Abstract
BACKGROUND:
Chronic exposure to socioeconomic or environmental stressors associates with greater stress-related neurobiological activity (ie, higher amygdalar activity [AmygA]) and higher risk of major adverse cardiovascular events (MACE). However, among individuals exposed to such stressors, it is unknown whether neurobiological resilience (NBResilience, defined as lower AmygA despite stress exposure) lowers MACE risk. We tested the hypotheses that NBResilience protects against MACE, and that it does so through decreased bone marrow activity and arterial inflammation.
METHODS:
Individuals underwent 18F-fluorodeoxyglucose positron emission tomography/computed tomography; AmygA, bone marrow activity, and arterial inflammation were quantified. Chronic socioeconomic and environmental stressors known to associate with AmygA and MACE (ie, transportation noise exposure, neighborhood median household income, and crime rate) were quantified. Heightened stress exposure was defined as exposure to at least one chronic stressor (ie, the highest tertile of noise exposure or crime or lowest tertile of income). MACE within 5 years of imaging was adjudicated. Relationships were evaluated using linear and Cox regression, Kaplan-Meier survival, and mediation analyses.
RESULTS:
Of 254 individuals studied (median age [interquartile range]: 57 years [46–67], 36.7% male), 166 were exposed to at least one chronic stressor. Among stress-exposed individuals, 12 experienced MACE over a median follow-up of 3.75 years. Among this group, higher AmygA (ie, lower resilience) associated with higher bone marrow activity (standardized β [95% CI]: 0.192 [0.030–0.353], P=0.020), arterial inflammation (0.203 [0.055–0.351], P=0.007), and MACE risk (standardized hazard ratio [95% CI]: 1.927 [1.370–2.711], P=0.001). The effect of NBResilience on MACE risk was significantly mediated by lower arterial inflammation (P<0.05).
CONCLUSIONS:
Among individuals who are chronically exposed to socioeconomic or environmental stressors, NBResilience (AmygA <1 SD above the mean) associates with a >50% reduction in MACE risk, potentially via reduced arterial inflammation. These data raise the possibility that enhancing NBResilience may decrease the burden of cardiovascular disease.
Keywords: bone marrow, cardiovascular diseases, crime, income, positron emission tomography
Chronic stress is associated with a higher risk of cardiovascular disease (CVD)1 with an attributable risk similar to that of traditional CVD risk factors. While one’s perception of personal stress is highly subjective, tomographic brain imaging allows objective quantitation of stress-associated neural activity.2 Individuals who experience high levels of chronic stress exposure but show lower levels of stress-related reactivity at a neural level may perceive stressful experiences as less threatening and, therefore, be less vulnerable to stress-related outcomes over time. This neurobiologically indexed capacity for resilience (NBResilience) may have downstream consequences for stress-related physical diseases such as CVD, although this association and its mediating mechanisms remains unexplored.
Animal studies demonstrate that chronic stress potentiates atherosclerosis by promoting leukopoiesis and atherosclerotic inflammation.3 Human multimodality imaging studies have further elucidated the role of stress-associated neural centers in eliciting CVD.2 Such studies have leveraged 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) imaging, which can simultaneously provide validated measures of metabolic activity in the bone marrow (a measure of leukopoiesis) and the arterial wall (a measure of atherosclerotic inflammation [ArtI]).2 Notably, 18F-FDG-PET/CT imaging additionally enables quantification of resting metabolic activity of the amygdala, a key component of the brain’s salience network.2 This measure of neurobiological activity associates with anxious temperament4 and with clinical manifestations of stress-related disorders.5 Moreover, using this imaging approach, the link between stress and CVD has been shown to involve a serial pathway of: ↑resting amygdalar activity→↑leukopoiesis→↑ArtI→↑CVD events.2,6 Accordingly, 18F-FDG-PET/CT imaging enables quantification of neurobiological activity as well as its downstream immune and atherosclerotic consequences, providing a measure that predicts subsequent CVD events.
Studying the impact of resilience on the risk of stress-associated physical diseases is facilitated by assessment of common, pathophysiologically consequential, and reproducibly quantifiable stressors. Socioeconomic stress and environmental noise represent such stressors. Both lower socioeconomic status (SES) and chronic environmental noise exposure increase psychological stress and associate with a heightened risk of CVD.7-12 Indeed, recent studies have shown that lower SES and chronic noise exposure link to CVD via the aforementioned neuro-inflammatory-arterial path.13,14
Accordingly, we sought to study the impact of resilience on the risk of CVD among individuals who are chronically exposed to socioeconomic and environmental stressors. To do so, socioeconomic and environmental stressors were quantified in individuals who underwent 18F-FDG-PET/CT imaging, after which the development of incident major adverse cardiovascular events (MACEs) was adjudicated. Greater NBResilience was defined as lower stress-related amygdalar metabolic activity despite the presence of chronic socioeconomic and or environmental stress. Subsequently, we tested the hypotheses that among individuals living under conditions of chronic stress: (1) NBResilience associates with a lower MACE risk and (2) the mechanism mediating that effect involves attenuation of neuro-inflammatory-arterial activity.
METHODS
Overview
The study employed a retrospective, longitudinal, observational design. The protocol was approved by the Partners Human Research Committee. Informed consent was not required for this retrospective study. The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Study Cohort Selection
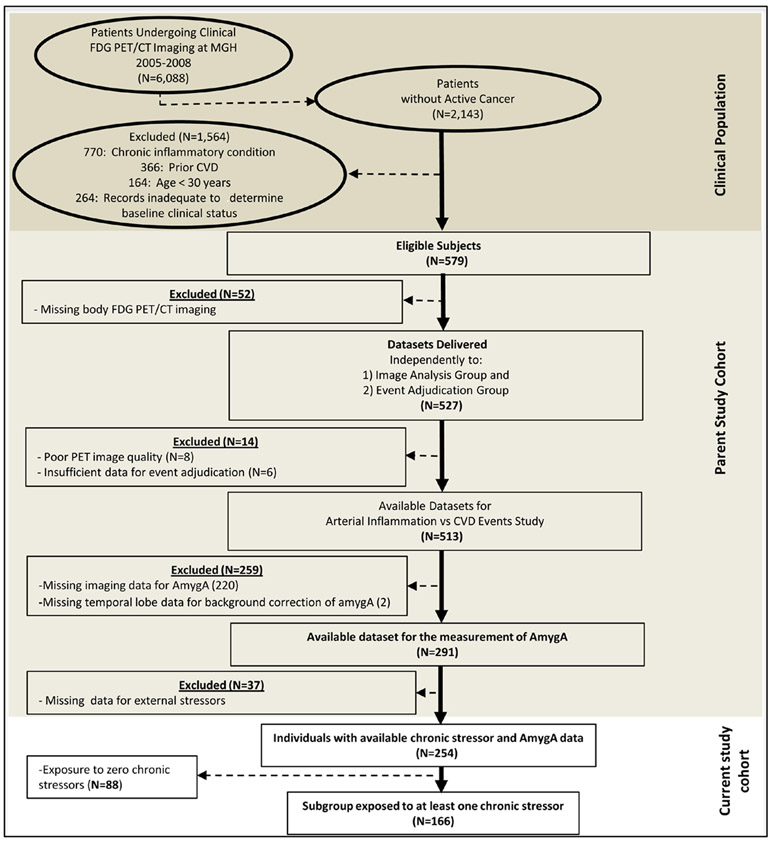
The study cohort has been described previously.13,14 In brief, patients who underwent clinical 18F-FDG-PET/CT imaging at the Massachusetts General Hospital (Boston, MA) from 2005 to 2008 were identified (Figure 1). Predefined exclusion criteria were (1) clinically diagnosed CVD, (2) clinical diagnosis of an inflammatory or autoimmune disease, (3) active diagnosis of cancer, imaging within 1 year of remission from malignancy, or cancer during the follow-up interval, and (4) age ≤30 years. Patient records were retrieved using hospital electronic medical records, and individual charts were reviewed to obtain individual home addresses as well as other variables of interest. Included subjects were also required to have (1) at least 3 follow-up visit notes over a period of ≥1 year after imaging to ensure adequate follow-up, (2) data available for socioeconomic and environmental stressors at their addresses, and (3) 18F-FDG-PET/CT brain images allowing the measurement of amygdalar activity (AmygA).
Figure 1. Study subject selection.
AmygA indicates amygdalar activity; CVD, cardiovascular disease; FDG PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; and MGH, Massachusetts General Hospital.
18F-FDG-PET/CT Imaging Protocol
Whole-body 18F-FDG-PET/CT imaging was performed using an integrated PET/CT scanner (eg, Biograph 64 Siemens Healthcare, Erlangen, Germany) with a protocol that includes an overnight fast followed by IV administration of 18F-FDG (≈370 MBq). Approximately 1-hour post-injection, low-dose, nongated, noncontrast CT (120 keV, ≈50 mAs) images were acquired followed by PET imaging.
Measurement of Arterial and Leukopoietic Tissue Activity
18F-FDG-PET/CT images were analyzed to yield to validated measures of arterial and leukopoietic activity as described previously.15-17 Additional details are provided in the Data Supplement. An investigator blinded to all clinical and stressor data conducted the analyses.
Measurement of Regional Brain Activity
A separate blinded radiologist analyzed 18F-FDG-PET/CT brain images using previously validated methods.2 Briefly, using CT images to identify brain regions, regions of interest were placed over the right and left amygdalae to derive the standardized uptake values of 18F-FDG accumulation. The maximum standardized uptake value of the right and left amygdalae were averaged and divided by the background cerebral activity (mean temporal lobe standardized uptake value)2,18 to yield background-adjusted resting amygdalar activitymeanofmax (AmygAmnmx). Additionally, the mean of the standardized uptake value of the right and left amygdalae were obtained and corrected for background cerebral activity to yield amygdalar activitymeanofmean (AmygAmnmn). Herein, AmygA refers to AmygAmnmx unless otherwise specified.
Assessment of Socioeconomic and Environmental Stressors
Socioeconomic measures (ie, neighborhood median income and total town crime rates) were derived from the US Census Bureau’s 2015 American Community Survey 5-Year Estimates (https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml) using each subject’s residential zip code and from the Massachusetts Uniform Crime Reporting database by the Federal Bureau of Investigation (https://ucr.fbi.gov/crime-in-the-u.s/2010/crime-in-the-u.s.-2010/tables/table-8/10tbl08ma.xls) using each subject’s town of residence. Such zip code and town-level SES measures, while less precise proxies for individual SES, are important determinants of SES-associated health outcomes and associate with AmygA.14,19
Environmental noise levels at subjects’ household addresses were estimated as described previously.13 The estimated average 24-hour equivalent ambient noise level for each individual home address was obtained from the US Department of Transportation US Road and Aviation Noise Map (https://maps.bts.dot.gov/arcgis).
Study End Points
The primary end point, major adverse cardiovascular events (MACEs), was defined as: CVD death, myocardial infarction, unstable angina, cerebrovascular accident, coronary artery revascularization, heart failure, or peripheral artery disease revascularization. Time-to-event was recorded as time between imaging and the index MACE event. Adjudication of MACE was performed by 2 cardiologists who were blinded to imaging and stressor data.
Statistical Analyses
Statistical analyses were performed using SPSS (Versions 25 and 26, IBM Corporation, Armonk, NY). Continuous variables are reported as mean (±SD) for normally distributed variables or as median with interquartile range (IQR, 25th–75th percentile) when skewed. Categorical variables were compared between groups using χ2 or Fisher exact tests. Continuous variables were compared using independent samples t-tests or Mann-Whitney U tests, depending on data distribution. Additionally, continuous variables were standardized. Associations between covariables and AmygA were assessed. Higher chronic stress exposure was defined as living in (1) neighborhoods with lower local median income (lowest tertile; median annual income <$66 370.00), (2) neighborhoods with higher crime rate (highest tertile, total crime rate >1327/y), or (3) a home with higher noise exposure (upper tertile or >45 dBA). Univariable and multivariable linear regression models were used to test for associations with continuous variables, as β and 95% CIs. Cox proportional hazards models (with and without multivariable adjustment) were implemented to derive hazard ratios. Kaplan-Meier event-free survival was used to test relationships between AmygA and MACE. Using mean +1 SD as a cutoff value, AmygA was dichotomized into high (AmygA ≥mean +1 SD) versus lower AmygA. In a supplemental analysis, the Youden index was used to establish an alternative cutoff for AmygA. Cases were censored on the date of last clinical follow-up or the first MACE-qualifying event within 5 years of imaging. Important covariables were selected based on clinical factors, those that associated with AmygA in univariable models, and those that differed between individuals with versus without MACE. The assumptions for all Cox models were verified using Schoenfeld residuals. Backward selection was implemented where appropriate.
Mediation analysis was performed using the SPSS PROCESS macro (IBM Corporation, Armonk, NY). Among the subgroup of individuals chronically exposed to at least one socioeconomic or environmental stressor, we estimated the effect of a hypothesized single mediator path: ↑AmygA →↑ArtI →↑MACE. Exact P-values are not available for path analyses with dichotomous dependent variables; CIs that do not cross zero indicate P<0.05.
RESULTS
Baseline Characteristics
Two hundred fifty-four individuals provided data for assessment of (1) chronic socioeconomic and environmental stressors, (2) AmygA, and (3) MACE. Baseline characteristics are summarized in Table 1 and Tables I and II in the Data Supplement. Of these, 166 individuals were chronically exposed to at least one socioeconomic or environmental stressor (ie, residing in low income or high crime neighborhoods or exposure to noise >45 dBA).
Table 1.
Baseline Characteristics of Individuals With “Low” AmygA vs “Higher” AmygA
| Variable | Entire Cohort (n=254) | Chronic Stress-Exposed (N=166) | P-Value | |||
|---|---|---|---|---|---|---|
| Higher AmygA (n=28) |
Lower AmygA (n=226) |
P-Value | Lower NBResilience (Higher AmygA; n=23) |
Higher NBResilience (Lower AmygA; n=143) |
||
| Demographics | ||||||
| Median age, y (IQR) | 60 (44–70) | 56 (46–65) | 0.147 | 63 (44–76) | 57 (46–66) | 0.074 |
| Male sex | 9 (32.1%) | 95 (42%) | 0.315 | 8 (34.8%) | 53 (37.1%) | 0.833 |
| White race | 23 (82.1 %) | 203 (89.8%) | 0.210 | 19 (82.6%) | 125 (87.4%) | 0.513 |
| Cardiovascular Factors | ||||||
| Current smoker | 3 (10.7%) | 19 (8.4%) | 0.719 | 2 (8.7%) | 15 (10.5%) | 1.00 |
| Hypertension | 11 (39.3%) | 74 (32.7%) | 0.489 | 9 (39.1%) | 50 (35.0%) | 0.698 |
| Diabetes mellitus | 4 (14.3%) | 19 (8.4%) | 0.297 | 4 (17.4%) | 14 (9.8%) | 0.282 |
| Hyperlipidemia | 7 (25%) | 68 (30.1%) | 0.578 | 6 (26.1%) | 44 (30.8%) | 0.650 |
| Mean total cholesterol (SD), mg/dL | 188.89 (51.83) | 187.31 (46.73) | 0.687 | 193.94 (54.12) | 188.00 (43.79) | 0.986 |
| Mean LDL cholesterol (SD), mg/dL | 106.53 (43.08) | 107.20 (38.68) | 0.695 | 110.25 (45.85) | 105.32 (34.76) | 0.923 |
| Median Framingham risk score (IQR) | 5 (2–12) | 3 (1–7) | 0.198 | 5 (2–15) | 3 (1–7) | 0.105 |
| Median body mass index (IQR), kg/m2 | 26.44 (23.29–31.74) | 26.44 (23.08–31.12) | 0.822 | 25.89 (22.9–31.94) | 27.00 (23.66–32.48) | 0.479 |
| Family history of coronary disease | 11 (39.3%) | 55 (24.3%) | 0.126 | 10 (43.5%) | 34 (23.7%) | 0.110 |
| Medications | ||||||
| Statin therapy | 7 (25%) | 46 (20.4%) | 0.568 | 6 (26.1%) | 33 (23.1%) | 0.752 |
| Antihypertensive therapy | 11 (39.3%) | 75 (33.2%) | 0.520 | 10 (39.1) | 50 (35.0) | 0.430 |
| β blockers | 5 (19.2%) | 25 (11.1%) | 0.214 | 3 (13%) | 16 (11.3%) | 0.724 |
| Selective serotonin reuptake inhibitors | 1 (3.6%) | 17 (7.5%) | 0.703 | 1 (4.3%) | 11 (7.8%) | 1.00 |
| Other antidepressant drug | 1 (3.6%) | 10 (4.4%) | 1.00 | 1 (4.3%) | 7 (4.9%) | 1.00 |
| Malignancy History | ||||||
| History of cancer | 24 (85.7%) | 198 (87.6%) | 0.764 | 19 (82.6%) | 124 (86.7%) | 0.530 |
| Previous chemotherapy or radiation | 22 (78.6%) | 180 (79.6%) | 0.894 | 17 (73.9 %) | 111 (77.6%) | 0.694 |
| Psychiatric History | ||||||
| Depression or anxiety | 4 (14.3%) | 23 (10.2%) | 0.499 | 3 (13%) | 12 (8.5%) | 0.430 |
| Socioeconomic and Environmental Stressors | ||||||
| Median average noise exposure (dBA) (IQR) | 45–50 (37.5–52.5) | 35–40 (<35–47.5) | 0.029 | 45–50 (42.5–57.5) | 45–50 (<35–52.5) | 0.255 |
| Median income (IQR), $ | 77347.50 (50 874.50–104 942.50) | 77 371.00 (61 525.50–96 480.50) | 0.442 | 61 919 (50 865–86 240) | 66 354.50 (51 464.25–81 577.25) | 0.891 |
| Median total annual crimes (IQR) | 1617 (369–3199) | 679 (274–1907) | 0.080 | 1990 (1203–3708) | 1327 (414–3708) | 0.182 |
| Other SES factors | ||||||
| Health insurance | 24 (85.7%) | 204 (90.3%) | 0.504 | 20 (87.0%) | 126 (88.1%) | 1.00 |
| In-state residence | 25 (89.3%) | 207 (91.6%) | 0.719 | 20 (87.0%) | 124 (86.7%) | 1.00 |
Categorical variables were compared using either χ2 or Fischer exact tests (depending on data distribution). AmygA indicates amygdalar activity; IQR, interquartile range; LDL, low-density lipoprotein; NBResilience, neurobiological resilience; and SES, socioeconomic status.
Amygdalar Activity And Chronic External Stressors
Across the entire cohort, age, race, marital status, and the number of stressors significantly associated with AmygA (Table III and Figure I in the Data Supplement). Notably, AmygA increased in proportion to the number of measured stressors (P=0.028 for linear trend, Figure 2).
Figure 2. AmygAmnmx vs number of stressors.
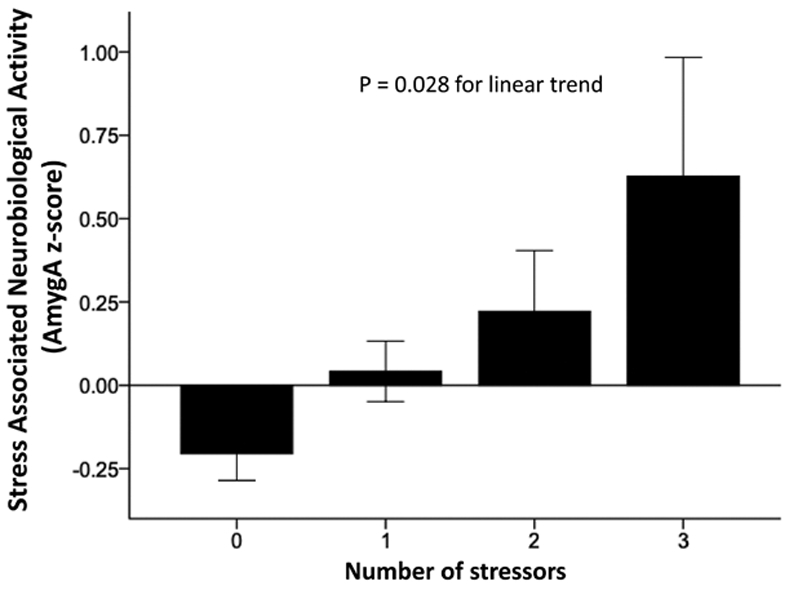
Amygdalar activity (AmygA) increased in proportion to the number of stressors (P=0.028; error bars represent ±1 SE). AmygAmnmx indicates resting amygdalar activitymeanofmax.
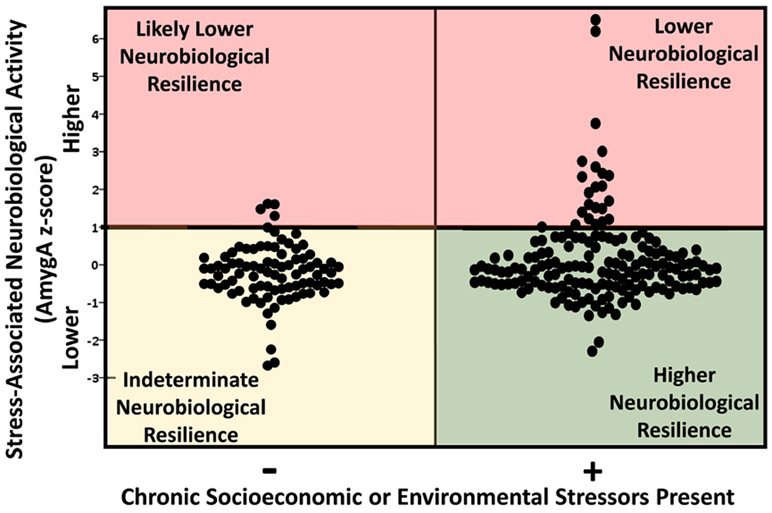
Neurobiological resilience (NBResilience) was defined as having lower AmygA despite exposure to at least one chronic stressor (Figure 3). In analyses that dichotomized AmygA (at ≥1 SD above the mean), lower AmygA was observed in 86% (143 of 166) of individuals exposed to at least one chronic socioeconomic or environmental stressor, versus 94% (83 of 88) of individuals who were not exposed to any of the measured stressors (P=0.047). Notably, individuals with lower AmygA who were not chronically exposed to at least stressor were deemed to have indeterminate resilience, as the presence of substantial stressors was not confirmed.
Figure 3. Neurobiological resilience.
Amygdalar activity (AmygA) varied substantially among stress-exposed individuals. Those with AmygA <1 SD above the mean in the context of at least one stressor were identified as more resilient. Those with lower AmygA without confirmed chronic stressors were defined as having indeterminate resilience.
Amygdalar Activity Predicts MACE Among Those Exposed to Chronic Stressors
Among individuals exposed to at least one chronic socioeconomic or environmental stressor (n=166), 12 (7.2%) developed MACE over a median follow-up of 3.75 (IQR: 2.80–4.60) years. Within this stress-exposed group, CVD risk factors were not significantly different between individuals with higher versus lower AmygA (ie, those without versus with NBResilience, respectively, Table 1). However, among this group, AmygA (as a continuous variable) associated with MACE risk in univariable (standardized HR [95% CI]: 1.624 [1.205, 2.188], P=0.001) and multivariable models (1.927 [1.370, 2.711], P<0.001; adjusted for age, sex, and CVD risk factors). Further, AmygA associated with MACE in both males (1.486 [1.019–2.168], P=0.039) and females (2.263 [1.109–4.619], P=0.025) and remained associated with MACE regardless of the number or type of chronic external stressors (Table 2).
Table 2.
Cox Proportional Hazard Ratios for MACE Risk Among Stress-Exposed Individuals Using AmygA as a Predictor (Standardized Continuous Variable)
| Stress-Exposed Subgroups |
Models | Hazard Ratio (95% CI) |
P-Value |
|---|---|---|---|
| Individuals exposed to ≥1 stressor* (n=166) | Model 1 | 1.624 (1.205–2.188) | 0.001 |
| Model 2 | 1.438 (1.026–2.016) | 0.035 | |
| Model 3 | 1.927 (1.370–2.711) | <0.001 | |
| Model 4 | 1.900 (1.347–2.680) | <0.001 | |
| Model 5 | 1.600 (1.173–2.182) | 0.003 | |
| Individuals exposed to ≥2 stressors* (n=66) | Model 1 | 1.604 (1.163–2.211) | 0.004 |
| Model 2 | 1.432 (0.830–2.470) | 0.197 | |
| Model 3 | 2.219 (1.346–3.658) | 0.002 | |
| Model 4 | 2.200 (1.335–3.623) | 0.002 | |
| Model 5 | 1.595 (1.136–2.240) | 0.007 | |
| Individuals exposed to high noise† (n=89) | Model 1 | 1.499 (1.073–2.094) | 0.018 |
| Model 2 | 1.467 (0.975–2.205) | 0.066 | |
| Model 3 | 1.962 (1.302–2.957) | 0.001 | |
| Model 4 | 2.099 (1.382–3.188) | 0.001 | |
| Model 5 | 1.477 (1.042–2.092) | 0.028 | |
| Individuals exposed to low income‡ (n=94) | Model 1 | 1.747 (1.289–2.369) | <0.001 |
| Model 2 | 1.468 (1.029–2.095) | 0.034 | |
| Model 3 | 2.242 (1.429–3.519) | <0.001 | |
| Model 4 | 4.075 (1.630–10.188) | 0.003 | |
| Model 5 | 1.747 (1.289–2.369) | <0.001 | |
| Individuals exposed to high crime§ (n=72) | Model 1 | 1.842 (1.272–2.668) | 0.001 |
| Model 2 | 1.827 (1.183–2.820) | 0.007 | |
| Model 3 | 2.023 (1.311–3.122) | 0.001 | |
| Model 4 | 1.623 (1.125–2.341) | 0.010 | |
| Model 5 | 1.770 (1.207–2.597) | 0.003 |
Number of subjects analyzed in each stressor subgroup indicated in parenthesis. Model 1—univariable; Model 2—age and sex; Model 3—CVD risk factors∥; Model 4-Factors differing between individuals with vs without MACE (age, current smoking, hypertension, diabetes mellitus, Framingham risk score, statin therapy, history of cancer, noise exposure); Model 5—Univariable predictors of AmygA (Race, marital status, and number of stressors). AmygA indicates amygdalar activity; CVD, cardiovascular diseases; and MACE, major adverse cardiovascular disease event.
Living in areas with environmental noise exposure >45 dbA, highest tertile crime rate, and/or lowest tertile household income
Living in areas with environmental noise exposure >45 dbA.
Living in areas with household income in the lowest tertile.
Living in areas with the highest tertile of crime rate.
CVD risk factors (age, sex, current smoking, diabetes mellitus, hypertension, hyperlipidemia).
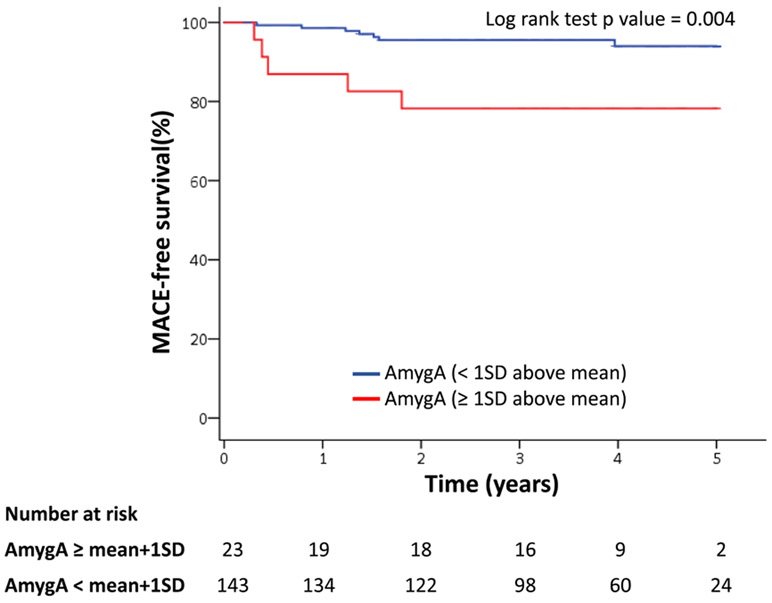
Furthermore, when AmygA was dichotomized (< versus >1 SD above the mean) among stress-exposed individuals, higher AmygA associated with increased MACE risk (HR [95% CI]:7.359 [2.163–25.038], P=0.001). In other words, lower AmygA (ie, higher NBResilience) associated with an 86% reduction in MACE risk (log-rank P=0.004, Figure 4). Differences in event-free survival between NBResilience and non-NBResilience individuals persisted regardless of the culprit stressor to which individuals were exposed (Figure IIA through IIC in the Data Supplement). Similarly, high AmygA associated with increased MACE risk when AmygA was dichotomized by the Youden index value (9.056 [2.872–28.558], P<0.001; log-rank P<0.001 Figure IID in the Data Supplement).
Figure 4. Kaplan-Meier major adverse cardiovascular event (MACE)-free survival.
Kaplan-Meier MACE-free survival for amygdalar activity (AmygA) (≥1 SD above mean vs <1 SD above mean) in patients exposed to at least one stressor hazard ratio (95% CI): 7.359 (2.163–25.038); P=0.001*.
*adjusted for cardiovascular disease risk factors.
Amygdalar Activity Versus Leukopoietic Tissue Activity and ArtI Among Stress-Exposed Individuals
Among stress-exposed individuals (n=166), lower AmygA associated with lower bone marrow (ie, leukopoietic) activity (standardized β [95% CI]: 0.192 [0.030–0.353], P=0.020) and lower ArtI (0.203 [0.055-0.351], P=0.007, Table 3). These associations remained significant regardless of the number or type of stressors (Table 3). Moreover, mediation analysis (within the stress-exposed subgroup) demonstrated that the link between higher AmygA (ie, lower NBResilience) and greater MACE risk was mediated in part by increased ArtI (standardized log odds ratio [95% CI]: 1.19 [0.11-3.32], P<0.05).
Table 3.
Associations Between AmygA and Imaging Measures of Inflammatory Activity Among Stress-Exposed Individuals
| Population Analyzed | Target Tissue Activity | |||
|---|---|---|---|---|
| Leukopoietic Activity | Arterial Inflammation | |||
| Standardized β (95% CI) | P-Value | Standardized β (95% CI) | P-Value | |
| Individuals exposed to ≥1 stressor* (n=166) | 0.192 (0.030 to 0.353) | 0.020 | 0.203 (0.055 to 0.351) | 0.007 |
| Individuals exposed to ≥2 stressors* (n=66) | 0.284 (0.062 to 0.506) | 0.013 | 0.240 (0.039 to 0.441) | 0.020 |
| Individuals exposed to high noise† (n=89) | 0.155 (−0.036 to 0.346) | 0.110 | 0.176 (0.001 to 0.352) | 0.049 |
| Individuals exposed to low income‡ (n=94) | 0.327 (0.124 to 0.530) | 0.002 | 0.290 (0.105 to 0.475) | 0.002 |
| Individuals exposed to high crime§ (n=72) | 0.322 (0.069 to 0.575) | 0.014 | 0.237 (0.011 to 0.463) | 0.040 |
Models are adjusted for age and sex. Number of subjects analyzed in each stressor subgroup indicated in parenthesis.
Living in areas with environmental noise exposure > 45 dbA, highest tertile crime rate, and/or lowest tertile household income
Living in areas with environmental noise exposure >45 dbA.
Living in areas with household income in the lowest tertile.
Living in areas with the highest tertile of crime rate.
DISCUSSION
The study yielded several novel observations. First, we observed that AmygA generally increases in proportion to the number of socioeconomic and environmental stressors (although a broad range of AmygA exists within each stratum of stress exposure). Second, we provide a novel conceptualization of resilience, whereby individuals manifesting lower AmygA for a given level of stress may be interpreted as being more neurobiologically resilient (NBResilience). Third, individuals with higher NBResilience had significantly lower MACE risk (>50% relative risk reduction). Fourth, the mechanism by which NBResilience leads to fewer MACEs likely involves decreased ArtI and may involve decreased leukopoietic activity. Accordingly, the study’s findings provide a framework for conceptualizing resilience through a neuro-immune-arterial path that substantially impacts cardiovascular health.
Psychosocial stress has long been known to be associated with increased risk of several psychiatric and physical conditions (including cancer and CVD).20-21 The perception of stress is highly variable; resilience is commonly conceptualized to some extent to reflect a balance of an external stressor and an organism’s interpretation of the threat of that stressor. A heightened perception of stress may result in excessive metabolic wear and tear, referred to as allostatic overloading, which carries with it, vulnerability to stress-related illnesses.22
Several studies suggest that resilience, traditionally defined by psychological assessment, may associate with lower CVD risk.23-25 Similarly, a recent meta-analysis demonstrated that optimism was associated with decreased cardiovascular risk.26 Prior studies relied upon subjective questionnaire-based scales to assess resilience or optimism and, therefore, lend themselves to an inherent bias. Moreover, the mechanisms that may explain the cardiovascular benefits associated with these behavioral traits have not previously been delineated. In the current study, we identified normative stress-associated AmygA in the context of stress exposure as a potentially important marker of NBResilience and provide insights into the mechanisms that may underlie the benefit of resilience on CVD.
Psychological resilience has been conceptualized as the ability to resist the development of stress-associated neuropsychiatric disorders27 and is often connected with the capacity to modulate emotion. Physiological resilience, on the other hand, is characterized by the capacity to buffer the downstream physiological consequences of stress (eg, sympathetic nervous system activity, hypothalamic-pituitary-adrenal axis activity, inflammatory response) that precipitate stress-associated diseases.28-31 There is increasing evidence, mainly from animal models, that resilience involves active behavioral, molecular, and neurobiological adaptations.32 18F-FDG-PET/CT and functional magnetic resonance imaging provide a means to investigate the intricate neurobiological mechanisms underlying resilience. Chronic stress has been associated with morphological changes, including increasing and persistent dendritic branching and spine formation in the amygdala, reversible dendritic atrophy and reduced neurogenesis in the hippocampus, and reversible dendritic atrophy the in medial prefrontal cortex,33-34 as well as increased activity in brain regions, including the amygdala, on functional magnetic resonance and 18F-FDG-PET/CT imaging.35 Stress has also been linked to accelerated atherosclerosis via increased leukopoietic activity and inflammatory cytokine production36; amygdalar activity seems to play a key role in linking external stressors to systemic inflammation via its modulation of the sympathetic nervous system and hypothalamic-pituitary-adrenal axis and the resultant perturbations in leukopoiesis.2,37
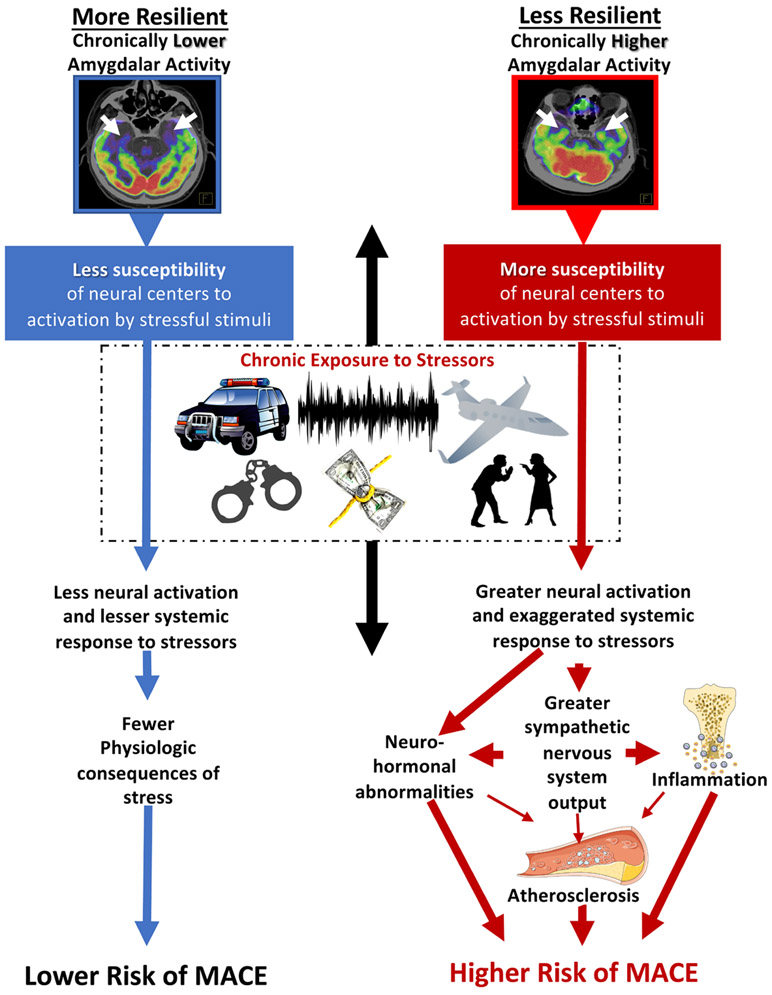
In humans, AmygA itself has been linked to MACE through a serial pathway involving increased bone marrow activity and ArtI. Accordingly, we implemented 18F-FDG-PET/CT imaging to objectively quantify the range of the neurobiological response (ie, AmygA) to a given quantum of external stress across a cohort of stress-exposed individuals to improve our understanding of NBResilience. AmygA is quantified as the ratio of metabolic activity of the amygdala, a subcortical structure involved in emotional response, to metabolic activity of regulatory regions in the cerebral cortex (ie, the temporal lobe). Within this construct, lower AmygA could result from either lower amygdalar activity or increased regulatory cortical activity. As such, more resilient individuals would have their response to stress modulated by higher relative cortical activity while less resilient individuals would have their response modulated by higher relative subcortical activity. This approach provided a unique opportunity to identify resilient individuals and to validate the protective impact of increased resilience on CVD outcomes. Accordingly, we suggest a pathway depicting the protective effect of NBResilience against MACE (Figure 5).
Figure 5. Mechanistic model.
Schematic showing the hypothesized impact of neurobiological resilience on different pathophysiologic pathways leading to increased (left) or decreased (right) major adverse cardiovascular event (MACE) risk.
Our results inform additional studies. These findings should be confirmed in a prospective study that also evaluates other neural centers involved in stress perception and NBResilience (eg, hippocampus, prefrontal cortex) and the genetic factors underlying NBResilience. Furthermore, research is needed to evaluate whether enhancing NBResilience (ie, decreasing AmygA) through behavioral or pharmacological therapies has salutary impacts. Investigations into the length of time that is required to develop increased AmygA (consequent to stress exposure) and the time needed to acquire NBResilience would also be of interest. Furthermore, cutoff values to define NBResilience are not clearly delineated by this study; the current findings provide a proof-of-construct that should be clarified by subsequent studies.
Limitations
The current study comes with the inherent limitations of a single-center retrospective cohort design. The data for median income and crime were based on zip code or town rather than individual information. Subject relocation or interval changes in SES or external stressor exposure between the time of imaging and outcomes as well as the total duration of stressor exposure could not be accounted for in our study. Due to the limitations of clinical whole-body 18F-FDG-PET/CT imaging, the assessment of the activities of all brain regions involved in stress perception was not feasible. Additionally, dedicated magnetic resonance neuroimaging was not available to assist with the identification of subcortical structures or perform partial volume correction. Neurovascular imaging and neurocognitive testing were not conducted, thus the presence of potentially confounding vascular or neurodegenerative disorders was not ascertained. Additionally, the modest sample size, small number of events, and lack of inflammatory markers are notable limitations. Future prospective studies assessing the relationship between NBResilience and CVD could leverage PET/MRI and robust laboratory and psychometric assessments in a larger cohort to overcome several of these limitations.
CONCLUSIONS
Among individuals exposed to chronic socioeconomic or environmental stressors, lower amygdalar activity was associated with a >50% relative risk reduction in subsequent MACE, in part through reductions in Art1. Accordingly, greater NBResilience may protect against cardiovascular disease, raising the possibility that methods to improve NBResilience in the context of chronic socioeconomic or environmental stressors may improve CVD outcomes.
Supplementary Material
CLINICAL PERSPECTIVE.
Psychosocial stress is highly prevalent in the general population and is an important risk factor for cardiovascular disease. In the current study, we observed that common, quantifiable chronic stressors (eg, exposure to conditions of lower income, higher crime, or higher noise) associate with greater stress-associated neurobiological activity (measured as amygdalar metabolic activity). Furthermore, we observe a broad range of stress-associated neurobiological activity among stress-exposed individuals and provide a novel definition of resilience (neurobiological resilience) to describe individuals with lower stress-associated neurobiological activity despite higher stressor exposure. Moreover, we observe that such neurobiological resilience associates with a lower risk of cardiovascular disease events. Accordingly, these data support efforts to improve neurobiological resilience, especially among individuals who are exposed to chronic stressors and/or have an elevated risk for developing cardiovascular disease. Prospective studies should test whether enhancing neurobiological resilience reduces cardiovascular disease risk.
Acknowledgments
This work was conducted with support from Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic healthcare centers, or the National Institutes of Health.
Sources of Funding
This work was partially supported by NIH grants P01HL131478 and KL2TR002542 and a grant from the Harvard Medical School/Osher Center for Integrative Medicine.
Footnotes
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.119.010337.
Disclosures
Dr Tawakol reports institutional research grants from Genentech and personal fees from Actelion and Esperion for work outside of this research. Dr Osborne has received consulting fees from Intrinsic Imaging, LLC for work outside of this research. The other authors report no conflicts.
Contributor Information
Tawseef Dar, Cardiovascular Imaging Research Center, Cardiology Division, Massachusetts General Hospital and Harvard Medical School, Boston..
Michael T. Osborne, Cardiovascular Imaging Research Center, Cardiology Division, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston..
Shady Abohashem, Cardiovascular Imaging Research Center, Cardiology Division, Massachusetts General Hospital and Harvard Medical School, Boston..
Taimur Abbasi, Cardiovascular Imaging Research Center, Cardiology Division, Massachusetts General Hospital and Harvard Medical School, Boston..
Karmel W. Choi, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston.; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.; Psychiatric and Neurodevelopmental Genetics Unit, Center for Genomic Medicine, Massachusetts General Hospital, Boston.; Stanley Center for Psychiatric Research, Broad Institute, Boston, MA..
Ahmed Ghoneem, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston..
Nicki Naddaf, Cardiovascular Imaging Research Center, Massachusetts General Hospital and Harvard Medical School, Boston..
Jordan W. Smoller, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston.; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.; Psychiatric and Neurodevelopmental Genetics Unit, Center for Genomic Medicine, Massachusetts General Hospital, Boston.; Stanley Center for Psychiatric Research, Broad Institute, Boston, MA..
Roger K. Pitman, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston..
John W. Denninger, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston..
Lisa M. Shin, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston.; Department of Psychology, Tufts University, Medford, MA..
Gregory Fricchione, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston..
Ahmed Tawakol, Cardiovascular Imaging Research Center, Cardiology Division, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston..
REFERENCES
- 1.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S; INTERHEART investigators. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0 [DOI] [PubMed] [Google Scholar]
- 2.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in post-traumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273 [DOI] [PubMed] [Google Scholar]
- 6.Goyal A, Dey AK, Chaturvedi A, Elnabawi YA, Aberra TM, Chung JH, Belur AD, Groenendyk JW, Lerman JB, Rivers JP, et al. Chronic stress-related neural activity associates with subclinical cardiovascular disease in psoriasis – a Prospective Cohort Study. JACC Cardiovasc Imaging. 2020;13:465–77. doi: 10.1016/j.jcmg.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grzywacz JG, Almeida DM, Neupert SD, Ettner SL. Socioeconomic status and health: a micro-level analysis of exposure and vulnerability to daily stressors. J Health Soc Behav 2004;45:1–16. doi: 10.1177/002214650404500101 [DOI] [PubMed] [Google Scholar]
- 8.Hatch SL, Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: a review of the research. Am J Community Psychol. 2007;40:313–332. doi: 10.1007/s10464-007-9134-z [DOI] [PubMed] [Google Scholar]
- 9.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basner M, Babisch W, Davis A, Brink M, Clark C, Janssen S, Stansfeld S. Auditory and non-auditory effects of noise on health. Lancet. 2014;383:1325–1332. doi: 10.1016/S0140-6736(13)61613-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halonen JI, Hansell AL, Gulliver J, Morley D, Blangiardo M, Fecht D, Toledano MB, Beevers SD, Anderson HR, Kelly FJ, et al. Road traffic noise is associated with increased cardiovascular morbidity and mortality and all-cause mortality in London. Eur Heart J. 2015;36:2653–2661. doi: 10.1093/eurheartj/ehv216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stansfeld SA, Haines MM, Burr M, Berry B, Lercher P A review of environmental noise and mental health. Noise Health. 2000;2:1–8. [PubMed] [Google Scholar]
- 13.Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, Tung B, Wang Y, Ishai A, Scott JA, et al. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. Eur Heart J. 2020;41:772–782. doi: 10.1093/eurheartj/ehz820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tawakol A, Osborne MT, Wang Y, Hammed B, Tung B, Patrich T, Oberfeld B, Ishai A, Shin LM, Nahrendorf M, et al. Stress-associated neurobiological pathway linking socioeconomic disparities to cardiovascular disease. J Am Coll Cardiol. 2019;73:3243–3255. doi: 10.1016/j.jacc.2019.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6:1250–1259. doi: 10.1016/j.jcmg.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 16.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardio-splenic axis in humans. JACC Cardiovasc Imaging. 2015;8:121–130. doi: 10.1016/j.jcmg.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britz-Cunningham SH, Millstine JW, Gerbaudo VH. Improved discrimination of benign and malignant lesions on FDG PET/CT, using comparative activity ratios to brain, basal ganglia, or cerebellum. Clin Nucl Med. 2008;33:681–687. doi: 10.1097/RLU.0b013e318184b435 [DOI] [PubMed] [Google Scholar]
- 19.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–1789. doi: 10.2105/ajph.91.11.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877 [DOI] [PubMed] [Google Scholar]
- 21.Dar T, Radfar A, Abohashem S, Pitman RK, Tawakol A, Osborne MT. Psychosocial Stress and Cardiovascular Disease. Curr Treat Options Cardiovasc Med. 2019;21:23. doi: 10.1007/s11936-019-0724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert K, Hunter RG, Bartlett AA, Lapp HE, Kent M. In search of optimal resilience ratios: differential influences of neurobehavioral factors contributing to stress-resilience spectra. Front Neuroendocrinol. 2020;56:100802. doi: 10.1016/j.yfrne.2019.100802 [DOI] [PubMed] [Google Scholar]
- 23.Crump C, Sundquist J, Winkleby MA, Sundquist K. Low stress resilience in late adolescence and risk of hypertension in adulthood. Heart. 2016;102:541–547. doi: 10.1136/heartjnl-2015-308597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergh C, Udumyan R, Fall K, Nilsagård Y, Appelros P, Montgomery S. Stress resilience in male adolescents and subsequent stroke risk: cohort study. J Neurol Neurosurg Psychiatry. 2014;85:1331–1336. doi: 10.1136/jnnp-2013-307485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergh C, Udumyan R, Fall K, Almroth H, Montgomery S. Stress resilience and physical fitness in adolescence and risk of coronary heart disease in middle age. Heart. 2015;101:623–629. doi: 10.1136/heartjnl-2014-306703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozanski A, Bavishi C, Kubzansky LD, Cohen R. Association of optimism with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e1912200. doi: 10.1001/jamanetworkopen.2019.12200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, Mathé AA. Understanding resilience. Front Behav Neurosci. 2013;7: 10. doi: 10.3389/fnbeh.2013.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195 [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatry. 2017;74:551–552. doi: 10.1001/jamapsychiatry.2017.0270 [DOI] [PubMed] [Google Scholar]
- 31.Seeman T, Gruenewald T, Karlamangla A, Sidney S, Liu K, McEwen B, Schwartz J. Modeling multisystem biological risk in young adults: The Coronary Artery Risk Development in Young Adults Study. Am J Hum Biol. 2010;22:463–472. doi: 10.1002/ajhb.21018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535–549. doi: 10.1515/RNS.2011.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651 [DOI] [PubMed] [Google Scholar]
- 35.Linden DE. How psychotherapy changes the brain-the contribution of functional neuroimaging. Mol Psychiatry. 2006;11:528–538. doi: 10.1038/sj.mp.4001816 [DOI] [PubMed] [Google Scholar]
- 36.Bernberg E, Ulleryd MA, Johansson ME, Bergström GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE−/− mice. Atherosclerosis. 2012;221:359–365. doi: 10.1016/j.atherosclerosis.2011.11.041 [DOI] [PubMed] [Google Scholar]
- 37.Lagraauw HM, Kuiper J, Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain Behav Immun. 2015;50:18–30. doi: 10.1016/j.bbi.2015.08.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.