venom factor (CVF) or genetic knock-out method is beneficial or detrimental for the outcome of ischemic stroke is still controversial.23,45 Our study supported the notion that C3 was up-regulated both at the early and late stage of ischemic stroke and associated with exacerbated ischemic brain injury and impaired tissue repair during the whole pathogenesis of ischemic stroke. EPC transplantation reduced C3 expression both at the early and late stage of ischemic stroke, contributing to reduced ischemic brain injury and neurological function deficiency. Contrary to our results of the detrimental role of C3 in ischemic stroke, another study showed that C3 was beneficial for functional recovery, because intranasal treatment of complement peptide C3a, an active fragment of C3,46 promoted neuronal plasticity.47 The discrepancy of the conclusions may due to different ischemic models. We used the tMCAO model, which has a different pathogenesis from the photothrombotic model. In addition, complement peptide C3a treatment could not imitate the ischemia-induced C3 elevation in the brain.
C3 could be expressed on neurons, microglia, and astrocytes in the brain under some pathological conditions.25,48 However, which cell types mediate specific aspects of C3 in ischemic stroke is still unclear. We found that C3 was predominantly expressed on astrocytes both at 3 and 14 days after cerebral ischemia. Fewer C3 was expressed on endothelial cells and injured neurons, but no C3 was found on microglia. EPC transplantation attenuated astrocyte proliferation and reduced astrocyte-derived C3 expression at 3 and 14 days after cerebral ischemia. C3aR is one of the receptor of C3, and the interaction between C3 and C3aR has been widely recognized to mediate inflammatory response and exacerbate tissue injury during pathological conditions including major depressive disorder (MDD),49 non-ischemic heart failure,50 perioperative neurocognitive disorders (PND),51 acute lung injury,52 traumatic brain injury34 and ischemic stroke.16,53 In mice models of ischemic stroke, depletion of C3 or C3aR contributed to reduced granulocyte infiltration, inflammatory response and brain injury within three days after ischemia.16,53 We found that C3aR was up-regulated in the brain both at the early and late stage of cerebral ischemia and was mainly expressed on microglia. EPC transplantation reduced C3aR expression at the late stage of cerebral ischemia both at mRNA and protein level. Furthermore, we wanted to clarify whether EPC transplantation alleviated inflammatory response after ischemic stroke. We detected the expression of TNF-a, IL-1β and IL-6 in ischemic brain using real-time PCR, and the results showed that EPC transplantation reduced TNF-a, IL-1β and IL-6 mRNA expression both at 3 and 14 days after cerebral ischemia. Despite more microglia were detected in the EPC-treated group, we demonstrated that EPC mainly enhanced anti-inflammatory microglia markers and reduced pro-inflammatory markers after ischemic stroke, indicating that it was more anti-inflammatory phenotype of microglia, which may exert protective function in ischemic brain. To determine whether EPC transplantation reduced inflammatory response via affecting C3/C3aR pathway in microglia, we performed real-time PCR experiment. We found that C3a enhanced TNF-α, IL-1β, and IL-6 expression in cultured microglia. C3a could increase CD16 and CD86 expression and reduce CD206 and Arginase expression. EPC-conditioned medium reversed TNF-α, IL-1β, IL-6, CD16 and CD86 expression and enhanced CD206 and Arginase expression during C3a treatment. These results suggested that EPCs could weaken inflammatory response and promote anti-inflammatory phenotype transformation of microglia. Although C3/C3aR and inflammatory response was not affected at one day after EPC transplantation, improved neurological function was still observed. This may be explained by EPCs could influence hemodynamics at day 0 after acute stroke, which was associated with functional recovery.52
We did not find out what pathway determined C3 synthesis and activity. NF-κB activation was reported to be involved in C3 synthesis in astrocytes after HIV infection or in Alzheimer's disease.31,48 We examined NF-κB activation using Western blot after tMCAO and did not find changes of pNF-KB/NF-KB expression between EPC and PBS groups (data not shown). We also did not detect changes in MAPK and AKT signals in the EPC-treated group, suggesting that EPCs reduced C3 synthesis may be via other pathways. Previous studies showed that twist basic helix-loop-helix transcription factor 1 (TWIST1) could bind to the C3 promoter and enhance its expression in tumor cells, contributing to pathologic and physiologic epithelial-mesenchymal transition in malignant tumors.54 Carboxypeptidase B1 (Cpb1), a complement-related peptidase, was found to modify C3 expression and to have played a critical role for Cpb1-C3-C3aR pathway in pro-inflammatory signaling, caspase-11 cell death, and sepsis severity.55 We demonstrated TWIST1 and Cpb1 expression using real-time PCR. The results showed that Cpb1 and TWIST1 expression were increased after tMCAO (Suppl. Figure 3). EPC transplantation alone did not affect TWIST1 expression at three days after tMCAO. It was noted that after 14 days of tMCAO, EPC transplantation did not affect Cpb1 expression but reduced TWIST1 expression (Suppl. Figure 3). These results suggested that TWIST1 and Cpb1 were partially involved in modulating C3 synthesis and C3 activity during brain ischemia. EPC transplantation could affect Cpb1 and TWIST1 expression at different stages of ischemic stroke to reduce C3 activation. Whether EPC transplantation reduced C3 activation via inhibiting Cpb1 and TWIST1 expression needs to be further studied.
In conclusion, our work demonstrated that C3 was up-regulated both at the early and late stage of ischemic stroke and the up-regulated C3 mainly derived from astrocytes in the brain. Furthermore, EPC transplantation reduced astrocyte-derived C3 expression and the expression of C3aR in ischemic brain, contributing to decreased inflammatory response, reduced brain injury and accelerated neurological function recovery. Our study provided a novel insight into the mechanism of EPC transplantation for ischemic stroke treatment. Attenuating astrocyte-derived C3 in the brain is possibly a potential therapeutic strategy for cerebral ischemia.
Supplemental Material
Supplemental material, JCB892777 Supplemental Material for Endothelial progenitor cell transplantation alleviated ischemic brain injury via inhibiting C3/C3aR pathway in mice by Yuanyuan Ma, Lu Jiang, Liping Wang, Yongfang Li, Yanqun Liu, Wenjing Lu, Rubing Shi, Linyuan Zhang, Zongjie Fu, Meijie Qu, Yingling Liu, Yongting Wang, Zhijun Zhang and Guo-Yuan Yang in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (81771244, ZJZ; 81771251, GYY; 81901185, YYM), the Science and Technology Commission of Shanghai Municipality (17ZR1413600, ZJZ), the National Key Research and Development Program of China (2016YFC1300600) and the K. C. Wong Education Foundation (GYY).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: MYY prepared the figures and wrote the manuscript; JL helped collect animal samples and contributed to the analysis of data; WLP and LYQ did the neurological function assessment. LYF helped transplant EPCs into mice; LWJ and QMJ helped culture EPCs. SRB helped collect EPC-conditioned medium and culture microglia; FZJ helped perform immunostaining; LYL and ZLY contributed to the FACS experiment; WYT helped analyze the results; ZZJ helped design and instruct the experiment; YGY conceived the study and revised the manuscript.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Hankey GJ.Stroke. Lancet 2017; 389: 641–654. [DOI] [PubMed] [Google Scholar]
- 2.Mangin G, Kubis N.Cell therapy for ischemic stroke: how to turn a promising preclinical research into a successful clinical story. Stem Cell Rev Rep 2019; 15: 176–193. [DOI] [PubMed] [Google Scholar]
- 3.Gervois P, Wolfs E, Ratajczak J, et al. Stem cell-based therapies for ischemic stroke: preclinical results and the potential of imaging-assisted evaluation of donor cell fate and mechanisms of brain regeneration. Med Res Rev 2016; 36: 1080–1126. [DOI] [PubMed] [Google Scholar]
- 4.Janowski M, Wagner DC, Boltze J.Stem cell-based tissue replacement after stroke: factual necessity or notorious fiction? Stroke 2015; 46: 2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao YH, Yuan B, Chen J, et al. Endothelial progenitor cells: therapeutic perspective for ischemic stroke. CNS Neurosci Ther 2013; 19: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Lin X, Wang J, et al. Effect of HMGB1 on the paracrine action of EPC promotes post-ischemic neovascularization in mice. Stem Cells 2014; 32: 2679–2689. [DOI] [PubMed] [Google Scholar]
- 7.Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol 2010; 67: 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapergue B, Mohammad A, Shuaib A.Endothelial progenitor cells and cerebrovascular diseases. Prog Neurobiol 2007; 83: 349–362. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 2004; 114: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung HS, Jeong SY, Yang J, et al. Neuroprotective effect of mesenchymal stem cell through complement component 3 downregulation after transient focal cerebral ischemia in mice. Neurosci Lett 2016; 633: 227–234. [DOI] [PubMed] [Google Scholar]
- 11.Cowell RM, Plane JM, Silverstein FS.Complement activation contributes to hypoxic-ischemic brain injury in neonatal rats. J Neurosci 2003; 23: 9459–9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajishengallis G, Reis ES, Mastellos DC, et al. Novel mechanisms and functions of complement. Nat Immunol 2017; 18: 1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsberg PJ, Ohman J, Lehto T, et al. Complement activation in the central nervous system following blood-brain barrier damage in man. Ann Neurol 1996; 40: 587–596. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Liu Y, Zhang Z, et al. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis 2019; 10: 429–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkinson C, Zhu H, Qiao F, et al. Complement-dependent P-selectin expression and injury following ischemic stroke. J Immunol 2006; 177: 7266–7274. [DOI] [PubMed] [Google Scholar]
- 16.Mocco J WJM, Andrew FD, Sergei AS, et al. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circul Res 2006; 99: 209–217. [DOI] [PubMed] [Google Scholar]
- 17.Ricklin D, Hajishengallis G, Yang K, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 2010; 11: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank MM, Fries LF.The role of complement in inflammation and phagocytosis. Immunol Today 1991; 12: 322–326. [DOI] [PubMed] [Google Scholar]
- 19.Stephan AH, Barres BA, Stevens B.The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 2012; 35: 369–389. [DOI] [PubMed] [Google Scholar]
- 20.Mocco J, Wilson DA, Komotar RJ, et al. Alterations in plasma complement levels after human ischemic stroke. Neurosurgery 2006; 59: 28–33; discussion 28–33. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Yang N, Gao C.Is plasma C3 and C4 levels useful in young cerebral ischemic stroke patients? Associations with prognosis at 3 months. J Thromb Thromb 2015; 39: 209–214. [DOI] [PubMed] [Google Scholar]
- 22.Cojocaru IM, Cojocaru M, Tanasescu R, et al. Changes in plasma levels of complement in patients with acute ischemic stroke. Rom J Intern Med 2008; 46: 77–80. [PubMed] [Google Scholar]
- 23.Alawieh A, Elvington A, Zhu H, et al. Modulation of post-stroke degenerative and regenerative processes and subacute protection by site-targeted inhibition of the alternative pathway of complement. J Neuroinflammation 2015; 12: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueroa E, Gordon LE, Feldhoff PW, et al. The administration of cobra venom factor reduces post-ischemic cerebral injury in adult and neonatal rats. Neurosci Lett 2005; 380: 48–53. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Ahn HN, Chang M, et al. Complement component 3 inhibition by an antioxidant is neuroprotective after cerebral ischemia and reperfusion in mice. J Neurochem 2013; 124: 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng J, Wang L, Qu M, et al. Endothelial progenitor cells transplantation attenuated blood-brain barrier damage after ischemia in diabetic mice via HIF-1alpha. Stem Cell Res Ther 2017; 8: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Li Y, Jiang L, et al. Macrophage depletion reduced brain injury following middle cerebral artery occlusion in mice. J Neuroinflammation 2016; 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CY, Wu RW, Tsai NW, et al. Increased circulating endothelial progenitor cells and improved short-term outcomes in acute non-cardioembolic stroke after hyperbaric oxygen therapy. J Transl Med 2018; 16: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fadini GP, Baesso I, Albiero M, et al. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis 2008; 197: 496–503. [DOI] [PubMed] [Google Scholar]
- 30.Gao M, Dong Q, Lu Y, et al. Induced neural stem cell-derived astrocytes modulate complement activation and mediate neuroprotection following closed head injury. Cell Death Dis 2018; 9: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitkiewicz J, Borjabad A, Morgello S, et al. HIV induces expression of complement component C3 in astrocytes by NF-kappaB-dependent activation of interleukin-6 synthesis. J Neuroinflammation 2017; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Beek J, Elward K, Gasque P.Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann N Y Acad Sci 2003; 992: 56–71. [DOI] [PubMed] [Google Scholar]
- 33.Lohman RJ, Hamidon JK, Reid RC, et al. Exploiting a novel conformational switch to control innate immunity mediated by complement protein C3a. Nat Commun 2017; 8: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammad A, Westacott L, Zaben M.The role of the complement system in traumatic brain injury: a review. J Neuroinflammation 2018; 15: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y, Wang J, Wang Y, et al. The biphasic function of microglia in ischemic stroke. Prog Neurobiol 2017; 157: 247–272. [DOI] [PubMed] [Google Scholar]
- 36.Acosta SA, Lee JY, Nguyen H, et al. Endothelial progenitor cells modulate inflammation-associated stroke vasculome. Stem Cell Rev Rep 2019; 15: 256–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Chang S, Li W, et al. cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res Ther 2018; 9: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan Y, Wang Y, Yuan F, et al. Effect of suture properties on stability of middle cerebral artery occlusion evaluated by synchrotron radiation angiography. Stroke 2012; 43: 888–891. [DOI] [PubMed] [Google Scholar]
- 39.Lin X, Miao P, Wang J, et al. Surgery-related thrombosis critically affects the brain infarct volume in mice following transient middle cerebral artery occlusion. PLoS One 2013; 8: e75561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan F, Tang Y, Lin X, et al. Optimizing suture middle cerebral artery occlusion model in C57BL/6 mice circumvents posterior communicating artery dysplasia. J Neurotrauma 2012; 29: 1499–1505. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Huang J, He X, et al. Postacute stromal cell-derived factor-1alpha expression promotes neurovascular recovery in ischemic mice. Stroke 2014; 45: 1822–1829. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Huang J, Ma Y, et al. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J Cereb Blood Flow Metab 2015; 35: 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang G, Liu Y, Zhang Z, et al. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells 2014; 32: 3150–3162. [DOI] [PubMed] [Google Scholar]
- 44.Lai W, Xie X, Zhang X, et al. Inhibition of complement drives increase in early growth response proteins and neuroprotection mediated by salidroside after cerebral ischemia. Inflammation 2018; 41: 449–463. [DOI] [PubMed] [Google Scholar]
- 45.Rahpeymai Y, Hietala MA, Wilhelmsson U, et al. Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J 2006; 25: 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawksworth OA, Li XX, Coulthard LG, et al. New concepts on the therapeutic control of complement anaphylatoxin receptors. Mol Immunol 2017; 89: 36–43. [DOI] [PubMed] [Google Scholar]
- 47.Stokowska A, Atkins AL, Moran J, et al. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain 2017; 140: 353–369. [DOI] [PubMed] [Google Scholar]
- 48.Lian H, Yang L, Cole A, et al. NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease. Neuron 2015; 85: 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crider A, Feng T, Pandya CD, et al. Complement component 3a receptor deficiency attenuates chronic stress-induced monocyte infiltration and depressive-like behavior. Brain Behav Immun 2018; 70: 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mueller KAL, Patzelt J, Sauter M, et al. Myocardial expression of the anaphylatoxin receptor C3aR is associated with cardiac inflammation and prognosis in patients with non-ischaemic heart failure. ESC Heart Fail 2018; 5: 846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Yang Z, Chavko M, et al. Complement inhibition ameliorates blast-induced acute lung injury in rats: potential role of complement in intracellular HMGB1-mediated inflammation. PLoS One 2018; 13: e0202594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sargento-Freitas J, Aday S, Nunes C, et al. Endothelial progenitor cells influence acute and subacute stroke hemodynamics. J Neurol Sci 2018; 385: 119–125. [DOI] [PubMed] [Google Scholar]
- 53.Andrew F, Ducruet BGH, William J, Sergei AS, et al. C3a receptor modulation of granulocyte infiltration after murine focal cerebral ischemia is reperfusion dependent. J Cereb Blood Flow Metab 2008; 28: 1048–1058. [DOI] [PubMed] [Google Scholar]
- 54.Cho MS, Rupaimoole R, Choi HJ, et al. Complement component 3 is regulated by TWIST1 and mediates epithelial-mesenchymal transition. J Immunol 2016; 196: 1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napier BA, Brubaker SW, Sweeney TE, et al. Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. J Exp Med 2016; 213: 2365–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 2000; 20: 1311–1319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JCB892777 Supplemental Material for Endothelial progenitor cell transplantation alleviated ischemic brain injury via inhibiting C3/C3aR pathway in mice by Yuanyuan Ma, Lu Jiang, Liping Wang, Yongfang Li, Yanqun Liu, Wenjing Lu, Rubing Shi, Linyuan Zhang, Zongjie Fu, Meijie Qu, Yingling Liu, Yongting Wang, Zhijun Zhang and Guo-Yuan Yang in Journal of Cerebral Blood Flow & Metabolism