Abstract
Background and purpose
Functional connectivity studies revealed alterations within thalamic, salience, and default mode networks in restless legs syndrome patients.
Methods
Eighty‐two patients with restless legs syndrome (untreated, n = 30; on dopaminergic medication, n = 42; on alpha‐2‐delta ligands as mono‐ or polytherapy combined with dopaminergic medication, n = 10), and 82 individually age‐ and gender‐matched healthy controls were studied with resting‐state functional magnetic resonance imaging. Connectivity of 12 resting‐state networks was investigated with independent component analysis, and network topology was studied with graph methods among 410 brain regions.
Results
Patients with restless legs syndrome showed significantly higher connectivity within salience (p = 0.029), executive (p = 0.001), and cerebellar (p = 0.041) networks, as well as significantly lower (p < 0.05) cerebello‐frontal communication compared to controls. In addition, they had a significantly higher (p < 0.05) clustering coefficient and local efficiency in motor and frontal regions; lower clustering coefficient in the central sulcus; and lower local efficiency in the central opercular cortex, temporal, parieto‐occipital, cuneus, and occipital regions compared to controls. Untreated patients had significantly lower (p < 0.05) cerebello‐parietal communication compared to healthy controls. Connectivity between the thalamus and frontal regions was significantly increased (p < 0.05) in patients on dopaminergic medication compared to untreated patients and controls.
Conclusions
Networks with higher intranetwork connectivity (i.e., salience, executive, cerebellar) and lower cerebello‐frontal connectivity in the restless legs syndrome patients, as well as lower cerebello‐parietal connectivity in untreated patients, correspond to regions associated with attention, response inhibitory control, and processing of sensory information. Intact cerebello‐parietal communication and increased thalamic connectivity to the prefrontal regions in patients on dopaminergic medication suggests a treatment effect on thalamus.
Keywords: brain connectivity, functional magnetic resonance imaging, restless legs syndrome, sleep wake disorders
This resting‐state functional magnetic resonance imaging study applied graph methods, which revealed dopaminergic‐related alterations in a broad array of brain networks in restless legs syndrome (RLS) patients. In particular, lower cerebello‐parietal connectivity was present in untreated RLS, but not in medicated patients. RLS patients treated solely with dopaminergic medication presented with higher thalamo‐frontal connectivity compared to untreated patients.
Introduction
Restless legs syndrome (RLS), also known as Willis‐Ekbom disease, is a sensorimotor disorder characterized by unpleasant sensations, mainly in the lower limbs, accompanied by an urge to move [1]. Symptoms worsen at rest and in the evening, and movement relieves them. Pathophysiological concepts of underlying mechanisms come from genome‐wide studies that report risk alleles, which is associated with synapse formation and neural network plasticity [2]. Imaging, cerebrospinal fluid, and autopsy studies demonstrated dysregulation of dopamine turnover and its circadian dynamics, as well as alterations of brain iron metabolism [3, 4].
Task‐based functional magnetic resonance imaging (fMRI) detected altered brain function in the cerebellum and thalamus [5, 6, 7], primary motor and somatosensory cortex [6, 7, 8], as well as prefrontal cortex [6, 7] in RLS patients. In contrast to task‐based fMRI, resting‐state fMRI (rs‐fMRI) enables detection of well‐established brain networks associated with functional domains [9]. RLS studies that applied seed‐based methods [10] detected changes in attentional (ventral, dorsal, salience), sensory (thalamic), and cognitive (default mode network [DMN]) networks [11, 12]. Previous RLS studies demonstrated altered connectivity in the thalamus and dopaminergic pathways [13], diurnal connectivity variations [14] and associations of connectivity changes with the emergence of RLS symptoms [15], as well as alterations of thalamic connectivity by dopamine agonist treatment [16]. In graph theoretical modeling, the entire brain is subdivided into nodes that are connected by edges. This provides a way to reflect changes in the topological architecture and localize highly connected regions, known as hubs [17]. To date, the only graph study in RLS found connectivity decreases in sensorimotor and visual networks, and increases in the affective cognitive and cerebellar–thalamic networks, as well as degree centrality (DC) alterations in DMN regions [18].
The aims of our study were to investigate changes in functional connectivity and topology in a large cohort of RLS patients compared to healthy controls (HCs) with independent component analysis (ICA) and graph methods, and to compare the brain connectivity of patients on dopaminergic medication with untreated patients.
Methods
Subjects
A total of 82 RLS patients were recruited at the Sleep Disorders Clinic, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria. Inclusion criteria were age between 18 and 75 years and a diagnosis of RLS according to the current International Restless Legs Syndrome Study Group criteria [19]. Exclusion criteria were secondary RLS, other neurological diseases identified through clinical examination and conventional magnetic resonance imaging (MRI), white matter lesions of Fazekas score >1 [20], iron supplementation during the previous 6 months, or current opioid medication. Eighty‐two HCs individually matched for gender and age (±5 years) were included in this case‐control study. Inclusion criteria for controls were age between 18 and 75 years and the absence of any relevant medical conditions. Exclusion criteria were a history of neurological or psychiatric diseases, structural MRI changes, white matter lesions of Fazekas score >1, iron supplementation during the previous 6 months, any symptoms of RLS (evaluated during a clinical interview), or a positive family history for RLS to limit possible inclusion of carriers of genetic risk factors.
Procedures
Restless legs syndrome patients underwent a structured interview performed by a board‐certified expert in sleep medicine, which included information about family history of RLS, age at onset, and medications. Levodopa equivalent daily doses (LEDD) were calculated. Validated severity scales were completed: International RLS Scale (IRLS) [21], clinical global impression (CGI) [22], and Restless Legs Syndrome‐6 Scale (RLS‐6) [23]. Anatomical and rs‐fMRI sequences were acquired with a 3T whole‐body MRI scanner (Magnetom Verio; Siemens) at daytime between 8 a.m. and 4 p.m. (Appendix S1). The study procedures were performed according to the Declaration of Helsinki and approved by the ethics committee of the Medical University of Innsbruck. Prior to inclusion to the study, all subjects signed an informed consent form.
Statistical analysis
Clinical parameters
Based on current medication, the RLS cohort was divided into therapeutic subgroups: untreated patients (n = 30), patients on dopaminergic treatment (n = 42), and patients on alpha‐2‐delta ligands as mono‐ or polytherapy in combination with dopaminergic medication (n = 10). Distributions of demographic data are presented as frequencies (percentage), means ± standard deviations, or median (interquartile range), accordingly. Gaussian distribution was confirmed by the Kolmogorov‐Smirnov test. Group differences of normally distributed data were analyzed by parametric tests (t test). Non‐Gaussian distributed variables were assessed by Mann‐Whitney U and Wilcoxon rank sum test (α < 0.05) using MATLAB (MathWorks) [24].
Independent component analysis
The preprocessed fMRI dataset (Appendix S1) was concatenated across the entire cohort and decomposed into 30 automatic components. Components with neural origin were identified as rs‐networks by visual inspection and comparison to earlier studies [9]. Dual regression [25] was used to generate subject‐specific spatial maps and associated time series (Appendix S1). After this, group differences in intranetwork connectivity (α < 0.05, threshold‐free cluster enhancement) were tested with the randomized permutation‐testing tool (5000 permutations).
Graph connectivity
Graph theory methods were applied on preprocessed data (Appendix S1) to investigate connectivity among regions. The Atlas of Intrinsic Connectivity of Homotopic Areas (AICHA), which consists of 384 brain regions, was selected for parcellation as it takes into account the intrinsic connectivity of homotopic areas [26]. Twenty‐six cerebellar regions were added from the Automated Anatomical Labeling Atlas [27], as these motor function related regions are not provided in the AICHA. To obtain graph matrices of 410 × 410, each parcellated region’s functional time series was averaged, thus accounting for differences in voxel sizes, and then Pearson correlated with the other regions. Then, the obtained correlation matrices were Fisher transformed according to z = ln((1 + r)/(1‐r))/2 with MATLAB [24]. Network‐based statistics [28] were used to perform the t test (α < 0.05, false discovery rate [FDR] corrected, 100,000 permutations) on every edge in the connectivity matrices between groups.
Correlations (α < 0.05) of connectivity differences to clinical scores (IRLS, CGI, and RLS‐6) were evaluated in patients.
Graph measures
To study graph measures, each individual connectivity matrix was thresholded over a wide range of densities (N = 41) between 0.1 and 0.5 with increments of 0.01, where a density of 0.1 preserves the top 10% of the edges. This was done to avoid that a selection of one specific threshold might affect the results. Graph measures were calculated with the Brain Connectivity Toolbox [17] on each node (N = 410) of each thresholded matrix (N = 41 per subject). The results were averaged to create individual graph values for each node.
Measures of centrality were studied. DC provides information on the importance of a node for functional performance, whereas betweenness centrality (BC) measures how many times a node acts as a bridge along the shortest paths between two other nodes [29]. In addition, the clustering coefficient (CC), and global and local efficiency were studied. The CC tells the tendency for dense interconnections of a node with its topological neighbors that form segregated and functionally specialized clusters [30]. Global and local efficiency measure the ability of a network to transmit information at the global and local level, and inform about the fault tolerance of a network [31].
To investigate hub nodes, all obtained measures (DC, BC, CC, and local efficiency) were thresholded (average + standard deviation) in RLS patients and HCs separately. In addition, group differences were estimated by two‐sample t test (α < 0.05, FDR corrected). Correlations of significant group differences to clinical scores of patients were studied (Spearman's, α < 0.05). Graph results were visualized with BrainNet Viewer (NeuroImaging Tools and Resources Collaboratory) [32].
Results
Clinical characteristics
Demographic and clinical data of study participants are presented in Table 1. There were no significant differences in age, sex, or disease duration between subgroups. IRLS scores were significantly higher (p = 0.048) (i.e., worse symptom severity) in patients on alpha‐2‐delta ligands as mono‐ or polytherapy with dopaminergic medication compared to patients on dopaminergic medication alone. Untreated patients presented with significantly higher (p = 0.025) RLS‐6 scores compared to patients on dopaminergic medication. No significant differences in symptom severity were evident in CGI scores between subgroups.
Table 1.
Demographics and clinical information
| Healthy controls | RLS patients | Patients on dopaminergic medication | Untreated patients | Patients on alpha‐2‐delta ligands a | p value b | p value c | |
|---|---|---|---|---|---|---|---|
| Sample size, n | 82 | 82 | 42 | 30 | 10 | — | — |
|
Age, years, mean ± SD |
50.2 ± 10.0 | 51.9 ± 10.8 | 53.1 ± 10.4 | 49.6 ± 11.4 | 53.9 ± 10.3 | 0.320 | 0.736 |
|
Gender, females, n (%) |
43 (52) | 43 (52) | 22 (52) | 14 (47) | 7 (70) | — | — |
|
Disease duration, years, mean ± SD |
— | 16.3 ± 12.9 | 15.2 ± 10.4 | 13.2 ± 10.6 | 21.3 ± 10.9 | 0.447 | 0.104 |
| LEDD medication, median (IQR) | — | 8.8 (0–36) | 30 (18–70) | — | 4.4 (0–64) | — | 0.070 |
| CGI, median (IQR) | — | 3 (3–4) | 3 (3–4) | 3 (3–4) | 4 (2.75–5) | 0.468 | 0.705 |
| IRLS, median (IQR) | — | 14.5 (22–7) | 12 (0–18.75) | 15.50 (9–24.25) | 20.50 (12–31) | 0.127 | 0.048* |
| RLS‐6, mean ± SD | — | 16.0 ± 10.7 | 13.1 ± 9.9 | 17.9 ± 8.7 | 22.7 ± 15.2 | 0.025* | 0.052 |
| Family history | — | 28 | 17 | 9 | 2 | — | — |
| Age of onset | — | 36.5 ± 14.1 | 37.7 ± 14.1 | 36.4 ± 14.5 | 31.2 ± 13.7 | 0.709 | 0.216 |
CGI, clinical global impression (0–7); IQR, interquartile range; IRLS, International Restless Legs Scale (0–40); LEDD, levodopa equivalent daily doses; RLS, restless legs syndrome; RLS‐6, Restless Legs Syndrome‐6 Scale (0–60); SD, standard deviation.
Patients on alpha‐2‐delta ligands as mono‐ or polytherapy with dopaminergic medication.
The p values shown are for patients on dopaminergic medication against untreated patients.
The p values shown are for patients on dopaminergic medication against patients on alpha‐2‐delta ligands as mono‐ or polytherapy with dopaminergic medication.
Significant differences.
Resting‐state networks
Twelve identified components that corresponded to known rs‐networks [9] were DMN, somatomotor network (SMN), medial and lateral visual, dorsal attention, left and right executive, salience, auditory, cerebellar, frontal, and working memory (Figure S1). RLS patients had significantly higher (p < 0.05) connectivity within three of these networks compared to HCs (Fig. 1, Table S1). The findings included two clusters in the salience network with the main cluster in left frontal pole (p = 0.029), six clusters in the left executive network with the main cluster in the left frontal pole (p = 0.001), and a cluster in the cerebellar network localized to the left cerebellum (p = 0.041).
Figure 1.
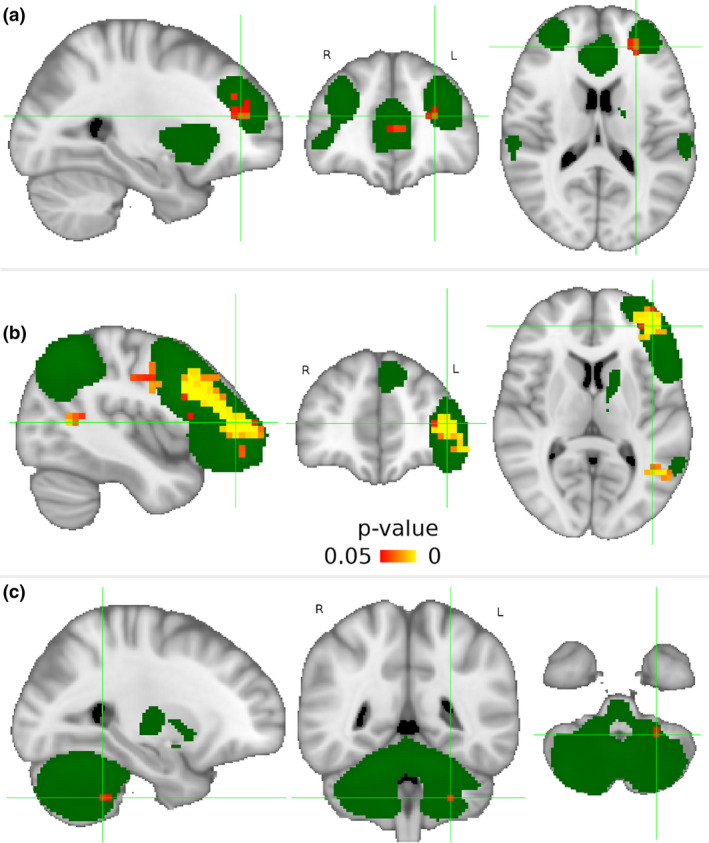
Three resting‐state networks with significantly higher (p < 0.05) intranetwork connectivity in restless legs syndrome (RLS) compared to healthy controls. Independent component analysis with dual regression (5000 permutations, threshold‐free cluster enhancement) found significantly higher (in orange) intranetwork connectivity in RLS patients compared to healthy controls in the following three networks (maps in green): (A) salience network with the main cluster in the left frontal pole (x = −26, y = 42, z = 16), (B) left executive network with the main cluster in the left frontal pole (x = −42, y = 46, z = 8), and (C) cerebellar network with the cluster in the left cerebellum (x = −26, y = −46, z = −44). Sagittal, axial, and coronal views are shown in radiological orientation in Montreal Neurological Institute (MNI) coordinates. The maps were thresholded with arbitrary threshold of Z = 4 for visualization purposes. L, left; R, right. [Colour figure can be viewed at wileyonlinelibrary.com]
The effects of disease and medications to functional connectivity [33] were studied in untreated and medicated patients compared to matched controls. There were no significant differences in intranetwork connectivity in subgroups compared to HCs. However, untreated patients had significantly higher (p = 0.003) connectivity in the working memory network with a cluster in the left cerebellum compared to patients on dopaminergic medication (Table S2).
Graph connectivity
Graph analysis revealed significantly lower (p < 0.05, corrected) cerebello‐frontal connectivity (i.e., between vermis 7, and the left and right inferior frontal sulcus 2) in RLS patients compared to HCs (Fig. 2A). Subgroup analysis showed significantly lower (p < 0.05, corrected) cerebello‐parietal connectivity (i.e., between vermis 7 and the right superior parietal gyrus 4 and left intraparietal sulcus 3, and between vermis 8 and left intraparietal sulcus 3) in untreated patients compared to HCs (Fig. 2B). Patients on dopaminergic medication had significantly higher (p < 0.05, corrected) connectivity between the right subcallosal gyrus 1 and left thalamus 1 (i.e., anterior nuclei) compared to HCs (Fig. 2C), and between the right orbital sulcus 1 and right thalamus 1 compared to untreated patients (Fig. 2D). Patients on alpha‐2‐delta ligands (as mono‐ or polytherapy with dopaminergic medication) had significantly higher (p < 0.05, corrected) connectivity between the right frontal inferior orbital gyrus 2 and left superior parietal gyrus 1 compared to HCs (Fig. 2E). Severity scores (IRLS, CGI, and RLS‐6), disease duration, and LEDD values did not correlate with connectivity alterations.
Figure 2.
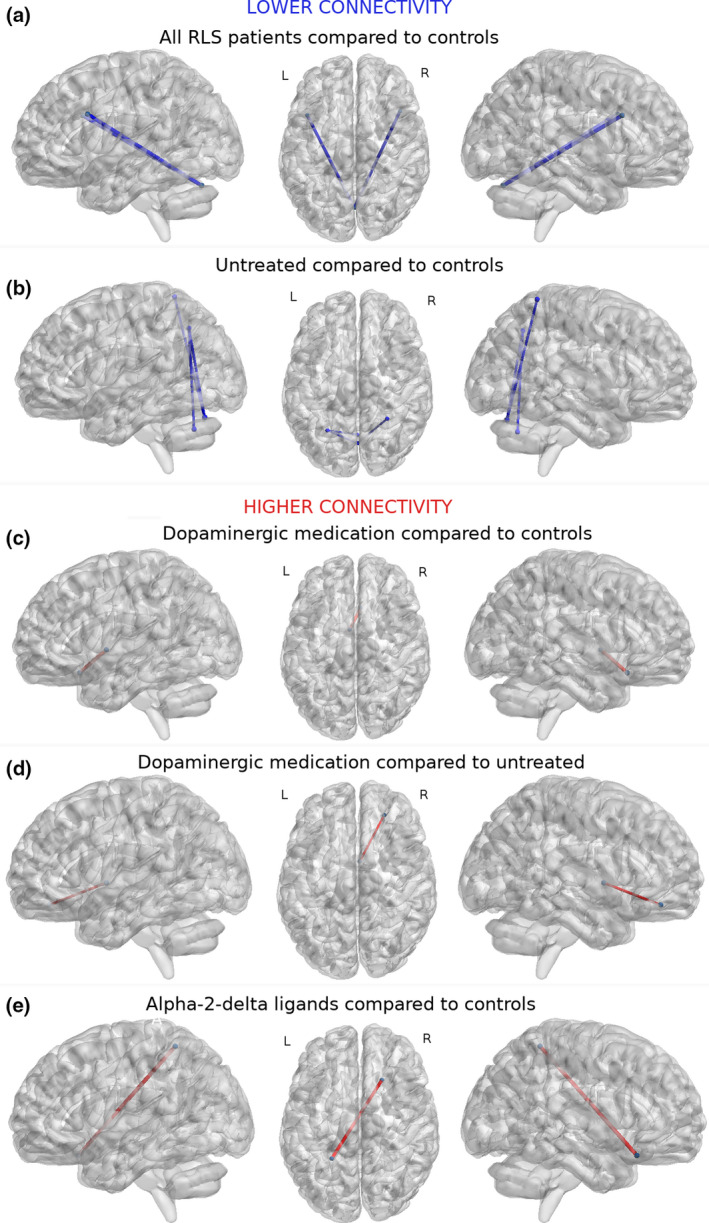
Functional connections with significant differences (p < 0.05, 100,000 permutations, t test, false discovery rate–corrected) in restless legs syndrome (RLS) patients. Lower connectivity (in blue) was found in (A) RLS patients compared to healthy controls between vermis 7 (x = 1, y = −72, z = −25) and left (x = −43, y = 15, z = 29) (Tstat: 4.41) and right (x = 44, y = 19, z = 28) (Tstat: 4.72) inferior frontal sulcus 2, and (B) in untreated patients compared to healthy controls between vermis 7 and right superior parietal gyrus 4 (x = 29, y = −49, z = 67) (Tstat: 5.18) and left intraparietal sulcus 3 (x = −27, y = −60, z = 43) (Tstat: 4.71), and between vermis 8 (x = 1, y = −64, z = −34) and left intraparietal sulcus 3 (Tstat: 5.09). Higher (in red) connectivity was found in patients on dopaminergic medication (C) between right subcallosal gyrus 1 (x = 6, y = 21, z = −16) and left thalamus 1 (x = −4, y = 0, z = 1) (Tstat: 4.71) compared to healthy controls, and (D) between right orbital sulcus 1 (x = 25, y = 41, z = −15) and right thalamus 1 (x = 4, y = 0, z = 1) (Tstat: 3.81) compared to untreated patients. (E) Patients on alpha‐2‐delta ligands as mono‐ or polytherapy with dopaminergic medication had higher connectivity between right frontal inferior orbital gyrus 2 (x = 21, y = 22, z = −20) and left superior parietal gyrus 1 (x = −24, y = −47, z = 59) (Tstat: 4.31) compared to healthy controls. Nodes are presented in their centroid stereotaxic coordinates (Montreal Neurological Institute) among 410 parcellated atlas regions from An Atlas of Intrinsic Connectivity of Homotopic Areas and Automated Anatomical Labeling. L, left; R, right. [Colour figure can be viewed at wileyonlinelibrary.com]
Hub nodes and nodal differences
There were no significant group differences in nodal measures of DC and BC. Both RLS patients and HCs revealed their highest DC and BC values (i.e., hubs) in precentral, occipital, insula, temporal, cingulum, paracentral, precuneus, parieto‐occipital, and calcarine regions (Figures S2 and S3). In addition, DC was high in the Rolando, postcentral, parietal, intraparietal, Rolandic operculum, and lingual regions; and BC in the frontal, hippocampal, parahippocampal, caudate, thalamic, and cerebellar regions in both groups.
Both groups revealed their highest CC and local efficiency in the precentral, Rolando, postcentral, supramarginal, cingulate, parietal, parieto‐occipital, occipital, cuneus, lingual, fusiform, and paracentral regions (Figures S4 and S5). In addition, high CC was found in the intraparietal and calcarine regions in both groups and in frontal regions in patients. In addition, high local efficiency was found in the intraoccipital, Rolandic operculum, and temporal regions in both groups.
Clustering coefficient (Fig. 3A, Table S3) was significantly higher (p < 0.05, FDR) in motor (i.e., left supplementary motor regions 2 and 3) and frontal regions (i.e., left superior frontal sulci 4 and 5, middle frontal gyrus 5, right superior frontal sulci 4 and 5, middle frontal gyrus 3, medial frontal superior 2) in RLS patients compared to HCs. Lower CC was present in the left central sulcus in RLS patients compared to HCs. There were no significant differences of CC in subgroups compared to HCs.
Figure 3.
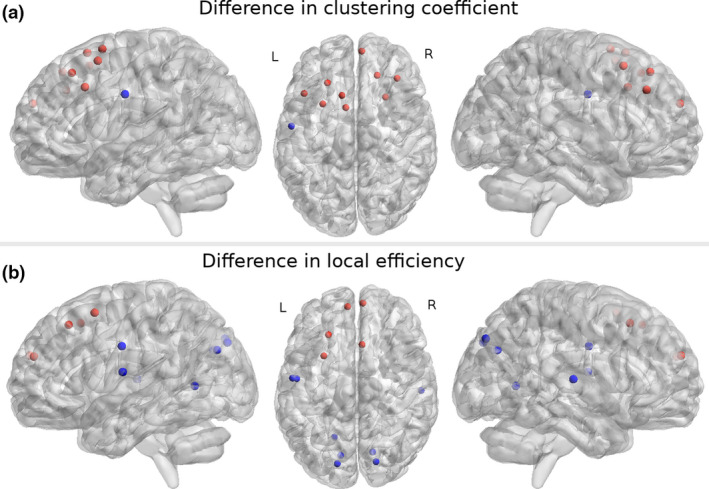
Significant (p < 0.05, two‐sample t test, false discovery rate) differences in graph measures in restless legs syndrome patients compared to healthy controls. Regions where significant differences were found in (A) clustering coefficient and (B) local efficiency. Red nodes represent regions where graph measures were significantly (p < 0.05) higher and blue nodes where measures were significantly lower in RLS patients compared to healthy controls. Left sagittal, axial, and right sagittal views are shown. The results were calculated on averaged nodal measures with graph densities between 0.1 and 0.5. Nodes present atlas regions from An Atlas of Intrinsic Connectivity of Homotopic Areas and Automated Anatomical Labeling that are presented in their centroid stereotaxic coordinates (Montreal Neurological Institute). L, left; R, right. [Colour figure can be viewed at wileyonlinelibrary.com]
In RLS patients, local efficiency (Fig. 3B, Table S4) was significantly higher (p < 0.05, FDR) in frontal (i.e., left superior frontal sulci 4 and 5, left superior frontal medial gyrus 2, right medial frontal superior 2) and motor (i.e., right supplementary motor region 1) regions compared to HCs. In turn, local efficiency was lower in Rolandic operculum (i.e., central opercular cortex), temporal, parieto‐occipital, cuneus, and occipital regions in RLS patients compared to HCs. No significant differences of local efficiency were evident in subgroups compared to HCs. None of the nodal differences correlated with clinical scores, disease duration, or LEDD values.
There was no significant difference of whole brain global efficiency in patients (0.2967 ± 0.0576) compared to HCs (0.2969 ± 0.0557).
Discussion
In this case–control rs‐fMRI study, we applied brain network analysis (i.e., ICA and graph methods) to investigate functional topology and communication in a large cohort of untreated and medicated RLS patients and individually matched HCs. Thus, age and gender as confounding factors were excluded. Our results showed significantly higher intranetwork connectivity localized to frontal regions in the salience and left executive networks and in the cerebellar network, as well as lower fronto‐cerebellar connectivity in RLS patients compared to HCs. Interestingly, in the cohort of RLS patients on dopaminergic medication, lower cerebello‐parietal connectivity, previously identified in untreated patients compared to HCs, was not evident. Instead, they presented with higher connectivity between the thalamus and the subcallosal gyrus compared to HCs, and between thalamus and the orbital sulcus compared to untreated patients.
Fronto‐cerebellar connectivity
We found higher connectivity within the salience network, as well as higher CC (i.e., functional segregation) and higher local efficiency values (i.e., ability to transmit information at the local level) within the frontal regions. Similarly, a seed‐based study in RLS patients found connectivity increases in the frontal regions of the salience network [11], which was suggested to serve as the functional substrate of altered attentional control of sensory inputs. The salience network comprises the anterior insula and anterior cingulate cortex, and is involved in the processing of external stimuli and the assignment of attentional resources such as the engagement of frontoparietal systems for working memory and higher‐order cognitive control [34].
We identified higher connectivity within the left executive network, which is located within the dorsolateral prefrontal and posterior parietal cortex [34], and is associated with control of attention and working memory, and higher cognitive functions such as planning and decision making in the context of goal‐directed behavior [35].
We found significantly higher intranetwork connectivity in the cerebellum and lower cerebello‐frontal connectivity (between vermis 7 and inferior frontal sulci) in RLS patients compared to HCs. Both salience and executive networks comprise regions that involve the inferior frontal sulcus, which has a role in response inhibitory control [36]. Therefore, this region could represent the functional substrate for the reported reflection impulsivity and perceptual decision making in RLS patients, which is disease‐related regardless of dopaminergic treatment [37].
In this regard, the higher intranetwork connectivity in the cerebellum, salience, and left executive networks might result from compensatory upregulation for the lower communication between these regions. Similar disconnection between cerebello‐frontal regions, as noted in our study, exists in attention deficit hyperactivity disorder (ADHD) [38]. RLS is common in children and adults with ADHD (44% and 35%, respectively), and 27% of RLS patients present with ADHD symptoms [39]. This is not surprising, as iron deficiency and dopamine dysfunction are common in both conditions. A diffusion tensor imaging study that comprised 94% of the participants of this study detected lower fractional anisotropy values in the anterior part of the internal capsule bilaterally [40]. This region comprises the frontopontine tract, which carries neuronal inputs from the frontal (and parietal) cortex to the cerebellum through the thalamus. Therefore, this finding supports the noted lower fronto‐cerebellar connectivity [41] and suggests communication alterations through the thalamus in RLS.
Motor networks
In addition, we found higher CC and local efficiency in supplementary motor regions, lower CC in the left central sulcus, and lower local efficiency in the Rolandic operculum in RLS patients compared to HCs. Alterations in motor regions could relate to the urge to move in RLS patients. Connectivity changes in the SMN were hypothesized in a study that reported fractional anisotropy reductions in proximity to primary and associate motor and somatosensory cortices [42]. In addition, a possible explanation for our results could be the involvement of cerebellar and motor regions in the generation of periodic limb movements (PLMs) [5, 6].
Cerebello‐parietal connectivity
In line with the noted cerebello‐frontal communication changes through the frontopontine tract, untreated RLS patients had lower cerebello‐parietal connectivity (between vermis 7 and 8, and the intraparietal sulcus) compared to HCs. Intraparietal sulci are functionally part of the left and right executive networks, which comprise parts of the neocerebellum. This, in turn, includes vermis 6 and 7 as well as corresponding crus I and II [43]. Functions of the parietal lobe include the integration of proprioceptive and mechanoreceptive information in the somatosensory cortex [14]. Therefore, lower cerebello‐parietal connectivity in untreated RLS patients might reflect an altered processing of sensory information, which may contribute to the generation of RLS symptoms. In addition, decreased local efficiency in temporal, parieto‐occipital, cuneus, and occipital regions in RLS patients compared to HCs suggests alterations in local information transfer. Interestingly, as such change was not found in the medicated patients, these results suggest a possible modulating effect of medications (both dopaminergic and alpha‐2‐delta ligands).
Thalamic connectivity
Graph analysis revealed higher thalamo‐frontal connectivity (between the thalamus and subcallosal gyrus and orbital sulcus) in RLS patients on dopaminergic medication compared to HCs and untreated patients. The thalamus is an important relay station that provides sensory information from subcortical structures to the cortex [44]. Previous RLS studies reported reduced iron content [45], metabolic changes [46, 47], and a higher (11C)FLB‐457‐binding potential to D2‐receptors [48] of the thalamus in RLS patients. In addition, task‐based fMRI revealed thalamic signal changes [5, 6, 7] that were associated with sensory leg discomfort [5] Furthermore, rs‐fMRI showed that thalamic connectivity was reduced in untreated RLS patients [15], was higher in patients on combined medication [11] and was altered by dopamine agonist treatment [16]. In this context, reduced thalamic connectivity was suggested to be the functional substrate of deficits in control and management of sensory information [15]. An increase following dopamine intake has been suggested as a compensation for otherwise insufficient mesocortical connectivity [13]. In our study, the connectivity changes in the anterior nuclei of the thalamus could be related to its function to receive inputs from the cerebellum and basal ganglia (i.e., globus pallidus and substantia nigra) that project through the internal capsule (thalamic radiation) to the cortex (i.e., motor cortices) [49]. These findings support the hypothesis that the thalamus plays a role in the processing and perception of sensory symptoms of RLS [10, 15, 46].
In untreated RLS patients, lower connectivity between the thalamus and the orbito‐frontal and subcallosal gyrus was previously proposed as a sign for weaker classification and regulation of processing the inner‐generated stimuli [12]. Another study suggested the cortico‐striatal‐thalamic‐cortical loop as the site of dysfunction in RLS [50]. Our results of higher thalamo‐frontal connectivity in RLS patients on dopaminergic medication suggest that treatment normalizes the communication between these regions.
Furthermore, untreated patients showed higher connectivity within the working memory network compared to patients on dopaminergic medication. However, the located region (i.e., cerebellum) was outside the main components of this network, and therefore the result might be trivial. Nevertheless, a previous study indicated the role of dopamine in working memory–related tasks [51] that could explain the noted differences.
Default mode network
Both patients and HCs showed high DC and BC values in regions that correspond to known brain hubs (e.g., occipital, insula, temporal, precuneus, cingulate, parahippocampal) [52, 53]. Although a previous study found DC differences in DMN regions, in drug‐naïve RLS patients [18] no significant differences in DC and BC were evident between any of the groups in our cohort. Differences in symptom severity might explain this disagreement, as our RLS cohort presented moderate in contrast to the severe RLS symptoms in the previous study [18]. DMN reflects a network that is active during rest. In a previous RLS study, connectivity between the DMN and thalamus was increased in the morning (between 9 a.m. and 12 p.m.) and reduced in the evening (between 6 p.m. and 8 p.m.) [14]. They suggested that RLS is a disorder of the disturbed sensory activation threshold associated with the dysfunction of the thalamic control mechanisms. Although we found alterations of thalamic connectivity, the wide range of scan times (between 8 a.m. and 4 p.m.) might have suppressed DMN changes in our cohort.
Medication effects across networks
Our study revealed medication‐dependent connectivity alterations in a broad array of networks. In particular, lower cerebello‐parietal connectivity was present in untreated RLS, but not in medicated patients, independently of type of drug treatment. RLS patients treated solely with dopaminergic medication presented with higher thalamo‐frontal connectivity compared to untreated patients. This is in line with the preponderance of medication‐induced connectivity changes of the mesocortical pathway [13]. Reported dopaminergic‐related connectivity alterations in RLS patients fit the pathophysiological concept of the altered pre‐ (hyper‐) and postsynaptic (hypo‐) dopaminergic system [4], which therefore might represent a compensatory treatment effect.
Limitations
As the rs‐scans were acquired as part of a larger protocol, the duration of the sequence was limited due to time concerns. In addition, we cannot entirely rule out whether sensory discomfort occurred during fMRI, and therefore it might have influenced the connectivity. To minimize this effect, scans were performed during the asymptomatic period during daytime. Therefore, further studies are required to investigate whether the noted thalamic differences are stronger at evening.
Conclusions
First, rs‐fMRI identified higher connectivity in three networks (i.e., salience, left executive, and cerebellar networks) in the entire RLS cohort compared to HCs. These changes were discussed with their related functions such as attention, cognition, response inhibitory control, generation of PLMs, processing of sensory information, and their relation to known disease characteristic symptoms. Second, we found lower cerebello‐frontal connectivity in RLS patients and cerebello‐parietal connectivity in untreated patients compared to HCs. As these changes were not noted in patients on dopaminergic medication, this suggests a treatment effect that normalized the altered processing of sensory information. Third, patients on dopaminergic medication had higher thalamo‐frontal connectivity compared to HCs and untreated patients, which was discussed in light of a medication‐induced effect on the thalamus that might mitigate the occurrence of RLS symptoms. Network analysis revealed drug treatment–dependent connectivity alterations in RLS. This supports the application of rs‐fMRI as a complementary tool to investigate the impact of existing and novel treatments in RLS.
Disclosure of conflicts of interest
N.T. reports no competing interests. She received personal funding from the Finnish Parkinson Foundation and Finnish Foundation for Cardiovascular Research outside the context of the submitted work. A.S. reports no competing interests. T.M. reports no disclosures related to this study. He received speakers honorary by UCB Pharma and AOP Orphan outside the context of the submitted work. A.H. reports no competing interests. B.F. reports no disclosures related to this study. She receives salary support from the Fonds de la Recherche du Québec (Boursier‐Clinicien junior 2) outside the context of the submitted work. She received honoraria/speaker engagements from UCB Pharma and Eisai outside the context of the submitted work. E.R.G. reports no competing interests. W.P. reports no competing interests. B.H. reports no disclosures related to this study. She received speaker honoraria from Novartis, AoP Orphan, and Inspire, and honoraria as a consultant from Axovant, Benevolent Bio, Jazz, Roche, Takeda, and ONO Pharma. C.S. reports no competing interests.
Supporting information
Figure S1. Twelve identified resting‐state networks in the entire study cohort.
Figure S2. Regions with the highest degree centrality (DC) in healthy controls and restless legs syndrome patients.
Figure S3. Regions with highest betweenness centrality in healthy controls and restless legs syndrome patients.
Figure S4. Regions with highest clustering coefficient in healthy controls and restless legs syndrome patients.
Figure S5. Regions with highest local efficiency in healthy controls and restless legs syndrome patients.
Table S1. Clusters of significantly higher (p < 0.05, 100,000 permutations, t test, false discovery rate ) intranetwork connectivity in resting‐state networks in restless legs syndrome patients compared to healthy controls.
Table S2. Clusters of significantly higher (p < 0.05, 100,000 permutations, t test, false discovery rate) intranetwork connectivity in resting‐state networks in untreated patients compared to patients on dopaminergic medication.
Table S3. Significant differences (p < 0.05, two‐sample t test, false discovery rate) in clustering coefficient in restless legs syndrome patients compared to healthy controls.
Table S4. Significant differences (p < 0.05, two=sample t test, false discovery rate ) in local efficiency in restless legs syndrome patients compared to healthy controls.
Acknowledgments
This study was supported by a grant from the Translational Research Fund of the government of Tirol, Austria to Dr. Birgit Högl.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Allen RP, Picchietti D, Hening WA, et al Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the national institutes of health. Sleep Med 2003; 4: 101–119. [DOI] [PubMed] [Google Scholar]
- 2. Schormair B, Zhao C, Bell S, et al Identification of novel risk loci for restless legs syndrome in genome‐wide association studies in individuals of European ancestry: a meta‐analysis. Lancet Neurol 2017; 16: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen RP. Restless leg syndrome/Willis‐Ekbom disease pathophysiology. Sleep Med Clin 2015; 10: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Earley CJ, Uhl GR, Clemens S, Ferre S. Connectome and molecular pharmacological differences in the dopaminergic system in restless legs syndrome (RLS): plastic changes and neuroadaptation that may contribute to augmentation. Sleep Med 2017; 31: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bucher SF, Seelos KC, Oertel WH, Reiser M, Trenkwalder C. Cerebral generators involved in the pathogenesis of the restless legs syndrome. Ann Neurol 1997; 41: 639–645. [DOI] [PubMed] [Google Scholar]
- 6. Margariti PN, Astrakas LG, Tsouli SG, Hadjigeorgiou GM, Konitsiotis S, Argyropoulou MI. Investigation of unmedicated early onset restless legs syndrome by voxel‐based morphometry, T2 relaxometry, and functional MR imaging during the night‐time hours. AJNR Am J Neuroradiol 2012; 33: 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Astrakas LG, Konitsiotis S, Margariti P, Tsouli S, Tzarouhi L, Argyropoulou MI. T2 relaxometry and fMRI of the brain in late‐onset restless legs syndrome. Neurology 2008; 71: 911–916. [DOI] [PubMed] [Google Scholar]
- 8. Spiegelhalder K, Feige B, Paul D, et al Cerebral correlates of muscle tone fluctuations in restless legs syndrome: a pilot study with combined functional magnetic resonance imaging and anterior tibial muscle electromyography. Sleep Med 2008; 9: 177–183. [DOI] [PubMed] [Google Scholar]
- 9. Van Dijk KR, Hedden A, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 2010; 103: 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rizzo G, Li X, Galantucci S, Filippi M, Cho YW. Brain imaging and networks in restless legs syndrome. Sleep Med 2017; 31: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gorges M, Rosskopf J, Müller HP, Lindemann K, Hornyak M, Kassubek J. Patterns of increased intrinsic functional connectivity in patients with restless legs syndrome are associated with attentional control of sensory inputs. Neurosci Lett 2016; 617: 264–269. [DOI] [PubMed] [Google Scholar]
- 12. Ku J, Lee YS, Chang H, Earley CJ, Allen RP, Cho YW. Default mode network disturbances in restless legs syndrome/Willis‐Ekbom disease. Sleep Med 2016; 23: 6–11. [DOI] [PubMed] [Google Scholar]
- 13. Kocar TD, Müller HP, Kassubek J. Differential functional connectivity in thalamic and dopaminergic pathways in restless legs syndrome: a meta‐analysis. Ther Adv Neurol Disord 2020; 13 10.1177/1756286420941670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ku J, Lee YS, Chang HW, Earley CJ, Allen RP, Cho YW. Diurnal variation of default mode network in patients with restless legs syndrome. Sleep Med 2018; 41: 1–8. [DOI] [PubMed] [Google Scholar]
- 15. Ku J, Cho YW, Lee YS, et al Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: a resting‐state connectivity study using functional magnetic resonance imaging. Sleep Med 2014; 15: 289–294. [DOI] [PubMed] [Google Scholar]
- 16. Lee YS, Ku J, Kim KT, et al Resting‐state connectivity and the effects of treatment in restless legs syndrome. Sleep Med 2020; 67: 33–38. [DOI] [PubMed] [Google Scholar]
- 17. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage 2010; 52: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 18. Liu C, Wang J, Hou Y, et al Mapping the changed hubs and corresponding functional connectivity in idiopathic restless legs syndrome. Sleep Med 2018; 45: 132–139. [DOI] [PubMed] [Google Scholar]
- 19. Allen RP, Picchietti DL, Garcia‐Borreguero D, et al Restless legs syndrome/Willis‐Ekbom disease diagnostic criteria: updated international restless legs syndrome study group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med 2014; 15: 860–873. [DOI] [PubMed] [Google Scholar]
- 20. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987; 149: 351–356. [DOI] [PubMed] [Google Scholar]
- 21. Walters AS, LeBrocq C, Dhar A, et al Validation of the international restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med 2003; 4: 121–132. [DOI] [PubMed] [Google Scholar]
- 22. Guy W . Clinical Global Impression (CGI). ECDEU assessment manual for psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare, 1976. [Google Scholar]
- 23. Kohnen R, Martinez‐Martin P, Benes H, et al Rating of daytime and nighttime symptoms in RLS: validation of the RLS‐6 scale of restless legs syndrome/Willis‐Ekbom disease. Sleep Med 2016; 20: 116–122. [DOI] [PubMed] [Google Scholar]
- 24. MATLAB and Statistics Toolbox Release R2017a, The MathWorks Inc, Natick, Massachusetts, United States.
- 25. Nickerson LD, Smith SM, Öngür D, Beckmann CF. Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front Neurosci. 2017; 11: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joliot M, Jobard G, Naveau M, et al AICHA: an atlas of intrinsic connectivity of homotopic areas. J Neurosci Methods 2015; 254: 46–59. [DOI] [PubMed] [Google Scholar]
- 27. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 2002; 15(1): 273–289. [DOI] [PubMed] [Google Scholar]
- 28. Zalesky A, Fornito A, Bullmore ET. Network‐based statistic: identifying differences in brain networks. NeuroImage 2010; 53: 1197–1207. [DOI] [PubMed] [Google Scholar]
- 29. Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012; 13: 336–349. [DOI] [PubMed] [Google Scholar]
- 30. Watts DJ, Strogatz SH. Collective dynamics of 'small‐world' networks. Nature 1998; 393: 440–442. [DOI] [PubMed] [Google Scholar]
- 31. Latora V, Marchiori M. Efficient behavior of small‐world networks. Phys Rev Lett 2001; 87: 198701. [DOI] [PubMed] [Google Scholar]
- 32. Xia M, Wang J, He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One 2013; 8: e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jech R, Mueller K, Schroeter ML, Růžička E. Levodopa increases functional connectivity in the cerebellum and brainstem in Parkinson's disease. Brain 2013; 136: e234. [DOI] [PubMed] [Google Scholar]
- 34. Menon V. Large‐scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011; 15: 483–506. [DOI] [PubMed] [Google Scholar]
- 35. Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci 2007; 11: 229–235. [DOI] [PubMed] [Google Scholar]
- 36. Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage 2003; 20: 351–358. [DOI] [PubMed] [Google Scholar]
- 37. Heim B, Pertl MT, Stefani A, et al Reflection impulsivity perceptual decision‐making in patients with restless legs syndrome. Ann Clin Transl Neurol 2018; 5: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolf RC, Plichta MM, Sambataro F, et al Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention‐deficit/hyperactivity disorder. Hum Brain Mapp 2009; 30: 2252–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bjorvatn B, Brevik EJ, Lundervold AJ, et al Adults with attention deficit hyperactivity disorder report high symptom levels of troubled sleep, restless legs, and cataplexy. Front Psychol 2017; 8: 1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stefani A, Mitterling T, Heidbreder A, et al Multimodal MRI reveals alterations of sensorimotor circuits in restless legs syndrome. Sleep 2019; 42: zsz171. [DOI] [PubMed] [Google Scholar]
- 41. Haines DE, Mihailoff GA. Fundamental neuroscience for basic and clinical applications, 5th edn Amsterdam, The Netherlands: Elsevier, 2018. [Google Scholar]
- 42. Unrath A, Müller HP, Ludolph AC, Riecker A, Kassubek J. Cerebral white matter alterations in idiopathic restless legs syndrome measured by diffusion tensor imaging. Mov Disord 2008; 23: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 43. Habas C, Kamdar N, Nguyen D, et al Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009; 29: 8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hwang K, Bertolero MA, Liu WB, D'Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci 2017; 37: 5594–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Connor JR, Patton SM, Oexle K, Allen RP. Iron and restless legs syndrome: treatment, genetics and pathophysiology. Sleep Med 2017; 31: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Allen RP, Barker PB, Horska A, Earley CJ. Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology 2013; 80: 2028–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rizzo G, Tonon C, Testa C, et al Abnormal medial thalamic metabolism in patients with idiopathic restless legs syndrome. Brain 2012; 135: 3712–3720. [DOI] [PubMed] [Google Scholar]
- 48. Cervenka S, Palhagen SE, Comley RA, et al Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2‐receptor binding. Brain 2006; 129: 2017–2028. [DOI] [PubMed] [Google Scholar]
- 49. Mitchell AS, Sherman SM, Sommer MA, Mair RG, Vertes RP, Chudasama Y. Advances in understanding mechanisms of thalamic relays in cognition and behavior. J Neurosci. 2014; 34: 15340–15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhuo Y, Wu Y, Xu Y, et al Combined resting state functional magnetic resonance imaging and diffusion tensor imaging study in patients with idiopathic restless legs syndrome. Sleep Med 2017; 38: 96–103. [DOI] [PubMed] [Google Scholar]
- 51. D'Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci USA 2012; 109: 19900–19909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci 2013; 17: 683–696. [DOI] [PubMed] [Google Scholar]
- 53. Zuo XN, Ehmke R, Mennes M, et al Network centrality in the human functional connectome. Cereb Cortex 2012; 22: 1862–1875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Twelve identified resting‐state networks in the entire study cohort.
Figure S2. Regions with the highest degree centrality (DC) in healthy controls and restless legs syndrome patients.
Figure S3. Regions with highest betweenness centrality in healthy controls and restless legs syndrome patients.
Figure S4. Regions with highest clustering coefficient in healthy controls and restless legs syndrome patients.
Figure S5. Regions with highest local efficiency in healthy controls and restless legs syndrome patients.
Table S1. Clusters of significantly higher (p < 0.05, 100,000 permutations, t test, false discovery rate ) intranetwork connectivity in resting‐state networks in restless legs syndrome patients compared to healthy controls.
Table S2. Clusters of significantly higher (p < 0.05, 100,000 permutations, t test, false discovery rate) intranetwork connectivity in resting‐state networks in untreated patients compared to patients on dopaminergic medication.
Table S3. Significant differences (p < 0.05, two‐sample t test, false discovery rate) in clustering coefficient in restless legs syndrome patients compared to healthy controls.
Table S4. Significant differences (p < 0.05, two=sample t test, false discovery rate ) in local efficiency in restless legs syndrome patients compared to healthy controls.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


