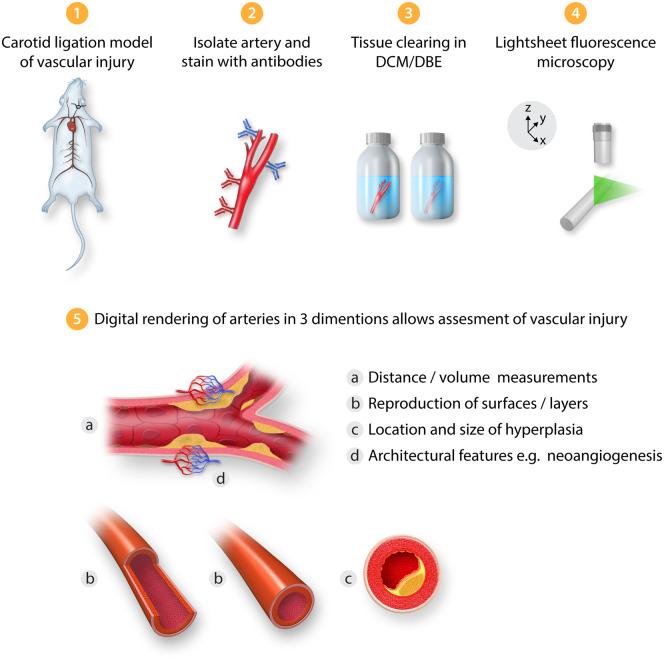
This editorial refers to ‘Light sheet fluorescence microscopy as a new method for unbiased three-dimensional analysis of vascular injury’ by N.E. Buglak et al., pp. 520–532.
Vascular pathologies create marked changes in the structure of the vessel wall, with pronounced alterations in the extracellular matrix and vascular cell organization together with infiltration of inflammatory cells. The result is a complex co-ordinated series of events that, depending on the nature of the condition, resulting in vascular repair or chronic injury and inflammation. In rodent models of vascular disease, ex vivo analysis of these changes is frequently performed using destructive or where possible, non-destructive methods. The most widely used method of assessing vascular morphology and spatial localization of cells and disease markers involves traditional histological methods, whereby tissue sections (typically 4–10 µm) are cut and stained either for morphological analysis/scoring or immunohistochemistry/immunofluorescence (IHC/IF) for identifying particular cells and markers of interest. While such tools are particularly useful for identifying the presence or absence of pathology, several factors converge to produce a final data set that is often only semiquantitative and prone to selection bias.1 These include the somewhat arbitrary number of tissue sections used for analysis, thus making like for like comparisons between animals problematic. This is an important concern in interpreting datasets since vascular pathologies consist of microenvironments with a non-homogenous distribution of cells and morphology. Since vascular remodelling in response to injury occurs in three dimensions, the above limitations can arise from the inherent two-dimensional (2D) approach of sectioning and imaging arterial cross-sections across a single axis (e.g. transverse or longitudinal). These limitations may be mitigated by three-dimensional (3D) imaging of the vessel in a non-destructive manner, thus allowing volumetric analysis, in addition to the more precise analysis of the geometry and topology throughout the site of vascular injury.
One such approach is light sheet fluorescent microscopy (LSFM), that allows 3D structural analysis of intact tissues following illumination with a sheet of laser light across multiple planes. The principles behind this technology are relatively simple,2 whereby the tissue of interest (typically several mms in diameter) is perfusion-fixed, mounted and stained with fluorescently labelled antibodies before undergoing tissue clearing (to induce transparency) and exposure to LSFM, followed by digital image analysis (Figure 1). Previously, LSFM has been performed to investigate the architecture and function of both zebrafish3 and mouse hearts,4–6 and only very recently to visualize atherosclerotic plaques and neointima formation in mice.7 Since optical sections can be acquired across multiple planes, both 2D and importantly 3D quantitative measurements of vessels can be extracted such as medial and intimal hyperplasia volumes.
Figure 1.
Workflow schematic summarizing the use of light sheet fluorescence microscopy for the assessment of vascular injury. DBE, dibenzyl ether; DCM, dichloromethane.
Buglak et al.8 provide further validation of the technique by quantitatively comparing LSFM with traditional 2D histological staining in two rodent models of vascular injury: the rat balloon angioplasty and the mouse carotid artery ligation. The authors utilize the inherent autofluorescence of the media layer alongside fluorescent antibodies specific for endothelial cells to define the architecture of the vessel. When comparing measurements of stenosis (intima:media ratio) between LSFM and haematoxylin and eosin stained histology slices, a greater degree of precision [lower coefficient of variation (CV%)] was observed for LSFM while mean values were similar. It was also noted that CV% values for stenosis in the rat model decreased as the number of optical sections used for analysis increased. Importantly, since the measurements are performed automatically along the entire section of the vessel, the results are less prone to selection bias than physical tissue sectioning. In addition, the 3D long spatial view of the injured vessel segment allowed the identification of regions of intimal hyperplasia and quantitative comparison with neighbouring regions where hyperplasia was absent. The ability to create both 2D slice images and 3D volumetric rendered images also allowed a more detailed and accurate view of vessel morphology. This is evidenced by the finding of neoangiogenesis in the adventitia of injured mouse carotid arteries, a feature also recently noted by Becher et al.7 Furthermore, by utilizing similar laser excitation wavelengths to classical confocal and multiphoton microscopy, the authors employed multi-channel LSFM to identify the presence of CD68+ macrophages within the same adventitial space. One could, therefore, imagine LSFM being used to identify the location of leukocyte aggregates in vascular pathology such as dendritic cell/T-cell interactions which have been shown to play an important role in experimental atherosclerosis.9–11 Moreover, as demonstrated by Buglak et al.,8 3D reconstruction can be very informative in delineating disease-specific changes in the endothelium. This has been expanded recently to assess endothelial erosion on atherosclerotic murine plaques and even small diameter human vessels.7
The advantages of LSFM in comparison to classical histology/microscopy can be summarized as follows: (i) greater precision; (ii) imaging in three dimensions and hence greater extraction of information; (iii) increased imaging depth; (iv) faster data capture and less photobleaching/phototoxicity compared with laser scanning fluorescence microscopy; (v) reduction in user bias; (vi) easier to standardize between experiments/laboratories than arbitrary physical sectioning. On the contrary, confocal and multi-photon microscopy display higher spatial resolution12–14 allowing more detailed examination of events at the cellular level but this limitation of LSFM may be mitigated to some degree by the ability to prepare physical sections and perform IHC/IF following LSFM.8 As noted by Buglak et al.,8 the process from vessel isolation to analysis is also longer (although less user intensive) than physical sectioning. It is also worth considering that LSFM cannot be applied in vivo and hence longitudinal imaging of evolving vascular pathologies cannot be performed. However, LSFM could act as a supplemental tool for post-analysis following in vivo imaging by other high-resolution modalities such as magnetic resonance imaging or ultrasound.
Increased experimental rigour and techniques that allow reproducibility are highly desired given the lack of reproducibility of many animal models.15 By extracting larger and more accurate datasets over comparable methods, LSFM may reduce the number of animals required per study and therefore be aligned with the principles of 3Rs (Replacement, Reduction, and Refinement), as well as improve the translation of new therapeutics for vascular disease.
Conflict of interest: none declared.
Funding
Our lab is supported by the British Heart Foundation [PG/12/81/29897 and PG/19/84/34771 to P.M., RE/13/5/30177 and FS/16/55/32731]; the Engineering and Physical Sciences Research Council (EPSRC) [EP/L014165/1 to P.M.]; and the Wellcome Trust [204820/Z/16/Z].
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP 3rd, Rosenfeld ME, Virmani R; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Basic Cardiovascular Sciences. Recommendation on design, execution, and reporting of animal atherosclerosis studies: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2017;37:e131–e157. [DOI] [PubMed] [Google Scholar]
- 2. Power RM, Huisken J.. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat Methods 2017;14:360–373. [DOI] [PubMed] [Google Scholar]
- 3. Packard RRS, Baek KI, Beebe T, Jen N, Ding Y, Shi F, Fei P, Kang BJ, Chen PH, Gau J, Chen M, Tang JY, Shih YH, Ding Y, Li D, Xu X, Hsiai TK.. Automated segmentation of light-sheet fluorescent imaging to characterize experimental doxorubicin-induced cardiac injury and repair. Sci Rep 2017;7:8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fei P, Lee J, Packard RR, Sereti KI, Xu H, Ma J, Ding Y, Kang H, Chen H, Sung K, Kulkarni R, Ardehali R, Kuo CC, Xu X, Ho CM, Hsiai TK.. Cardiac light-sheet fluorescent microscopy for multi-scale and rapid imaging of architecture and function. Sci Rep 2016;6:22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding Y, Lee J, Hsu JJ, Chang CC, Baek KI, Ranjbarvaziri S, Ardehali R, Packard RRS, Hsiai TK.. Light-sheet imaging to elucidate cardiovascular injury and repair. Curr Cardiol Rep 2018;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merz SF, Korste S, Bornemann L, Michel L, Stock P, Squire A, Soun C, Engel DR, Detzer J, Lorchner H, Hermann DM, Kamler M, Klode J, Hendgen-Cotta UB, Rassaf T, Gunzer M, Totzeck M.. Contemporaneous 3D characterization of acute and chronic myocardial I/R injury and response. Nat Commun 2019;10:2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becher T, Riascos-Bernal DF, Kramer DJ, Almonte VM, Chi J, Tong T, Oliveira-Paula GH, Koleilat I, Chen W, Cohen P, Sibinga N.. Three-dimensional imaging provides detailed atherosclerotic plaque morphology and reveals angiogenesis after carotid artery ligation. Circ Res 2020;126:619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buglak NE, Lucitti J, Ariel P, Maiocchi S, Miller FJ, Bahnson ESM.. Light sheet fluorescence microscopy as a new method for unbiased three-dimensional analysis of vascular injury. Cardiovasc Res 2021;117:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacRitchie N, Grassia G, Noonan J, Cole JE, Hughes CE, Schroeder J, Benson RA, Cochain C, Zernecke A, Guzik TJ, Garside P, Monaco C, Maffia P.. The aorta can act as a site of naive CD4+ T-cell priming. Cardiovasc Res 2020;116:306–316. [DOI] [PubMed] [Google Scholar]
- 10. Cole JE, Park I, Ahern DJ, Kassiteridi C, Danso Abeam D, Goddard ME, Green P, Maffia P, Monaco C.. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res 2018;114:1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, Burton FL, Ialenti A, Sabir SR, McInnes IB, Brewer JM, Garside P, Weber C, Lehmann T, Teupser D, Habenicht L, Beer M, Grabner R, Maffia P, Weih F, Habenicht AJ.. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin beta receptors. Immunity 2015;42:1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baek KI, Ding Y, Chang CC, Chang M, Sevag Packard RR, Hsu JJ, Fei P, Hsiai TK.. Advanced microscopy to elucidate cardiovascular injury and regeneration: 4D light-sheet imaging. Prog Biophys Mol Biol 2018;138:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maffia P, Zinselmeyer BH, Ialenti A, Kennedy S, Baker AH, McInnes IB, Brewer JM, Garside P.. Images in cardiovascular medicine. Multiphoton microscopy for 3-dimensional imaging of lymphocyte recruitment into apolipoprotein-E-deficient mouse carotid artery. Circulation 2007;115:e326–e328. [DOI] [PubMed] [Google Scholar]
- 14. Gibson VB, Benson RA, Bryson KJ, McInnes IB, Rush CM, Grassia G, Maffia P, Jenkinson EJ, White AJ, Anderson G, Brewer JM, Garside P.. A novel method to allow noninvasive, longitudinal imaging of the murine immune system in vivo. Blood 2012;119:2545–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Voelkl B, Vogt L, Sena ES, Wurbel H.. Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol 2018;16:e2003693. [DOI] [PMC free article] [PubMed] [Google Scholar]