Abstract
Background
Malaria is a severe global public health challenge that causes significant morbidity and mortality worldwide, particularly in sub-Saharan Africa. This study was designed to determine the prevalence, parasite density, and risk factors associated with malaria infection transmission among residents of two urban communities of Ibadan, southwestern Nigeria.
Materials and methods
A cross-sectional hospital-based study was carried out on 300 participants. Blood samples were obtained. Thick and thin blood films were prepared and viewed using the standard parasitological technique of microscopy. Moreover, data on sociodemographic and environmental variables were obtained using a pre-tested standard questionnaire.
Results
Of the 300 participants examined, a total of 165 (55.0%) were found positive for Plasmodium falciparum with a mean (S.D) parasite density of 1814.70 (1829.117) parasite/μL of blood. The prevalence and parasite density of malaria infection vary significantly (P < 0.05) with age group. Children <5 years old were more likely to have malaria infection and high parasite densities than adults (p < 0.05). Similarly, in relation to gender, males significantly (P < 0.05) had a higher prevalence (60.2%) and mean (S.D) parasite density of malaria infection [2157.73 (1659.570) parasite/μL of blood] compared to females. Additionally, those without formal education had the highest prevalence (73.0%) and mean (S.D) parasite density of infection [2626.96 (2442.195) parasite/μL of blood]. The bivariate logistic regression analysis shows that age group 6–10 (Crude Odds Ratio, COR 0.066, 95% CI: 0.007–0.635), presence of streams/rivers (COR 0.225, 95% CI: 0.103–0.492), distance from streams/rivers within ≤1 km (COR 0.283, 95% CI: 0.122–0.654) and travel to rural area (COR 4.689, 95% CI: 2.430–9.049) were the significant risk factors.
Conclusions
Malaria infection is prevalent in the study area and was greatly influenced by traveling activities from the rural areas to urban centers and vice versa. Multifaceted and integrated control strategy should be adopted. Health education on mosquito prevention and chemoprophylaxis before and during travel to rural areas are essential.
Keywords: Ibadan, Malaria infection, Plasmodium falciparum, Prevalence, Risk factors, Urban areas
Ibadan, Malaria infection, Plasmodium falciparum, Prevalence, Risk factors, Urban areas.
1. Introduction
Malaria is an important disease of public health problem caused by Plasmodium parasite belonging to the Apicomplexans [1]. It is spread when an infected female Anopheles mosquito feeds on human blood [2]. It is majorly infecting people in the world's tropical and subtropical countries, particularly in sub-Saharan Africa [3]. The four major malaria parasites causing disease in humans include Plasmodium (P). falciparum, P. vivax, P. malariae, and P. ovale, while P. knowlesi is a zoonotic species found in Southeast Asia [4]. P. falciparum is considered the most pathogenic of all, and it is most prevalent in Africa [5, 6]. Though malaria is a curable and preventable disease, malaria continues to have an overwhelming effect on people's health globally, particularly among pregnant women and children in rural and urban areas [3]. Globally, it is estimated that 3.2 billion people are at risk of contracting malaria annually [1]. Furthermore, about 219 million cases which led to approximately 435,000 deaths, were reported in 2017 [3]. In Nigeria, malaria is transmitted throughout the year, with more than 194 million people predisposed to contracting malaria infection. Thus, Nigeria reported the highest malaria prevalence among all of the world's countries in 2007 [7]. This has led to an increased level of poverty due to unexpected expenses on treatment, control, and prevention. Moreover, time expected to be at work and school is wasted on ill-health due to malaria infection thereby further aggravating poor conditions in rural and urban areas [8].
Major risk factors enhancing malaria transmission include demographic factors, environmental factors, and socioeconomic factors. Demographic factors include age and gender, while environmental factors include the presence or absence of bushes and forests which enhance mosquito breeding. Meanwhile, climatic factors include temperature, humidity, and rainfall that may support rapid growth and development of mosquito vectors. Lastly, socioeconomic factors such as education, occupation and income which can directly affect human exposure and treatment pattern. These factors have been well reported, particularly in rural and peri-urban communities in previous studies [9]. Other studies have compared malaria parasite prevalence in rural and urban areas. Govoetchan and colleagues observed that malaria prevalence was 5.5 times higher in rural Kandi than to urban Kandi in Northeastern Benin [10]. Despite better conditions in urban areas such as the availability of health facilities and low mosquito breeding sites, studies have shown that malaria parasites are prevalent in urban areas [11, 12]. Meanwhile, factors enhancing malaria infections in these urban areas are yet to be fully unraveled. Thus, to align with the target to reduce malaria parasite by 90% between 2016 and 2030 by World Health Organization's Global Technical Strategy for malaria, there is a need to understand and document adequate epidemiological data upon which malaria management and control could be based, particularly in urban settings. Thus, this study sought to investigate the prevalence and risk factors enhancing malaria parasites transmission in two urban areas in Ibadan, Oyo State, Nigeria.
2. Materials and Methods
2.1. Study area
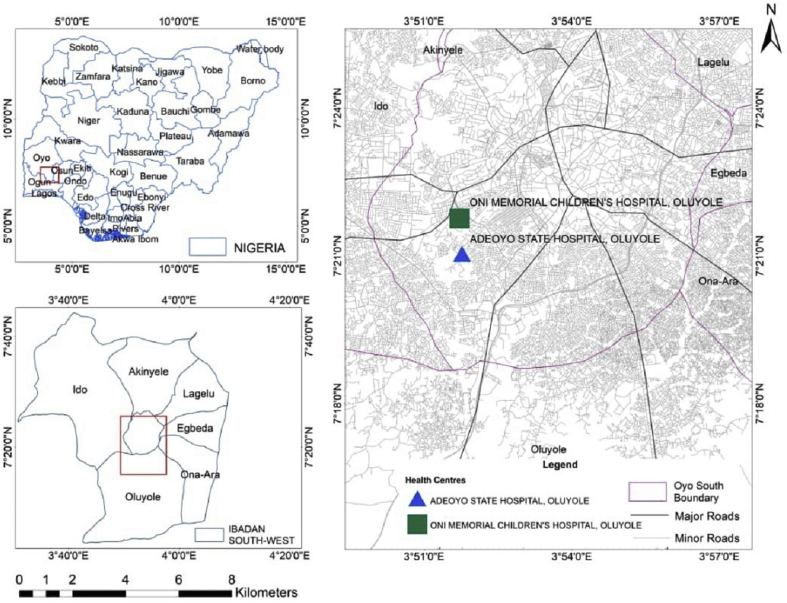
This study was carried out in Adeoyo State Hospital and Oni Memorial Children's Hospital. Both hospitals are in Ibadan South West Local Government Area of Oyo State, Nigeria. Ibadan South West Local Government Area is situated between Latitude 7°21′2.48″N and Longitude 3°51′55.84″E (Figure 1). The population is projected to be 397,700 in 2016 [13]. Generally, Ibadan city is the third most populous city in Nigeria, with over 6 million people. Thus, Ibadan is categorized as an urban area. An urban area has a high population density with well-designed infrastructure. It could be classified as cities, towns, and suburbs. The climatic condition is typical of tropical regions consisting of rainy and dry seasons that spans through April to October and November to March, respectively. The average annual rainfall is about 2100 mm, while the temperature is about 27 °C [14]. There are rivers running through the city of Ibadan, which includes Ogunpa, Kudeti, and Ona river, among others. The population consists of civil servants, traders, students, artisans, and farmers. While some of the residents live and settle in the urban City of Ibadan, they still visit rural areas or their home village for some activities. Many come from rural areas to Ibadan, thereby hosting different people from various parts of the country. Nonetheless, Ibadan residents are predominantly of the Yoruba ethnic group.
Figure 1.
Map of Adeoyo State Hospital and Oni Memorial Children's Hospital in Ibadan, Nigeria.
2.2. Study design
This is a hospital-based and randomized cross-sectional survey. The study was conducted from May to August 2019 in two different hospitals, which are Adeoyo State Hospital and Oni Memorial Children's Hospital, Ibadan. The quantitative method of data collection was employed, and data was collected with the aid of a pre-tested structured questionnaire from each patient visiting the health facilities. A face-to-face interview was conducted to collect their data such as age, sex, occupation, education, use of mosquito nets, and presence or absence of stream/river within ≤1 km of the participant's home. Other data, such as blood group and haemoglobin genotype were obtained from their medical records. Recruitment of participants was done in the outpatient section of the health facilities. Out of the 310 potential participants initially selected, only 300 eventually participated given a response rate of 96.7%.
2.3. Sample and sampling
The sample size was computed using a previous malaria parasite prevalence of 78% [15] at a confidence interval (CI) of 95% and a precision of 0.05 (or 5%) following the formula of Araoye, 2004 for calculating sample size [16]. This gives rise to a total of 300 subjects who were recruited for this study. Criteria for inclusion encompass feelings of headache, fever with temperature ≥38 °C, which was screened at the hospital by one of the health officials, completion of questionnaires, being a resident of Ibadan, blood samples submission, and willingness to provide written or oral informed consent. Those who declined to participate were excluded.
2.4. Blood collection and laboratory procedures
Samples of blood were obtained intravenously with the assistance of a trained Laboratory Technologist. A 3 mL blood was obtained from each participant. After collection, blood samples were transferred into an ethylenediaminetetraacetic acid (EDTA) tube to prevent blood coagulation. Next, thick and thin smears were made on well cleaned and sterilized slides. The thin smear was fixed in absolute ethanol. Subsequently, 3% Giemsa stain was added to the thick and thin smears for 30 min. The slides were later viewed under x100 objective lens of the light microscope to confirm the presence or absence of Plasmodium parasites and the species present. When about 200 microscopic fields have been observed and no parasite discovered, it is considered negative. The mean parasite density was classified according to the recommendations of Atroosh et al. [17]. Parasite density was recorded as the number of Parasite/μL of blood, assuming an average leucocyte count of 8,000/μL of blood for an average individual [1]. The formula used is stated as follows:
2.5. Statistical analysis
Data collected were analyzed using SPSS version 20.0 (IBM Corporation, NY, USA). The presence or absence of malaria parasite was computed and the differences in prevalence between age groups and sex were calculated using chi-square test at a 95% level of confidence. The malaria parasite density was computed using the student's t-test for the dichotomous variable while ANOVA was used to determine categorical variables. Malaria-associated risk factors were determined by Bivariate Logistic Model and Multivariate Logistic Regression Analysis. P-values of ≤0.05 were recognized as significant.
2.6. Ethical approval
The protocol for this study was approved by the Ondo State Ministry of Health (protocol number OSHREC/09/04/2018/046), the Ethical Review Committee, Federal University of Technology, Akure, Nigeria. Meanwhile, permission was sought from the hospital management board before study commencement. Written Informed consent was obtained from each adult subject. However, for children, accent was obtained from few while caregivers or guardians provided the informed consent for other younger ones.
3. Results
Of the total 300 individuals selected for this study, males represent 44.3%, while females represent 55.7%. The mean (S.D) age is 28.03 (17.52). Furthermore, the individuals examined in Adeoyo State hospital and Oni Memorial hospital were 196 (65.3%) and 104 (34.7%), respectively (Table 1). All malaria infections in this study area were observed to be caused by P. falciparum and most (56.4%) of the malaria infections were classified as low (<1000 parasites/μL of blood). Meanwhile, 43.6% were classified as moderate infections, which ranges between 1000 to ≤9999 parasites/μL of blood.
Table 1.
Prevalence and density of Plasmodium falciparum infection stratified by sociodemographic variables in Ibadan South West Local Government Area of Oyo State, Nigeria.
| Variables | Number of subjects | Number positive (%) | Geometric Mean (±S.D) Parasite Density (parasite/μL of blood) |
|---|---|---|---|
|
Age group (years) | |||
| ≤5 | 30 | 23 (76.7) | 2433.43 (2547.742) |
| 6–10 | 31 | 22 (71.0) | 2211.45 (1623.279) |
| 11–20 | 49 | 26 (53.1) | 1478.00 (1498.845) |
| 21–30 | 62 | 32 (51.6) | 2327.03 (2156.780) |
| 31–40 | 44 | 19 (43.2) | 1527.11 (1330.635) |
| 41–50 | 42 | 19 (45.2) | 1575.89 (1447.093) |
| >50 | 42 | 24 (57.1) | 956.42 (1262.708) |
| P- value | 0.03 | 0.38 | |
|
Gender | |||
| Male | 133 | 80 (60.2) | 2157.73 (1659.570) |
| Female | 167 | 85 (50.9) | 1491.85 (209.320) |
| P- value | 0.110 | 0.19 | |
|
Occupation | |||
| Trading | 74 | 37 (50.0) | 1407.78 (1349.310) |
| Civil servants | 63 | 29 (46.0) | 1592.24 (1622.752) |
| Farming | 20 | 10 (50.0) | 2280.80 (1675.544) |
| Students | 57 | 33 (57.9) | 2064.94 (1739.525) |
| Artisans | 42 | 24 (57.1) | 1748.17 (2126.970) |
| Others | 44 | 32 (24.2) | 2132.97 (2328.753) |
| P- value | 0.115 | 0.481 | |
|
Education | |||
| No Formal Education | 37 | 27 (73.0) | 2626.96 (2442.195) |
| Primary | 110 | 60 (54.5) | 1894.52 (1775.649) |
| Secondary | 109 | 58 (53.2) | 1419.16 (1414.614) |
| Tertiary | 44 | 20 (45.5) | 1625.75 (1857.351) |
| P- value | 0.086 | 0.38 | |
|
Hospital visited | |||
| Adeoyo State Hospital | 196 | 106 (54.1) | 2207.38 (2045.230) |
| Oni Memorial Hospital | 104 | 59 (56.7) | 1109.20 (1048.542) |
| P- value | 0.661 | <0.0001 | |
|
Travel to rural area/village last month | |||
| Yes | 125 | 93 (74.4) | 2367.51 (2098.600) |
| No | 175 | 72 (41.1) | 1100.65 (1050.686) |
| P- value | <0.0001 | <0.0001 | |
| Total | 300 | 165 (55.0) | 1814.70 (1829.117) |
In all, a total of 165 participants (55.0%) had malaria infection with mean (S.D) parasite density of 1814.70 (1829.117) parasite/μL of blood. The association between prevalence and density of P. falciparum and sociodemographic factors are presented in Table 1. Age group ≤5 years has the highest malaria prevalence of 76.7% while the lowest malaria prevalence of 43.2% is noted among the 31–40 year old participants. Generally, malaria infection in this study significantly (P < 0.05) decreases with increasing age and cumulates at 40 years old (Table 1). The highest mean (S.D) parasite density of infection was recorded among participants ≤5 years old [2433.43 (2547.742) parasite/μL of blood] while the least was recorded among participants >50 years old [ 956.42 (1262.708) parasite/μL of blood] (P > 0.05).
In relation to gender, males have a higher prevalence (60.2%) and mean (S.D) parasite density of infection [2157.73 (1659.570) parasite/μL of blood] compared to their female counterparts with malaria prevalence of 50.9% and mean (S.D) parasite density of 1491.85 (209.320) parasite/μL of blood. Infection was significant at P < 0.05.
Furthermore, those without formal education had an infection that almost doubled than those who attained tertiary education (P < 0.05). Similarly travelling to rural areas or villages highly contributed to malaria prevalence and parasite density. Those who travel to rural areas or villages had higher malaria prevalence of 74.4% and mean (S.D) parasite density of 2367.51 (2098.600) parasite/μL of blood compared to those who did not travel to rural areas or villages in the previous month (P < 0.05) (Table 1).
Table 2 details the prevalence and parasite density of P. falciparum infection stratified by haemoglobin genotype and blood group. Genotype HbAA has the highest malaria prevalence of 62.6% and mean (S.D) parasite density of 1937.33 (1627.828) parasite/μL of blood, while genotype HbSS has the least malaria prevalence of 12.5%. The result is statistically significant (P < 0.05). Also, while blood group O significantly (P < 0.05) has the highest prevalence of 68.8%, blood group AB has the least malaria prevalence (Table 2).
Table 2.
Prevalence and density of Plasmodium falciparum infection stratified by haemoglobin genotype and blood group in Ibadan South West Local Government Area of Oyo State, Nigeria.
| Variables | Number of subjects | Number positive (%) | Geometric Mean (±S.D) Parasite Density (parasite/μL of blood) |
|---|---|---|---|
|
Genotype | |||
| AA | 147 | 92 (62.6) | 1937.33 (1627.828) |
| AS | 133 | 67 (50.4) | 1713.76 (2113.703) |
| AC | 9 | 4 (44.4) | 1256.75 (1488.377) |
| SS | 8 | 1 (12.5) | - |
| SC | 3 | 1 (33.3) | - |
| P- value | 0.023 | 0.776 | |
|
Blood group | |||
| A | 97 | 54 (55.7) | 2118.30 (1862.318) |
| B | 11 | 5 (45.5) | 1240.20 (1244.996) |
| AB | 48 | 7 (14.6) | 1768.14 (3087.814) |
| O | 144 | 99 (68.8) | 1681.40 (1729.292) |
| P- value | <0.0001 | 0.477 | |
| Total | 300 | 165 (55.0) | 1814.70 (1829.117) |
Table 3 presents environmental variables and their association with malaria infection prevalence and density. The presence of streams and distance from streams are significantly (P < 0.05) related to malaria infection prevalence. Those who live nearby rivers/streams within the distance of ≤1 km are more likely to have malaria infection. Similarly, sleeping under the mosquito net could significantly (P < 0.05) reduce malaria infection (Table 4).
Table 3.
Prevalence and density of Plasmodium falciparum infection stratified by environmental variables in Ibadan South West Local Government Area of Oyo State, Nigeria.
| Variables | Number of subjects | Number positive (%) | Geometric Mean (±S.D) Parasite Density (parasite/μL of blood) |
|---|---|---|---|
|
Presence of Vegetation | |||
| Yes | 128 | 75 (58.6) | 1909.12 (1884.169) |
| No | 172 | 90 (52.3) | 1736.01 (1788.719) |
| P- value | 0.280 | 0.547 | |
|
Distance from vegetation | |||
| ≤1 km | 61 | 39 (63.9) | 1793.31 (1750.687) |
| >1 km | 67 | 36 (53.7) | 2034.58 (2036.458) |
| No Vegetation | 172 | 90 (52.3) | 1736.01 (1788.719) |
| P- value | 0.285 | 0.710 | |
|
Presence of open water/stream | |||
| Yes | 187 | 91 (48.7) | 1784.27 (1955.472) |
| No | 113 | 74 (65.5) | 1852.11 (1673.128) |
| P- value | 0.005 | 0.814 | |
|
Distance from open water/Stream | |||
| ≤1 km | 75 | 48 (64.0) | 1576.69 (1938.798) |
| >1 km | 112 | 43 (38.4) | 2016.00 (1970.623) |
| Others | 113 | 74 (65.5) | 1852.11 (1673.128) |
| P- value | <0.0001 | 0.508 | |
|
Environmental Sanitation | |||
| Yes | 188 | 97 (51.6) | 1783.96 (1760.957) |
| No | 112 | 68 (60.7) | 1858.54 (1934.656) |
| P- value | 0.125 | 0.797 | |
| Total | 300 | 165 (55.0) | 1814.70 (1829.117) |
Table 4.
Prevalence and density of Plasmodium falciparum infection stratified by ownership and use of mosquito nets in Ibadan South West Local Government Area of Oyo State, Nigeria.
| Variables | Number of subjects | Number positive (%) | Geometric Mean (±S.D) parasite density (parasite/μL of blood) |
|---|---|---|---|
|
Own mosquito net | |||
| Yes | 215 | 129 (60.0) | 1904.27 (1954.169) |
| No | 85 | 36 (42.4) | 1493.72 (1255.188) |
| P- value | 0.006 | 0.235 | |
|
Slept under mosquito net last night | |||
| Yes | 41 | 19 (46.3) | 1623.58 (1547.594) |
| No | 259 | 146 (56.37) | 1728.14 (1639.740) |
| P- value | 0.002 | 0.432 | |
|
Reason for not sleeping under bed net | |||
| Causes heat | 88 | 54 (61.4) | 2448.94 (2189.184) |
| Disfigure room | 41 | 21 (51.2) | 1129.05 (1256.053) |
| Not effective | 40 | 30 (75.0) | 1340.40 (1689.851) |
| Use alternative | 6 | 2 (33.3) | 2015.50 (1738.776) |
| No mosquito net | 125 | 58 (68.8) | 1710.84 (1557.363) |
| P- value | 0.001 | 0.018 | |
| Total | 300 | 165 (55.0) | 1814.70 (1829.117) |
Additionally, the Bivariate Logistic Regression Model analysis results show the risk factors associated with malaria infection (Table 5). Age group between 6-10 years old, group O blood type, presence of rivers or streams, distance from rivers or streams and travel to rural areas or villages the previous month were observed to be associated with malaria infection prevalence.
Table 5.
Bivariate Logistic Regression Rodel for crude odd ratio (CORs) of factors associated with malaria infection prevalence in Ibadan South West Local Government Area of Oyo State, Nigeria.
| Variables | Crude Odd Ratio, COR (95% CI) | P-value |
|---|---|---|
|
Age group | ||
| ≤5 | 0.13 (0.01–2.37) | 0.170 |
| 6–10 | 0.07 (0.01–0.64) | 0.099 |
| 11–20 | 0.49 (0.09–2.62) | 0.407 |
| 21–30 | 0.87 (0.27–2.79) | 0.818 |
| 31–40 | 1.12 (0.30–4.13) | 0.868 |
| 41–50 | 1.11 (0.33–3.78) | 0.866 |
| >50 | 1 | 0.143 |
|
Gender | ||
| Male | 1.81 (0.92–3.55) | 0.086 |
| Female | 1 | |
|
Occupation | ||
| Trading | 0.91 (0.18–4.71) | 0.908 |
| Civil servants | 1.49 (0.29–7.65) | 0.632 |
| Farming | 1.47 (0.19–10.95) | 0.707 |
| Students | 7.11 (0.74–68.17) | 0.089 |
| Artisans | 1.19 (0.19–7.32) | 0.853 |
| Others | 1 | 0.268 |
|
Education | ||
| No Formal Education | 2.16 (0.18–26.06) | 0.546 |
| Primary | 1.17 (0.33–4.10) | 0.811 |
| Secondary | 1.12 (0.38–3.33) | 0.834 |
| Tertiary | 1 | 0.947 |
|
Genotype | ||
| AA | 0.75 (0.04–14.89) | 0.849 |
| AS | 0.86 (0.05–16.46) | 0.923 |
| AC | 0.39 (0.01–11.35) | 0.585 |
| SS | 7.37 (0.15–382.63) | 0.322 |
| SC | 1 | 0.456 |
|
Blood group | ||
| A | 1.88 (0.96–3.69) | 0.066 |
| B | 3.09 (0.64–14.97) | 0.160 |
| AB | 15.40 (5.25–45.21) | 0.000 |
| O | 1 | 0.000 |
|
Presence of Vegetation | ||
| Yes | 1.26 (0.47–3.38) | 0.640 |
| No | 1 | |
|
Distance from vegetation | ||
| ≤1 km | 0.44 (0.15–1.25) | 0.122 |
| >1 km | 1 | |
|
Presence of stream | ||
| Yes | 0.23 (0.10–0.49) | 0.001 |
| No | 1 | |
|
Distance from Stream | ||
| ≤1 km | 0.28 (0.12–0.65) | 0.003 |
| >1 km | 1 | |
|
Environmental Sanitation | ||
| Yes | 0.61 (0.32–1.17) | 0.137 |
| No | 1 | |
|
Have mosquito net | ||
| Yes | 14.74 (3.12–69.63) | 0.001 |
| No | 1 | |
|
Slept under mosquito net last night | ||
| Yes | 0.35 (0.12–1.06) | 0.064 |
| No | 1 | |
|
Travel to rural area last month | ||
| Yes | 4.69 (2.43–9.05) | <0.0001 |
| No | 1 | |
Multivariate logistic regression analysis of the independent variables is detailed in Table 6. Presence of rivers or streams, distance of rivers or streams from home, travel to rural areas and having blood group A and AB are significant risk factors.
Table 6.
Multivariate Logistic Regression analysis of factors associated with malaria infection prevalence in Ibadan South West Local Government Area of Oyo State, Nigeria.
| Variables | Adjusted Odd Ratio (95% CI) | P-value |
|---|---|---|
|
Blood group | ||
| A | 0.44 (0.22–0.91) | 0.026 |
| B | 0.45 (0.09–2.39) | 0.351 |
| AB | 0.05 (0.02–0.16) | <0.0001 |
| O | 1 | |
|
Presence of stream | ||
| Yes | 0.20 (0.09–0.47) | <0.0001 |
| No | 1 | |
|
Distance from Stream | ||
| ≤1 km | 4.26 (1.79–10.18) | 0.001 |
| >1 km | 1 | |
|
Travel to rural area last month | ||
| Yes | 4.96 (2.49–9.87) | <0.0001 |
| No | 1 | |
4. Discussion
This study shows a strong evidence that malaria is still highly prevalent in many urban communities including Ibadan South West Local Government Area of Oyo State, Nigeria. The high prevalence of 55% with mean (S.D) parasite density of 1814.70 (1829.117) parasite/μL of blood is an indication that Ibadan is a high-risk area for malaria transmission, since it falls within the Nigerian malaria risk map estimates of less than 20% in certain zone to more than 70% in other zones [18]. This is supported by other studies reported from Ibadan [8, 19, 20]. Similarly, the current prevalence of 55% from urban area of Ibadan is significantly (P < 0.05) lower than those reported from many rural areas. This notion is supported by the reports of Wang et al. [21] and Baragatti et al. [22] who reported lower malaria prevalence of 24.1% and 26.1% in urban areas of the Republic of Benin and Burkina Faso respectively. In some rural settings, prevalence as high as 74% and 71.4% have been reported [9, 23]. Thus, while evidence abounds on malaria prevalence in urban areas, prevalence is generally significantly (P < 0.05) lower than in rural areas [11, 24]. This lower malaria prevalence in urban areas could result from better access to health facilities, well-designed houses that can protect against mosquito vectors, improved basic amenities, and reduced mosquito breeding sites [25].
Our findings on age-specific malaria prevalence patterns and mean parasite density shows that age group ≤5 years has the highest malaria infection. Similar findings have been reported in previous studies [26, 27]. The World Health Organization has emphasized the fact that children between the age of 5 years and below are the most vulnerable group of people, particularly in Africa [3]. This can be attributed to the gradual loss of maternal immunity, coupled with a low level of acquired immunity among children compared to adults. Thus, as age and exposure increase, malaria infection decreases except among the elderly and the immunocompromised. Thus, the focus should be on these children between the age five years and below, even in urban centers. Prevention against mosquito bites should be intensified through the provision of mosquito nets to such households with children. Additionally, the sex pattern of infection in this study shows that males have higher malaria prevalence and mean parasite density than their female colleagues. This is related to previous reports from other studies in malaria-endemic areas such as Ethiopia and Chile [28, 29]. This could be because males usually get involved in outdoor activities, stay late until night outside, have a lackadaisical attitude towards malaria prevention and farming, which inadvertently exposes them to high mosquito bites than females. Furthermore, those without formal education had an infection that almost doubled than those who attained tertiary education though no association was reported. This is in line with previous studies which show that people can be acquainted with the knowledge of malaria transmission, prevention and control irrespective of their educational status [30, 31]. This is, however, in contrast to the report of Adedotun et al., [32] Eteng et al., [33] and Dawaki et al., [34] who noted that the level of education significantly influences the knowledge, attitude, and practices of people which in turn can lead to reduced malaria infection. Similarly, those who travelled to rural areas or villages in this study area significantly (P < 0.05) had higher malaria prevalence and density. This is corroborated by studies conducted in malaria-endemic zones [21, 35]. Generally, people are at greater risks when they travel from urban areas to rural areas due to the high mosquito vectors present in rural areas. This is further aggravated by the low immunity of urban dwellers [36]. Chemoprophylactic drugs are recommended for use before and during such visits to rural areas to prevent malaria infection.
Furthermore, our findings show that genetic factors such as haemoglobin genotype and blood group also influenced malaria parasite distribution in this study area. Having blood group O is significantly associated with higher malaria infection. This is corroborated by Akhigbe et al. [37] and Afoakwah et al. [38], who recorded higher malaria prevalence for blood group O in Ghana. Another study suggested that the ABO blood group does not hinder the development of uncomplicated falciparum malaria but severe malaria [39]. This variation could be due to the different geographical regions [40]. In the same vein, haemoglobin AA is significantly associated with malaria infection in this study. This is consistent with previous findings [41, 42] but inconsistent with the report by Suchdev et al. [43] who found no significant association between genotype AA, AS and SS in Kenya.
Finally, the Bivariate Logistic Regression analysis shows that age group 6–10 years, the presence of streams, living near streams within ≤1 km and travel to rural areas were the major risk factors that often increase the odds of malaria infection in this study area. These findings are in agreement with previous studies in Nigeria and other malaria-endemic regions [22, 27, 35, 44, 45]. Therefore, the government should provide awareness to urban residents on mosquito breeding site identification and removal from time to time. Additionally, government should ensure the provision of additional mosquito bed net, vaccination, fumigation, indoor residual spray, and enforcement of law on frequent public sanitation. Encouraging travelers to use chemoprophylaxis before and during travel to malaria-endemic zone is highly imperative for management control of malaria in Nigeria.
5. Conclusion
Malaria is endemic in Ibadan city, and this is a glaring evidence indicating malaria as a public health challenge even in urban areas. Major risk factors influencing transmission include age, stream within ≤1 km from home, and, most importantly, travel to rural areas. Health education on mosquito prevention and use of chemoprophylaxis before and during travel to rural areas is important and recommended.
5.1. Limitation
Malaria prevalence in this study area may have been underestimated since the study was a hospital-based study and not a community-based study. Also, the sample size is not large enough, thus likelihood of sampling error. The study did not include vector surveillance to validate the malaria vector species in the study area. In spite of this, the study provides relevant information that can help in making pertinent policies in the study area.
Declarations
Author contribution statement
O. Awosolu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Z. Yahaya, M. T. Farah Haziqah and I. Simon-Oke: Analyzed and interpreted the data; Wrote the paper.
C. Fakunle: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.World Health Organization . World Health Organization; 2015. Microscopy for the Detection, Identification and Quantification of Malaria Parasites on Stained Thick and Thin Blood Films in Research Settings (Version 1.0): Procedure: Methods Manual; p. 32. [Google Scholar]
- 2.Besansky N.J., Hill C.A., Costantini C. No accounting for taste: host preference in malaria vectors. Trends Parasitol. 2004;20:249–251. doi: 10.1016/j.pt.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2018. World Malaria Report 2018.https://www.who.int/malaria/publications/world-malaria-report-2018/en/ [Google Scholar]
- 4.Abeysinghe Rabindra. Present. Malar. Policy Advis. Comm. Meet. 22–24 March 2017, Geneva. 2017. Outcomes from the evidence review group on Plasmodium knowlesi. [Google Scholar]
- 5.Gething P.W., Elyazar I.R., Moyes C.L., Smith D.L., Battle K.E., Guerra C.A. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001814. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . World Health Organization; Geneva: 2017. World Malaria Report 2017. [Google Scholar]
- 7.FMOH. National Malaria Control Programme. Abuja Nigeria. June 2008. Strategic Plan 2009–2013. A Road Map for Malaria Control in Nigeria.http://www.nationalplanningcycles.org/sites/default/fles/country_docs/Nigeria/nigeria_draft_malaria_strategic_plan_2009-2013 Draft 16. [Google Scholar]
- 8.Okonko I.O., Donbraye-Emmanuel O.O.B., Donbraye E., Abubakar M.J., Fowotade A., Fadeyi A., Babalola E.T., Ojezele M.O., Adeyi A.O. Malaria parasitaemia among patients in Ibadan, Southwestern Nigeria. J. Appl. Biosci. 2010;29:1774–1780. [Google Scholar]
- 9.Awosolu O.B., David M.C., Lawal A.O., Ikuesan F.A. Pattern of Malaria Parasitaemia and Genotype Among Residents of Orita Obele, Akure South Local Government Area of Ondo State, Nigeria. South Asian J. Parasitol. 2019;3(2):1–5. [Google Scholar]
- 10.Govoetchan R., Gnanguenon V., Azondékon R. Evidence for perennial malaria in rural and urban areas under the Sudanian climate of Kandi, Northeastern Benin. Parasites Vectors. 2014;7:79. doi: 10.1186/1756-3305-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anumudu C.I., Adepoju A., Adeniran M., Adeoye O., Kassim A., Oyewole I., Nwuba R.I. Malaria prevalence and treatment seeking behaviour of young Nigerian adults. Ann. Afr. Med. 2006;15:82–88. [Google Scholar]
- 12.Olusegun-Joseph T.S., Oboh M.A., Godwin O., Ovioma G.O., Fagbohun I.K., Okorafor U., Aina D.D. Differential prevalence of malaria infection in rural and urban out-patient clinics in Lagos state, Nigeria. Pan Afr. J. Life Sci. 2019;2:79–84. [Google Scholar]
- 13.Demographia . eleventh ed. 2015. Demographia World Urban Areas. Retrieved 2 December, 2020. [Google Scholar]
- 14.Ogolo, Adeyemi Variations and trends of some meteorological parameters at Ibadan, Nigeria. Pac. J. Sci. Technol. 2009;10(2):981–987. [Google Scholar]
- 15.Anumudu C.I., Okafor C.M.F., Ngwumohaike V., Afolabi K.A., Nwuba R.I., Nwagwu M. Epidemiological factors that promote the development of severe malaria anaemia in children in Ibadan. Afr. Health Sci. 2007;7(2):80–85. doi: 10.5555/afhs.2007.7.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araoye M.O. first ed. Nathadex Publishers; Ilorin: 2004. Sample Size Determination. Research Methodology with Statistics for Health and Social Sciences; pp. 115–120. [Google Scholar]
- 17.Atroosh W.M., Al-Mekhlaf H.M., Al-Jasari A., Sady H., Al-Delaimy A.K., Nasr N.A. Genetic variation of pfhrp2 in Plasmodium falciparum isolates from Yemen and the performance of HRP2-based malaria rapid diagnostic test. Parasites Vectors. 2015;8:388. doi: 10.1186/s13071-015-1008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onyiri N. Estimating malaria burden in Nigeria: a geostatistical modelling approach. Geospat Health. 2015;10:306. doi: 10.4081/gh.2015.306. [DOI] [PubMed] [Google Scholar]
- 19.Okonko I.O., Adejuwon O.A., Okerentugba P.O., Innocent-Adiele H.C. Circulating Plasmodium falciparum and HIV 1/2 as coinfections among blood donors in Ibadan, Southwestern Nigeria. Nat. Sci. 2012;10(9):42–47. [Google Scholar]
- 20.Bello F.A., Ayede A.I. Prevalence of malaria parasitaemia and the use of malaria prevention measures in pregnant women in Ibadan, Nigeria. Ann. Ib. Postgrad. Med. 2019;17(2):124–129. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S.J., Lengeler C., Smith T.A. Rapid urban malaria appraisal (RUMA) I: epidemiology of urban malaria in Ouagadougou. Malar. J. 2005;4:43. doi: 10.1186/1475-2875-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baragatti M., Fournet F., Henry M. Social and environmental malaria risk factors in urban areas of Ouagadougou, Burkina Faso. Malar. J. 2009;8:13. doi: 10.1186/1475-2875-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woldearegai T.G., Lalremruata A., Nguyen T.T., Gmeiner M., Veletzky L., TazemdaKuitsouc G.B. Characterization of Plasmodium infections among inhabitants of rural areas in Gabon. Sci. Rep. 2019;9:9784. doi: 10.1038/s41598-019-46194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pond B.S. Malaria indicator surveys demonstrate a markedly lower prevalence of malaria in large cities of sub-Saharan Africa. Malar. J. 2013;12:313. doi: 10.1186/1475-2875-12-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinkenberg E., McCall P.J., Wilson M.D., Amerasinghe F.P., Donnelly M.J. Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar. J. 2008;7:151. doi: 10.1186/1475-2875-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hailemariam M., Gebre S. Trend analysis of malaria prevalence in Arsi Negelle health center, Southern Ethiopia. J. Infect. Dis. 2015;7(1):1–6. [Google Scholar]
- 27.Zgambo M., Mbakaya B.C., Kalembo F.W. Prevalence and factors associated with malaria parasitaemia in children under the age of five years in Malawi: a comparison study of the 2012 and 2014 Malaria Indicator Surveys (MISs) PloS One. 2017;12(4) doi: 10.1371/journal.pone.0175537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebretsadik D., Feleke D.G., Fiseha M. Eight-year trend analysis of malaria prevalence in Kombolcha, South Wollo, north-central Ethiopia: a retrospective study. Parasites Vectors. 2018;11(1):55. doi: 10.1186/s13071-018-2654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escobar D.F., Lucchi N.W., Abdallah R. Molecular and epidemiological characterization of imported malaria cases in Chile. Malar. J. 2020;19:289. doi: 10.1186/s12936-020-03353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muchena G., Dube B., Chikodzore R., Pasipamire J., Murugasampillay S., Mberikunashe J. A review of progress towards sub-national malaria elimination in Matabeleland South Province, Zimbabwe (2011–2015): a qualitative study. Malar. J. 2018;17(1):146. doi: 10.1186/s12936-018-2299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dube B., Mberikunashe J., Dhliwayo P., Tangwena A., Shambira G., Chimusoro A., Gambinga B. How far is the journey before malaria is knocked out malaria in Zimbabwe: results of the malaria indicator survey 2016. Malar. J. 2019;18(1):171. doi: 10.1186/s12936-019-2801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adedotun A.A., Morenikeji O.A., Odaibo A.B. Knowledge, attitudes and practices about malaria in an urban community in south-western Nigeria. J. Vector Borne Dis. 2010;47:155–159. [PubMed] [Google Scholar]
- 33.Eteng M., Mitchell S., Garba L., Ana O., Liman M., Cockcroft A. Socioeconomic determinants of ownership and use of treated bed nets in Nigeria: results from a cross-sectional study in Cross River and Bauchi States in 2011. Malar. J. 2014;13:316. doi: 10.1186/1475-2875-13-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawaki S., Al-Mekhlafi H.M., Ithoi I., Ibrahim J., Atroosh W.M., Abdulsalam A.M. Is Nigeria winning the battle against malaria? Prevalence, risk factors and KAP assessment among Hausa communities in Kano state. Malar. J. 2016;15:351. doi: 10.1186/s12936-016-1394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siri J.G., Wilson M.L., Murray S., Rosen D.H., Vulule J.M., Slutsker L., Lindblade K.A. Significance of travel to rural areas as a risk factor for malarial anemia in an urban setting. Am. J. Trop. Med. Hyg. 2010;82:391–397. doi: 10.4269/ajtmh.2010.09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carme B. Reducing the risk of malaria Acquisition by urban dwellers of sub-Saharan Africa during travel in malaria-endemic areas. J. Infect. Dis. July 1994;170(1):257–258. doi: 10.1093/infdis/170.1.257. [DOI] [PubMed] [Google Scholar]
- 37.Akhigbe R.E., Ige S.F., Adegunlola G.J., Adewumi M.O., Azeez O.M. Malaria, haemoglobin genotypes and ABO blood groups in Ogbomoso, Nigeria. Int. J. Trop. Med. 2011;6(4):73–76. [Google Scholar]
- 38.Afoakwah R., Aubyn E., Prah J., Nwaefuna E.K., Boampong J.N. Relative susceptibilities of ABO blood groups to Plasmodium falciparum malaria in Ghana. Adv Hematol. 2016:5368793. doi: 10.1155/2016/5368793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degarege A., Gebrezgi M.T., Beck-Sague C.M., Wahlgren M., de Mattos L.C., Madhivanan P. Effect of ABO blood group on asymptomatic, uncomplicated and placental Plasmodium falciparum infection: systematic review and meta-analysis. BMC Infect. Dis. 2019;19(1):86. doi: 10.1186/s12879-019-3730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manjurano A., Sepulveda N., Nadjm B., Mtove G., Wangai H., Maxwell C., Olomi R., Reyburn H., Riley E.M., Drakeley C.J. African glucose-6-phosphate dehydrogenase alleles associated with protection from severe malaria in heterozygous females in Tanzania. PLoS Genet. 2015;11(2) doi: 10.1371/journal.pgen.1004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komba A.N., Makani J., Sadarangani M., Ajala-Agbo T., Berkley J.A., Newton C.R., Marsh K., Williams T.N. Malaria as a cause of morbidity and mortality in children with homozygous sickle cell disease on the coast of Kenya. Clin. Infect. Dis. 2009;49(2):216–222. doi: 10.1086/599834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAuley C.F., Webb C., Makani J., Macharia A., Uyoga S., Opi D.H., Ndila C., Ngatia A., Scott J.A., Marsh K. High mortality from Plasmodium falciparum malaria in children living with sickle cell anemia on the coast of Kenya. Blood. 2010;116(10):1663–1668. doi: 10.1182/blood-2010-01-265249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suchdev P.S., Ruth L.J., Earley M., Macharia A., Williams T.N. The burden and consequences of inherited blood disorders among young children in western Kenya. Matern. Child Nutr. 2014;10(1):135–144. doi: 10.1111/j.1740-8709.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ceesay S.J., Bojang K.A., Nwakanma D., Conway D.J., Koita O.A., Doumbia S.O., Ndiaye D., Coulibaly T.F., Diakité M., Traoré S.F., Coulibaly M., Ndiaye J.L., Sarr O., Gaye O., Konaté L., Sy N., Faye B., Faye O., Sogoba N., Jawara M., Dao A., Poudiougou B., Diawara S., Okebe J., Sangaré L., Abubakar I., Sissako A., Diarra A., Kéita M., Kandeh B., Long C.A., Fairhurst R.M., Duraisingh M., Perry R., Muskavitch M.A., Valim C., Volkman S.K., Wirth D.F., Krogstad D.J. Sahel, savana, riverine and urban malaria in West Africa: similar control policies with different outcomes. Acta Trop. 2012;121:166–174. doi: 10.1016/j.actatropica.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awosolu O., Adesina F., Afolabi O., Ogunsanya D. Malaria parasite distribution and knowledge among students of Federal University of Technology, Akure, Nigeria. Anim. Res. Int. 2020;17(3):3903–3910. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.