Abstract
Ischaemic heart disease (IHD) is a complex disorder and a leading cause of death and morbidity in both men and women. Sex, however, affects several aspects of IHD, including pathophysiology, incidence, clinical presentation, diagnosis as well as treatment and outcome. Several diseases or risk factors frequently associated with IHD can modify cellular signalling cascades, thus affecting ischaemia/reperfusion injury as well as responses to cardioprotective interventions. Importantly, the prevalence and impact of risk factors and several comorbidities differ between males and females, and their effects on IHD development and prognosis might differ according to sex. The cellular and molecular mechanisms underlying these differences are still poorly understood, and their identification might have important translational implications in the prediction or prevention of risk of IHD in men and women. Despite this, most experimental studies on IHD are still undertaken in animal models in the absence of risk factors and comorbidities, and assessment of potential sex-specific differences are largely missing. This ESC WG Position Paper will discuss: (i) the importance of sex as a biological variable in cardiovascular research, (ii) major biological mechanisms underlying sex-related differences relevant to IHD risk factors and comorbidities, (iii) prospects and pitfalls of preclinical models to investigate these associations, and finally (iv) will provide recommendations to guide future research. Although gender differences also affect IHD risk in the clinical setting, they will not be discussed in detail here.
Keywords: Cardioprotection Sex differences Ischaemic heart disease Ischaemia and reperfusion Translational research Comorbidities
1. Introduction
Ischaemic heart disease (IHD) is the leading cause of death and morbidity in both men and women in Europe, even if age-standardized incidence and prevalence of IHD are lower in females than males.1 Several differences in pathophysiology, clinical manifestations, treatment, and effect of cardiovascular drugs due to sex have been reported as recently reviewed.2–8
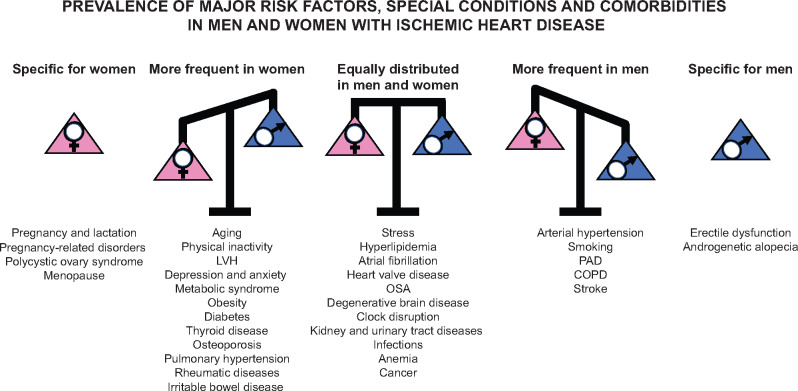
Apart from genetic predisposition and age, risk factors including abnormal lipid profile, smoking, hypertension, diabetes, abdominal obesity, psychosocial factors, alcohol intake, and lack of regular physical activity are associated with occurrence of myocardial infarction (MI) worldwide in both sexes and at all ages.9 However, several other diseases and lifestyle-related factors are also frequently associated with IHD, even if mechanistic links to IHD risk have not been proved yet.10–12 The prevalence of some cardiovascular risk factors and comorbidities is different in male or female IHD patients (Figure 1), and these conditions, as well as their treatments, can also differently impact IHD risk according to sex.13–15 Thus, sex-specific health promotion efforts may be needed to improve IHD prognosis in both women and men.15
Figure 1.
Distribution of major risk factors, special conditions, and comorbidities in patients with IHD according to divergence (or lack of this) between males and females. Sex-specific prevalence represented in this figure was derived from epidemiological data available in the literature. LVH, left ventricular hypertrophy; OSA, obstructive sleep apnoea; PAD, peripheral artery disease; COPD, chronic obstructive pulmonary disease.
It is well known that the presence of risk factors, comorbidities, or specific health behaviours may also differently affect myocardial response to ischaemia and reperfusion (IR) in males and females. Indeed, several animal models can be used to investigate either the mechanisms underlying sex differences, or the effects of risk factors, comorbidities, and their medications.16,17 Consistent with clinical observations, sex-specific responses to myocardial IR injury have been observed in preclinical studies.18 Several sex-related changes have been implicated in these differences, including androgens,19 oestrogens, nitric oxide, calcium handling (including mitochondrial permeability transition),20–22 reactive oxygen species formation,23 which leads to changes in apoptosis and autophagy24 as well as programmed necrosis,25 to name some of them.18 Unfortunately, current pharmacological approaches directed at attenuation of IR injury have failed to translate into clinical treatments in both males and females.26 Possible explanation for these disappointing results is that IHD is a complex disorder depending on a number of etiologic factors, and is frequently associated with other systemic disease states.17,27 Furthermore, these conditions might exert different effects in males and females. Despite this evidence, preclinical studies usually only include young and healthy male animals and/or derived tissues and cells, thus neglecting the possible effects of sex-related variables.
This ESC WG Position Paper will (i) discuss biological mechanisms underlying the interaction between sex and most common IHD risk factors or comorbidities; (ii) discuss the advantages and challenges of preclinical studies investigating the interplay between sex, IHD, risk factors, comorbidities, and associated co-medications; (iii) provide recommendations on strategies to enhance identification, characterization, validation, and publication of studies addressing sex-related differences in comorbidities and IHD.
2. Mechanisms underlying sex-related differences in IHD
Sex classification of sexually reproducing organisms is made according to their chromosomal complements, functional reproductive organs, and levels of sex steroids.28 Whether sex differences in IHD are due to sex, hormones, or sex and hormone interactions at various life stages is still not well known.3,28 Additional factors like prenatal environment may also be crucial. In addition to sex, defined by biological factors, gender differences related to social, environmental, and community factors can also affect IHD risk.2,29 For example, gender can account for differences in health-seeking behaviours and thus clinical outcomes in women affected by IHD.2 Since gender recapitulates the social and cultural role of individuals within a given society, it is usually developed in response to environment and cultural settings (including family interactions, media, peers, and education), it can change among different societies,30 and it is very complicated to dissect and study gender differences by using preclinical studies. However, in a Canadian study of young adults with acute coronary syndromes using a newly developed composite measure of gender, feminine gender was associated with increased risk of recurrent events independent of female sex.31 Since it is beyond the scope of this manuscript, mechanisms underlying gender-related differences will not be discussed further in the current article.
2.1 Sex chromosomes
2.1.1 Y chromosome
Compared to the X and autosomal chromosomes, the Y chromosome encodes for very few genes, divided into male-specific genes and genes with an X chromosome analogue. So far, only 71 protein-coding genes have been described, and the best known is Sry, gene coding for testis determining factor, a transcription factor needed for testis development and testosterone production in male foetal life. Knowledge of the function of the additional male-specific Y chromosome-derived genes is scarce.32,33 Sex-related difference in IHD epidemiology makes it reasonable to ask what role the non-gonadal effects of the Y-chromosome play. Importantly, the up-regulation of inflammatory genes and down-regulation of autoimmunity promoting atherosclerosis in men, has been linked to Y chromosome genes.34,35 In addition, gene and chromosome manipulation in mice has made it possible to move testis determining gene Sry from the Y chromosome to an autosome, and thereafter produce offspring with gonadal sex uncoupled from sex chromosome identity. Cardioprotection studies in these mice have shown that XY combination results in smaller MIs compared to XX combination independent of gonadal sex and hormonal status through development.36
2.1.2 X chromosome
Despite the difference between males and females in total number of genes due to the much larger X chromosome, dosage compensation is secured by inactivation of one of the X chromosomes in female cells. Some genes, however, seem to escape inactivation, thereby partially explaining phenotypic diversity. Random inactivation of one X chromosome makes the female heart a mosaic of two different cardiomyocytes (one with the maternal X chromosome and one with the paternal X chromosome)37–39. When it comes to the question of whether genes on the X chromosome have a role in IHD, associations between different forms of ischaemic injury, specific X chromosomal gene variants or dosing remain to be studied.40 In contrast to large studies of sets of single nucleotide polymorphisms on defined chromosome loci of autosomal chromosomes, studies so far found no association between IHD and X chromosomal variants40 However, most studies had limited power to detect sex differences, since they mainly enrolled males41
2.2 Gonadal hormones and their receptors
Systemic or tissue-specific levels of gonadal hormones (oestrogens, progestogens, androgens) change through different stages of life in a sex-specific pattern and are believed to have significant impact on IHD. Several experiments involving gonadectomy prior to IR demonstrated that both female and male hearts benefit from exogenous supplementation of oestradiol or testosterone, respectively.42–45 Oestradiol protects the isolated heart against IR injury via non-genomic oestrogen receptors either by stimulating G protein-coupled oestrogen receptors, resulting in activation of phosphoinositol 3 kinase and mitochondrial adenosine triphosphate-sensitive potassium channel-dependent cell signalling survival pathways,46–47 or through non-nuclear oestrogen receptors leading to endothelial nitric oxide synthase activation and cardioprotective S-nitrosylation of key mitochondrial proteins.48 Preclinical studies indicate that acute administration of progesterone has a non-genomic cardio-depressive effect involving modulation of calcium handling, including sarco-endoplasmic reticulum calcium adenosine triphosphatease expressionr49 and action potential duration50; anti-apoptotic effects have also been suggested, and might provide cardioprotection.51 The role of testosterone has been controversial, and synergistic effects or co-dependency of oestradiol and testosterone might also be crucial.52,53 Non-gonadal expression of aromatase is higher in males than females,54,55 and significant conversion of androgens to oestrogens takes place in the heart. Recent experimental studies indicate a dose-dependent cardioprotective effect of testosterone, but also additive cardioprotection when combined oestrogen and testosterone treatment is used.42 However, results from clinical studies of IHD after testosterone supplementation to elderly men with low endogenous levels of testosterone are inconclusiveref52,56,57
2.3 Pre-natal environment and foetal programming
Preclinical and epidemiological studies suggest that susceptibility to IHD can be the result of foetal programming via limitation of the final cell number in the heart, reduced vessel density, and by epigenetic modification of gene expression. Sex dimorphisms could be due to foetal hormonal differences (testosterone in males) and other less well-characterized dissimilarities.58–62 Pre- and perinatal complications like hypoxia, foetal malnutrition, and maternal hypothyroidism have repeatedly been linked experimentally to increased susceptibility to IR injury of the adult heart.62–65 Later studies confirmed the presence of DNA hypermethylation leading to reduced expression of cardioprotective protein kinase Cε, endothelial nitric oxide synthase, adenosine monophosphate kinase, and heat-shock protein 70.66,67 Reduced adult expression of heart mitochondrial respiratory chain proteins has also been reported after prenatal hypoxia,68 potentially increasing vulnerability to ischaemia. A limited number of studies included both sexes, and some but not all of these reported larger MI in adult male compared to female hearts after pre- or perinatal stress.62,65,69
3. Sex-specific effects of comorbidities and other confounding factors in IHD
According to sex distribution, comorbidities can be considered ‘general’ when similarly distributed among men and women or sex-related when disproportionately represented in or exclusively limited to one sex. Divergence in prevalence (or lack of this) between males and females for major comorbidities and confounding factors is schematically indicated in Figure 1 and discussed below. In the general population, association of IHD to single or frequently multiple diseases (and relative treatments) can impact on IHD development, IR injury, and protection from it. However, much less information is currently available regarding the role of sex, and in particular whether the effects of comorbidities in IHD differ between men and women, and if so what are the underlying mechanisms. Importantly, prevalence of comorbidities and their sex-specific prognostic effect on IHD might change after stratification for age. For several risk factors or comorbidities common to males and females, no data are currently available regarding sex-specific effects of them on IHD risk (Table 1). Moreover, there are significant differences in the clinical treatment of several comorbidities in men and women that may be further complicated by the different efficacy profile of some drugs used for treatment of these comorbidities as recently extensively reviewed,4,70–72 and by the confounding effect of drugs that are indicated only for women (e.g. contraceptives, menopausal hormone therapy).
Table 1.
Effects of general risk factors or comorbidities on IHD risk in women
| Increasing risk | Decreasing risk | Unknown or unclear |
|---|---|---|
| Ageing | Physical activity | Thyroid diseases |
| Smoking | Osteoporosis | |
| Stress | LVH | |
| Obesity | Pulmonary hypertension | |
| Hyperlipidaemia | Atrial fibrillation | |
| Hypertension | Heart valve diseases | |
| Diabetes | PAD | |
| Depression | COPD | |
| HIV | OSA | |
| Inflammatory diseases | Brain diseases | |
| Clock disruption | ||
| Gastro-intestinal diseases | ||
| Kidney diseases | ||
| Anaemia | ||
| Cancer |
LVH, left ventricular hypertrophy; OSA, obstructive sleep apnoea; PAD, peripheral artery disease; COPD, chronic obstructive pulmonary disease, HIV, human immunodeficiency virus.
Various preclinical models have been used to study most comorbid diseases possibly affecting IHD risk and prognosis. However, there is a critical information gap between preclinical and clinical research in this area since the majority of animal experiments is conducted on young and healthy animals of one sex only, even though the confounding effect of several risk factors and comorbidities on IHD has been known for decades.12,27,73 Even more, in most animal models of comorbidities, drug treatments as done in humans are lacking. The combination of multidisciplinary approaches in both male and female experimental models has the potential to unravel novel mechanisms underlying sex-related differences, but it has been rarely attempted.
3.1 Age and lifestyle
3.1.1 Age
Women are affected by IHD at a later age than men.74 On the other hand, young women have a particularly high risk of mortality following MIref74 More women than men die each year of IHD, and the hearts of postmenopausal women are more vulnerable to ischaemic insults compared to premenopausal women, suggesting that ageing has an effect on sex-specific differences in IHD. Ovariectomy significantly increases infarct size, but it increases by ageing in female rats, independent of plasma oestradiol levels.75 Ischaemic preconditioning is well known to reduce infarct size in young male rats, but both in aged hearts and female hearts the protective effect is less evident.27 There are also age-dependent, sex-specific differences in extracellular matrix and coronary resistance vessels, which may affect adaptation to work load.76–78
3.1.2 Smoking
Smoking is currently more common in males compared to females, but it has been repeatedly reported to increase IHD risk more in females than males.79–81 In addition, passive smoking exposure since birth increases risk of higher cholesterol levels in late adolescence especially in females.82 Experimental studies on IHD and smoking including both sexes are few; however, a nicotine-induced reduction in oestrogen levels has been proposed as an explanation for the increased ischaemic brain damage in females.83
3.1.3 Physical inactivity
Although most studies have been undertaken in men, women benefit at least as much as men from being physically active both prior to cardiac events and as part of rehabilitation.84–88 Unfortunately, available data are limited due to adjustment for age and sex prior to presentation of clinical trial results.86 After short-term forced exercise, sex-dependent differences in cardioprotection have been observed in preclinical models.89 In sedentary female rats, infarct size was smaller than in age-matched sedentary males, and males benefitted more from the preischaemic exercise protocol.89
3.1.4 Stress
Psychosocial and metabolic chronic stresses modify the atherosclerotic process, the related acute cardiovascular events,90 and other disorders such as Takotsubo cardiomyopathy differently in males and females.91 The underlying mechanisms involve, among possible other factors, enhanced haematopoiesis and different responses of immune cells to glucocorticoid release,92 with consequent changes in leucocyte homing to atherosclerotic plaques in response to enhanced sympathetic activation.90 In addition, young women post-MI have a 2-fold higher likelihood of developing mental stress-induced myocardial ischaemia, presumably due to increased proclivity to microcirculatory abnormalities.93
3.2 Endocrine and metabolic diseases
3.2.1 Obesity, metabolic syndrome, and diabetes
Although prevalence of obesity varies greatly within and between countries, overall, more women are obese than men, but an increased body mass index has the same deleterious effects on IHD risk in women and men across diverse populations.94 In contrast, sex may modify the prevalence and incidence of IHD in the context of type 1 and 2 diabetes and metabolic syndrome.95–98 Sexual disparity in the diagnosis of cardiovascular risk factors for IHD as well as the management and treatment of acute coronary syndromes are involved in the loss of ‘female advantage’ in metabolic disorders,96,98 beside any significant sex difference in the effects and complications of diabetes itself.99–106
3.2.2 Hyperlipidaemia
The management of dyslipidaemia is known to be different in men and women.107 Interestingly, in a community-based study conducted in USA among subjects with high risk for IHD, hyperlipidaemia was more aggressively treated in white men compared to white women or black men and women.108 In the community-based Tromsø Study in Norway, higher serum total cholesterol implied higher relative risk of MI in men than women.109 Various experimental models of hyperlipidaemia confirm increased myocardial injury due to ischaemia, but the cofounding role of sex differences has not been studied yet.
3.2.3 Thyroid disease
Although observational and experimental studies suggest that thyroid hormones might have a possible therapeutic role modifying the course of IHD,110,111 it remains yet unknown whether such effect translate into efficacy and safety in the clinical setting and whether they vary by sex.112 Thyroid hormones have inotropic actions mediated through the modulation of calcium re-uptake and, in particular triiodothyronine, modulates inflammatory response, apoptosis, mitochondrial function, and hence progression to heart failurer113,114 Under experimental conditions, thyroid status markedly affects the acute response to myocardial IR.115
3.2.4 Osteoporosis
IHD and osteoporosis have been seen as two independent conditions, but recent evidences may change this view.116–118 Proposed shared mechanisms are reduced sex hormone production, elevated follicle stimulating hormone in women, hyperlipidaemia, inflammation, reduced blood flow in intraosseous and coronary vascular beds, increased homocysteine level, and reduced vitamin K or D levels.119–124 The most commonly used animal models of induced osteoporosis are based on gonadal hormone deficiency in rats or mice, addition of glucocorticoids125 aged or female gonadectomized Apo E−/− mice. All these models also increase susceptibility to myocardial IR.
3.3 Cardiopulmonary and vascular diseases
3.3.1 Hypertension
3.3.1.1 Arterial hypertension
Hypertension approximately doubles the risk of IHD. Although recent reports have found that overall hypertension is more prevalent in men, its sex-specific prevalence varies according to age, and while in subjects <40 years old it is more prevalent in men, in subjects older than 65 years it is more prevalent in women.126 Specific relations between IHD, hypertension, and sex are also influenced by age. Surprisingly, in perspective of human clinical data, the number of experimental studies examining IR in hypertensive hearts in both sexes is limited.127,128
Left ventricular hypertrophy (LVH) is more prevalent in women when the recommended definitions of LVH are currently used.129,130 Patients with LVH are more vulnerable to IR,131–133 and some therapeutic strategies reducing LVH, including antihypertensive drugs, may exert beneficial effects not completely related to their hypertension-lowering effect.131,134 Male and female hypertrophic rat cardiac myocytes exhibit different responses to experimental IR, suggesting that sex-specific strategies should be attempted to optimize post-ischaemic treatment of male and female patients with LVH.135
3.3.1.2 Pulmonary hypertension
Recent studies highlight the high prevalence of mechanical left coronary artery compression by a dilated pulmonary artery in patients with pulmonary arterial hypertension, an effect which would explain, at least in part, the angina and angina-like symptoms observed in a large number of patients with the disease.136 The difference in prevalence of pulmonary hypertension may be explained by chromosomal, sexual hormone and/or immune system differences. Preclinical studies have identified a partly paradoxical role of oestrogen and/or testosterone depending on experimental model and sex.137,138
3.3.2 Atrial fibrillation
Atrial fibrillation and IHD are frequently associated in the ageing population. Men have a 1.5- to 2-fold higher lifetime risk of incident atrial fibrillation than women, and major risk factors for atrial fibrillation are IHD, hypertension, and obesity.139,140 Myocardial ischaemia can trigger atrial fibrillation, and atrial fibrosis can sustain re-entry circuits.141,142 Moreover, atrial fibrillation can induce or aggravate myocardial ischaemia through several mechanisms, including microcirculatory abnormalities.143 Significant sex differences in pulmonary veins and left atrium action potential characteristics have been reported in rabbits, and they may contribute to sex-related arrhythmogenesis144 Available experimental models in this area of research might be used to test susceptibility to electrical induction of atrial fibrillation in conjunction with acute myocardial ischaemia or post-infarct remodelling, however, the role of sex in these models is still unclear.145,146
3.3.3 Heart valve disease
Aortic stenosis is frequently associated with IHD and its risk factors.147 Compared to men, women with severe aortic stenosis have less valve calcification and more valve fibrosis, suggesting that pathophysiology of aortic stenosis and potential drug targets may differ according to sex.148 In contrast, men with aortic stenosis develop more myocardial fibrosis, maladaptive hypertrophy and ventricular dilatation than women.149,150 Several small and large animal models of calcific aortic valve diseases are currently available that might be useful to improve understanding of the basic biology, determine the contributions of comorbidities to IHD development and the efficacy of early interventions.151
3.3.4 Peripheral arterydisease
As with IHD, the prevalence of peripheral artery disease at younger ages is higher in men compared to women, but increases after menopause.61 Preclinical studies of peripheral artery disease as comorbidity to IHD are limited, as is the inclusion of both sexes in such studies.
3.3.5 Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is frequently associated with IHD.152 Their coexistence is associated with worse outcomes than either condition alone. Pathophysiological links between COPD and IHD include common risk factors, predominantly smoking, and systemic inflammation during COPD exacerbations. Sex-specific knowledge about the influence of COPD and its treatments on IHD and vice-versa remains incomplete.153 Information from preclinical models is also limited.
3.3.6 Obstructive sleep apnoea
Obstructive sleep apnoea (OSA) increases cardiovascular risk, including IHD.154 Intermittent hypoxia due to OSA may promote atherosclerosis,155–157 and it seems to increase the risk of IHD in men, with an apparently weaker relationship in women.158,159 Information from preclinical models is limited.
3.4 Neuro-psychological diseases
3.4.1 Stroke
A relationship between endogenous sex hormones (oestrogens and androgens) and ischaemic stroke or IHD has been suspected. Similar to experimental MI, in animal models of stroke, premenopausal female rodents show reduced infarct size compared to male or menopausal female rodents, and oestrogen administration reduces infarct size. Oestrogen supplementation immediately after ovariectomy exerts neuroprotective effects, whereas it shows no beneficial effects when administered 10 weeks after ovariectomy.160 Protective effects are mediated via oestrogen receptors-α and downstream cellular signalling161 or increase in astrocyte-specific insulin-like growth factor-1 expression and improved mitochondrial metabolism.162 Information from preclinical models combining IHD and stroke is limited.
3.4.2 Degenerative brain disease
IHD is a risk factor for dementia or cognitive impairment, with an increased risk of dementia in women with IHD.163,164 In addition, prevalence of dementia subtypes and cognitive impairment differ between men and women.165 It has been hypothesized that anti-platelet/anti-thrombotic therapies could reduce the risk of dementia in IHD patients.166 However, the protective effect of anti-platelet agents was not the same in men and women, reinforcing the importance of sex-related pathophysiological differences.
3.4.3 Clock disruption
Circadian rhythms are driven by internal molecular clocks regulating sleep–wake cycles, heart rate, feeding, body temperature, blood pressure, hormone secretion, metabolism, and bone marrow function167,168 reflected in diurnal clinical manifestation of diseases like MI with increased incidence of in the early morning.169,170 Disturbances of the normal activity and resting phase have adverse effects on cardiovascular parameters, healing responses, and remodelling.171–173 Sex- and oestrogen cycle-dependent variations in circadian rhythmicity of plasma corticosterone levels in rats have been reported.174 Female clock mutant mice were found to be protected from the development of metabolic changes and cardiomyopathy that was observed in male mice with the same mutation.175 This protection could be mediated by ovarian hormones via differentially regulated metabolic pathways, but its importance in IHD remains to be determined.
3.4.4 Depression and anxiety
Depression and anxiety disorders are common in male and female IHD patients, are linked to higher mortality and morbidity rates176 and increased mortality in coronary artery disease patients.177 Depression represented a cardiovascular risk factor comparable to obesity and high cholesterol levels in a study focusing on males only.178 With respect to mechanisms, an experimental study in rats revealed a sexual dimorphism in the molecular response to stress, involving sex-specific differences in brain-derived neurotrophic factor (BDNF) and cyclic adenosine monophosphate response element-binding protein.179 A point mutation of the BDNF protein caused a defect in the coagulation cascade in mice and was significantly associated to MI.180 Interestingly, occurrence of a polymorphism in BDNF is associated to either depressive symptoms or female sex181 therefore suggesting a direct link between change in BDNF activity and increased susceptibility to IHD in women carrying this specific variant.
3.5 Gastro-intestinal tract diseases
Inflammatory bowel disease has been consistently associated with an increased risk of IHD.182 In addition, the correlation between alterations in gut microbiota composition and IHD is gaining increasing attention.183,184 Interestingly, comorbidities such as obesity and type 2 diabetes are associated with alterations in gut microbiota.185 Animal models of intestinal inflammation might be extremely helpful to dissect the molecular mechanisms underlying these interactions.186 Several animal and human studies have shown sex-related differences in gut microbiota composition.187–189 However, whether gut symbiosis can attenuate the effects of risk factors or reduce post-ischaemic events,190 and whether sex plays a role in these processes is still unclear.
3.6 Kidney and urinary tract diseases
Disorders of the kidney and urinary tract are comorbidities with sex-specific effects in cardiovascular diseases (CVD).191,192 In patients with decreased glomerular filtration rate, IHD is the most common cardiovascular cause of death whereby men are more often affected than women.193 Interestingly, uric acid levels together with glomerular filtration rate levels are strong predictors of IHD, particularly in women.194–197 However, a Korean study of renal function and clinical outcomes after ST-segment elevated MI revealed no sex difference in 1-year mortality.198 Although many animal models have been developed to study the causes and treatments of chronic kidney disease in humans,199 most of them do not develop chronic kidney disease-associated CVD200 except for the adenine diet model that produces rapid-onset kidney disease and CVD.201 Subtotal nephrectomy plus permanent coronary ligation in rats resulted in more organ damage than each condition separately,202 however, nephrectomy did not affect the cardioprotective effect of preconditioning.203 The role of sex in these conditions is still unknown.
3.7 Immune system and blood diseases
3.7.1 Infection(s)
Infectious agents, including viruses, bacteria, and parasites, can be associated with atherosclerosis and IHD. While the association for some, like helicobacter pylori, chlamydia pneumonia, and cytomegalovirus is strong, others like influenza still need clarification. Nevertheless, large randomized prospective trials, evaluating the efficacy of antibiotic treatment for the secondary prevention of IHD have not demonstrated a reduction in the rate of events. Differences between sex in the association between infections and IHD and in response to treatment remain largely unknown.204
3.7.2 Human immunodeficiency virus
Infection by human immunodeficiency virus (HIV) and the use of some antiretroviral drugs are associated with an increased risk of CVD that goes beyond the risk explained by traditional cardiovascular risk factors including social status. Although most studies in HIV-positive patients mainly included male subjects, HIV infection has been associated with up to twice as high risk of IHD in females as in males.205–207 Lower body weight, slower drug metabolism, and hormonal control may explain sex-related differences in antiretroviral associated toxicities and contribute to differences in outcome of co-existing IHD.208 Furthermore, the use of IHD-related therapeutic interventions is lower in HIV-positive females than males with similar risk profiles.209
3.7.3 SARS-CoV-2 virus
COVID-19 pandemic caused by SARS-CoV-2 with debut in 2019 is another example of infective disease with remarkable sex-related differences. Although similar numbers of affected have been reported in men and women, for still unknown reasons, men seem more vulnerable compared to women.210 The mechanisms underlying these findings as well as their connections to CVD and IHD in particular remain to be investigated, and might include differences in cardiovascular risk factors, comorbidities, and lifestyles.211–213 Obviously, long-term recovery and risk of IHD are still unknown and will need further investigations in both men and women.
3.7.4 Inflammation and rheumatic diseases
Several systemic inflammatory diseases are associated with increased risk of IHD.214–218 Chronic inflammatory diseases can promote coronary microvascular dysfunction and hereby contribute to the development of myocardial ischaemia and cardiovascular events even in the absence of obstructive epicardial IHD.219,220 Autoimmune diseases are on average more frequent in women,221 and are also characterized by cardiovascular inflammation-promoting development of hypertension, LVH as well as atherosclerosis.222,223 These cardiovascular changes may regress in response to immunomodulatory therapy.224 Inducible, spontaneous, or engineered mouse models of chronic inflammatory diseases are available, reflecting the sex bias in susceptibility to the specific diseases,225–229 and the higher vulnerability to atherosclerosis.230–232 Among those mouse models, only one spontaneously develops MI,233 and the incidence of degenerative coronary vascular disease with MI is more pronounced in male vs. female mice.234 To the best of our knowledge, no studies are available evaluating the outcome of MI or IR in models of chronic inflammatory diseases, neither including evaluation of sex, even if clinical studies suggest sex-specific impact of rheumatic diseases on cardiovascular risk.235,240
3.7.5 Anaemia
In a cohort study including over 17 000 patients undergoing elective percutaneous coronary interventions, pre-procedural anaemia was associated with higher prevalence of bleeding and stroke, while post-procedural anaemia had higher incidence of death, MI, target vessel revascularization, bleeding, and major adverse cardiovascular events. However, no sex-related differences in outcome were found in anaemic patients compared to non-anaemic patients of either sex.223
3.8 Cancer
Oncological patients are susceptible to experience CVD,240,241 due to the clustering of cardiovascular risk factors in cancer242,243 or cardiovascular toxicity of anticancer therapies.244,245 Proposed mechanisms linking IHD, sex hormones, and cancer are obtained from preclinical and cellular studies, for example by regulation of hypoxia-inducible factor 1α.246–249 Experimental models combining cancer with anti-cancer therapies are needed beyond observational cohort studies. Although experimental cancer models exist, reflecting the sex bias in prevalence or severity of the specific cancer,250,251 so far they only focused on tumour effects, without addressing the occurrence of IHD. Mouse models of anti-cancer therapies associated with cardiotoxicity, but not specifically with IHD, are available and illustrate sex bias in susceptibility to cardiac toxicity.252
3.9 Special conditions exclusive for a specific sex
3.9.1 Pregnancy, lactation, and contraceptives
IHD is usually rare in pregnancy, although it is becoming more common for several factors, including lifestyle changes and increased maternal age, associated with stress, smoking, diabetes, and chronic hypertension.253 MI in pregnancy or the early postpartum period is associated with higher risk,253,254 while data on the effects of pregnancy after MI are scarce.255 Consistent with these clinical observations, hearts of late pregnant rodents are more prone to IR injury compared to non-pregnant rodents.256,257 Despite this, some cardioprotective mechanisms are activated during pregnancy. For example, the pregnancy-related hormone relaxin has been shown to exert multiple beneficial cardiovascular effects during MI, including suppression of arrhythmias and inflammation and reversal of fibrosis,258 while amniotic fluid stem cells play a cardioprotective role following MI.259 While higher parity is associated with a higher risk of IHD later in life, breastfeeding duration inversely impacts on IHD risk.260,261 Oxytocin, a main breastfeeding hormone, is cardioprotective against IR injury, mainly through the activation of pro-survival pathways.262–264
Oral contraceptive therapies based on oestrogens are known to increase thrombotic events, however, there is scant evidence related to the adverse effects of contraception types among women with already existing IHD.265,266 Moreover, little is known on the confounding effects of contraceptives in women with comorbidities such as, for example, obesity on cardiovascular risk.267
3.10 Comorbid diseases exclusive for a specific sex
3.10.1 Pregnancy-related disorders
Women with a history of common pregnancy complications or pregnancy-related disorders, including hypertensive disorders or gestational diabetes, peri-partum cardiomyopathy, and persistence of weight gain after delivery are at increased risk for CVD later in life.268,269 Since a large proportion of women worldwide become pregnant once or twice over their lives,269 evaluation of pregnancy outcome and in general reproductive factors may provide an unique and early opportunity to prevent IHD in women.270 Abnormal placental development and function underlie most pregnancy disorders, including spontaneous preterm birth, foetal growth restriction, and preeclampsia. Even women between 45 and 55 years of age with former preeclampsia show severe subclinical atherosclerosis.271 In addition to its crucial role in maternal and foetal circulatory systems, the placenta is hormonally, metabolically, and immunologically active.272 Several animal models involving rodents, guinea pigs, sheep, and non-human primates have been useful to address the role of placenta in foetal growth disorders, preeclampsia, or other maternal diseases during pregnancy.272–275 Using surgical, genetic, and pharmacological approaches, animal models have been also developed to recapitulate maternal symptoms of preeclampsia and other hypertensive disorders of pregnancy,276 as well as gestational diabetes.277–279 To our knowledge, combination of these systems with IHD models has never been systematically attempted.
3.10.2 Endocrine-related conditions and disorders
3.10.2.1 Polycystic ovary syndrome
Women with polycystic ovary syndrome are characterized by hyperandrogenism, infertility, and an unfavourable cardiometabolic profile in early life.280 Data on IHD and mortality in peri- and post-menopausal women with polycystic ovary syndrome appear to be controversial, even if they seem to be at an elevated risk.281–284 Available animal models of hyperandrogenism and ovarian morphology changes can be used to investigate polycystic ovary syndrome,285 and might be crucial to determine the molecular mechanisms underpinning these effects.
3.10.2.2 Menopause
Similar to humans, rats and mice cease oestrus cycling with ageing, but the age may vary with strain or other variables. To investigate the mechanisms underlying menopause and pre-menopause, 4-vinylcyclohexene diepoxide (VCD), a chemical toxin that causes ovarian failure by targeting pre-antral follicles can be used.286,287 VCD treatment blocks the production of female ovarian hormones, while production of androgens is preserved, representing a better model to analyse menopause rather than the loss of all ovarian hormones as would result from ovariectomy. VCD can be also administered to young adult animals to mimic early ovarian failure. Timing of gonads removal in animal models (indicated as castration if shortly after birth, prior to sexual development, or gonadectomy if performed after puberty) may be critical in the development or progression of IHD. Menopausal hormone replacement therapies to prevent and treat symptoms of menopause have a complex risk-benefit pattern as they may also modify the risk for IHD in certain subpopulations of women.288,289 Sufficient clinical data for individual risk-benefit considerations of these treatments are missing.290
3.10.2.3 Erectile dysfunction
Vascular erectile dysfunction is a strong predictor of IHD, and cardiovascular evaluation of patients presenting with erectile dysfunction is now recommended.291 Erectile dysfunction shares common pathways and risk factors with IHD.292 Phosphodiesterase-5 (PDE5) inhibitors, usually reserved as treatments of erectile dysfunction and pulmonary arterial hypertension, have been shown to reduce MI size and suppress ischaemia-induced ventricular arrhythmias.293
3.10.2.4 Androgenetic alopecia
Alopecia has been associated with an increased IHD risk and there appears to be a greater risk with degree of baldness.294–296 Alopecia is also associated with an increased risk of hypertension, hyperinsulinemia, metabolic syndrome, and dyslipidemia.294–296 The precise mechanisms underlying these effects are currently unknown and deserve further investigation.
4. Preclinical research to assess sex-specific effects of comorbidities in IHD: opportunities and challenges
Preclinical models are crucial to test hypotheses on sex differences in cardiovascular research and to study the importance of specific signalling cascades.297,298 Similar to humans, animal models display cardiac remodelling and sexually dimorphic characteristics with respect to IR injury.297 Here, mitochondria—which are mainly derived from the mother only—play an important role in mediating IR injury and protection from it, but also to explain the biology of sex differences.299,300 Experimental animal studies have reported sex differences in various aspects of mitochondrial function, some of which may explain, in part, the cardioprotection against IHD observed in pre-menopausal women. Cardiac mitochondria from female animals show decreased uptake of calcium,301,302 improved respiratory function,303,304 less oxidative stress,303,305,306 greater resistance to calcium-induced mitochondrial permeability transition pore opening307,308 and less mitochondrial fragmentation,309 when compared to mitochondria from male animals. Post-translational modification of mitochondrial proteins (such as aldehyde dehydrogenase and α-ketoglutarate dehydrogenase) modify reactive oxygen species handling and play an important role in female cardioprotection.306
While animal studies are of utmost importance for a better understanding of the underlying causes for sex differences in IHD, current research approaches present major limitations (summarized in Table 2). To more easily allow translation of animal data, inclusion of males and females and the use of a wider range of models, incorporating more realistic environmental and comorbid conditions are required.27,310 Moreover, unbiased studies can provide a general overview and avoid reductionist approaches.311,312 Species-specificity issues and technical/methodological caveats should be also considered, to allow a better alignment of animal studies with IHD patients’ real world, and a focus on human biology and therapeutic goals. Whenever possible, global or tissue-specific knockout mice or overexpression of crucial genes involved in the modulation of gonadal sex or sex hormones should be considered to study the mechanisms underlying sex-dimorphic effects of comorbidities on IHD. The following sections will address opportunities and challenges related to these aims.
Table 2.
Major limitations of current research approaches to investigate the role of sex and comorbidities in IHD
|
|
|
|
|
|
|
|
4.1 Use of male and female cells, tissues, organs, or organisms
Although the study of both sexes individually is important to validate scientific hypothesis or test novel therapeutic approaches, direct comparison of results in both sexes might present even greater advantages. While most signalling pathways might be commonly shared in cells or tissues derived from male or female animals, specific gene and protein expression or modifications might be affected by sex.313 Therefore, focusing on only one sex might prevent the identification of important biological effects or promote their misinterpretation.
4.2 Comorbidity models
Several animal models are currently available to reproduce comorbidities as well as sex-related conditions such as peri-menopause and menopause, to test novel therapeutic interventions and health-promoting strategies.314–316 Combination of these models might allow the identification of sex-dimorphic effects of specific comorbid diseases on IR injury and protection from it and their underlying mechanisms. Unfortunately, not all comorbidities identified in humans can be currently mimicked in animal models, and in almost all animal studies on the effects of comorbidities in IR injury and protection from it, adequate treatment of comorbidities by state-of-the-art therapy is lacking.27
4.3 Sex-related candidate mechanisms
Once sex dimorphisms on the effects of comorbidities on IR injury and protection from it are identified, the relative contributions of sex hormones and sex chromosomes should be determined.317,318 Since peripheral or ‘activational’ effects of gonadal hormones cause the majority of sex differences, gonadectomy is usually the first experiment performed in this context, preferably in both sexes. Gonadectomy allows to determine whether the sex difference depends on the secretion of gonadal hormones in adulthood. Then, further experiments will be needed to determine relevant hormones and their downstream mechanisms of action. In addition to the exogenous administration of sex hormones, oestrogen and androgen receptor knockout mice are also available.319–321 For example, oestrogen receptor-beta knockout mice have been widely used to investigate the effects of these hormones on IHD.320,322–325
In case sex differences persist after gonadectomy, then permanent changes caused by gonadal hormones eventually acting at early stages of development (long-lasting, differentiating ‘organizational’ effects) need to be assessed. If these effects also do not explain the sex difference, then extra-gonadal mechanisms related to sex chromosomes might be considered. This simplified sequential experimental approach addresses essential questions and provides the first steps for finding the mechanisms explaining sex-biased effects of diseases in preclinical models. To determine whether a phenotype depends on gonadal hormones or sex chromosomes different mouse models could also be used, including the Four Core Genotypes and the XY* mouse model (advantages and limitations have been previously reviewed elsewhere).317,326
4.4 Species differences
Results obtained from animal species may not translate directly to humans for several reasons. Firstly, the frequency of oestrous cycle in female experimental animals is species dependent. In particular, rodents present different duration of oestrous cycle and very different oestrogen levels, they are poly-ovulatory while women are mono-ovulatory. Moreover, although the initial stages of follicular growth seem to be comparable between humans and rodents, differences in the later stages cannot be excluded.327 Among small mammals, mice are the most commonly used because of the possibility to perform in vivo genetic modifications.328 As outlined above, mice also allow the manipulation of the hormonal state and specific sex-chromosome genes and thus to discriminate between sex chromosomes, gonadal status, and hormonal effects.28
Rats have also been used to study sex differences. However, oestradiol levels do not fall as low in female rats after cessation of oestrous cycling as in women following menopause, and this represents a critical issue when using rats as a model of menopause.329 In addition, remarkable differences have been described after MI between mice and rats, when comparing males and females.330,331
In large animals provided by commercial suppliers (in particular pigs), the presence of gonads should be confirmed, since some male animals may be castrated at birth. In other cases, animals might be sexually immature at the time of study (for example piglets smaller than 100 kg used in research), making extrapolation of data to adult animals problematic. Moreover, mostly female pigs are used for studies of IHD due to easier handling of these animals.332 Finally, while preclinical models may identify biological sex differences when they exist, the complex social, psychological, environmental, community factors, and constraints leading to gender peculiarities are impossible to examine in animal models.
4.5 Technical caveats
The bias deriving from the preferential use of only animals of one sex is often based on practical rather than scientific concerns. Since in many fields there is a significantly larger body of literature and data sets on male mice, this further encourages the use of this sex in preclinical studies. In addition, male mice are larger and easier to be surgically manipulated, and they lack oestrous cycles. In contrast, females are smaller (requiring lower weight-adjusted drug dosages), less aggressive, easier to handle, and they generally are less expensive. However, the use of female mice with synchronized oestrus cycles strongly complicates research design.
Although most primary or stabilized cell lines are derived from animals of unknown sex, the sex of the cell/tissue donor can be determined identifying specific fragments of the X and Y chromosomes. With respect to cardiomyocyte-like cell lines, H9C2 are rat female myoblasts, while HL-1 are myocyte-like cells from female mice. In addition, it is important to consider the hormonal environment of cultured cells, in particular culture media composition, since it might contain sex steroid hormones and in vitro exposure of cells to hormones may affect cellular pathways/signals of interest over several passages. Conversely, charcoal treatment could be used to eliminate or reduce hormones levels.
Sex steroid hormones initiate rapid actions that do not require gene transcription (non-genomic actions) as well as effects on gene transcription (genomic actions). Thus, duration of hormone exposure is a critical consideration in study design. Moreover, since systemic actions of hormones might significantly affect hemodynamic state, the use of in vivo animal models followed up by isolated heart perfusion studies might be helpful to eliminate in vivo confounding factors related to extracardiac hemodynamic, particularly in the pregnancy state.
Several conditions related to animal feeding, housing, or breeding need accurate evaluation. Retired breeder females may be used for studies of ageing, but this approach has some limitations, since it is currently unknown whether presence and number of previous pregnancies can affect over time cardiovascular function. Thus, comparisons between multiparous animals and age-matched nulliparous females or males might be inaccurate.
Housing conditions, including light/dark cycles, temperature, absence of vibrations, or external noise, are crucial to maintain oestrous cycling in female rats and mice. Females housed together frequently synchronize their cycles. Disruption of sleep/wake cycles, isolation, lack of physical activity, or handling conditions may increase stress imposed on animals, influence sex hormone-related pathways and therefore should be taken into account. Finally, chow composition and the possible presence of phytoestrogens should be ruled out.
4.6 Documentation, costs, and duration of research
ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting animal research propose to include sex of the animals among the items to be described as the minimum information in all scientific publications.333 Similarly, revised recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals clearly report the importance of describing variables of the source population including sex.334 However, these recommendations are not always fulfilled, even if requested by most scientific journals.
While preliminary studies can identify sex-dependent effects of comorbidities on IHD, only subsequent more complex, long, and costly studies may identify the precise mechanisms underlying observed sexual dimorphisms. Combination of several available animal models will require time and a learning curve to identify the best conditions and segments of investigation. It is possible that new animal models will be needed, and these requirements might further increase costs and prolong duration of research.
Furthermore, experimental preclinical studies involving ageing or pregnant animals usually present several ethical and regulatory difficulties in most countries, and duration of research in these cases is usually longer. In addition, although studies in non-human primates represent a pre-requisite of studies in humans, costs and hurdles related to project managing are even higher and make them prohibitive for most basic science investigators and small companies developing novel therapies for IHD. These considerations should be taken into account by investigators, scientific societies and funding agencies in order to provide financing through dedicated calls or considering rewards/bonuses/incentives covering higher costs and longer duration of research.
5. Conclusions and recommendations
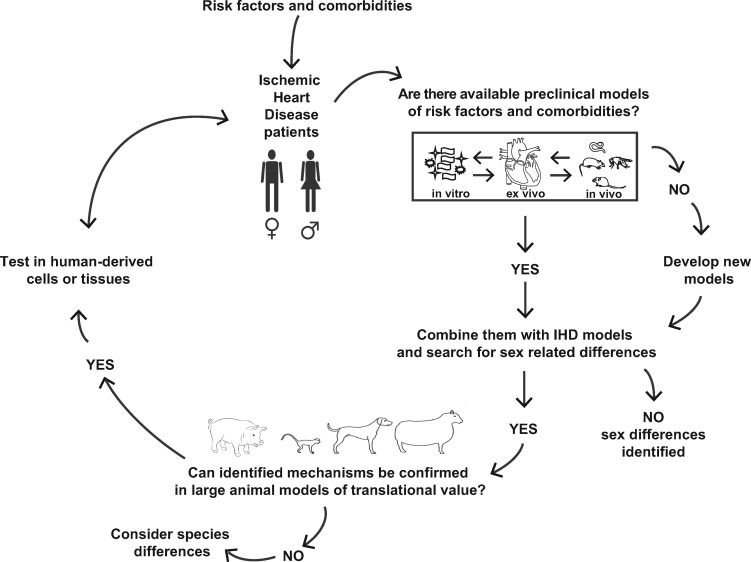
IHD is an epidemic and global disease affecting men and women, frequently associated with multi-morbidity in the adult and ageing population. Within scientific and medical communities, there is now increasing awareness that many IHD mechanisms differ between sexes, and sex differences in IHD risk factors and types of IHD have been identified. Despite this evidence, studies specifically investigating sex-specific implications of comorbidities in IHD are largely missing at all levels of research. Extremely narrowly focused studies may bias research directions and eventually miss essential aspects of human disease, including sex-related differences and their relation to comorbid disease. To overcome these hurdles, it would be necessary to account for sex, comorbidities, and their treatments in a virtuous circle tightly linking preclinical, translational, and clinical research (schematically illustrated in Figure 2). According to this hypothetical model, relevant clinical questions could be addressed through available preclinical models, investigating the presence of sexual dimorphisms and their underlying mechanisms. Next, the relevance of obtained results should be tested in larger animals or using human-derived cells or tissues, in order to finally translate results into large real-world populations of IHD patients.
Figure 2.
Proposed flow-chart to investigate the role of sex and comorbidities in IHD in a virtuous circle tightly linking preclinical, translational, and clinical research.
Based on these considerations, the ESC WG on Cellular Biology of the Heart and invited experts provide the following Recommendations (Table 3):
Table 3.
Recommendations
| 1 | Correct nomenclature should be always used when describing sex- or gender-related differences in IHD. |
| 2 | Experimental studies investigating IHD should include subjects from both sexes and, if not possible, results should be cautiously interpreted. |
| 3 | For any observed sexual dimorphic phenotype in IHD, it should be determined whether it is dependent on the hormonal state and if it is specific to or modified by genetic sex. |
| 4 | All relevant experimental details including age, strain, and sex should be clearly provided, preferably also in the searchable parts of the MS, for example, abstract and title. |
| 5 | Combination of IHD and comorbidities in preclinical models in male and female animals should be encouraged. |
| 6 | Peer-review of studies investigating IHD and comorbidities should always consider whether potential sex-specific effects have been accounted for. |
| 7 | Educational programmes in Cardiology and basic cardiovascular research should include elements addressing sex differences in Biology and Medicine. |
| 8 | Research should include a wide spectrum of diseases present in an adult population of both sexes and consider the sex-related effects of comedications. |
| 9 | Scientific Societies and Funding agencies should provide financing through dedicated calls or consider rewards/bonuses/incentives covering higher costs and longer duration of research in this area. |
Some confusion regarding sex or gender nomenclature still exists in the literature, and the two terms are sometimes incorrectly considered interchangeable. Proper terminology should be always used, particularly in preclinical research involving animals, cells and tissues that can explore biological mechanisms related to sex, but are unable to address the complex socio-cultural phenomena underlying gender differences.
To test whether sex is an independent biological variable, experimental protocols should include both sexes, possibly analysed simultaneously (not separately or under different conditions or timing). If not possible, results should be cautiously interpreted, or this should be highlighted as a study limitation.
In order to facilitate comparisons between published data, all relevant experimental details (including age, strain, sex, anaesthesia, model, timing of intervention) should be clearly provided, preferentially in parts of the text searchable in databases (e.g. title and abstract). Publishers and Editors should require a report on sex and age of experimental animals or cell lines included in full papers of biomedical research.
Since several preclinical models are currently available to reproduce most conditions, risk factors and comorbid diseases that might affect IHD risk and prognosis differently according to sex, an interdisciplinary approach could be useful, combining IHD and comorbidities preclinical models in male and female animals.
Reviewers of grant applications and manuscripts for studies addressing IHD and the different comorbidities should consider whether a potential sex-specific effect has been accounted for. If the Authors propose to generalize results based on investigations in only one sex, this should be very well motivated and potential limitations should be discussed.
Educational programmes in cardiology and basic cardiovascular research should include elements encouraging students and young doctors to be aware of the sex differences in biology and medicine.
Considering the widespread, global presence of IHD and multimorbidity in the adult and ageing population, research should not be limited only to the most common comorbidities in IHD but address a wider spectrum of diseases present in an adult population of both sexes and their relative comedications. Such research adds to the basic understanding of IHD independently from the role of sex and comorbidities.
Research addressing sex-specific effects of comorbidities in IHD is expected to have great scientific and clinical impact, but presents several technical, methodological, economical, and scientific challenges. These considerations should be taken into account by Investigators, Scientific Societies and funding agencies in order to provide financing through dedicated calls or considering rewards/bonuses/incentives covering higher costs and longer duration of research to reach this goal.
Funding
C.P. was supported by Ministero dell’Istruzione, Università e Ricerca Scientifica (2015583WMX) and by Programma STAR, financially supported by Federico II University (Unina) and Compagnia di San Paolo grants. P.F. is the vice chair of the European Cooperation in Science and Technology (COST action CA16225, EU-Cardioprotection). P.F. was supported by the National Research, Development and Innovation Office of Hungary (OTKA KH_17 125570, the National Heart Program NVKP 16-1-2016-0017) and by the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic program of the Semmelweis University. H.E.B. was supported by the Danish Council for Strategic Research (11-115818), the Novo Nordisk Foundation (NNF13OC0007447, NNF14OC0013337, and NNF15OC0016674), and TrygFonden (109624). F.B.E. is supported by the German Research Foundation (DFG, EN 453/12-1 to F.B.E.). R.S. was funded by the German Research Foundation (Project ID: 268555672-SFB-B05) and the Cardio-Pulmonary Institute (CPI), EXC 2026 (Project ID: 390649896). S.L. is supported by the National research Foundation (111801), CANSA and the South African Department of Science and Technology (for COST). E.G. was supported by the European Regional Development Fund (ERDF) through the Operational Program for Competitiveness Factors (COMPETE) (under the projects PAC ‘NETDIAMOND’ POCI‐01‐0145‐FEDER‐016385; HealthyAging2020 CENTRO‐01‐0145‐FEDER‐000012‐N2323; POCI‐01‐0145‐FEDER‐007440, CENTRO‐01‐0145‐FEDER‐032179, CENTRO‐01‐0145‐FEDER‐032414, and FCTUID/NEU/04539/2013 to CNC.IBILI). S.M.D. is supported by the Biomedical Research Council (BRC233/CM/SD/101320) and the British Heart Foundation (PG/18/44/33790). B.J.J.M.B. is supported by CVON-STW2016-14728 AFFIP grant by Cardiovascular Research The Netherlands. D.J.H. was supported by the British Heart Foundation (CS/14/3/31002), the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke-National University Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). R.M. was supported by a Cardio-Oncology grants from Incyte s.r.l. and funds from Ministero dell’Istruzione, Università e Ricerca Scientifica (549901_2020_Madonna: Ateneo). J.P.G.S. was supported by the Project EVICARE (No. 725229) of the European Research Council (ERC) and PPS grant (No. 2018B014) of the Dutch Heart Foundation. C.G.T. was supported by the Austrian Science Fund (P 32821).
Conflict of interest: P.F. is the founder and CEO of Pharmahungary Group, a group of R&D companies.
References
- 1. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P ESC Scientific Document Group. European Society of Cardiology: Cardiovascular Disease Statistics 2019 (executive summary). Eur Heart J Qual Care Clin Outcomes 2020;6:7–9. [DOI] [PubMed] [Google Scholar]
- 2. Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, Mendelson MA, Wood MJ, Volgman AS, Mieres JH.. Sex differences in ischemic heart disease: advances. Circ Cardiovasc Qual Outcomes 2018;11:e004437. [DOI] [PubMed] [Google Scholar]
- 3. Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K.. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol 2017;37:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski JC, Ceconi C, Drexel H, Kjeldsen K, Savarese G, Torp-Pedersen C, Atar D, Lewis BS, Agewall S.. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother 2017;3:163–182. [DOI] [PubMed] [Google Scholar]
- 5. Wei J, Cheng S, Merz C.. Coronary microvascular dysfunction causing cardiac ischemia in women. Jama 2019;322:2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waheed N, Elias-Smale S, Malas W, Maas AH, Sedlak TL, Tremmel J, Mehta PK.. Sex differences in non-obstructive coronary artery disease. Cardiovasc Res 2020;116:829–840. [DOI] [PubMed] [Google Scholar]
- 7. Gerdts E, Regitz-Zagrosek V.. Sex differences in cardiometabolic disorders. Nat Med 2019;25:1657–1666. [DOI] [PubMed] [Google Scholar]
- 8. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA.. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. [DOI] [PubMed] [Google Scholar]
- 9. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 10. Kendir C, van den Akker M, Vos R, Metsemakers J.. Cardiovascular disease patients have increased risk for comorbidity: a cross-sectional study in the Netherlands. Eur J Gen Pract 2018;24:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R, Working Group of Cellular Biology of Heart of European Society of Cardiology. Postconditioning and protection from reperfusion injury: where do we stand? Position Paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2010;87:406–423. [DOI] [PubMed] [Google Scholar]
- 12. Ferdinandy P, Schulz R, Baxter GF.. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 2007;59:418–458. [DOI] [PubMed] [Google Scholar]
- 13. Bairey Merz CN, Ramineni T, Leong D.. Sex-specific risk factors for cardiovascular disease in women-making cardiovascular disease real. Curr Opin Cardiol 2018;33:500–505. [DOI] [PubMed] [Google Scholar]
- 14. Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R, Working Group on Coronary Pathophysiology and Microcirculation. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position Paper from the Working Group on Coronary Pathophysiology and Microcirculation of the European Society of Cardiology. Cardiovasc Res 2011;90:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peters SAE, Muntner P, Woodward M.. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation 2019;139:1025–1035. [DOI] [PubMed] [Google Scholar]
- 16. Mahmoodzadeh S, Fliegner D, Dworatzek E.. Sex differences in animal models for cardiovascular diseases and the role of estrogen. Handb Exp Pharmacol 2012;23–48. [DOI] [PubMed] [Google Scholar]
- 17. Sack MN, Murphy E.. The role of comorbidities in cardioprotection. J Cardiovasc Pharmacol Ther 2011;16:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruiz-Meana M, Boengler K, Garcia-Dorado D, Hausenloy DJ, Kaambre T, Kararigas G, Perrino C, Schulz R, Ytrehus K.. Ageing, sex, and cardioprotection. Br J Pharmacol 2019;doi:10.1111/bph.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le TY, Ashton AW, Mardini M, Stanton PG, Funder JW, Handelsman DJ, Mihailidou AS.. Role of androgens in sex differences in cardiac damage during myocardial infarction. Endocrinology 2014;155:568–575. [DOI] [PubMed] [Google Scholar]
- 20. Mendoza L, Kaufman L, Standard PG.. Immunodiffusion test for diagnosing and monitoring pythiosis in horses. J Clin Microbiol 1986;23:813–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bienvenu LA, Morgan J, Reichelt ME, Delbridge LMD, Young MJ.. Chronic in vivo nitric oxide deficiency impairs cardiac functional recovery after ischemia in female (but not male) mice. J Mol Cell Cardiol 2017;112:8–15. [DOI] [PubMed] [Google Scholar]
- 22. Shao Q, Fallica J, Casin KM, Murphy E, Steenbergen C, Kohr MJ.. Characterization of the sex-dependent myocardial S-nitrosothiol proteome. Am J Physiol Heart Circ Physiol 2016;310:H505–H515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ciocci Pardo A, Scuri S, Gonzalez Arbelaez LF, Caldiz C, Fantinelli J, Mosca SM.. Survival kinase-dependent pathways contribute to gender difference in the response to myocardial ischemia-reperfusion and ischemic post-conditioning. Cardiovasc Pathol 2018;33:19–26. [DOI] [PubMed] [Google Scholar]
- 24. Chen C, Hu LX, Dong T, Wang GQ, Wang LH, Zhou XP, Jiang Y, Murao K, Lu SQ, Chen JW, Zhang GX.. Apoptosis and autophagy contribute to gender difference in cardiac ischemia-reperfusion induced injury in rats. Life Sci 2013;93:265–270. [DOI] [PubMed] [Google Scholar]
- 25. Garvin AM, Jackson MA, Korzick DH.. Inhibition of programmed necrosis limits infarct size through altered mitochondrial and immune responses in the aged female rat heart. Am J Physiol Heart Circ Physiol 2018;315:H1434–H1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davidson SM, Ferdinandy P, Andreadou I, Botker HE, Heusch G, Ibanez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D, Action CC.. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol 2019;73:89–99. [DOI] [PubMed] [Google Scholar]
- 27. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R.. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 28. Miller VM, Kaplan JR, Schork NJ, Ouyang P, Berga SL, Wenger NK, Shaw LJ, Webb RC, Mallampalli M, Steiner M, Taylor DA, Merz CN, Reckelhoff JF.. Strategies and methods to study sex differences in cardiovascular structure and function: a guide for basic scientists. Biol Sex Dif 2011;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norris CM, Yip CYY, Nerenberg KA, Clavel MA, Pacheco C, Foulds HJA, Hardy M, Gonsalves CA, Jaffer S, Parry M, Colella TJF, Dhukai A, Grewal J, Price JAD, Levinsson ALE, Hart D, Harvey PJ, Van Spall HGC, Sarfi H, Sedlak TL, Ahmed SB, Baer C, Coutinho T, Edwards JD, Green CR, Kirkham AA, Srivaratharajah K, Dumanski S, Keeping-Burke L, Lappa N, Reid RD, Robert H, Smith G, Martin-Rhee M, Mulvagh SL.. State of the science in women’s cardiovascular disease: a Canadian perspective on the influence of sex and gender. J Am Heart Assoc 2020;9:e015634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manandhar M, Hawkes S, Buse K, Nosrati E, Magar V.. Gender, health and the 2030 agenda for sustainable development. Bull World Health Organ 2018;96:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, Lavoie KL, Daskupta K, Rabi D, Humphries KH, Norris CM, Thanassoulis G, Behlouli H, Pilote L, GENESIS-PRAXY Investigators. Sex versus gender-related characteristics: which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol 2016;67:127–135. [DOI] [PubMed] [Google Scholar]
- 32. Maan AA, Eales J, Akbarov A, Rowland J, Xu X, Jobling MA, Charchar FJ, Tomaszewski M.. The Y chromosome: a blueprint for men’s health? Eur J Hum Genet 2017;25:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prokop JW, Deschepper CF.. Chromosome Y genetic variants: impact in animal models and on human disease. Physiol Genomics 2015;47:525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haitjema S, Kofink D, van Setten J, van der Laan SW, Schoneveld AH, Eales J, Tomaszewski M, de Jager SCA, Pasterkamp G, Asselbergs FW, den Ruijter HM, Asselbergs FW, den Ruijter HM. Loss of Y chromosome in blood is associated with major cardiovascular events during follow-up in men after carotid endarterectomy. Circ Cardiovasc Genet 2017;10:e001544. [DOI] [PubMed] [Google Scholar]
- 35. Voskarides K, Hadjipanagi D, Papazachariou L, Griffin M, Panayiotou AG.. Evidence for contribution of the y chromosome in atherosclerotic plaque occurrence in men. Genet Test Mol Biomarkers 2014;18:552–556. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Chen X, Mcclusky R, Ruiz-Sundstrom M, Itoh Y, Umar S, Arnold A P, Eghbali M. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovascular Research 2014;102:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tukiainen T, Villani A-C, Yen A, Rivas M A, Marshall J L, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings B B, Castel S E, Karczewski K J, Aguet F, Byrnes A, Lappalainen T, Regev A, Ardlie K G, Hacohen N, Macarthur D G. Landscape of X chromosome inactivation across human tissues. Nature 2017;550:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viggiano E, Ergoli M, Picillo E, Politano L.. Determining the role of skewed X-chromosome inactivation in developing muscle symptoms in carriers of Duchenne muscular dystrophy. Hum Genet 2016;135:685–698. [DOI] [PubMed] [Google Scholar]
- 39. Mercer TR, Dinger ME, Mattick JS.. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009;10:155–159. [DOI] [PubMed] [Google Scholar]
- 40. Loley C, Alver M, Assimes T L, Bjonnes A, Goel A, Gustafsson S, Hernesniemi J, Hopewell J C, Kanoni S, Kleber M E, Lau K W, Lu Y, Lyytikäinen L-P, Nelson C P, Nikpay M, Qu L, Salfati E, Scholz M, Tukiainen T, Willenborg C, Won H-H, Zeng L, Zhang W, Anand S S, Beutner F, Bottinger E P, Clarke R, Dedoussis G, Do R, Esko T, Eskola M, Farrall M, Gauguier D, Giedraitis V, Granger C B, Hall A S, Hamsten A, Hazen S L, Huang J, Kähönen M, Kyriakou T, Laaksonen R, Lind L, Lindgren C, Magnusson P K E, Marouli E, Mihailov E, Morris A P, Nikus K, Pedersen N, Rallidis L, Salomaa V, Shah S H, Stewart A F R, Thompson J R, Zalloua P A, Chambers J C, Collins R, Ingelsson E, Iribarren C, Karhunen P J, Kooner J S, Lehtimäki T, Loos R J F, März W, Mcpherson R, Metspalu A, Reilly M P, Ripatti S, Sanghera D K, Thiery J, Watkins H, Deloukas P, Kathiresan S, Samani N J, Schunkert H, Erdmann J, König I R. No Association of Coronary Artery Disease with X-Chromosomal Variants in Comprehensive International Meta-Analysis. Scientific Reports Sci Rep 2016;6:35278. 10.1038/srep35278PMC: 27731410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erdmann J, Kessler T, Munoz Venegas L, Schunkert H. A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovascular Research Cardiovasc Res 2018;114:1241–1257. [DOI] [PubMed] [Google Scholar]
- 42. Pongkan W, Chattipakorn S C, Chattipakorn N. Roles of Testosterone Replacement in Cardiac Ischemia–Reperfusion Injury. J Cardiovasc Pharmacol Ther 2016;21:27–43. [DOI] [PubMed] [Google Scholar]
- 43. Tsang S, Wu S, Liu J, Wong TM.. Testosterone protects rat hearts against ischaemic insults by enhancing the effects of alpha(1)-adrenoceptor stimulation. Br J Pharmacol 2008;153:693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moolman JA. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc Res 2006;69:777–780. [DOI] [PubMed] [Google Scholar]
- 45. Zhai P, Eurell TE, Cotthaus R, Jeffery EH, Bahr JM, Gross DR.. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am J Physiol Heart Circ Physiol 2000;279:H2766–H2775. [DOI] [PubMed] [Google Scholar]
- 46. Rocca C, Femmino S, Aquila G, Granieri MC, Francesco D, Pasqua EM, Rigiracciolo T, Fortini DC, Cerra F, Maggiolini MC, Pagliaro M, Rizzo P, Angelone T, Penna C.. Notch1 mediates preconditioning protection induced by GPER in normotensive and hypertensive female rat hearts. Front Physiol 2018;9:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sovershaev MA, Egorina EM, Andreasen TV, Jonassen AK, Ytrehus K.. Preconditioning by 17beta-estradiol in isolated rat heart depends on PI3-K/PKB pathway, PKC, and ROS. Am J Physiol Heart Circ Physiol 2006;291:H1554–H1562. [DOI] [PubMed] [Google Scholar]
- 48. Menazza S, Sun J, Appachi S, Chambliss KL, Kim SH, Aponte A, Khan S, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW, Murphy E.. Non-nuclear estrogen receptor alpha activation in endothelium reduces cardiac ischemia-reperfusion injury in mice. J Mol Cell Cardiol 2017;107:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moshal KS, Zhang Z, Roder K, Kim TY, Cooper L, Patedakis Litvinov B, Lu Y, Reddy V, Terentyev D, Choi BR, Koren G.. Progesterone modulates SERCA2a expression and function in rabbit cardiomyocytes. Am J Physiol Cell Physiol 2014;307:C1050–C1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feridooni HA, MacDonald JK, Ghimire A, Pyle WG, Howlett SE.. Acute exposure to progesterone attenuates cardiac contraction by modifying myofilament calcium sensitivity in the female mouse heart. Am J Physiol Heart Circ Physiol 2017;312:H46–H59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morrissy S, Xu B, Aguilar D, Zhang J, Chen QM.. Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell 2010;9:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herring MJ, Oskui PM, Hale SL, Kloner RA.. Testosterone and the cardiovascular system: a comprehensive review of the basic science literature. J Am Heart Assoc 2013;2:e000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Koeverden ID, de Bakker M, Haitjema S, van der Laan SW, de Vries JPM, Hoefer IE, de Borst GJ, Pasterkamp G, den Ruijter HM.. Testosterone to oestradiol ratio reflects systemic and plaque inflammation and predicts future cardiovascular events in men with severe atherosclerosis. Cardiovasc Res 2019;115:453–462. [DOI] [PubMed] [Google Scholar]
- 54. Biegon A. In vivo visualization of aromatase in animals and humans. Front Neuroendocrinol 2016;40:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jazbutyte V, Stumpner J, Redel A, Lorenzen JM, Roewer N, Thum T, Kehl F.. Aromatase inhibition attenuates desflurane-induced preconditioning against acute myocardial infarction in male mouse heart in vivo. PLoS One 2012;7:e42032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bianchi VE. Testosterone, myocardial function, and mortality. Heart Fail Rev 2018;23:773–788. [DOI] [PubMed] [Google Scholar]
- 57. Kloner RA, Carson C III, Dobs A, Kopecky S, Mohler ER. III. Testosterone and cardiovascular disease. J Am Coll Cardiol 2016;67:545–557. [DOI] [PubMed] [Google Scholar]
- 58. Rodrigo S, Fauste E, de la Cuesta M, Rodríguez L, Álvarez-Millán JJ, Panadero MI, Otero P, Bocos C.. Maternal fructose induces gender-dependent changes in both LXRalpha promoter methylation and cholesterol metabolism in progeny. J Nutr Biochem 2018;61:163–172. [DOI] [PubMed] [Google Scholar]
- 59. Camm EJ, Botting KJ, Sferruzzi-Perri AN.. Near to one’s heart: the intimate relationship between the placenta and fetal heart. Front Physiol 2018;9:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Botting KJ, Loke XY, Zhang S, Andersen JB, Nyengaard JR, Morrison JL.. IUGR decreases cardiomyocyte endowment and alters cardiac metabolism in a sex- and cause-of-IUGR-specific manner. Am J Physiol Regul Integr Comp Physiol 2018;315:R48–R67. [DOI] [PubMed] [Google Scholar]
- 61. Upadhyaya B, Larsen T, Barwari S, Louwagie EJ, Baack ML, Dey M.. Prenatal exposure to a maternal high-fat diet affects histone modification of cardiometabolic genes in newborn rats. Nutrients 2017;9:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghanbari M, Jeddi S, Bagheripuor F, Ghasemi A.. The effect of maternal hypothyroidism on cardiac function and tolerance to ischemia-reperfusion injury in offspring male and female rats. J Endocrinol Invest 2015;38:915–922. [DOI] [PubMed] [Google Scholar]
- 63. Xiong F, Lin T, Song M, Ma Q, Martinez SR, Lv J, MataGreenwood E, Xiao D, Xu Z, Zhang L.. Antenatal hypoxia induces epigenetic repression of glucocorticoid receptor and promotes ischemic-sensitive phenotype in the developing heart. J Mol Cell Cardiol 2016;91:160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L.. Chronic prenatal hypoxia induces epigenetic programming of PKC{epsilon} gene repression in rat hearts. Circ Res 2010;107:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xue Q, Zhang L.. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther 2009;330:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L.. Foetal nicotine exposure causes PKCepsilon gene repression by promoter methylation in rat hearts. Cardiovasc Res 2011;89:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L.. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther 2008;324:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Al-Hasan YM, Pinkas GA, Thompson LP.. Prenatal hypoxia reduces mitochondrial protein levels and cytochrome c oxidase activity in offspring guinea pig hearts. Reprod Sci 2014;21:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST.. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res 2011;90:285–294. [DOI] [PubMed] [Google Scholar]
- 70. Raparelli V, Elharram M, Moura CS, Abrahamowicz M, Bernatsky S, Behlouli H, Pilote L.. Sex differences in cardiovascular effectiveness of newer glucose-lowering drugs added to metformin in type 2 diabetes mellitus. J Am Heart Assoc 2020;9:e012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Farkouh A, Riedl T, Gottardi R, Czejka M, Kautzky-Willer A.. Sex-related differences in pharmacokinetics and pharmacodynamics of frequently prescribed drugs: a review of the literature. Adv Ther 2020;37:644–655. [DOI] [PubMed] [Google Scholar]
- 72. Moyer AM, Matey ET, Miller VM.. Individualized medicine: sex, hormones, genetics, and adverse drug reactions. Pharmacol Res Perspect 2019;7:e00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ferdinandy P, Szilvassy Z, Baxter GF.. Adaptation to myocardial stress in disease states: is preconditioning a healthy heart phenomenon? Trends Pharmacol Sci 1998;19:223–229. [DOI] [PubMed] [Google Scholar]
- 74. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK on behalf of the American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 75. Korzick DH, Lancaster TS.. Age-related differences in cardiac ischemia-reperfusion injury: effects of estrogen deficiency. Pflugers Arch Eur J Physiol 2013;465:669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dworatzek E, Baczko I, Kararigas G.. Effects of aging on cardiac extracellular matrix in men and women. Prot Clin Appl 2016;10:84–91. [DOI] [PubMed] [Google Scholar]
- 77. Leblanc AJ, Chen B, Dougherty PJ, Reyes RA, Shipley RD, Korzick DH, Muller-Delp JM.. Divergent effects of aging and sex on vasoconstriction to endothelin in coronary arterioles. Microcirculation 2013;20:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moro L, Pedone C, Mondi A, Nunziata E, Antonelli Incalzi R.. Effect of local and remote ischemic preconditioning on endothelial function in young people and healthy or hypertensive elderly people. Atherosclerosis 2011;219:750–752. [DOI] [PubMed] [Google Scholar]
- 79. Iversen B, Jacobsen BK, Lochen ML.. Active and passive smoking and the risk of myocardial infarction in 24,968 men and women during 11 year of follow-up: the Tromso Study. Eur J Epidemiol 2013;28:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huxley RR, Woodward M.. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 2011;378:1297–1305. [DOI] [PubMed] [Google Scholar]
- 81. Bosetti C, Negri E, Tavani A, Santoro L, La Vecchia C.. Smoking and acute myocardial infarction among women and men: a case-control study in Italy. Prev Med 1999;29:343–348. [DOI] [PubMed] [Google Scholar]
- 82. Le-Ha C, Beilin LJ, Burrows S, Huang RC, Oddy WH, Hands B, Mori TA.. Gender difference in the relationship between passive smoking exposure and HDL-cholesterol levels in late adolescence. J Clin Endocrinol Metab 2013;98:2126–2135. [DOI] [PubMed] [Google Scholar]
- 83. d’Adesky ND, de Rivero Vaccari JP, Bhattacharya P, Schatz M, Perez-Pinzon MA, Bramlett HM, Raval AP.. Nicotine alters estrogen receptor-beta-regulated inflammasome activity and exacerbates ischemic brain damage in female rats. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bellettiere J, LaMonte MJ, Evenson KR, Rillamas-Sun E, Kerr J, Lee I-M, Di C, Rosenberg DE, Stefanick ML, Buchner DM, Hovell MF, LaCroix AZ, Rossouw J, Ludlam S, Burwen D, McGowan J, Ford L, Geller N, Anderson G, Prentice R, Kooperberg C, Manson JE, Howard BV, Jackson R, Thomson CA, Wactawski-Wende J, Limacher M, Wallace R, Kuller L, Shumaker S, Shumaker S.. Sedentary behavior and cardiovascular disease in older women: the Objective Physical Activity and Cardiovascular Health (OPACH) Study. Circulation 2019;139:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, Kaur A, Smith Wilkins FC, Rayner E, Roberts N, Scarborough P.. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc 2016:e002495.doi: 10.1161/JAHA.115.002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM, Lancet Physical Activity Series 2 Executive Committe; Lancet Sedentary Behaviour Working Group. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016;388:1302–1310. [DOI] [PubMed] [Google Scholar]
- 87. Ford ES, Caspersen CJ.. Sedentary behaviour and cardiovascular disease: a review of prospective studies. Int J Epidemiol 2012;41:1338–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS.. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009;54:25–33. [DOI] [PubMed] [Google Scholar]
- 89. Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, Moore RL.. Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol 2005;564:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nahrendorf M, Swirski FK.. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res 2015;116:884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, S YH, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C.. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kemeny ME, Schedlowski M.. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun 2007;21:1009–1018. [DOI] [PubMed] [Google Scholar]
- 93. Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA, Raggi P.. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mongraw-Chaffin ML, Peters SAE, Huxley RR, Woodward M.. The sex-specific association between BMI and coronary heart disease: a systematic review and meta-analysis of 95 cohorts with 1.2 million participants. Lancet Diabetes Endocrinol 2015;3:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Madonna R, Balistreri CR, De Rosa S, Muscoli S, Selvaggio S, Selvaggio G, Ferdinandy P, De Caterina R.. Impact of sex differences and diabetes on coronary atherosclerosis and ischemic heart disease. J Clin Med 2019:98. doi: 10.3390/jcm8010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Manzo-Silberman S, Couturaud F, Charpentier S, Auffret V, El Khoury C, Le Breton H, Belle L, Marliere S, Zeller M, Cottin Y, Danchin N, Simon T, Schiele F, Gilard M.. Influence of gender on delays and early mortality in ST-segment elevation myocardial infarction: insight from the first French Metaregistry, 2005-2012 patient-level pooled analysis. Int J Cardiol 2018;262:1–8. [DOI] [PubMed] [Google Scholar]
- 97. Beckman JA, Paneni F, Cosentino F, Creager MA.. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J 2013;34:2444–2452. [DOI] [PubMed] [Google Scholar]
- 98. Brown RA, Walsh MF, Ren J.. Influence of gender and diabetes on vascular and myocardial contractile function. Endocr Res 2001;27:399–408. [DOI] [PubMed] [Google Scholar]
- 99. Peters SA, Huxley RR, Sattar N, Woodward M.. Sex differences in the excess risk of cardiovascular diseases associated with type 2 diabetes: potential explanations and clinical implications. Curr Cardiovasc Risk Rep 2015;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wannamethee SG, Papacosta O, Lawlor DA, Whincup PH, Lowe GD, Ebrahim S, Sattar N.. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012;55:80–87. [DOI] [PubMed] [Google Scholar]
- 101. Garaulet M, Perex-Llamas F, Fuente T, Zamora S, Tebar FJ.. Anthropometric, computed tomography and fat cell data in an obese population: relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur J Endocrinol 2000;143:657–666. [DOI] [PubMed] [Google Scholar]
- 102. Steinberg HO, Paradisi G, Cronin J, Crowde K, Hempfling A, Hook G, Baron AD.. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation 2000;101:2040–2046. [DOI] [PubMed] [Google Scholar]
- 103. Howard BV, Cowan LD, Go O, Welty TK, Robbins DC, Lee ET.. Adverse effects of diabetes on multiple cardiovascular disease risk factors in women. The Strong Heart Study. Diabetes Care 1998;21:1258–1265. [DOI] [PubMed] [Google Scholar]
- 104. Ossei-Gerning N, Wilson IJ, Grant PJ.. Sex differences in coagulation and fibrinolysis in subjects with coronary artery disease. Thromb Haemost 1998;79:736–740. [PubMed] [Google Scholar]
- 105. Mansfield MW, Heywood DM, Grant PJ.. Sex differences in coagulation and fibrinolysis in white subjects with non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 1996;16:160–164. [DOI] [PubMed] [Google Scholar]
- 106. Evans RW, Orchard TJ.. Oxidized lipids in insulin-dependent diabetes mellitus: a sex-diabetes interaction? Metabolism 1994;43:1196–1200. [DOI] [PubMed] [Google Scholar]
- 107. Cooke CE, Hammerash WJ Jr.. Retrospective review of sex differences in the management of dyslipidemia in coronary heart disease: an analysis of patient data from a Maryland-based health maintenance organization. Clin Ther 2006;28:591–599. [DOI] [PubMed] [Google Scholar]
- 108. Safford MM, Gamboa CM, Durant RW, Brown TM, Glasser SP, Shikany JM, Zweifler RM, Howard G, Muntner P.. Race-sex differences in the management of hyperlipidemia: the REasons for Geographic and Racial Differences in Stroke study. Am J Prev Med 2015;48:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Albrektsen G, Heuch I, Lochen ML, Thelle DS, Wilsgaard T, Njolstad I, Bonaa KH.. Risk of incident myocardial infarction by gender: interactions with serum lipids, blood pressure and smoking. The Tromso Study 1979-2012. Atherosclerosis 2017;261:52–59. [DOI] [PubMed] [Google Scholar]
- 110. Pantos C, Mourouzis I, Saranteas T, Clave G, Ligeret H, Noack-Fraissignes P, Renard PY, Massonneau M, Perimenis P, Spanou D, Kostopanagiotou G, Cokkinos DV.. Thyroid hormone improves postischaemic recovery of function while limiting apoptosis: a new therapeutic approach to support hemodynamics in the setting of ischaemia-reperfusion? Basic Res Cardiol 2009;104:69–77. [DOI] [PubMed] [Google Scholar]
- 111. Chen YF, Kobayashi S, Chen J, Redetzke RA, Said S, Liang Q, Gerdes AM.. Short term triiodo-L-thyronine treatment inhibits cardiac myocyte apoptosis in border area after myocardial infarction in rats. J Mol Cell Cardiol 2008;44:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jabbar A, Ingoe L, Pearce S, Zaman A, Razvi S.. Thyroxine in acute myocardial infarction (ThyrAMI) – levothyroxine in subclinical hypothyroidism post-acute myocardial infarction: study protocol for a randomised controlled trial. Trials 2015;16:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S.. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 2017;14:39–55. [DOI] [PubMed] [Google Scholar]
- 114. Forini F, Kusmic C, Nicolini G, Mariani L, Zucchi R, Matteucci M, Iervasi G, Pitto L.. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology 2014;155:4581–4590. [DOI] [PubMed] [Google Scholar]
- 115. Seara FAC, Maciel L, Barbosa RAQ, Rodrigues NC, Silveira ALB, Marassi MP, Carvalho AB, Nascimento JHM, Olivares EL.. Cardiac ischemia/reperfusion injury is inversely affected by thyroid hormones excess or deficiency in male Wistar rats. PLoS One 2018;13:e0190355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ye C, Xu M, Wang S, Jiang S, Chen X, Zhou X, He R.. Decreased bone mineral density is an independent predictor for the development of atherosclerosis: a systematic review and meta-analysis. PLoS One 2016;11:e0154740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Prasad M, Reriani M, Khosla S, Gossl M, Lennon R, Gulati R, Prasad A, Lerman LO, Lerman A.. Coronary microvascular endothelial dysfunction is an independent predictor of development of osteoporosis in postmenopausal women. Vasc Health Risk Manag 2014;10:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Farhat GN, Cauley JA.. The link between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab 2008;5:19–34. [PMC free article] [PubMed] [Google Scholar]
- 119. Chen Z, Qureshi AR, Brismar TB, Ripsweden J, Haarhaus M, Barany P, Heimburger O, Lindholm B, Stenvinkel P.. Differences in association of lower bone mineral density with higher coronary calcification in female and male end-stage renal disease patients. BMC Nephrol 2019;20:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhu D, Li X, Macrae VE, Simoncini T, Fu X.. Extragonadal effects of follicle-stimulating hormone on osteoporosis and cardiovascular disease in women during menopausal transition. Trends Endocrinol Metab 2018;29:571–580. [DOI] [PubMed] [Google Scholar]
- 121. Warnefors M, Mossinger K, Halbert J, Studer T, VandeBerg JL, Lindgren I, Fallahshahroudi A, Jensen P, Kaessmann H.. Sex-biased microRNA expression in mammals and birds reveals underlying regulatory mechanisms and a role in dosage compensation. Genome Res 2017;27:1961–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Campos-Obando N, Kavousi M, Roeters van Lennep JE, Rivadeneira F, Hofman A, Uitterlinden AG, Franco OH, Zillikens MC.. Bone health and coronary artery calcification: the Rotterdam Study. Atherosclerosis 2015;241:278–283. [DOI] [PubMed] [Google Scholar]
- 123. Weilner S, Schraml E, Redl H, Grillari-Voglauer R, Grillari J.. Secretion of microvesicular miRNAs in cellular and organismal aging. Exp Gerontol 2013;48:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, Tourkova IL, Li J, Davies TF, Sun L, Bian Z, Rosen C, Zallone A, New MI, Zaidi M.. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A 2012;109:14574–14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang Z, Ren H, Shen G, Qiu T, Liang D, Yang Z, Yao Z, Tang J, Jiang X, Wei Q.. Animal models for glucocorticoid-induced postmenopausal osteoporosis: an updated review. Biomed Pharmacother 2016;84:438–446. [DOI] [PubMed] [Google Scholar]
- 126. Gillis EE, Sullivan JC.. Sex differences in hypertension: recent advances. Hypertension 2016;68:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Neckář J, Svatoňová A, Weissová R, Drahota Z, Zajíčková P, Brabcová I, Kolář D, Alánová P, Vašinová J, Šilhavý J, Hlaváčková M, Tauchmannová K, Milerová M, Ošťádal B, Červenka L, Žurmanová J, Kalous M, Nováková O, Novotný J, Pravenec M, Kolář F.. Selective replacement of mitochondrial DNA increases the cardioprotective effect of chronic continuous hypoxia in spontaneously hypertensive rats. Clin Sci (Lond) 2017;131:865–881. [DOI] [PubMed] [Google Scholar]
- 128. Ledvényiová-Farkašová V, Bernátová I, Balis P, Puzserova A, Barteková M, Gablovsky I, Ravingerová T.. Effect of crowding stress on tolerance to ischemia-reperfusion injury in young male and female hypertensive rats: molecular mechanisms. Can J Physiol Pharmacol 2015;93:793–802. [DOI] [PubMed] [Google Scholar]
- 129. Gerdts E, Izzo R, Mancusi C, Losi MA, Manzi MV, Canciello G, De Luca N, Trimarco B, de Simone G.. Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the Campania Salute Network). Int J Cardiol 2018;258:257–261. [DOI] [PubMed] [Google Scholar]
- 130. Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K, Devereux RB.. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension 2008;51:1109–1114. [DOI] [PubMed] [Google Scholar]
- 131. Pagliaro P, Penna C.. Hypertension, hypertrophy, and reperfusion injury. J Cardiovasc Med (Hagerstown) 2017;18:131–135. [DOI] [PubMed] [Google Scholar]
- 132. Anderson PG, Allard MF, Thomas GD, Bishop SP, Digerness SB.. Increased ischemic injury but decreased hypoxic injury in hypertrophied rat hearts. Circ Res 1990;67:948–959. [DOI] [PubMed] [Google Scholar]
- 133. Batist G, Mersereau W, Malashenko BA, Chiu RC.. Response to ischemia-reperfusion injury in hypertrophic heart. Role of free-radical metabolic pathways. Circulation 1989;80:III10–III13. [PubMed] [Google Scholar]
- 134. Amoni M, Kelly-Laubscher R, Petersen M, Gwanyanya A.. Cardioprotective and anti-arrhythmic effects of magnesium pretreatment against ischaemia/reperfusion injury in isoprenaline-induced hypertrophic rat heart. Cardiovasc Toxicol 2017;17:49–57. [DOI] [PubMed] [Google Scholar]
- 135. Bell JR, Curl CL, Harding TW, Vila Petroff M, Harrap SB, Delbridge L.. Male and female hypertrophic rat cardiac myocyte functional responses to ischemic stress and beta-adrenergic challenge are different. Biol Sex Differ 2016;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Galie N, Saia F, Palazzini M, Manes A, Russo V, Bacchi Reggiani ML, Dall’Ara G, Monti E, Dardi F, Albini A, Rinaldi A, Gotti E, Taglieri N, Marrozzini C, Lovato L, Zompatori M, Marzocchi A.. Left main coronary artery compression in patients with pulmonary arterial hypertension and angina. J Am Coll Cardiol 2017;69:2808–2817. [DOI] [PubMed] [Google Scholar]
- 137. van de Veerdonk MC, Bogaard HJ, Voelkel NF.. The right ventricle and pulmonary hypertension. Heart Fail Rev 2016;21:259–271. [DOI] [PubMed] [Google Scholar]
- 138. Lahm T, Tuder RM, Petrache I.. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014;307:L7–26. [DOI] [PubMed] [Google Scholar]
- 139. Sharashova E, Wilsgaard T, Ball J, Morseth B, Gerdts E, Hopstock LA, Mathiesen EB, Schirmer H, Lochen ML.. Long-term blood pressure trajectories and incident atrial fibrillation in women and men: the Tromso Study. Eur Heart J 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ball J, Lochen ML, Wilsgaard T, Schirmer H, Hopstock LA, Morseth B, Mathiesen EB, Njolstad I, Tiwari S, Sharashova E.. Sex differences in the impact of body mass index on the risk of future atrial fibrillation: insights from the longitudinal population-based Tromso study. J Am Heart Assoc 2018:e008414. doi: 10.1161/JAHA.117.008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lieder H, Breithardt G, Heusch G.. Fatal attraction – a brief pathophysiology of the interaction between atrial fibrillation and myocardial ischemia. Int J Cardiol 2018;254:132–135. [DOI] [PubMed] [Google Scholar]
- 142. Alasady M, Shipp NJ, Brooks AG, Lim HS, Lau DH, Barlow D, Kuklik P, Worthley MI, Roberts-Thomson KC, Saint DA, Abhayaratna W, Sanders P.. Myocardial infarction and atrial fibrillation: importance of atrial ischemia. Circ Arrhythm Electrophysiol 2013;6:738–745. [DOI] [PubMed] [Google Scholar]
- 143. Bukowska A, Hammwohner M, Sixdorf A, Schild L, Wiswedel I, Rohl FW, Wolke C, Lendeckel U, Aderkast C, Bochmann S, Chilukoti RK, Mostertz J, Bramlage P, Goette A.. Dronedarone prevents microcirculatory abnormalities in the left ventricle during atrial tachypacing in pigs. Br J Pharmacol 2012;166:964–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tsai WC, Chen YC, Lin YK, Chen SA, Chen YJ.. Sex differences in the electrophysiological characteristics of pulmonary veins and left atrium and their clinical implication in atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:550–559. [DOI] [PubMed] [Google Scholar]
- 145. Riley G, Syeda F, Kirchhof P, Fabritz L.. An introduction to murine models of atrial fibrillation. Front Physiol 2012;3:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Nishida K, Michael G, Dobrev D, Nattel S.. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace 2010;12:160–172. [DOI] [PubMed] [Google Scholar]
- 147. Paradis JM, Fried J, Nazif T, Kirtane A, Harjai K, Khalique O, Grubb K, George I, Hahn R, Williams M, Leon MB, Kodali S.. Aortic stenosis and coronary artery disease: what do we know? What don’t we know? A comprehensive review of the literature with proposed treatment algorithms. Eur Heart J 2014;35:2069–2082. [DOI] [PubMed] [Google Scholar]
- 148. Simard L, Cote N, Dagenais F, Mathieu P, Couture C, Trahan S, Bosse Y, Mohammadi S, Page S, Joubert P, Clavel MA.. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: is valvular fibrosis the explanation? Circ Res 2017;120:681–691. [DOI] [PubMed] [Google Scholar]
- 149. Petrov G, Dworatzek E, Schulze TM, Dandel M, Kararigas G, Mahmoodzadeh S, Knosalla C, Hetzer R, Regitz-Zagrosek V.. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc Imaging 2014;7:1073–1080. [DOI] [PubMed] [Google Scholar]
- 150. Petrov G, Regitz-Zagrosek V, Lehmkuhl E, Krabatsch T, Dunkel A, Dandel M, Dworatzek E, Mahmoodzadeh S, Schubert C, Becher E, Hampl H, Hetzer R.. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation 2010;122:S23–28. [DOI] [PubMed] [Google Scholar]
- 151. Huefner DS. Another point of the suspension and expulsion cases. Except Child 1991;57:364–360. Discussion 364–368. [PubMed] [Google Scholar]
- 152. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM, Clayton JA, Cooper LS, Crea F, Di Carli M, Douglas PS, Galis ZS, Gurbel P, Handberg EM, Hasan A, Hill JA, Hochman JS, Iturriaga E, Kirby R, Levine GN, Libby P, Lima J, Mehta P, Desvigne-Nickens P, Olive M, Pearson GD, Quyyumi AA, Reynolds H, Robinson B, Sopko G, Taqueti V, Wei J, Wenger N.. Ischemia and No Obstructive Coronary Artery Disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Aryal S, Diaz-Guzman E, Mannino DM.. COPD and gender differences: an update. Transl Res 2013;162:208–218. [DOI] [PubMed] [Google Scholar]
- 154. Fan J, Wang X, Ma X, Somers VK, Nie S, Wei Y.. Association of obstructive sleep apnea with cardiovascular outcomes in patients with acute coronary syndrome. J Am Heart Assoc 2019;8:e010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mandal S, Kent BD.. Obstructive sleep apnoea and coronary artery disease. J Thorac Dis 2018;10:S4212–S4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Mokhlesi B, Ham SA, Gozal D.. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J 2016;47:1162–1169. [DOI] [PubMed] [Google Scholar]
- 157. Kent BD, Ryan S, McNicholas WT.. Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respir Physiol Neurobiol 2011;178:475–481. [DOI] [PubMed] [Google Scholar]
- 158. Dong JY, Zhang YH, Qin LQ.. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis 2013;229:489–495. [DOI] [PubMed] [Google Scholar]
- 159. Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK.. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012;5:720–728. [DOI] [PubMed] [Google Scholar]
- 160. Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM.. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A 2007;104:6013–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM.. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A 2001;98:1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD.. Gender-linked brain injury in experimental stroke. Stroke 1998;29:159–165. discussion 166. [DOI] [PubMed] [Google Scholar]
- 163. Ikram MA, van Oijen M, de Jong FJ, Kors JA, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM.. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke 2008;39:1421–1426. [DOI] [PubMed] [Google Scholar]
- 164. Aronson MK, Ooi WL, Morgenstern H, Hafner A, Masur D, Crystal H, Frishman WH, Fisher D, Katzman R.. Women, myocardial infarction, and dementia in the very old. Neurology 1990;40:1102–1106. [DOI] [PubMed] [Google Scholar]
- 165. Podcasy JL, Epperson CN.. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci 2016;18:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kim MY, Noh Y, Son SJ, Shin S, Paik HY, Lee S, Jung YS.. Effect of cilostazol on incident dementia in elderly men and women with ischemic heart disease. J Alzheimers Dis 2018;63:635–644. [DOI] [PubMed] [Google Scholar]
- 167. Chen L, Yang G.. Recent advances in circadian rhythms in cardiovascular system. Front Pharmacol 2015;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Feng D, Lazar MA.. Clocks, metabolism, and the epigenome. Mol Cell 2012;47:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Crnko S, Du Pre BC, Sluijter JPG, Van Laake LW.. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol 2019;16:437–447. [DOI] [PubMed] [Google Scholar]
- 170. Crnko S, Ernens I, Van Laake LW.. New dimensions in circadian clock function: the role of biological sex. Cardiovasc Res 2018;114:203–204. [DOI] [PubMed] [Google Scholar]
- 171. McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK.. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 2019;566:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hulsegge G, Gupta N, Proper KI, van Lobenstein N, W IJ, Hallman DM, Holtermann A, van der Beek AJ.. Shift work is associated with reduced heart rate variability among men but not women. Int J Cardiol 2018;258:109–114. [DOI] [PubMed] [Google Scholar]
- 173. McAlpine CS, Swirski FK.. Circadian influence on metabolism and inflammation in atherosclerosis. Circ Res 2016;119:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Atkinson HC, Waddell BJ.. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology 1997;138:3842–3848. [DOI] [PubMed] [Google Scholar]
- 175. Alibhai FJ, Reitz CJ, Peppler WT, Basu P, Sheppard P, Choleris E, Bakovic M, Martino TA.. Female ClockDelta19/Delta19 mice are protected from the development of age-dependent cardiomyopathy. Cardiovasc Res 2018;114:259–271. [DOI] [PubMed] [Google Scholar]
- 176. Hare DL, Toukhsati SR, Johansson P, Jaarsma T.. Depression and cardiovascular disease: a clinical review. Eur Heart J 2014;35:1365–1372. [DOI] [PubMed] [Google Scholar]
- 177. Watkins LL, Koch GG, Sherwood A, Blumenthal JA, Davidson JR, O’Connor C, Sketch MH.. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc 2013;2:e000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ladwig KH, Baumert J, Marten-Mittag B, Lukaschek K, Johar H, Fang X, Ronel J, Meisinger C, Peters A, Investigators K.. Room for depressed and exhausted mood as a risk predictor for all-cause and cardiovascular mortality beyond the contribution of the classical somatic risk factors in men. Atherosclerosis 2017;257:224–231. [DOI] [PubMed] [Google Scholar]
- 179. Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C.. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex 2009;19:1978–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Amadio P, Colombo GI, Tarantino E, Gianellini S, Ieraci A, Brioschi M, Banfi C, Werba JP, Parolari A, Lee FS, Tremoli E, Barbieri SS.. BDNFVal66met polymorphism: a potential bridge between depression and thrombosis. Eur Heart J 2017;38:1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Bozzini S, Gambelli P, Boiocchi C, Schirinzi S, Falcone R, Buzzi P, Storti C, Falcone C.. Coronary artery disease and depression: possible role of brain-derived neurotrophic factor and serotonin transporter gene polymorphisms. Int J Mol Med 2009;24:813–818. [DOI] [PubMed] [Google Scholar]
- 182. Feng W, Chen G, Cai D, Zhao S, Cheng J, Shen H.. Inflammatory bowel disease and risk of ischemic heart disease: an updated meta-analysis of cohort studies. J Am Heart Assoc 2017:e005892.doi: 10.1161/JAHA.117.005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Tang WH, Kitai T, Hazen SL.. Gut microbiota in cardiovascular health and disease. Circ Res 2017;120:1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Emoto T, Yamashita T, Kobayashi T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata KI.. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels 2017;32:39–46. [DOI] [PubMed] [Google Scholar]
- 185. Schiattarella GG, Sannino A, Esposito G, Perrino C.. Diagnostics and therapeutic implications of gut microbiota alterations in cardiometabolic diseases. Trends Cardiovasc Med 2019;29:141–147. [DOI] [PubMed] [Google Scholar]
- 186. Bamias G, Arseneau KO, Cominelli F.. Mouse models of inflammatory bowel disease for investigating mucosal immunity in the intestine. Curr Opin Gastroenterol 2017;33:411–416. [DOI] [PubMed] [Google Scholar]
- 187. Kim YS, Unno T, Kim BY, Park MS.. Sex differences in gut microbiota. World J Mens Health 2020;38:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ.. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016;7:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Turley EA. Hyaluronic acid stimulates protein kinase activity in intact cells and in an isolated protein complex. J Biol Chem 1989;264:8951–8955. [PubMed] [Google Scholar]
- 190. Zununi Vahed S, Barzegari A, Zuluaga M, Letourneur D, Pavon-Djavid G.. Myocardial infarction and gut microbiota: an incidental connection. Pharmacol Res 2018;129:308–317. [DOI] [PubMed] [Google Scholar]
- 191. Vallianou NG, Mitesh S, Gkogkou A, Geladari E.. Chronic kidney disease and cardiovascular disease: is there any relationship? Curr Cardiol Rev 2018;15:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Hopper I, Kotecha D, Chin KL, Mentz RJ, von Lueder TG.. Comorbidities in heart failure: are there gender differences? Curr Heart Fail Rep 2016;13:1–12. [DOI] [PubMed] [Google Scholar]
- 193. Runesson B, Qureshi AR, Xu H, Gasparini A, Lindholm B, Barany P, Elinder CG, Carrero JJ.. Causes of death across categories of estimated glomerular filtration rate: the Stockholm CREAtinine Measurements (SCREAM) project. PLoS One 2019;14:e0209440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Braga F, Pasqualetti S, Ferraro S, Panteghini M.. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med 2016;54:7–15. [DOI] [PubMed] [Google Scholar]
- 195. Puddu PE, Menotti A.. The U-shaped risk of estimated glomerular filtration rate for all-cause mortality and the role of serum uric acid. Int J Cardiol 2014;174:737–738. [DOI] [PubMed] [Google Scholar]
- 196. Puddu PE, Bilancio G, Terradura Vagnarelli O, Lombardi C, Mancini M, Zanchetti A, Menotti A.. Serum uric acid and eGFR_CKDEPI differently predict long-term cardiovascular events and all causes of deaths in a residential cohort. Int J Cardiol 2014;171:361–367. [DOI] [PubMed] [Google Scholar]
- 197. Puddu PE, Lanti M, Menotti A, Mancini M, Zanchetti A, Cirillo M, Angeletti M, Panarelli W, Gubbio Study RG. Serum uric acid for short-term prediction of cardiovascular disease incidence in the Gubbio population Study. Acta Cardiol 2001;56:243–251. [DOI] [PubMed] [Google Scholar]
- 198. Choi JS, Kim MJ, Kang YU, Kim CS, Bae EH, Ma SK, Ahn Y-K, Jeong MH, Kim YJ, Cho MC, Kim CJ, Kim SW, Korea Acute Myocardial Infarction Registry. Does gender influence the impact of impaired renal function on prognosis after ST-segment elevated myocardial infarction? Cardiol J 2013;20:526–532. [DOI] [PubMed] [Google Scholar]
- 199. Neugarten J. Gender and the progression of renal disease. J Am Soc Nephrol 2002;13:2807–2809. [DOI] [PubMed] [Google Scholar]
- 200. Liu S. Heart-kidney interactions: mechanistic insights from animal models. Am J Physiol Renal Physiol 2019;316:F974–F985. [DOI] [PubMed] [Google Scholar]
- 201. Diwan V, Brown L, Gobe GC.. Adenine-induced chronic kidney disease in rats. Nephrology (Carlton )2018;23:5–11. [DOI] [PubMed] [Google Scholar]
- 202. Bongartz LG, Joles JA, Verhaar MC, Cramer MJ, Goldschmeding R, Tilburgs C, Gaillard CA, Doevendans PA, Braam B.. Subtotal nephrectomy plus coronary ligation leads to more pronounced damage in both organs than either nephrectomy or coronary ligation. Am J Physiol Heart Circ Physiol 2012;302:H845–H854. [DOI] [PubMed] [Google Scholar]
- 203. Kocsis GF, Sarkozy M, Bencsik P, Pipicz M, Varga ZV, Paloczi J, Csonka C, Ferdinandy P, Csont T.. Preconditioning protects the heart in a prolonged uremic condition. Am J Physiol Heart Circ Physiol 2012;303:H1229–H1236. [DOI] [PubMed] [Google Scholar]
- 204. Stassen FR, Vainas T, Bruggeman CA.. Infection and atherosclerosis. An alternative view on an outdated hypothesis. Pharmacol Rep 2008;60:85–92. [PubMed] [Google Scholar]
- 205. Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, Budoff M, Butt AA, Gibert C, Goetz MB, Gottdiener J, Gottlieb S, Justice AC, Leaf D, McGinnis K, Rimland D, Rodriguez-Barradas MC, Sico J, Skanderson M, Tindle H, Tracy RP, Warner A, Freiberg MS.. HIV infection and cardiovascular disease in women. J Am Heart Assoc 2014;3:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL.. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr 2011;57:245–253. [DOI] [PubMed] [Google Scholar]
- 207. Aboud M, Elgalib A, Pomeroy L, Panayiotakopoulos G, Skopelitis E, Kulasegaram R, Dimian C, F CL, Duncan A, Wierzbicki AS, Peters BS.. Cardiovascular risk evaluation and antiretroviral therapy effects in an HIV cohort: implications for clinical management: the CREATE 1 study. Int J Clin Pract 2010;64:1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Nicolson TJ, Mellor HR, Roberts RR.. Gender differences in drug toxicity. Trends Pharmacol Sci 2010;31:108–114. [DOI] [PubMed] [Google Scholar]
- 209. Hatleberg CI, Ryom L, El-Sadr W, Mocroft A, Reiss P, de Wit S, Dabis F, Pradier C, Monforte A, Rickenbach M, Law M, Lundgren J, Sabin C.. Gender differences in HIV-positive persons in use of cardiovascular disease-related interventions: D:A:D study. J Int AIDS Soc 2014;17:19516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Spagnolo PA, Manson JE, Joffe H.. Sex and gender differences in health: what the COVID-19 pandemic can teach us. Ann Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Sharma G, Volgman AS, Michos ED.. Sex differences in mortality from COVID-19 pandemic: are men vulnerable and women protected? JACC Case Rep 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP, Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. Jama 2020;323:2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, Kvien TK, Dougados M, Radner H, Atzeni F, Primdahl J, Sodergren A, Wallberg Jonsson S, van Rompay J, Zabalan C, Pedersen TR, Jacobsson L, de Vlam K, Gonzalez-Gay MA, Semb AG, Kitas GD, Smulders YM, Szekanecz Z, Sattar N, Symmons DP, Nurmohamed MT.. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- 215. Mason JC, Libby P.. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015;36:482–489c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Gargiulo P, Marsico F, Parente A, Paolillo S, Cecere M, Casaretti L, Pellegrino AM, Formisano T, Fabiani I, Soricelli A, Trimarco B, Perrone-Filardi P.. Ischemic heart disease in systemic inflammatory diseases. An appraisal. Int J Cardiol 2014;170:286–290. [DOI] [PubMed] [Google Scholar]
- 217. Schnell O, Cappuccio F, Genovese S, Standl E, Valensi P, Ceriello A.. Type 1 diabetes and cardiovascular disease. Cardiovasc Diabetol 2013;12:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, Goodyear CS, Hestad KA, Kahaleh B, Riggio M, Shields K, Wasko MC.. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev 2013;12:1004–1015. [DOI] [PubMed] [Google Scholar]
- 219. Gianturco L, Bodini BD, Atzeni F, Colombo C, Stella D, Sarzi-Puttini P, Drago L, Galaverna S, Turiel M.. Cardiovascular and autoimmune diseases in females: the role of microvasculature and dysfunctional endothelium. Atherosclerosis 2015;241:259–263. [DOI] [PubMed] [Google Scholar]
- 220. Faccini A, Kaski JC, Camici PG.. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J 2016;37:1799–1806. [DOI] [PubMed] [Google Scholar]
- 221. Ngo ST, Steyn FJ, McCombe PA.. Gender differences in autoimmune disease. Front Neuroendocrinol 2014;35:347–369. [DOI] [PubMed] [Google Scholar]
- 222. Midtbo H, Semb AG, Matre K, Kvien TK, Gerdts E.. Disease activity is associated with reduced left ventricular systolic myocardial function in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:371–376. [DOI] [PubMed] [Google Scholar]
- 223. Midtbo H, Gerdts E, Kvien TK, Olsen IC, Lonnebakken MT, Davidsen ES, Rollefstad S, Semb AG.. The association of hypertension with asymptomatic cardiovascular organ damage in rheumatoid arthritis. Blood Press 2016;25:298–304. [DOI] [PubMed] [Google Scholar]
- 224. Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D.. Effect of 1-year anti-TNF-alpha therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens 2012;25:644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS.. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013;339:1084–1088. [DOI] [PubMed] [Google Scholar]
- 226. Taneja V, Behrens M, Mangalam A, Griffiths MM, Luthra HS, David CS.. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis Rheum 2007;56:69–78. [DOI] [PubMed] [Google Scholar]
- 227. Kim S, Voskuhl RR.. Decreased IL-12 production underlies the decreased ability of male lymph node cells to induce experimental autoimmune encephalomyelitis. J Immunol 1999;162:5561–5568. [PubMed] [Google Scholar]
- 228. Le Hir M, Martin M, Haas CA.. syndrome resembling human systemic sclerosis (scleroderma) in MRL/lpr mice lacking interferon-gamma (IFN-gamma) receptor (MRL/lprgammaR-/-). Clin Exp Immunol 1999;115:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Roubinian J, Talal N, Siiteri PK, Sadakian JA.. Sex hormone modulation of autoimmunity in NZB/NZW mice. Arthritis Rheum 1979;22:1162–1169. [DOI] [PubMed] [Google Scholar]
- 230. Wang X, Huang R, Zhang L, Li S, Luo J, Gu Y, Chen Z, Zheng Q, Chao T, Zheng W, Qi X, Wang L, Wen Y, Liang Y, Lu L.. A severe atherosclerosis mouse model on the resistant NOD background. Dis Model Mech 2018:dmm033852. doi: 10.1242/dmm.033852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Dragoljevic D, Kraakman MJ, Nagareddy PR, Ngo D, Shihata W, Kammoun HL, Whillas A, Lee MKS, Al-Sharea A, Pernes G, Flynn MC, Lancaster GI, Febbraio MA, Chin-Dusting J, Hanaoka BY, Wicks IP, Murphy AJ.. Defective cholesterol metabolism in haematopoietic stem cells promotes monocyte-driven atherosclerosis in rheumatoid arthritis. Eur Heart J 2018;39:2158–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Ma Z, Choudhury A, Kang SA, Monestier M, Cohen PL, Eisenberg RA.. Accelerated atherosclerosis in ApoE deficient lupus mouse models. Clin Immunol 2008;127:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Sanghera C, Wong LM, Panahi M, Sintou A, Hasham M, Sattler S.. Cardiac phenotype in mouse models of systemic autoimmunity. Dis Model Mech 2019:dmm036947. doi: 10.1242/dmm.036947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Hang LM, Izui S, Dixon FJ. ( NZW x BXSB)F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med 1981;154:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Targońska-Stępniak B, Biskup M, Biskup W, Majdan M.. Gender differences in cardiovascular risk profile in rheumatoid arthritis patients with low disease activity. Biomed Res Int 2019;2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Targońska-Stępniak B, Biskup M, Biskup W, Majdan M.. Diastolic dysfunction in rheumatoid arthritis patients with low disease activity. Clin Rheumatol 2019;38:1131–1137. [DOI] [PubMed] [Google Scholar]
- 237. Jiang L, Gao Z, Song Y, Xu J, Tang X, Wang H, Liu R, Jiang P, Xu B, Yuan J. Impact of anemia on percutaneous coronary intervention in Chinese patients: A large single center data. J Interven Cardiol 2018;31:826–833. [DOI] [PubMed] [Google Scholar]
- 238. Lee W-C, Fang H-Y, Chen H-C, Chen C-J, Yang C-H, Hang C-L, Wu C-J, Fang C-Y. Anemia: A significant cardiovascular mortality risk after ST-segment elevation myocardial infarction complicated by the comorbidities of hypertension and kidney disease. PLoS ONE 2017;12:e0180165 10.1371/journal.pone.0180165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Thompson L E, Masoudi F A, Gosch K L, Peterson P N, Jones P G, Salisbury A C, Kosiborod M, Daugherty S L. Gender differences in the association between discharge hemoglobin and 12-month mortality after acute myocardial infarction. Clin Cardiol 2017;40:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK.. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077–1084. [DOI] [PubMed] [Google Scholar]
- 241. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T.. Cardio-oncology – strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol 2019;280:163–175. [DOI] [PubMed] [Google Scholar]
- 242. Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, Douglas PS, Bhatia S, Chao C.. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol 2016;34:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, Stovall M, Chow EJ, Sklar CA, Mulrooney DA, Mertens AC, Border W, Durand JB, Robison LL, Meacham LR.. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31:3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 245. Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS.. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004;109:3122–3131. [DOI] [PubMed] [Google Scholar]
- 246. Docherty CK, Nilsen M, MacLean MR.. Influence of 2-methoxyestradiol and sex on hypoxia-induced pulmonary hypertension and hypoxia-inducible factor-1-alpha. J Am Heart Assoc 2019;8:e011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Shimoda LA. Let’s talk about sex: a novel mechanism by which estrogen receptor beta limits hypoxia-inducible factor expression in pulmonary endothelial cells. Am J Respir Cell Mol Biol 2018;59:11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC.. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep 2015;42:841–851. [DOI] [PubMed] [Google Scholar]
- 249. Zampino M, Yuzhakova M, Hansen J, McKinney RD, Goldspink PH, Geenen DL, Buttrick PM.. Sex-related dimorphic response of HIF-1 alpha expression in myocardial ischemia. Am J Physiol Heart Circ Physiol 2006;291:H957–H964. [DOI] [PubMed] [Google Scholar]
- 250. Caetano MS, Hassane M, Van HT, Bugarin E, Cumpian AM, McDowell CL, Cavazos CG, Zhang H, Deng S, Diao L, Wang J, Evans SE, Behrens C, Wistuba II, Fuqua SAW, Lin H, Stabile LP, Watowich SS, Kadara H, Moghaddam SJ.. Sex specific function of epithelial STAT3 signaling in pathogenesis of K-ras mutant lung cancer. Nat Commun 2018;9:4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Antico-Arciuch VG, Dima M, Liao XH, Refetoff S, Di Cristofano A.. Cross-talk between PI3K and estrogen in the mouse thyroid predisposes to the development of follicular carcinomas with a higher incidence in females. Oncogene 2010;29:5678–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Zhang J, Knapton A, Lipshultz SE, Cochran TR, Hiraragi H, Herman EH.. Sex-related differences in mast cell activity and doxorubicin toxicity: a study in spontaneously hypertensive rats. Toxicol Pathol 2014;42:361–375. [DOI] [PubMed] [Google Scholar]
- 253. Cauldwell M, Baris L, Roos-Hesselink JW, Johnson MR.. Ischaemic heart disease and pregnancy. Heart 2019;105:189–195. [DOI] [PubMed] [Google Scholar]
- 254. Cauldwell M, Steer PJ, von Klemperer K, Kaler M, Grixti S, Hale J, O’Heney J, Warriner D, Curtis S, Mohan AR, Dockree S, Mackillop L, Head CEG, Sterrenberg M, Wallace S, Freeman LJ, Patridge G, Baalman JH, McAuliffe FM, Simpson M, Walker N, Girling J, Siddiqui F, Bolger AP, Bredaki F, Walker F, Vause S, Gatzoulis MA, Johnson MR, Roberts A.. Maternal and neonatal outcomes in women with history of coronary artery disease. Heart 2020;106:380–386. [DOI] [PubMed] [Google Scholar]
- 255. Janion-Sadowska A, Sadowski M, Kurzawski J, Zandecki L, Janion M.. Pregnancy after acute coronary syndrome: a proposal for patients’ management and a literature review. Biomed Res Int 2013;2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Li J, Ruffenach G, Kararigas G, Cunningham CM, Motayagheni N, Barakai N, Umar S, Regitz-Zagrosek V, Eghbali M.. Intralipid protects the heart in late pregnancy against ischemia/reperfusion injury via Caveolin2/STAT3/GSK-3beta pathway. J Mol Cell Cardiol 2017;102:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Li J, Umar S, Iorga A, Youn JY, Wang Y, Regitz-Zagrosek V, Cai H, Eghbali M.. Cardiac vulnerability to ischemia/reperfusion injury drastically increases in late pregnancy. Basic Res Cardiol 2012;107:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Martin B, Romero G, Salama G.. Cardioprotective actions of relaxin. Mol Cell Endocrinol 2019;487:45–53. [DOI] [PubMed] [Google Scholar]
- 259. Bollini S, Cheung KK, Riegler J, Dong X, Smart N, Ghionzoli M, Loukogeorgakis SP, Maghsoudlou P, Dube KN, Riley PR, Lythgoe MF, De Coppi P.. Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev 2011;20:1985–1994. [DOI] [PubMed] [Google Scholar]
- 260. Rajaei S, Rigdon J, Crowe S, Tremmel J, Tsai S, Assimes TL.. Breastfeeding duration and the risk of coronary artery disease. J Womens Health (Larchmt) 2019;28:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Peters SA, van der Schouw YT, Wood AM, Sweeting MJ, Moons KG, Weiderpass E, Arriola L, Benetou V, Boeing H, Bonnet F, Butt ST, Clavel-Chapelon F, Drake I, Gavrila D, Key TJ, Klinaki E, Krogh V, Kuhn T, Lassale C, Masala G, Matullo G, Merritt M, Molina-Portillo E, Moreno-Iribas C, Nost TH, Olsen A, Onland-Moret NC, Overvad K, Panico S, Redondo ML, Tjonneland A, Trichopoulou A, Tumino R, Turzanski-Fortner R, Tzoulaki I, Wennberg P, Winkvist A, Thompson SG, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J, Butterworth AS.. Parity, breastfeeding and risk of coronary heart disease: a pan-European case-cohort study. Eur J Prev Cardiolog 2016;23:1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Gonzalez-Reyes A, Menaouar A, Yip D, Danalache B, Plante E, Noiseux N, Gutkowska J, Jankowski M.. Molecular mechanisms underlying oxytocin-induced cardiomyocyte protection from simulated ischemia-reperfusion. Mol Cell Endocrinol 2015;412:170–181. [DOI] [PubMed] [Google Scholar]
- 263. Ondrejcakova M, Barancik M, Bartekova M, Ravingerova T, Jezova D.. Prolonged oxytocin treatment in rats affects intracellular signaling and induces myocardial protection against infarction. Gen Physiol Biophys 2012;31:261–270. [DOI] [PubMed] [Google Scholar]
- 264. Anvari MA, Imani A, Faghihi M, Karimian SM, Moghimian M, Khansari M.. The administration of oxytocin during early reperfusion, dose-dependently protects the isolated male rat heart against ischemia/reperfusion injury. Eur J Pharmacol 2012;682:137–141. [DOI] [PubMed] [Google Scholar]
- 265. Roos-Hesselink JW, Cornette J, Sliwa K, Pieper PG, Veldtman GR, Johnson MR.. Contraception and cardiovascular disease. Eur Heart J 2015;36:1728–1734, 1734a–1734b. [DOI] [PubMed] [Google Scholar]
- 266. Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM.. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev 2015;CD011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Horton LG, Simmons KB, Curtis KM.. Combined hormonal contraceptive use among obese women and risk for cardiovascular events: a systematic review. Contraception 2016;94:590–604. [DOI] [PubMed] [Google Scholar]
- 268. Canoy D, Cairns BJ, Balkwill A, Wright FL, Khalil A, Beral V, Green J, Reeves G, Million Women Study Collaborators. Hypertension in pregnancy and risk of coronary heart disease and stroke: a prospective study in a large UK cohort. Int J Cardiol 2016;222:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM.. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev 2014;36:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, Lewis CE, Loucks EB, Parker DR, Rillamas-Sun E, Ryckman KK, Waring ME, Schenken RS, Johnson KC, Edstedt-Bonamy AK, Allison MA, Howard BV.. Reproductive risk factors and coronary heart disease in the women’s health initiative observational study. Circulation 2016;133:2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Zoet GA, Benschop L, Boersma E, Budde RPJ, Fauser BCJM, van der Graaf Y, de Groot CJM, Maas AHEM, Roeters van Lennep JE, Steegers EAP, Visseren FL, van Rijn BB, Velthuis BK, Franx A, Appelman YE, Baart SJ, Brouwers L, Cannegieter SC, Dam V, Eijkemans MCJ, Ferrari MD, Gunning MN, Hoek A, Koffijberg E, Koster MPH, Kruit M, Lagerwij GR, Lambalk CB, Laven JS, Linstra K, van der Lugt A, Maassen van den Brink A, Meun C, Middeldorp S, Moons KGM, Roos-Hesselink JW, Scheres LJJ, Steegers-Theunissen RPM, Terwindt GM, Wermer MJH CREW Consortium. Prevalence of subclinical coronary artery disease assessed by coronary computed tomography angiography in 45- to 55-year-old women with a history of preeclampsia. Circulation 2018;137:877–879. [DOI] [PubMed] [Google Scholar]
- 272. Thornburg KL, O’Tierney PF, Louey S.. Review: the placenta is a programming agent for cardiovascular disease. Placenta 2010;31:S54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Carter AM. Animal models of human placentation – a review. Placenta 2007;28:S41–47. [DOI] [PubMed] [Google Scholar]
- 274. Grigsby PL. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med 2016;34:011– 016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Lip SV, van der Graaf AM, Wiegman MJ, Scherjon SA, Boekschoten MV, Plösch T, Faas MM.. Experimental preeclampsia in rats affects vascular gene expression patterns. Sci Rep 2017;7:14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Cushen SC, Goulopoulou S.. New models of pregnancy-associated hypertension. Am J Hypertens 2017;30:1053–1062. [DOI] [PubMed] [Google Scholar]
- 277. Abdul Aziz SH, John CM, Mohamed Yusof NI, Nordin M, Ramasamy R, Adam A, Mohd Fauzi F.. Animal model of gestational diabetes mellitus with pathophysiological resemblance to the human condition induced by multiple factors (nutritional, pharmacological, and stress) in rats. Biomed Res Int 2016;2016:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Jawerbaum A, White V.. Animal models in diabetes and pregnancy. Endocr Rev 2010;31:680–701. [DOI] [PubMed] [Google Scholar]
- 279. Kiss AC, Lima PH, Sinzato YK, Takaku M, Takeno MA, Rudge MV, Damasceno DC.. Animal models for clinical and gestational diabetes: maternal and fetal outcomes. Diabetol Metab Syndr 2009;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Gunning MN, Fauser B.. Are women with polycystic ovary syndrome at increased cardiovascular disease risk later in life? Climacteric 2017;20:222–227. [DOI] [PubMed] [Google Scholar]
- 281. Ding DC, Tsai IJ, Wang JH, Lin SZ, Sung FC.. Coronary artery disease risk in young women with polycystic ovary syndrome. Oncotarget 2018;9:8756–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Vryonidou A, Papatheodorou A, Tavridou A, Terzi T, Loi V, Vatalas IA, Batakis N, Phenekos C, Dionyssiou-Asteriou A.. Association of hyperandrogenemic and metabolic phenotype with carotid intima-media thickness in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005;90:2740–2746. [DOI] [PubMed] [Google Scholar]
- 283. Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF II, Fitzpatrick LA.. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:2562–2568. [DOI] [PubMed] [Google Scholar]
- 284. Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev 2003;24:302–312. [DOI] [PubMed] [Google Scholar]
- 285. Paixao L, Ramos RB, Lavarda A, Morsh DM, Spritzer PM.. Animal models of hyperandrogenism and ovarian morphology changes as features of polycystic ovary syndrome: a systematic review. Reprod Biol Endocrinol 2017;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Van Kempen TA, Milner TA, Waters EM.. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res 2011;1379:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Hoyer PB, Devine PJ, Hu X, Thompson KE, Sipes IG.. Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model. Toxicol Pathol 2001;29:91–99. [DOI] [PubMed] [Google Scholar]
- 288. Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D.. Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause 2018;25:1286–1290. [DOI] [PubMed] [Google Scholar]
- 289. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB.. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. Jama 2013;310:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Langer RD, Simon JA, Pines A, Lobo RA, Hodis HN, Pickar JH, Archer DF, Sarrel PM, Utian WH.. Menopausal hormone therapy for primary prevention: why the USPSTF is wrong. Climacteric 2017;20:402–413. [DOI] [PubMed] [Google Scholar]
- 291. Shamloul R, Ghanem H.. Erectile dysfunction. Lancet 2013;381:153–165. [DOI] [PubMed] [Google Scholar]
- 292. Katsiki N, Wierzbicki AS, Mikhailidis DP.. Erectile dysfunction and coronary heart disease. Curr Opin Cardiol 2015;30:416–421. [DOI] [PubMed] [Google Scholar]
- 293. Hutchings DC, Anderson SG, Caldwell JL, Trafford AW.. Phosphodiesterase-5 inhibitors and the heart: compound cardioprotection? Heart 2018;104:1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Trieu N, Eslick GD.. Alopecia and its association with coronary heart disease and cardiovascular risk factors: a meta-analysis. Int J Cardiol 2014;176:687–695. [DOI] [PubMed] [Google Scholar]
- 295. Shahar E, Heiss G, Rosamond WD, Szklo M.. Baldness and myocardial infarction in men: the atherosclerosis risk in communities study. Am J Epidemiol 2007;167:676–683. [DOI] [PubMed] [Google Scholar]
- 296. Matilainen VA, Makinen PK, Keinanen-Kiukaanniemi SM.. Early onset of androgenetic alopecia associated with early severe coronary heart disease: a population-based, case-control study. J Cardiovasc Risk 2001;8:147–151. [DOI] [PubMed] [Google Scholar]
- 297. Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA.. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res 2016;118:1294–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Bøtker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, Efentakis P, Femminò S, García-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhäuser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schlüter K-D, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G.. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 2018;113:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Vona R, Ascione B, Malorni W, Straface E.. Mitochondria and sex-specific cardiac function. Adv Exp Med Biol 2018;1065:241–256. [DOI] [PubMed] [Google Scholar]
- 300. Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A.. Mitochondria: a central target for sex differences in pathologies. Clin Sci (Lond) 2017;131:803–822. [DOI] [PubMed] [Google Scholar]
- 301. Chweih H, Castilho RF, Figueira TR.. Tissue and sex specificities in Ca2+ handling by isolated mitochondria in conditions avoiding the permeability transition. Exp Physiol 2015;100:1073–1092. [DOI] [PubMed] [Google Scholar]
- 302. Arieli Y, Gursahani H, Eaton MM, Hernandez LA, Schaefer S.. Gender modulation of Ca(2+) uptake in cardiac mitochondria. J Mol Cell Cardiol 2004;37:507–513. [DOI] [PubMed] [Google Scholar]
- 303. Colom B, Oliver J, Roca P, Garcia-Palmer FJ.. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res 2007;74:456–465. [DOI] [PubMed] [Google Scholar]
- 304. Yan L, Ge H, Li H, Lieber SC, Natividad F, Resuello RR, Kim SJ, Akeju S, Sun A, Loo K, Peppas AP, Rossi F, Lewandowski ED, Thomas AP, Vatner SF, Vatner DE.. Gender-specific proteomic alterations in glycolytic and mitochondrial pathways in aging monkey hearts. J Mol Cell Cardiol 2004;37:921–929. [DOI] [PubMed] [Google Scholar]
- 305. Barba I, Miró-Casas E, Torrecilla JL, Pladevall E, Tejedor S, Sebastián-Pérez R, Ruiz-Meana M, Berrendero JR, Cuevas A, García-Dorado D.. High-fat diet induces metabolic changes and reduces oxidative stress in female mouse hearts. J Nutr Biochem 2017;40:187–193. [DOI] [PubMed] [Google Scholar]
- 306. Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E.. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res 2010;106:1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Ribeiro RF Jr, Ronconi KS, Morra EA, Do Val Lima PR, Porto ML, Vassallo DV, Figueiredo SG, Stefanon I.. Sex differences in the regulation of spatially distinct cardiac mitochondrial subpopulations. Mol Cell Biochem 2016;419:41–51. [DOI] [PubMed] [Google Scholar]
- 308. Milerová M, Drahota Z, Chytilová A, Tauchmannová K, Houštěk J, Ošťádal B.. Sex difference in the sensitivity of cardiac mitochondrial permeability transition pore to calcium load. Mol Cell Biochem 2016;412:147–154. [DOI] [PubMed] [Google Scholar]
- 309. Khalifa ARM, Abdel-Rahman EA, Mahmoud AM, Ali MH, Noureldin M, Saber SH, Mohsen M, Ali SS.. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol Rep 2017;5:e13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Hausenloy DJ, Garcia-Dorado D, Botker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, Van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P.. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:564–585. [DOI] [PubMed] [Google Scholar]
- 311. Harrington J, Fillmore N, Gao S, Yang Y, Zhang X, Liu P, Stoehr A, Chen Y, Springer D, Zhu J, Wang X, Murphy E.. A systems biology approach to investigating sex differences in cardiac hypertrophy. J Am Heart Assoc 2017:e005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Perrino C, Barabasi AL, Condorelli G, Davidson SM, De Windt L, Dimmeler S, Engel FB, Hausenloy DJ, Hill JA, Van Laake LW, Lecour S, Leor J, Madonna R, Mayr M, Prunier F, Sluijter JPG, Schulz R, Thum T, Ytrehus K, Ferdinandy P.. Epigenomic and transcriptomic approaches in the post-genomic era: path to novel targets for diagnosis and therapy of the ischaemic heart? Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA.. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res 2001;88:1020–1027. [DOI] [PubMed] [Google Scholar]
- 314. Brooks HL, Pollow DP, Hoyer PB.. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda )2016;31:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology 2012;153:3571–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Koebele SV, Bimonte-Nelson HA.. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas 2016;87:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Arnold AP. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Exp Neurol 2014;259:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E.. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 2005;146:1650–1673. [DOI] [PubMed] [Google Scholar]
- 319. Kerkhofs S, Denayer S, Haelens A, Claessens F.. Androgen receptor knockout and knock-in mouse models. J Mol Endocrinol 2009;42:11–17. [DOI] [PubMed] [Google Scholar]
- 320. Arias-Loza PA, Jazbutyte V, Pelzer T.. Genetic and pharmacologic strategies to determine the function of estrogen receptor alpha and estrogen receptor beta in cardiovascular system. Gend Med 2008;5:S34–45. [DOI] [PubMed] [Google Scholar]
- 321. Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C.. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A 2002;99:13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Lin J, Steenbergen C, Murphy E, Sun J.. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation 2009;120:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Pelzer T, Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, Couse JF, Korach KS, Neyses L, Ertl G.. Increased mortality and aggravation of heart failure in estrogen receptor-beta knockout mice after myocardial infarction. Circulation 2005;111:1492–1498. [DOI] [PubMed] [Google Scholar]
- 324. Toutain CE, Brouchet L, Raymond-Letron I, Vicendo P, Bergès H, Favre J, Fouque M-J, Krust A, Schmitt A-M, Chambon P, Gourdy P, Arnal JF, Lenfant F.. Prevention of skin flap necrosis by estradiol involves reperfusion of a protected vascular network. Circ Res 2009;104:245–254, 212p following 254. [DOI] [PubMed] [Google Scholar]
- 325. van Rooij E, Fielitz J, Sutherland LB, Thijssen VL, Crijns HJ, Dimaio MJ, Shelton J, De Windt LJ, Hill JA, Olson EN.. Myocyte enhancer factor 2 and class II histone deacetylases control a gender-specific pathway of cardioprotection mediated by the estrogen receptor. Circ Res 2010;106:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Arnold AP, Chen X.. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 2009;30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Paixao TS, Leao RV, de Souza Maciel Rocha Horvat N, Viana PC, Da Costa Leite C, de Azambuja RL, Damasceno RS, Ortega CD, de Menezes MR, Cerri GG.. Abdominal manifestations of fishbone perforation: a pictorial essay. Abdom Radiol 2017;42:1087–1095. [DOI] [PubMed] [Google Scholar]
- 328. Perrino C, Rockman HA.. Reversal of cardiac remodeling by modulation of adrenergic receptors: a new frontier in heart failure. Curr Opin Cardiol 2007;22:443–449. [DOI] [PubMed] [Google Scholar]
- 329. Yanes LL, Reckelhoff JF.. Postmenopausal hypertension. Am J Hypertens 2011;24:740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Chen YF, Redetzke RA, Sivertson RM, Coburn TS, Cypher LR, Gerdes AM.. Post-myocardial infarction left ventricular myocyte remodeling: are there gender differences in rats? Cardiovasc Pathol 2011;20:e189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Cavasin MA, Tao Z, Menon S, Yang XP.. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci 2004;75:2181–2192. [DOI] [PubMed] [Google Scholar]
- 332. Baranyai T, Giricz Z, Varga ZV, Koncsos G, Lukovic D, Makkos A, Sarkozy M, Pavo N, Jakab A, Czimbalmos C, Vago H, Ruzsa Z, Toth L, Garamvolgyi R, Merkely B, Schulz R, Gyongyosi M, Ferdinandy P.. In vivo MRI and ex vivo histological assessment of the cardioprotection induced by ischemic preconditioning, postconditioning and remote conditioning in a closed-chest porcine model of reperfused acute myocardial infarction: importance of microvasculature. J Transl Med 2017;15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG.. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Persson PB. Good publication practice in physiology 2017: current revisions of the recommendations for the conduct, reporting, editing and publication of scholarly work in medical journals. Acta Physiol 2017;221:283–284. [DOI] [PubMed] [Google Scholar]