Abstract
White matter hyperintensity (WMH) is a common finding in aging population and considered to be a contributor to cognitive decline. Our study aimed to characterize the spatial patterns of WMH in different severities and explore its impact on cognition and brain microstructure in non-demented elderly. Lesions were both qualitatively (Fazekas scale) and quantitatively assessed among 321 community-dwelled individuals with MRI scanning. Voxel- and atlas-based analyses of the whole-brain white matter microstructure were performed. The WMH of the same severities was found to occur uniformly with a specific pattern of lesions. The severity of WMH had a significant negative association with the performance of working and episodic memory, beginning to appear in Fazekas 3 and 4. The white matter tracts presented significant impairments in Fazekas 3, which showed brain-wide changes above Fazekas 4. Lower FA in the superior cerebellar peduncle and left posterior thalamic radiation was mainly associated with episodic memory, and the middle cerebellar peduncle was significantly associated with working memory. These results support that memory is the primary domain to be affected by WMH, and the effect may potentially be influenced by tract-specific WM abnormalities. Fazekas scale 3 might be the critical stage predicting a future decline in cognition.
Keywords: Cognitive impairment, diffusion tensor imaging, magnetic resonance imaging, non-demented elderly, white matter hyperintensities
Introduction
White matter hyperintensities (WMHs) are considered to be associated with an increased risk of ischaemic and haemorrhagic stroke, dementia and depression,1,2 as one of the most common and prominent changes seen in MRI of elderly individuals,3,4 and are indeed associated with microstructural damage, brain atrophy and cognitive decline on a global level.5 Therefore, it is important to determine the underlying pathologic mechanisms of WMH.
Mounting evidence showed that the spatial location and volume of WMH may have significant neuropathological and clinical associations with cognitive impairment.6–8 Regional, tract-specific lesions might accelerate the process of brain ageing, as a mediator and an independent factor in the general population.9 Recent studies using diffusion tensor imaging (DTI) techniques indicated that global fractional anisotropy (FA) decreases in patients with WMH,10–12 and changes in white matter microstructures may occur more than a decade earlier than WMH which can be seen on imaging.13,14 However, little is known about how the microstructure of white matter changes on a local level as the severity of WMH increases and whether it affects cognitive functions independently.
Despite being a subjective visual assessment, Fazekas score is convenient in primary health care services. High correlation between Fazekas scale scores and volumetric WML assessment has previously been demonstrated in the Leukoaraiosis and Disability study (LADIS) and the Sunnybrook Dementia Study.15,16 Therefore, we adopted both Fazekas scale and WMH volume as criteria for evaluating the severity of white matter hyperintensities to detect the microstructural changes of white matter as well as their location-specific impact on cognition, and whether these effects vary among the degree of WMH in community-dwelling non-demented elderly.
Materials and methods
Participants
The present sample consisted of 321 clinically normal, community-dwelling older adults from the Beijing Aging Brain Rejuvenation Initiative Study Group (BABRI), an ongoing longitudinal study. All enrolled participants (1) scored ≥24 on the Mini-Mental Status Examination; (2) had no history of coronary disease, nephritis, tumours, neurological or psychiatric disorders, or addiction; (3) had no conditions known to affect cerebral function, including alcoholism, current depression, Parkinson’s disease, or epilepsy; and (4) had no large vessel diseases such as cortical or subcortical infarcts or watershed infarcts. Demographic information for each group is presented in Table 1. This protocol was approved by the Institutional Review Board of the Beijing Normal University Imaging Center for Brain Research (approval number: ICBIR_A_0041_002.02) and was in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant.
Table 1.
Characteristics of the cross-sectional study.
|
Fazekas score |
F /x2 | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |||
| N (%) | 84 (26.2) | 34 (10.6) | 67 (20.9) | 80 (24.9) | 38 (11.8) | 11 (3.4) | 7 (2.2) | ||
| Mean age (SD) | 62.4 (6.4) | 66.2 (6.3) | 66.6 (6.3) | 68.2 (7.1) | 68.8 (6.6) | 71.9 (7.8) | 72.5 (7.0) | 52.0 | <0.001 |
| Gender (male/female) | 24/60 | 17/17 | 24/43 | 33/47 | 16/22 | 5/6 | 5/2 | 9.5 | 0.15 |
| Education (SD) | 12.0 (3.1) | 11.9 (3.6) | 11.2 (3.1) | 11.4 (3.1) | 11.7 (2.9) | 12.1 (4.1) | 12.3 (2.9) | 41.0 | <0.001 |
| Smoking ever (%) | 22.4 | 23.5 | 16.4 | 20.0 | 18.4 | 18.2 | 14.3 | 2.0 | 0.92 |
| Diabetes (%) | 28.6 | 26.5 | 19.4 | 25 | 44.7 | 36.4 | 14.3 | 9.9 | 0.13 |
| Arterial hypertension (%) | 34.5 | 44.1 | 43.4 | 70 | 63.2 | 81.8 | 57.1 | 29.3 | <0.001 |
| Lacunar infarcts (%) | 13.1 | 47.1 | 70.1 | 81.3 | 97.4 | 100 | 100 | 131.5 | <0.001 |
| WMH volume (SD, mL) | 0 | 1.0 (0.9) | 2.1 (1.8) | 4.4 (2.7) | 10.3 (4.7) | 20.2 (4.8) | 34.4 (12.7) | 397.8 | <0.001 |
| MMSE (SD) | 28.2 (1.4) | 27.8 (1.7) | 27.9 (1.5) | 28.0 (1.5) | 27.9 (1.3) | 28.4 (1.7) | 26.1 (1.3) | 2.7 | 0.104 |
| MoCA (SD) | 24.4 (3.0) | 21.9 (3.8) | 23.4 (2.9) | 23.3 (2.8) | 22.5 (2.7) | 23.9 (2.7) | 21.4 (2.5) | 5.9 | 0.016 |
| Episodic memory (SD) | 0.2 (0.7) | 0.0 (1.0) | 0.2 (0.8) | −0.1 (0.8) | 0.2 (0.8) | −0.1 (0.7) | 0.8 (0.5) | 11.8 | 0.001 |
| Working memory (SD) | 0.3 (1.0) | 0.1 (1.0) | −0.1 (0.9) | −0.1 (1.0) | −0.2 (0.9) | −0.5 (0.5) | −0.3 (0.8) | 11 | 0.001 |
| Language (SD) | 0.2 (0.8) | −0.2 (0.8) | 0.0 (0.9) | 0.0 (0.8) | −0.2 (0.9) | −0.3 (0.7) | −0.5 (0.8) | 8.4 | 0.004 |
| Processing speed (SD) | 0.2 (0.5) | 0.0 (0.6) | −0.1 (0.6) | −0.1 (0.4) | 0.0 (0.5) | −0.1 (0.5) | −0.2 (0.7) | 8.8 | 0.003 |
| Executive function (SD) | −0.3 (0.8) | 0.1 (0.8) | 0.0 (0.8) | 0.0 (0.8) | 0.2 (0.5) | −0.2 (0.7) | 0.9 (0.9) | 10.4 | 0.001 |
| Spatial processing (SD) | 0.1 (0.6) | 0.0 (0.8) | 0.0 (0.7) | −0.1 (0.8) | −0.1 (0.8) | 0.0 (1.0) | −0.3 (0.8) | 4.6 | 0.033 |
Clinical and neuropsychological examination
All participants were subjected to a battery of neuropsychological tests that assessed several cognitive domains. The comprehensive neuropsychological battery was comprised of the following seven cognitive domains (the tests used to assess each domain are in parentheses): 1. general mental status (the Chinese version of the Mini-Mental-Status Examination [MMSE]17 and the Montreal Cognitive Assessment [MoCA]18); 2. Episodic memory (the Auditory Verbal Learning Test [AVLT]19 and the Rey–Osterrieth Complex Figure test [ROCF] (recall)20); 3. Working memory (the Digit Span test, which was a sub-test of the Wechsler Adult Intelligence Scale-Chinese revision); 4. Spatial processing (ROCF-copy20) and the Clock-Drawing Test [CDT]21); 5. Language (the Category Verbal Fluency Test [CVFT] and the Boston Naming Test [BNT]22); 6. Processing speed (the Trail Making Test [TMT] A23 and the Symbol Digit Modalities Test [SDMT]24); and 7. Executive function (the TMT-B23 and the Stroop Color and Word Test C [SCWT]22). The mean z-scores were used for the representation of each cognitive domain.
Image acquisition
All subjects were scanned using a Siemens Trio 3 T MRI scanner in the Imaging Center for Brain Research at Beijing Normal University. Diffusion tensor images were acquired using a twice-refocused spin-echo diffusion-weighted EPI sequence with the following parameters: 70 axial sections, section thickness [ST] = 2 mm, no section gap, 30 diffusion directions with a b-value of 1000 s/mm2 and an additional image with a b-value of 0 s/mm2, field of view [FOV] = 256 mm × 256 mm, acquisition matrix [AM] = 128 × 128, number of signals acquired = 3. T1-weighted brain structural images were acquired using 3D magnetization-prepared rapid gradient echo (MP-RAGE) sequence with the following parameters: 176 sagittal slices, TR = 1900 ms, TE = 3.44 ms, ST = 1 mm, FA = 9°, FOV = 256 mm × 256 mm, AM = 256 × 256. A T2-weighed fluid-attenuated inversion recovery (T2w-FLAIR) sequence was also applied to measure WM hyperintensities with the following parameters: TR = 9000 ms, TE = 81 ms, slice thickness = 3 mm, flip angle = 150°, number of slices = 25.
WMH visual assessment
Three hundred and twenty-one subjects who had T2w-FLAIR images were assessed and scored their WMH on the Fazekas scale by two experienced neurologists who were blind to the clinical data. In the Fazekas rating scale, periventricular and deep WMH are rated separately,25 and periventricular WMH was graded according to the following patterns: 0 = absent; 1 = caps or pencil-thin lining; 2 = smooth halo; and 3 = irregular periventricular WMH extending into a deep WMH. Deep WMH was graded according to the following patterns: 0 = absent; 1 = punctate foci; 2 = beginning confluence of foci; and 3 = large fused areas. A total Fazekas score, ranging from 0 to 6, was acquired by summing the periventricular and deep WMH scores.
Lesion segmentation and registration
Lesions were segmented using the lesion growth algorithm, as implemented in the LST toolbox version 2.0.15 (www.statistical-modelling.de/lst.html) for SPM.26 Briefly, this instrument segments the T1 images into CSF, WM and GM; then, it coregisters the FLAIR images with them and generates lesion belief maps. A threshold of 0.2 was used for obtaining the lesion probability map and lesion volume. We used a nonlinear registration tool in FSL, FNIRT27 to register the subject-specific images to the Montreal Neurological Institute (MNI) space via T1-structural image: (1) Start by getting an initial affine transform mapping structural onto the MNI152 template with flirt; (2) The next step we used that as initial guess for fnirt; (3) And finally we used that to resample the flair scan into the MNI152 space. Then, we overlapped the images of each group in order to get the lesion probability maps.
Diffusion tensor imaging preprocessing and tract-based spatial statistics
Preprocessing and analyses were performed using the FMRIB Diffusion Toolbox (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, http://www.fmrib.ox.ac.uk/fsl). Briefly, all the diffusion-weighted images were eddy current corrected by applying affine alignment using the b = 0 image as reference. A binary brain mask was then calculated for each subject using the Brain Extraction Tool. Finally, a fractional anisotropy (FA) map was calculated using DTIfit.
The resulting FA maps for all participants were then fed into the TBSS pipeline.28 First, each subject’s FA map was aligned into the MNI standard space by affine registration. The images were further averaged to generate a mean FA image and its skeleton from all subjects. The mean FA skeleton was subject to a threshold of 0.3, and all subjects’ images were projected onto this skeleton.
Statistical analysis
The means and standard deviations are presented for the continuous variables (age, education, neuropsychological scores, WMH volume, intercranial volume), and frequencies and percentages of the total population were calculated for categorical variables (gender, diabetes, arterial hypertension, lacunar infarcts and smoking prevalence). Group differences were tested by independent sample t test (continuous variables) and Chi-squared test (categorical variables). The distribution of the WMH scores in the study was positively skewed, as expected, and the agreement between Fazekas scores and WMH volume was assessed by using Spearman correlation. Linear regression models with different covariates were used to examine the influence of the following variables on relationship between the white matter hyperintensities and cognitive functions. And we added the covariates gradually to see that which factor influenced the correlation between WMH and cognitive functions. In the beginning, we added no covariates in Model 1. Clear evidence exists that aging leads to cognitive decline and in association with the prevalence and severity of WMH.29,30 So, we first added age as a covariate in Model 2. Furthermore, hypertension and diabetes are related to cognitive impairment, and are major risk factors for the development of WMH.31–33 Lacunar infarcts, which is also a primary MRI representation of cerebral small vessel disease, are found to be associated with WMH progression and cognitive deficits.34,35 Therefore, we added lacunar infarcts, hypertension, diabetes, age, and some other demographic data (gender and education) as covariates in the Model 3.
For the voxel-wise analysis of WMH versus no WMH group differences, we applied a permutation-based statistical interference tool (randomise) with a standard general linear model for Fazekas scores 1 to 6 in FSL with 5000 random permutations. The significance threshold was set at family-wise error corrected p < 0.05 after adjusted by age, sex, education, lacunar infarcts, hypertension and diabetes using the threshold-free cluster enhancement option. The skeleton areas showing significant differences were located and labelled using the digital WM atlas JHUICBMDTI-81 (http://cmrm.med.jhmi.edu/). And we did statistical analyses of group comparisons of atlas-based tracts, applying false discovery rate (FDR) for multiple comparison correction. The mean FA of the WM tracts with significant statistical group differences was extracted for further correlation analyses.
For each tract, linear regression analyses were performed to assess the relationship between the white matter microstructural alteration and cognitive functions in all participants. Then, we did further analysis to investigate whether there was tract-specific effect on cognition. We created modified global mask that contained all tracts except for the tract under investigation, and examined the effects of specific white matter tracts (resulted from correlation analysis) while controlling for the remaining tracts. Mean FA was extracted from the skeleton of each of these modified global masks. The statistical threshold was set as p < 0.05 (controlled for age, sex, education, lacunar infarcts, hypertension and diabetes).
Results
Demographic and clinical characteristics
Demographic and clinical characteristics of the study cohort (n = 321) are shown in Table 1. The mean age of the population was 66.4 years, and age was significantly related to the WMH severity (p < 0.001). There were no significant differences in gender or education among the seven groups. The prevalence of smoking did not differ significantly among all the groups in our sample. As expected, participants with WMH were more likely to be affected by hypertension and lacunar infarcts (p < 0.001), though the prevalence of diabetes exhibited no significance among groups in our study (p = 0.13). WMH volume was highly correlated with Fazekas score (p < 0.001, Supplementary Figure 1).
Distribution of WMH
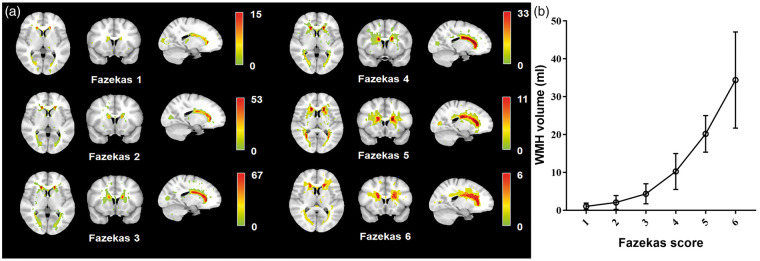
Figure 1 illustrates both the lesion quantity and frequency distributions from Fazekas scores 1 to 6. In our study, the volume and extent of WMH increased with the Fazekas score, with more regions being involved. The figure shows a specific pattern of lesions that spreads from the periventricular areas in mild groups (Fazekas 1 and 2) to frequent periventricular lesions with a larger spatial extent and higher probabilities of lesions in the basal ganglia and frontal lobes in moderate and severe groups (Fazekas 4 to 6).
Figure 1.
Distribution and volume of white matter hyperintensities, stratified by Fazekas score. (a) Prevalence of WMH stratified by WMH severity, colour-coded by the number of patients with a lesion for each voxel. WMH severity is determined by Fazekas score scale (1–6). (b) The graph depicts means and standard mean errors of WHM volume for each of the six Fazekas groups.
Association between the severity of WMH and cognition
We used correlation models to analyse the relationship between WMH and cognition, and we found that in the unadjusted model, the severity of WMH was significantly associated with all six cognitive domains (executive function, working memory, episodic memory, language, spatial processing and processing speed) and MoCA (Table 2, Model 1). After adjustments for age, episodic memory, working memory, spatial processing and processing speed remained significant (Model 2), which was expected, as WMH was responsible for age-related cognitive decline. Episodic memory (r = −0.127, p = 0.045) and working memory (r = −0.144, p = 0.022) remained decreasing with the progress of WMH after additional adjustment for sex, education, hypertension, diabetes and lacunar infarcts (Model 3).
Table 2.
Relationship between the white matter hyperintensities and cognitive functions.
|
Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| r1 | p value | r2 | p value | r4 | p value | |
| MMSE | −0.091 | 0.104 | −0.043 | 0.490 | −0.024 | 0.701 |
| MoCA | −0.140 | 0.016 | −0.101 | 0.141 | −0.028 | 0.663 |
| Episodic memory | −0.192 | 0.001 | −0.147 | 0.017 | −0.127 | 0.045 |
| Working memory | −0.183 | 0.001 | −0.140 | 0.024 | −0.144 | 0.022 |
| Spatial processing | −0.119 | 0.033 | −0.132 | 0.034 | −0.098 | 0.121 |
| Language | −0.161 | 0.004 | −0.114 | 0.067 | −0.035 | 0.581 |
| Processing speed | −0.168 | 0.003 | −0.151 | 0.015 | −0.059 | 0.35 |
| Executive function | 0.184 | 0.001 | 0.077 | 0.217 | −0.004 | 0.948 |
Note: Model 1: No covariate; Model 2: Age; Model 3: age, sex, education, lacunar infarcts, hypertension, diabetes.
MMSE: mini-mental state examination; MoCA: Montreal cognitive assessment.
Group comparisons of white matter microstructure
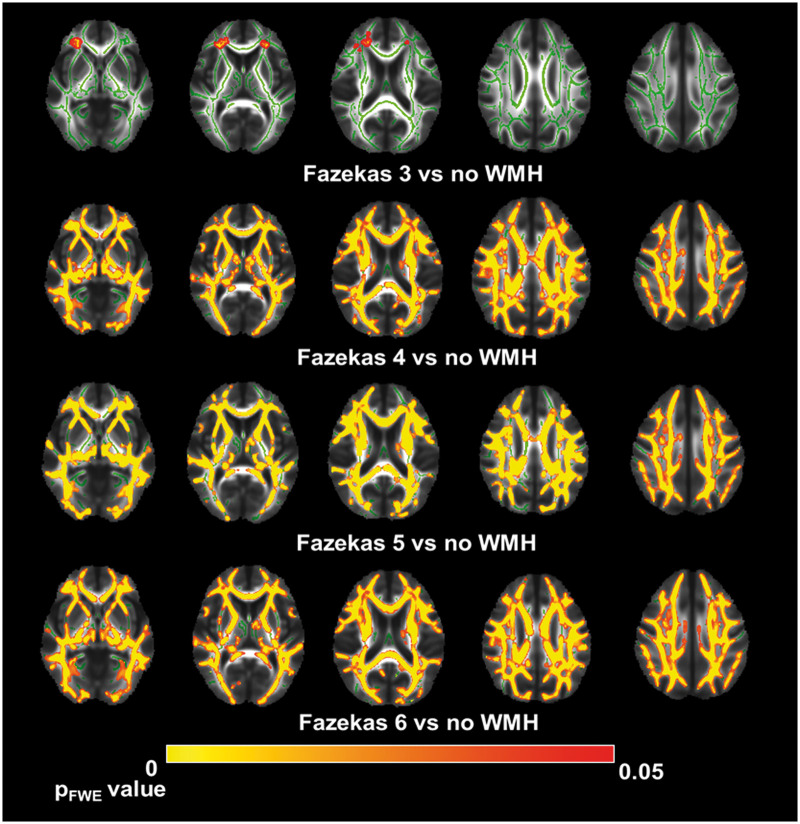
Higher Fazekas scores were highly correlated with more WM tracts showing lower FA in the entire brain. While mild WMH (Fazekas 1 and 2) had no significant differences in FA value, Fazekas group 3 showed white matter abnormalities in the anterior corona radiata and superior fronto-occipital fasciculus (p < 0.05 after FDR correction). In patients with high WMH volume (Fazekas 4 and 5), we found a widespread reduction of FA value across multi-WM fibres, including the corpus callosum, superior longitudinal fasciculus, external capsule, corona radiata and sagittal stratum. In the medial lemniscus, inferior cerebellar peduncle, posterior limb of internal capsule, superior cerebellar and left cingulate gyrus, we could only find significant differences between the control group and Fazekas 6 group (Figure 2, Supplementary Table 1 and Figure 2).
Figure 2.
Tract-based spatial statistics results between white matter hyperintensities and no WMH groups. A TBSS-derived t-map of decreased fractional anisotropy (FA) in the moderate to severe (Fazekas 3 to 6, while Fazekas 1 and 2 showed no significant changes) white matter hyperintensities (WMH) groups relative to the no WMH group is shown in red-yellow (p < 0.05; corrected for family-wise error).
Association between white matter microstructural changes and cognition
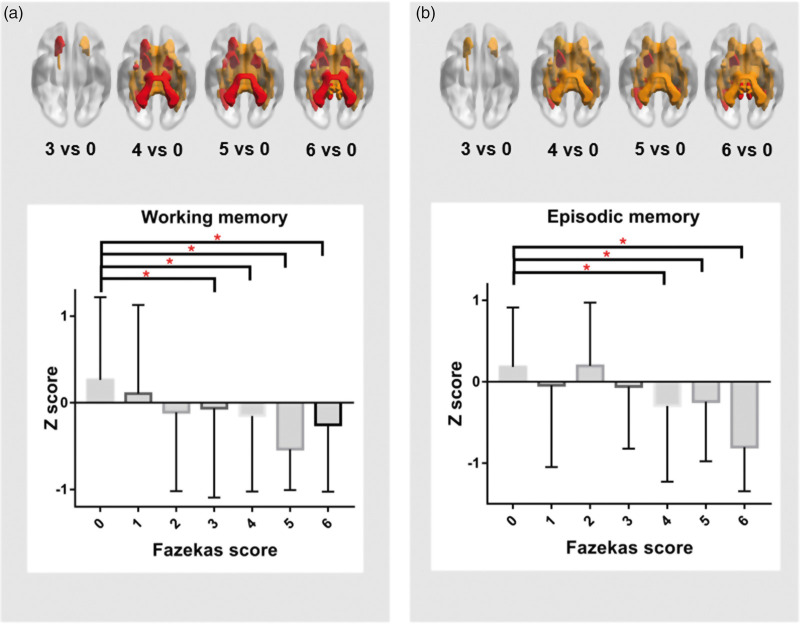
We then examined the correlation between the loss of white matter microstructural changes and cognitive domains, which we found was related to WMH in all participants. The microstructural changes of the right (r = 0.142, p = 0.014) and left (r = 0.141, p = 0.015) superior cerebellar peduncles were associated with episodic memory, as well as to the left anterior limbic of internal capsule (r = 0.119, p = 0.042), left posterior corona radiata (r = 0.131, p = 0.024), left posterior thalamic radiation (r = 0.142, p = 0.015) and right tapetum (r = 0.137, p = 0.019). Participants with lower working memory scores also showed impairments in several WM tracts compared with the no WMH group, including the middle cerebellar peduncle (r = 0.188, p = 0.001), left superior cerebellar peduncle (r = 0.133, p = 0.020), right cerebral peduncle (r = 0.146, p = 0.011), left cerebral peduncle (r = 0.058, p = 0.006), right anterior limbic of internal capsule (r = 0.115, p = 0.044), left anterior limbic of internal capsule (r = 0.143, p = 0.012), left anterior corona radiata (r = 0.131, p = 0.023), left posterior thalamic radiation (r = 0.120, p = 0.037), left external capsule (r = 0.126, p = 0.029) and left uncinate fasciculus (r = 0.141, p = 0.014) (Figure 3). After controlling for global FA, we could only find the tract-specific correlation between middle cerebellar peduncle and working memory (r = 0.178, p = 0.002).
Figure 3.
Relationship between white matter microstructural changes and memory function. The significant tracts differences are shown in the upper panel (including red and yellow). Among them, the red ones presented tracts which were found to be associated with working memory (a) or episodic memory (b). The bottom panel depicts means and standard mean errors of the working memory (a)/episodic memory (b) for each of the seven groups. P-values indicate the main effect of Fazekas score on the respective memory domain. *p < 0.05.
Discussion
In our community-based study of elderly adults free of dementia, we observed that participants with a higher Fazekas score presented worse performances in several cognitive domains, most notably in memory. The accumulation of white matter lesions occurred in periventricular areas, mainly in the bilateral frontal horn, which then extended to the frontal and parietal lobes, and the lesions ultimately appeared in the inferior parietal and superior frontal areas. Participants with severe WMH showed extensive losses of microstructural integrity, while participants with mild WMH exhibited no significant differences compared with the no WMH group. We found a region-specific association between white matter diffusion characteristics and cognitive functions, and we further suggest that these changes might be synchronized. We found that Fazekas score 3 was an important watershed point, and from this level, the participants began to show significant impairments in both cognitive functions and white matter microstructure. To our knowledge, this is one of the few reports investigating the contribution of various severities of WMH to microstructural integrity and cognitive decline.
A multitude of evidence, from both population- and patient-based studies, have suggested that small vessel diseases, including WMH, cause cognitive decline and that WMH is especially associated with reductions in processing speed, working memory, and executive function.36–40 Our results, in line with these studies, suggest a relationship between WMH and cognitive deficits in multiple domains, including working memory and episodic memory, which become apparent at progressed stages of white matter lesions (beginning at Fazekas score 3). Compared to the performance of individuals without WMH, Fazekas scores 1 and 2 barely exhibited any differences in any cognitive domains. Consistent with previous studies, our results indicate a late effect of WMH on cognitive functions, meaning that mild WMH might not cause cognitive decline. However, we were unable to detect the expected decline in executive function, which is frequently impaired in VCI.41 This may partly be due to the participants we observed being community elderly with relatively preserved cognition and the strong effect of age; additionally, the executive function tests we used may lack the sensitivity for detecting this subtle variability.
In contrast to prior studies, which presented age-related spatial distribution of white matter lesions, our study applied a clinically broadly used assessment tool to infer the severity of white matter lesions.7,42 Given that we observed a high level of individual consistency in all six stages, especially in periventricular areas, we assume that this cross-sectional data can represent the progression of the disease burden. Our results showed that in the cognitively normal elderly, the visual white matter abnormalities originated from the anterior horn of the periventricular WM, and in patients with moderate WMH (Fazekas 3 & 4), WMH with high frequencies expanded to the inferior parietal, occipital, as well as parts of the medial temporal areas; moderate WMH was followed by higher volumes and expansion in the parietal-temporal as well as superior frontal areas in the Fazekas 6 group. This result might reflect an underlying association between WMH and neurodegenerative processes, which could be accelerated by ageing. The spatial patterns of WMH could also be linked with our findings on cognitive functions, as patients in Fazekas 3 and 4 also exhibited deficits in working memory, which might be associated with their lesions expanding to frontal areas, as well as episodic memory, which might be the consequence of damage in parietal-temporal areas.
DTI is now widely used in investigating WM microstructural changes in vivo, as it utilizes measurement of the restriction of water movement.5,14 Restricted mobility of water molecules, which could be caused by myelin loss or axonal dysfunction, indicates reduction of white matter integrity. When examining the microstructural integrity (as measured by FA) among different stages of WMH and no WMH group, we consistently found a relationship between lower FA and higher-grade WMH, which implied the underlying pathology of demyelination and axonal loss. Recent longitudinal studies suggested that the process of white matter integrity loss could be detected years before visible WMH appeared on conventional MRI,14,43,44 which partially explained the lack of significant difference observed between mild WMH (Fazekas 1 and 2) groups and no WMH controls, as potential microstructural changes in normal appearing white matter (NAWM) might exist in these groups of similar ages. Of note, we observed impaired microstructural integrity in the anterior corona radiata and superior fronto-occipital fasciculus in participants with Fazekas score 3, which encompassed the highest average WHM volume in the population.45 Instead of the gradually expanding microstructural white matter damage expected with the progression of WMH, we observed a significantly widespread, decreased FA in Fazekas 4 group. And some areas of the white matter skeleton, such as middle temporal WM and Superior frontal WM, showed a decrease in FA value, but were normal appearing tissue in FLAIR image in Fazekas 4 group. These results further suggest that WMH might also affect brain areas beyond the visual abnormalities on MRI by inducing a series of pathological changes, which probably contribute to the clinical outcomes.46
With respect to the relationship between cognitive decline and white matter changes, our study demonstrated significant associations with episodic and working memory. The strongest relationships between microstructural integrity and episodic memory performance were located in the posterior corona radiata, thalamic radiation and cerebellar peduncles, thus disrupting the thalamocortical pathway, as well as the connections in the ventral posterior parietal cortex. Working memory depends upon the coordination of multiple brain regions, including the sensory cortices, areas of prefrontal and parietal cortices, as well as the medial temporal lobe.47 In our study, we consistently found associations with the posterior thalamus, uncinate fasciculi, inferior occipito-frontal fasciculi, and superior longitudinal fasciculi. Given that the general effect of sampling might lead to findings of specific white matter tracts on cognitive functions, we controlled for global white matter microstructure and found remarkable association between working memory and middle cerebellar peduncle, which provided support for the relationship between tract-specific WM changes and memory.
The results of this study implicate that region-specific WMH relates to declines in cognitive functions and that there might be a tract-specific link between white matter microstructural changes and cognitive impairment, which is independent of age and global white matter. We found that significant declines of cognitive function and tract-specific white matter changes synchronously arose at certain levels of WMH, which were in line with the “disconnection hypothesis” and suggested that potential treatments for preventing WMH from reaching this level might reduce the loss of cognitive function.
There were some limitations in our study. First, we adopted a cross-sectional design with limitations in causal inferences. Longitudinal data are needed for detecting this dynamic processing. Furthermore, we investigated the effects of different severities of WMH in a large sample size from community elderly with normal cognition, which limited the number of participants exhibiting scores of Fazekas 5 and Fazekas 6. However, the significant changes we had found already existed when compared to Fazekas 3 and Fazekas 4 groups. Another limitation is that we used the average FA value for each white matter tract that we examined, which might conceal the region-specific effects. Finally, participants with cognitive impairments were not included in this study, which may cause particular cognitive performances caused by WMH in mild cognitive impairment and dementia to be ignored.
Our findings may provide additional pathological support for both region- and tract-specific effects of WMH on various cognitive domains, which are independent of age and vascular risk factors. Episodic and working memory were predominantly impaired at moderate severities of white matter damage, which indicates potential treatment strategies to prevent the progression of WMH at these levels might delay brain ageing. Further studies are needed to investigate the role of lesion location and lesion load on longitudinal cognitive changes, especially when comorbid with other neuropathology.
Supplemental Material
Supplemental material, JCB893600 Supplemental Material for Severity of white matter hyperintensities: Lesion patterns, cognition, and microstructural changes by Weiyi Zeng, Yaojing Chen, Zhibao Zhu, Shudan Gao, Jianan Xia, Xiaochun Chen, Jianjun Jia and Zhanjun Zhang in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors thank the staff and senior citizen participants in the BABRI study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of the article: This research is supported by the National Key Research and Development Project of China (grant number 2018YFC1315200), National Science Fund for Distinguished Young Scholars (grant number 81625025), Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (grant number 81820108034), National Natural Science Foundation of China (grant number 31700997),and the Fundamental Research Funds for the Central Universities (grant number 2017XTCX04).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s contributions: Design and conceptualized study: ZZ, JJ; analyzed the data; drafted the manuscript for intellectual content: WZ, YC, ZBZ; data collection, literature research: all authors; verified the analytical methods: JX, YC; interpreted the data, revised the manuscript for intellectual content: ZZ, JJ, XC; and manuscript final version approval: all authors.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Weiyi Zeng https://orcid.org/0000-0003-2516-9710
Zhanjun Zhang https://orcid.org/0000-0001-7266-4218
References
- 1.Bos D, Wolters FJ, Darweesh SKL, et al. Cerebral small vessel disease and the risk of dementia: a systematic review and meta-analysis of population-based evidence. Alzheimers Dement 2018; 14: 1482–1492. [DOI] [PubMed] [Google Scholar]
- 2.Rensma S, Sloten TV, Launer L, Stehouwer C.Cerebral small vessel disease and risk of stroke, dementia, depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev 2018; 90: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilal S, Mok V, Youn YC, et al. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry 2017; 88: 669–674. [DOI] [PubMed] [Google Scholar]
- 4.Ylikoski A, Erkinjuntti T, Raininko R, et al. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995; 26: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 5.Prins ND, Scheltens P.White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015; 11: 157–165. [DOI] [PubMed] [Google Scholar]
- 6.Lampe L, Kharabian-Masouleh S, Kynast J, et al. Lesion location matters: the relationships between white matter hyperintensities on cognition in the healthy elderly. J Cereb Blood Flow Metab 2019; 39: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habes M, Sotiras A, Erus G, et al. White matter lesions: spatial heterogeneity, links to risk factors, cognition, genetics, and atrophy. Neurology 2018; 91: e964–e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hase Y, Horsburgh K, Ihara M, et al. White matter degeneration in vascular and other ageing-related dementias. J Neurochem 2018; 144: 617–633. [DOI] [PubMed] [Google Scholar]
- 9.Habes M, Erus G, Toledo JB, et al. Regional tract-specific white matter hyperintensities are associated with patterns to aging-related brain atrophy via vascular risk factors, but also independently. Alzheimers Dement Diagnos Assess Dis Monitor 2018; 10: 278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maillard P, Carmichael O, Harvey D, et al. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol 2013; 34: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juan José SR, Júlia M, Elena LC, et al. Tract-specific fractional anisotropy predicts cognitive outcome in a community sample of middle-aged participants with white matter lesions. J Cereb Blood Flow Metab 2014; 34: 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang Y, Sun X, Xu S, et al. Preclinical cerebral network connectivity evidence of deficits in mild white matter lesions. Front Aging Neurosci 2016; 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marius dG, Benjamin FJV, Renske dB, et al. Changes in normal-appearing white matter precede development of white matter lesions. Stroke 2013; 44: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 14.Leijsen EMCV, Bergkamp MI, Uden IWMV, et al. Progression of white matter hyperintensities preceded by heterogeneous decline of microstructural integrity. Stroke 2018; 49: 1386–1393. [DOI] [PubMed] [Google Scholar]
- 15.Gao FQ, Swartz RH, Scheltens P, et al. Complexity of MRI white matter hyperintensity assessments in relation to cognition in aging and dementia from the Sunnybrook Dementia Study. J Alzheimers Dis 2011; 26(Suppl 3): 379–388. [DOI] [PubMed] [Google Scholar]
- 16.Gouw AA, Van der Flier WM, van Straaten EC, et al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. J Neurol 2006; 253: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 17.Zhang MY, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol 2010; 27: 428–437. [DOI] [PubMed] [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bedirian V.The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SJ, Ryan JJ, Prifitera A.Rey auditory-verbal learning test performance of patients with and without memory impairment. J Clin Psychol 1984; 40: 785–787. [DOI] [PubMed] [Google Scholar]
- 20.Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique (Les problems) [The psychological examination in cases of traumatic encepholopathy (Problems)]. Archives de Psychologie 1941; 28: 215–285.
- 21.Rouleau I, Salmon DP, Butters N, et al. Quantitative and qualitative analyses of clock drawings in Alzheimer's and Huntington's disease. Brain Cogn 1992; 18: 70–87. [DOI] [PubMed] [Google Scholar]
- 22.Guo QH.Boston naming test in Chinese elderly, patient with mild cognitive impairment and Alzheimer's dementia. Chin Mental Health J 2006; 20: 81–84. [Google Scholar]
- 23.Reitan RM.Validity of the trail making test as an indicator of organic brain damage. Percept Motor Skills 1958; 8: 271–276. [Google Scholar]
- 24.Sheridan LK, Fitzgerald HE, Adams KM, et al. Normative symbol digit modalities test performance in a community-based sample. Arch Clin Neuropsychol 2006; 21: 23–28. [DOI] [PubMed] [Google Scholar]
- 25.Fazekas F, Niederkom K, Schmidt R, et al. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke 1988; 19: 1285–1288. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 2012; 59: 3774–3783. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. Neuroimage 2012; 62: 782–790. [DOI] [PubMed] [Google Scholar]
- 28.Smith SM, Mark J, Heidi JB, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 29.Prins ND, Scheltens P.White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015; 11: 157–165. [DOI] [PubMed] [Google Scholar]
- 30.Perry A, Wen W, Kochan NA, et al. The independent influences of age and education on functional brain networks and cognition in healthy older adults. Hum Brain Mapp 2017; 38: 5094–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uiterwijk R, Staals J, Huijts M, et al. MRI progression of cerebral small vessel disease and cognitive decline in patients with hypertension. J Hypertens 2017; 35: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 32.Tamura Y, Kimbara Y, Yamaoka T, et al. White matter hyperintensity in elderly patients with diabetes mellitus is associated with cognitive impairment, functional disability, and a high glycoalbumin/glycohemoglobin ratio. Front Aging Neurosci 2017; 9: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudre CH, Smith L, Atkinson D, et al. Cardiovascular risk factors and white matter hyperintensities: difference in susceptibility in South Asians compared with Europeans. J Am Heart Assoc 2018; 7: e010533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin P, Trippier S, Lawrence AJ, et al. Lacunar infarcts, but not perivascular spaces, are predictors of cognitive decline in cerebral small-vessel disease. Stroke 2018; 49: 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Gao Y, Liu R, et al. Progression of white matter hyperintensities contributes to lacunar infarction. Aging Dis 2018; 9: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Berg E, Geerlings MI, Biessels GJ, et al. White matter hyperintensities and cognition in mild cognitive impairment and Alzheimer's disease: a domain-specific meta-analysis. J Alzheimers Dis 2018; 63: 515–527. [DOI] [PubMed] [Google Scholar]
- 37.Dong C, Nabizadeh N, Caunca M, et al. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology 2015; 85: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X, Liang Y, Wang J, et al. Early frontal structural and functional changes in mild white matter lesions relevant to cognitive decline. J Alzheimers Dis 2014; 40: 123–134. [DOI] [PubMed] [Google Scholar]
- 39.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging 2015; 36: 2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee G, Jang H, Kim HJ, et al. Total MRI small vessel disease burden correlates with cognitive performance, cortical atrophy, and network measures in a memory clinic population. J Alzheimers Dis. 2018; 63: 1485–1497. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien JT, Thomas A.Vascular dementia. Lancet 2015; 386: 1698–1706. [DOI] [PubMed] [Google Scholar]
- 42.Lindemer ER, Greve DN, Fischl BR, Augustinack JC, Salat DH.Regional staging of white matter signal abnormalities in aging and Alzheimer's disease. Neuroimage Clin 2017; 14: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Groot M, Verhaaren BF, de Boer R, et al. Changes in normal-appearing white matter precede development of white matter lesions. Stroke 2013; 44: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 44.Griffanti L, Jenkinson M, Suri S, et al. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: a study in older adults. Neuroimage 2018; 170: 174–181. [DOI] [PubMed] [Google Scholar]
- 45.Habes M, Erus G, Toledo JB, et al. Regional tract-specific white matter hyperintensities are associated with patterns to aging-related brain atrophy via vascular risk factors, but also independently. Alzheimers Dement 2018; 10: 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ter Telgte A, van Leijsen EMC, Wiegertjes K, et al. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol 2018; 14: 387–398. [DOI] [PubMed] [Google Scholar]
- 47.Eriksson J, Vogel EK, Lansner A, et al. Neurocognitive architecture of working memory. Neuron 2015; 88: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JCB893600 Supplemental Material for Severity of white matter hyperintensities: Lesion patterns, cognition, and microstructural changes by Weiyi Zeng, Yaojing Chen, Zhibao Zhu, Shudan Gao, Jianan Xia, Xiaochun Chen, Jianjun Jia and Zhanjun Zhang in Journal of Cerebral Blood Flow & Metabolism